Sugiol Masters Apoptotic Precision to Halt Gastric Cancer Cell Proliferation
Abstract
:1. Introduction
2. Results and Discussion
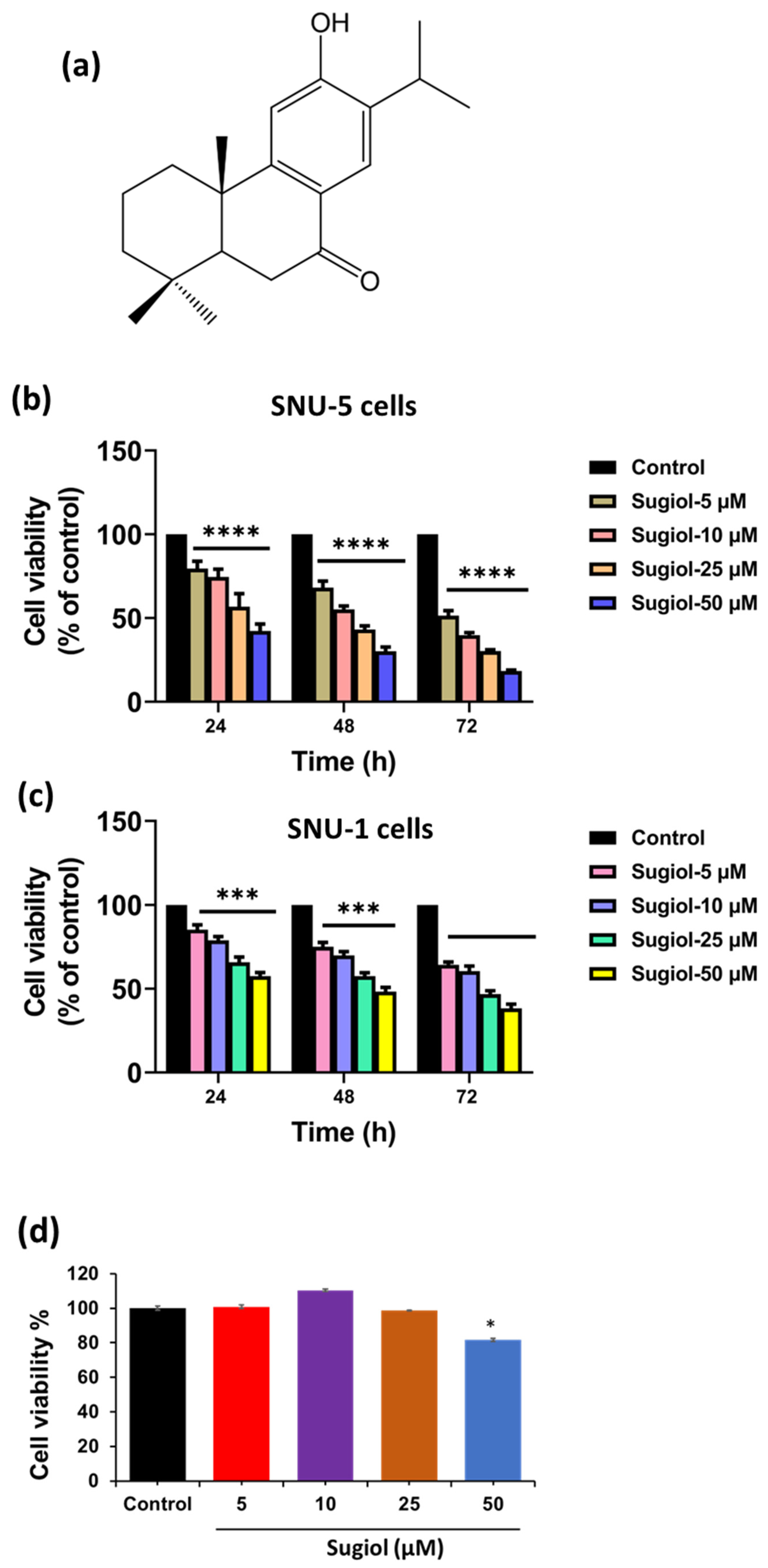
2.1. Sugiol Exhibits Anticancer Potential against Human Gastric Cancer Cells
2.2. Morphological Analysis—Effect of Sugiol on SNU-5 Cells via SEM
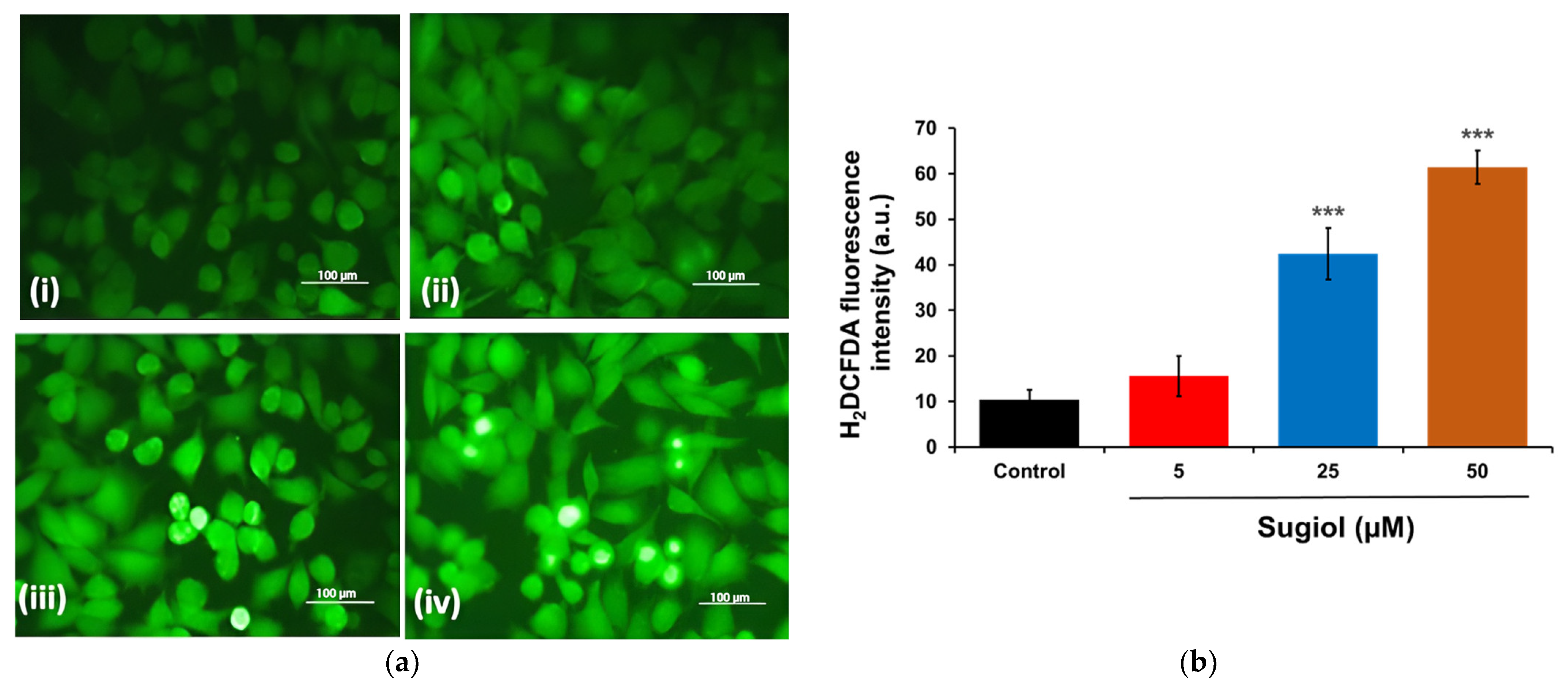
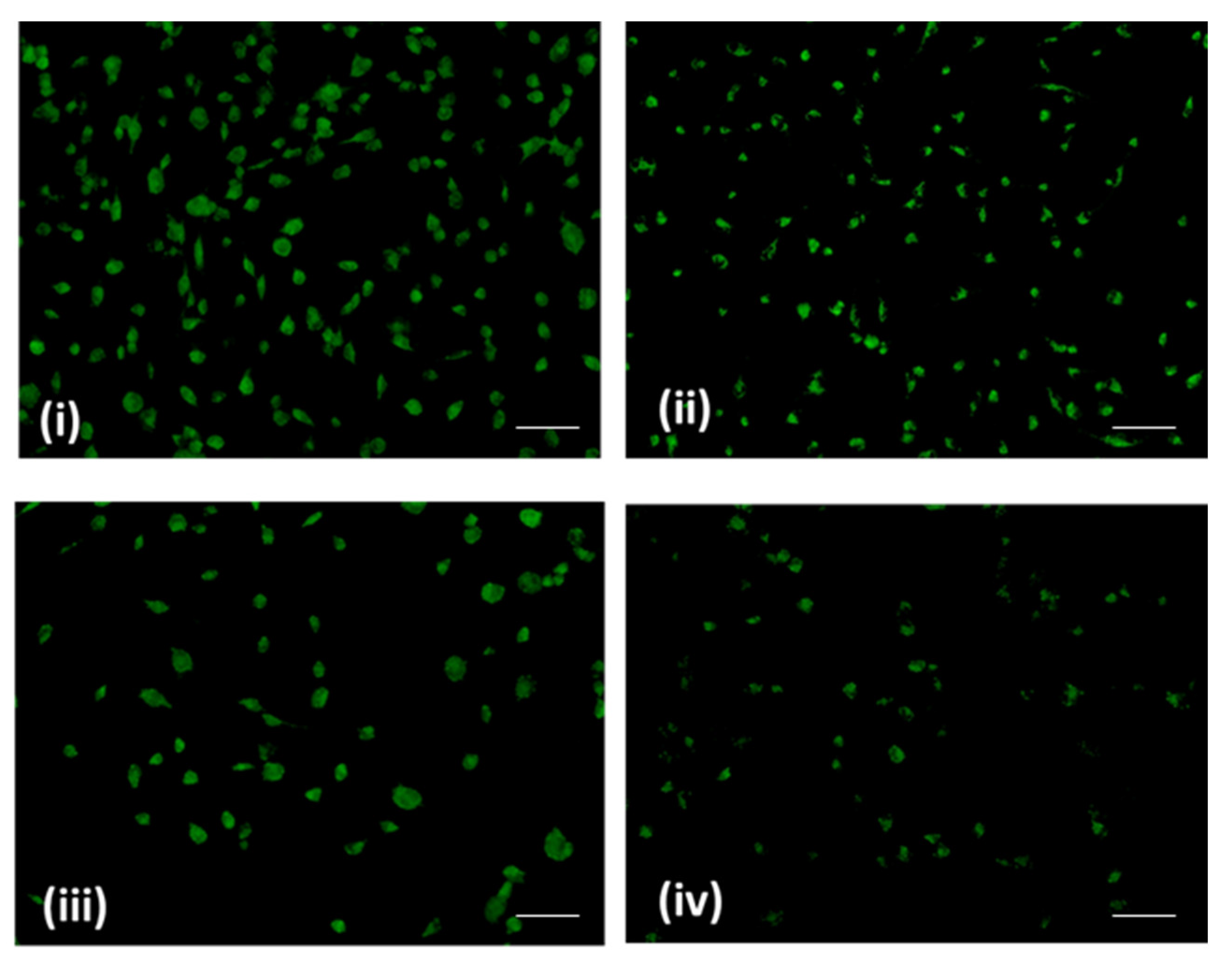
2.3. Sugiol Treatment Increases the ROS Generation and Membrane Potential
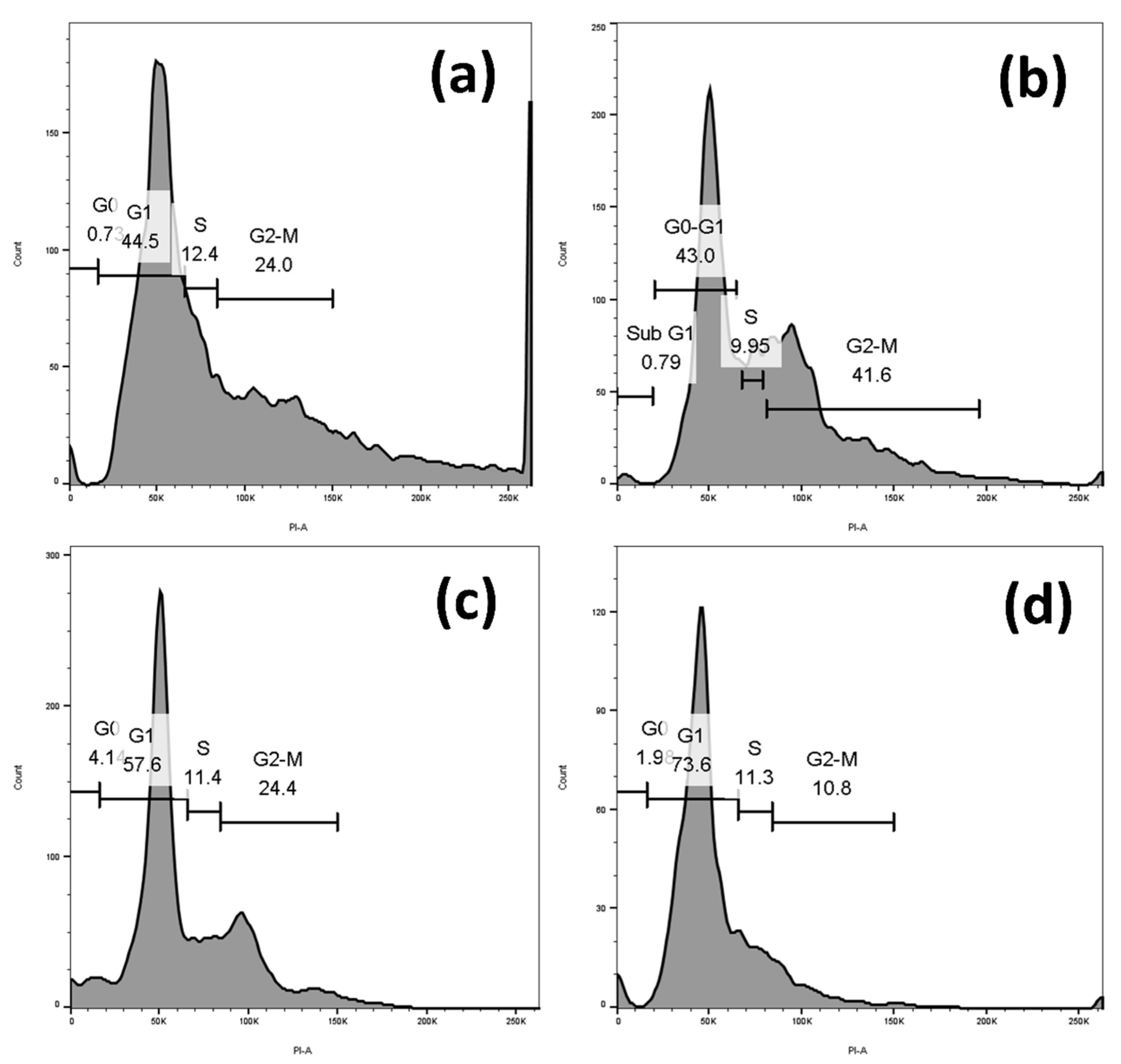
2.4. Sugiol Treatment Induced Cell-Cycle Arrest at G1 Phase
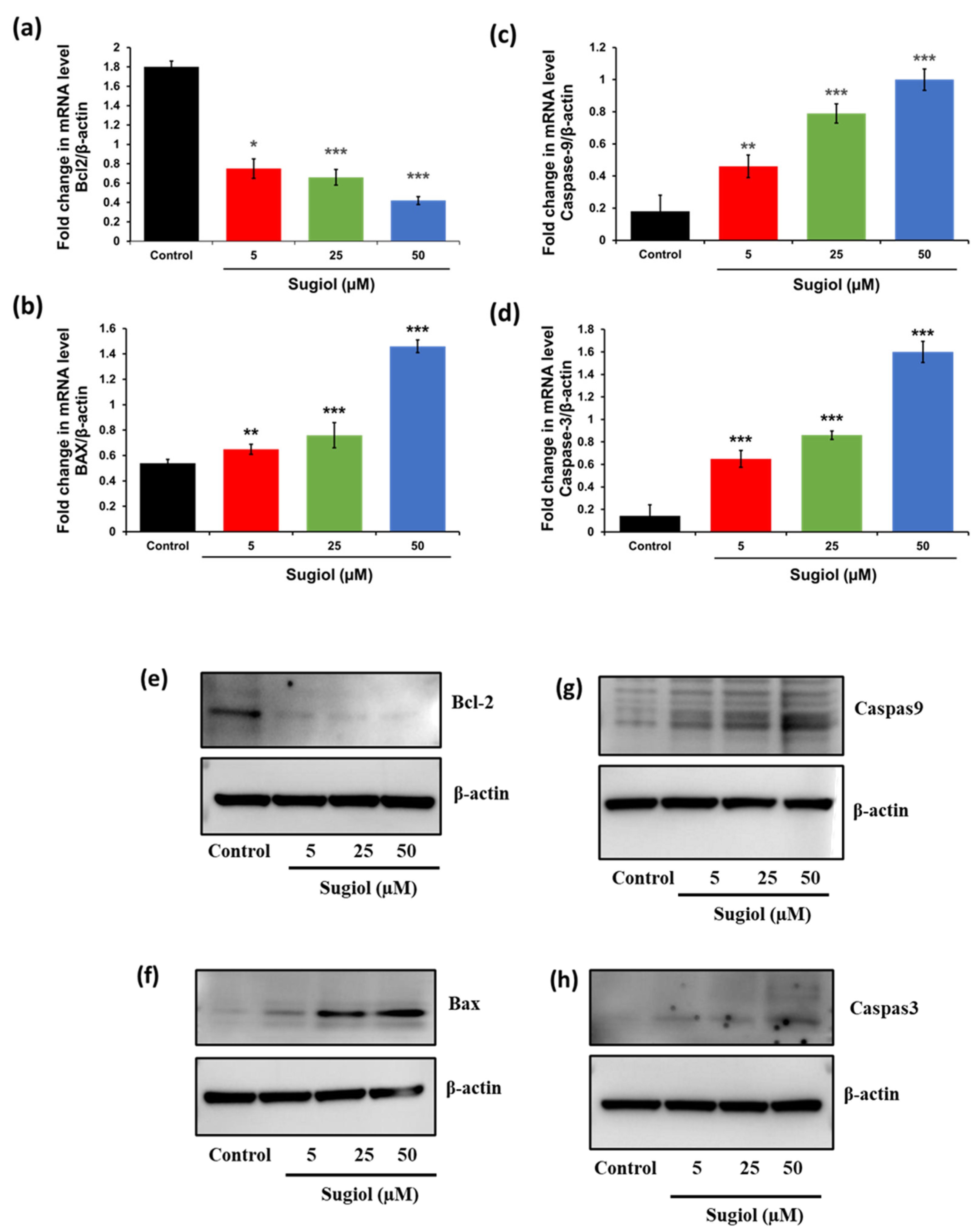
2.5. Sugiol Altered the mRNA and Protein Levels of Apoptosis-Associated Genes in SNU-5 Cells
2.6. Sugiol Inhibits STAT3 Signaling in SNU-5 Cells
2.7. In Silico ADMET Studies
2.7.1. Toxicity Analysis
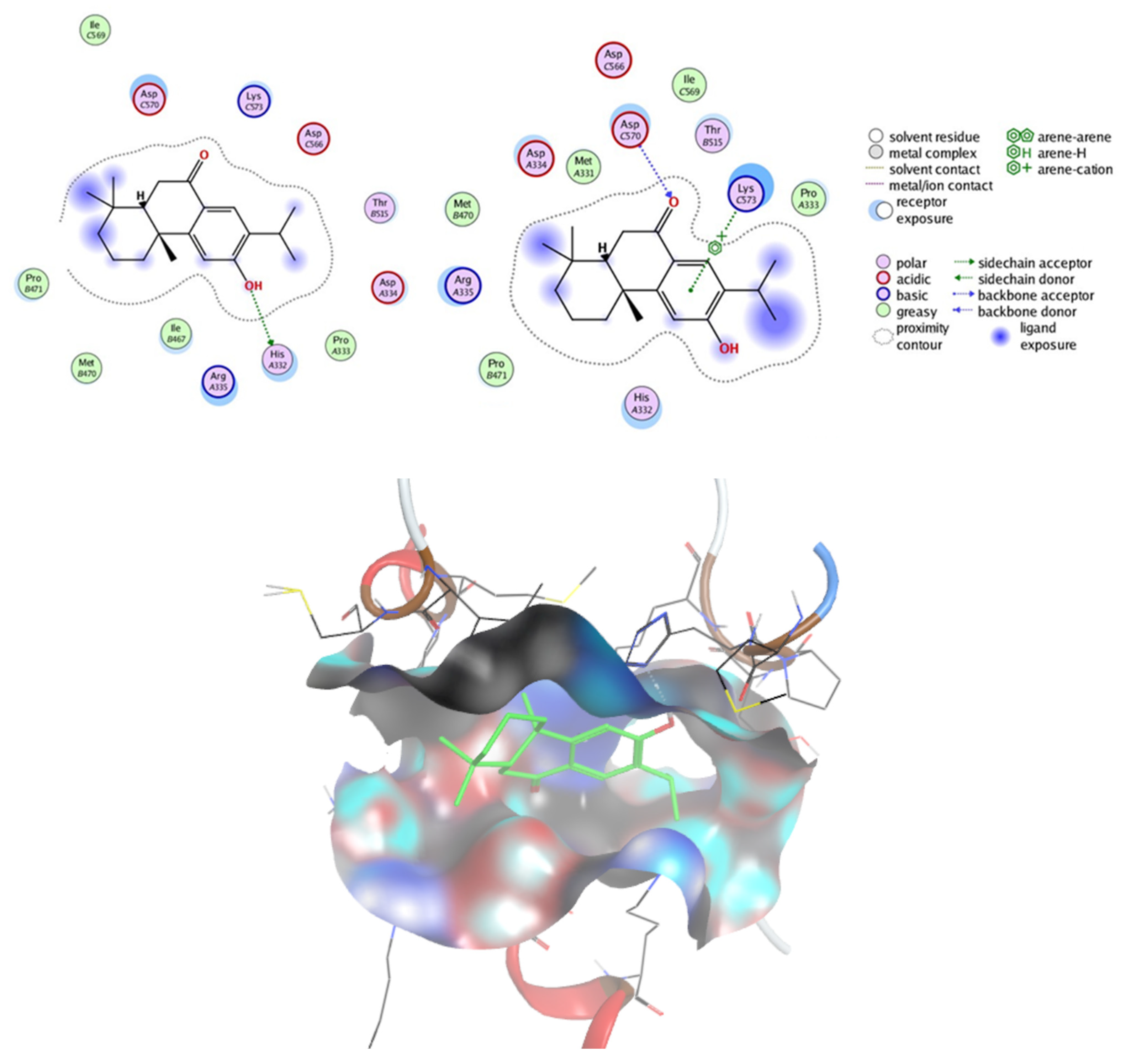
2.7.2. Prediction of Targets and Docking Studies
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Cell Culture and Maintenance
3.3. Cell Viability Analysis
3.4. Confirmation of Cell Death via SEM Morphology Analysis
3.5. H2DCHFDA Staining
3.6. Mitochondrial Membrane Potential (∆ψm)
3.7. Cell-Cycle Profiling
3.8. Quantitative PCR Analysis
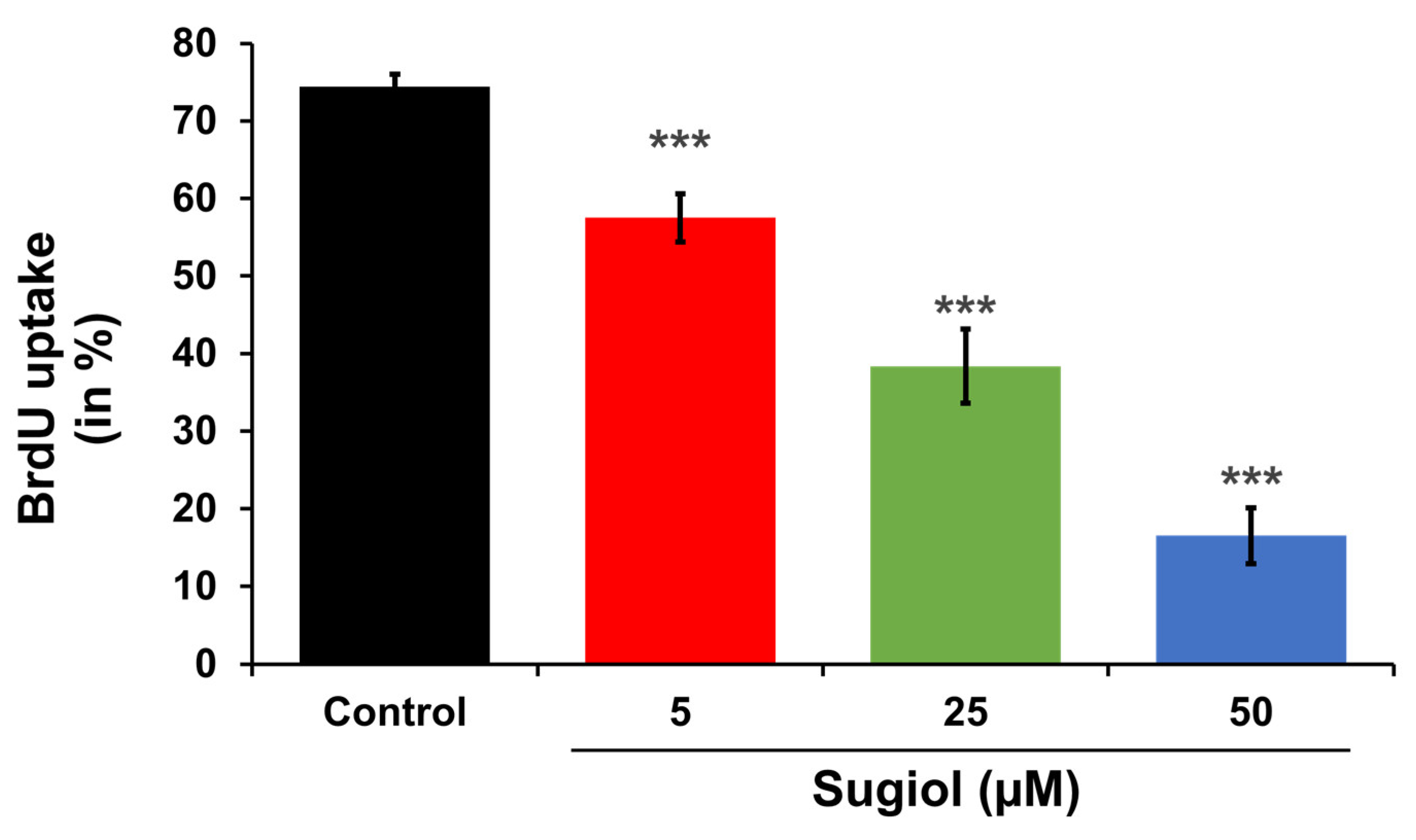
3.9. Analysis of Bromodeoxyuridine (BrdU) Uptake
3.10. Immunoblotting
3.11. Apoptosis Assay
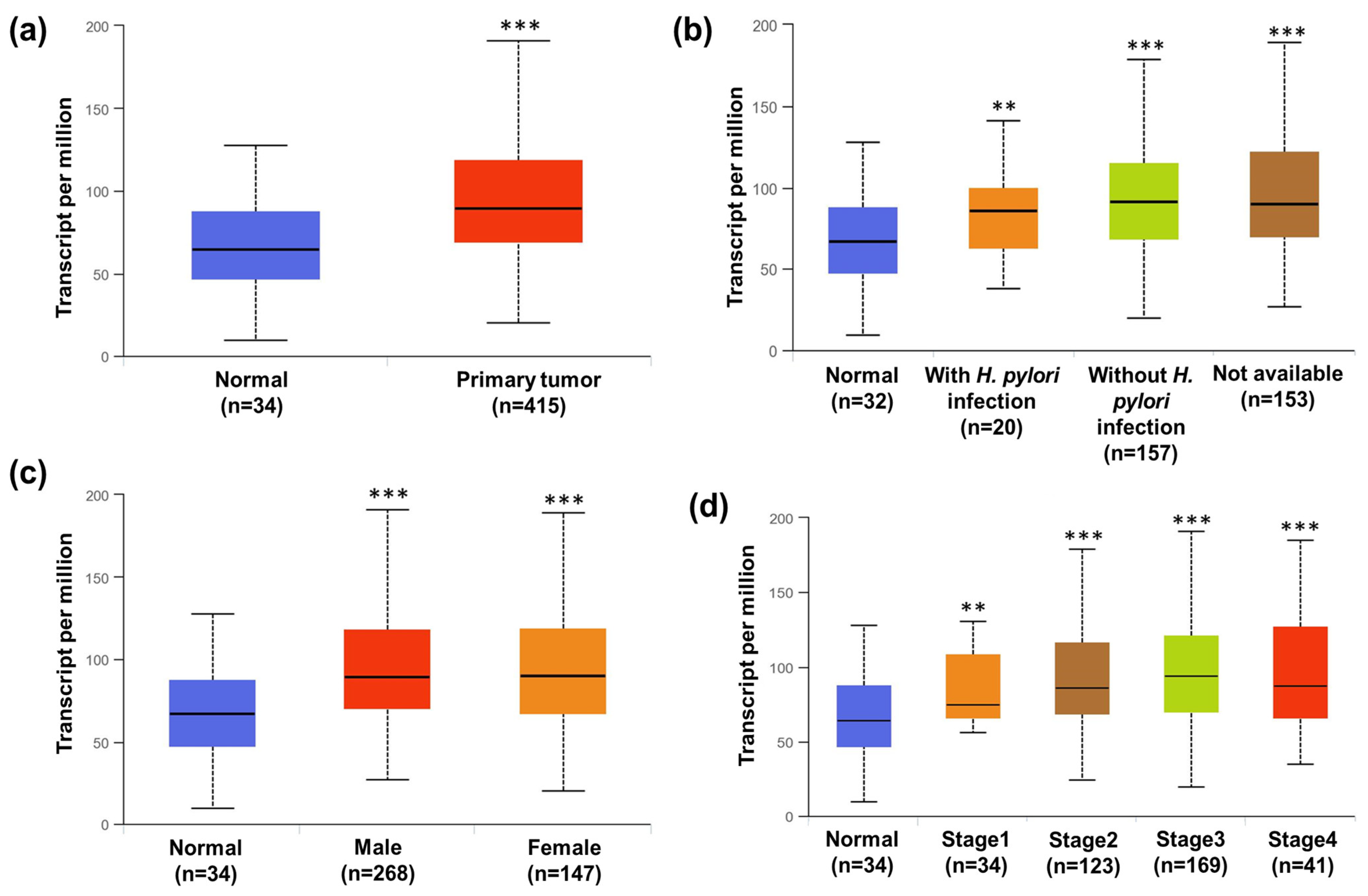
3.12. STATs Transcriptional Level in Human Gastric Cancer Patients
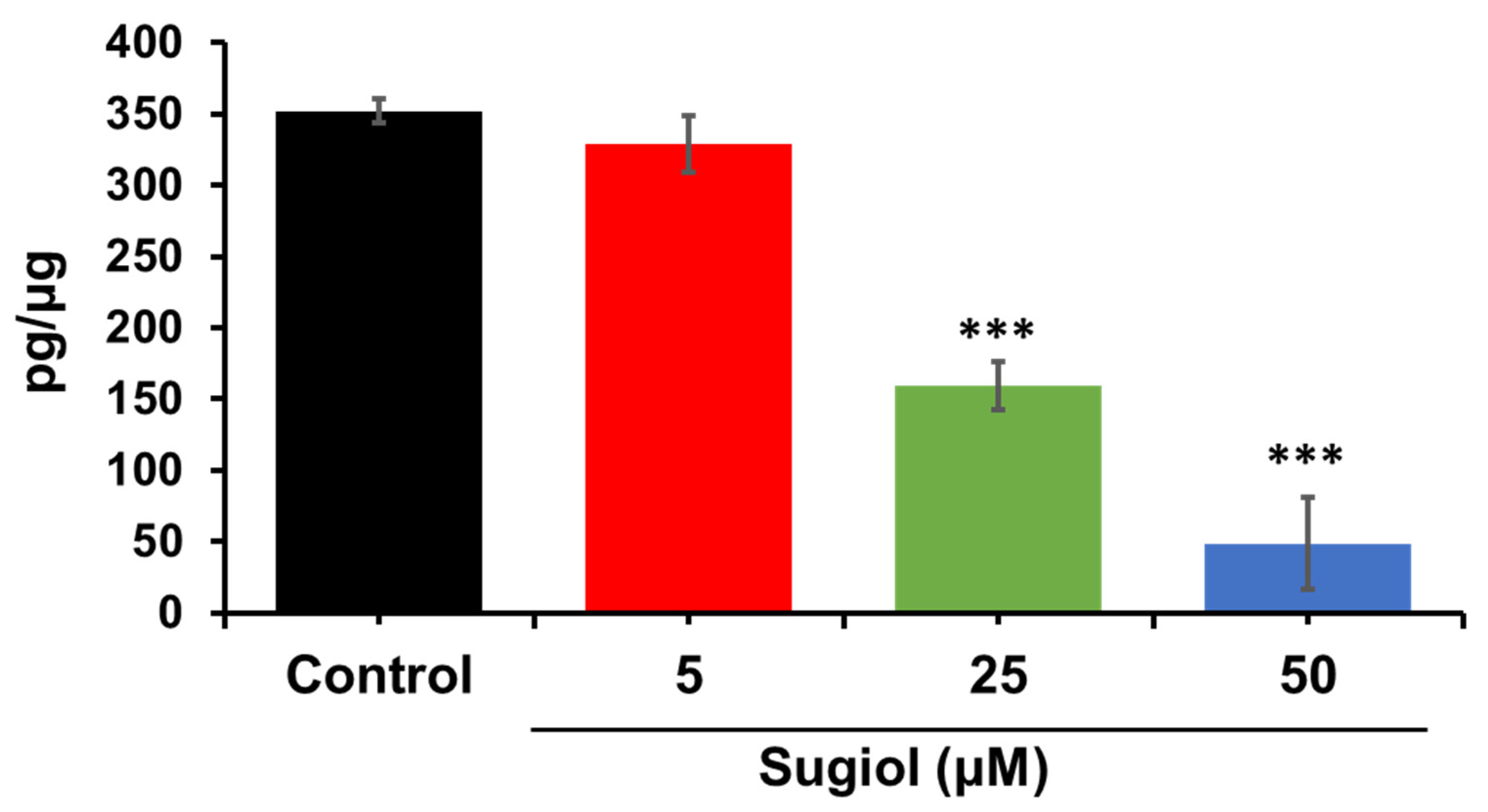
3.13. ELISA Assay
