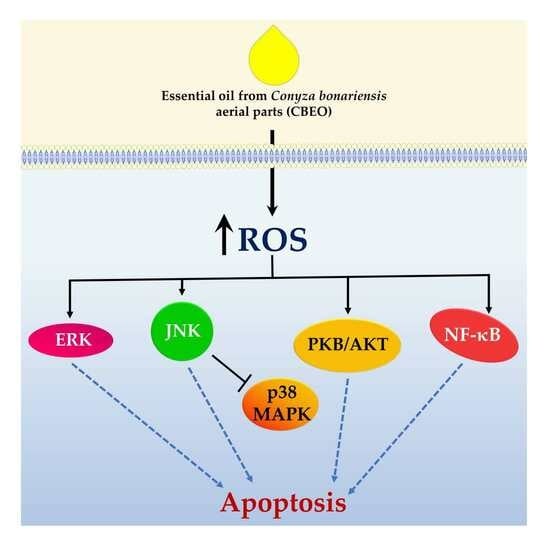
The Essential Oil from Conyza bonariensis (L.) Cronquist (Asteraceae) Exerts an In Vitro Antimelanoma Effect by Inducing Apoptosis and Modulating the MAPKs, NF-κB, and PKB/AKT Signaling Pathways
Abstract
:1. Introduction
2. Results
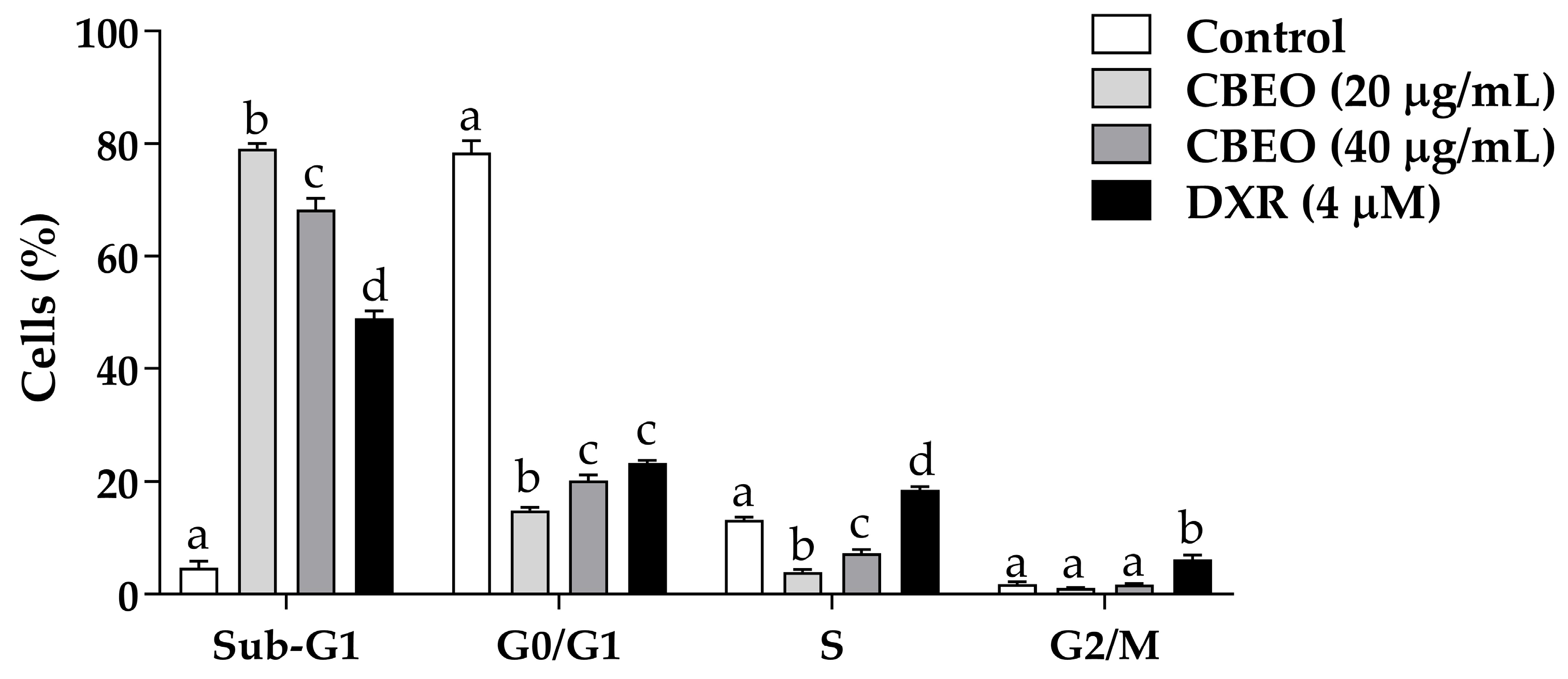
2.1. CBEO Treatment Increases the Percentage of Cells in the Sub-G1 Peak
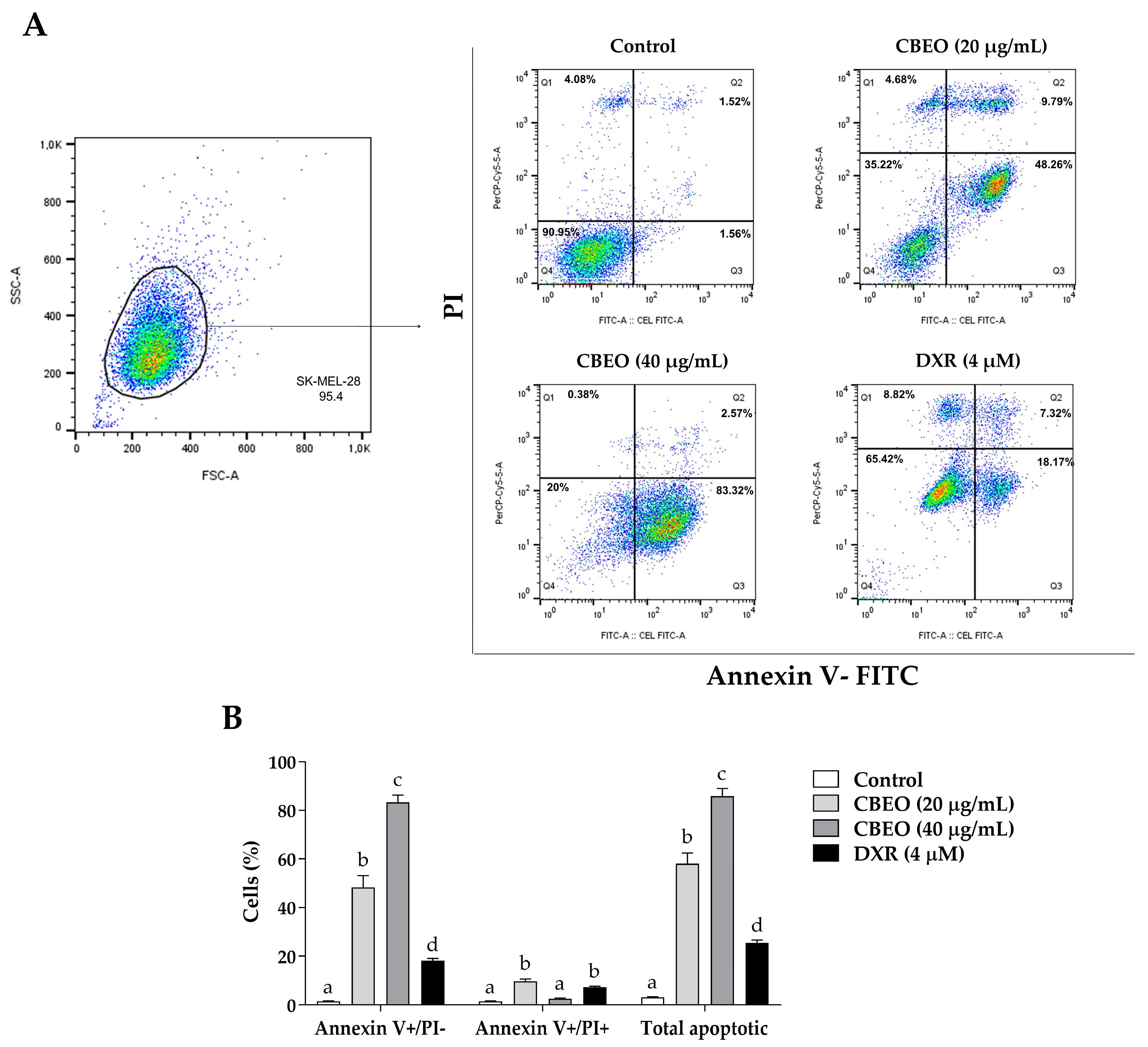
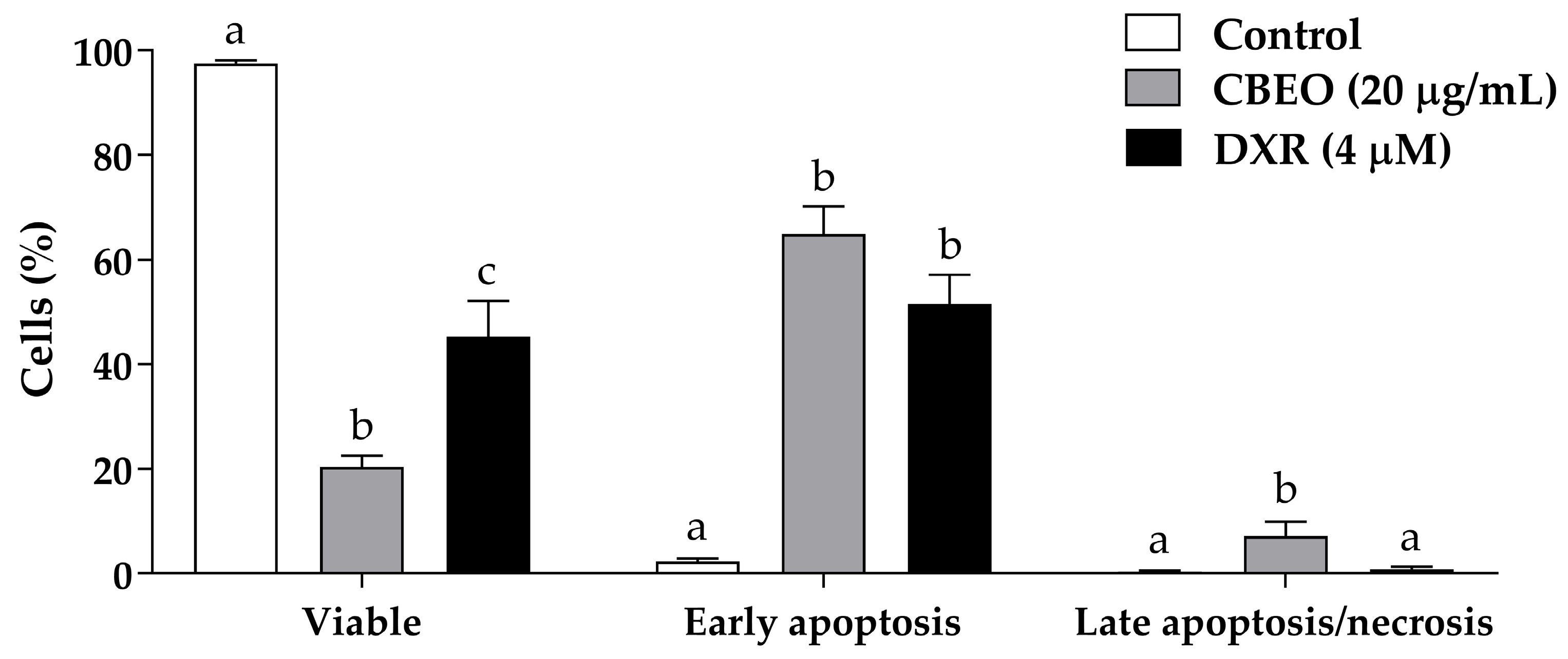
2.2. CBEO Treatment Induces Apoptosis
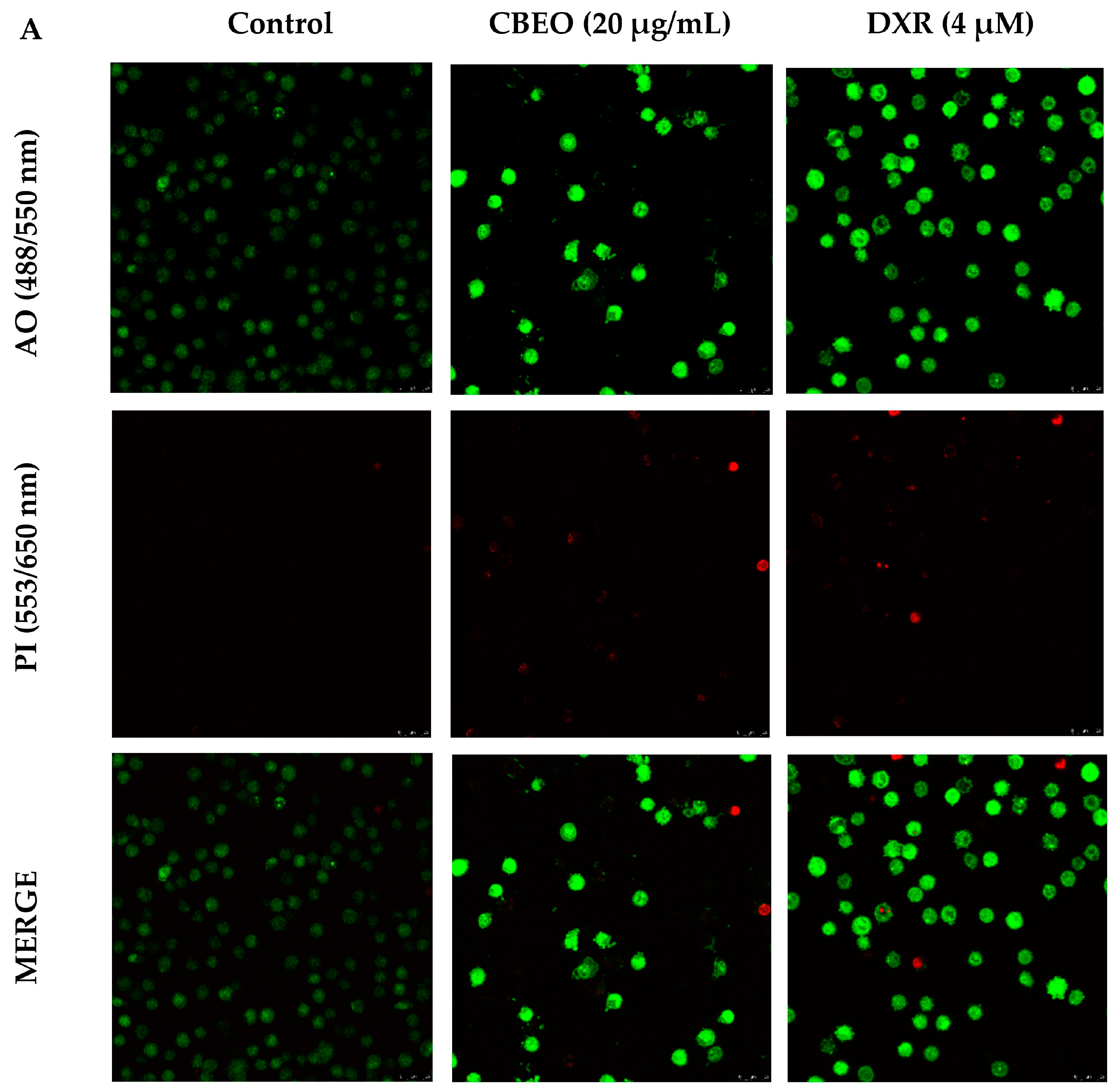
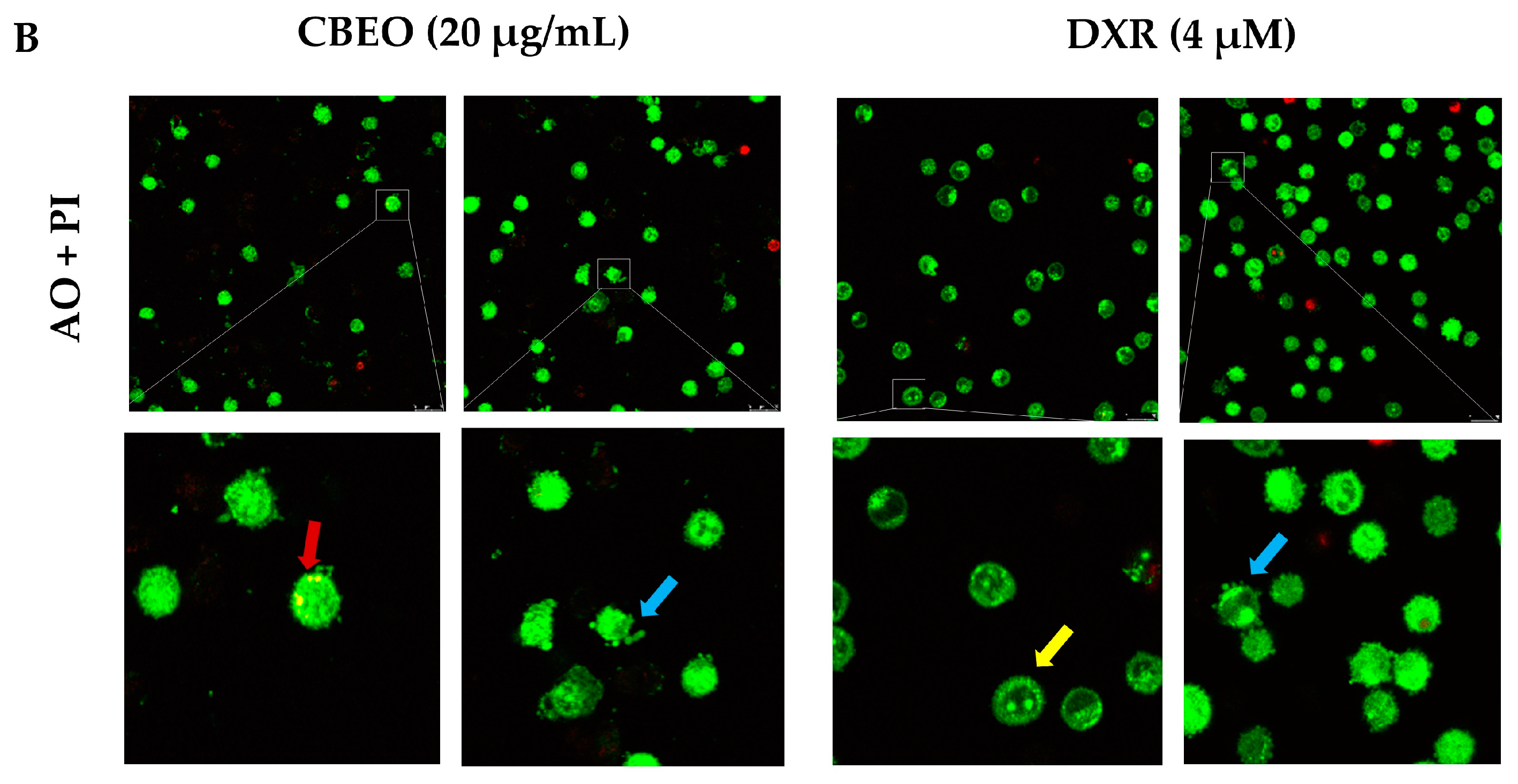
2.3. CBEO-Treated Cells Exhibit Morphological Characteristics of Apoptosis in Confocal Microscopy
2.4. Docking Prediction
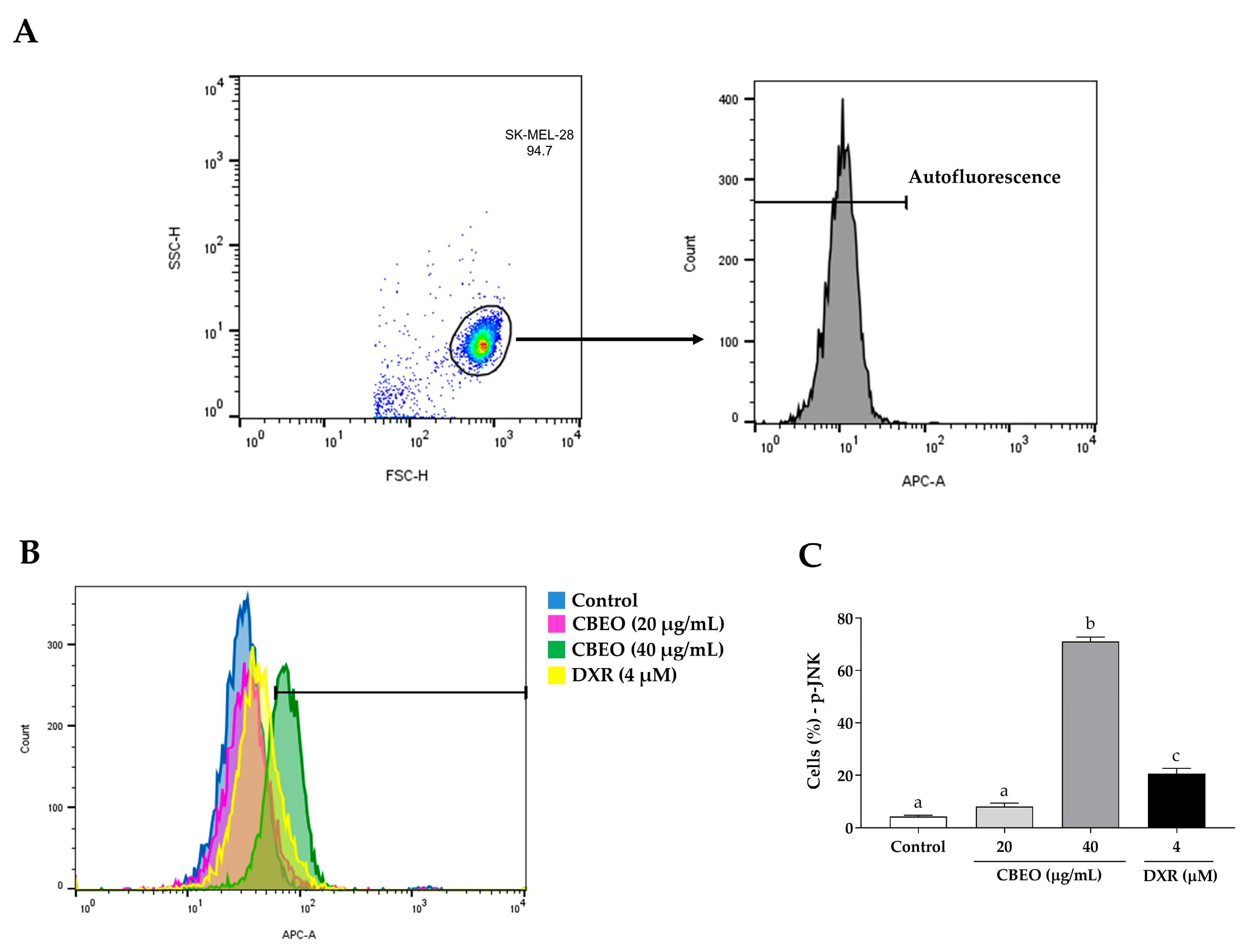
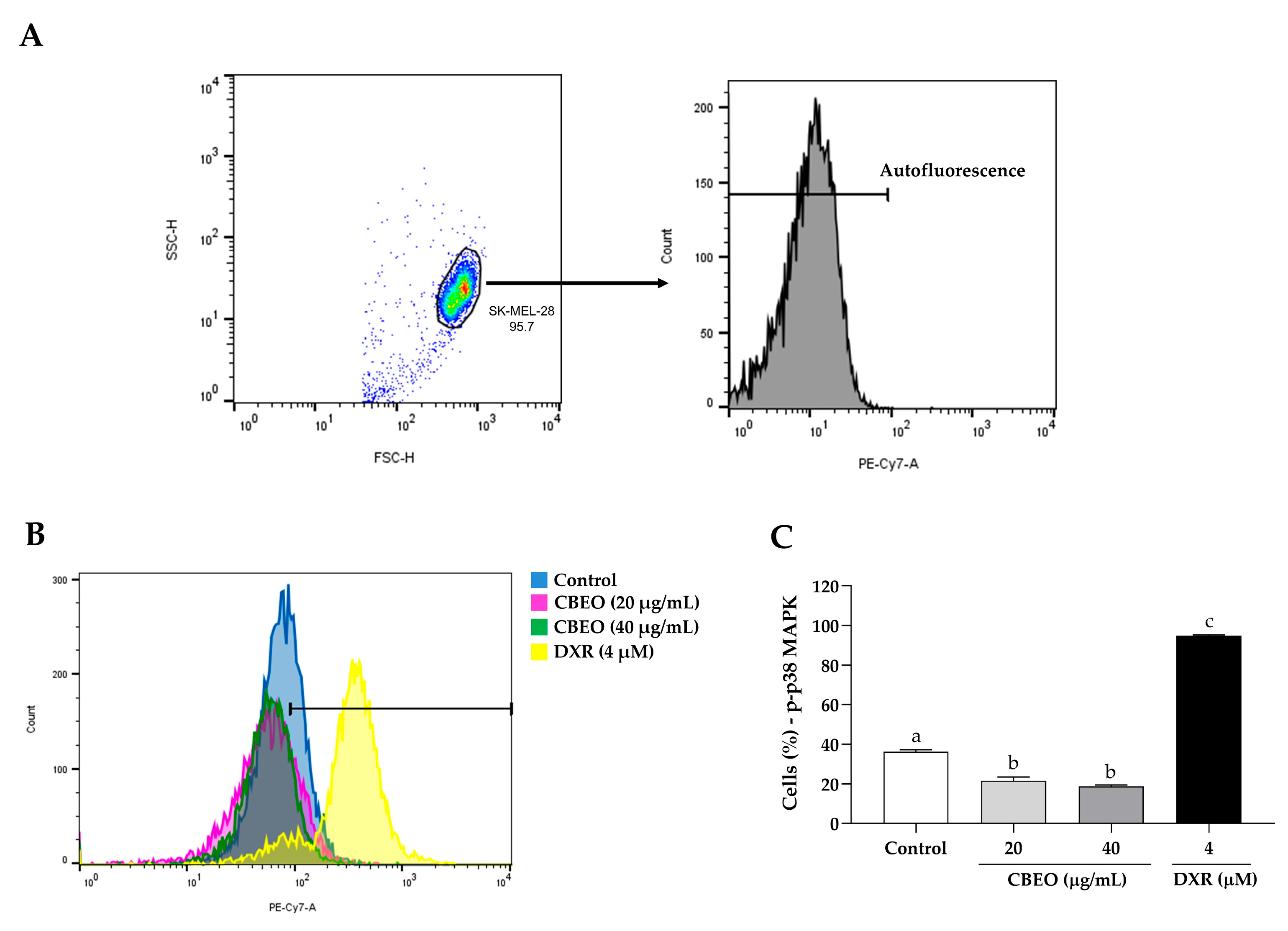
2.5. CBEO Treatment Modulates the Activity of MAPKs (Mitogen-Activated Protein Kinases)
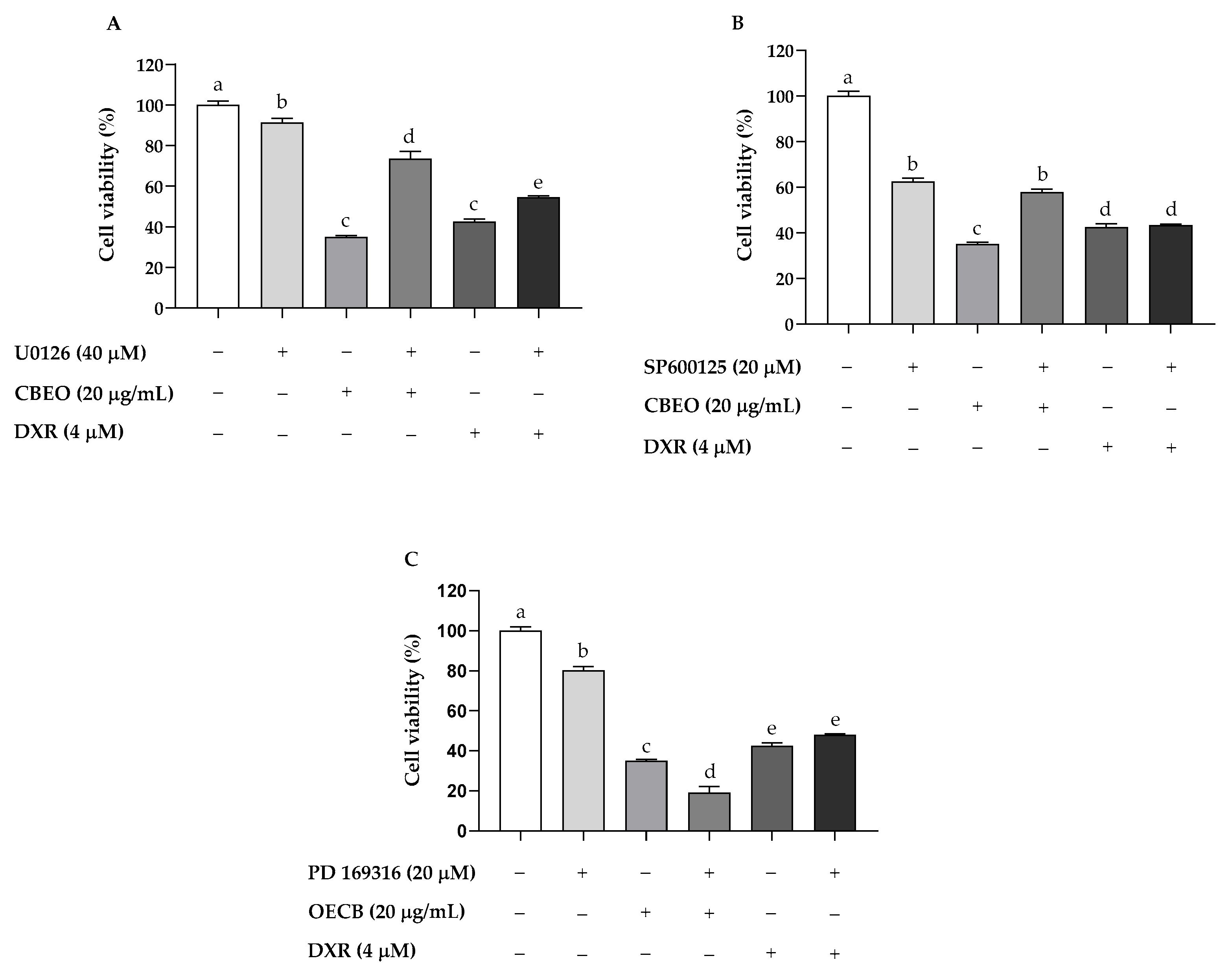
2.6. CBEO Cytotoxicity in SK-MEL-28 Cells Is MAPK-Dependent
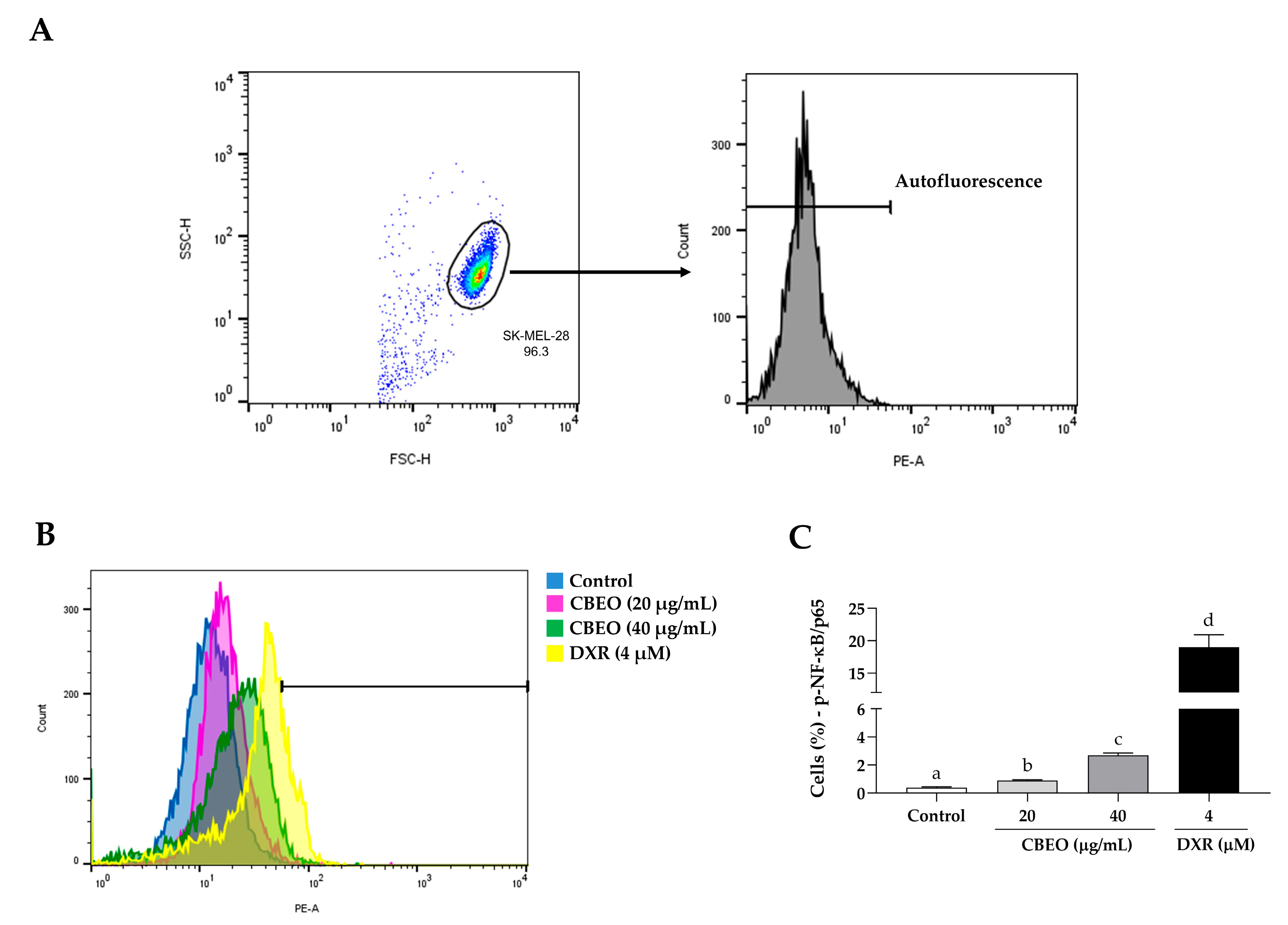
2.7. CBEO Treatment Modulates the Activity of NF-κB (Nuclear Factor kappa B)
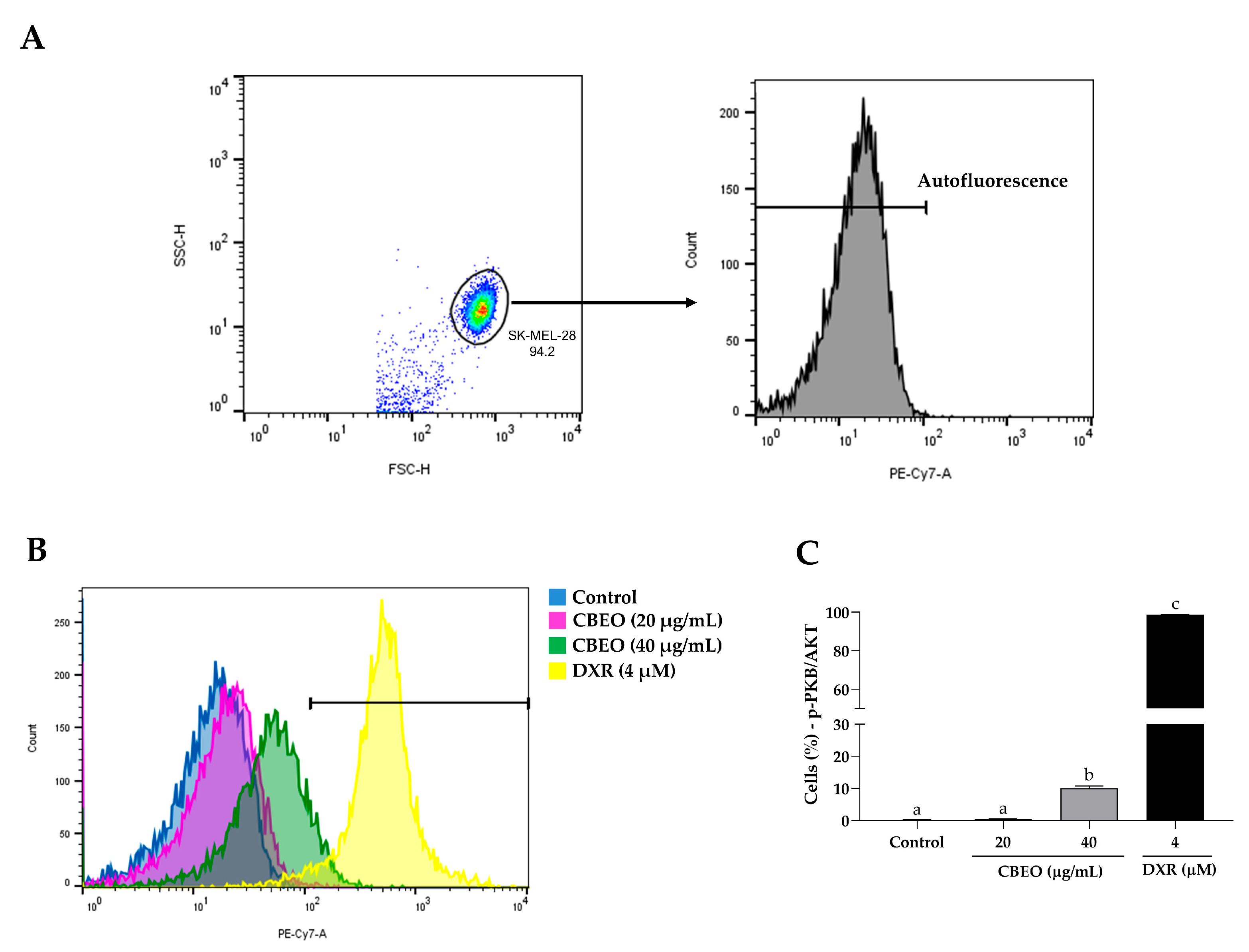
2.8. CBEO Treatment Modulates the Activity of PKB/AKT (Protein Kinase B)
3. Discussion
4. Materials and Methods
4.1. Drugs and Reagents
4.2. Human Tumor Cell Line
4.3. Cell Cycle Analysis
4.4. Apoptosis Analysis by Flow Cytometry
4.5. Apoptosis Analysis by Laser Confocal Microscopy
4.6. Docking Prediction
4.7. MAPK Signaling Analysis by Flow Cytometry
4.8. Evaluation of CBEO Cytotoxicity in the Presence or Absence of MAPKs Inhibitors
4.9. NF-κB and PKB/AKT Signaling Analysis by Flow Cytometry
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Switzer, B.; Puzanov, I.; Skitzki, J.J.; Hamad, L.; Ernstoff, M.S. Managing metastatic melanoma in 2022: A clinical review. J. Oncol. Pract. 2022, 18, 335–351. [Google Scholar] [CrossRef]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of melanoma. Med. Sci. 2021, 9, 63. [Google Scholar] [CrossRef]
- Arnold, M.; Singh, D.; Laversanne, M.; Vignat, J.; Vaccarella, S.; Meheus, F.; Cust, A.E.; Vries, E.; Whiteman, D.C.; Bray, F. Global burden of cutaneous melanoma in 2020 and projections to 2040. JAMA Dermatol. 2022, 158, 495–503. [Google Scholar] [CrossRef]
- El-Hussein, A.; Manoto, S.L.; Ombinda-Lemboumba, S.; Alrowaili, Z.A.; Mthunzi-Kufa, P. A review of chemotherapy and photodynamic therapy for lung cancer treatment. Anti-Cancer Agents Med. Chem. 2021, 21, 149–161. [Google Scholar] [CrossRef]
- Rosen, C.; Mayes, T.; Overholt, C.; Lucke-Wold, B. Treatment of melanoma metastasis: Surgical, chemotherapy, and innovation. Med. Discov. 2023, 2, 4. [Google Scholar]
- Bahar, E.; Han, S.Y.; Kim, J.Y.; Yoon, H. Chemotherapy resistance: Role of mitochondrial and autophagic components. Cancers 2022, 14, 1462. [Google Scholar] [CrossRef]
- Marei, H.E.; Hasan, A.; Pozzoli, G.; Cenciarelli, C. Cancer immunotherapy with immune checkpoint inhibitors (ICIs): Potential, mechanisms of resistance, and strategies for reinvigorating T cell responsiveness when resistance is acquired. Cancer Cell Int. 2023, 23, 64. [Google Scholar] [CrossRef]
- Said, S.S.; Ibrahim, W.N. Cancer resistance to immunotherapy: Comprehensive insights with future perspectives. Pharmaceutics 2023, 15, 1143. [Google Scholar] [CrossRef]
- Malyarenko, O.S.; Malyarenko, T.V.; Usoltseva, R.V.; Kicha, A.A.; Ivanchina, N.V.; Ermakova, S.P. Combined radiomodifying effect of fucoidan from the brown alga Saccharina cichorioides and pacificusoside D from the starfish Solaster pacificus in the model of 3D melanoma cells. Biomolecules 2023, 13, 419. [Google Scholar] [CrossRef]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signaling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar]
- Kashyap, N.; Kumari, A.; Raina, N.; Zakir, F.; Gupta, M. Prospects of essential oil loaded nanosystems for skincare. Phytomed. Plus. 2022, 2, 100198. [Google Scholar] [CrossRef]
