Pyrrole-Doped Polydopamine-Pyrrole (PDA-nPY) Nanoparticles with Tunable Size and Improved NIR Absorption for Photothermal Therapy
Abstract
:1. Introduction
2. Results and Discussion
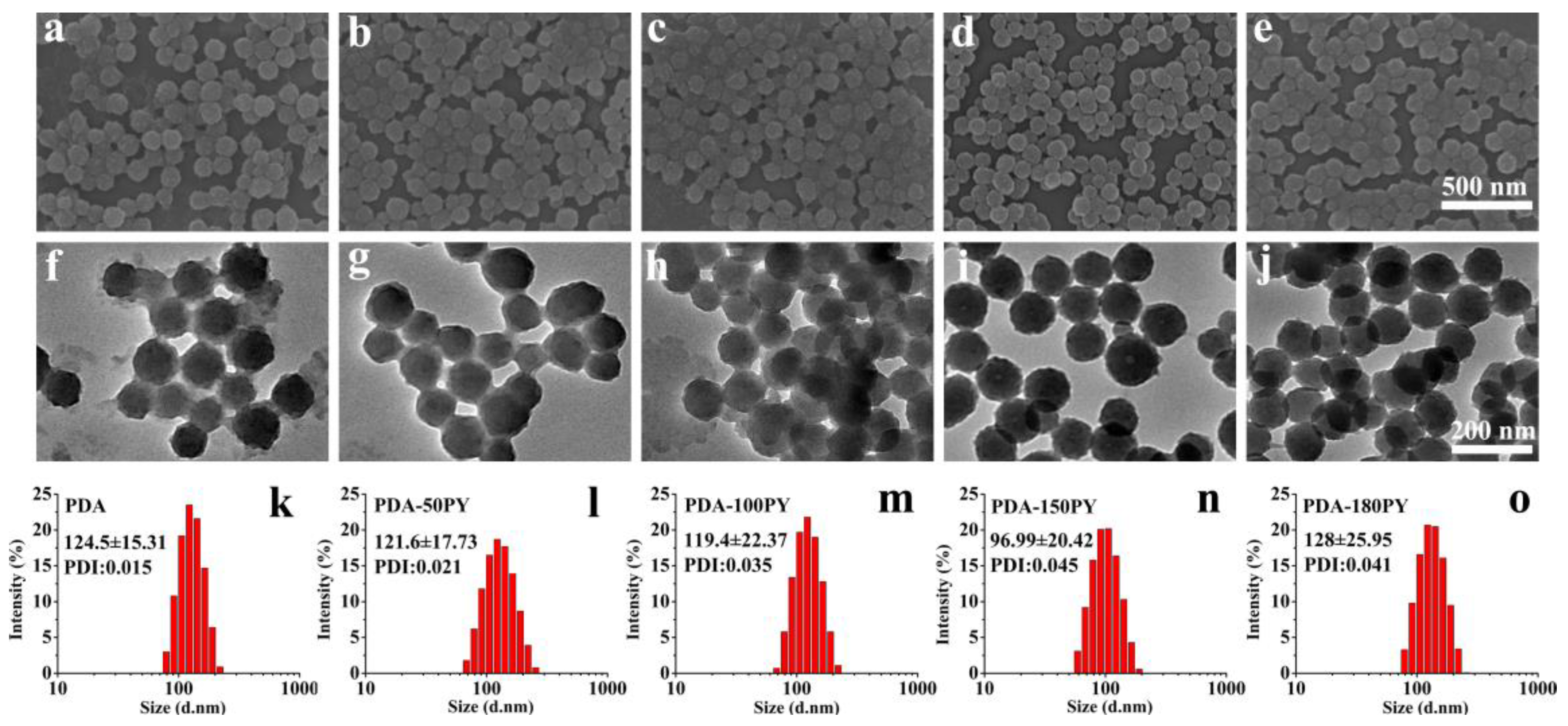
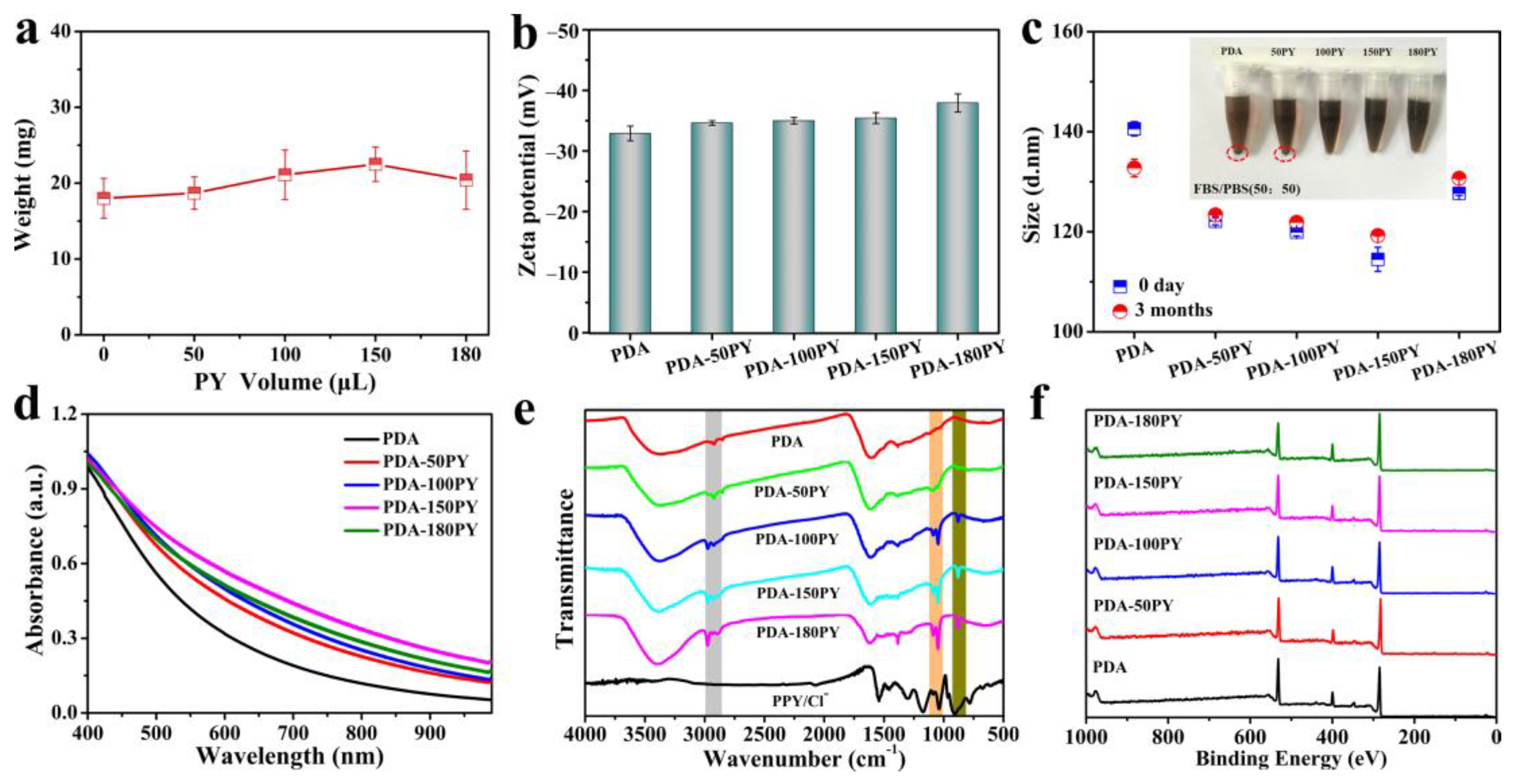
2.1. Synthesis and Characteristics of PDA-nPY NPs
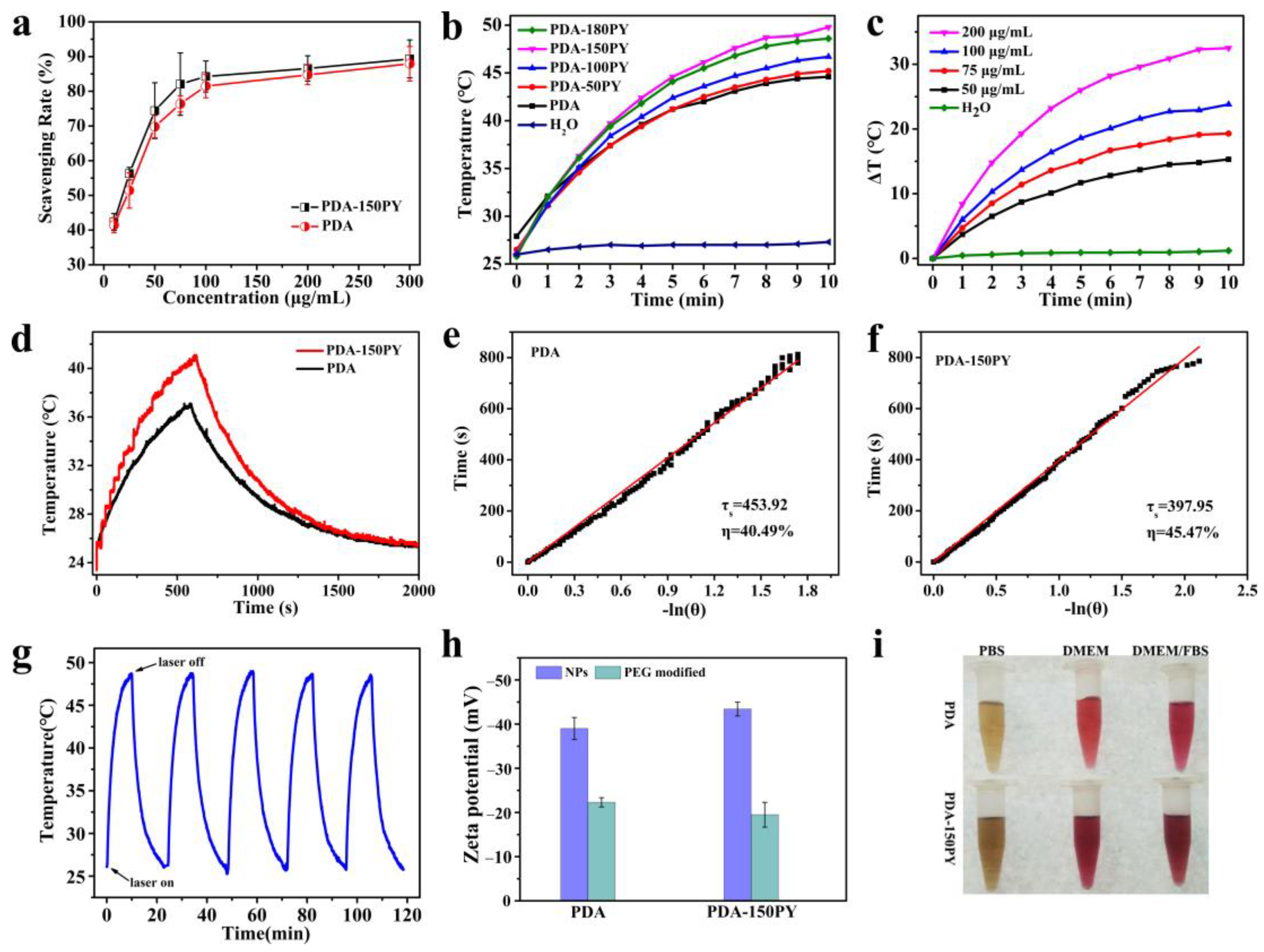
2.2. DPPH Radical Scavenging Capacity of PDA-PY
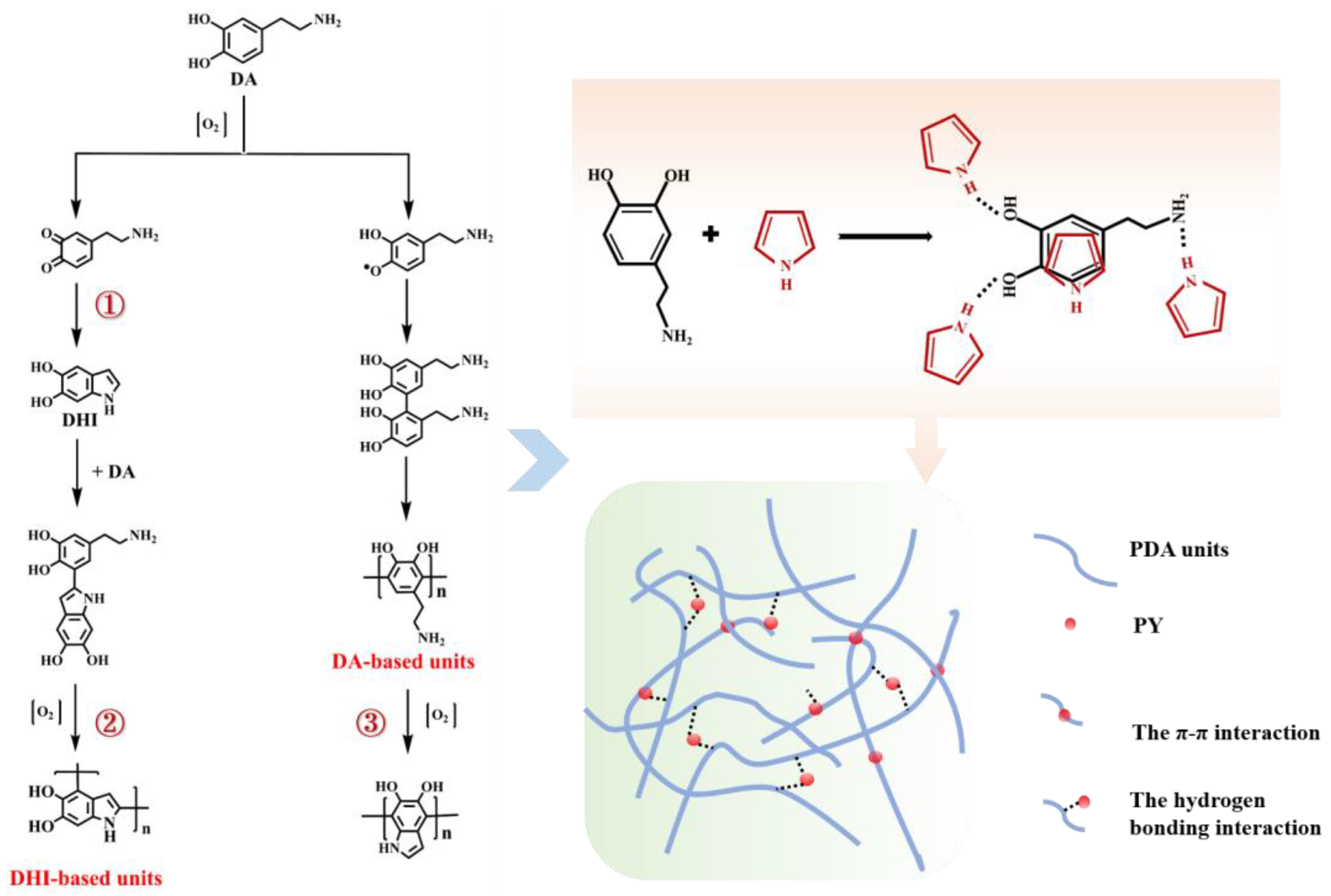
2.3. The Reaction Mechanism of DA–PY
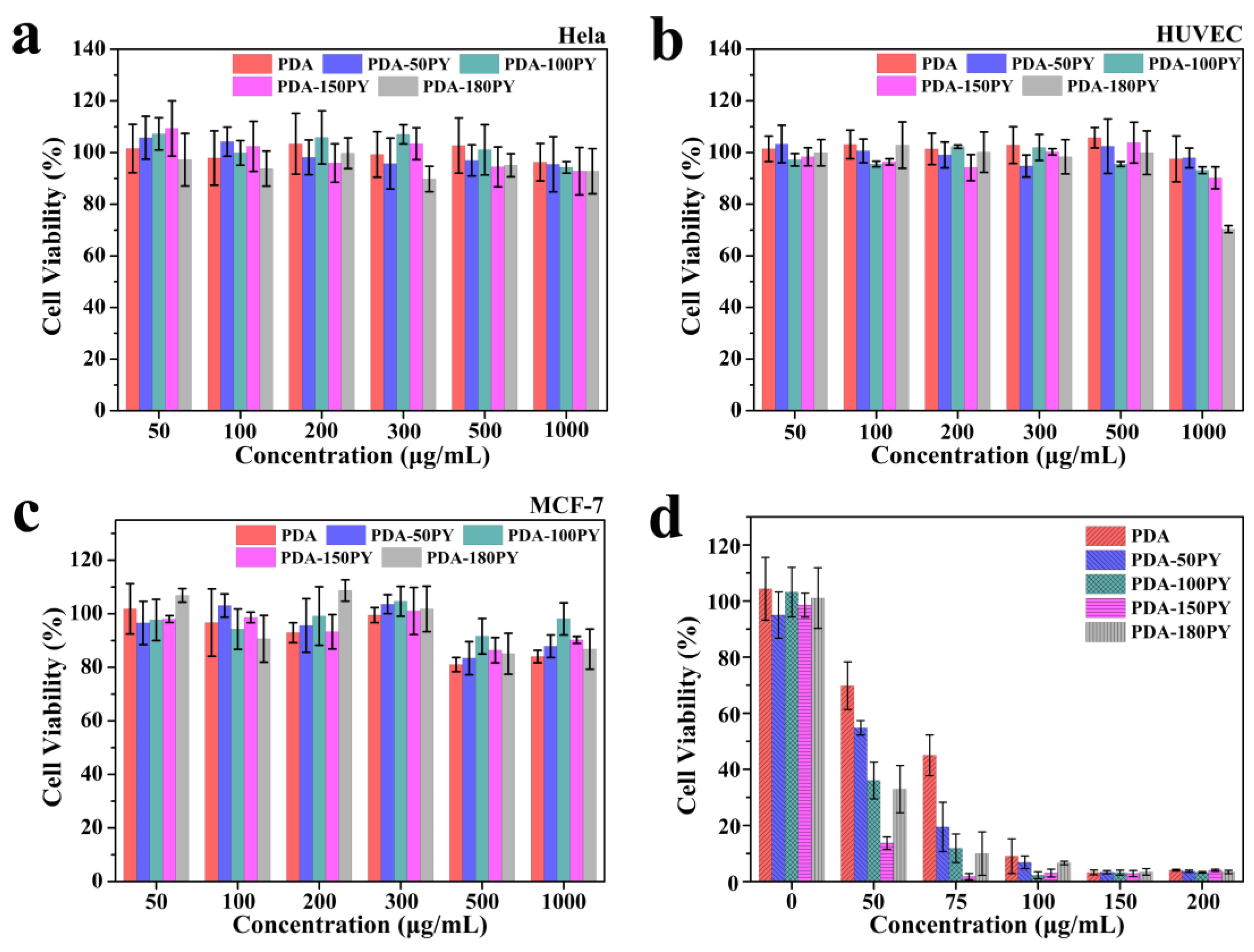
2.4. In Vitro Biocompatibility and Photothermal Effect
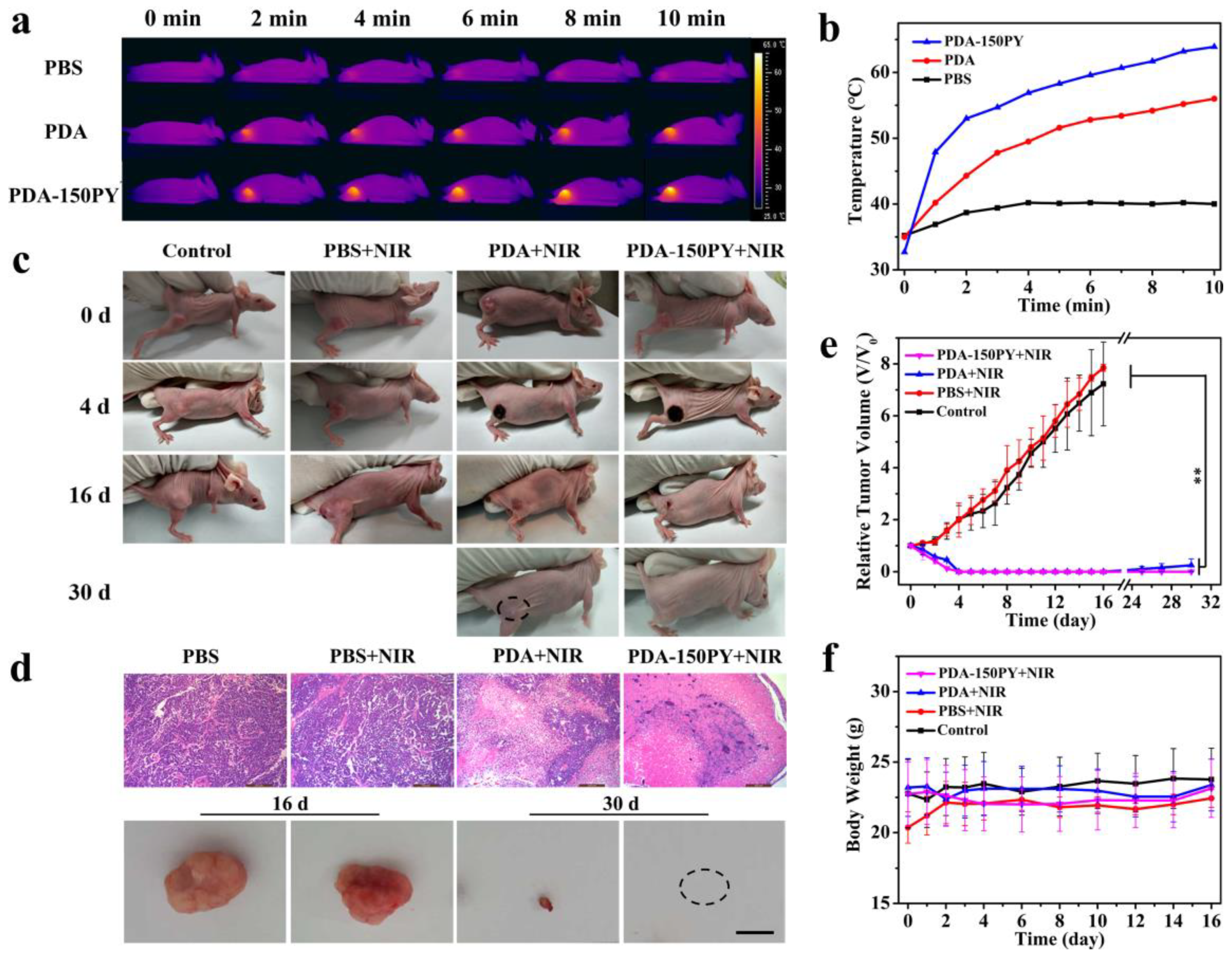
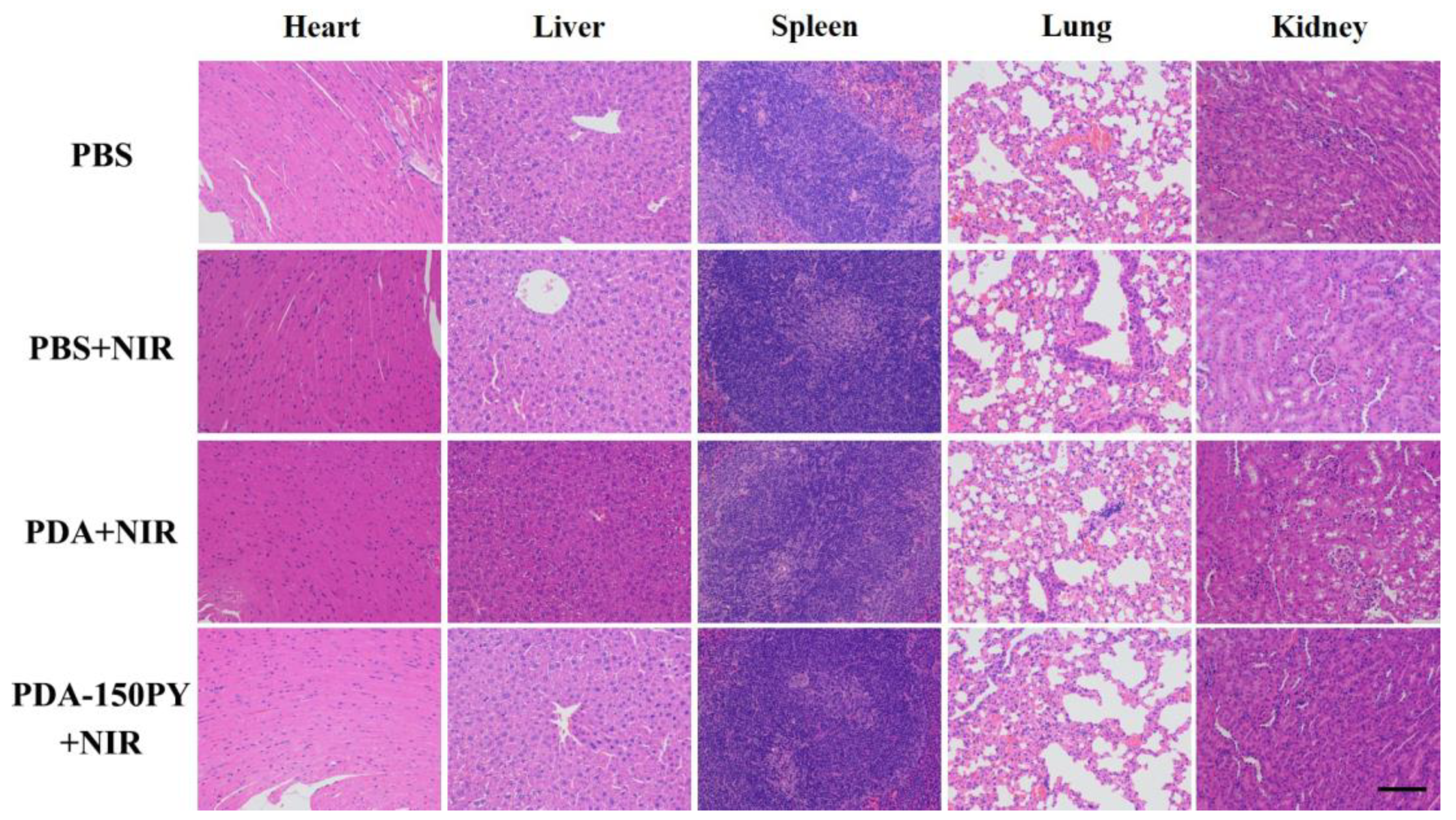
2.5. In Vivo Photothermal (PT) Treatment Performance
3. Materials and Methods
3.1. Materials
3.2. Synthesis of PDA-nPY NPs
3.3. Characterization
3.4. DPPH Radical Scavenging Test
3.5. Photothermal Performance of PDA-nPY NPs
3.6. Cell Culture
3.7. In Vitro Cytotoxicity Assay
3.8. In Vitro Photothermal Killing of Cancer Cells
3.9. In Vivo Photothermal Treatment for Tumor Ablation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mrówczyński, R.; Markiewicz, R.; Liebscher, J. Chemistry of polydopamine analogues. Polym. Int. 2016, 65, 1288–1299. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, L.; Zhang, J.; Hu, J.; Duan, G.; Liu, X.; Li, Y.; Gu, Z. Polydopamine antibacterial materials. Mater. Horiz. 2021, 8, 1618–1633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ou, Y.; Lei, W.X.; Wan, L.S.; Ji, J.; Xu, Z.K. CuSO4/H2O2-induced rapid deposition of polydopamine coatings with high uniformity and enhanced stability. Angew. Chem. Int. Ed. 2016, 55, 3054–3057. