Advances in Neuropathic Pain Research: Selected Intracellular Factors as Potential Targets for Multidirectional Analgesics
Abstract
1. Neuropathic Pain
2. MAPKs and Neuropathic Pain
3. MMPs and Neuropathic Pain
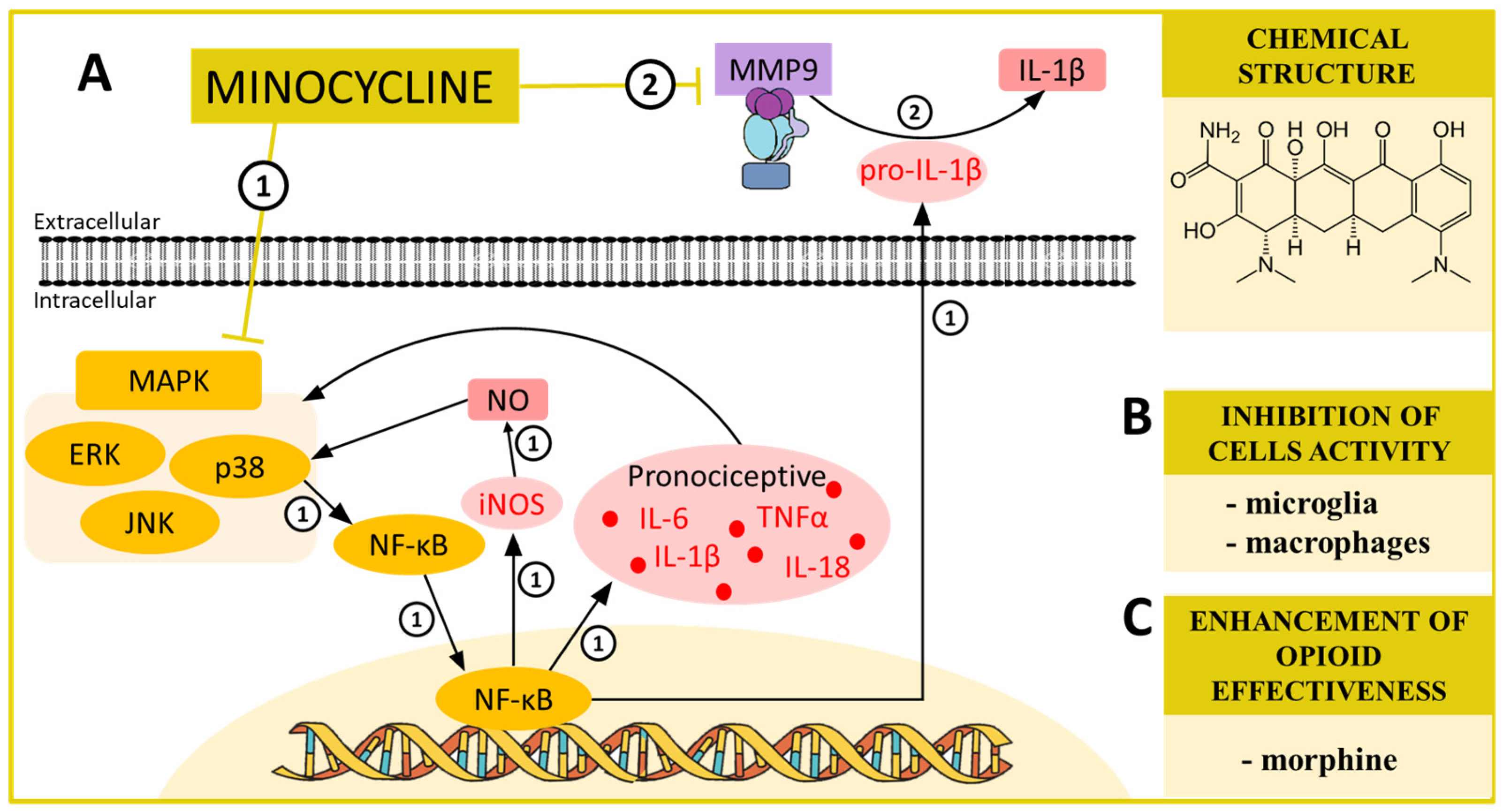
4. MINOCYCLINE—A MAPK and MMP Modulator in Neuropathic Pain
5. NRF2 and Neuropathic Pain
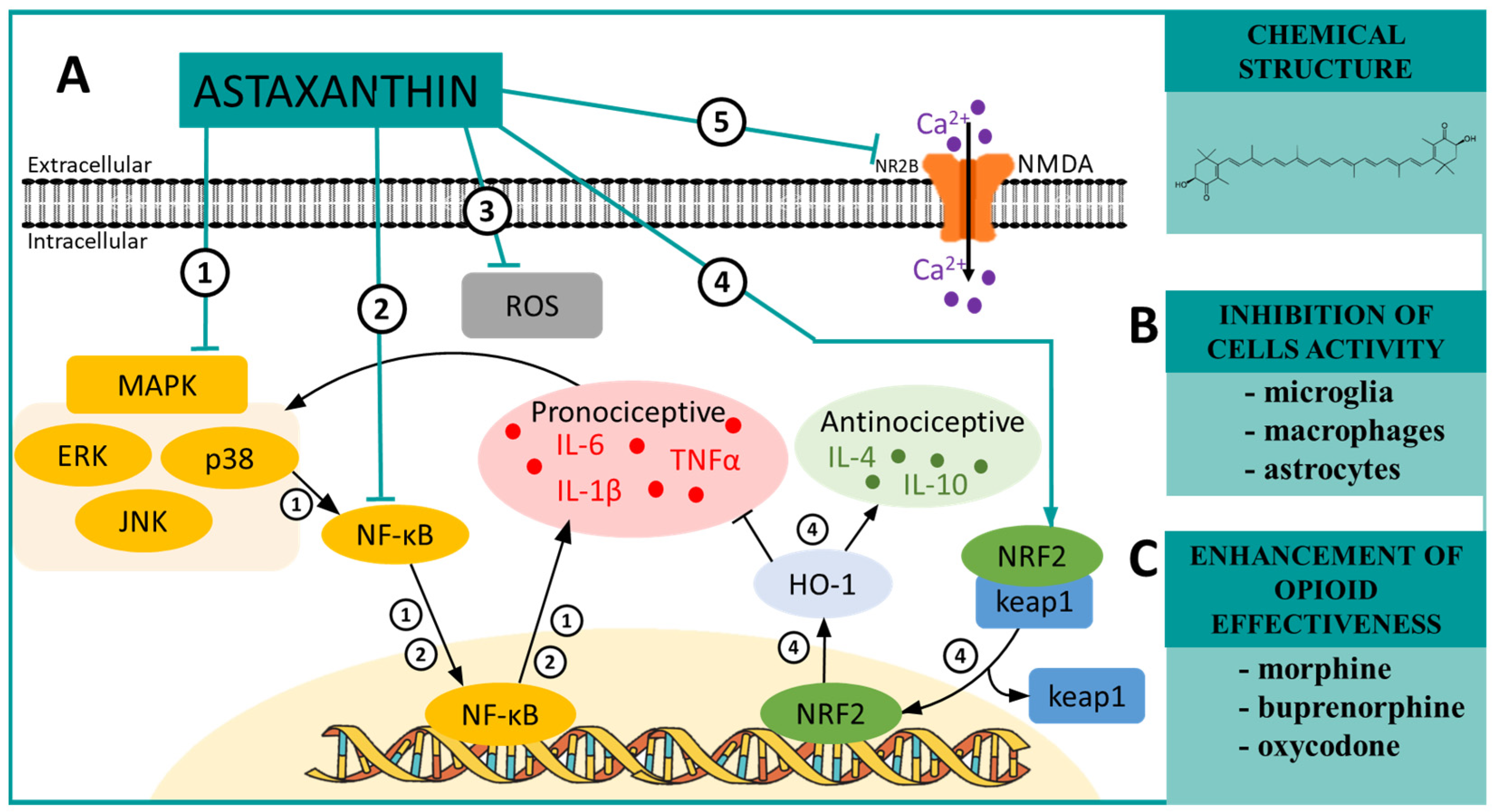
6. ASTAXANTHIN—A MAPK and NRF2 Modulator in Neuropathic Pain
7. NF-κB and Neuropathic Pain
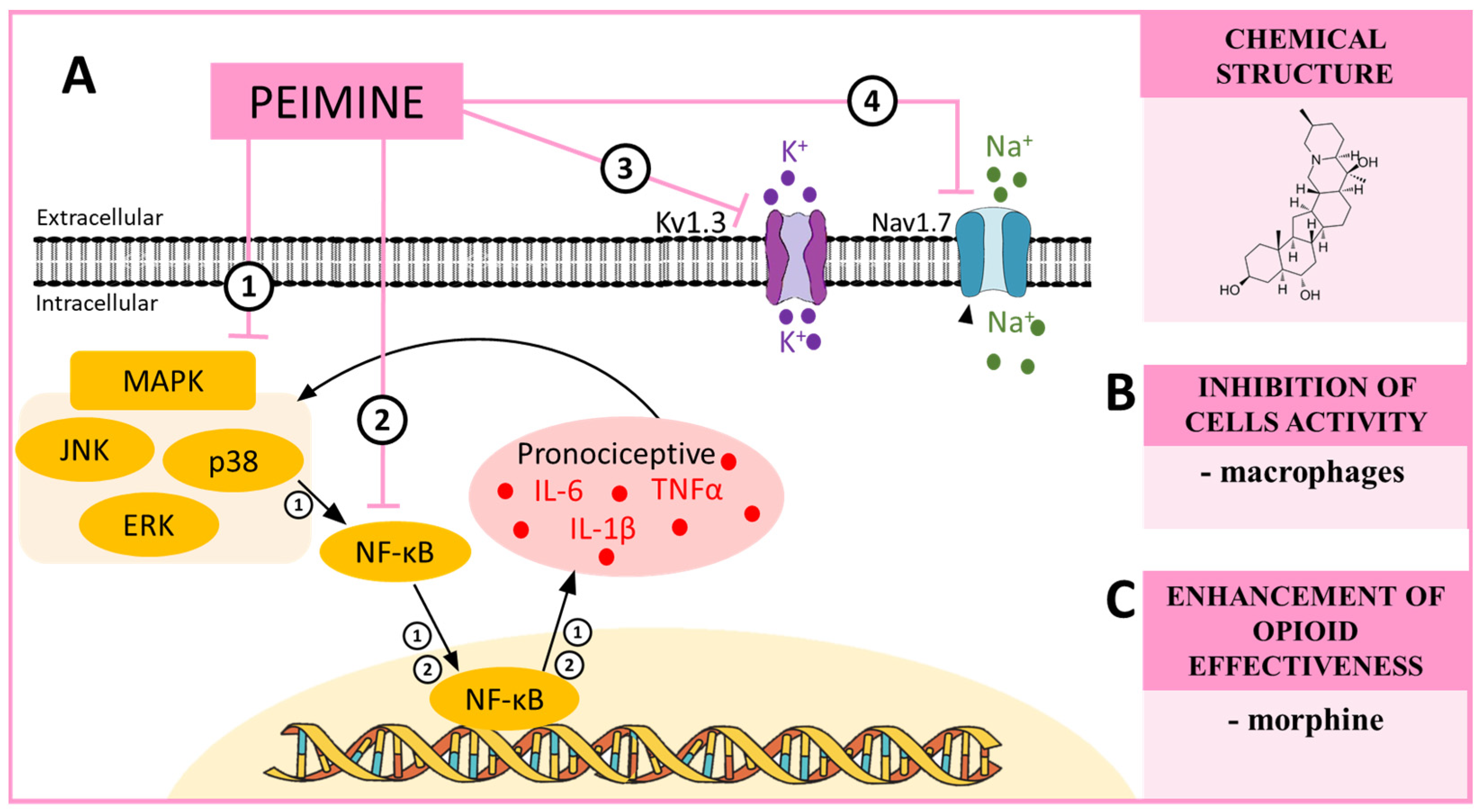
8. PEIMINE—A MAPK and NF-κB Modulator in Neuropathic Pain
9. PI3K/AKT and Neuropathic Pain
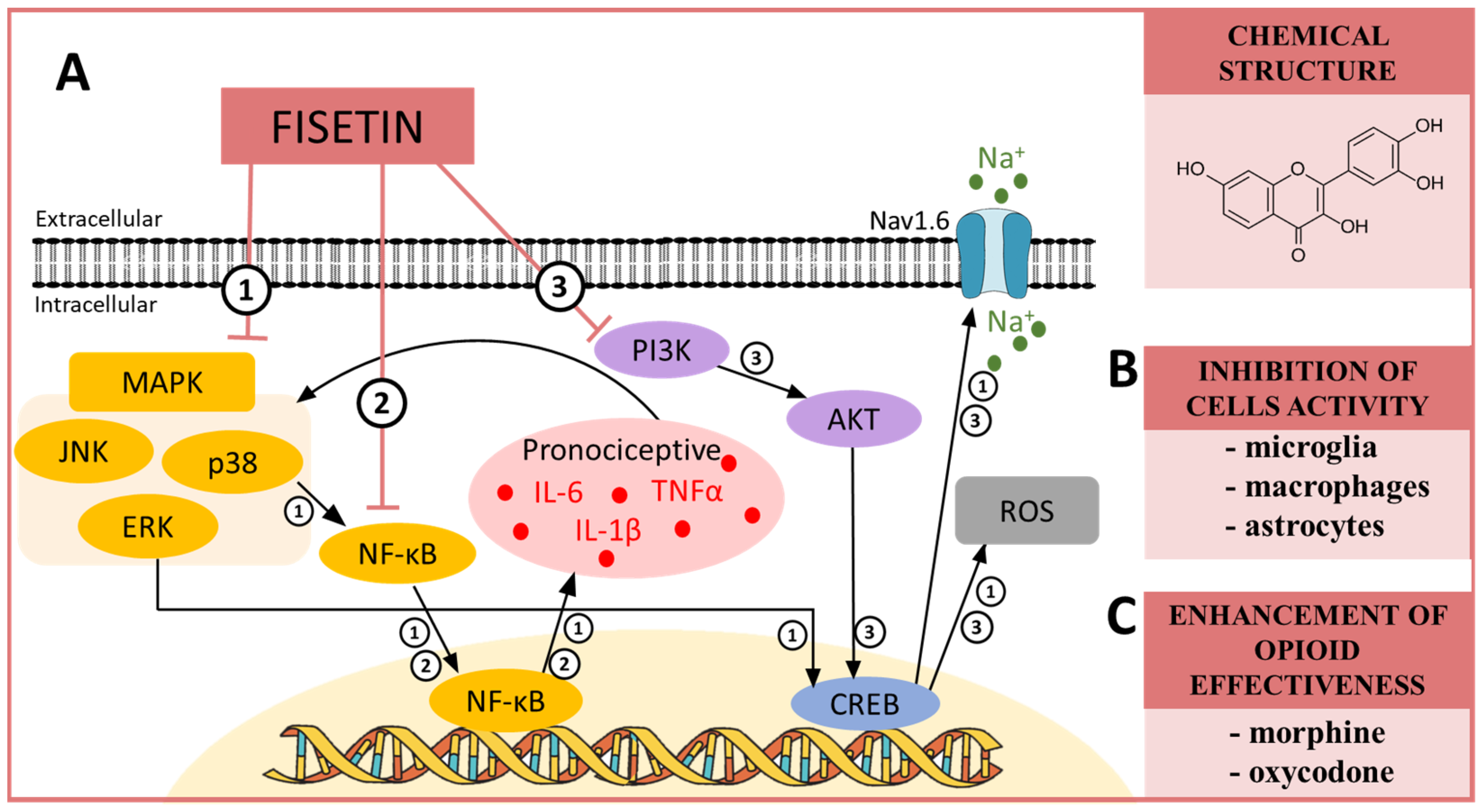
10. FISETIN—A MAPK, NF-κB and PI3K Modulator in Neuropathic Pain
11. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schembri, E. Are Opioids Effective in Relieving Neuropathic Pain? SN Compr. Clin. Med. 2019, 1, 30–46. [Google Scholar] [CrossRef]
- Van Hecke, O.; Austin, S.K.; Khan, R.A.; Smith, B.H.; Torrance, N. Neuropathic Pain in the General Population: A Systematic Review of Epidemiological Studies. Pain 2014, 155, 654–662. [Google Scholar] [CrossRef]
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic Pain. Nat. Rev. Dis. Prim. 2017, 3, 17002. [Google Scholar] [CrossRef] [PubMed]
- Fallon, M.T. Neuropathic Pain in Cancer. Br. J. Anaesth. 2013, 111, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Baba, H.; Brenner, G.J.; Woolf, C.J. Nociceptive-Specific Activation of ERK in Spinal Neurons Contributes to Pain Hypersensitivity. Nat. Neurosci. 1999, 2, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Samad, T.A.; Jin, S.X.; Schmoll, R.; Woolf, C.J. p38 MAPK Activation by NGF in Primary Sensory Neurons after Inflammation Increases TRPV1 Levels and Maintains Heat Hyperalgesia. Neuron 2002, 36, 57–68. [Google Scholar] [CrossRef]
- Zhuang, Z.-Y.; Wen, Y.-R.; Zhang, D.-R.; Borsello, T.; Bonny, C.; Strichartz, G.R.; Decosterd, I.; Ji, R.-R. A Peptide C-Jun N-Terminal Kinase (JNK) Inhibitor Blocks Mechanical Allodynia after Spinal Nerve Ligation: Respective Roles of JNK Activation in Primary Sensory Neurons and Spinal Astrocytes for Neuropathic Pain Development and Maintenance. J. Neurosci. 2006, 26, 3551–3560. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, B.-R.; Wang, X.; Kuang, F.; Duan, X.-L.; Jiao, X.-Y.; Ju, G. ERK1/2 and p38 Mitogen-Activated Protein Kinase Mediate INOS-Induced Spinal Neuron Degeneration after Acute Traumatic Spinal Cord Injury. Life Sci. 2006, 79, 1895–1905. [Google Scholar] [CrossRef]
- Karim, F.; Wang, C.C.; Gereau, R.W. Metabotropic Glutamate Receptor Subtypes 1 and 5 Are Activators of Extracellular Signal-Regulated Kinase Signaling Required for Inflammatory Pain in Mice. J. Neurosci. 2001, 21, 3771–3779. [Google Scholar] [CrossRef]
- Pezet, S.; Malcangio, M.; Lever, I.J.; Perkinton, M.S.; Thompson, S.W.N.; Williams, R.J.; McMahon, S.B. Noxious Stimulation Induces Trk Receptor and Downstream ERK Phosphorylation in Spinal Dorsal Horn. Mol. Cell. Neurosci. 2002, 21, 684–695. [Google Scholar] [CrossRef]
- Hains, B.C.; Waxman, S.G. Activated Microglia Contribute to the Maintenance of Chronic Pain after Spinal Cord Injury. J. Neurosci. 2006, 26, 4308–4317. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.-R.; Suter, M.R. p38 MAPK, Microglial Signaling, and Neuropathic Pain. Mol. Pain 2007, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Quirion, R. Partial Sciatic Nerve Ligation Induces Increase in the Phosphorylation of Extracellular Signal-Regulated Kinase (ERK) and c-Jun N-Terminal Kinase (JNK) in Astrocytes in the Lumbar Spinal Dorsal Horn and the Gracile Nucleus. Pain 2002, 99, 175–184. [Google Scholar] [CrossRef]
- Ma, W.; Quirion, R. The ERK/MAPK Pathway, as a Target for the Treatment of Neuropathic Pain. Expert Opin. Ther. Targets 2005, 9, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Mizokoshi, A.; Shigemoto-Mogami, Y.; Koizumi, S.; Inoue, K. Activation of p38 Mitogen-Activated Protein Kinase in Spinal Hyperactive Microglia Contributes to Pain Hypersensitivity Following Peripheral Nerve Injury. Glia 2004, 45, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.Y.; Gerner, P.; Woolf, C.J.; Ji, R.R. ERK Is Sequentially Activated in Neurons, Microglia, and Astrocytes by Spinal Nerve Ligation and Contributes to Mechanical Allodynia in This Neuropathic Pain Model. Pain 2005, 114, 149–159. [Google Scholar] [CrossRef]
- Morrison, D.K. MAP Kinase Pathways. Cold Spring Harb. Perspect. Biol. 2012, 4, a011254. [Google Scholar] [CrossRef]
- Pearson, G.; Robinson, F.; Beers Gibson, T.; Xu, B.; Karandikar, M.; Berman, K.; Cobb, M.H. Mitogen-Activated Protein (MAP) Kinase Pathways:Regulation and Physiological Functions. Endocr. Rev. 2001, 22, 153–183. [Google Scholar] [CrossRef]
- Jin, S.-X.; Zhuang, Z.-Y.; Woolf, C.J.; Ji, R.-R. p38 Mitogen-Activated Protein Kinase Is Activated After a Spinal Nerve Ligation in Spinal Cord Microglia and Dorsal Root Ganglion Neurons and Contributes to the Generation of Neuropathic Pain. J. Neurosci. 2003, 23, 4017–4022. [Google Scholar] [CrossRef]
- Zhou, T.T.; Wu, J.R.; Chen, Z.Y.; Liu, Z.X.; Miao, B. Effects of Dexmedetomidine on P2X4Rs, p38-MAPK and BDNF in Spinal Microglia in Rats with Spared Nerve Injury. Brain Res. 2014, 1568, 21–30. [Google Scholar] [CrossRef]
- Popiolek-Barczyk, K.; Kolosowska, N.; Piotrowska, A.; Makuch, W.; Rojewska, E.; Jurga, A.M.; Pilat, D.; Mika, J. Parthenolide Relieves Pain and Promotes M2 Microglia/Macrophage Polarization in Rat Model of Neuropathy. Neural Plast. 2015, 2015, 676473. [Google Scholar] [CrossRef]
- Rojewska, E.; Popiolek-Barczyk, K.; Kolosowska, N.; Piotrowska, A.; Zychowska, M.; Makuch, W.; Przewlocka, B.M.J. PD98059 Influences Immune Factors and Enhances Opioid Analgesia in Model of Neuropathy. PLoS ONE 2015, 10, e0138583. [Google Scholar] [CrossRef]
- Zhou, X.; Cheng, H.; Xu, D.; Yin, Q.; Cheng, L.; Wang, L.; Song, S.; Zhang, M. Attenuation of Neuropathic Pain by Saikosaponin a in a Rat Model of Chronic Constriction Injury. Neurochem. Res. 2014, 39, 2136–2142. [Google Scholar] [CrossRef]
- Wen, Y.R.; Suter, M.R.; Kawasaki, Y.; Huang, J.; Pertin, M.; Kohno, T.; Berde, C.B.; Decosterd, I.; Ji, R.R. Nerve Conduction Blockade in the Sciatic Nerve Prevents but Does Not Reverse the Activation of p38 Mitogen-Activated Protein Kinase in Spinal Microglia in the Rat Spared Nerve Injury Model. Anesthesiology 2007, 107, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Taves, S.; Berta, T.; Liu, D.L.; Gan, S.; Chen, G.; Kim, Y.H.; Van de Ven, T.; Laufer, S.; Ji, R.R. Spinal Inhibition of p38 MAP Kinase Reduces Inflammatory and Neuropathic Pain in Male but Not Female Mice: Sex-Dependent Microglial Signaling in the Spinal Cord. Brain. Behav. Immun. 2016, 55, 70–81. [Google Scholar] [CrossRef]
- Rojewska, E.; Popiolek-Barczyk, K.; Jurga, A.M.; Makuch, W.; Przewlocka, B.; Mika, J. Involvement of Pro- and Antinociceptive Factors in Minocycline Analgesia in Rat Neuropathic Pain Model. J. Neuroimmunol. 2014, 277, 57–66. [Google Scholar] [CrossRef]
- Chen, L.; Wang, H.; Xing, J.; Shi, X.; Huang, H.; Huang, J.; Xu, C.; Chen, L.; Wang, H.; Xing, J.; et al. Silencing P2X7R Alleviates Diabetic Neuropathic Pain Involving TRPV1 via PKCε/p38MAPK/NF-κB Signaling Pathway in Rats. Int. J. Mol. Sci. 2022, 23, 14141. [Google Scholar] [CrossRef] [PubMed]
- Milligan, E.D.; Twining, C.; Chacur, M.; Biedenkapp, J.; O’Connor, K.; Poole, S.; Tracey, K.; Martin, D.; Maier, S.F.; Watkins, L.R. Spinal Glia and Proinflammatory Cytokines Mediate Mirror-Image Neuropathic Pain in Rats. J. Neurosci. 2003, 23, 1026. [Google Scholar] [CrossRef] [PubMed]
