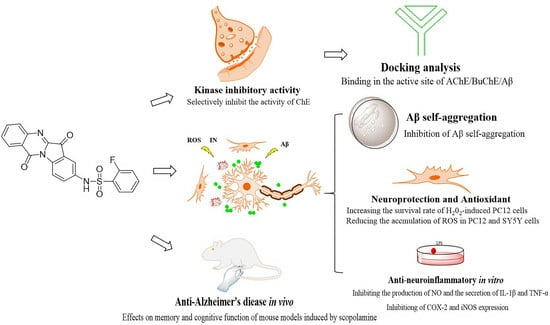
Discovery of Novel Tryptanthrin Derivatives with Benzenesulfonamide Substituents as Multi-Target-Directed Ligands for the Treatment of Alzheimer’s Disease
Abstract
:1. Introduction
2. Results
2.1. Cholinesterase Inhibition Activity
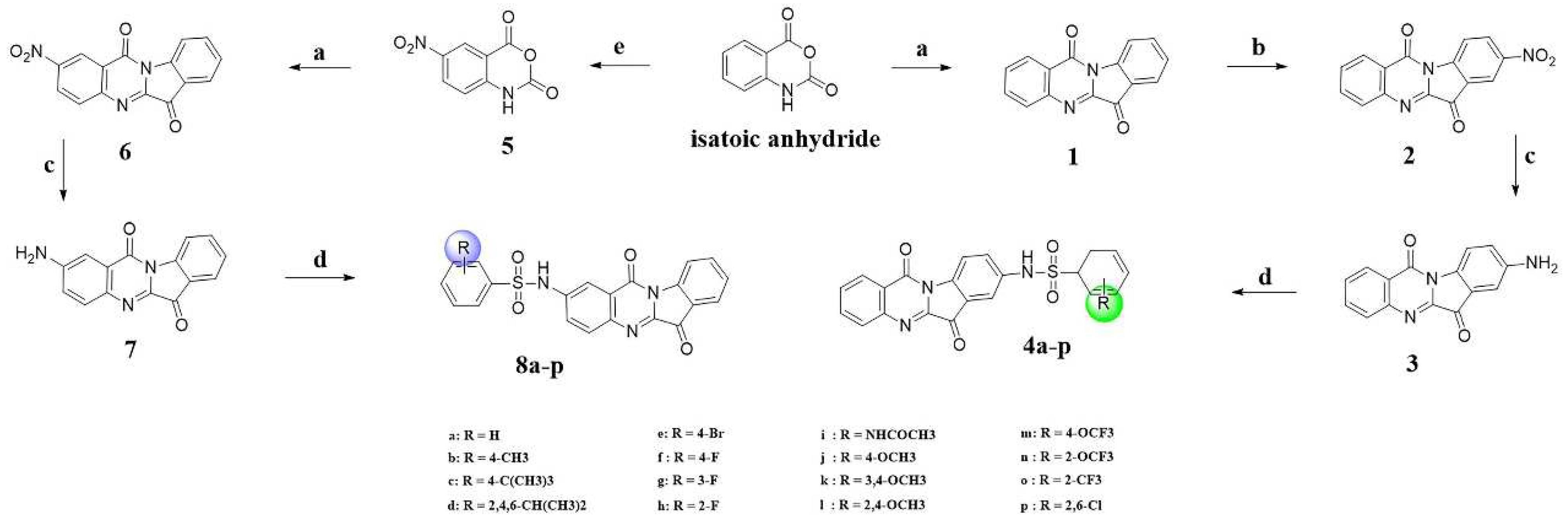
2.2. Reversibility Studies of 4h for AChE/BuChE Inhibition
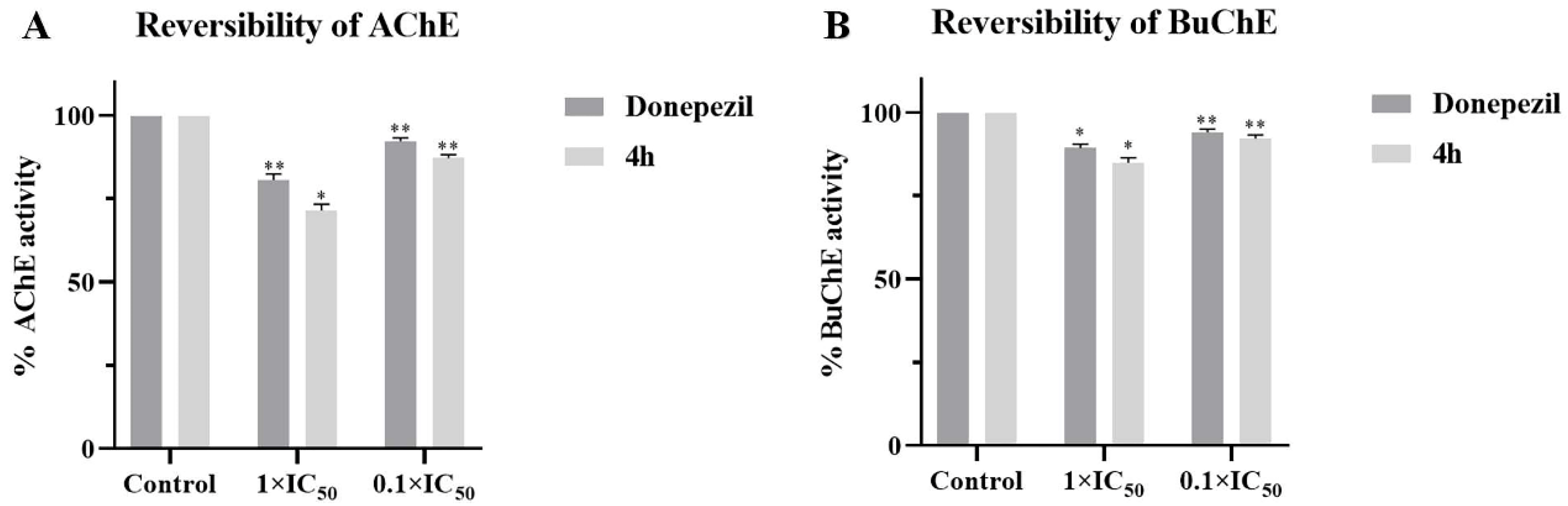
2.3. Kinetic Study of AChE and BuChE Inhibition
2.4. Docking Analysis of 4h with AChE and BuChE
2.5. Cell Viability and Neuroprotection Study
2.6. Effect on Aβ1-42 Self-Aggregation
2.7. Molecular Docking to Aβ1-42
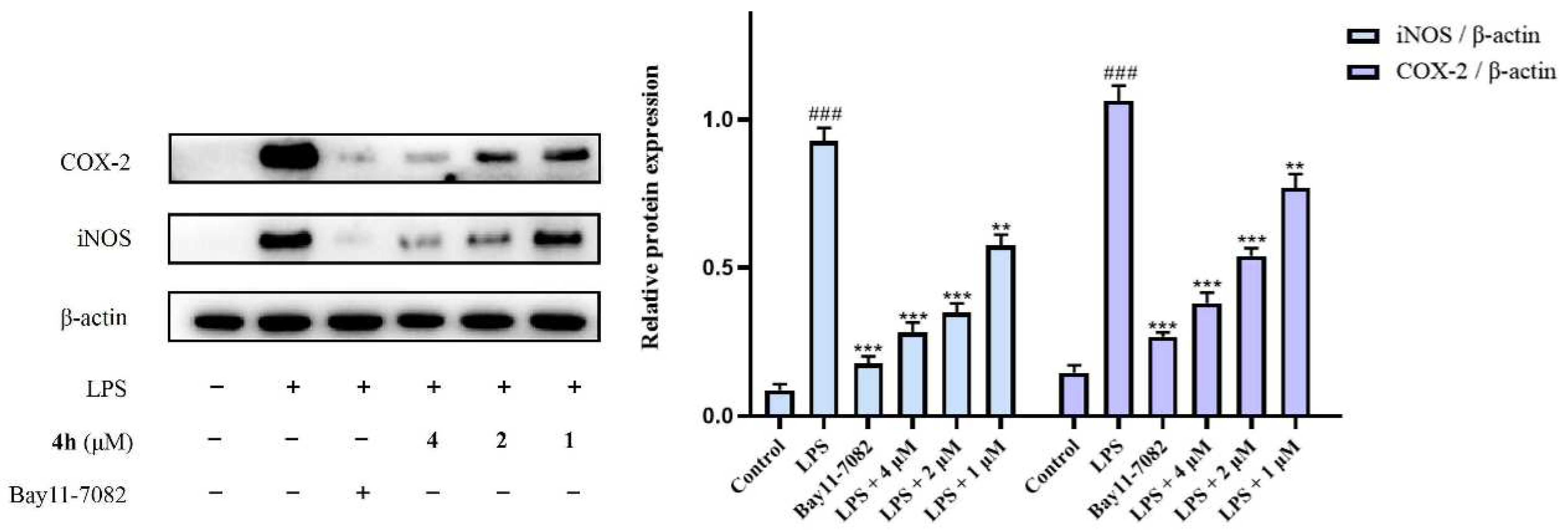
2.8. Inhibition of Proinflammatory Cytokines
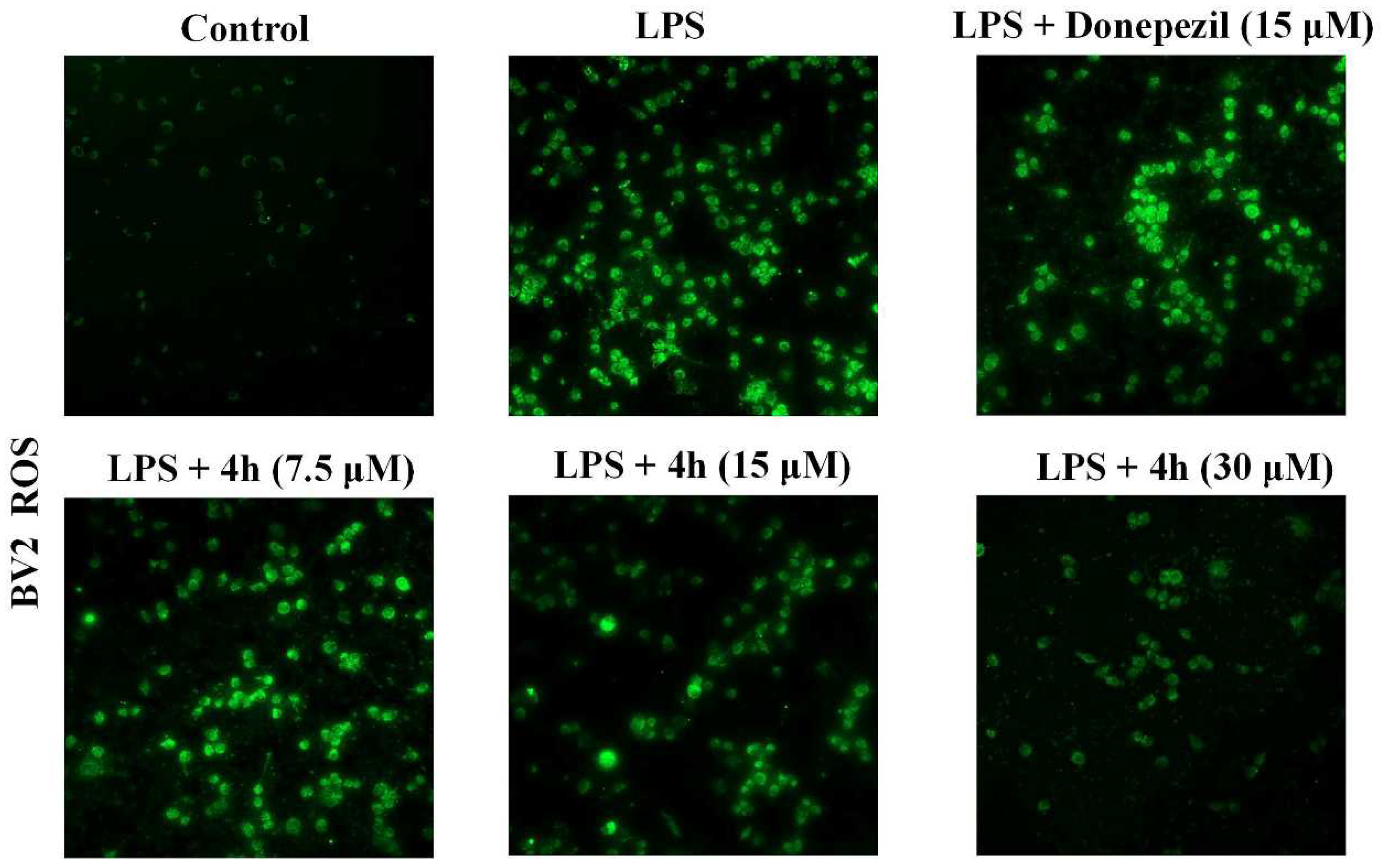
2.9. Effects of LPS-Induced ROS Production on BV2 Cells
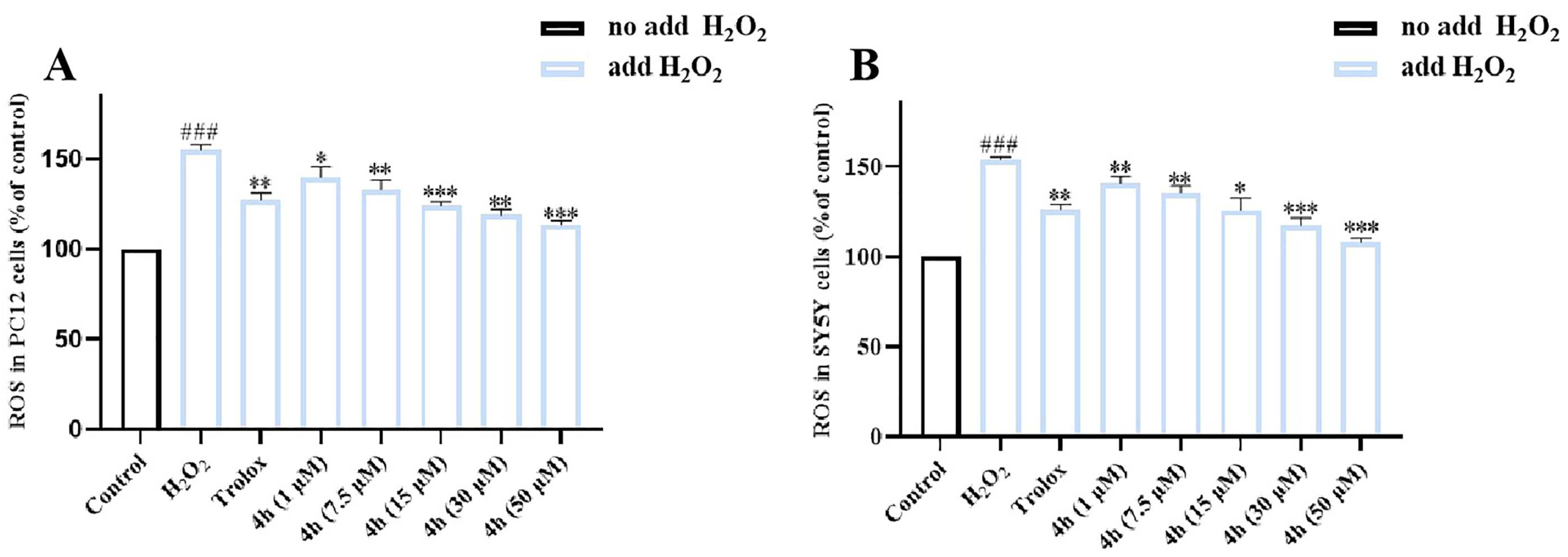
2.10. Effects on H2O2-Induced Intracellular Reactive Oxygen Species Production
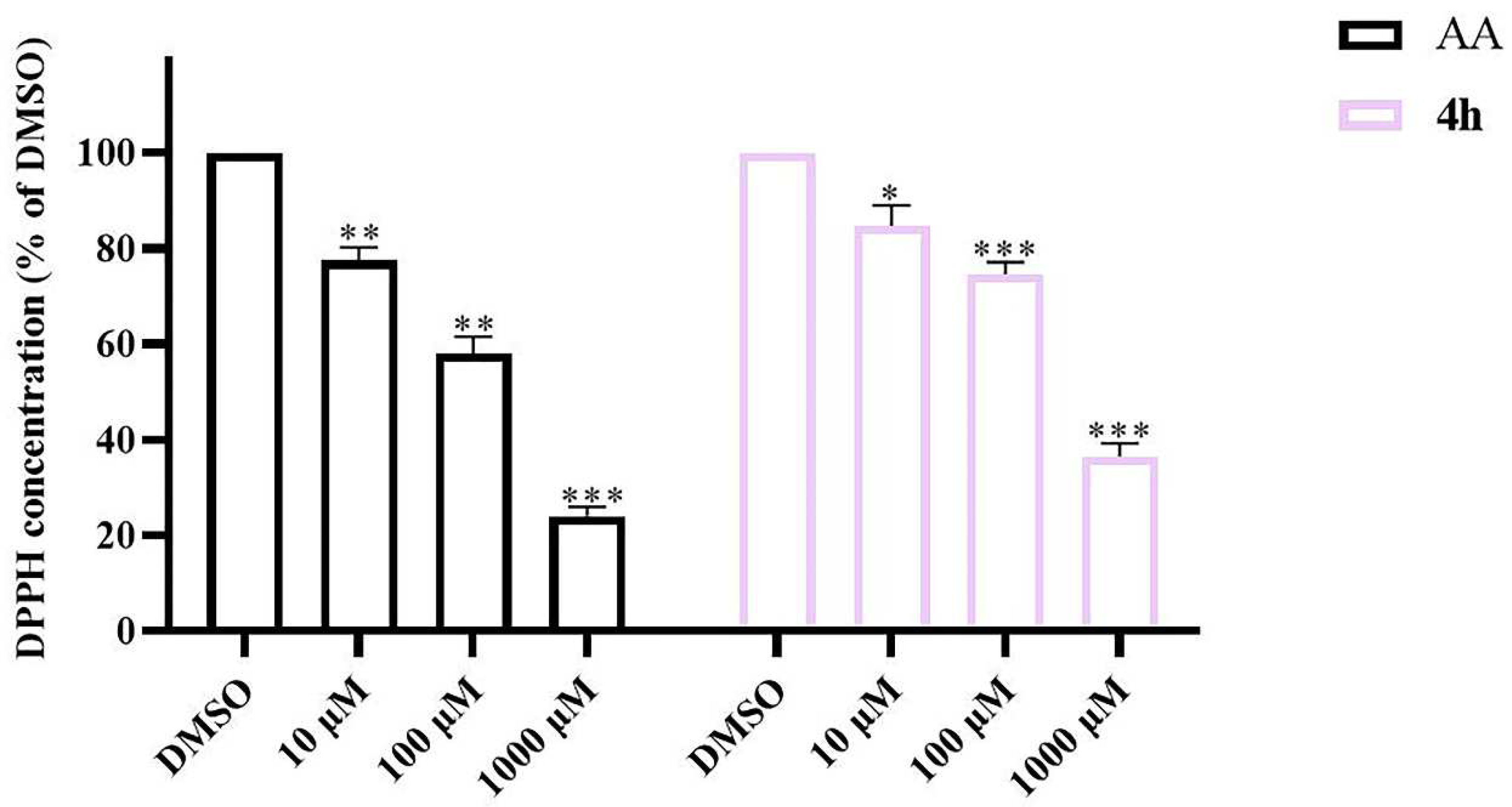
2.11. DPPH Radical Scavenging Activity of 4h
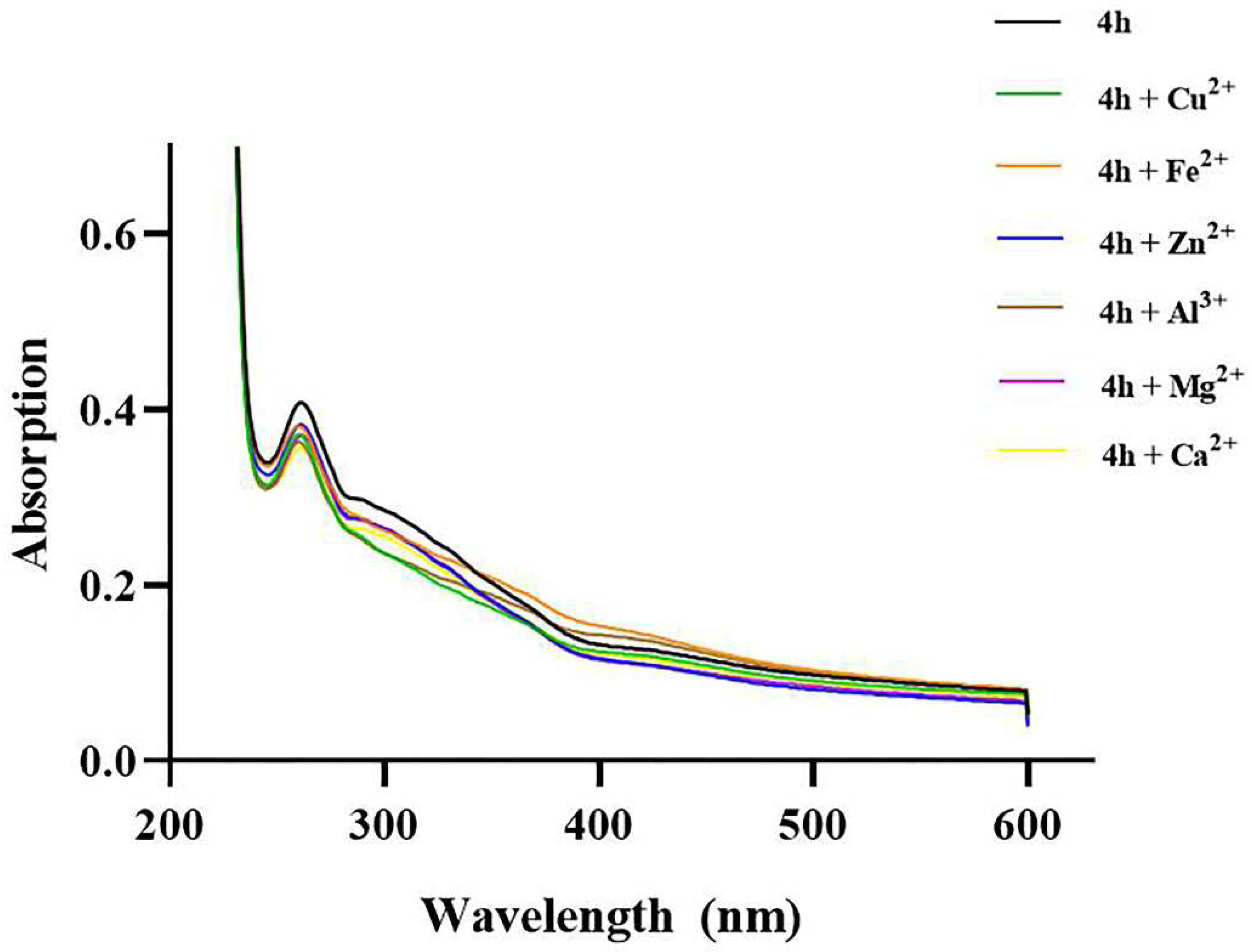
2.12. Chelating Properties of 4h
2.13. Blood–Brain Barrier (BBB) Permeability
2.14. Metabolic Stability in Liver Microsomes of SD Rats
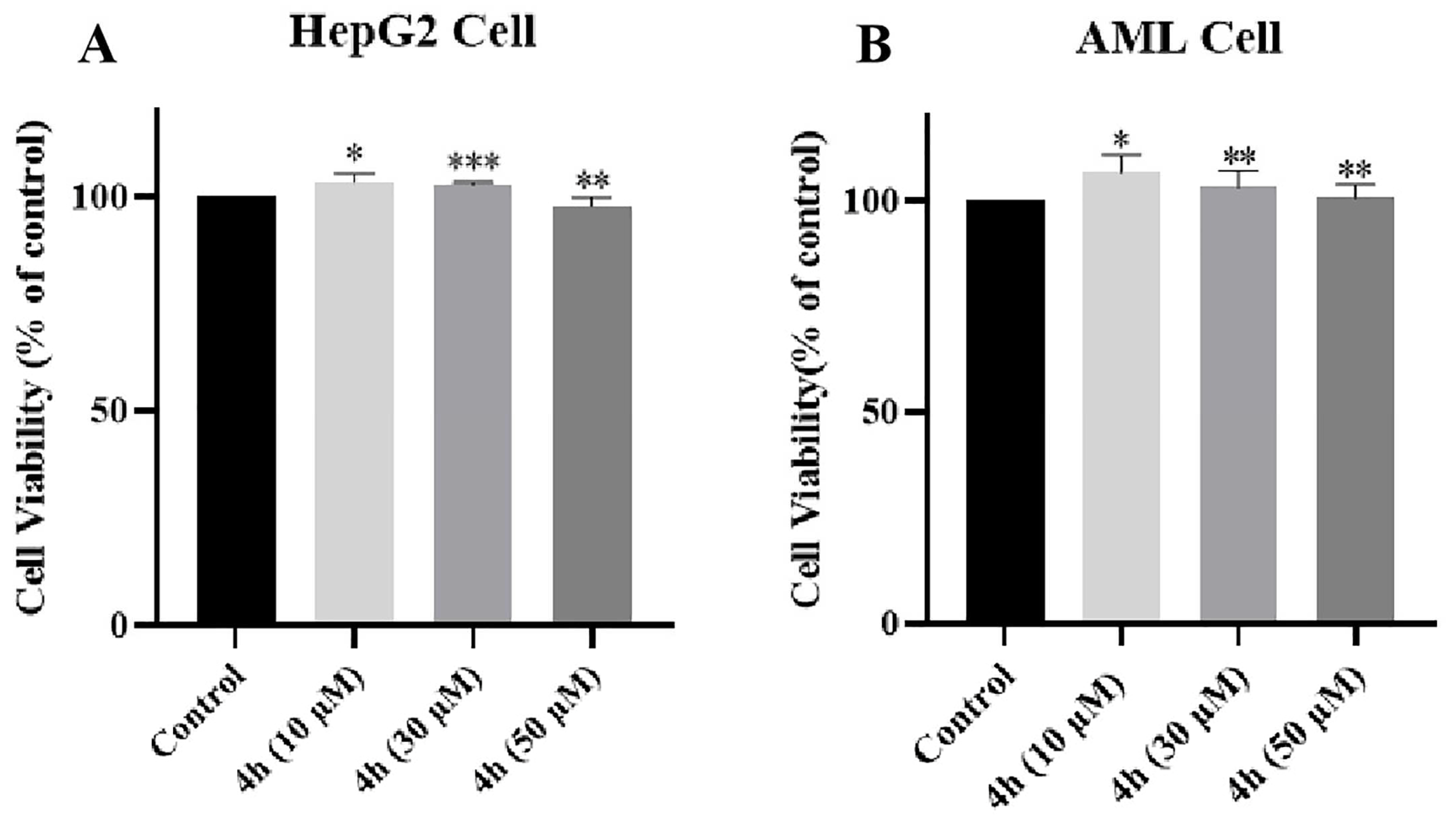
2.15. Evaluation of Hepatotoxicity of 4h in AML-12 and HepG2 Cells
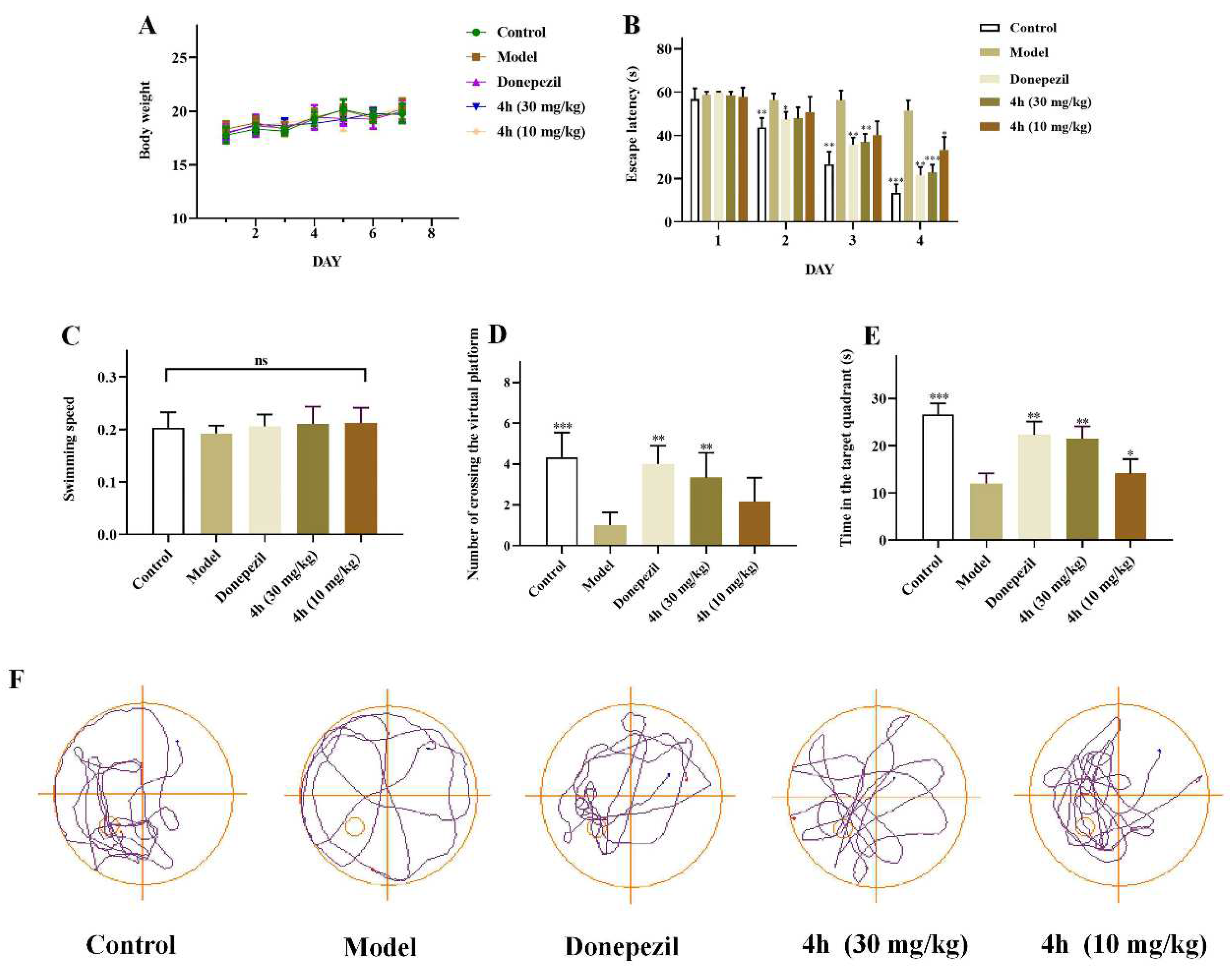
2.16. In Vivo Anti-AD Activity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Chemistry
4.3. AChE and BuChE Inhibition Experiments
4.4. Inhibition Reversibility of AChE/BuChE
4.5. Kinetic Studies for AChE/BuChE
4.6. Molecular Modeling
4.7. MTT Assay and Neuroprotective Effect against H2O2-Induced Toxicity
4.8. Effect on Aβ1-42 Peptide Aggregation
4.9. In Vitro Determination of NO, IL-1β, and TNF-α Contents
4.10. Western Blot
4.11. ROS Measurement in BV2 Cell
4.12. Determination of Intracellular ROS Production
4.13. Radical Scavenging Activity (DPPH Assay)
4.14. Metal Chelating Property
4.15. Bidirectional Transport Studies
4.16. Metabolic Stability in Liver Microsomes
4.17. Hepatotoxicity Assays
4.18. In Vivo Assays
4.18.1. Animals and Treatments
4.18.2. Determination of LD50 of 4h
4.18.3. Treatment and Modeling
4.18.4. Morris Water Maze Test
4.19. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abeysinghe, A.; Deshapriya, R.; Udawatte, C. Alzheimer’s disease; a review of the pathophysiological basis and therapeutic interventions. Life Sci. 2020, 256, 117996. [Google Scholar] [CrossRef] [PubMed]
- Barnett, R. Alzheimer’s disease. Lancet 2019, 393, 1589. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Srivastava, P.; Seth, A.; Tripathi, P.N.; Banerjee, A.G.; Shrivastava, S.K. Comprehensive review of mechanisms of pathogenesis involved in Alzheimer’s disease and potential therapeutic strategies. Prog. Neurobiol. 2019, 174, 53–89. [Google Scholar] [CrossRef] [PubMed]
- Vaz, M.; Silvestre, S. Alzheimer’s disease: Recent treatment strategies. Eur. J. Pharmacol. 2020, 887, 173554. [Google Scholar] [CrossRef]
- Du, X.; Wang, X.; Geng, M. Alzheimer’s disease hypothesis and related therapies. Transl. Neurodegener. 2018, 7, 2. [Google Scholar] [CrossRef]
- Anand, P.; Singh, B. A review on cholinesterase inhibitors for Alzheimer’s disease. Arch. Pharm. Res. 2013, 36, 375–399. [Google Scholar] [CrossRef]
- Bortolami, M.; Rocco, D.; Messore, A.; Di Santo, R.; Costi, R.; Madia, V.N.; Scipione, L.; Pandolfi, F. Acetylcholinesterase inhibitors for the treatment of Alzheimer’s disease—A patent review (2016–present). Expert. Opin. Ther. Pat. 2021, 31, 399–420. [Google Scholar] [CrossRef]