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Qu, X.; Tan, H.; Song, J.; Lei, M.; Kim, E.; Payne, G.F.; Liu, C. Role of polydopamine’s redox-activity on its pro-oxidant, radical-scavenging, and antimicrobial activities. Acta Biomater. 2019, 88, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, Y.; Liu, Y.; Pan, J.; Wang, J.; Man, F.; Zhang, W.; Liu, G. Melanin-like nanomaterials for advanced biomedical applications: A versatile platform with extraordinary promise. Adv. Sci. 2020, 7, 1903129. [Google Scholar] [CrossRef]
- Acter, S.; Moreau, M.; Ivkov, R.; Viswanathan, A.; Ngwa, W. Polydopamine nanomaterials for overcoming current challenges in cancer treatment. Nanomaterials 2023, 13, 1656. [Google Scholar] [CrossRef]
- Chinchulkar, S.A.; Patra, P.; Dehariya, D.; Yu, A.; Rengan, A.K. Polydopamine nanocomposites and their biomedical applications: A review. Polym. Adv. Technol. 2022, 33, 3935–3956. [Google Scholar] [CrossRef]
- Pan, J.; Xia, Z.; Deng, N.; Chen, L.; Zhang, H.; Lu, Y.; Liu, Y.; Gao, H. Eumelanin-inspired nanomaterials in electrochemical energy storage devices: A review. Chem. Eng. J. 2023, 452, 138607. [Google Scholar] [CrossRef]
- Yan, J.; Yang, L.P.; Lin, M.F.; Ma, J.; Lu, X.T.; Lee, P.S. Polydopamine Spheres as active templates for convenient synthesis of various nanostructures. Small 2013, 9, 596–603. [Google Scholar] [CrossRef]
- Ho, C.C.; Ding, S.J. The pH-controlled nanoparticles size of polydopamine for anti-cancer drug delivery. J. Mater. Sci. Mater. Med. 2013, 24, 2381–2390. [Google Scholar] [CrossRef]