- Gilhotra, N.; Sharma, A.; Singh, M.; Dhingra, D. Involvement of p38 MAPkinase in Attenuation of Antinociceptive Effect of Morphine in Diabetic Mice. Indian J. Exp. Biol. 2007, 45, 654–656. [Google Scholar]
- Wang, Z.; Chabot, J.G.; Quirion, R. On the Possible Role of ERK, p38 and CaMKII in the Regulation of CGRP Expression in Morphine-Tolerant Rats. Mol. Pain 2011, 7, 1744–8069. [Google Scholar] [CrossRef] [PubMed]
- Sweitzer, S.M.; Medicherla, S.; Almirez, R.; Dugar, S.; Chakravarty, S.; Shumilla, J.A.; Yeomans, D.C.; Protter, A.A. Antinociceptive Action of a p38alpha MAPK Inhibitor, SD-282, in a Diabetic Neuropathy Model. Pain 2004, 109, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Shenoy, R.; Palmer, J.E.; Baines, A.J.; Lai, R.Y.K.; Robertson, J.; Bird, N.; Ostenfeld, T.; Chizh, B.A. Clinical Trial of the p38 MAP Kinase Inhibitor Dilmapimod in Neuropathic Pain Following Nerve Injury. Eur. J. Pain 2011, 15, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Ostenfeld, T.; Krishen, A.; Lai, R.Y.; Bullman, J.; Green, J.; Anand, P.; Scholz, J.; Kelly, M. A Randomized, Placebo-Controlled Trial of the Analgesic Efficacy and Safety of the p38 MAP Kinase Inhibitor, Losmapimod, in Patients with Neuropathic Pain from Lumbosacral Radiculopathy. Clin. J. Pain 2015, 31, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Willemen, H.L.D.M.; Campos, P.M.; Lucas, E.; Morreale, A.; GIL-Redondo, R.; Agut, J.; González, F.V.; Ramos, P.; Heijnen, C.; Mayor, F.; et al. A Novel p38 MAPK Docking Groove-Targeted Compound Is a Potent Inhibitor of Inflammatory Hyperalgesia. Biochem. J. 2014, 459, 427. [Google Scholar] [CrossRef]
- Visseq, A.; Descheemaeker, A.; Pinto-Pardo, N.; Nauton, L.; Théry, V.; Giraud, F.; Abrunhosa-Thomas, I.; Artola, A.; Anizon, F.; Dallel, R.; et al. Pyridin-2(1H)One Derivatives: A Possible New Class of Therapeutics for Mechanical Allodynia. Eur. J. Med. Chem. 2020, 187, 111917. [Google Scholar] [CrossRef]
- Svensson, C.I.; Hua, X.-Y.; Protter, A.A.; Powell, H.C.; Yaksh, T.L. Spinal p38 MAP Kinase Is Necessary for NMDA-Induced Spinal PGE(2) Release and Thermal Hyperalgesia. NeuroReport 2003, 14, 1153–1157. [Google Scholar] [CrossRef]
- Cui, Y.; Liao, X.X.; Liu, W.; Guo, R.X.; Wu, Z.Z.; Zhao, C.M.; Chen, P.X.; Feng, J.Q. A Novel Role of Minocycline: Attenuating Morphine Antinociceptive Tolerance by Inhibition of p38 MAPK in the Activated Spinal Microglia. Brain. Behav. Immun. 2008, 22, 114–123. [Google Scholar] [CrossRef]
- Zhuang, Z.Y.; Kawasaki, Y.; Tan, P.H.; Wen, Y.R.; Huang, J.; Ji, R.R. Role of the CX3CR1/p38 MAPK Pathway in Spinal Microglia for the Development of Neuropathic Pain Following Nerve Injury-Induced Cleavage of Fractalkine. Brain. Behav. Immun. 2007, 21, 642–651. [Google Scholar] [CrossRef]
- Kumar, S.; Boehm, J.; Lee, J.C. p38 MAP Kinases: Key Signalling Molecules as Therapeutic Targets for Inflammatory Diseases. Nat. Rev. Drug Discov. 2003, 2, 717–726. [Google Scholar] [CrossRef]
- Sung, C.-S.; Wen, Z.-H.; Chang, W.-K.; Chan, K.-H.; Ho, S.-T.; Tsai, S.-K.; Chang, Y.-C.; Wong, C.-S. Inhibition of p38 Mitogen-Activated Protein Kinase Attenuates Interleukin-1beta-Induced Thermal Hyperalgesia and Inducible Nitric Oxide Synthase Expression in the Spinal Cord. J. Neurochem. 2005, 94, 742–752. [Google Scholar] [CrossRef]
- Mei, X.-P.; Sakuma, Y.; Xie, C.; Wu, D.; Ho, I.; Kotani, J.; Xu, L.-X. Depressing Interleukin-1β Contributed to the Synergistic Effects of Tramadol and Minocycline on Spinal Nerve Ligation-Induced Neuropathic Pain. Neurosignals. 2013, 710032, 30–42. [Google Scholar] [CrossRef]
- Piotrowska, A.; Popiolek-Barczyk, K.; Pavone, F.; Mika, J. Comparison of the Expression Changes after Botulinum Toxin Type A and Minocycline Administration in Lipopolysaccharide-Stimulated Rat Microglial and Astroglial Cultures. Front. Cell. Infect. Microbiol. 2017, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, V.; Tanga, F.; Deleo, J.A. Inhibition of Microglial Activation Attenuates the Development but Not Existing Hypersensitivity in a Rat Model of Neuropathy. J. Pharmacol. Exp. Ther. 2003, 306, 624–630. [Google Scholar] [CrossRef]
- Miyoshi, K.; Obata, K.; Kondo, T.; Okamura, H.; Noguchi, K. Interleukin-18-Mediated Microglia/Astrocyte Interaction in the Spinal Cord Enhances Neuropathic Pain Processing after Nerve Injury. J. Neurosci. 2008, 28, 12775–12787. [Google Scholar] [CrossRef]
- Daigo, E.; Sakuma, Y.; Miyoshi, K.; Noguchi, K.; Kotani, J. Increased Expression of Interleukin-18 in the Trigeminal Spinal Subnucleus Caudalis after Inferior Alveolar Nerve Injury in the Rat. Neurosci. Lett. 2012, 529, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.J.; Moalem-Taylor, G. The Neuro-Immune Balance in Neuropathic Pain: Involvement of Inflammatory Immune Cells, Immune-like Glial Cells and Cytokines. J. Neuroimmunol. 2010, 229, 26–50. [Google Scholar] [CrossRef]
- Pilat, D.; Piotrowska, A.; Rojewska, E.; Jurga, A.; Ślusarczyk, J.; Makuch, W.; Basta-Kaim, A.; Przewlocka, B.; Mika, J. Blockade of IL-18 Signaling Diminished Neuropathic Pain and Enhanced the Efficacy of Morphine and Buprenorphine. Mol. Cell. Neurosci. 2016, 71, 114–124. [Google Scholar] [CrossRef]
- Pilat, D.; Rojewska, E.; Jurga, A.M.; Piotrowska, A.; Makuch, W.; Przewlocka, B.; Mika, J. IL-1 Receptor Antagonist Improves Morphine and Buprenorphine Efficacy in a Rat Neuropathic Pain Model. Eur. J. Pharmacol. 2015, 764, 240–248. [Google Scholar] [CrossRef]
- Ren, K.; Dubner, R. Neuron-Glia Crosstalk Gets Serious: Role in Pain Hypersensitivity. Curr. Opin. Anaesthesiol. 2008, 21, 570. [Google Scholar] [CrossRef]
- Bouhassira, D. Neuropathic Pain: Definition, Assessment and Epidemiology. Rev. Neurol. 2019, 175, 16–25. [Google Scholar] [CrossRef]
- Bading, H.; Greenberg, M.E. Stimulation of Protein Tyrosine Phosphorylation by NMDA Receptor Activation. Science 1991, 253, 912–914. [Google Scholar] [CrossRef] [PubMed]
- Fiore, R.S.; Murphy, T.H.; Sanghera, J.S.; Pelech, S.L.; Baraban, J.M. Activation of P42 Mitogen-Activated Protein Kinase by Glutamate Receptor Stimulation in Rat Primary Cortical Cultures. J. Neurochem. 1993, 61, 1626–1633. [Google Scholar] [CrossRef] [PubMed]
- Rosen, L.B.; Ginty, D.D.; Weber, M.J.; Greenberg, M.E. Membrane Depolarization and Calcium Influx Stimulate MEK and MAP Kinase via Activation of Ras. Neuron 1994, 12, 1207–1221. [Google Scholar] [CrossRef] [PubMed]
- Slusarczyk, J.; Trojan, E.; Glombik, K.; Piotrowska, A.; Budziszewska, B.; Kubera, M.; Popiolek-Barczyk, K.; Lason, W.; Mika, J.; Basta-Kaim, A. Anti-Inflammatory Properties of Tianeptine on Lipopolysaccharide-Induced Changes in Microglial Cells Involve Toll-like Receptor-Related Pathways. J. Neurochem. 2015, 136, 958–970. [Google Scholar] [CrossRef]
- Morozov, A.; Muzzio, I.A.; Bourtchouladze, R.; Van-Strien, N.; Lapidus, K.; Yin, D.; Winder, D.G.; Adams, J.P.; Sweatt, J.D.; Kandel, E.R. Rap1 Couples CAMP Signaling to a Distinct Pool of P42/44MAPK Regulating Excitability, Synaptic Plasticity, Learning, and Memory. Neuron 2003, 39, 309–325. [Google Scholar] [CrossRef]
- Fitzgerald, E.M. Regulation of Voltage-Dependent Calcium Channels in Rat Sensory Neurones Involves a Ras—Mitogen-Activated Protein Kinase Pathway. J. Physiol. 2000, 527, 433–444. [Google Scholar] [CrossRef]
- Han, M.; Huang, R.-Y.; Du, Y.-M.; Zhao, Z.-Q.; Zhang, Y.-Q. Early Intervention of ERK Activation in the Spinal Cord Can Block Initiation of Peripheral Nerve Injury-Induced Neuropathic Pain in Rats. Sheng Li Xue Bao 2011, 63, 106–114. [Google Scholar]
- Dai, Z.K.; Lin, T.C.; Liou, J.C.; Cheng, K.I.; Chen, J.Y.; Chu, L.W.; Chen, I.J.; Wu, B.N. Xanthine Derivative KMUP-1 Reduces Inflammation and Hyperalgesia in a Bilateral Chronic Constriction Injury Model by Suppressing MAPK and NF-κB Activation. Mol. Pharm. 2014, 11, 1621–1631. [Google Scholar] [CrossRef]