3.14. Docking Studies
3.15. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, M.C.S.; Huang, J.; Chan, P.S.F.; Choi, P.; Lao, X.Q.; Chan, S.M.; Teoh, A.; Liang, P. Global Incidence and Mortality of Gastric Cancer, 1980–2018. JAMA Netw. Open 2021, 4, e2118457. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Barsouk, A. Epidemiology of Gastric Cancer: Global Trends, Risk Factors and Prevention. Prz. Gastroenterol. 2019, 14, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Correa, P.; Piazuelo, M.B. The Gastric Precancerous Cascade. J. Dig. Dis. 2012, 13, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Q.; Zheng, G.F.; Sumanac, K.; Irvine, E.J.; Hunt, R.H. Meta-Analysis of the Relationship between CagA Seropositivity and Gastric Cancer. Gastroenterology 2003, 125, 1636–1644. [Google Scholar] [CrossRef] [PubMed]
- Aziz, F.; Khan, I.; Shukla, S.; Dey, D.K.; Yan, Q.; Chakraborty, A.; Yoshitomi, H.; Hwang, S.K.; Sonwal, S.; Lee, H.; et al. Partners in Crime: The Lewis Y Antigen and Fucosyltransferase IV in Helicobacter Pylori-Induced Gastric Cancer. Pharmacol. Ther. 2022, 232, 107994. [Google Scholar] [CrossRef]
- GLOBOCAN International Agency for Research on Cancer. GLOBOCAN: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2018; GLOBOCAN International Agency for Research on Cancer: Lyon, France, 2018. [Google Scholar]
