Imipramine Administration in Brucella abortus 2308-Infected Mice Restores Hippocampal Serotonin Levels, Muscle Strength, and Mood, and Decreases Spleen CFU Count
Abstract
:1. Introduction
2. Results
2.1. Orogastric Administration of Imipramine Restores Mood and Hippocampal Serotonin Levels in Im6Ba14 and Im20Ba28 Mice
2.2. Orogastric Administration of Imipramine Restores Equilibrium and Muscular Strength in ImBa Mice
2.3. Imipramine Administration Decreases IL-6 Serum Levels in ImBa Mice Group
2.4. Treatment with Imipramine Did Not Modify the Number of Macrophages and Dendritic Cells in the Spleen of Infected Animals
2.5. After Treatment with Imipramine, Mice in the ImBa Group Had Lower Spleen CFU Counts
3. Discussion
Limitations
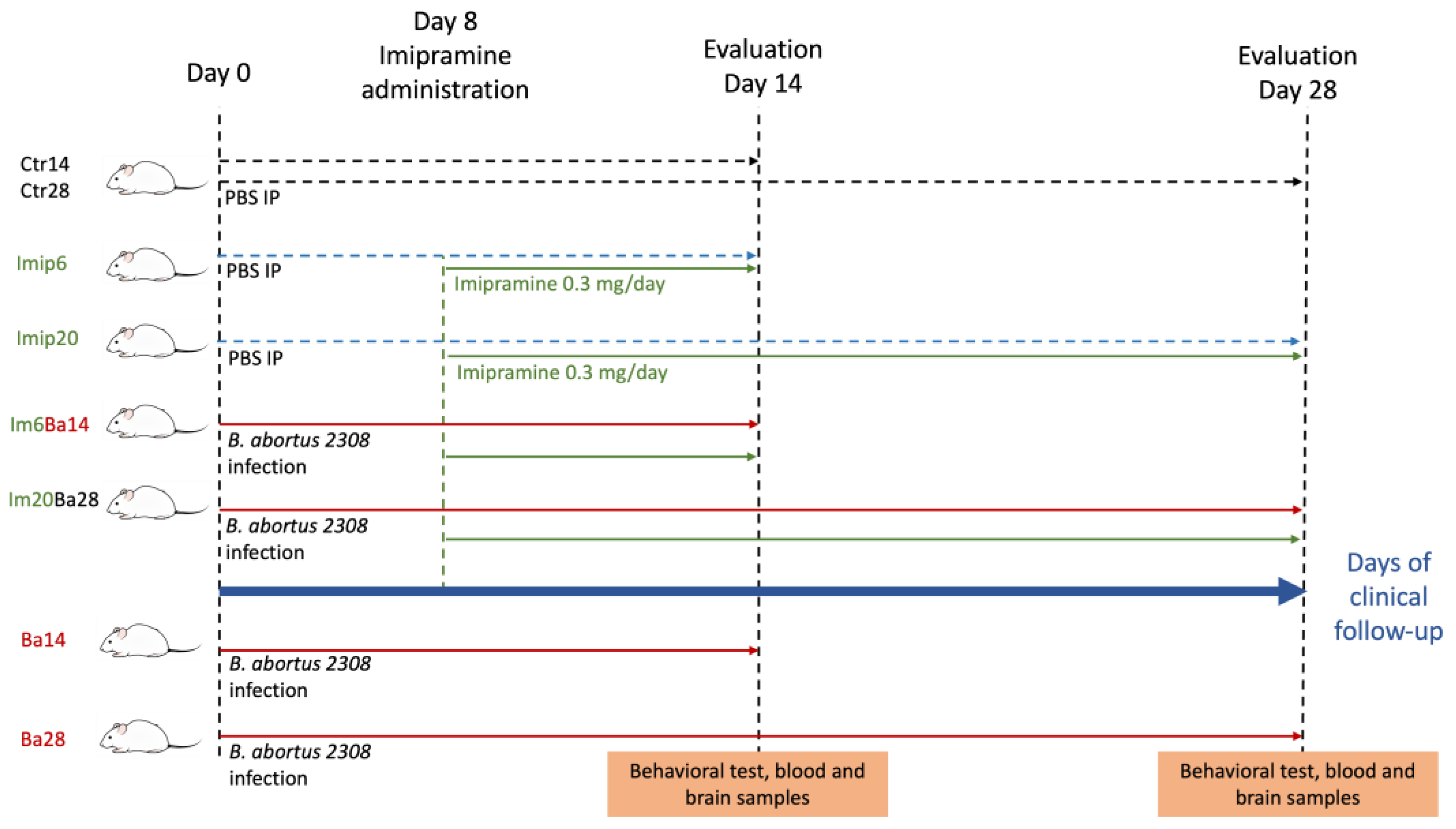
4. Materials and Methods
4.1. Mice
4.2. Imipramine Administration
4.3. Behavioral Test
4.4. Collection of Serum, Spleen, and Brain Samples
4.5. Determination of Spleen Brucella Colony-Forming Units (CFU)
4.6. Dendritic Cells and Macrophages Determination in Flow Cytometry
4.7. Cytokine Quantification
4.8. Serotonin Quantification by High Performance Liquid Chromatography (HPLC)
4.8.1. Hippocampal Tissue Processing
4.8.2. Solid Phase Extraction (SPE) of Serotonin
4.8.3. Chromatographic Runs
4.9. Statical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goodwin, R.D. Association between Infection Early in Life and Mental Disorders among Youth in the Community: A Cross-Sectional Study. BMC Public Health 2011, 11, 878. [Google Scholar] [CrossRef]
- Coughlin, S.S. Anxiety and Depression: Linkages with Viral Diseases. Public Health Rev. 2012, 34, 7. [Google Scholar] [CrossRef]
- Canli, T. Reconceptualizing Major Depressive Disorder as an Infectious Disease. Biol. Mood Anxiety Disord. 2014, 4, 10. [Google Scholar] [CrossRef]
- Müller, N. Infectious Diseases and Mental Health. Key Issues Ment. Health 2015, 179, 99–113. [Google Scholar] [CrossRef]
- Nadeem, A.; Ahmad, S.F.; Al-Harbi, N.O.; Fardan, A.S.; El-Sherbeeny, A.M.; Ibrahim, K.E.; Attia, S.M. IL-17A Causes Depression-like Symptoms via NFκB and P38MAPK Signaling Pathways in Mice: Implications for Psoriasis Associated Depression. Cytokine 2017, 97, 14–24. [Google Scholar] [CrossRef]
- Doyle, C.; Swain, W.A.; Ewald, H.A.S.; Ewald, P.W. Inflammation, Infection and Depression: An Evolutionary Perspective. Evol. Hum. Sci. 2019, 1, e14. [Google Scholar] [CrossRef]
- Nothdurfter, C.; Milenkovic, V.M.; Sarubin, N.; Hilbert, S.; Manook, A.; Weigl, J.; Almeqbaali, K.; Wetzel, C.H.; Rupprecht, R.; Baghai, T.C. The Cytokine IL-17A as a Marker of Treatment Resistance in Major Depressive Disorder? Eur. J. Neurosci. 2021, 53, 172–182. [Google Scholar] [CrossRef]