- Popiolek-Barczyk, K.; Makuch, W.; Rojewska, E.; Pilat, D.; Mika, J. Inhibition of Intracellular Signaling Pathways NF-κB and MEK1/2 Attenuates Neuropathic Pain Development and Enhances Morphine Analgesia. Pharmacol. Rep. 2014, 66, 845–851. [Google Scholar] [CrossRef]
- Ciruela, A.; Dixon, A.K.; Bramwell, S.; Gonzalez, M.I.; Pinnock, R.D.; Lee, K. Identification of MEK1 as a Novel Target for the Treatment of Neuropathic Pain. Br. J. Pharmacol. 2003, 138, 751–756. [Google Scholar] [CrossRef]
- Shao, J.; Yu, W.; Wei, W.; Wang, S.; Zheng, Z.; Li, L.; Sun, Y.; Zhang, J.; Li, Z.; Ren, X.; et al. MAPK-ERK-CREB Signaling Pathway Upregulates Nav1.6 in Oxaliplatin-Induced Neuropathic Pain in the Rat. Toxicol. Lett. 2023, 384, 149–160. [Google Scholar] [CrossRef]
- Sanna, M.D.; Manassero, G.; Vercelli, A.; Herdegen, T.; Galeotti, N. The Isoform-Specific Functions of the c-Jun N-Terminal Kinase (JNK) in a Mouse Model of Antiretroviral-Induced Painful Peripheral Neuropathy. Eur. J. Pharmacol. 2020, 880, 173161. [Google Scholar] [CrossRef]
- Wang, J.; Van De Water, T.R.; Bonny, C.; De Ribaupierre, F.; Puel, J.L.; Zine, A. A Peptide Inhibitor of C-Jun N-Terminal Kinase Protects against Both Aminoglycoside and Acoustic Trauma-Induced Auditory Hair Cell Death and Hearing Loss. J. Neurosci. 2003, 23, 8596–8607. [Google Scholar] [CrossRef]
- Tezel, G.; Yang, X.; Yang, J.; Wax, M.B. Role of Tumor Necrosis Factor Receptor-1 in the Death of Retinal Ganglion Cells Following Optic Nerve Crush Injury in Mice. Brain Res. 2004, 996, 202–212. [Google Scholar] [CrossRef]
- Yamasaki, T.; Kawasaki, H.; Nishina, H. Diverse Roles of JNK and MKK Pathways in the Brain. J. Signal Transduct. 2012, 2012, 459265. [Google Scholar] [CrossRef] [PubMed]
- Ip, Y.T.; Davis, R.J. Signal Transduction by the C-Jun N-Terminal Kinase (JNK)—From Inflammation to Development. Curr. Opin. Cell Biol. 1998, 10, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Borsello, T.; Clarkel, P.G.H.; Hirt, L.; Vercelli, A.; Repici, M.; Schorderet, D.F.; Bogousslavsky, J.; Bonny, C. A Peptide Inhibitor of C-Jun N-Terminal Kinase Protects against Excitotoxicity and Cerebral Ischemia. Nat. Med. 2003, 9, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhu, C.; Li, Z.; Liu, X.; Sun, S.; Zhang, T.; Luo, Z.; Zhang, H.; Li, W. Inhibition of the Spinal Astrocytic JNK/MCP-1 Pathway Activation Correlates with the Analgesic Effects of Tanshinone IIA Sulfonate in Neuropathic Pain. J. Neuroinflammation 2015, 12, 57. [Google Scholar] [CrossRef] [PubMed]
- Sanna, M.D.; Ghelardini, C.; Galeotti, N. Blockade of the Spinal BDNF-Activated JNK Pathway Prevents the Development of Antiretroviral-Induced Neuropathic Pain. Neuropharmacology 2016, 105, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.P.; Xu, H.; Xie, C.; Ren, J.; Zhou, Y.; Zhang, H.; Xu, L.X. Post-Injury Administration of Minocycline: An Effective Treatment for Nerve-Injury Induced Neuropathic Pain. Neurosci. Res. 2011, 70, 305–312. [Google Scholar] [CrossRef]
- Hua, X.Y.; Svensson, C.I.; Matsui, T.; Fitzsimmons, B.; Yaksh, T.L.; Webb, M. Intrathecal Minocycline Attenuates Peripheral Inflammation-Induced Hyperalgesia by Inhibiting p38 MAPK in Spinal Microglia. Eur. J. Neurosci. 2005, 22, 2431–2440. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.H.; Lee, M.J.; Jang, M.; Gwak, N.G.; Lee, K.Y.; Jung, H.S. Minocycline Markedly Reduces Acute Visceral Nociception via Inhibiting Neuronal ERK Phosphorylation. Mol. Pain 2012, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Niimi, N.; Kohyama, K.; Matsumoto, Y. Minocycline Suppresses Experimental Autoimmune Encephalomyelitis by Increasing Tissue Inhibitors of Metalloproteinases. Neuropathology 2013, 33, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Mika, J.; Wawrzczak-Bargiela, A.; Osikowicz, M.; Makuch, W.; Przewlocka, B. Attenuation of Morphine Tolerance by Minocycline and Pentoxifylline in Naive and Neuropathic Mice. Brain Behav. Immun. 2009, 23, 75–84. [Google Scholar] [CrossRef]
- Mika, J.; Osikowicz, M.; Makuch, W.; Przewlocka, B. Minocycline and Pentoxifylline Attenuate Allodynia and Hyperalgesia and Potentiate the Effects of Morphine in Rat and Mouse Models of Neuropathic Pain. Eur. J. Pharmacol. 2007, 560, 142–149. [Google Scholar] [CrossRef]
- Mika, J.; Rojewska, E.; Makuch, W.; Przewlocka, B. Minocycline Reduces the Injury-Induced Expression of Prodynorphin and Pronociceptin in the Dorsal Root Ganglion in a Rat Model of Neuropathic Pain. Neuroscience 2010, 165, 1420–1428. [Google Scholar] [CrossRef]
- Rojewska, E.; Korostynski, M.; Przewlocki, R.; Przewlocka, B.; Mika, J. Expression Profiling of Genes Modulated by Minocycline in a Rat Model of Neuropathic Pain. Mol. Pain 2014, 10, 47. [Google Scholar] [CrossRef]
- Qiao, L.; Tang, Q.; An, Z.; Qi, J. Minocycline Relieves Neuropathic Pain in Rats with Spinal Cord Injury via Activation of Autophagy and Suppression of PI3K/Akt/MTOR Pathway. J. Pharmacol. Sci. 2023, 153, 12–21. [Google Scholar] [CrossRef]
- Sun, J.S.; Yang, Y.J.; Zhang, Y.Z.; Huang, W.; Li, Z.S.; Zhang, Y. Minocycline Attenuates Pain by Inhibiting Spinal Microglia Activation in Diabetic Rats. Mol. Med. Rep. 2015, 12, 2677–2682. [Google Scholar] [CrossRef]
- Zychowska, M.; Rojewska, E.; Kreiner, G.; Nalepa, I.; Przewlocka, B.; Mika, J. Minocycline Influences the Anti-Inflammatory Interleukins and Enhances the Effectiveness of Morphine under Mice Diabetic Neuropathy. J. Neuroimmunol. 2013, 262, 35–45. [Google Scholar] [CrossRef]
- Makuch, W.; Mika, J.; Rojewska, E.; Zychowska, M.; Przewlocka, B. Effects of Selective and Non-Selective Inhibitors of Nitric Oxide Synthase on Morphine- and Endomorphin-1-Induced Analgesia in Acute and Neuropathic Pain in Rats. Neuropharmacology 2013, 75, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Rojewska, E.; Makuch, W.; Przewlocka, B.; Mika, J. Minocycline Prevents Dynorphin-Induced Neurotoxicity during Neuropathic Pain in Rats. Neuropharmacology 2014, 86, 301–310. [Google Scholar] [CrossRef]
- Ciechanowska, A.; Rojewska, E.; Piotrowska, A.; Barut, J.; Pawlik, K.; Ciapała, K.; Kreiner, G.; Mika, J. New Insights into the Analgesic Properties of the XCL1/XCR1 and XCL1/ITGA9 Axes Modulation under Neuropathic Pain Conditions—Evidence from Animal Studies. Front. Immunol. 2022, 13, 1058204. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Fu, S.; Shi, X.; Liu, R. Microglial BDNF, PI3K, and p-ERK in the Spinal Cord Are Suppressed by Pulsed Radiofrequency on Dorsal Root Ganglion to Ease SNI-Induced Neuropathic Pain in Rats. Pain Res. Manag. 2019, 2019, 5948686. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Tao, X.; Song, T. Astaxanthin Alleviates Neuropathic Pain by Inhibiting the MAPKs and NF-κB Pathways. Eur. J. Pharmacol. 2021, 912, 174575. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, L.; Aggarwal, A. Astaxanthin Inhibits Cytokines Production and Inflammatory Gene Expression by Suppressing IκB Kinase-Dependent Nuclear Factor ΚB Activation in Pre and Postpartum Murrah Buffaloes during Different Seasons. Vet. World 2018, 11, 782–788. [Google Scholar] [CrossRef]
- Fakhri, S.; Dargahi, L.; Abbaszadeh, F.; Jorjani, M. Astaxanthin Attenuates Neuroinflammation Contributed to the Neuropathic Pain and Motor Dysfunction Following Compression Spinal Cord Injury. Brain Res. Bull. 2018, 143, 217–224. [Google Scholar] [CrossRef]