- Li, Q.; Chen, Y.; Xing, S.; Liao, Q.; Xiong, B.; Wang, Y.; Lu, W.; He, S.; Feng, F.; Liu, W.; et al. Highly Potent and Selective Butyrylcholinesterase Inhibitors for Cognitive Improvement and Neuroprotection. J. Med. Chem. 2021, 64, 6856–6876. [Google Scholar] [CrossRef]
- Mesulam, M.M.; Guillozet, A.; Shaw, P.; Levey, A.; Duysen, E.G.; Lockridge, O. Acetylcholinesterase knockouts establish central cholinergic pathways and can use butyrylcholinesterase to hydrolyze acetylcholine. Neuroscience 2002, 110, 627–639. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, Y.; Yin, W.; Li, J.; Wang, W.; Bai, F.; Xu, S.; Gong, Q.; Peng, T.; Hong, Y.; et al. Kinetics-Driven Drug Design Strategy for Next-Generation Acetylcholinesterase Inhibitors to Clinical Candidate. J. Med. Chem. 2021, 64, 1844–1855. [Google Scholar] [CrossRef]
- Greig, N.H.; Utsuki, T.; Yu, Q.; Zhu, X.; Holloway, H.W.; Perry, T.; Lee, B.; Ingram, D.K.; Lahiri, D.K. A new therapeutic target in Alzheimer’s disease treatment: Attention to butyrylcholinesterase. Curr. Med. Res. Opin. 2001, 17, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarnia, S.; Teimuri-Mofrad, R.; Rashidi, M.R. Design, synthesis and biological evaluation of 2,3-dihydro-5,6-dimethoxy-1H-inden-1-one and piperazinium salt hybrid derivatives as hAChE and hBuChE enzyme inhibitors. Eur. J. Med. Chem. 2020, 191, 112140. [Google Scholar] [CrossRef] [PubMed]
- Namdaung, U.; Athipornchai, A.; Khammee, T.; Kuno, M.; Suksamrarn, S. 2-Arylbenzofurans from Artocarpus lakoocha and methyl ether analogs with potent cholinesterase inhibitory activity. Eur. J. Med. Chem. 2018, 143, 1301–1311. [Google Scholar] [CrossRef]
- Zhou, B.; Li, H.; Cui, Z.; Li, D.; Geng, H.; Gao, J.; Zhou, L. Simple analogues of natural product chelerythrine: Discovery of a novel anticholinesterase 2-phenylisoquinolin-2-ium scaffold with excellent potency against acetylcholinesterase. Eur. J. Med. Chem. 2020, 200, 112415. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.O.C.; Alarcón, R.; Garrido, J.; Inestrosa, N.C. Acetylcholinesterase promotes the aggregation of amyloid-beta-peptide fragments by forming a complex with the growing fibrils. J. Mol. Biol. 1997, 272, 348–361. [Google Scholar] [CrossRef]
- De, S.; Whiten, D.R.; Ruggeri, F.S.; Hughes, C.; Rodrigues, M.; Sideris, D.I.; Taylor, C.G.; Aprile, F.A.; Muyldermans, S.; Knowles, T.P.J.; et al. Soluble aggregates present in cerebrospinal fluid change in size and mechanism of toxicity during Alzheimer’s disease progression. Acta Neuropathol. Commun. 2019, 7, 120. [Google Scholar] [CrossRef]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef]
- D’Acunto, C.W.; Kaplanek, R.; Gbelcova, H.; Kejik, Z.; Briza, T.; Vasina, L.; Havlik, M.; Ruml, T.; Kral, V. Metallomics for Alzheimer’s disease treatment: Use of new generation of chelators combining metal-cation binding and transport properties. Eur. J. Med. Chem. 2018, 150, 140–155. [Google Scholar] [CrossRef]