- Elsayed, H.E.; El-Deeb, E.M.; Taha, H.; Taha, H.S.; Elgindi, M.R.; Moharram, F.A. Essential oils of Psidium cattleianum Sabine leaves and flowers: Anti-inflammatory and cytotoxic activities. Front. Chem. 2023, 11, 1120432. [Google Scholar] [CrossRef]
- García-Sánchez, E.; Solano, R.; Maciel-Amador, O.; Lagunez-Rivera, L. Composition of essential oil from the bark and leaves of two morphotypes of Salmea scandens (Asteraceae), a medicinal plant in Oaxaca, Mexico. Nat. Prod. Res. 2023, 1–5. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Chem. Res. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Basavegowda, N.; Baek, K. Synergistic Antioxidant and Antibacterial Advantages of Essential Oils for Food Packaging Applications. Biomolecules 2021, 11, 1267. [Google Scholar] [CrossRef]
- Adande, K.; Eloh, K.; Simalou, O.; Bakaï, M.F.; Caboni, P. Chemical composition of different extracts of Conyza bonariensis: Insecticidal and nematicidal activities. Am. J. Anal. Chem. 2023, 14, 95–120. [Google Scholar] [CrossRef]
- Araujo, L.; Moujir, L.M.; Rojas, J.; Rojas, L.; Carmona, J.; Rondón, M. Chemical composition and biological activity of Conyza bonariensis essential oil collected in Mérida, Venezuela. Nat. Prod. Commun. 2013, 8, 1175–1178. [Google Scholar] [CrossRef]
- Elgamal, A.M.; Ahmed, R.F.; Abd-Elgawad, A.M.; El Gendy, A.E.N.G.; Elshamy, A.I.; Nassar, M.I. Chemical profiles, anti-cancer, and anti-aging activities of essential oils of Pluchea dioscoridis (L.) DC. and Erigeron bonariensis L. Plants 2021, 10, 667. [Google Scholar] [CrossRef]
- Ferreira, R.C.; Nascimento, Y.M.D.; Loureiro, P.B.D.A.; Martins, R.X.; Maia, M.E.D.S.; Farias, D.F.; Tavares, J.F.; Gonçalves, J.C.R.; Silva, M.S.; Sobral, M.V. Chemical composition, in vitro antitumor effect, and toxicity in zebrafish of the essential oil from Conyza bonariensis (L.) Cronquist (Asteraceae). Biomolecules 2023, 13, 1439. [Google Scholar] [CrossRef]
- Zhao, S.; Tang, Y.; Wang, R.; Najafi, M. Mechanisms of cancer cell death induction by paclitaxel: An updated review. Apoptosis 2022, 27, 647–667. [Google Scholar] [CrossRef]
- Di Martile, M.; Garzoli, S.; Ragno, R.; Del Bufalo, D. Essential oils and their main chemical components: The past 20 years of preclinical studies in melanoma. Cancers 2020, 12, 2650. [Google Scholar] [CrossRef]
- Ahmadian, E.; Eftekhari, A.; Babaei, H.; Nayebi, A.M.; Eghbal, M.A. Anti-cancer effects of citalopram on hepatocellular carcinoma cells occur via cytochrome C release and the activation of NF-κB. Anti-Cancer Agents Med. Chem. 2017, 17, 1570–1577. [Google Scholar] [CrossRef]
- Hussain, A.R.; Uddin, S.; Bu, R.; Khan, O.S.; Ahmed, S.O.; Ahmed, M.; Al-Kuraya, K.S. Resveratrol suppresses constitutive activation of AKT via generation of ROS and induces apoptosis in diffuse large B cell lymphoma cell lines. PLoS ONE 2011, 6, e24703. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Elbadawi, M.; Efferth, T. Multiple cell death modalities and their key features. World Acad. Sci. J. 2020, 2, 39–48. [Google Scholar] [CrossRef]
- Patergnani, S.; Danese, A.; Bouhamida, E.; Aguiari, G.; Previati, M.; Pinton, P.; Giorgi, C. Various aspects of calcium signaling in the regulation of apoptosis, autophagy, cell proliferation, and cancer. Int. J. Mol. Sci. 2020, 21, 8323. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.-K.; Becker, A.; Park, J.-I. Growth inhibitory signaling of the Raf/MEK/ERK pathway. Int. J. Mol. Sci. 2020, 21, 5436. [Google Scholar] [CrossRef]
- Buchbinder, E.I.; Giobbie-Hurder, A.; Ott, P.A. A phase I/II study of MCS110 with BRAF/MEK inhibition in patients with melanoma after progression on BRAF/MEK inhibition. Investig. New Drugs. 2023, 41, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Ouyang, W.; Zhang, X.; Liao, L.; Pi, X.; Yang, R.; Mei, B.; Xu, H.; Xiang, S.; Li, J. UTRN inhibits melanoma growth by suppressing p38 and JNK/c-Jun signaling pathways. Cancer Cell Int. 2021, 21, 88. [Google Scholar] [CrossRef]
