Diagnostic Accuracy of [68Ga]Ga Labeled Fibroblast-Activation Protein Inhibitors in Detecting Head and Neck Cancer Lesions Using Positron Emission Tomography: A Systematic Review and a Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol
2.2. Strategy for Literature Research and Information Sources
2.3. Eligibility Criteria
2.4. Selection Method
2.5. Process of Data Collection and Data Extraction
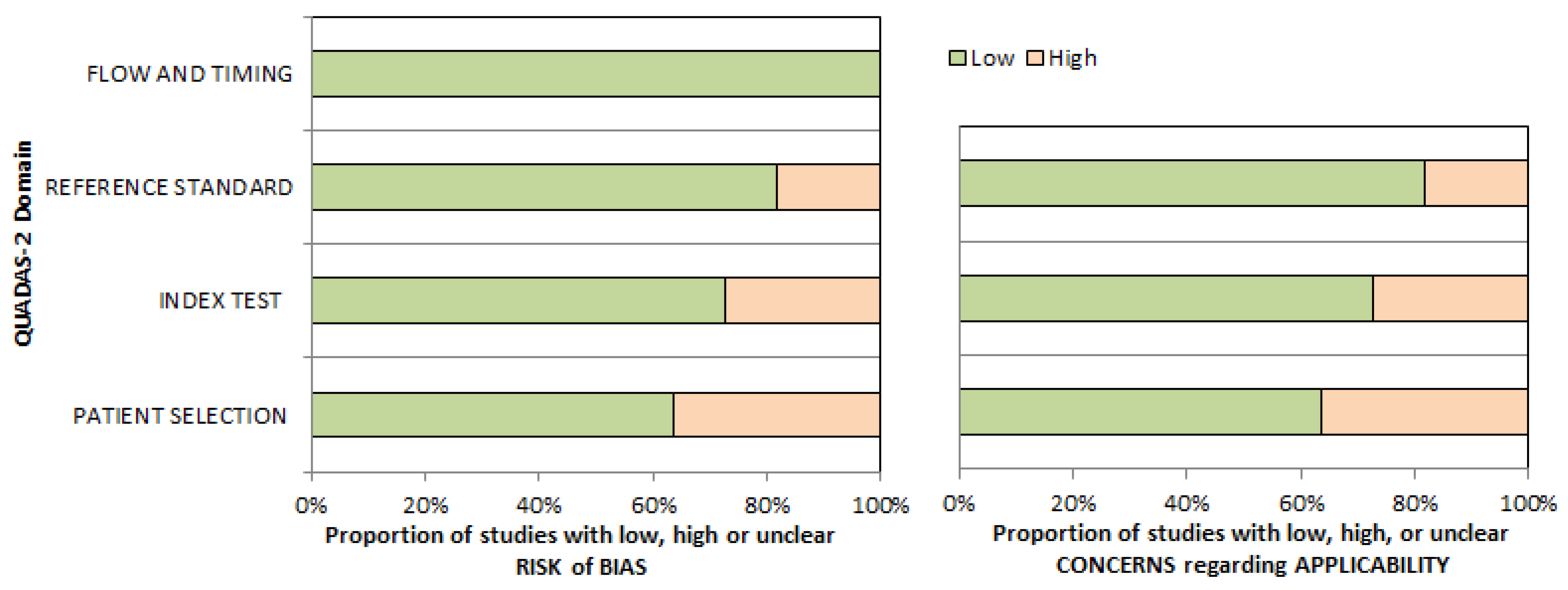
2.6. Quality Assessment (Risk of Bias Assessment)
2.7. Effects Metrics
2.8. Statistical Analysis
2.9. Additional Analyses
3. Results
3.1. Literature Search and Study Selection
3.2. Study Characteristics
3.3. Risk of Bias and Applicability
3.4. Results of Individual Studies (Qualitative Synthesis)
3.5. Meta-Analysis (Quantitative Synthesis)
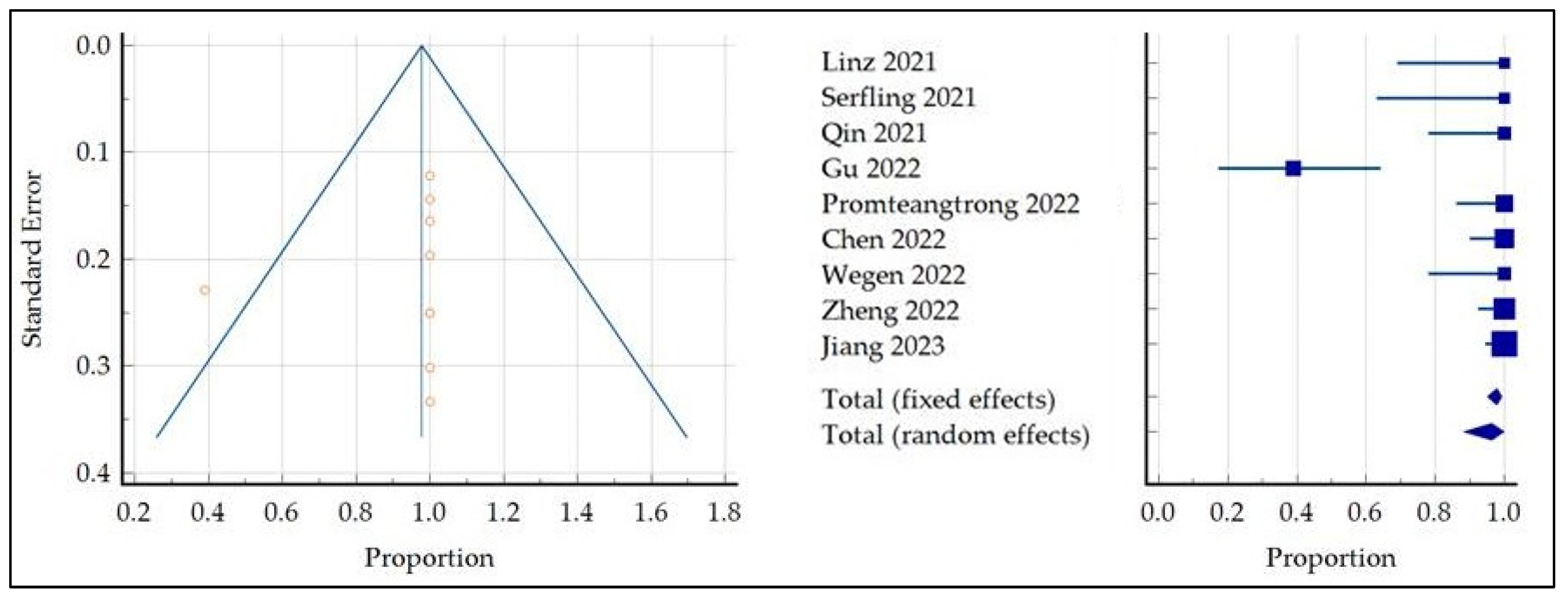
3.5.1. Detection Rate of Primary Tumor
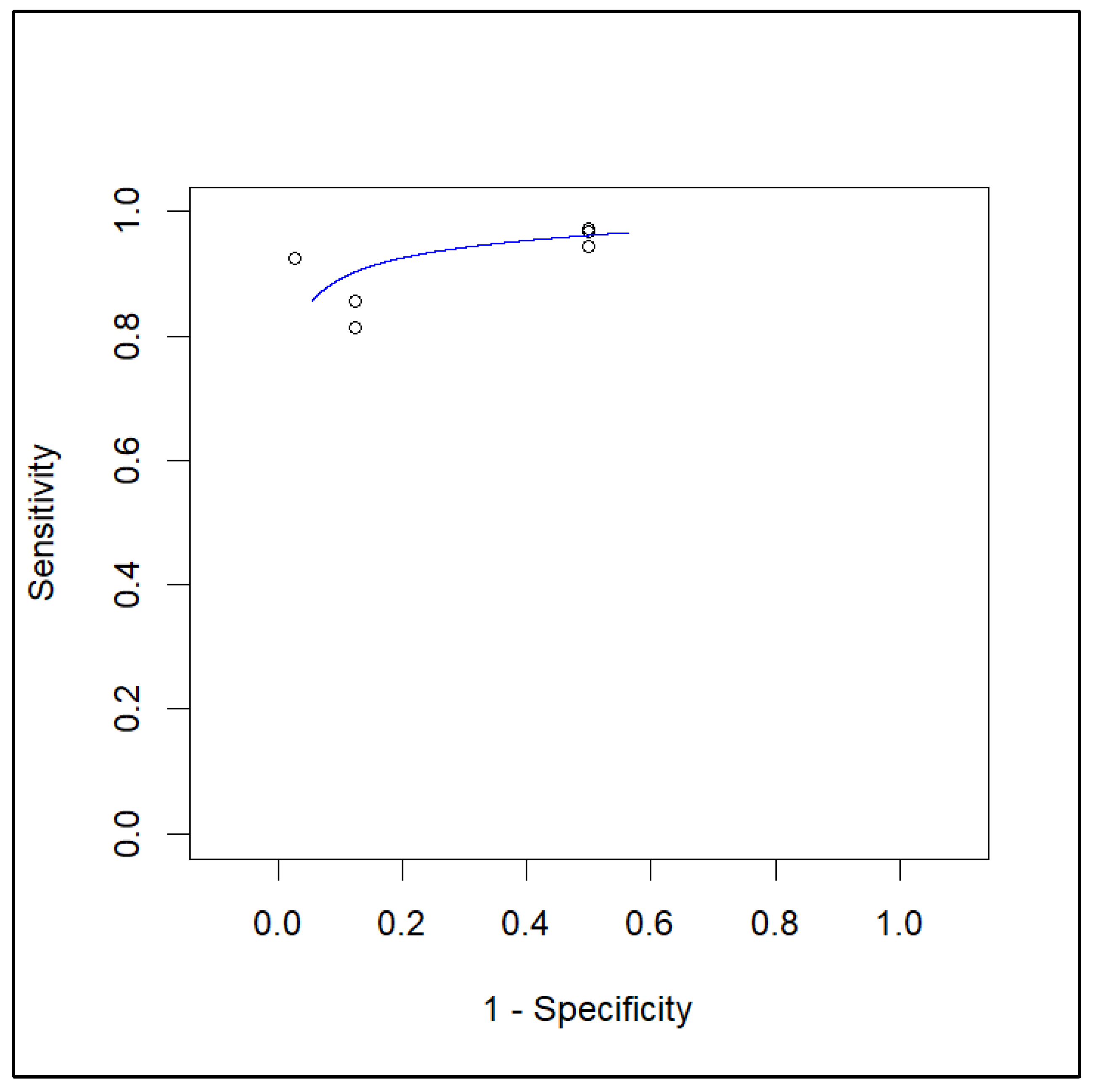
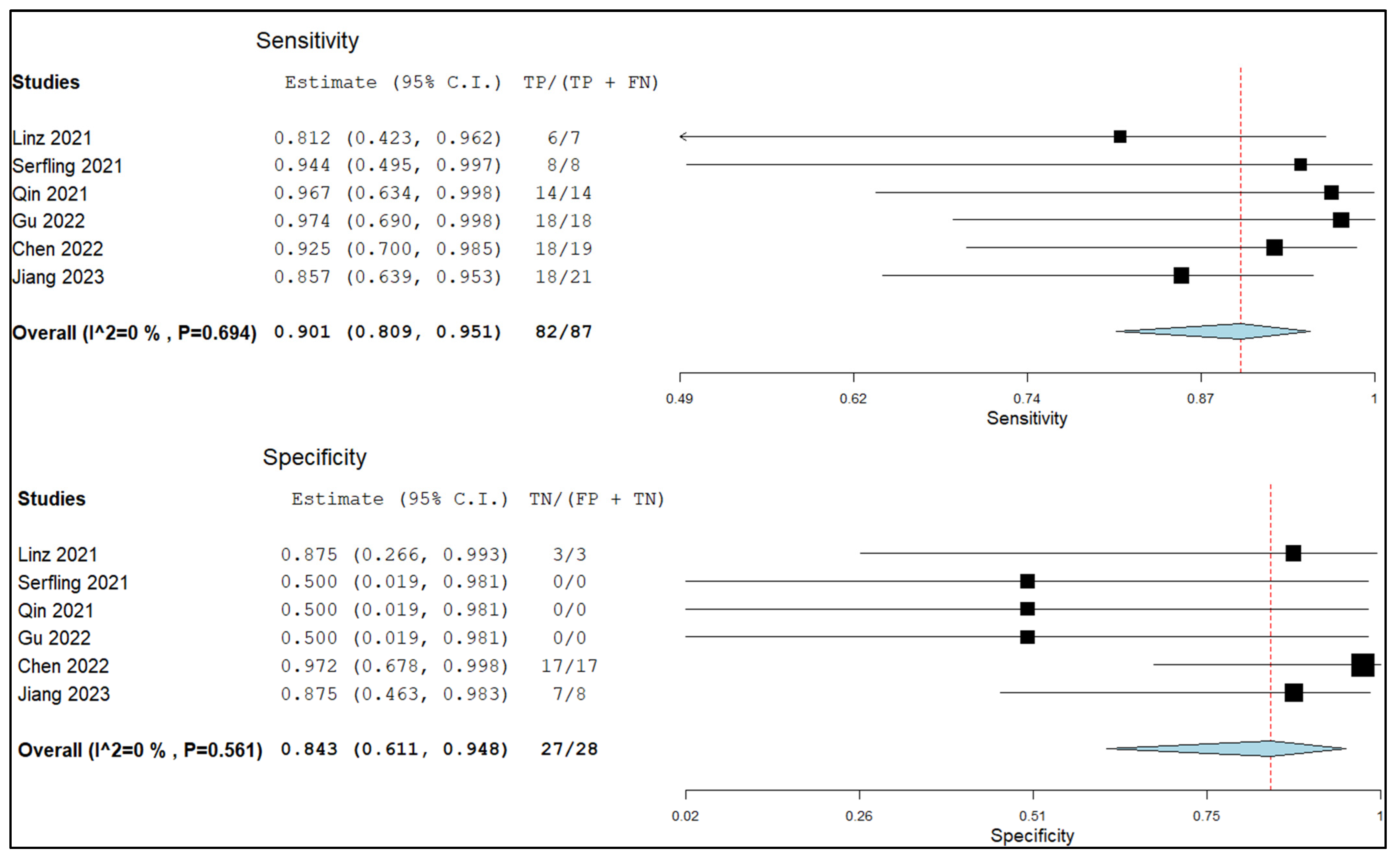
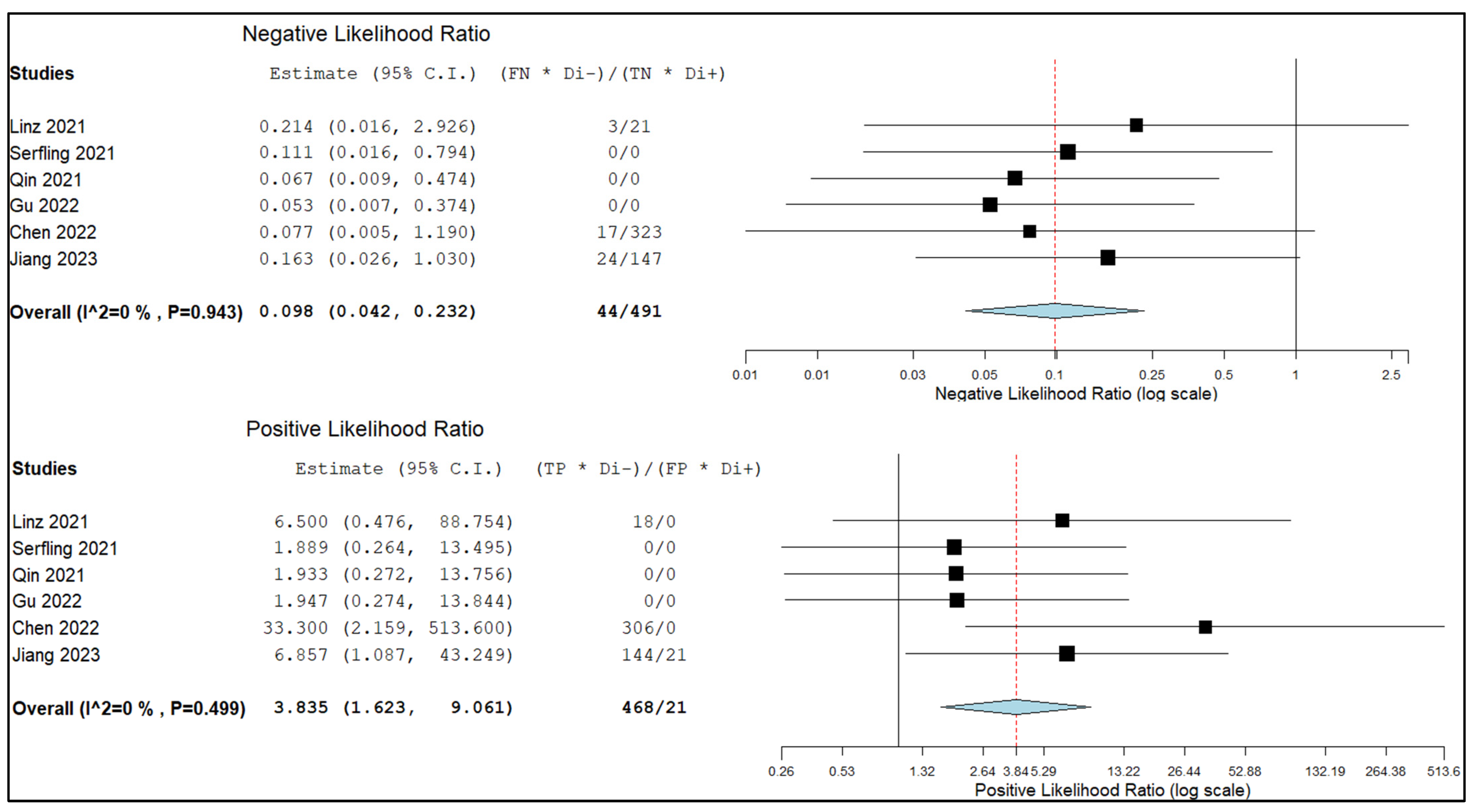
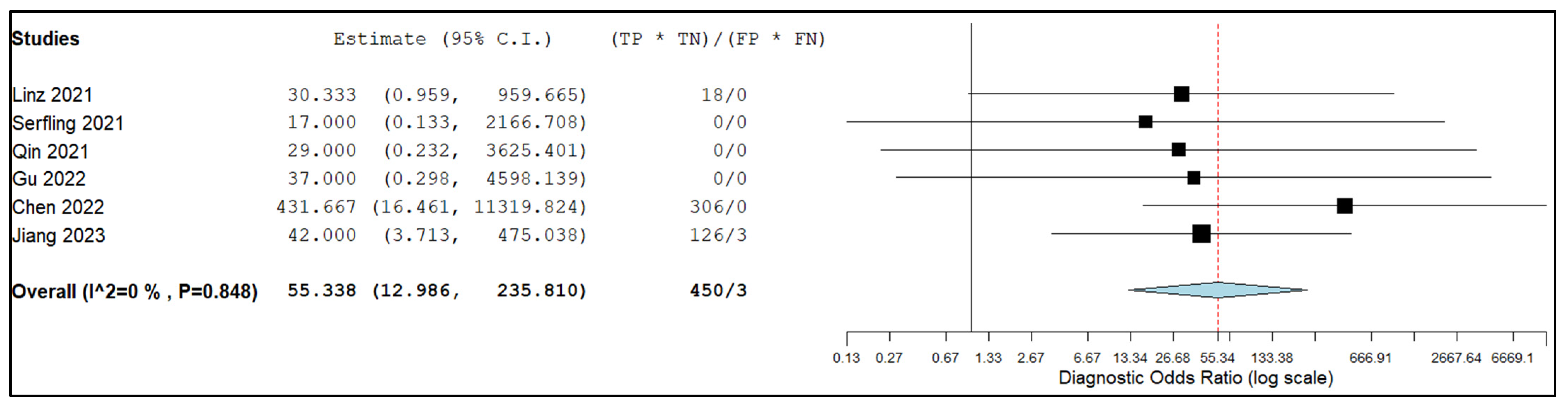
3.5.2. Sensitivity and Specificity in Lymph Node Metastasis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef]
- Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries–Bray–2018–CA: A Cancer Journal for Clinicians—Wiley Online Library. Available online: https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21492 (accessed on 31 October 2023).
- Galizia, D.; Minei, S.; Maldi, E.; Chilà, G.; Polidori, A.; Merlano, M.C. How Risk Factors Affect Head and Neck Squamous Cell Carcinoma (HNSCC) Tumor Immune Microenvironment (TIME): Their Influence on Immune Escape Mechanisms and Immunotherapy Strategy. Biomedicines 2022, 10, 2498. [Google Scholar] [CrossRef] [PubMed]
- Junn, J.C.; Soderlund, K.A.; Glastonbury, C.M. Imaging of Head and Neck Cancer with CT, MRI, and US. Semin. Nucl. Med. 2021, 51, 3–12. [Google Scholar] [CrossRef]
- Sheikhbahaei, S.; Marcus, C.; Subramaniam, R.M. 18F FDG PET/CT and Head and Neck Cancer: Patient Management and Outcomes. PET Clin. 2015, 10, 125–145. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.; Shantha, S.; Muralitharan, S.; Sudhakar, U.; Thamizhchelvan, H.; Parvathi, V.D. Role of Tissue Markers Associated with Tumor Microenvironment in the Progression and Immune Suppression of Oral Squamous Cell Carcinoma. Med. Oncol. 2023, 40, 303. [Google Scholar] [CrossRef] [PubMed]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A Framework for Advancing Our Understanding of Cancer-Associated Fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef]
- Chen, Y.; McAndrews, K.M.; Kalluri, R. Clinical and Therapeutic Relevance of Cancer-Associated Fibroblasts. Nat. Rev. Clin. Oncol. 2021, 18, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Treglia, G.; Albano, D. FAPI PET/CT in Infectious, Inflammatory, and Rheumatological Diseases: “Watch It like a Hawk” or “One Swallow Does Not Make a Summer”? Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1848–1850. [Google Scholar] [CrossRef]