- Phillips, A.C.; Carroll, D.; Khan, N.; Moss, P. Cytomegalovirus Is Associated with Depression and Anxiety in Older Adults. Brain. Behav. Immun. 2008, 22, 52–55. [Google Scholar] [CrossRef]
- Evrensel, A.; Ceylan, M.E. The Gut-Brain Axis: The Missing Link in Depression. Clin. Psychopharmacol. Neurosci. 2015, 13, 239. [Google Scholar] [CrossRef]
- Nanthakumaran, S.; Sridharan, S.; Somagutta, M.R.; Arnold, A.A.; May, V.; Pagad, S.; Malik, B.H. The Gut-Brain Axis and Its Role in Depression. Cureus 2020, 12, e10280. [Google Scholar] [CrossRef]
- Jang, S.H.; Woo, Y.S.; Lee, S.Y.; Bahk, W.M. The Brain–Gut–Microbiome Axis in Psychiatry. Int. J. Mol. Sci. 2020, 21, 7122. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Miller, A.H. The Evolutionary Significance of Depression in Pathogen Host Defense (PATHOS-D). Mol. Psychiatry 2013, 18, 15–37. [Google Scholar] [CrossRef]
- Raison, C.L.; Miller, A.H. Pathogen-Host Defense in the Evolution of Depression: Insights into Epidemiology, Genetics, Bioregional Differences and Female Preponderance. Neuropsychopharmacology 2017, 42, 5–27. [Google Scholar] [CrossRef]
- Andersson, N.W.; Goodwin, R.D.; Okkels, N.; Gustafsson, L.N.; Taha, F.; Cole, S.W.; Munk-Jørgensen, P. Depression and the Risk of Severe Infections: Prospective Analyses on a Nationwide Representative Sample. Int. J. Epidemiol. 2016, 45, 131–139. [Google Scholar] [CrossRef]
- Witthauer, C.; Gloster, A.T.; Meyer, A.H.; Goodwin, R.D.; Lieb, R. Comorbidity of Infectious Diseases and Anxiety Disorders in Adults and Its Association with Quality of Life: A Community Study. Front. Public Health 2014, 2, 80. [Google Scholar] [CrossRef]
- Ajdacic-Gross, V.; Aleksandrowicz, A.; Rodgers, S.; Müller, M.; Kawohl, W.; Rössler, W.; Castelao, E.; Vandeleur, C.; von Känel, R.; Mutsch, M.; et al. Social Phobia Is Associated with Delayed Onset of Chickenpox, Measles, and Mumps Infections. Front. Psychiatry 2016, 7, 203. [Google Scholar] [CrossRef]
- Ellul, P.; Mariotti-Ferrandiz, E.; Leboyer, M.; Klatzmann, D. Regulatory T Cells As Supporters of Psychoimmune Resilience: Toward Immunotherapy of Major Depressive Disorder. Front. Neurol. 2018, 9, 167. [Google Scholar] [CrossRef]
- Maldonado-García, J.L.; Pérez-Sánchez, G.; Becerril Villanueva, E.; Alvarez-Herrera, S.; Pavón, L.; Gutiérrez-Ospina, G.; López-Santiago, R.; Maldonado-Tapia, J.O.; Pérez-Tapia, S.M.; Moreno-Lafont, M.C. Behavioral and Neurochemical Shifts at the Hippocampus and Frontal Cortex Are Associated to Peripheral Inflammation in Balb/c Mice Infected with Brucella Abortus 2308. Microorganisms 2021, 9, 1937. [Google Scholar] [CrossRef]