- Naffa, R.; Vogel, L.; Hegedűs, L.; Pászty, K.; Tóth, S.; Kelemen, K.; Singh, N.; Reményi, A.; Kállay, E.; Cserepes, M.; et al. P38 MAPK promotes migration and metastatic activity of BRAF mutant melanoma cells by inducing degradation of PMCA4b. Cells 2020, 9, 1209. [Google Scholar] [CrossRef]
- Martínez-Limón, A.; Joaquin, M.; Caballero, M.; Posas, F.; Nadal, E. The p38 pathway: From biology to cancer therapy. Int. J. Mol. Sci. 2020, 21, 1913. [Google Scholar] [CrossRef]
- Pranteda, A.; Piastra, V.; Stramucci, L.; Fratantonio, D.; Bossi, G. The p38 MAPK signaling activation in colorectal cancer upon therapeutic treatments. Int. J. Mol. Sci. 2020, 21, 2773. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, R.; Satoh, R.; Takasaki, T. ERK: A double-edged sword in cancer. ERK-dependent apoptosis as a potential therapeutic strategy for cancer. Cells 2021, 10, 2509. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Jiang, X.; Xie, H.; He, J.; Xiao, S. The Jun N-terminal kinases signaling pathway plays a “seesaw” role in ovarian carcinoma: A molecular aspect. J. Ovarian Res. 2019, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Mo, S.; Wang, Z.; Xu, J.; Fu, X.; Tian, Y. UXT at the crossroads of cell death, immunity and neurodegenerative diseases. Front. Oncol. 2023, 13, 1179947. [Google Scholar] [CrossRef] [PubMed]
- Mussbacher, M.; Derler, M.; Basílio, J.; Schmid, J.A. NF-κB in monocytes and macrophages-an inflammatory master regulator in multitalented immune cells. Front. Immunol. 2023, 14, 1134661. [Google Scholar] [CrossRef]
- Deka, K.; Li, Y. Transcriptional regulation during aberrant activation of NF-κB signaling in cancer. Cells 2023, 12, 788. [Google Scholar] [CrossRef]
- Ashikawa, K.; Shishodia, S.; Fokt, I.; Priebe, W.; Aggarwal, B.B. Evidence that activation of nuclear factor-κB is essential for the cytotoxic effects of doxorubicin and its analogues. Biochem. Pharmacol. 2004, 67, 353–364. [Google Scholar] [CrossRef]
- Jin, F.; Liu, X.; Zhou, Z.; Yue, P.; Lotan, R.; Khuri, F.R.; Chung, L.W.K.; Sun, S.Y. Activation of Nuclear Factor-KB contributes to induction of death receptors and apoptosis by the synthetic retinoid CD437 in DU145 human prostate cancer cells. Cancer Res. 2005, 64, 16. [Google Scholar]
- Riganti, C.; Doublier, S.; Costamagna, C.; Aldieri, E.; Pescarmona, G.; Ghigo, D.; Bosia, A. Activation of nuclear factor-κB pathway by simvastatin and RhoA silencing increases doxorubicin cytotoxicity in human colon cancer HT29 cells. Mol. Pharmacol. 2008, 74, 476–484. [Google Scholar] [CrossRef]
- Yuan, Y.; Long, H.; Zhou, Z.; Fu, Y.; Jiang, B. PI3K–AKT-Targeting breast cancer treatments: Natural products and synthetic compounds. Biomolecules 2023, 13, 93. [Google Scholar] [CrossRef]
- Kumar, H.B.; Manandhar, S.; Rathi, E.; Kabekkodu, S.P.; Mehta, C.H.; Nayak, U.Y.; Kini, S.G.; Pai, K.S.R. Identification of potential Akt activators: A ligand and structure-based computational approach. Mol. Divers. 2023, 1–19. [Google Scholar] [CrossRef]
- Dong, L.; Jin, L.; Tseng, H.Y.; Wang, C.Y.; Wilmott, J.S.; Yosufi, B.; Guo, S.T. Oncogenic suppression of PHLPP1 in human melanoma. Oncogene 2014, 33, 4756–4766. [Google Scholar]
- Ma, J.; Wang, H.; Guo, S.; Yi, X.; Zhao, T.; Liu, Y.; Shi, Q.; Gao, T.; Li, C.; Guo, W. A20 promotes melanoma progression via the activation of Akt pathway. Cell Death Dis. 2020, 11, 794. [Google Scholar] [PubMed]
- Sun, P.; Gu, L.; Luo, J.; Qin, Y.; Sun, L.; Jiang, S. ROS-mediated JNK pathway critically contributes to PFOS-triggered apoptosis in SH-SY5Y cells. Neurotoxicol. Teratol. 2019, 75, 106821. [Google Scholar] [PubMed]
- Rayan, A.; Raiyn, J.; Falah, M. Nature is the best source of anticancer drugs: Indexing natural products for their anticancer bioactivity. PLoS ONE 2017, 12, e0187925. [Google Scholar]