- Qu, X.; Zhang, Z.; Hu, W.; Lou, M.; Zhai, B.; Mei, S.; Hu, Z.; Zhang, L.; Liu, D.; Liu, Z.; et al. Attenuation of the Na/K-ATPase/Src/ROS Amplification Signaling Pathway by Astaxanthin Ameliorates Myocardial Cell Oxidative Stress Injury. Mol. Med. Rep. 2020, 22, 5125–5134. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, D.; Sharma, M.; Sharma, N.; Bidve, P.; Prajapati, N.; Kalia, K.; Tiwari, V. Astaxanthin Ameliorates Behavioral and Biochemical Alterations in In-Vitro and in-Vivo Model of Neuropathic Pain. Neurosci. Lett. 2018, 674, 162–170. [Google Scholar] [CrossRef]
- Ciapała, K.; Rojewska, E.; Pawlik, K.; Ciechanowska, A.; Mika, J. Analgesic Effects of Fisetin, Peimine, Astaxanthin, Artemisinin, Bardoxolone Methyl and 740 Y-P and Their Influence on Opioid Analgesia in a Mouse Model of Neuropathic Pain. Int. J. Mol. Sci. 2023, 24, 9000. [Google Scholar] [CrossRef]
- Chen, K.; Lv, Z.T.; Zhou, C.H.; Liang, S.; Huang, W.; Wang, Z.G.; Zhu, W.T.; Wang, Y.T.; Jing, X.Z.; Lin, H.; et al. Peimine Suppresses Interleukin-1β-Induced Inflammation via MAPK Downregulation in Chondrocytes. Int. J. Mol. Med. 2019, 43, 2241–2251. [Google Scholar] [CrossRef] [PubMed]
- Yi, P.F.; Wu, Y.C.; Dong, H.B.; Guo, Y.; Wei, Q.; Zhang, C.; Song, Z.; Qin, Q.Q.; Lv, S.; Wu, S.C.; et al. Peimine Impairs Pro-Inflammatory Cytokine Secretion through the Inhibition of the Activation of NF-κB and MAPK in LPS-Induced RAW264.7 Macrophages. Immunopharmacol. Immunotoxicol. 2013, 35, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhao, W.; Pan, L.; Zhang, A.; Chen, Q.; Xu, K.; Lu, H.; Chen, Y. Peimine, a Main Active Ingredient of Fritillaria, Exhibits Anti-Inflammatory and Pain Suppression Properties at the Cellular Level. Fitoterapia 2016, 111, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chuang, J.Y.; Chang, P.C.; Shen, Y.C.; Lin, C.; Tsai, C.F.; Chen, J.H.; Yeh, W.L.; Wu, L.H.; Lin, H.Y.; Liu, Y.S.; et al. Regulatory Effects of Fisetin on Microglial Activation. Molecules 2014, 19, 8820–8839. [Google Scholar] [CrossRef]
- Wang, T.H.; Wang, S.Y.; Wang, X.D.; Jiang, H.Q.; Yang, Y.Q.; Wang, Y.; Cheng, J.L.; Zhang, C.T.; Liang, W.W.; Feng, H.L. Fisetin Exerts Antioxidant and Neuroprotective Effects in Multiple Mutant HSOD1 Models of Amyotrophic Lateral Sclerosis by Activating ERK. Neuroscience 2018, 379, 152–166. [Google Scholar] [CrossRef]
- Hassan, S.S.U.; Samanta, S.; Dash, R.; Karpiński, T.M.; Habibi, E.; Sadiq, A.; Ahmadi, A.; Bungau, S. The Neuroprotective Effects of Fisetin, a Natural Flavonoid in Neurodegenerative Diseases: Focus on the Role of Oxidative Stress. Front. Pharmacol. 2022, 13, 1015835. [Google Scholar] [CrossRef]
- Ren, Q.; Guo, F.; Tao, S.; Huang, R.; Ma, L.; Fu, P. Flavonoid Fisetin Alleviates Kidney Inflammation and Apoptosis via Inhibiting Src-Mediated NF-κB P65 and MAPK Signaling Pathways in Septic AKI Mice. Biomed. Pharmacother. 2020, 122, 109772. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, C.; Cui, W.G.; Ma, Q.; Zhou, W.H. Fisetin Exerts Antihyperalgesic Effect in a Mouse Model of Neuropathic Pain: Engagement of Spinal Serotonergic System. Sci. Rep. 2015, 5, srep09043. [Google Scholar] [CrossRef]
- Zhao, X.; Li, X.L.; Liu, X.; Wang, C.; Zhou, D.S.; Ma, Q.; Zhou, W.H.; Hu, Z.Y. Antinociceptive Effects of Fisetin against Diabetic Neuropathic Pain in Mice: Engagement of Antioxidant Mechanisms and Spinal GABAA Receptors. Pharmacol. Res. 2015, 102, 286–297. [Google Scholar] [CrossRef]
- Sandireddy, R.; Yerra, V.G.; Komirishetti, P.; Areti, A.; Kumar, A. Fisetin Imparts Neuroprotection in Experimental Diabetic Neuropathy by Modulating Nrf2 and NF-κB Pathways. Cell. Mol. Neurobiol. 2016, 36, 883–892. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Myers, R.R.; Janes, J.; Shubayev, V. Cytokine Regulation of MMP-9 in Peripheral Glia: Implications for Pathological Processes and Pain in Injured Nerve. Brain. Behav. Immun. 2007, 21, 561–568. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Xu, Z.Z.; Wang, X.; Park, J.Y.; Zhuang, Z.Y.; Tan, P.H.; Gao, Y.J.; Roy, K.; Corfas, G.; Lo, E.H.; et al. Distinct Roles of Matrix Metalloproteases in the Early- and Late-Phase Development of Neuropathic Pain. Nat. Med. 2008, 14, 331–336. [Google Scholar] [CrossRef]
- Kobayashi, H.; Chattopadhyay, S.; Kato, K.; Dolkas, J.; Kikuchi, S.; Myers, R.R.; Shubayev, V.I. MMPs Initiate Schwann Cell-Mediated MBP Degradation and Mechanical Nociception after Nerve Damage. Mol. Cell. Neurosci. 2008, 39, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Chandler, S.; Coates, R.; Gearing, A.; Lury, J.; Wells, G.; Bone, E. Matrix Metalloproteinases Degrade Myelin Basic Protein. Neurosci. Lett. 1995, 201, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Devor, M. Sodium Channels and Mechanisms of Neuropathic Pain. J. Pain 2006, 7, S3. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Khanna, D.; Kalra, S. Minocycline and Doxycycline: More Than Antibiotics. Curr. Mol. Pharmacol. 2021, 14, 1046–1065. [Google Scholar] [CrossRef]
- Kim, H.S.; Suh, Y.H. Minocycline and Neurodegenerative Diseases. Behav. Brain Res. 2009, 196, 168–179. [Google Scholar] [CrossRef]
- Garrido-Mesa, N.; Zarzuelo, A.; Gálvez, J. Minocycline: Far beyond an Antibiotic. Br. J. Pharmacol. 2013, 169, 337–352. [Google Scholar] [CrossRef]
- Garrido-Mesa, N.; Zarzuelo, A.; Gálvez, J. What Is behind the Non-Antibiotic Properties of Minocycline? Pharmacol. Res. 2013, 67, 18–30. [Google Scholar] [CrossRef]
- Yrjänheikki, J.; Tikka, T.; Keinänen, R.; Goldsteins, G.; Chan, P.H.; Koistinaho, J. A Tetracycline Derivative, Minocycline, Reduces Inflammation and Protects against Focal Cerebral Ischemia with a Wide Therapeutic Window. Proc. Natl. Acad. Sci. USA 1999, 96, 13496–13500. [Google Scholar] [CrossRef]
- Golub, L.M.; Ramamurthy, N.S.; McNamara, T.F.; Greenwald, R.A.; Rifkin, B.R. Tetracyclines Inhibit Connective Tissue Breakdown: New Therapeutic Implications for an Old Family of Drugs. Crit. Rev. Oral Biol. Med. 1991, 2, 297–321. [Google Scholar] [CrossRef]
- Festoff, B.W.; Ameenuddin, S.; Arnold, P.M.; Wong, A.; Santacruz, K.S.; Citron, B.A. Minocycline Neuroprotects, Reduces Microgliosis, and Inhibits Caspase Protease Expression Early after Spinal Cord Injury. J. Neurochem. 2006, 97, 1314–1326. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, H.S.; Shin, K.Y.; Kim, E.M.; Kim, M.; Kim, H.S.; Park, C.H.; Jeong, Y.H.; Yoo, J.; Lee, J.P.; et al. Minocycline Attenuates Neuronal Cell Death and Improves Cognitive Impairment in Alzheimer’s Disease Models. Neuropsychopharmacology 2007, 32, 2393–2404. [Google Scholar] [CrossRef]
- Thomas, M.; Le, W. Minocycline: Neuroprotective Mechanisms in Parkinson’s Disease. Curr. Pharm. Des. 2004, 10, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Ashizawa, T.; Jankovic, J. Minocycline in Huntington’s Disease: A Pilot Study. Mov. Disord. 2004, 19, 692–695. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Stavrovskaya, I.G.; Drozda, M.; Kim, B.Y.S.; Ona, V.; Li, M.; Sarang, S.; Liu, A.S.; Hartley, D.M.; Wu, D.C.; et al. Minocycline Inhibits Cytochrome c Release and Delays Progression of Amyotrophic Lateral Sclerosis in Mice. Nature 2002, 417, 74–78. [Google Scholar] [CrossRef]
- Brundula, V.; Rewcastle, N.B.; Metz, L.M.; Bernard, C.C.; Yong, V.W. Targeting Leukocyte MMPs and Transmigration: Minocycline as a Potential Therapy for Multiple Sclerosis. Brain 2002, 125, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Huang, X.; Pan, X.; Zhang, T.; Hou, C.; Su, W.J.; Liu, L.L.; Li, J.M.; Wang, Y.X. Minocycline Prevents the Depressive-like Behavior through Inhibiting the Release of HMGB1 from Microglia and Neurons. Brain. Behav. Immun. 2020, 88, 132–143. [Google Scholar] [CrossRef]