- Gilardi, L.; Airò Farulla, L.S.; Demirci, E.; Clerici, I.; Omodeo Salè, E.; Ceci, F. Imaging Cancer-Associated Fibroblasts (CAFs) with FAPi PET. Biomedicines 2022, 10, 523. [Google Scholar] [CrossRef]
- Röhrich, M. Fibroblast Activation Protein Inhibitor PET Imaging in Head and Neck Cancer. PET Clin. 2023, 18, 315–323. [Google Scholar] [CrossRef]
- Syed, M.; Flechsig, P.; Liermann, J.; Windisch, P.; Staudinger, F.; Akbaba, S.; Koerber, S.A.; Freudlsperger, C.; Plinkert, P.K.; Debus, J.; et al. Fibroblast Activation Protein Inhibitor (FAPI) PET for Diagnostics and Advanced Targeted Radiotherapy in Head and Neck Cancers. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2836–2845. [Google Scholar] [CrossRef] [PubMed]
- Linz, C.; Brands, R.C.; Kertels, O.; Dierks, A.; Brumberg, J.; Gerhard-Hartmann, E.; Hartmann, S.; Schirbel, A.; Serfling, S.; Zhi, Y.; et al. Targeting Fibroblast Activation Protein in Newly Diagnosed Squamous Cell Carcinoma of the Oral Cavity—Initial Experience and Comparison to [18F]FDG PET/CT and MRI. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3951–3960. [Google Scholar] [CrossRef] [PubMed]
- Serfling, S.; Zhi, Y.; Schirbel, A.; Lindner, T.; Meyer, T.; Gerhard-Hartmann, E.; Lapa, C.; Hagen, R.; Hackenberg, S.; Buck, A.K.; et al. Improved Cancer Detection in Waldeyer’s Tonsillar Ring by 68Ga-FAPI PET/CT Imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1178–1187. [Google Scholar] [CrossRef]
- Röhrich, M.; Syed, M.; Liew, D.P.; Giesel, F.L.; Liermann, J.; Choyke, P.L.; Wefers, A.K.; Ritz, T.; Szymbara, M.; Schillings, L.; et al. 68Ga-FAPI-PET/CT Improves Diagnostic Staging and Radiotherapy Planning of Adenoid Cystic Carcinomas—Imaging Analysis and Histological Validation. Radiother. Oncol. 2021, 160, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Liu, F.; Huang, J.; Ruan, W.; Liu, Q.; Gai, Y.; Hu, F.; Jiang, D.; Hu, Y.; Yang, K.; et al. A Head-to-Head Comparison of 68Ga-DOTA-FAPI-04 and 18F-FDG PET/MR in Patients with Nasopharyngeal Carcinoma: A Prospective Study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3228–3237. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Xu, X.; Zhang, J.; Ou, X.; Xia, Z.; Guan, Q.; Hu, S.; Yang, Z.; Song, S. The Added Value of 68Ga-FAPI PET/CT in Patients with Head and Neck Cancer of Unknown Primary with 18F-FDG-Negative Findings. J. Nucl. Med. 2022, 63, 875–881. [Google Scholar] [CrossRef]
- Promteangtrong, C.; Siripongsatian, D.; Jantarato, A.; Kunawudhi, A.; Kiatkittikul, P.; Yaset, S.; Boonkawin, N.; Chotipanich, C. Head-to-Head Comparison of 68Ga-FAPI-46 and 18F-FDG PET/CT for Evaluation of Head and Neck Squamous Cell Carcinoma: A Single-Center Exploratory Study. J. Nucl. Med. 2022, 63, 1155–1161. [Google Scholar] [CrossRef]