- Abdoul-Latif, F.M.; Ainane, A.; Aboubaker, I.H.; Mohamed, J.; Ainane, T. Exploring the potent anticancer activity of essential oils and their bioactive compounds: Mechanisms and prospects for future cancer therapy. Pharmaceuticals 2023, 16, 1086. [Google Scholar]
- Rawat, P.S.; Jaiswal, A.; Khurana, A.; Bhatti, J.S.; Navik, U. Doxorubicin-induced cardiotoxicity: An update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed. Pharmacother. 2021, 139, 111708. [Google Scholar]
- Sritharan, S.; Sivalingam, N. A comprehensive review on time-tested anticancer drug doxorubicin. Life Sci. 2021, 278, 119527. [Google Scholar]
- Brantley-Finley, C.; Lyle, C.S.; Du, L.; Goodwin, M.E.; Hall, T.; Szwedo, D.; Kaushal, G.P.; Chambers, T.C. The JNK, ERK and p53 pathways play distinct roles in apoptosis mediated by the antitumor agents vinblastine, doxorubicin, and etoposide. Biochem. Pharmacol. 2003, 66, 459–469. [Google Scholar]
- Choi, J.; Yip-Schneider, M.; Albertin, F.; Wiesenauer, C.; Wang, Y.; Schmidt, C.M. The effect of doxorubicin on MEK-ERK signaling predicts its efficacy in HCC. J. Surg. Res. 2008, 150, 219–226. [Google Scholar]
- Wei, T.; Xiaojun, X.; Peilong, C. Magnoflorine improves sensitivity to doxorubicin (DOX) of breast cancer cells via inducing apoptosis and autophagy through AKT/mTOR and p38 signaling pathways. Biomed. Pharmacother. 2020, 121, 109139. [Google Scholar]
- Chougule, M.B.; Patel, A.R.; Jackson, T.; Singh, M. Antitumor activity of Noscapine in combination with doxorubicin in triple negative breast cancer. PLoS ONE 2011, 6, e17733. [Google Scholar]
- Li, X.; Lu, Y.; Liang, K.; Liu, B.; Fan, Z. Differential responses to doxorubicin-induced phosphorylation and activation of Akt in human breast cancer cells. Breast Cancer Res. 2005, 7, R589–R597. [Google Scholar] [PubMed]
- Panda, S.K.; Ray, S.; Nayak, S.; Behera, S.; Bhanja, S.; Acharya, V. A review on cell cycle checkpoints in relation to cancer. J. Med. Sci. 2019, 5, 88–95. [Google Scholar]
- Zhang, M.; Lai, J.; Wu, Q.; Lai, J.; Su, J.; Zhu, B.; Li, Y. Naringenin induces hepg2 cell apoptosis via ROS-mediated JAK-2/STAT-3 signaling pathways. Molecules 2023, 28, 4506. [Google Scholar]
- Bhagat, J.; Zang, L.; Nishimura, N.; Shimada, Y. Zebrafish: An emerging model to study microplastic and nanoplastic toxicity. Sci. Total Environ. 2020, 728, 138707. [Google Scholar]
- Hashem-Dabaghian, F.; Shojaii, A.; Asgarpanah, J.; Entezari, M. Anti-mutagenicity and apoptotic effects of Teucrium polium L. essential oil in HT29 cell line. Jundishapur J. Nat. Pharm. Prod. 2020, 15, 3. [Google Scholar] [CrossRef]
- Trang, D.T.; Hoang, T.K.V.; Nguyen, T.T.M.; Van Cuong, P.; Dang, N.H.; Dang, H.D.; Quang, T.N.; Dat, N.T. Essential oils of lemongrass (Cymbopogon citratus Stapf) induces apoptosis and cell cycle arrest in A549 lung cancer cells. Biomed. Res. Int. 2020, 2020, 5924856. [Google Scholar]
- Bock, F.J.; Tait, S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar]
- Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [PubMed]
- Jan, R.; Chaudhry, G.-S. Understanding apoptosis and apoptotic pathways targeted cancer therapeutics. Adv. Pharm. Bull. 2019, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Manjamalai, A.; Kumar, M.J.; Grace, V.M. Essential oil of Tridax procumbens L. induces apoptosis and suppresses angiogenesis and lung metastasis of the B16F-10 cell line in C57BL/6 mice. Asian Pac. J. Cancer 2012, 13, 5887–5895. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Graziano, A.; Bruno, M.; Cardile, V.; Rigano, D. Apoptosis induction of essential oils from Artemisia arborescens L. in human prostate cancer cells. J. Ethnopharmacol. 2023, 303, 115929. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Pan, L.H.; Lin, L.; Zhang, R.; Du, Y.C.; Chen, H.; Huang, M.; Guo, K.W.; Yang, X.-Z. Essential oil from Carpesium abrotanoides L. Induces apoptosis via activating mitochondrial pathway in hepatocellular carcinoma cells. Curr. Med. Sci. 2018, 38, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Si, C.; Ou, Y.; Ma, D.; Hei, L.; Wang, X.; Du, R.; Zhao, J. Cytotoxic effect of the essential oils from Erigeron canadensis L. on human cervical cancer HeLa cells in vitro. Chem. Biodivers. 2022, 19, e202200436. [Google Scholar] [CrossRef]