- Akundi, R.S.; Huang, Z.; Eason, J.; Pandya, J.D.; Zhi, L.; Cass, W.A.; Sullivan, P.G.; Bueler, H. Increased mitochondrial calcium sensitivity and abnormal expression of innate immunity genes precede dopaminergic defects in Pink1-deficient mice. PLoS ONE 2011, 6, e16038. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Rekatsina, M.; Paladini, A.; Piroli, A.; Zis, P.; Pergolizzi, J.V.; Varrassi, G. Pathophysiology and Therapeutic Perspectives of Oxidative Stress and Neurodegenerative Diseases: A Narrative Review. Adv. Ther. 2020, 37, 113–139. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.W.; Zhang, X.; Huang, W.J. Role of neuroinflammation in neurodegenerative diseases (Review). Mol. Med. Rep. 2016, 13, 3391–3396. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Perez, J.M.; Morillas-Ruiz, J.M. A review: Inflammatory process in Alzheimer’s disease, role of cytokines. ScientificWorldJournal 2012, 2012, 756357. [Google Scholar] [CrossRef]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. 2018, 4, 575–590. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Zhou, J.W. Neuroinflammation in the central nervous system: Symphony of glial cells. Glia 2019, 67, 1017–1035. [Google Scholar] [CrossRef]
- Marttinen, M.; Takalo, M.; Natunen, T.; Wittrahm, R.; Gabbouj, S.; Kemppainen, S.; Leinonen, V.; Tanila, H.; Haapasalo, A.; Hiltunen, M. Molecular Mechanisms of Synaptotoxicity and Neuroinflammation in Alzheimer’s Disease. Front. Neurosci. 2018, 12, 963. [Google Scholar] [CrossRef]
- Umar, T.; Hoda, N. Alzheimer’s Disease: A Systemic Review of Substantial Therapeutic Targets and the Leading Multi-functional Molecules. Curr. Top. Med. Chem. 2017, 17, 3370–3389. [Google Scholar] [CrossRef]
- de Freitas Silva, M.; Dias, K.S.T.; Gontijo, V.S.; Ortiz, C.J.C.; Viegas, C., Jr. Multi-Target Directed Drugs as a Modern Approach for Drug Design towards Alzheimer’s Disease: An Update. Curr. Med. Chem. 2018, 25, 3491–3525. [Google Scholar] [CrossRef]
- Barnham, K.J.; Bush, A.I. Biological metals and metal-targeting compounds in major neurodegenerative diseases. Chem. Soc. Rev. 2014, 43, 6727–6749. [Google Scholar] [CrossRef]
- Morphy, R.; Rankovic, Z. Designed multiple ligands. An emerging drug discovery paradigm. J. Med. Chem. 2005, 48, 6523–6543. [Google Scholar] [CrossRef] [PubMed]
- Andersson, C.D.; Forsgren, N.; Akfur, C.; Allgardsson, A.; Berg, L.; Engdahl, C.; Qian, W.; Ekstrom, F.; Linusson, A. Divergent structure-activity relationships of structurally similar acetylcholinesterase inhibitors. J. Med. Chem. 2013, 56, 7615–7624. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Lu, C.; Sun, Y.; Mao, F.; Luo, Z.; Su, T.; Jiang, H.; Shan, W.; Li, X. Multitarget-directed benzylideneindanone derivatives: Anti-beta-amyloid (Abeta) aggregation, antioxidant, metal chelation, and monoamine oxidase B (MAO-B) inhibition properties against Alzheimer’s disease. J. Med. Chem. 2012, 55, 8483–8492. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, S.; Prajapati, S.K.; Majumdar, S.; Raza, M.K.; Gabr, M.T.; Kumar, S.; Pal, K.; Rashid, H.; Kumar, S.; Krishnamurthy, S.; et al. Discovery of new phenyl sulfonyl-pyrimidine carboxylate derivatives as the potential multi-target drugs with effective anti-Alzheimer’s action: Design, synthesis, crystal structure and in-vitro biological evaluation. Eur. J. Med. Chem. 2021, 215, 113224. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, H.; Song, Q.; Yu, G.; Liu, Z.; Zhong, F.; Tan, Z.; Liu, X.; Deng, Y. Development of novel 2-aminoalkyl-6-(2-hydroxyphenyl)pyridazin-3(2H)-one derivatives as balanced multifunctional agents against Alzheimer’s disease. Eur. J. Med. Chem. 2022, 230, 114098. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Lan, H.Y.; Peng, W.; Rahman, K.; Liu, Q.C.; Luan, X.; Zhang, H. Isatis indigotica: A review of phytochemistry, pharmacological activities and clinical applications. J. Pharm. Pharmacol. 2021, 73, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Jahng, Y. Progress in the studies on tryptanthrin, an alkaloid of history. Arch. Pharm. Res. 2013, 36, 517–535. [Google Scholar] [CrossRef]