- Kwon, M.J.; Kang, H.S.; Kim, J.-H.; Kim, J.H.; Kim, S.H.; Kim, N.Y.; Nam, E.S.; Min, K.-W.; Choi, H.G. Association between Statin Use and Gastric Cancer: A Nested Case-Control Study Using a National Health Screening Cohort in Korea. Pharmaceuticals 2021, 14, 1283. [Google Scholar] [CrossRef]
- Hazard, L.; O’Connor, J.; Scaife, C. Role of radiation therapy in gastric adenocarcinoma. World J. Gastroenterol. 2006, 12, 1511–1520. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Sonwal, S.; Hwang, S.K.; Shukla, S.; Khan, I.; Dey, D.K.; Chen, L.; Simal-Gandara, J.; Xiao, J.; Huh, Y.S.; et al. Sugiol, a Diterpenoid: Therapeutic Actions and Molecular Pathways Involved. Pharmacol. Res. 2021, 163, 105313. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, L.Y.; Qi, W.H.; Yang, J.; Qi, Y. Anticancer Activity of Sugiol against Ovarian Cancer Cell Line SKOV3 Involves Mitochondrial Apoptosis, Cell Cycle Arrest and Blocking of the RAF/MEK/ERK Signalling Pathway. Arch. Med. Sci. 2020, 16, 428–435. [Google Scholar] [CrossRef]
- Hao, C.; Zhang, X.; Zhang, H.; Shang, H.; Bao, J.; Wang, H.; Li, Z. Sugiol (12-Hydroxyabieta-8,11,13-Trien-7-One) Targets Human Pancreatic Carcinoma Cells (Mia-PaCa2) by Inducing Apoptosis, G2/M Cell Cycle Arrest, ROS Production and Inhibition of Cancer Cell Migration. J. BUON 2018, 23, 205–210. [Google Scholar]
- Jung, S.N.; Shin, D.S.; Kim, H.N.; Jeon, Y.J.; Yun, J.; Lee, Y.J.; Kang, J.S.; Han, D.C.; Kwon, B.M. Sugiol Inhibits STAT3 Activity via Regulation of Transketolase and ROS-Mediated ERK Activation in DU145 Prostate Carcinoma Cells. Biochem. Pharmacol. 2015, 97, 38–50. [Google Scholar] [CrossRef]