- Ju, K.-Y.; Lee, Y.; Lee, S.; Park, S.B.; Lee, J.-K. Bioinspired polymerization of dopamine to generate melanin-like nanoparticles having an excellent free-radical-scavenging property. Biomacromolecules 2011, 12, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-melanin colloidal nanospheres: An efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, Y.; Li, M. Selecting water-alcohol mixed solvent for synthesis of polydopamine nano-spheres using solubility parameter. Sci. Rep. 2014, 4, 6070. [Google Scholar] [CrossRef]
- Alfieri, M.L.; Panzella, L.; Oscurato, S.L.; Salvatore, M.; Avolio, R.; Errico, M.E.; Maddalena, P.; Napolitano, A.; D’Ischia, M. The chemistry of polydopamine film formation: The amine-quinone interplay. Biomimetics 2018, 3, 26. [Google Scholar] [CrossRef]
- Zangmeister, R.A.; Morris, T.A.; Tarlov, M.J. Characterization of polydopamine thin films deposited at short times by autoxidation of dopamine. Langmuir 2013, 29, 8619–8628. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Na, Y.S.; Choi, S.; Song, I.T.; Kim, W.Y.; Lee, H. Non-covalent self-assembly and covalent polymerization co-contribute to polydopamine formation. Adv. Funct. Mater. 2012, 22, 4711–4717. [Google Scholar] [CrossRef]
- Hong, S.; Wang, Y.; Park, S.Y.; Lee, H. Progressive fuzzy cation-π assembly of biological catecholamines. Sci. Adv. 2018, 4, eaat7457. [Google Scholar] [CrossRef]