- Zhao, X.; Sui, T.; Fu, Z.; Zhang, L.; Gao, Y.; Wang, L.; Zhang, H. Anticancer activity and mechanisms of action of Taisui fermentation broth in human colorectal cancer HCT116 cells in vitro and in vivo. J. Funct. Foods 2023, 106, 105592. [Google Scholar]
- Ullah, F.; Dima, D.; Omar, N.; Ogbue, O.; Ahmed, S. Advances in the treatment of Hodgkin lymphoma: Current and future approaches. Front. Oncol. 2023, 13, 1067289. [Google Scholar]
- Stefani, C.; Miricescu, D.; Stanescu-Spinu, I.I.; Nica, R.I.; Greabu, M.; Totan, A.R.; Jinga, M. Growth factors, PI3K/AKT/mTOR and MAPK signaling pathways in colorectal cancer pathogenesis: Where are we now? Int. J. Mol. Sci. 2021, 22, 10260. [Google Scholar]
- Lu, Y.; Liu, B.; Liu, Y.; Yu, X.; Cheng, G. Dual effects of active ERK in cancer: A potential target for enhancing radiosensitivity. Oncol. Lett. 2020, 20, 993–1000. [Google Scholar] [CrossRef]
- Nagalakshmamma, V.; Venkataswamy, M.; Pasala, C.; Maheswari, A.U.; Raju, K.T.; Nagaraju, C.; Chalapathi, P.V. A study on MAPK/ERK and CDK2-cyclin-E signal switch “on and off” in cell proliferation by bis urea derivatives of 1, 4-diisocyanatobenzene. Bioorg. Chem. 2021, 112, 104940. [Google Scholar] [CrossRef] [PubMed]
- Sugara, T.H.; Solikhah, E.N.; Pranowo, H.D. QSAR and molecular docking approaches for development of haloxanthones as the anticancer agent against MCF-7 and HepG2. Rasayan J. Chem. 2021, 14, 3. [Google Scholar] [CrossRef]
- Barančík, M.; Boháčová, V.; Kvačkajová, J.; Hudecová, S.; Križanová, O.G.; Breier, A. SB203580, a specific inhibitor of p38-MAPK pathway, is a new reversal agent of P-glycoprotein-mediated multidrug resistance. Eur. J. Pharm. Sci. 2001, 14, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.; Gopinath, S.M.; Kollur, S.P.; Sushma, P.; Jain, A.S.; Patil, S.S.; Srinivasa, C.; Shivamallu, C. Structural diversity and role of phytochemicals against p38-α Mitogen-Activated Protein Kinase and Epidermal Growth Factor Receptor Kinase domain: A privileged computational approach. J. Pure Appl. Microbiol. 2021, 15, 2263–2269. [Google Scholar] [CrossRef]
- Wang, Q.; Fang, X.; Sun, B.; Zhu, K.; Yao, M.; Wei, S.; Zhang, A. Rosa roxburghii Tratt juice inhibits NF-κB and increases IL-2 to alleviates the Foxp3-mediated Tregs imbalance in the peripheral blood of arseniasis patients. Food Sci. Biotechnol. 2023, 1–10. [Google Scholar] [CrossRef]
- Wu, X.; Sun, L.; Xu, F. NF-κB in Cell deaths, therapeutic resistance and nanotherapy of tumors: Recent advances. J. Pharm. 2023, 16, 783. [Google Scholar] [CrossRef]
- Shiroma, Y.; Fujita, G.; Yamamoto, T.; Takahashi, R.U.; Kumar, A.; Zhang, K.Y.; Ito, A.; Osada, H.; Yoshida, M.; Tahara, H. Identification of a selective RelA inhibitor based on DSE-FRET screening methods. Int. J. Mol. Sci. 2020, 21, 9150. [Google Scholar] [CrossRef]
- Zazeri, G.; Povinelli, A.P.R.; Le Duff, C.S.; Tang, B.; Cornelio, M.L.; Jones, A.M. Synthesis and spectroscopic analysis of piperine-and piperlongumine-inspired natural product scaffolds and their molecular docking with IL-1β and NF-κB proteins. Molecules 2020, 25, 2841. [Google Scholar] [CrossRef]
- Kumar, A.; Saini, K.; Kumari, R. A brief literature review on Piper longum with special references to different Ayurvedic samhitas. World J. Pharm. Res. 2022, 11, 683–696. [Google Scholar]
- Quijia, C.R.; Chorilli, M. Piperine for treating breast cancer: A review of molecular mechanisms, combination with anticancer drugs, and nanosystems. Phytother. Res. 2022, 36, 147–163. [Google Scholar] [CrossRef]