- Tolomeo, M.; Cascio, A. The Multifaced Role of STAT3 in Cancer and Its Implication for Anticancer Therapy. Int. J. Mol. Sci. 2021, 22, 603. [Google Scholar] [CrossRef]
- Ihle, J.N. The Janus Kinase Family and Signaling Through Members of the Cytokine Receptor Superfamily. Exp. Biol. Med. 1994, 206, 268–272. [Google Scholar] [CrossRef]
- Chonov, D.C.; Ignatova, M.M.K.; Ananiev, J.R.; Gulubova, M.V. IL-6 Activities in the Tumour Microenvironment. Part 1. Open Access Maced. J. Med. Sci. 2019, 7, 2391–2398. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, J.; Liu, W.; Zhang, X.; Xue, L. The Diagnostic Value of Interleukin 6 as a Biomarker for Gastric Cancer. Medicine 2021, 100, e27945. [Google Scholar] [CrossRef]
- Lee, H.; Oh, C.; Kim, S.; Dey, D.K.; Kim, H.K.; Bajpai, V.K.; Han, Y.K.; Huh, Y.S. Metasequoia Glyptostroboides Potentiates Anticancer Effect against Cervical Cancer via Intrinsic Apoptosis Pathway. Sci. Rep. 2021, 11, 894. [Google Scholar] [CrossRef]
- Wenzel, U.; Nickel, A.; Daniel, H. α-Lipoic Acid Induces Apoptosis in Human Colon Cancer Cells by Increasing Mitochondrial Respiration with a Concomitant O2-.-Generation. Apoptosis 2005, 10, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Moiseeva, O.; Bourdeau, V.; Roux, A.; Deschênes-Simard, X.; Ferbeyre, G. Mitochondrial Dysfunction Contributes to Oncogene-Induced Senescence. Mol. Cell. Biol. 2009, 29, 4495–4507. [Google Scholar] [CrossRef]