- Alfieri, M.L.; Micillo, R.; Panzella, L.; Crescenzi, O.; Oscurato, S.L.; Maddalena, P.; Napolitano, A.; Ball, V.; d’Ischia, M. Structural basis of polydopamine film formation: Probing 5,6-dihydroxyindole-based eumelanin type units and the porphyrin issue. ACS Appl. Mater. Interfaces 2018, 10, 7670–7680. [Google Scholar] [CrossRef]
- Wu, M.; Wang, T.; Müller, L.; Müller, F.A. Adjustable synthesis of polydopamine nanospheres and their nucleation and growth. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125196. [Google Scholar] [CrossRef]
- Chen, J.; Xia, L.; Kong, M.; He, Y.; Lv, Y.; Huang, Y.; Li, G. Optimized design of environmentally-friendly polydopamine nanoparticles for the stabilization of both thermo- and photo-oxidation of polypropylene: Size effects. Polym. Test. 2022, 116, 107795. [Google Scholar] [CrossRef]
- Carmignani, A.; Battaglini, M.; Sinibaldi, E.; Marino, A.; Vighetto, V.; Cauda, V.; Ciofani, G. In vitro and ex vivo investigation of the effects of polydopamine nanoparticle size on their antioxidant and photothermal properties: Implications for biomedical applications. ACS Appl. Nano Mater. 2022, 5, 1702–1713. [Google Scholar] [CrossRef]
- Nieto, C.; Marcelo, G.; Vega, M.; Martín del Valle, E.M. Antineoplastic behavior of polydopamine nanoparticles prepared in different water/alcohol media. Colloids Surf. B Biointerfaces 2021, 199, 111506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, X.; Xu, Q.; Wang, Y.; Ji, J. Polydopamine nanoparticles with different sizes for NIR-promoted gene delivery and synergistic photothermal therapy. Colloids Surf. B Biointerfaces 2021, 208, 112125. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Z.; Yang, P.; Hu, J.; Wang, Z.; Li, Y. Size control synthesis of melanin-like polydopamine nanoparticles by tuning radicals. Polym. Chem. 2019, 10, 4194–4200. [Google Scholar] [CrossRef]
- Huang, C.; Wang, X.; Yang, P.; Shi, S.; Duan, G.; Liu, X.; Li, Y. Size regulation of polydopamine nanoparticles by boronic acid and lewis base. Macromol. Rapid Commun. 2022, 44, e2100916. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, S.; Zhang, N.; Wang, Y.; Zhong, J.; Sun, X.; Qi, Y.; Chen, X.; Li, Z.; Li, Y. Tailoring Synthetic Melanin Nanoparticles for Enhanced Photothermal Therapy. ACS Appl. Mater. Interfaces 2019, 11, 42671–42679. [Google Scholar] [CrossRef]
- Zou, Y.; Chen, X.; Yang, P.; Liang, G.; Yang, Y.; Gu, Z.; Li, Y. Regulating the absorption spectrum of polydopamine. Sci. Adv. 2020, 6, eabb4696. [Google Scholar] [CrossRef]
- Sardoiwala, M.N.; Nagpal, S.; Bhatt, B.; Roy Choudhury, S.; Karmakar, S. Improved melatonin delivery by a size-controlled polydopamine nanoformulation attenuates preclinical diabetic retinopathy. Mol. Pharm. 2023, 20, 2899–2910. [Google Scholar] [CrossRef]
- Qi, X.; Xiang, Y.; Cai, E.; Ge, X.X.; Chen, X.; Zhang, W.; Li, Z.; Shen, J. Inorganic-organic hybrid nanomaterials for photothermal antibacterial therapy. Coord. Chem. Rev. 2023, 496, 215426. [Google Scholar] [CrossRef]
- Sharma, S.; Zvyagin, A.V.; Roy, I. Theranostic applications of nanoparticle-mediated photoactivated therapies. J. Nanotheranostics 2021, 2, 131–156. [Google Scholar] [CrossRef]