- Chang, H.N.; Yeh, Y.C.; Chueh, H.Y.; Pang, J.S. The anti-angiogenic effect of tryptanthrin is mediated by the inhibition of apelin promoter activity and shortened mRNA half-life in human vascular endothelial cells. Phytomedicine 2019, 58, 152879. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Wang, R.; Cui, M.; Liu, W.; Yang, Q.; Kuang, C. Synthesis of novel tryptanthrin derivatives as dual inhibitors of indoleamine 2,3-dioxygenase 1 and tryptophan 2,3-dioxygenase. Bioorganic Med. Chem. Lett. 2020, 30, 127159. [Google Scholar] [CrossRef]
- Zhang, S.; Qi, F.; Fang, X.; Yang, D.; Hu, H.; Huang, Q.; Kuang, C.; Yang, Q. Tryptophan 2,3-dioxygenase inhibitory activities of tryptanthrin derivatives. Eur. J. Med. Chem. 2018, 160, 133–145. [Google Scholar] [CrossRef]
- Du, J.; Liu, P.; Zhu, Y.; Wang, G.; Xing, S.; Liu, T.; Xia, J.; Dong, S.; Lv, N.; Li, Z. Novel tryptanthrin derivatives with benzenesulfonamide substituents: Design, synthesis, and anti-inflammatory evaluation. Eur. J. Med. Chem. 2023, 246, 114956. [Google Scholar] [CrossRef]
- Ellman, G.L.; Andres, K.D.C.V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Erdogan, M.; Kilic, B.; Sagkan, R.I.; Aksakal, F.; Ercetin, T.; Gulcan, H.O.; Dogruer, D.S. Design, synthesis and biological evaluation of new benzoxazolone/benzothiazolone derivatives as multi-target agents against Alzheimer’s disease. Eur. J. Med. Chem. 2021, 212, 113124. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, H.; Wang, Y.; Liu, W.; Yin, G.; Wang, D.; Li, J.; Shi, T.; Wang, Z. Design, synthesis, and biological evaluation of carbamate derivatives of N-salicyloyl tryptamine as multifunctional agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2022, 229, 114044. [Google Scholar] [CrossRef]
- Czarnecka, K.; Chufarova, N.; Halczuk, K.; Maciejewska, K.; Girek, M.; Skibinski, R.; Jonczyk, J.; Bajda, M.; Kabzinski, J.; Majsterek, I.; et al. Tetrahydroacridine derivatives with dichloronicotinic acid moiety as attractive, multipotent agents for Alzheimer’s disease treatment. Eur. J. Med. Chem. 2018, 145, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Han, J.; Liu, Z.; Zhang, Y.; Huang, C.; Li, J.; Li, Z. Identification of novel CDK 9 inhibitors based on virtual screening, molecular dynamics simulation, and biological evaluation. Life Sci. 2020, 258, 118228. [Google Scholar] [CrossRef] [PubMed]
- Abdullaha, M.; Nuthakki, V.K.; Bharate, S.B. Discovery of methoxy-naphthyl linked N-(1-benzylpiperidine) benzamide as a blood-brain permeable dual inhibitor of acetylcholinesterase and butyrylcholinesterase. Eur. J. Med. Chem. 2020, 207, 112761. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef]
- Yao, H.; Uras, G.; Zhang, P.; Xu, S.; Yin, Y.; Liu, J.; Qin, S.; Li, X.; Allen, S.; Bai, R.; et al. Discovery of Novel Tacrine-Pyrimidone Hybrids as Potent Dual AChE/GSK-3 Inhibitors for the Treatment of Alzheimer’s Disease. J. Med. Chem. 2021, 64, 7483–7506. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Cagide, F.; Teixeira, J.; Amorim, R.; Sequeira, L.; Mesiti, F.; Silva, T.; Garrido, J.; Remiao, F.; Vilar, S.; et al. Hydroxybenzoic Acid Derivatives as Dual-Target Ligands: Mitochondriotropic Antioxidants and Cholinesterase Inhibitors. Front. Chem. 2018, 6, 126. [Google Scholar] [CrossRef]