- Ziegler, D.V.; Wiley, C.D.; Velarde, M.C. Mitochondrial Effectors of Cellular Senescence: Beyond the Free Radical Theory of Aging. Aging Cell 2015, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.K.; Kang, S.C. CopA3 Peptide Induces Permanent Cell-Cycle Arrest in Colorectal Cancer Cells. Mech. Ageing Dev. 2021, 196, 111497. [Google Scholar] [CrossRef]
- Fronza, M.; Lamy, E.; Günther, S.; Heinzmann, B.; Laufer, S.; Merfort, I. Abietane Diterpenes Induce Cytotoxic Effects in Human Pancreatic Cancer Cell Line MIA PaCa-2 through Different Modes of Action. Phytochemistry 2012, 78, 107–119. [Google Scholar] [CrossRef]
- Al Zaid Siddiquee, K.; Turkson, J. STAT3 as a Target for Inducing Apoptosis in Solid and Hematological Tumors. Cell Res. 2008, 18, 254–267. [Google Scholar] [CrossRef]
- Shuai, K.; Horvath, C.M.; Huang, L.H.T.; Qureshi, S.A.; Cowburn, D.; Darnell, J.E. Interferon Activation of the Transcription Factor Stat91 Involves Dimerization through SH2-Phosphotyrosyl Peptide Interactions. Cell 1994, 76, 821–828. [Google Scholar] [CrossRef]
- Turkson, J.; Ryan, D.; Kim, J.S.; Zhang, Y.; Chen, Z.; Haura, E.; Laudano, A.; Sebti, S.; Hamilton, A.D.; Jove, R. Phosphotyrosyl Peptides Block Stat3-Mediated DNA Binding Activity, Gene Regulation, and Cell Transformation. J. Biol. Chem. 2001, 276, 45443–45455. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xu, Q.; Zeng, L.; Xie, L.; Zhao, Q.; Xu, H.; Wang, X.; Jiang, N.; Fu, P.; Sang, M. Resveratrol Suppresses the Growth and Metastatic Potential of Cervical Cancer by Inhibiting STAT3Tyr705 Phosphorylation. Cancer Med. 2020, 9, 8685–8700. [Google Scholar] [CrossRef]