- Lin, T.H.; Kuo, C.H.; Zhang, Y.S.; Chen, P.T.; Chen, S.H.; Li, Y.Z.; Lee, Y.R. Piperlongumine induces cellular apoptosis and autophagy via the ROS/AKT signaling pathway in human follicular thyroid cancer cells. Int. J. Mol. Sci. 2023, 24, 8048. [Google Scholar] [CrossRef] [PubMed]
- Ginzburg, S.; Golovine, K.V.; Makhov, P.B.; Uzzo, R.G.; Kutikov, A.; Kolenko, V.M. Piperlongumine inhibits NF-κB activity and attenuates aggressive growth characteristics of prostate cancer cells. Prostate 2014, 74, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Iida, M.; Harari, P.M.; Wheeler, D.L.; Toulany, M. Targeting AKT/PKB to improve treatment outcomes for solid tumors. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2020, 819, 111690. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, S.; Li, H.; Chou, H. FGFR3 promotes the growth and malignancy of melanoma by influencing EMT and the phosphorylation of ERK, AKT, and EGFR. BMC Cancer 2019, 19, 1–12. [Google Scholar] [CrossRef]
- Ramdhani, D.; Mustarichie, R. Anticancer activity of brevilin a by molecular docking method. World J. Pharm. Res. 2021, 10, 142–149. [Google Scholar]
- Vallathol, D.H.; Babu, T.; Kutty, S.V.; Ntk, T.; Warrier, N. Small cell neuroendocrine carcinoma of the breast-case series of a common tumour at a rare location. Braz. J. Oncol. 2023, 19, e20230415. [Google Scholar] [CrossRef]
- Ramdhani, D.; Mustarichie, R. Anticancer mechanism of epirucibin by molecular docking method. World J. Pharm. Res. 2023, 12, 12–19. [Google Scholar]
- Ramdhani, D.; Mustarichie, R. Anticancer mechanism of pelargonidin compound from berry fruits by molecular docking method. World J. Pharm. Res. 2023, 13, 12–19. [Google Scholar]
- Cha, J.-D.; Kim, Y.-H.; Kim, J.-Y. Essential oil and 1, 8-cineole from Artemisia lavandulaefolia induces apoptosis in KB cells via mitochondrial stress and caspase activation. Food Sci. Biotechnol. 2010, 19, 185–191. [Google Scholar] [CrossRef]
- Jo, J.R.; Park, J.S.; Park, Y.K.; Chae, Y.Z.; Lee, G.H.; Park, G.Y.; Jang, B.C. Pinus densiflora leaf essential oil induces apoptosis via ROS generation and activation of caspases in YD-8 human oral cancer cells. Int. J. Oncol. 2012, 40, 1238–1245. [Google Scholar] [CrossRef]
- Guesmi, F.; Prasad, S.; Tahri, W.; Dridi, I.; Ali, M.B.; Hedfi, A.; Ismail, A.I.; Landoulsi, A. Volatile oil of Teucrium alopecurus sensitizes colon cancer cells to TRAIL-induced cell death. Front. Biosci.-Sch. 2021, 13, 1–13. [Google Scholar]
- Guesmi, F.; Prasad, S.; Ali, M.B.; Ismail, I.A.; Landoulsi, A. Thymus hirtus sp. algeriensis Boiss. and Reut. volatile oil enhances Trail/Apo2l induced apoptosis and inhibits colon carcinogenesis through upregulation of death receptor pathway. Aging 2021, 13, 21975. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wu, P.; Qin, S.; Deng, Y.; Han, P.; Li, X.; Fan, C.; Xu, Y. Curcin C inhibit osteosarcoma cell line U2OS proliferation by ROS induced apoptosis, autophagy and cell cycle arrest through activating JNK signal pathway. Int. J. Biol. Macromol. 2022, 195, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Navarra, M.; Ferlazzo, N.; Cirmi, S.; Trapasso, E.; Bramanti, P.; Lombardo, G.E.; Minciullo, P.L.; Calapai, G.; Gangemi, S.; Gangemi, S. Effects of bergamot essential oil and its extractive fractions on SH-SY5Y human neuroblastoma cell growth. J. Pharm. Pharmacol. 2015, 67, 1042–1053. [Google Scholar] [CrossRef]
- Lin, X.; Peng, Z.; Fu, X.; Liu, C.; Xu, Y.; Ji, W.; Fan, J.; Chen, L.; Fang, L.; Huang, Y.; et al. Volatile oil from Saussurea lappa exerts antitumor efficacy by inhibiting epithelial growth factor receptor tyrosine kinase-mediated signaling pathway in hepatocellular carcinoma. Oncotarget 2016, 7, 79761. [Google Scholar] [CrossRef]
- Yue, J.; López, J.M. Understanding MAPK signaling pathways in apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef]