- Chen, S.; Chen, Z.; Zou, G.; Zheng, S.; Zheng, K.; Zhang, J.; Huang, C.; Yao, S.; Miao, W. Accurate Preoperative Staging with [68Ga]Ga-FAPI PET/CT for Patients with Oral Squamous Cell Carcinoma: A Comparison to 2-[18F]FDG PET/CT. Eur. Radiol. 2022, 32, 6070–6079. [Google Scholar] [CrossRef]
- Wegen, S.; van Heek, L.; Linde, P.; Claus, K.; Akuamoa-Boateng, D.; Baues, C.; Sharma, S.J.; Schomäcker, K.; Fischer, T.; Roth, K.S.; et al. Head-to-Head Comparison of [68 Ga]Ga-FAPI-46-PET/CT and [18F]F-FDG-PET/CT for Radiotherapy Planning in Head and Neck Cancer. Mol. Imaging Biol. 2022, 24, 986–994. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, F.; Lin, K.; Zhang, L.; Huang, N.; Zheng, W.; Zhang, J.; Yao, S.; Miao, W. [68Ga]Ga-FAPI PET/CT Improves the T Staging of Patients with Newly Diagnosed Nasopharyngeal Carcinoma: A Comparison with [18F]F-FDG. Mol. Imaging Biol. 2022, 24, 973–985. [Google Scholar] [CrossRef]
- Jiang, Y.; Wen, B.; Li, C.; Tian, Y.; Xiao, Z.; Xu, K.; Xing, D.; Yu, Z.; Huang, J.; Jia, J.; et al. The Performance of 68Ga-FAPI-04 PET/CT in Head and Neck Squamous Cell Carcinoma: A Prospective Comparison with 18F-FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2114–2126. [Google Scholar] [CrossRef]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, L.; Ruan, D.; Pang, Y.; Hao, B.; Dai, Y.; Wu, X.; Guo, W.; Fan, C.; Wu, J.; et al. Usefulness of [68Ga]Ga-DOTA-FAPI-04 PET/CT in Patients Presenting with Inconclusive [18F]FDG PET/CT Findings. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Racca, M.; Garrou, F.; Fenocchio, E.; Pellegrino, L.; Albano, D.; Dondi, F.; Bertagna, F.; Annunziata, S.; Treglia, G. Diagnostic Performance of Positron Emission Tomography with Fibroblast-Activating Protein Inhibitors in Gastric Cancer: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 10136. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Dahlbom, M.; Lindner, T.; Vauclin, S.; Mona, C.; Slavik, R.; Czernin, J.; Haberkorn, U.; Calais, J. Radiation Dosimetry and Biodistribution of 68Ga-FAPI-46 PET Imaging in Cancer Patients. J. Nucl. Med. 2020, 61, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Jin, J.; Krishnamachary, B.; Mironchik, Y.; Wildes, F.; Vesuna, F.; Barnett, J.D.; Bhujwalla, Z.M. Functional Roles of FAP-α in Metabolism, Migration and Invasion of Human Cancer Cells. Front. Oncol. 2023, 13, 1068405. [Google Scholar] [CrossRef]
- Jia, J.; Martin, T.A.; Ye, L.; Jiang, W.G. FAP-α (Fibroblast Activation Protein-α) Is Involved in the Control of Human Breast Cancer Cell Line Growth and Motility via the FAK Pathway. BMC Cell Biol. 2014, 15, 16. [Google Scholar] [CrossRef]