- Kim, S.-J.; Cho, N.-C.; Hahn, Y.-I.; Kim, S.H.; Fang, X.; Surh, Y.-J. STAT3 as a Potential Target for Tumor Suppressive Effects of 15-Deoxy-δ 12,14 -Prostaglandin J 2 in Triple Negative Breast Cancer. J. Cancer Prev. 2021, 26, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Kunigal, S.; Lakka, S.S.; Sodadasu, P.K.; Estes, N.; Rao, J.S. Stat3-SiRNA Induces Fas-Mediated Apoptosis In Vitro and In Vivo in Breast Cancer. Int. J. Oncol. 2009, 34, 1209–1220. [Google Scholar]
- Gritsko, T.; Williams, A.; Turkson, J.; Kaneko, S.; Bowman, T.; Huang, M.; Nam, S.; Eweis, I.; Diaz, N.; Sullivan, D.; et al. Persistent Activation of Stat3 Signaling Induces Survivin Gene Expression and Confers Resistance to Apoptosis in Human Breast Cancer Cells. Clin. Cancer Res. 2006, 12, 11–19. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Khan, I.; Shukla, S.; Kang, S.M.; Aziz, F.; Tripathi, K.M.; Saini, D.; Cho, H.J.; Heo, N.S.; Sonkar, S.K.; et al. Multifunctional N-P-Doped Carbon Dots for Regulation of Apoptosis and Autophagy in B16F10 Melanoma Cancer Cells and In Vitro Imaging Applications. Theranostics 2020, 10, 7841. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.K.; Kang, S.C. Aflatoxin B1 Induces Reactive Oxygen Species-Dependent Caspase-Mediated Apoptosis in Normal Human Cells, Inhibits Allium Cepa Root Cell Division, and Triggers Inflammatory Response in Zebrafish Larvae. Sci. Total Environ. 2020, 737, 139704. [Google Scholar] [CrossRef]