- Zhou, L.C.; Liang, Y.F.; Huang, Y.; Yang, G.X.; Zheng, L.L.; Sun, J.M.; Li, Y.; Zhu, F.L.; Qian, H.W.; Wang, R.; et al. Design, synthesis, and biological evaluation of diosgenin-indole derivatives as dual-functional agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2021, 219, 113426. [Google Scholar] [CrossRef] [PubMed]
- Sang, Z.; Song, Q.; Cao, Z.; Deng, Y.; Tan, Z.; Zhang, L. Design, synthesis and evaluation of novel dimethylamino chalcone-O-alkylamines derivatives as potential multifunctional agents against Alzheimer’s disease. Eur. J. Med. Chem. 2021, 216, 113310. [Google Scholar] [CrossRef] [PubMed]
- Sang, Z.; Wang, K.; Shi, J.; Cheng, X.; Zhu, G.; Wei, R.; Ma, Q.; Yu, L.; Zhao, Y.; Tan, Z.; et al. Apigenin-rivastigmine hybrids as multi-target-directed liagnds for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2020, 187, 111958. [Google Scholar] [CrossRef]
- Sang, Z.; Wang, K.; Shi, J.; Liu, W.; Cheng, X.; Zhu, G.; Wang, Y.; Zhao, Y.; Qiao, Z.; Wu, A.; et al. The development of advanced structural framework as multi-target-directed ligands for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2020, 192, 112180. [Google Scholar] [CrossRef]
- Zhou, W.; Zhong, G.; Fu, S.; Xie, H.; Chi, T.; Li, L.; Rao, X.; Zeng, S.; Xu, D.; Wang, H.; et al. Microglia-Based Phenotypic Screening Identifies a Novel Inhibitor of Neuroinflammation Effective in Alzheimer’s Disease Models. ACS Chem. Neurosci. 2016, 7, 1499–1507. [Google Scholar] [CrossRef]
- Zhang, J.; Yi, S.; Xiao, C.; Li, Y.; Liu, C.; Jiang, W.; Yang, C.; Zhou, T. Asperosaponin VI inhibits LPS-induced inflammatory response by activating PPAR-gamma pathway in primary microglia. Saudi J. Biol. Sci. 2020, 27, 3138–3144. [Google Scholar] [CrossRef] [PubMed]
- Pawate, S.; Shen, Q.; Fan, F.; Bhat, N.R. Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J. Neurosci. Res. 2004, 77, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Jana, A.; Yatish, K.; Freidt, M.B.; Fung, Y.K.; Martinson, J.A.; Pahan, K. Reactive oxygen species up-regulate CD11b in microglia via nitric oxide: Implications for neurodegenerative diseases. Free Radic. Biol. Med. 2008, 45, 686–699. [Google Scholar] [CrossRef]
- Purgatorio, R.; de Candia, M.; Catto, M.; Carrieri, A.; Pisani, L.; De Palma, A.; Toma, M.; Ivanova, O.A.; Voskressensky, L.G.; Altomare, C.D. Investigating 1,2,3,4,5,6-hexahydroazepino [4,3-b]indole as scaffold of butyrylcholinesterase-selective inhibitors with additional neuroprotective activities for Alzheimer’s disease. Eur. J. Med. Chem. 2019, 177, 414–424. [Google Scholar] [CrossRef]
- Chand, K.; Rajeshwari; Hiremathad, A.; Singh, M.; Santos, M.A.; Keri, R.S. A review on antioxidant potential of bioactive heterocycle benzofuran: Natural and synthetic derivatives. Pharmacol. Rep. 2017, 69, 281–295. [Google Scholar] [CrossRef]
- Wu, M.; Ma, J.; Ji, L.; Wang, M.; Han, J.; Li, Z. Design, synthesis, and biological evaluation of rutacecarpine derivatives as multitarget-directed ligands for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2019, 177, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Wichur, T.; Wieckowska, A.; Wieckowski, K.; Godyn, J.; Jonczyk, J.; Valdivieso, A.D.R.; Panek, D.; Pasieka, A.; Sabate, R.; Knez, D.; et al. 1-Benzylpyrrolidine-3-amine-based BuChE inhibitors with anti-aggregating, antioxidant and metal-chelating properties as multifunctional agents against Alzheimer’s disease. Eur. J. Med. Chem. 2020, 187, 111916. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Ajazuddin; Tripathi, D.K.; Saraf, S.; Antimisiaris, S.G.; Mourtas, S.; Hammarlund-Udenaes, M.; Alexander, A. Recent advancements in liposomes targeting strategies to cross blood-brain barrier (BBB) for the treatment of Alzheimer’s disease. J. Control Release 2017, 260, 61–77. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, H.; Ashby, C.R., Jr.; Assaraf, Y.G.; Chen, Z.S.; Liu, H.M. Chemical molecular-based approach to overcome multidrug resistance in cancer by targeting P-glycoprotein (P-gp). Med. Res. Rev. 2021, 41, 525–555. [Google Scholar] [CrossRef]
- Hu, H.H.; Bian, Y.C.; Liu, Y.; Sheng, R.; Jiang, H.D.; Yu, L.S.; Hu, Y.Z.; Zeng, S. Evaluation of blood-brain barrier and blood-cerebrospinal fluid barrier permeability of 2-phenoxy-indan-1-one derivatives using in vitro cell models. Int. J. Pharm. 2014, 460, 101–107. [Google Scholar] [CrossRef]
- Hellinger, E.; Veszelka, S.; Toth, A.E.; Walter, F.; Kittel, A.; Bakk, M.L.; Tihanyi, K.; Hada, V.; Nakagawa, S.; Duy, T.D.; et al. Comparison of brain capillary endothelial cell-based and epithelial (MDCK-MDR1, Caco-2, and VB-Caco-2) cell-based surrogate blood-brain barrier penetration models. Eur. J. Pharm. Biopharm. 2012, 82, 340–351. [Google Scholar] [CrossRef]
- Navarro, C.; Gonzalez-Alvarez, I.; Gonzalez-Alvarez, M.; Manku, M.; Merino, V.; Casabo, V.G.; Bermejo, M. Influence of polyunsaturated fatty acids on Cortisol transport through MDCK and MDCK-MDR1 cells as blood-brain barrier in vitro model. Eur. J. Pharm. Sci. 2011, 42, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, I.; Krizbai, I.A. In vitro models of the blood-brain barrier for the study of drug delivery to the brain. Mol. Pharm. 2014, 11, 1949–1963. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.-x.; Huang, M.; Cui, W.; Feng, L.-J.; Wu, Y.; Cai, Y.; Li, Z.; Zhu, X.; Liu, P.; Wan, Y.; et al. Discovery of a Phosphodiesterase 9A Inhibitor as a Potential Hypoglycemic Agent. J. Med. Chem. 2014, 57, 10304–10313. [Google Scholar] [CrossRef]
- Liew, K.-F.; Chan, K.-L.; Lee, C.-Y. Blood–brain barrier permeable anticholinesterase aurones: Synthesis, structure–activity relationship, and drug-like properties. Eur. J. Med. Chem. 2015, 94, 195–210. [Google Scholar] [CrossRef]
- Klinkenberg, I.; Blokland, A. The validity of scopolamine as a pharmacological model for cognitive impairment: A review of animal behavioral studies. Neurosci. Biobehav. Rev. 2010, 34, 1307–1350. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lin, H.Z.; Zhu, J.; Gu, K.; Li, Q.; He, S.Y.; Lu, X.; Tan, R.X.; Pei, Y.Q.; Wu, L.; et al. Design, synthesis, in vitro and in vivo evaluation of tacrine-cinnamic acid hybrids as multi-target acetyl-and butyrylcholinesterase inhibitors against Alzheimer’s disease. Rsc Adv. 2017, 7, 33851–33867. [Google Scholar] [CrossRef]
- Inestrosa, N.C.; Sagal, J.P.; Colombres, M. Acetylcholinesterase interaction with Alzheimer amyloid beta. Subcell. Biochem. 2005, 38, 299–317. [Google Scholar] [CrossRef]
- Nalivaeva, N.N.; Turner, A.J. AChE and the amyloid precursor protein (APP)—Cross-talk in Alzheimer’s disease. Chem. Biol. Interact. 2016, 259, 301–306. [Google Scholar] [CrossRef]