- Klein Nulent, T.J.W.; van Es, R.J.J.; Willems, S.M.; Braat, A.J.A.T.; Devriese, L.A.; de Bree, R.; de Keizer, B. First Experiences with 177Lu-PSMA-617 Therapy for Recurrent or Metastatic Salivary Gland Cancer. EJNMMI Res. 2021, 11, 126. [Google Scholar] [CrossRef]
- Rizzo, A.; Dall’Armellina, S.; Pizzuto, D.A.; Perotti, G.; Zagaria, L.; Lanni, V.; Treglia, G.; Racca, M.; Annunziata, S. PSMA Radioligand Uptake as a Biomarker of Neoangiogenesis in Solid Tumours: Diagnostic or Theragnostic Factor? Cancers 2022, 14, 4039. [Google Scholar] [CrossRef]
- Verena, A.; Zhang, Z.; Kuo, H.-T.; Merkens, H.; Zeisler, J.; Wilson, R.; Bendre, S.; Wong, A.A.W.L.; Bénard, F.; Lin, K.-S. Synthesis and Preclinical Evaluation of Three Novel 68Ga-Labeled Bispecific PSMA/FAP-Targeting Tracers for Prostate Cancer Imaging. Molecules 2023, 28, 1088. [Google Scholar] [CrossRef]
- Taralli, S.; Martino, A.; Cancellieri, A.; Calandriello, L.; Lococo, F.; Caldarella, C. Adenoid Cystic Carcinoma of the Parotid Gland: A First Case Report on 11C-Methionine PET/CT Detection of Histologically Confirmed Pulmonary Metastases. Acta Oncol. 2022, 61, 669–671. [Google Scholar] [CrossRef]
- Toubaru, S.; Yoshikawa, K.; Ohashi, S.; Tanimoto, K.; Hasegawa, A.; Kawaguchi, K.; Saga, T.; Kamada, T. Accuracy of Methionine-PET in Predicting the Efficacy of Heavy-Particle Therapy on Primary Adenoid Cystic Carcinomas of the Head and Neck. Radiat. Oncol. 2013, 8, 143. [Google Scholar] [CrossRef] [PubMed]
- Pandruvada, S.; Kessler, R.; Thai, A. Head and Neck Cancer Treatment in the Era of Molecular Medicine. Adv. Cancer Res. 2023, 160, 205–252. [Google Scholar] [CrossRef] [PubMed]
- Rong, X.; Lv, J.; Liu, Y.; Wang, Z.; Zeng, D.; Li, Y.; Li, S.; Wu, J.; Shen, Z.; Shi, M.; et al. PET/CT Imaging of Activated Cancer-Associated Fibroblasts Predict Response to PD-1 Blockade in Gastric Cancer Patients. Front. Oncol. 2021, 11, 802257. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]

| First Authors [Ref.] | Year | Country | Study Design/Number of Involved Centers | Funding Sources |
|---|---|---|---|---|
| Syed [12] | 2020 | Germany | Retrospective/Monocentric | Open Access funded by Projekt DEAL. No funding declared concerning the development of the study. |
| Linz [13] | 2021 | Germany | Prospective/monocentric | None declared |
| Serfling [14] | 2021 | Germany | Retrospective/Monocentric | Open Access funded by Projekt DEAL. No funding was declared concerning the development of the study. |
| Röhrich [15] | 2021 | Germany | Retrospective/Monocentric | Funded by the Federal Ministry of Education and Research (grant number 13N 13341). |
| Qin [16] | 2021 | China | Prospective/Monocentric | Funded by the Key Project of the National Natural Science Foundation of China (No. 81630049, 82030052) and National Key R&D Program of China (No. 2019 YFC 1316204). |