| Properties | Parameters | |
|---|---|---|
| Physicochemical Properties | Formula | C20H28O2 |
| Molecular weight | 300.44 g/mol | |
| Num. heavy atoms | 22 | |
| Num. arom. heavy atoms | 6 | |
| Fraction Csp3 | 0.65 | |
| Num. rotatable bonds | 1 | |
| Num. H-bond acceptors | 2 | |
| Num. H-bond donors | 1 | |
| Molar Refractivity | 92.06 | |
| TPSA | 37.30 Å2 | |
| Lipophilicity | Consensus Log Po/w | 4.64 |
| Water Solubility | Log S (ESOL) | −5.38 |
| Pharmacokinetics | GI absorption | High |
| Log Kp (skin permeation) | −4.14 cm/s | |
| Drug Likeness | Lipinski | Yes; 0 violation |
| Bioavailability Score | 0.55 | |
| Medicinal Chemistry | PAINS | 0 alert |
| Leadlikeness | No; 1 violation: XLOGP3 > 3.5 | |
| Synthetic accessibility | 3.45 | |
| Classification | Target | Prediction | Probability |
|---|---|---|---|
| Organ toxicity | Hepatotoxicity | Inactive | 0.71 |
| Toxicity end points | Carcinogenicity | Inactive | 0.73 |
| Immunotoxicity | Inactive | 0.88 | |
| Mutagenicity | Inactive | 0.92 | |
| Cytotoxicity | Inactive | 0.87 | |
| Tox21—nuclear receptor signaling pathways | Aryl Hydrocarbon Receptor (AhR) | Inactive | 0.93 |
| Androgen Receptor (AR) | Inactive | 0.77 | |
| Androgen Receptor Ligand Binding Domain (AR-LBD) | Inactive | 0.86 | |
| Aromatase | Inactive | 0.96 | |
| Estrogen Receptor Alpha (ER) | Inactive | 0.58 | |
| Estrogen Receptor Ligand Binding Domain (ER-LBD) | Inactive | 0.60 | |