- Cha, J.D.; Moon, S.E.; Kim, H.Y.; Cha, I.H.; Lee, K.Y. Essential oil of Artemisia capillaris induces apoptosis in KB cells via mitochondrial stress and caspase activation mediated by MAPK-stimulated signaling pathway. J. Food Sci. 2009, 74, T75–T81. [Google Scholar] [CrossRef]
- Manuele, M.G.; Barreiro Arcos, M.L.; Davicino, R.; Ferraro, G.; Cremaschi, G.; Anesini, C. Limonene exerts antiproliferative effects and increases nitric oxide levels on a lymphoma cell line by dual mechanism of the ERK pathway: Relationship with oxidative stress. Cancer Investig. 2009, 28, 135–145. [Google Scholar] [CrossRef]
- Jeong, J.B.; Choi, J.; Lou, Z.; Jiang, X.; Lee, S.H. Patchouli alcohol, an essential oil of Pogostemon cablin, exhibits anti-tumorigenic activity in human colorectal cancer cells. Int. Immunopharmacol. 2013, 16, 184–190. [Google Scholar] [CrossRef]
- Wei, B.; Huang, Q.; Huang, S.; Mai, W.; Zhong, X. Trichosanthin-induced autophagy in gastric cancer cell MKN-45 is dependent on reactive oxygen species (ROS) and NF-κB/p53 pathway. J. Pharmacol. Sci. 2016, 131, 77–83. [Google Scholar] [CrossRef]
- Ni, X.; Suhail, M.M.; Yang, Q.; Cao, A.; Fung, K.M.; Postier, R.G.; Woolley, C.; Young, G.; Zhang, J.; Lin, H.K. Frankincense essential oil prepared from hydrodistillation of Boswellia sacra gum resins induces human pancreatic cancer cell death in cultures and in a xenograft murine model. BMC Complement. Altern. Med. 2012, 12, 253. [Google Scholar] [CrossRef] [PubMed]
- Shiau, J.P.; Chuang, Y.T.; Tang, J.Y.; Yang, K.H.; Chang, F.R.; Hou, M.F.; Chang, H.W. The impact of oxidative stress and AKT pathway on cancer cell functions and its application to natural products. Antioxidants 2022, 11, 1845. [Google Scholar] [CrossRef] [PubMed]
- Jayat, C.; Ratinaud, M.H. Cell cycle analysis by flow cytometry: Principles and applications. Biol. Cell 1993, 78, 15–25. [Google Scholar] [CrossRef]
- Sousa, V.M.; Duarte, S.S.; Silva, D.K.F.; Ferreira, R.C.; Moura, R.O.; Segundo, M.A.S.P.; Farias, D.; Vieira, L.; Gonçalves, J.C.R.; Sobral, M.V. Cytotoxicity of a new spiro-acridine derivative: Modulation of cellular antioxidant state and induction of cell cycle arrest and apoptosis in HCT-116 colorectal carcinoma. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E. Manilkara zapota (L.) P. Royen leaf water extract triggered apoptosis and activated caspase-dependent pathway in HT-29 human colorectal cancer cell line. Biomed. Pharmacother. 2018, 110, 748–757. [Google Scholar] [PubMed]
- Robbins, E.; Marcus, P. Interrelationships of acridine orange particles and cytoplasmic reddening. J. Cell Biol. 1963, 21, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Renvoizé, C.; Biola, A.; Pallardy, M.; Breard, J. Apoptosis: Identification of dying cells. Cell Biol. Toxicol. 1998, 14, 111–120. [Google Scholar] [CrossRef]
- Gorlick, R.; Maris, J.M.; Houghton, P.J.; Lock, R.; Carol, H.; Kurmasheva, R.T.; Kolb, E.A.; Keir, S.T.; Reynolds, P.; Kang, M.H.; et al. Testing of the Akt/PKB inhibitor MK-2206 by the pediatric preclinical testing program. Pediatr. Blood Cancer 2012, 59, 518–524. [Google Scholar] [CrossRef]
- Homme, R.P.; Anewesha, L.; Majumder, A.; George, A.; Singh, M.; Tyagi, S. NF-κB p65 subunit inhibitor: JSH-23 mitigates diabetic retinopathy via reducing oxidative stress. FASEB J. 2019, 33, 685.5. [Google Scholar] [CrossRef]
- Paw, M.; Wnuk, D.; Nit, K.; Bobis-Wozowicz, S.; Szychowski, R.; Ślusarczyk, A.; Michalik, M. SB203580—A Potent p38 MAPK inhibitor reduces the profibrotic bronchial fibroblasts transition associated with asthma. Int. J. Mol. Sci. 2021, 22, 12790. [Google Scholar] [CrossRef]
- Fernandes, O.L.G.; Tizziani, T.; Dambrós, B.P.; Sousa, N.F.; Pontes, C.L.M.; Silva, L.A.; Pollo, L.A.E.; Assis, F.F.; Scotti, M.T.; Scotti, L.; et al. Studies of cytotoxicity effects, SARS-CoV-2 main protease inhibition, and in silico interactions of synthetic chalcones. Chem. Biodivers. 2023, 20, e202201151. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, J.; Walter, F. MolDock applied to structure-based virtual screening. Curr. Drug Targets 2010, 11, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, R.; Christensen, M.H. MolDock: A new technique for high-accuracy molecular docking. J. Med. Chem. 2006, 49, 3315–3321. [Google Scholar] [CrossRef] [PubMed]