| Gu [17] | 2022 | China | Prospective/Monocentric | Funded by the National Natural Science Foundation of China (No. 81771861, 81971648, and 81901778) and Shanghai Anticancer Association Program (No. HYXH2021004). |
| Promteangtrong [18] | 2022 | Thailand | Prospective/Monocentric | NSofie iTheranostics Inc. provided the 68Ga-FAPI-46 precursor. |
| Chen [19] | 2022 | China | Prospective/Monocentric | National Natural Science Foundation of China (No. 81971651, No. 82171928), Natural Science Foundation of Fujian Province (No. 2019J01454, No. 2020J05249), Fujian Provincial Health Technology Project (No. 2020GGA045, No. 2020QNA054) and Startup Fund for Scientific Research of Fujian Medical University (No. 2017XQ1099) |
| Wegen [20] | 2022 | Germany | Retrospective/Monocentric | Open Access funded by Projekt DEAL. No funding was declared concerning the development of the study. |
| Zheng [21] | 2022 | China | Prospective/Monocentric | National Natural Science Foundation of China (No. 81971651) |
| Jiang [22] | 2023 | China | Prospective/Monocentric | National Natural Science Foundation of China (No. 82171986); Improvement Project for Theranostic Ability on Miscellaneous Disease (ZLYNXM202007); fundamental research funds for the central universities, Wuhan University (2042021kf0160); research fund from medical Sci-Tech innovation platform of Zhongnan Hospital, Wuhan University (PTXM2021021). |
| First Authors [Ref.] | Sample Size (No. of Patients) | Mean/Median Age (Years) | Gender (Male %) | No. of Patients and Clinical Setting | Location of Primary Tumor (No. of Patients) | HNC Subtype (No. of Patients) | Comparative Imaging |
|---|---|---|---|---|---|---|---|
| Syed [12] | 14 | Median: 68.5 | 86% | 14 RT planning | n.a. | 12 SCC 1 Mucoepidermoid carcinoma 1 Undifferentiated | CT; MRI |
| Linz [13] | 10 | Mean: 62 | 80% | 10 Staging | 5 Mouth floor 2 Tongue 2 Mandible alveolar process 1 Maxillary mucosa | 10 SCC | [18F]FDG PET/CT; MRI |
| Serfling [14] | 8 | Mean: 62 | 75% | 8 Staging | 8 Waldeyer’s tonsillar ring | 8 SCC (6 HPV+) | [18F]FDG PET/CT |
| Röhrich [15] | 12 | Mean: 57.8 | 33% | 7 Staging 5 Restaging | 12 Salivary glands | 12 ACC | CT; MRI |
| Qin [16] | 15 | Mean: 51.2 | 53% | 14 Staging 1 Restaging | 15 Nasopharynx | 3 K SCC 2 nK SCC 8 nK undifferentiaded carcinoma 2 Unknown | [18F]FDG PET/MRI |
| Gu [17] | 18 | Median: 55 | 89% | 18 Staging | 18 HNCUP | 16 SCC 2 ADC | [18F]FDG PET/CT |
| Promteangtrong * [18] | 40 | Mean: 57 | 68% | 12 Staging 28 Restaging | 17 Nasopharynx 10 Tongue 5 Pyriform fossa 1 Lip 1 External ear canal 1 Nasal cavity 1 Oropharynx 1 Retromolar trigone 1 Oral mucosa 1 Glottis 1 Mouth floor | 40 SCC | [18F]FDG PET/CT |
| Chen ** [19] | 36 | Mean: 62 | 81% | 17 Staging 8 Restaging | 15 Tongue 6 Floor of the mouth 5 Buccal mucosa 5 Gingiva 3 Root of the tongue 3 Palate | 36 SCC | [18F]FDG PET/CT |