- Zeng, W.; Wu, X.; Chen, T.; Sun, S.; Shi, Z.; Liu, J.; Ji, X.; Zeng, X.; Guan, J.; Mei, L.; et al. Renal-clearable untrasmal polypyrrole nanoparticles with size-regulated property for second near-infrared light-mediated photothermal therapy. Adv. Funct. Mater. 2021, 31, 2008362. [Google Scholar] [CrossRef]
- Lin, Q.; Yang, Y.; Ma, Y.; Zhang, R.; Wang, J.; Chen, X.; Shao, Z. Bandgap engineered polypyrrole-polydopamine hybrid with intrinsic raman and photoacoustic imaging contrasts. Nano Lett. 2018, 18, 7485–7493. [Google Scholar] [CrossRef]
- Liedel, C.; Wang, X.; Antonietti, M. Biobased polymer cathodes with enhanced charge storage. Nano Energy 2018, 53, 536–543. [Google Scholar] [CrossRef]
- Sui, M.X.; Lu, X.L.; Xie, A.; Xu, W.D.; Rong, X.H.; Wu, G.J. The synthesis of three-dimensional (3D) polydopamine-functioned carbonyl iron powder@polypyrrole (CIP@PPy) aerogel composites for excellent microwave absorption. Synth. Met. 2015, 210, 156–164. [Google Scholar] [CrossRef]
- Rell, S.; Mazzotta, E.; Caroli, A.; Luca, M.D.; Bucci, C.; Malitesta, C. Investigation of polydopamine coatings by X-ray Photoelectron Spectroscopy as an effective tool for improving biomolecule conjugation. Appl. Surf. Sci. 2018, 447, 31–39. [Google Scholar] [CrossRef]
- Ding, Y.; Weng, L.T.; Yang, M.; Yang, Z.; Lu, X.; Huang, N.; Leng, Y. Insights into the aggregation/deposition and structure of a polydopamine film. Langmuir 2014, 30, 12258–12269. [Google Scholar] [CrossRef]