- Pan, Y.D.; Guo, Q.L.; Wang, E.; Ye, Z.; He, Z.H.; Zou, W.Y.; Cheng, Z.G.; Wang, Y.-J. Intrathecal Infusion of Pyrrolidine Dithiocarbamate for the Prevention and Reversal of Neuropathic Pain in Rats Using a Sciatic Chronic Constriction Injury Model. Reg. Anesth. Pain Med. 2010, 35, 231–237. [Google Scholar] [CrossRef]
- Wang, P.; Farmer, J.P.; Rullo, J. Minocycline-Induced Hyperpigmentation. JAMA Dermatol. 2021, 157, 992. [Google Scholar] [CrossRef]
- Budni, J.; Garcez, M.L.; de Medeiros, J.; Cassaro, E.; Bellettini-Santos, T.; Mina, F.; Quevedo, J. The Anti-Inflammatory Role of Minocycline in Alzheimers Disease. Curr. Alzheimer Res. 2016, 13, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Yong, V.W.; Wells, J.; Giuliani, F.; Casha, S.; Power, C.; Metz, L.M. The Promise of Minocycline in Neurology. Lancet Neurol. 2004, 3, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Yamanaka, H.; Fukuoka, T.; Dai, Y.; Obata, K.; Noguchi, K. P2Y12 Receptor Upregulation in Activated Microglia Is a Gateway of p38 Signaling and Neuropathic Pain. J. Neurosci. 2008, 28, 2892–2902. [Google Scholar] [CrossRef] [PubMed]
- Koistinaho, M.; Malm, T.M.; Kettunen, M.I.; Goldsteins, G.; Starckx, S.; Kauppinen, R.A.; Opdenakker, G.; Koistinaho, J. Minocycline Protects against Permanent Cerebral Ischemia in Wild Type but Not in Matrix Metalloprotease-9-Deficient Mice. J. Cereb. Blood Flow Metab. 2005, 25, 460–467. [Google Scholar] [CrossRef]
- Sanchez Mejia, R.O.; Ona, V.O.; Li, M.; Friedlander, R.M. Minocycline Reduces Traumatic Brain Injury-Mediated Caspase-1 Activation, Tissue Damage, and Neurological Dysfunction. Neurosurgery 2001, 48, 1393–1401. [Google Scholar] [CrossRef]
- Saikali, Z.; Singh, G. Doxycycline and Other Tetracyclines in the Treatment of Bone Metastasis. Anticancer Drugs 2003, 14, 773–778. [Google Scholar] [CrossRef]
- Campbell, J.H.; Burdo, T.H.; Autissier, P.; Bombardier, J.P.; Westmoreland, S.V.; Soulas, C.; González, R.G.; Ratai, E.M.; Williams, K.C. Minocycline Inhibition of Monocyte Activation Correlates with Neuronal Protection in SIV NeuroAIDS. PLoS ONE 2011, 6, e18688. [Google Scholar] [CrossRef]
- Osikowicz, M.; Skup, M.; Mika, J.; Makuch, W.; Czarkowska-Bauch, J.; Przewlocka, B. Glial Inhibitors Influence the MRNA and Protein Levels of MGlu2/3, 5 and 7 Receptors and Potentiate the Analgesic Effects of Their Ligands in a Mouse Model of Neuropathic Pain. Pain 2009, 147, 175–186. [Google Scholar] [CrossRef]
- Popiolek-Barczyk, K.; Rojewska, E.; Jurga, A.M.; Makuch, W.; Zador, F.; Borsodi, A.; Piotrowska, A.; Przewlocka, B.; Mika, J. Minocycline Enhances the Effectiveness of Nociceptin/Orphanin FQ during Neuropathic Pain. Biomed Res. Int. 2014, 2014, 762930. [Google Scholar] [CrossRef]
- Mika, J.; Popiolek-Barczyk, K.; Rojewska, E.; Makuch, W.; Starowicz, K.; Przewlocka, B. Delta-Opioid Receptor Analgesia Is Independent of Microglial Activation in a Rat Model of Neuropathic Pain. PLoS ONE 2014, 9, e104420. [Google Scholar] [CrossRef]
- Shin, D.A.; Kim, T.U.; Chang, M.C. Minocycline for Controlling Neuropathic Pain: A Systematic Narrative Review of Studies in Humans. J. Pain Res. 2021, 14, 139–145. [Google Scholar] [CrossRef]
- Mika, J.; Zychowska, M.; Popiolek-Barczyk, K.; Rojewska, E.; Przewlocka, B. Importance of Glial Activation in Neuropathic Pain. Eur. J. Pharmacol. 2013, 716, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Popiolek-Barczyk, K.; Mika, J. Targeting the Microglial Signaling Pathways: New Insights in the Modulation of Neuropathic Pain. Curr. Med. Chem. 2016, 23, 2908–2928. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, K.; Mika, J. The Importance of Chemokines in Neuropathic Pain Development and Opioid Analgesic Potency. Pharmacol. Rep. 2018, 70, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Rojewska, E.; Piotrowska, A.; Popiolek-Barczyk, K.; Mika, J. Botulinum Toxin Type A—A Modulator of Spinal Neuron–Glia Interactions under Neuropathic Pain Conditions. Toxins 2018, 10, 145. [Google Scholar] [CrossRef]
- Johnston, I.N.; Milligan, E.D.; Wieseler-Frank, J.; Frank, M.G.; Zapata, V.; Campisi, J.; Langer, S.; Martin, D.; Green, P.; Fleshner, M.; et al. A Role for Proinflammatory Cytokines and Fractalkine in Analgesia, Tolerance, and Subsequent Pain Facilitation Induced by Chronic Intrathecal Morphine. J. Neurosci. 2004, 24, 7353. [Google Scholar] [CrossRef]
- Zajączkowska, R.; Pawlik, K.; Ciapała, K.; Piotrowska, A.; Ciechanowska, A.; Rojewska, E.; Kocot-Kępska, M.; Makuch, W.; Wordliczek, J.; Mika, J. Mirogabalin Decreases Pain-like Behaviors by Inhibiting the Microglial/Macrophage Activation, p38MAPK Signaling, and Pronociceptive CCL2 and CCL5 Release in a Mouse Model of Neuropathic Pain. Pharmaceuticals 2023, 16, 1023. [Google Scholar] [CrossRef]
- Kwiatkowski, K.; Piotrowska, A.; Rojewska, E.; Makuch, W.; Jurga, A.; Slusarczyk, J.; Trojan, E.; Basta-Kaim, A.; Mika, J. Beneficial Properties of Maraviroc on Neuropathic Pain Development and Opioid Effectiveness in Rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 64, 68–78. [Google Scholar] [CrossRef]
- Wen, Y.R.; Tan, P.H.; Cheng, J.K.; Liu, Y.C.; Ji, R.R. Microglia: A Promising Target for Treating Neuropathic and Postoperative Pain, and Morphine Tolerance. J. Formos. Med. Assoc. 2011, 110, 487–494. [Google Scholar] [CrossRef]
- Piotrowska, A.; Kwiatkowski, K.; Rojewska, E.; Makuch, W.; Mika, J. Maraviroc Reduces Neuropathic Pain through Polarization of Microglia and Astroglia—Evidence from in Vivo and in Vitro Studies. Neuropharmacology 2016, 108, 207–219. [Google Scholar] [CrossRef]
- Kwiatkowski, K.; Pawlik, K.; Ciapała, K.; Piotrowska, A.; Makuch, W.; Mika, J. Bidirectional Action of Cenicriviroc, a CCR2/CCR5 Antagonist, Results in Alleviation of Pain-Related Behaviors and Potentiation of Opioid Analgesia in Rats with Peripheral Neuropathy. Front. Immunol. 2020, 11, 615327. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, K.; Piotrowska, A.; Kwiatkowski, K.; Ciapała, K.; Popiolek-Barczyk, K.; Makuch, W.; Mika, J. The Blockade of CC Chemokine Receptor Type 1 Influences the Level of Nociceptive Factors and Enhances Opioid Analgesic Potency in a Rat Model of Neuropathic Pain. Immunology 2020, 159, 413–428. [Google Scholar] [CrossRef] [PubMed]
- Sobot, N.M.; Sobot, T.S.; Jeremic, J.N.; Bolevich, S.B.; Bolevich, S.S.; Mitrovic, S.L.; Fisenko, V.P.; Inic, S.G.; Samanovic, A.D.M.; Rankovic, M.R.; et al. Minocycline as Heart Conditioning Agent in Experimental Type 2 Diabetes Mellitus—An Antibacterial Drug in Heart Protection. Naunyn. Schmiedebergs. Arch. Pharmacol. 2022, 395, 429–444. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, X.; Zheng, W.; Zhou, H.; Zhang, B.Y.; Zhao, D. Minocycline Attenuates Kidney Injury in a Rat Model of Streptozotocin-Induced Diabetic Nephropathy. Biol. Pharm. Bull. 2016, 39, 1231–1237. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shaughnessy, K.K.; Bouchard, S.M.; Mohr, M.R.; Herre, J.M.; Salkey, K.S. Minocycline-Induced Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome with Persistent Myocarditis. J. Am. Acad. Dermatol. 2010, 62, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, J.E.; Perez-Atayde, A.R.; Cohen, M.; Bousvaros, A.; Jonas, M.M. Minocycline-Related Autoimmune Hepatitis: Case Series and Literature Review. Arch. Pediatr. Adolesc. Med. 1998, 152, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 Signaling in Oxidative and Reductive Stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Robledinos-Antón, N.; Fernández-Ginés, R.; Manda, G.; Cuadrado, A. Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development. Oxid. Med. Cell. Longev. 2019, 2019, 9372182. [Google Scholar] [CrossRef]