| Peroxisome Proliferator Activated Receptor Gamma (PPAR-Gamma) | Inactive | 0.99 | |
| Tox21—stress response pathways | Nuclear factor (erythroid-derived 2)-like 2/antioxidant responsive element (nrf2/ARE) | Inactive | 0.94 |
| Heat shock factor response element (HSE) | Inactive | 0.94 | |
| Mitochondrial Membrane Potential (MMP) | Active | 0.60 | |
| Phosphoprotein (Tumor Suppressor) p53 | Inactive | 0.87 | |
| ATPase family AAA domain-containing protein 5 (ATAD5) | Inactive | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakhsh, T.; Abuzahrah, S.S.; Qahl, S.H.; Akela, M.A.; Rather, I.A. Sugiol Masters Apoptotic Precision to Halt Gastric Cancer Cell Proliferation. Pharmaceuticals 2023, 16, 1528. https://doi.org/10.3390/ph16111528
Bakhsh T, Abuzahrah SS, Qahl SH, Akela MA, Rather IA. Sugiol Masters Apoptotic Precision to Halt Gastric Cancer Cell Proliferation. Pharmaceuticals. 2023; 16(11):1528. https://doi.org/10.3390/ph16111528
Chicago/Turabian StyleBakhsh, Tahani, Samah Sulaiman Abuzahrah, Safa H. Qahl, Mohamed A. Akela, and Irfan A. Rather. 2023. "Sugiol Masters Apoptotic Precision to Halt Gastric Cancer Cell Proliferation" Pharmaceuticals 16, no. 11: 1528. https://doi.org/10.3390/ph16111528
APA StyleBakhsh, T., Abuzahrah, S. S., Qahl, S. H., Akela, M. A., & Rather, I. A. (2023). Sugiol Masters Apoptotic Precision to Halt Gastric Cancer Cell Proliferation. Pharmaceuticals, 16(11), 1528. https://doi.org/10.3390/ph16111528