- Thal, D.R.; Walter, J.; Saido, T.C.; Fandrich, M. Neuropathology and biochemistry of Abeta and its aggregates in Alzheimer’s disease. Acta Neuropathol. 2015, 129, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Calsolaro, V.; Edison, P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimer’s Dement. 2016, 12, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Shityakov, S.S.R.; Salvador, E.; Roewer, N.; Broscheit, J.; Förster, C. Evaluation of the potential toxicity of unmodified and modified cyclodextrins on murine blood-brain barrier endothelial cells. J. Toxicol. Sci. 2016, 41, 175–184. [Google Scholar] [CrossRef]
- Zhao, Y.; Gan, L.; Ren, L.; Lin, Y.; Ma, C.; Lin, X. Factors influencing the blood-brain barrier permeability. Brain Res. 2022, 1788, 147937. [Google Scholar] [CrossRef]
- Kurz, C.; Walker, L.; Rauchmann, B.S.; Perneczky, R. Dysfunction of the blood-brain barrier in Alzheimer’s disease: Evidence from human studies. Neuropathol. Appl. Neurobiol. 2022, 48, e12782. [Google Scholar] [CrossRef]
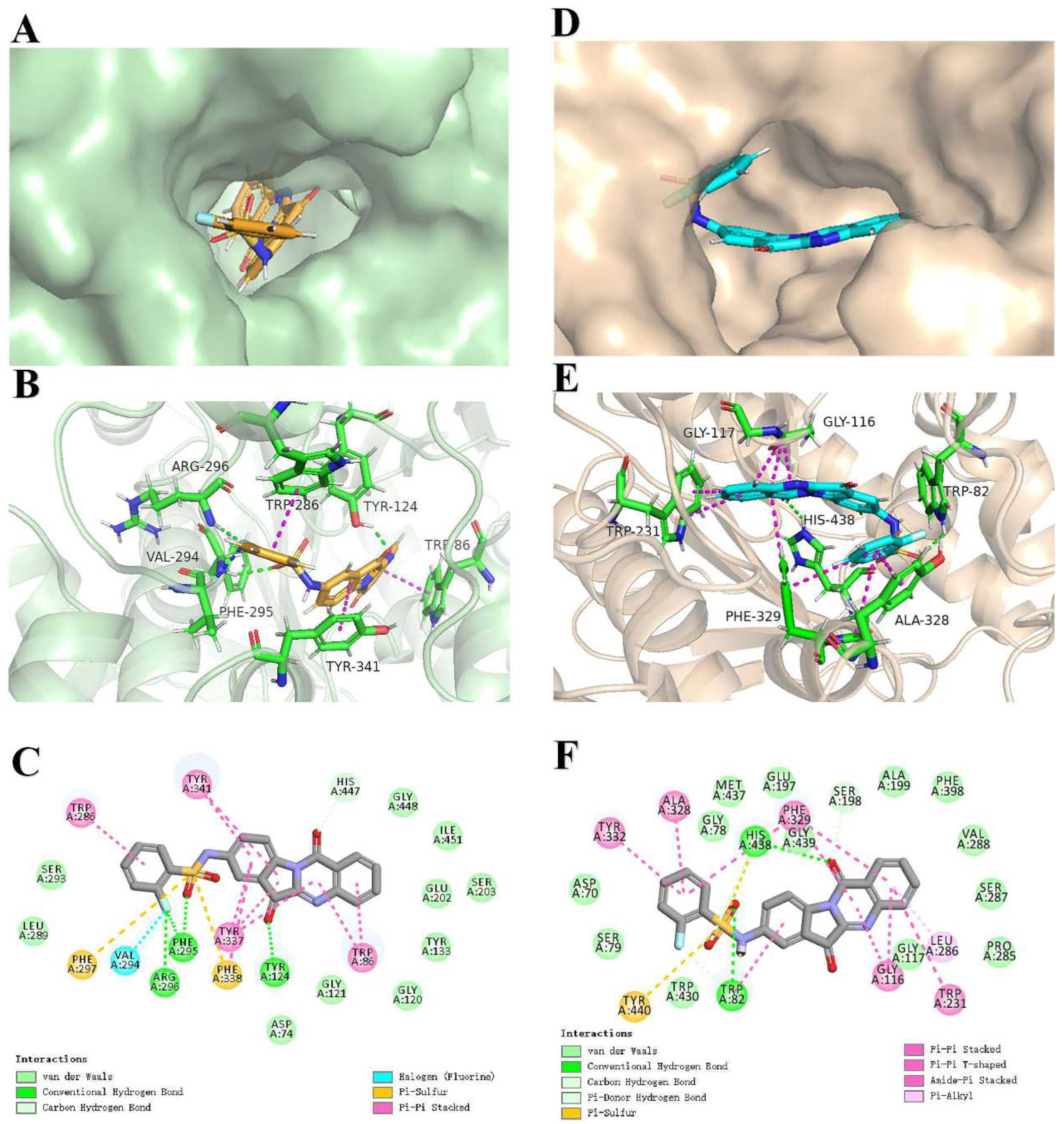
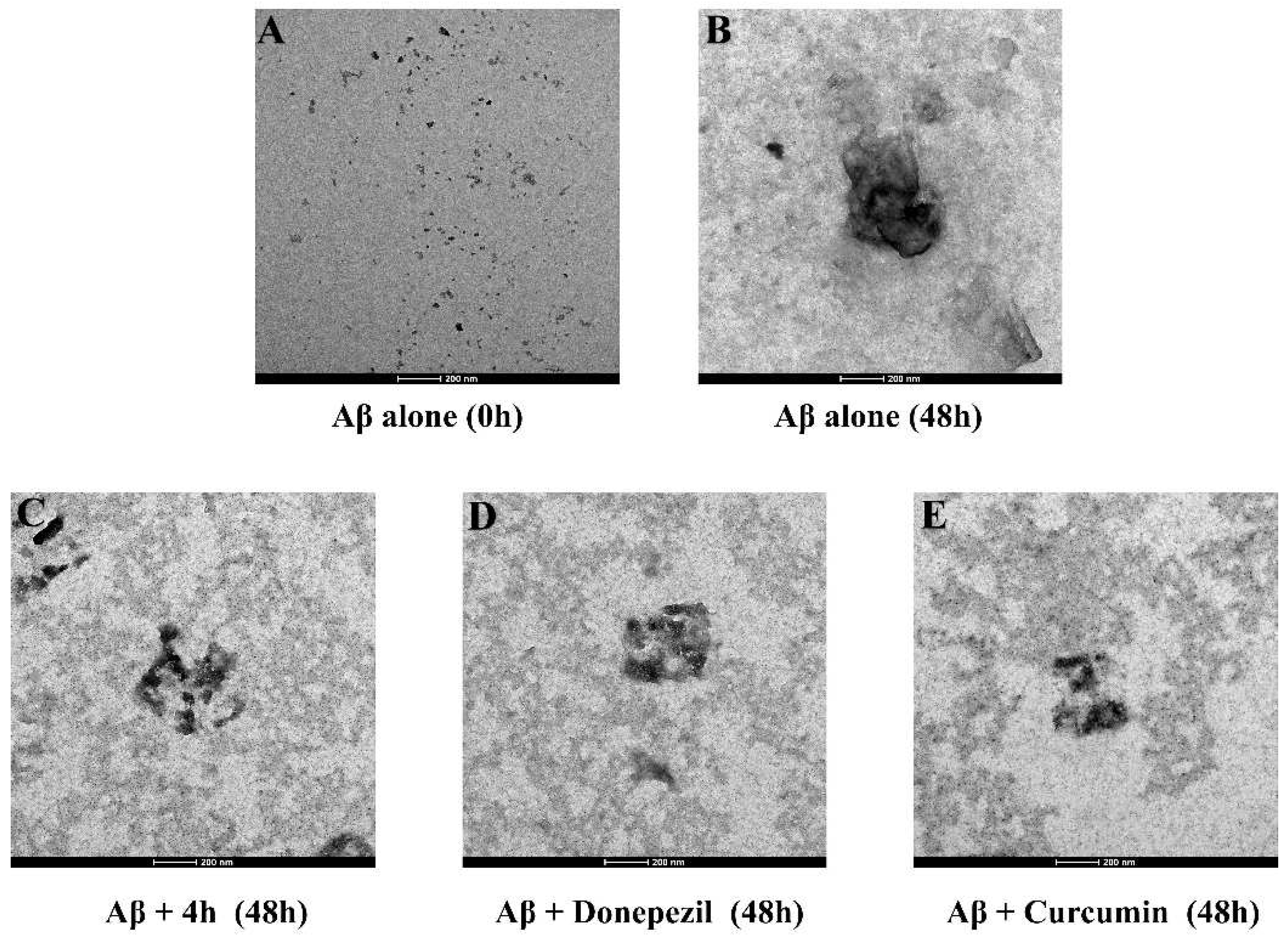
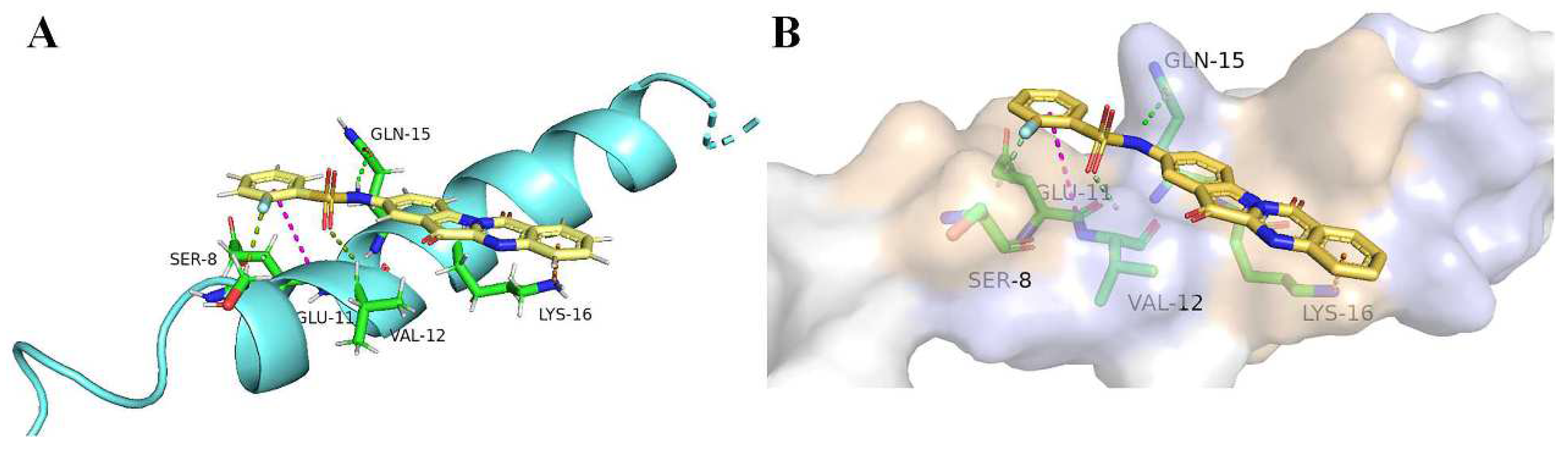
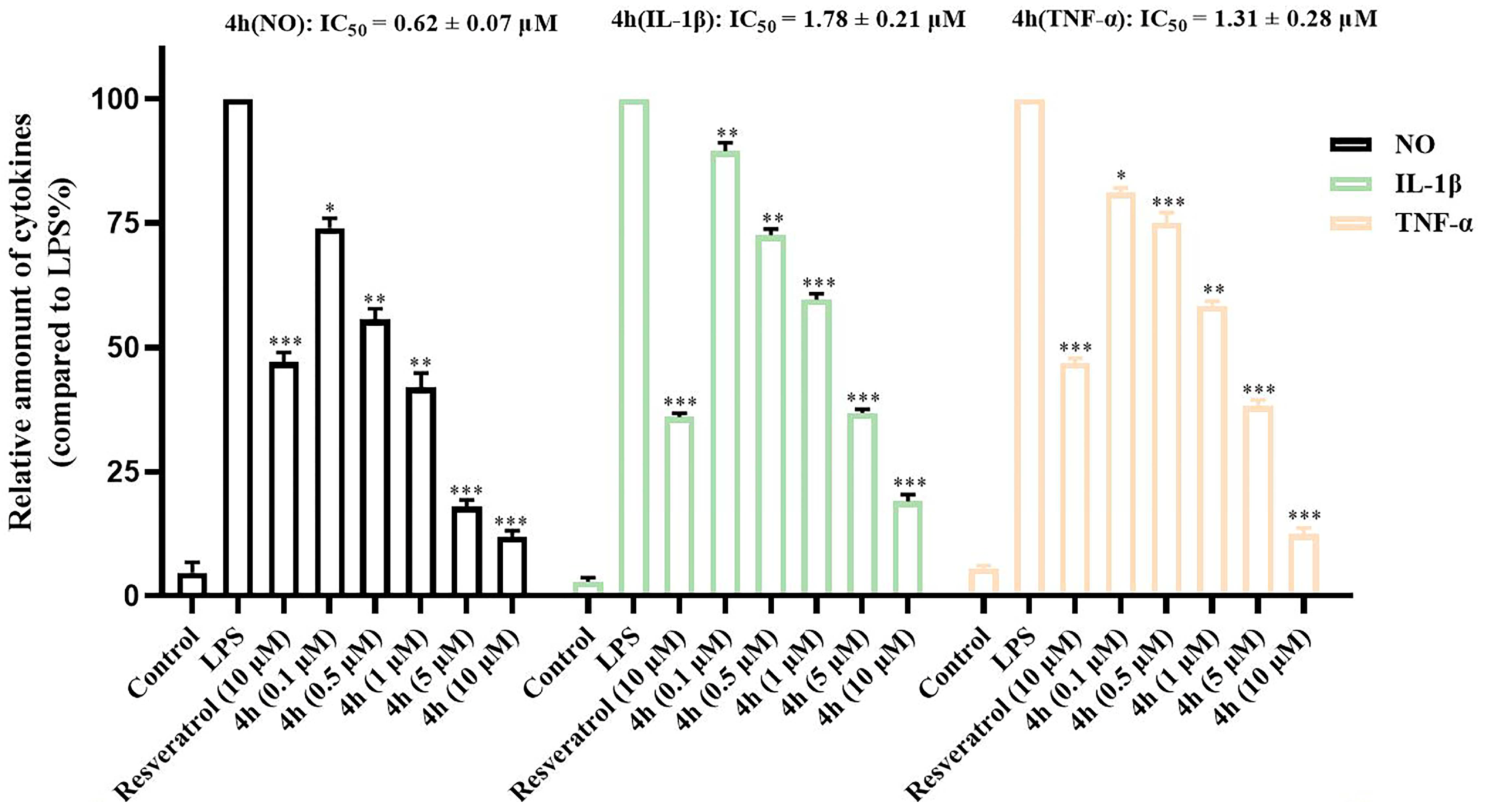
| Compd | AChE Inhibition, (IC50, μM) a | BuChE Inhibition, (IC50, μM) b | Selective Index c |
|---|---|---|---|
| 4a | 0.97 ± 0.16 | 9.98 ± 0.64 | 10.3 |
| 4b | 5.75 ± 0.27 | 8.61 ± 1.2 | 1.5 |
| 4c | 2.56 ± 0.32 | 8.13 ± 1.21 | 3.2 |
| 4d | >50 | >50 | N/A |
| 4e | 33.01 ± 1.91 | >50 | N/A |
| 4f | 0.56 ± 0.07 | 11.87 ± 1.86 | 21.2 |
| 4g | 3.21 ± 0.39 | 21.58 ± 1.79 | 6.7 |
| 4h | 0.13 ± 0.04 | 6.11 ± 0.15 | 47 |
| 4i | 8.8 ± 1.23 | >50 | N/A |
| 4j | >50 | >50 | N/A |
| 4k | 25.41 ± 1.53 | >50 | N/A |
| 4l | 18.56 ± 1.77 | >50 | N/A |
| 4m | 7.75 ± 0.21 | 10.19 ± 1.24 | 1.7 |
| 4n | 5.92 ± 0.56 | 4.93 ± 0.35 | 0.6 |
| 4o | 2.45 ± 0.25 | 8.61 ± 1.2 | 3.5 |
| 4p | 0.62 ± 0.09 | >50 | N/A |
| 8a | 14.02 ± 1.67 | >50 | N/A |
| 8b | 4.55 ± 0.8 | >50 | N/A |
| 8c | 15.84 ± 2.6 | >50 | N/A |
| 8d | >50 | >50 | N/A |
| 8e | >50 | >50 | N/A |
| 8f | 43 ± 1.12 | >50 | N/A |
| 8g | >50 | >50 | N/A |
| 8h | >50 | >50 | N/A |
| 8i | >50 | 25.87 ± 2.38 | N/A |
| 8j | >50 | 10.19 ± 2.41 | N/A |
| 8k | >50 | >50 | N/A |
| 8l | >50 | >50 | N/A |
| 8m | >50 | >50 | N/A |
| 8n | 42.02 ± 1.7 | >50 | N/A |
| 8o | 8.86 ± 0.76 | >50 | N/A |
| 8p | >50 | >50 | N/A |
| Donepezil | 0.021 ± 0.005 | 7.29 ± 0.6 | 347.1 |
| Tacrine | 0.270 ± 0.064 | 0.035 ± 0.05 | 0.12 |
| AChE | BuChE | |||
|---|---|---|---|---|
| Donepezil | 4h | Donepezil | 4h | |
| Control | 100 ± 0.01 | 100 ± 0.01 | 100 ± 0.01 | 100 ± 0.01 |
| 1 × IC50 | 83.1 ± 1.27 | 72.3 ± 1.03 | 89.4 ± 1.49 | 84.9 ± 1.2 |
| 0.1 × IC50 | 92.3 ± 0.74 | 87.2 ± 0.92 | 94.1 ± 0.61 | 92.2 ± 0.52 |
| Compd | H2O2-Induced PC12 Cell Viability (% of Control) a | Cell Viability (% of Control) a | |||
|---|---|---|---|---|---|
| H2O2 | 10 μM | 30 μM | 30 μM | 50 μM | |
| 4a | 32.1 ± 0.9 | 43.29 ± 3.1 | 48.5 ± 1.5 | 99.5 ± 1.7 | 98.3 ± 3.0 |
| 4b | 32.41 ± 1.1 | 47.36 ± 1.7 | 59.09 ± 2.5 | 99.7 ± 0.9 | 99.5 ± 1.5 |
| 4c | 30.3 ± 1.6 | 45.59 ± 0.7 | 56.4 ± 1.2 | 99.5 ± 3.1 | 98.2 ± 1.7 |
| 4d | 32.12 ± 1.2 | 42.53 ± 2.7 | 50.1 ± 0.8 | 99.4 ± 2.4 | 97.3 ± 3.6 |
| 4e | 38.17 ± 1.6 | 42.8 ± 1.5 | 57.51 ± 0.8 | 99.6 ± 2.1 | 99.5 ± 2.3 |
| 4f | 34.69 ± 1.4 | 45.23 ± 2.2 | 55.26 ± 0.5 | 99.4 ± 1.6 | 98.7 ± 1.1 |
| 4g | 36.26 ± 1.9 | 59.88 ± 0.9 | 68.83 ± 2.7 | 100 ± 1.3 | 99.7 ± 0.8 |
| 4h | 30.1 ± 2.1 | 61.66 ± 1.3 | 71.31 ± 1.9 | 99.6 ± 0.9 | 99.2 ± 1.5 |
| 4i | 33.92 ± 3.1 | 34.43 ± 5.5 | 40.93 ± 2.9 | 99.6 ± 1.7 | 98.3 ± 3.0 |
| 4j | 35.4 ± 2.3 | 38.24 ± 3.1 | 41.69 ± 1.7 | 99.7 ± 1.6 | 99.1 ± 1.2 |
| 4k | 34.22 ± 1.7 | 44.35 ± 1.5 | 46.23 ± 0.6 | 98.8 ± 1.9 | 98.2 ± 3.4 |