- Brandes, M.S.; Gray, N.E. NRF2 as a Therapeutic Target in Neurodegenerative Diseases. ASN Neuro 2020, 12. [Google Scholar] [CrossRef]
- Sun, J.; Li, J.Y.; Zhang, L.Q.; Li, D.Y.; Wu, J.Y.; Gao, S.J.; Liu, D.Q.; Zhou, Y.Q.; Mei, W. Nrf2 Activation Attenuates Chronic Constriction Injury-Induced Neuropathic Pain via Induction of PGC-1 α -Mediated Mitochondrial Biogenesis in the Spinal Cord. Oxid. Med. Cell. Longev. 2021, 2021, 9577874. [Google Scholar] [CrossRef] [PubMed]
- Basu, P.; Averitt, D.L.; Maier, C.; Basu, A. The Effects of Nuclear Factor Erythroid 2 (NFE2)-Related Factor 2 (Nrf2) Activation in Preclinical Models of Peripheral Neuropathic Pain. Antioxidants 2022, 11, 430. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, C. Nrf2-Mediated Anti-Inflammatory Polarization of Macrophages as Therapeutic Targets for Osteoarthritis. Front. Immunol. 2022, 13, 967193. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C. Anti-Nociceptive and Anti-Inflammatory Actions of Sulforaphane in Chronic Constriction Injury-Induced Neuropathic Pain Mice. Inflammopharmacology 2017, 25, 99–106. [Google Scholar] [CrossRef]
- Klomparens, E.; Ding, Y. The Neuroprotective Mechanisms and Effects of Sulforaphane. Brain Circ. 2019, 5, 74. [Google Scholar] [CrossRef]
- Ferreira-Chamorro, P.; Redondo, A.; Riego, G.; Leánez, S.; Pol, O. Sulforaphane Inhibited the Nociceptive Responses, Anxiety- and Depressive-Like Behaviors Associated with Neuropathic Pain and Improved the Anti-Allodynic Effects of Morphine in Mice. Front. Pharmacol. 2018, 9, 1332. [Google Scholar] [CrossRef]
- Li, S.; Yang, C.; Fang, X.; Zhan, G.; Huang, N.; Gao, J.; Xu, H.; Hashimoto, K.; Luo, A. Role of Keap1-Nrf2 Signaling in Anhedonia Symptoms in a Rat Model of Chronic Neuropathic Pain: Improvement with Sulforaphane. Front. Pharmacol. 2018, 9, 887. [Google Scholar] [CrossRef]
- Li, J.; Ma, J.; Lacagnina, M.J.; Lorca, S.; Odem, M.A.; Walters, E.T.; Kavelaars, A.; Grace, P.M. Oral Dimethyl Fumarate Reduces Peripheral Neuropathic Pain in Rodents via NFE2L2 Antioxidant Signaling. Anesthesiology 2020, 132, 343–356. [Google Scholar] [CrossRef]
- Garcia-Mesa, Y.; Xu, H.N.; Vance, P.; Gruenewald, A.L.; Garza, R.; Midkiff, C.; Alvarez-Hernandez, X.; Irwin, D.J.; Gill, A.J.; Kolson, D.L. Dimethyl Fumarate, an Approved Multiple Sclerosis Treatment, Reduces Brain Oxidative Stress in SIV-Infected Rhesus Macaques: Potential Therapeutic Repurposing for HIV Neuroprotection. Antioxidants 2021, 10, 416. [Google Scholar] [CrossRef]
- Dodson, M.; De La Vega, M.R.; Cholanians, A.B.; Schmidlin, C.J.; Chapman, E.; Zhang, D.D. Modulating NRF2 in Disease: Timing Is Everything. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 555–575. [Google Scholar] [CrossRef]
- Singh, J.; Thapliyal, S.; Kumar, A.; Paul, P.; Kumar, N.; Bisht, M.; Naithani, M.; Rao, S.; Handu, S.S. Dimethyl Fumarate Ameliorates Paclitaxel-Induced Neuropathic Pain in Rats. Cureus 2022, 14, e28818. [Google Scholar] [CrossRef] [PubMed]
- Kalvala, A.K.; Kumar, R.; Sherkhane, B.; Gundu, C.; Arruri, V.K.; Kumar, A. Bardoxolone Methyl Ameliorates Hyperglycemia Induced Mitochondrial Dysfunction by Activating the Keap1-Nrf2-ARE Pathway in Experimental Diabetic Neuropathy. Mol. Neurobiol. 2020, 57, 3616–3631. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.X.; Xiong, F. Astaxanthin and Its Effects in Inflammatory Responses and Inflammation-Associated Diseases: Recent Advances and Future Directions. Molecules 2020, 25, 5342. [Google Scholar] [CrossRef]
- Nishida, Y.; Nawaz, A.; Hecht, K.; Tobe, K. Astaxanthin as a Novel Mitochondrial Regulator: A New Aspect of Carotenoids, beyond Antioxidants. Nutrients 2022, 14, 107. [Google Scholar] [CrossRef] [PubMed]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Pourbagher-Shahri, A.M.; Samarghandian, S. Anti-Inflammatory Action of Astaxanthin and Its Use in the Treatment of Various Diseases. Biomed. Pharmacother. 2022, 145, 112179. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yan, Q.; Liu, F.; Jing, C.; Ding, L.; Zhang, L.; Pang, C. Chronic Trans-Astaxanthin Treatment Exerts Antihyperalgesic Effect and Corrects Co-Morbid Depressive like Behaviors in Mice with Chronic Pain. Neurosci. Lett. 2018, 662, 36–43. [Google Scholar] [CrossRef]
- Fakhri, S.; Dargahi, L.; Abbaszadeh, F.; Jorjani, M. Effects of Astaxanthin on Sensory-Motor Function in a Compression Model of Spinal Cord Injury: Involvement of ERK and AKT Signalling Pathway. Eur. J. Pain 2019, 23, 750–764. [Google Scholar] [CrossRef]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Samarghandian, S. Nrf2 a Molecular Therapeutic Target for Astaxanthin. Biomed. Pharmacother. 2021, 137, 111374. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Yaribeygi, H.; Sathyapalan, T.; Sahebkar, A. Astaxanthin and Nrf2 Signaling Pathway: A Novel Target for New Therapeutic Approaches. Mini Rev. Med. Chem. 2021, 22, 312–321. [Google Scholar] [CrossRef]
- Zhao, L.; Tao, X.; Wan, C.; Dong, D.; Wang, C.; Xi, Q.; Liu, Y.; Song, T. Astaxanthin Alleviates Inflammatory Pain by Regulating the p38 Mitogen-Activated Protein Kinase and Nuclear Factor-Erythroid Factor 2-Related Factor/Heme Oxygenase-1 Pathways in Mice. Food Funct. 2021, 12, 12381–12394. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Fakhri, S.; Mohammadi-Farani, A.; Farzaei, M.H.; Abbaszadeh, F. Astaxanthin Engages the l -Arginine/NO/CGMP/KATPchannel Signaling Pathway toward Antinociceptive Effects. Behav. Pharmacol. 2021, 32, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Hill, W.S.; Dohnalek, M.H.; Ha, Y.; Kim, S.J.; Jung, J.C.; Kang, S.B. A Multicenter, Randomized, Double-Blinded, Placebo-Controlled Clinical Trial to Evaluate the Efficacy and Safety of a Krill Oil, Astaxanthin, and Oral Hyaluronic Acid Complex on Joint Health in People with Mild Osteoarthritis. Nutrients 2023, 15, 3769. [Google Scholar] [CrossRef]
- Urakaze, M.; Kobashi, C.; Satou, Y.; Shigeta, K.; Toshima, M.; Takagi, M.; Takahashi, J.; Nishida, H. The Beneficial Effects of Astaxanthin on Glucose Metabolism and Modified Low-Density Lipoprotein in Healthy Volunteers and Subjects with Prediabetes. Nutrients 2021, 13, 4381. [Google Scholar] [CrossRef] [PubMed]
- Gowd, V.; Xiao, J.; Wang, M.; Chen, F.; Cheng, K.W. Multi-Mechanistic Antidiabetic Potential of Astaxanthin: An Update on Preclinical and Clinical Evidence. Mol. Nutr. Food Res. 2021, 65, 2100252. [Google Scholar] [CrossRef] [PubMed]
- Faraone, I.; Sinisgalli, C.; Ostuni, A.; Armentano, M.F.; Carmosino, M.; Milella, L.; Russo, D.; Labanca, F.; Khan, H. Astaxanthin Anticancer Effects Are Mediated through Multiple Molecular Mechanisms: A Systematic Review. Pharmacol. Res. 2020, 155, 104689. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, J.J.; Lee, B.J.; Joo, M.K.; Chun, H.J.; Lee, S.W.; Bak, Y.T. Astaxanthin Inhibits Proliferation of Human Gastric Cancer Cell Lines by Interrupting Cell Cycle Progression. Gut Liver 2016, 10, 369–374. [Google Scholar] [CrossRef]
- Wu, H.; Niu, H.; Shao, A.; Wu, C.; Dixon, B.J.; Zhang, J.; Yang, S.; Wang, Y. Astaxanthin as a Potential Neuroprotective Agent for Neurological Diseases. Mar. Drugs 2015, 13, 5750–5766. [Google Scholar] [CrossRef]
- Zaafan, M.A.; Abdelhamid, A.M. The Cardioprotective Effect of Astaxanthin against Isoprenaline-Induced Myocardial Injury in Rats: Involvement of TLR4/NF-κB Signaling Pathway. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4099–4105. [Google Scholar] [CrossRef]
- Kaltschmidt, B.; Kaltschmidt, C. NF-KappaB in the Nervous System. Cold Spring Harb. Perspect. Biol. 2009, 1, a001271. [Google Scholar] [CrossRef]