- Gandhia, G.R.; Sharanya, C.S.; Jayanandan, A.; Haridas, M.; Hillary, V.E.; Gandhi, S.R.; Sridharan, G.; Sivasubramanian, R.; Vasconcelos, A.B.S.; Montalvão, M.M.; et al. Multitargeted molecular docking and dynamics simulation studies of flavonoids and volatile components from the peel of Citrus sinensis L. (Osbeck) against specific tumor protein markers. J. Biomol. Struct. Dyn. 2023, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 53–55. [Google Scholar] [CrossRef]
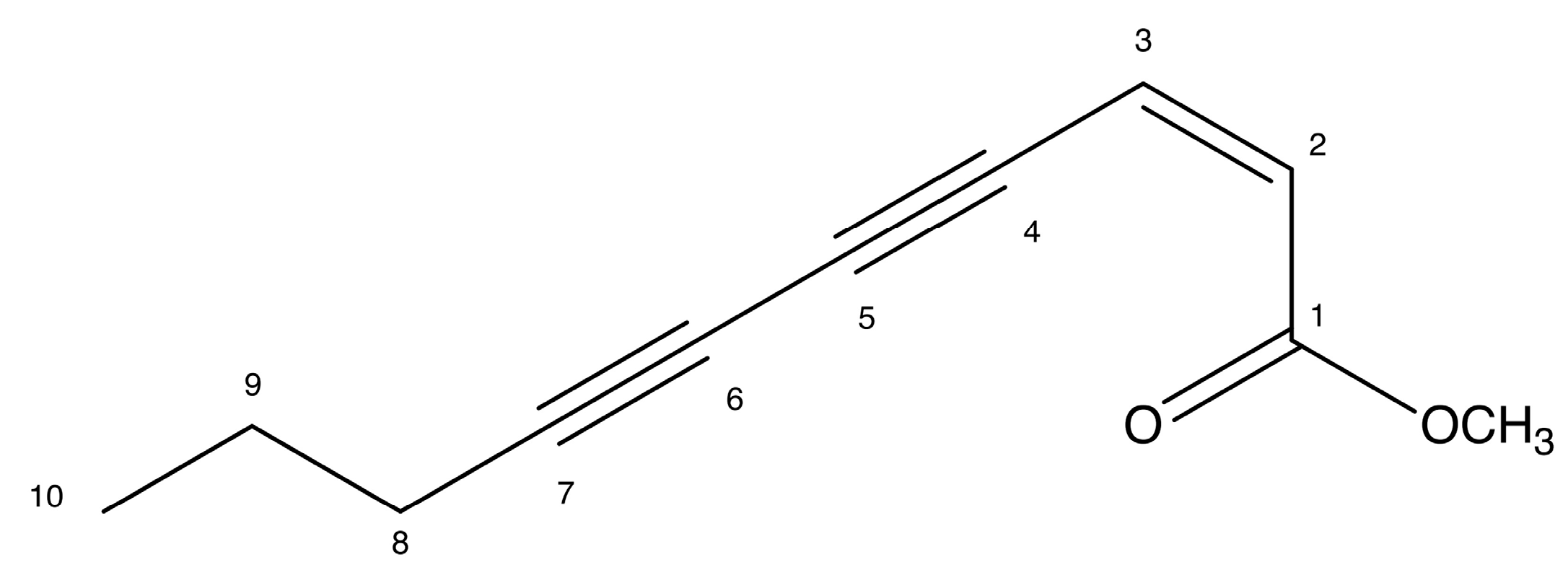
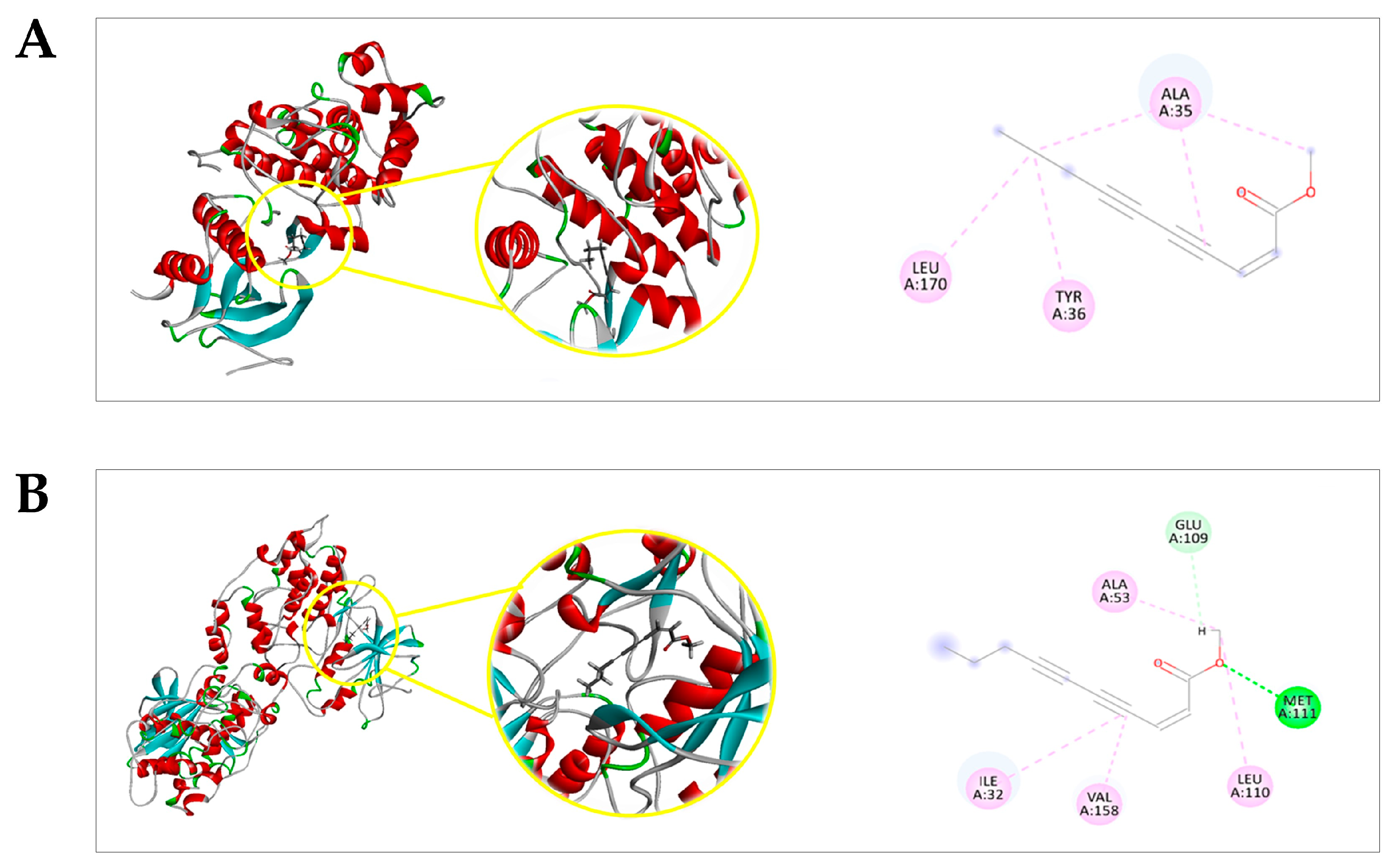
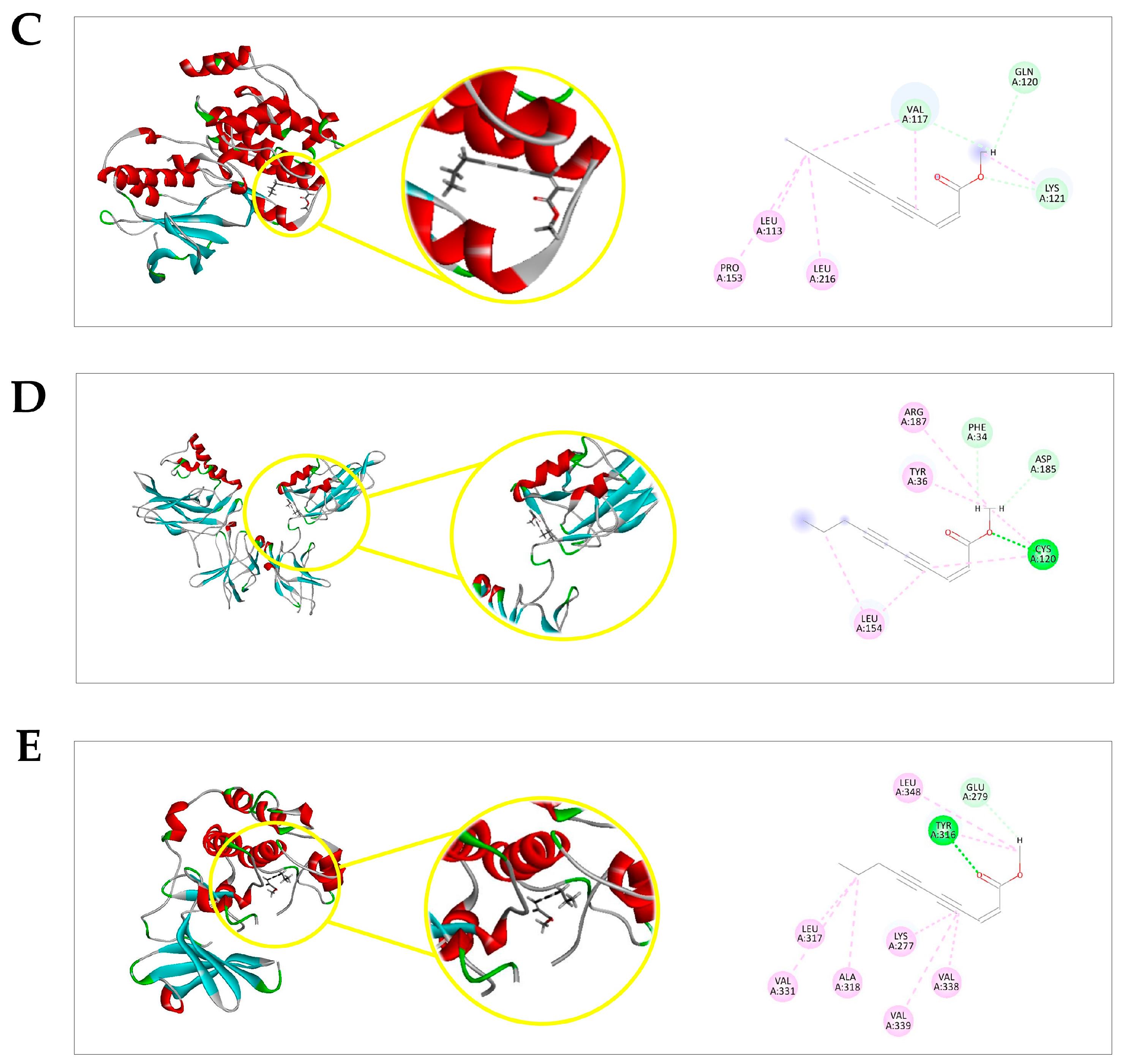

| Protein | Ligand | MolDock Score * | (p) † MolDock Score | Rerank Score * | (p) † Rerank Score | (p) † Total |
|---|---|---|---|---|---|---|
| ERK1 a | EZ | −63.2634 | 0.5106 | −52.153 | 0.7704558 | 0.6405279 |
| DXR | −110.085 | 0.8886 | −67.6911 | 1 | 0.9443 | |
| PDBL f | −123.881 | 1 | −66.5335 | 0.9828988 | 0.9914494 | |
| JNK1 b | EZ | −70.0612 | 0.5941 | −61.538 | 0.7443 | 0.6692 |
| DXR | −117.919 | 1 | −82.6781 | 1 | 1 | |
| PDBL g | −67.8571 | 0.5754 | −57.9951 | 0.7014 | 0.6384 | |
| p38α MAPK c | EZ | −76.5248 | 0.739384 | −62.4631 | 0.987851 | 0.863618 |
| DXR | −100.213 | 0.96826 | −63.2313 | 1 | 0.98413 | |
| CTL h | −103.498 | 1 | −59.1131 | 0.934871 | 0.967435 | |
| NF-κB (p50/p65) d | EZ | −83.0295 | 0.720536 | −69.7697 | 0.844223 | 0.782379 |
| DXR | −115.233 | 1 | −82.6437 | 1 | 1 | |
| CTL i | −100.634 | 0.873309 | 15.8197 | 0 | 0.436654 | |
| PKB/AKT e | EZ | −107.102 | 0.727012 | −90.3473 | 0.806392 | 0.766702 |
| DXR | −142.547 | 0.967614 | −112.039 | 1 | 0.983807 | |
| CTL j | −147.318 | 1 | −83.2668 | 0.743195 | 0.871597 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, R.C.; Duarte, S.S.; de Sousa, V.M.; de Souza, R.R.M.; Marques, K.K.G.; de Abrantes, R.A.; do Nascimento, Y.M.; de Sousa, N.F.; Scotti, M.T.; Scotti, L.; et al. The Essential Oil from Conyza bonariensis (L.) Cronquist (Asteraceae) Exerts an In Vitro Antimelanoma Effect by Inducing Apoptosis and Modulating the MAPKs, NF-κB, and PKB/AKT Signaling Pathways. Pharmaceuticals 2023, 16, 1553. https://doi.org/10.3390/ph16111553
Ferreira RC, Duarte SS, de Sousa VM, de Souza RRM, Marques KKG, de Abrantes RA, do Nascimento YM, de Sousa NF, Scotti MT, Scotti L, et al. The Essential Oil from Conyza bonariensis (L.) Cronquist (Asteraceae) Exerts an In Vitro Antimelanoma Effect by Inducing Apoptosis and Modulating the MAPKs, NF-κB, and PKB/AKT Signaling Pathways. Pharmaceuticals. 2023; 16(11):1553. https://doi.org/10.3390/ph16111553
Chicago/Turabian StyleFerreira, Rafael Carlos, Sâmia Sousa Duarte, Valgrícia Matias de Sousa, Ramon Ramos Marques de Souza, Karinne Kelly Gadelha Marques, Renata Albuquerque de Abrantes, Yuri Mangueira do Nascimento, Natália Ferreira de Sousa, Marcus Tullius Scotti, Luciana Scotti, and et al. 2023. "The Essential Oil from Conyza bonariensis (L.) Cronquist (Asteraceae) Exerts an In Vitro Antimelanoma Effect by Inducing Apoptosis and Modulating the MAPKs, NF-κB, and PKB/AKT Signaling Pathways" Pharmaceuticals 16, no. 11: 1553. https://doi.org/10.3390/ph16111553
APA StyleFerreira, R. C., Duarte, S. S., de Sousa, V. M., de Souza, R. R. M., Marques, K. K. G., de Abrantes, R. A., do Nascimento, Y. M., de Sousa, N. F., Scotti, M. T., Scotti, L., Tavares, J. F., Gonçalves, J. C. R., da Silva, M. S., & Sobral, M. V. (2023). The Essential Oil from Conyza bonariensis (L.) Cronquist (Asteraceae) Exerts an In Vitro Antimelanoma Effect by Inducing Apoptosis and Modulating the MAPKs, NF-κB, and PKB/AKT Signaling Pathways. Pharmaceuticals, 16(11), 1553. https://doi.org/10.3390/ph16111553