| Wegen [20] | 15 | Median: 66 | 80% | 15 RT planning | 3 Nasopharynx 8 Oropharynx 1 Hypopharynx 3 Larynx | 14 SCC (5 HPV+) 1 ADC | [18F]FDG PET/CT |
| Zheng [21] | 47 | Mean: 52 | 68% | 47 Staging | 47 Nasopharynx | 47 NPC (30 EBV+) | [18F]FDG PET/CT; MRI |
| Jiang [22] | 77 | Median: 58 | 79% | 67 Staging 10 Restaging | 18 Nasopharynx 19 Oral cavity 14 Oropharynx 16 Larynx 7 Hypopharynx 3 Nasal cavities and paranasal sinuses | 77 SCC | [18F]FDG PET/CT |
| First Authors [Ref.] | Tracer | Hybrid Imaging | Tomograph | Administered Activity | Uptake Time(Minutes) | Image Analysis |
|---|---|---|---|---|---|---|
| Syed [12] | [68Ga]Ga-FAPi (pharmaceutical form not specified) | PET/CT | Biograph mCT Flow (Siemens ®) | n.a. | 30 | Qualitative, semiquantitative (SUVmax, SUVmean, GTV, TBR) |
| Linz [13] | [68Ga]Ga-DOTA-FAPi-04 | PET/CT | Biograph mCT 64 (Siemens ®) | Mean: 119 MBq | n.a. | Qualitative, semiquantitative (SUVmax, SUVpeak) |
| Serfling [14] | [68Ga]Ga-DOTA-FAPi-04 | PET/CT | Biograph mCT 64 (Siemens ®) | Mean: 145 MBq | 60 | Qualitative, semiquantitative (SUVmax) |
| Röhrich [15] | [68Ga]Ga-DOTA-FAPi-04; [68Ga]Ga-DOTA-FAPi-74 | PET/CT | Biograph mCT Flow (Siemens ®) | n.a. | 10; 60; 180 | Qualitative, semiquantitative (SUVmax, SUVmean, GTV) |
| Qin [16] | [68Ga]Ga-FAPi (pharmaceutical form not specified) | PET/MRI | SIGNA(GE Healthcare ®) | 1.85–3.7 MBq/kg | 30–60 | Qualitative, semiquantitative (SUVmax) |
| Gu [17] | [68Ga]Ga-DOTA-FAPi-04 | PET/CT | Biograph mCT flow scanner (Siemens ®) | Mean: 143.71 MBq | 60 | Qualitative, semiquantitative (SUVmax, SUVmean, TBR) |
| Promteangtrong [18] | [68Ga]Ga-FAPi-46 | PET/CT | Biograph Vision (Siemens ®) | 2.0 MBq/kg | 60 | Qualitative, semiquantitative (SUVmax, FTV, TLA, TBR) |
| Chen [19] | [68Ga]Ga-DOTA-FAPi-04 | PET/CT | Biograph mCT 64 (Siemens ®) | 1.85–2.2 MBq/Kg | 60 | Qualitrative, semiquantitative (SUVmax, TBR) |
| Wegen [20] | [68Ga]Ga-DOTA-FAPi-46 | PET/CT | Biograph mCT Flow–Edge 128 (Siemens ®) | Mean: 147 MBq | Following tracer administration | Qualitative, semiquantitative (SUVmax, SUVmean, GTV, TBR) |
| Zheng [21] | [68Ga]Ga-DOTA-FAPi-04 | PET/CT | Biograph mCT 64 (Siemens ®) | Mean: 106.9 MBq | 44 | Qualitative, semiquantitative (SUVmax, TBR) |
| Jiang [22] | [68Ga]Ga-DOTA-FAPi-04 | PET/CT | n.a. | n.a. | n.a. | Qualitative, semiquantitative (SUVmax, TBR) |
| First Authors [Ref.] | Aim of the Study | Primary Lesion SUVmax | Metastatic Lesions SUVmax | Immunohistochemistry for FAP in Tumor Struma | Outcome |
|---|---|---|---|---|---|
| Syed [12] | Investigate the use of FAPi PET/CT to detect and delineate HNCs for RT planning. | Mean: 14.6 ± 4.4 | Lymph nodes: 9.4 ± 5.7 Bone: 7.5 ± 1.8 | n.a. | Tumor segmentation based on RT planning on FAPi PET resulted in larger treatment volumes, including FAPi-avid regions not covered by CT. |