- Tan, J.L.; Zhang, Z.; He, Y.; Yue, Q.H.; Xie, Z.; Ji, H.R.; Sun, Y.N.; Shi, W.; Ge, D.T. Electrochemical synthesis of conductive, superhydrophobic and adhesive polypyrrole-polydopamine nanowires. Synth. Met. 2017, 234, 86–94. [Google Scholar] [CrossRef]
- Yang, J.; Cohen Stuart, M.A.; Kamperman, M. Jack of all trades: Versatile catechol crosslinking mechanisms. Chem. Soc. Rev. 2014, 43, 8271–8298. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Xu, L.; Sun, W.; Liang, L.; Qi, X.; Zhao, Y. Mild photothermal therapy prevents posterior capsule opacification through cytoskeletal remodeling. Adv. Healthc. Mater. 2023, 2300470. [Google Scholar] [CrossRef]
- Sharma, S.; Chakraborty, N.; Jha, D.; Gautam, H.K.; Roy, I. Robust dual modality antibacterial action using silver-prussian blue nanoscale coordination polymer. Mater. Sci. Eng. C 2020, 113, 110982. [Google Scholar] [CrossRef]
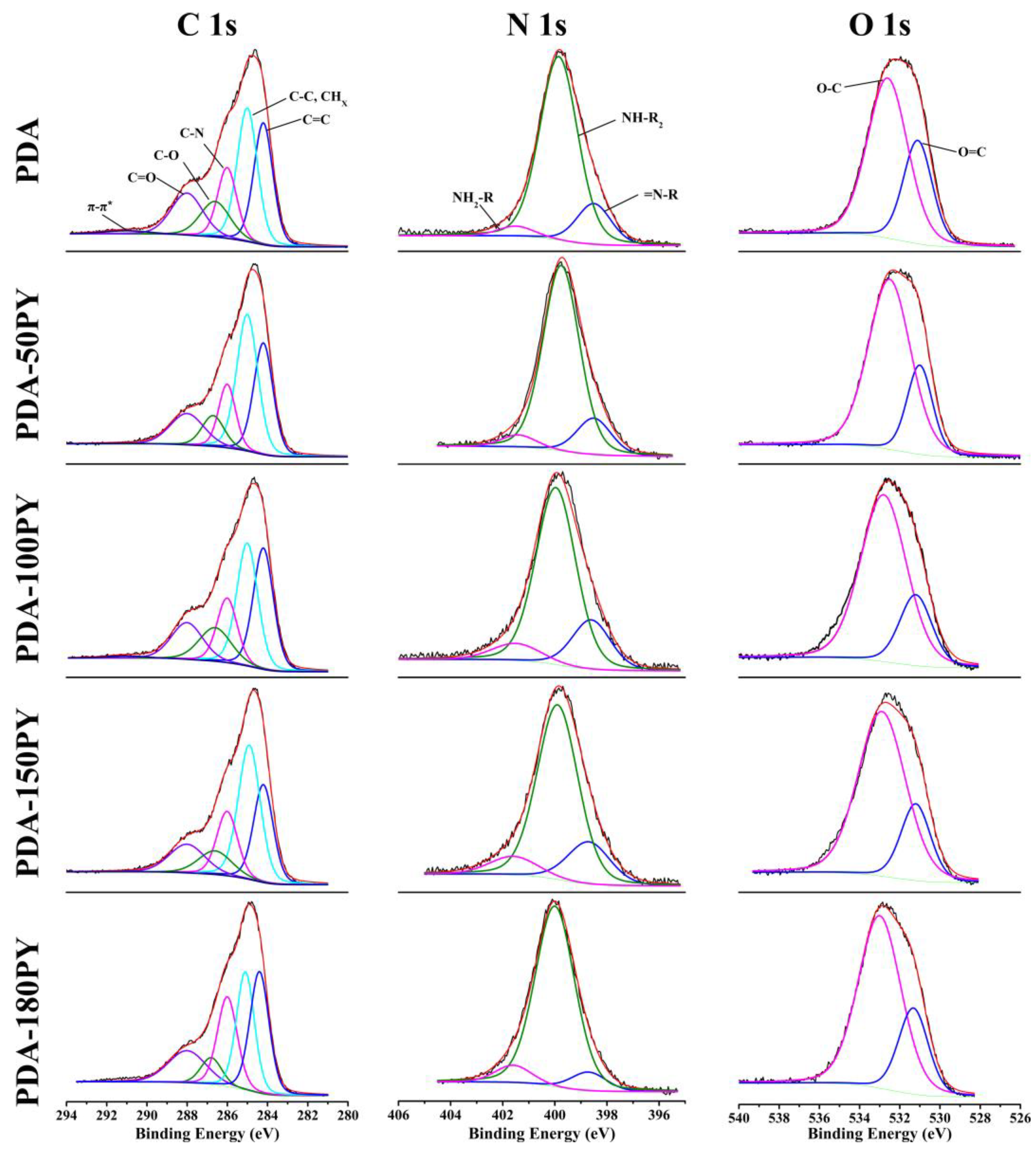
| C/% | N/% | O/% | N/C | |
|---|---|---|---|---|
| PDA | 67.5 | 9.34 | 23.16 | 0.138 |
| PDA-50PY | 68.14 | 9.48 | 22.38 | 0.139 |
| PDA-100PY | 68.58 | 10.01 | 21.41 | 0.146 |
| PDA-150PY | 70.08 | 9.92 | 20.01 | 0.142 |
| PDA-180PY | 70.72 | 10.7 | 18.58 | 0.151 |
| Peak Position eV | Area Ratio/% | |||||
|---|---|---|---|---|---|---|
| PDA | PDA-50PY | PDA-100PY | PDA-150PY | PDA-180PY | ||
| C1s | C=C (248.2 ± 0.1) | 26.05 | 27.35 | 27.94 | 23.90 | 18.55 |
| C–Hx, C–C (285) | 30.17 | 36.36 | 32.46 | 36.67 | 37.42 | |
| C–N (286) | 15.2 | 13.70 | 19.77 | 16.59 | 16.59 | |
| C–O (286.7 ± 0.1) | 11.93 | 8.69 | 6.55 | 9.84 | 10.62 | |
| C=O (288) | 14.96 | 13.46 | 12.58 | 12.72 | 14.24 | |