- Shih, R.-H.; Wang, C.-Y.; Yang, C.-M. NF-KappaB Signaling Pathways in Neurological Inflammation: A Mini Review. Front. Mol. Neurosci. 2015, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Meunier, A.; Latrémolière, A.; Dominguez, E.; Mauborgne, A.; Philippe, S.; Hamon, M.; Mallet, J.; Benoliel, J.; Pohl, M. Lentiviral-Mediated Targeted NF-κB Blockade in Dorsal Spinal Cord Glia Attenuates Sciatic Nerve Injury—Induced Neuropathic Pain in the Rat. Mol. Ther. 2007, 15, 687–697. [Google Scholar] [CrossRef]
- Tchivileva, I.E.; Nackley, A.G.; Qian, L.; Wentworth, S.; Conrad, M.; Diatchenko, L.B. Characterization of NF-κB -Mediated Inhibition of Catechol-O-Methyltransferase. Mol. Pain 2009, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Bisby, M. A Increased Activation of Nuclear Factor Kappa B in Rat Lumbar Dorsal Root Ganglion Neurons Following Partial Sciatic Nerve Injuries. Brain Res. 1998, 797, 243–254. [Google Scholar] [CrossRef] [PubMed]
- GYa, B.; Yakovleva, T.; Terenius, L. NF-Kappa B-like Factors in the Murine Brain. Developmentally-Regulated and Tissue-Specific Expression. Brain Res. Mol. Brain Res. 1993, 20, 137–146. [Google Scholar]
- Kaltschmidt, C.; Kaltschmidt, B.; Baeuerle, P.A. Brain Synapses Contain Inducible Forms of the Transcription Factor NF-Kappa, B. Mech. Dev. 1993, 43, 135–147. [Google Scholar] [CrossRef]
- Albensi, B.C. What Is Nuclear Factor Kappa B (NF-κB) Doing in and to the Mitochondrion? Front. Cell Dev. Biol. 2019, 7, 154. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-κB Family of Transcription Factors and Its Regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Lee, K.M.; Kang, B.S.; Lee, H.L.; Son, S.J.; Hwang, S.H.; Kim, D.S.; Park, J.S.; Cho, H.J. Spinal NF-κB Activation Induces COX-2 Upregulation and Contributes to Inflammatory Pain Hypersensitivity. Eur. J. Neurosci. 2004, 19, 3375–3381. [Google Scholar] [CrossRef]
- Lee, M.K.; Han, S.R.; Park, M.K.; Kim, M.J.; Bae, Y.C.; Kim, S.K.; Park, J.S.; Ahn, D.K. Behavioral Evidence for the Differential Regulation of p-p38 MAPK and p-NF-κB in Rats with Trigeminal Neuropathic Pain. Mol. Pain 2011, 7, 57. [Google Scholar] [CrossRef]
- Zhou, C.; Shi, X.; Huang, H.; Zhu, Y.; Wu, Y. Montelukast Attenuates Neuropathic Pain through Inhibiting p38 Mitogen-Activated Protein Kinase and Nuclear Factor-Kappa B in a Rat Model of Chronic Constriction Injury. Anesth. Analg. 2014, 118, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Bai, H.; Zhang, R.; Chen, B.; Wang, J.; Xue, B.; Ren, X.; Wang, J.; Jia, Y.; Zang, W.; et al. Systematic Analysis of Critical Genes and Pathways Identified a Signature of Neuropathic Pain after Spinal Cord Injury. Eur. J. Neurosci. 2022, 56, 3991–4008. [Google Scholar] [CrossRef]
- Kan, L.; Zhao, W.; Pan, L.; Xu, J.; Chen, Q.; Xu, K.; Xiao, L.; Chen, Y. Peimine Inhibits HERG Potassium Channels through the Channel Inactivation States. Biomed. Pharmacother. 2017, 89, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zhang, J.; Guo, Q.; Chen, L.; Zhang, W.; Kang, W. Pharmacological Effects of Verticine: Current Status. Evid. Based Complement. Altern. Med. 2019, 2019, 2394605. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, B.; Kim, H.K.; Kim, E.Y.; Kim, J.H.; Min, J.H.; Kim, S.; Sohn, Y.; Jung, H.S. Peimine Inhibits the Production of Proinflammatory Cytokines through Regulation of the Phosphorylation of NF-κB and MAPKs in HMC-1 Cells. Pharmacogn. Mag. 2017, 13, S359–S364. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, J.; Kong, X.; Guo, Q. Peimine Inhibits MCF-7 Breast Cancer Cell Growth by Modulating Inflammasome Activation: Critical Roles of MAPK and NF-κB Signaling. Anticancer. Agents Med. Chem. 2023, 23, 317–327. [Google Scholar] [CrossRef]
- Qian, B.C.; Xu, H.J. Studies on the Antitussive and Sedative Activities of Peimine and Peiminine. Acta Pharm. Sin. 1985, 20, 306–308. [Google Scholar]
- Alberola-Die, A.; Encinar, J.A.; Cobo, R.; Fernández-Ballester, G.; González-Ros, J.M.; Ivorra, I.; Morales, A. Peimine, an Anti-Inflammatory Compound from Chinese Herbal Extracts, Modulates Muscle-Type Nicotinic Receptors. Int. J. Mol. Sci. 2021, 22, 11287. [Google Scholar] [CrossRef]
- Cai, Z.H.; Tian, Y.G.; Li, J.Z.; Zhao, P.; Li, J.S.; Mei, X.; Bai, Y.P. Peimine Ameliorates Pulmonary Fibrosis via the Inhibition of M2-Type Macrophage Polarization through the Suppression of p38/Akt/STAT6 Signals. Biosci. Rep. 2022, 42, BSR20220986. [Google Scholar] [CrossRef]
- Samakova, A.; Gazova, A.; Sabova, N.; Valaskova, S.; Jurikova, M.; Kyselovic, J. The Pi3k/Akt Pathway Is Associated with Angiogenesis, Oxidative Stress and Survival of Mesenchymal Stem Cells in Pathophysiologic Condition in Ischemia. Physiol. Res. 2019, 68, S131–S138. [Google Scholar] [CrossRef]
- Davis, W.J.; Lehmann, P.Z.; Li, W. Nuclear PI3K Signaling in Cell Growth and Tumorigenesis. Front. Cell Dev. Biol. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Hemmings, B.A.; Restuccia, D.F. PI3K-PKB/Akt Pathway. Cold Spring Harb. Perspect. Biol. 2012, 4, a011189. [Google Scholar] [CrossRef] [PubMed]
- Kelly, Á.; Lynch, M. Long-Term Potentiation in Dentate Gyrus of the Rat Is Inhibited by the Phosphoinositide 3–Kinase Inhibitor, Wortmannin. Neuropharmacology 2000, 39, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Bohle, A.S.; Dohrmann, P.; Leuschner, I.; Schulz, A.; Kremer, B.; Fandrich, F. Overexpression of Phosphatidylinositol 3-Kinase in Human Lung Cancer. Langenbecks Arch. Surg. 2001, 386, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Man, H.Y.; Wang, Q.; Lu, W.Y.; Ju, W.; Ahmadian, G.; Liu, L.; D’Souza, S.; Wong, T.P.; Taghibiglou, C.; Lu, J.; et al. Activation of PI3-Kinase Is Required for AMPA Receptor Insertion during LTP of MEPSCs in Cultured Hippocampal Neurons. Neuron 2003, 38, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Yan, J.; Willis, W.D. Activation of Protein Kinase B/Akt in the Periphery Contributes to Pain Behavior Induced by Capsaicin in Rats. Neuroscience 2007, 144, 286–294. [Google Scholar] [CrossRef]
- Xu, J.-T.; Tu, H.-Y.; Xin, W.-J.; Liu, X.-G.; Zhang, G.-H.; Zhai, C.-H. Activation of Phosphatidylinositol 3-Kinase and Protein Kinase B/Akt in Dorsal Root Ganglia and Spinal Cord Contributes to the Neuropathic Pain Induced by Spinal Nerve Ligation in Rats. Exp. Neurol. 2007, 206, 269–279. [Google Scholar] [CrossRef]
- Carvalho, T.T.; Flauzino, T.; Otaguiri, E.S.; Batistela, A.P.; Zarpelon, A.C.; Cunha, T.M.; Ferreira, S.H.; Cunha, F.Q.; Verri, W.A. Granulocyte-Colony Stimulating Factor (G-CSF) Induces Mechanical Hyperalgesia via Spinal Activation of MAP Kinases and PI3K in Mice. Pharmacol. Biochem. Behav. 2011, 98, 188–195. [Google Scholar] [CrossRef]
- Pezet, S.; Marchand, F.; D’Mello, R.; Grist, J.; Clark, A.K.; Malcangio, M.; Dickenson, A.H.; Williams, R.J.; McMahon, S.B. Phosphatidylinositol 3-Kinase Is a Key Mediator of Central Sensitization in Painful Inflammatory Conditions. J. Neurosci. 2008, 28, 4261–4270. [Google Scholar] [CrossRef]
- Yu, L.-N.; Zhou, X.-L.; Yu, J.; Huang, H.; Jiang, L.-S.; Zhang, F.-J.; Cao, J.-L.; Yan, M. PI3K Contributed to Modulation of Spinal Nociceptive Information Related to EphrinBs/EphBs. PLoS ONE 2012, 7, e40930. [Google Scholar] [CrossRef]
- Chen, S.-P.; Zhou, Y.-Q.; Liu, D.-Q.; Zhang, W.; Manyande, A.; Guan, X.-H.; Tian, Y.; Ye, D.-W.; Omar, D.M. PI3K/Akt Pathway: A Potential Therapeutic Target for Chronic Pain. Curr. Pharm. Des. 2017, 23, 1860–1868. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wu, S.; Wu, X.; Zhong, J.; Lv, A.; Jiao, J.; Chen, Z. Blocking Mammalian Target of Rapamycin Alleviates Bone Cancer Pain and Morphine Tolerance via Μ-Opioid Receptor. Int. J. Cancer 2015, 138, 2013–2020. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.P.; Zhang, Z.D.; Kang, L.M.; Wang, Q.H.; Zhang, L.; Chen, H.P. Celecoxib Reverts Oxaliplatin-Induced Neuropathic Pain through Inhibiting PI3K/Akt2 Pathway in the Mouse Dorsal Root Ganglion. Exp Neurol. 2016, 275, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yang, Y.; Zhou, F.; Xiao, Y.; Shi, L. Inhibition of PI3K/AKT/MTOR Signaling Pathway Promotes Autophagy and Relieves Hyperalgesia in Diabetic Rats. Neuroreport 2020, 31, 644–649. [Google Scholar] [CrossRef]