| Linz [13] | Assess diagnostic accuracy of FAPi PET. | Mean: 20.8 ± 6.4 | Lymph nodes: 10.7 ± 6.9 | All primary tumors and lymph node metastases showed positive FAP immunostaining. | FAPi PET has superior specificity over FDG PET and might prevent potential overtreatment. |
| Serfling [14] | Investigate the use of FAPi PET/CT to detect and delineate ACC for RT planning. | Mean: 15.9 ± 6.3 | n.a. | Most of the primary tumors and lymph node metastases showed positive FAP immunostaining. | The differentiation between the primary tumor and surrounding physiologic tissues is improved by FAPi PET when compared to FDG PET. |
| Röhrich [15] | Assess diagnostic accuracy of FAPi PET. | Mean: 12.8 ± 2.1 | Mean of all sites: 4.3 ± 1.2 | ACC-stroma was variably positive for FAP immunostaining. | FAPi PET allowed a more accurate tumor segmentation than CT.Moreover, FAPi PET revealed additional lesions in 5/12 ACC patients. |
| Qin [16] | Assess diagnostic accuracy of FAPi PET. | Mean: 13.9 ± 5.1 | Lymph nodes: 8.8 ± 3.8 | n.a. | FAPi PET is superior in delineating primary tumors and detecting distant metastases because of its low background level in the brain. FAPi PET detected fewer lymph node metastases than FDG PET, but no histologic confirmation exists. |
| Gu [17] | Detection of primary tumor in patients with HNCUP. | Median: 8.79 | Lymph nodes: 9.1 ± 4.7 Bone: 6.96 ± 5.87 | n.a. | FAPi PET improves the primary tumor detection rate in HNCUP with negative FDG PET. |
| Promteangtrong [18] | Comparison of diagnostic accuracy between FAPi PET and FDG PET. | Mean: 19.28 ± 7.45 | Lymph nodes: 15.04 ± 10.25 Distant metastases: 13.59 ± 7.64 | n.a. | FAPi PET and FDG PET have comparable diagnostic performance for initial staging and recurrence detection in HNSCC patients. |
| Chen [19] | Assess diagnostic accuracy of FAPi PET. | Mean: 12.74 ± 3.51 | Lymph nodes: 4.86 ± 2.51 | n.a. | FAPi PET could detect primary HNSCCs and showed superior accuracy in detecting nodal metastases compared to FDG PET. |
| Wegen [20] | Investigate the use of FAPi PET/CT to detect and delineate HNCs for RT planning. | Median: 5.2 | Lymph nodes: 6.8Bone and visceral metastases: 6.5Uncommon sites: 6.0 | n.a. | FAPi PET had greater sensitivity and accuracy than FDG PET. |
| Zheng [21] | Comparison of diagnostic accuracy between FAPi PET and FDG PET. | Mean: 11.3 ± 5.3 | Lymph nodes: 7.1 ± 3.6 Bone: 8.3 ± 4.4 | Most primary tumors and lymph node metastases showed positive FAP immunostaining; however, FAP immunostaining did not correlate to SUV measured on FAPi PET images. | FAPi PET performed better than FDG PET in detecting intracranial invasion of primary tumors. However, FAPi PET performance was less accurate in lymph node staging. |