| π-π* (291) | 1.69 | 0.44 | 0.70 | 0.28 | 2.58 | |
| N1s | =N–R (398.6 ± 0.1) | 16.06 | 14.91 | 19.34 | 18.26 | 7.82 |
| NH–R2 (399.9 ± 0.1) | 79.79 | 80.04 | 72.10 | 72.57 | 84.75 | |
| NH2–R (401.5 ± 0.1) | 4.15 | 5.05 | 8.56 | 9.17 | 7.43 | |
| O1s | O=C (531.1 ± 0.1) | 31.71 | 23.99 | 23.03 | 22.53 | 25.75 |
| O–C (532.8 ± 0.2) | 68.29 | 76.01 | 76.97 | 77.47 | 74.25 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Li, Z.; Su, H.; Sun, Y.; Shi, W.; Yi, Y.; Ge, D.; Fan, Z. Pyrrole-Doped Polydopamine-Pyrrole (PDA-nPY) Nanoparticles with Tunable Size and Improved NIR Absorption for Photothermal Therapy. Pharmaceuticals 2023, 16, 1642. https://doi.org/10.3390/ph16121642
He Y, Li Z, Su H, Sun Y, Shi W, Yi Y, Ge D, Fan Z. Pyrrole-Doped Polydopamine-Pyrrole (PDA-nPY) Nanoparticles with Tunable Size and Improved NIR Absorption for Photothermal Therapy. Pharmaceuticals. 2023; 16(12):1642. https://doi.org/10.3390/ph16121642
Chicago/Turabian StyleHe, Yuan, Ziyang Li, Huiling Su, Yanan Sun, Wei Shi, Yunfeng Yi, Dongtao Ge, and Zhongxiong Fan. 2023. "Pyrrole-Doped Polydopamine-Pyrrole (PDA-nPY) Nanoparticles with Tunable Size and Improved NIR Absorption for Photothermal Therapy" Pharmaceuticals 16, no. 12: 1642. https://doi.org/10.3390/ph16121642
APA StyleHe, Y., Li, Z., Su, H., Sun, Y., Shi, W., Yi, Y., Ge, D., & Fan, Z. (2023). Pyrrole-Doped Polydopamine-Pyrrole (PDA-nPY) Nanoparticles with Tunable Size and Improved NIR Absorption for Photothermal Therapy. Pharmaceuticals, 16(12), 1642. https://doi.org/10.3390/ph16121642