- Saponaro, C.; Cianciulli, A.; Calvello, R.; Dragone, T.; Iacobazzi, F.; Panaro, M.A. The PI3K/Akt Pathway Is Required for LPS Activation of Microglial Cells. Immunopharmacol. Immunotoxicol. 2012, 34, 858–865. [Google Scholar] [CrossRef]
- Park, H.Y.; Kim, N.D.; Kim, G.-Y.; Hwang, H.J.; Kim, B.-W.; Kim, W.J.; Choi, Y.H. Inhibitory Effects of Diallyl Disulfide on the Production of Inflammatory Mediators and Cytokines in Lipopolysaccharide-Activated BV2 Microglia. Toxicol. Appl. Pharmacol. 2012, 262, 177–184. [Google Scholar] [CrossRef]
- Horvath, R.J.; DeLeo, J.A. Morphine Enhances Microglial Migration through Modulation of P2X4 Receptor Signaling. J. Neurosci. 2009, 29, 998–1005. [Google Scholar] [CrossRef]
- Sutherland, T.C.; Geoffroy, C.G. The Influence of Neuron-Extrinsic Factors and Aging on Injury Progression and Axonal Repair in the Central Nervous System. Front. Cell Dev. Biol. 2020, 8, 190. [Google Scholar] [CrossRef]
- Ravula, A.R.; Teegala, S.B.; Kalakotla, S.; Pasangulapati, J.P.; Perumal, V.; Boyina, H.K. Fisetin, Potential Flavonoid with Multifarious Targets for Treating Neurological Disorders: An Updated Review. Eur. J. Pharmacol. 2021, 910, 174492. [Google Scholar] [CrossRef]
- Fazel Nabavi, S.; Braidy, N.; Habtemariam, S.; Sureda, A.; Manayi, A.; Mohammad Nabavi, S. Neuroprotective Effects of Fisetin in Alzheimer’s and Parkinson’s Diseases: From Chemistry to Medicine. Curr. Top. Med. Chem. 2016, 16, 1910–1915. [Google Scholar] [CrossRef]
- Hemanth Kumar, B.; Arun Reddy, R.; Mahesh Kumar, J.; Dinesh Kumar, B.; Diwan, P.V. Effects of Fisetin on Hyperhomocysteinemia-Induced Experimental Endothelial Dysfunction and Vascular Dementia. Can. J. Physiol. Pharmacol. 2017, 95, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.J.; Feng, Y.; Liu, T.; Wu, T.T.; Chen, Y.J.; Li, X.; Li, Q.; Wu, Y.C. Fisetin Regulates Gut Microbiota and Exerts Neuroprotective Effect on Mouse Model of Parkinson’s Disease. Front. Neurosci. 2020, 14, 549037. [Google Scholar] [CrossRef] [PubMed]
- Maher, P.; Dargusch, R.; Bodai, L.; Gerard, P.E.; Purcell, J.M.; Lawrence Marsh, J. ERK Activation by the Polyphenols Fisetin and Resveratrol Provides Neuroprotection in Multiple Models of Huntington’s Disease. Hum. Mol. Genet. 2011, 20, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, D.; Wu, H.; Jia, H.; Yang, C.; Zhang, L. Fisetin Prolongs Therapy Window of Brain Ischemic Stroke Using Tissue Plasminogen Activator: A Double-Blind Randomized Placebo-Controlled Clinical Trial. Clin. Appl. Thromb. Hemost. 2019, 25. [Google Scholar] [CrossRef]
- Yousefzadeh, M.J.; Zhu, Y.; McGowan, S.J.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.Y.; Melos, K.I.; Pirtskhalava, T.; Inman, C.L.; et al. Fisetin Is a Senotherapeutic That Extends Health and Lifespan. EBioMedicine 2018, 36, 18–28. [Google Scholar] [CrossRef]
- Zhan, J.Q.; Chen, C.N.; Wu, S.X.; Wu, H.J.; Zou, K.; Xiong, J.W.; Wei, B.; Yang, Y.J. Flavonoid Fisetin Reverses Impaired Hippocampal Synaptic Plasticity and Cognitive Function by Regulating the Function of AMPARs in a Male Rat Model of Schizophrenia. J. Neurochem. 2021, 158, 413–428. [Google Scholar] [CrossRef]
- Cheong, H.; Ryu, S.Y.; Oak, M.H.; Cheon, S.H.; Yoo, G.S.; Kim, K.M. Studies of Structure Activity Relationship of Flavonoids for the Anti-Allergic Actions. Arch. Pharm. Res. 1998, 21, 478–480. [Google Scholar] [CrossRef]
- Ravichandran, N.; Suresh, G.; Ramesh, B.; Vijaiyan Siva, G. Fisetin, a Novel Flavonol Attenuates Benzo(a)Pyrene-Induced Lung Carcinogenesis in Swiss Albino Mice. Food Chem. Toxicol. 2011, 49, 1141–1147. [Google Scholar] [CrossRef]
- Patel, M.Y.; Panchal, H.V.; Ghribi, O.; Benzeroual, K.E. The Neuroprotective Effect of Fisetin in the MPTP Model of Parkinson’s Disease. J. Parkinsons. Dis. 2012, 2, 287–302. [Google Scholar] [CrossRef]
- Yadav, M.; Pradhan, D.; Singh, R.P. Integrated Analysis and Identification of Nine-Gene Signature Associated to Oral Squamous Cell Carcinoma Pathogenesis. 3 Biotech 2021, 11, 215. [Google Scholar] [CrossRef]
- Prasath, G.S.; Subramanian, S.P. Antihyperlipidemic Effect of Fisetin, a Bioflavonoid of Strawberries, Studied in Streptozotocin-Induced Diabetic Rats. J. Biochem. Mol. Toxicol. 2014, 28, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Prasath, G.S.; Subramanian, S.P. Modulatory Effects of Fisetin, a Bioflavonoid, on Hyperglycemia by Attenuating the Key Enzymes of Carbohydrate Metabolism in Hepatic and Renal Tissues in Streptozotocin-Induced Diabetic Rats. Eur. J. Pharmacol. 2011, 668, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Miao, L.; Zhang, H.; Tan, L.; Zhao, Y.; Tu, Y.; Angel Prieto, M.; Simal-Gandara, J.; Chen, L.; He, C.; et al. Anti-Inflammatory Activity of Flavonols via Inhibiting MAPK and NF-κB Signaling Pathways in RAW264.7 Macrophages. Curr. Res. Food Sci. 2022, 5, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Nan, W.; Yao, F.; Li, K.; Lanlan, Z.; Yin, G.; Du, M.; Wu, B. Fisetin Regulates Astrocyte Migration and Proliferation in Vitro. Int. J. Mol. Med. 2017, 39, 783–790. [Google Scholar] [CrossRef]
| NEUROPATHIC PAIN | ||||
|---|---|---|---|---|
| SUBSTANCE | SUGGESTED DIRECT TARGETS | NEUROPATHIC PAIN ANIMAL MODELS | INFLUENCE ON IMPORTANT FACTORS IN NOCICEPTION | EFFECT ON OPIOID TREATMENT EFFECTIVENESS |
| Minocycline | p38 [42,70,71] ERK [72] MMP9 [73] | CCI (mouse) [74,75] CCI (rats) [76,77] SCI (rats) [78] STZ (rats) [79] SNL (rats) [70] STZ (mouse) [80] | iNOS ↓ [81] IL-1β [82] IL-6 ↓ [26] IL-18 ↓ [26] MMP9 ↓ [26] MMP2 ↓ [26] XCL1 ↓ [83] pERK ↓ [72,84] PI3K ↓ [84] | morphine ↑ [26,74] |
| Astaxanthin | p38 [85] ERK [85] NF-κB [86] NR2B [87] ROS [88] | SNL (mouse) [85] CCI (rat) [89] SCI (rat) [87] CCI (mouse) [90] | IL-1β ↓ [85] IL-6 ↓ [85] IL-4 ↑ [85] IL-10 ↑ [85] pERK ↓ [85] pp38 ↓ [85] NF-κB ↓ [85] | morphine ↑ [90] buprenorphine ↑ [90] oxycodone ↑ [90] |
| Peimine | p38 [91] ERK [92] JNK [92] NF-κB [92] Kv1.3 [93] Nav1.7 [93] | CCI (mouse) [90] | __________ | morphine ↑ [90] |
| Fisetin | p38 [94] ERK [95] Pi3K [96] NF-κB [97] 5HT7 [98] GABA [99] | CCI (mouse) [98] CCI (mouse) [90] STZ (mouse) [99] STZ (rat) [100] | IL-6 ↓ [100] TNFα ↓ [100] NF-κB↓ [100] NRF2 ↑ [100] | morphine ↑ [90] oxycodone ↑ [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciapała, K.; Mika, J. Advances in Neuropathic Pain Research: Selected Intracellular Factors as Potential Targets for Multidirectional Analgesics. Pharmaceuticals 2023, 16, 1624. https://doi.org/10.3390/ph16111624
Ciapała K, Mika J. Advances in Neuropathic Pain Research: Selected Intracellular Factors as Potential Targets for Multidirectional Analgesics. Pharmaceuticals. 2023; 16(11):1624. https://doi.org/10.3390/ph16111624
Chicago/Turabian StyleCiapała, Katarzyna, and Joanna Mika. 2023. "Advances in Neuropathic Pain Research: Selected Intracellular Factors as Potential Targets for Multidirectional Analgesics" Pharmaceuticals 16, no. 11: 1624. https://doi.org/10.3390/ph16111624
APA StyleCiapała, K., & Mika, J. (2023). Advances in Neuropathic Pain Research: Selected Intracellular Factors as Potential Targets for Multidirectional Analgesics. Pharmaceuticals, 16(11), 1624. https://doi.org/10.3390/ph16111624