| 4l | 30.9 ± 1.2 | 36.35 ± 1.6 | 39.84 ± 2.8 | 100.2 ± 3.1 | 99.8 ± 2.7 |
| 4m | 31.6 ± 2.3 | 37.89 ± 4.3 | 44.98 ± 2.5 | 99.6 ± 0.8 | 99.3 ± 2.1 |
| 4n | 31.5 ± 0.4 | 46.53 ± 1.2 | 52.84 ± 1.8 | 99.9 ± 0.7 | 99.7 ± 1.8 |
| 4o | 29.31 ± 1.5 | 42.32 ± 0.7 | 50.5 ± 0.5 | 99.6 ± 2.7 | 98.2 ± 1.7 |
| 4p | 33.9 ± 1.7 | 48.49 ± 4.5 | 65.6 ± 2.2 | 99.6 ± 2.7 | 99.3 ± 2.9 |
| 8a | 33.77 ± 1.8 | 39.65 ± 3.1 | 50.98 ± 1.8 | 100.1 ± 1.5 | 99.8 ± 1.7 |
| 8b | 32.9 ± 2.1 | 42.01 ± 1.7 | 51.37 ± 2.1 | 101.2 ± 3.1 | 99.8 ± 2.4 |
| 8c | 32.16 ± 2.2 | 39.10 ± 4.5 | 49.64 ± 4.1 | 98.4 ± 1.9 | 96.7 ± 1.9 |
| 8d | 35.33 ± 1.9 | 39.65 ± 5.2 | 53.26 ± 1.4 | 99.8 ± 0.7 | 98.7 ± 2.6 |
| 8e | 32.6 ± 0.6 | 41.78 ± 1.2 | 55.86 ± 0.9 | 99.8 ± 0.7 | 99.7 ± 3.1 |
| 8f | 30.72 ± 0.9 | 34.24 ± 3.5 | 42.25 ± 1.7 | 99.4 ± 1.7 | 98.3 ± 3.0 |
| 8g | 34.41 ± 0.8 | 44.37 ± 0.5 | 52.28 ± 2.3 | 99.6 ± 1.2 | 99.5 ± 2.7 |
| 8h | 32.91 ± 1.4 | 36.03 ± 3.0 | 46.73 ± 3.5 | 99.4 ± 1.4 | 98.3 ± 2.7 |
| 8i | 29.9 ± 2.3 | 48.46 ± 1.6 | 59.63 ± 2.5 | 99.8 ± 1.2 | 99.4 ± 1.3 |
| 8j | 31.7 ± 0.3 | 38.02 ± 3.1 | 47.36 ± 6.1 | 99.9 ± 1.5 | 98.9 ± 2.1 |
| 8k | 35.44 ± 0.8 | 44.37 ± 0.5 | 52.28 ± 2.3 | 99.6 ± 1.2 | 99.5 ± 2.7 |
| 8l | 32.83 ± 1.7 | 34.61 ± 3.7 | 38.1 ± 2.7 | 99.4 ± 1.1 | 98.7 ± 1.3 |
| 8m | 30.13 ± 1.5 | 44.67 ± 2.3 | 53.92 ± 3.9 | 99.5 ± 3.1 | 98.2 ± 1.7 |
| 8n | 32.21 ± 2.1 | 38.94 ± 1.5 | 49.96 ± 0.8 | 99.2 ± 1.9 | 98.3 ± 2.7 |
| 8o | 35.1 ± 3.1 | 42.56 ± 2.1 | 54.6 ± 3.8 | 99.7 ± 2.4 | 99.4 ± 1.1 |
| 8p | 29.33 ± 1.5 | 42.32 ± 0.7 | 50.5 ± 0.5 | 99.6 ± 2.7 | 98.2 ± 1.7 |
| Quercetin | 30.4 ± 0.7 | 55.21 ± 1.6 | 63.27 ± 2.1 | 99.6 ± 1.1 | 99.5 ± 1.3 |
| Compd | Inhibition of Self-Induced Aβ Aggregation (%) a |
|---|---|
| 4h | 63.16 ± 2.33 |
| Curcumin | 55.41 ± 2.31 |
| Donepezil | 41.21 ± 1.87 |
| Compd | Papp AP (×10−5cm/s) a | Papp BL (×10−5cm/s) a | ER b Papp BL/Papp AP |
|---|---|---|---|
| 4h | 1.81 ± 0.27 | 1.74 ± 0.22 | 0.96 |
| Diazepam | 1.36 ± 0.17 | 1.19 ± 0.15 | 0.89 |
| FD4 | 0.47 ± 0.15 | 0.32 ± 0.14 | 0.65 |
| Compd. | K (min−1) | T1/2 (min) a |
|---|---|---|
| Testosterone b | 0.2593 ± 0.04409 | 2.4 ± 0.5 |
| Donepezil b | 0.00834 ± 0.00044 | 74.3 ± 5.3 |
| 4h | 0.00574 ± 0.00037 | 108.3 ± 4.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Du, J.; Ma, J.; Liu, P.; Xing, S.; Xia, J.; Dong, S.; Li, Z. Discovery of Novel Tryptanthrin Derivatives with Benzenesulfonamide Substituents as Multi-Target-Directed Ligands for the Treatment of Alzheimer’s Disease. Pharmaceuticals 2023, 16, 1468. https://doi.org/10.3390/ph16101468
Wang G, Du J, Ma J, Liu P, Xing S, Xia J, Dong S, Li Z. Discovery of Novel Tryptanthrin Derivatives with Benzenesulfonamide Substituents as Multi-Target-Directed Ligands for the Treatment of Alzheimer’s Disease. Pharmaceuticals. 2023; 16(10):1468. https://doi.org/10.3390/ph16101468
Chicago/Turabian StyleWang, Guoxing, Jiyu Du, Jie Ma, Peipei Liu, Siqi Xing, Jucheng Xia, Shuanghong Dong, and Zeng Li. 2023. "Discovery of Novel Tryptanthrin Derivatives with Benzenesulfonamide Substituents as Multi-Target-Directed Ligands for the Treatment of Alzheimer’s Disease" Pharmaceuticals 16, no. 10: 1468. https://doi.org/10.3390/ph16101468
APA StyleWang, G., Du, J., Ma, J., Liu, P., Xing, S., Xia, J., Dong, S., & Li, Z. (2023). Discovery of Novel Tryptanthrin Derivatives with Benzenesulfonamide Substituents as Multi-Target-Directed Ligands for the Treatment of Alzheimer’s Disease. Pharmaceuticals, 16(10), 1468. https://doi.org/10.3390/ph16101468