| Jiang [22] | Comparison of diagnostic accuracy between FAPi PET and FDG PET. | Mean: 17.7 ± 7.0 | Lymph nodes: 7.83 ± 7.55 Bone: 17.64 ± 10.32 | Most primary tumors and lymph node metastases showed positive FAP immunostaining; FAP strongly correlated to SUV measured on FAPi PET images. | FAPi PET performs similarly to FDG PET in detecting primary tumors and has higher specificity in preoperative lymph node staging. |
| First Authors [Ref.] | Sample Size | Detection Rate (%) | 95% CI | Weight (%) | |
|---|---|---|---|---|---|
| Fixed | Random | ||||
| Linz [13] | 10 | 100 | 69.1 to 100 | 4.76 | 4.76 |
| Serfling [14] | 8 | 100 | 63.1 to 100 | 3.90 | 3.90 |
| Qin [16] | 15 | 100 | 78.2 to 100 | 6.93 | 6.93 |
| Gu [17] | 18 | 38.89 | 17.3 to 64.2 | 7.6 | 10.97 |
| Promteangtrong * [18] | 25 | 100 | 86.3 to 100 | 11.26 | 11.26 |
| Chen [19] | 36 | 100 | 90.3 to 100 | 16.02 | 16.02 |
| Wegen [20] | 15 | 100 | 78.2 to 100 | 6.93 | 6.93 |
| Zheng [21] | 47 | 100 | 92.4 to 100 | 20.78 | 20.78 |
| Jiang * [22] | 67 | 100 | 94.6 to 100 | 29.44 | 29.44 |
| Total (fixed effects) | 241 | 97.8 | 95.1 to 99.2 | 100.00 | 100.00 |
| Total (random effects) | 241 | 96.3 | 88.3 to 99.8 | 100.00 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzo, A.; Miceli, A.; Racca, M.; Bauckneht, M.; Morbelli, S.; Albano, D.; Dondi, F.; Bertagna, F.; Galizia, D.; Muoio, B.; et al. Diagnostic Accuracy of [68Ga]Ga Labeled Fibroblast-Activation Protein Inhibitors in Detecting Head and Neck Cancer Lesions Using Positron Emission Tomography: A Systematic Review and a Meta-Analysis. Pharmaceuticals 2023, 16, 1664. https://doi.org/10.3390/ph16121664
Rizzo A, Miceli A, Racca M, Bauckneht M, Morbelli S, Albano D, Dondi F, Bertagna F, Galizia D, Muoio B, et al. Diagnostic Accuracy of [68Ga]Ga Labeled Fibroblast-Activation Protein Inhibitors in Detecting Head and Neck Cancer Lesions Using Positron Emission Tomography: A Systematic Review and a Meta-Analysis. Pharmaceuticals. 2023; 16(12):1664. https://doi.org/10.3390/ph16121664
Chicago/Turabian StyleRizzo, Alessio, Alberto Miceli, Manuela Racca, Matteo Bauckneht, Silvia Morbelli, Domenico Albano, Francesco Dondi, Francesco Bertagna, Danilo Galizia, Barbara Muoio, and et al. 2023. "Diagnostic Accuracy of [68Ga]Ga Labeled Fibroblast-Activation Protein Inhibitors in Detecting Head and Neck Cancer Lesions Using Positron Emission Tomography: A Systematic Review and a Meta-Analysis" Pharmaceuticals 16, no. 12: 1664. https://doi.org/10.3390/ph16121664
APA StyleRizzo, A., Miceli, A., Racca, M., Bauckneht, M., Morbelli, S., Albano, D., Dondi, F., Bertagna, F., Galizia, D., Muoio, B., Annunziata, S., & Treglia, G. (2023). Diagnostic Accuracy of [68Ga]Ga Labeled Fibroblast-Activation Protein Inhibitors in Detecting Head and Neck Cancer Lesions Using Positron Emission Tomography: A Systematic Review and a Meta-Analysis. Pharmaceuticals, 16(12), 1664. https://doi.org/10.3390/ph16121664