- CDC Brucellosis Reference Guide: Exposures, Testing and Prevention. Available online: https://www.cdc.gov/brucellosis/pdf/brucellosi-reference-guide.pdf (accessed on 1 August 2023).
- Gul, H.C.; Erdem, H.; Bek, S. Overview of Neurobrucellosis: A Pooled Analysis of 187 Cases. Int. J. Infect. Dis. 2009, 13, e339–e343. [Google Scholar] [CrossRef]
- Guven, T.; Ugurlu, K.; Ergonul, O.; Celikbas, A.K.; Gok, S.E.; Comoglu, S.; Baykam, N.; Dokuzoguz, B. Neurobrucellosis: Clinical and Diagnostic Features. Clin. Infect. Dis. 2013, 56, 1407–1412. [Google Scholar] [CrossRef]
- Elbehiry, A.; Aldubaib, M.; Al Rugaie, O.; Marzouk, E.; Abaalkhail, M.; Moussa, I.; El-Husseiny, M.H.; Abalkhail, A.; Rawway, M. Proteomics-Based Screening and Antibiotic Resistance Assessment of Clinical and Sub-Clinical Brucella Species: An Evolution of Brucellosis Infection Control. PLoS ONE 2022, 17, e0262551. [Google Scholar] [CrossRef] [PubMed]
- del Pozo, J.S.G.; Solera, J. Treatment of Human Brucellosis—Review of Evidence from Clinical Trials. In Updates on Brucellosis; Baddour, M.M., Ed.; InTech: London, UK, 2015; pp. 185–199. [Google Scholar]
- Alavi, S.M.; Alavi, L. Treatment of Brucellosis: A Systematic Review of Studies in Recent Twenty Years. Casp. J. Intern. Med. 2013, 4, 636–641. [Google Scholar]
- Hearn, L.; Wiffen, P.J.; Moore, R.A.; Derry, S. Imipramine for Neuropathic Pain and Fibromyalgia in Adults. Cochrane Database Syst. Rev. 2013, 2013, 30. [Google Scholar] [CrossRef]
- Sallee, F.R.; Pollock, B.G. Clinical Pharmacokinetics of Imipramine and Desipramine. Clin. Pharmacokinet. 1990, 18, 346–364. [Google Scholar] [CrossRef]
- Mukherjee, S.; Pradhan, S.; Ghosh, S.; Sundar, S.; Das, S.; Mukherjee, B.; Roy, S. Short-Course Treatment With Imipramine Entrapped in Squalene Liposomes Results in Sterile Cure of Experimental Visceral Leishmaniasis Induced by Antimony Resistant Leishmania Donovani With Increased Efficacy. Front. Cell Infect. Microbiol. 2020, 10, 595415. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mukherjee, B.; Mukhopadhyay, R.; Naskar, K.; Sundar, S.; Dujardin, J.C.; Das, A.K.; Roy, S. Imipramine Is an Orally Active Drug against Both Antimony Sensitive and Resistant Leishmania Donovani Clinical Isolates in Experimental Infection. PLoS Negl. Trop. Dis. 2012, 6, e1987. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mukherjee, B.; Mukhopadhyay, R.; Naskar, K.; Sundar, S.; Dujardin, J.-C.; Roy, S. Imipramine Exploits Histone Deacetylase 11 To Increase the IL-12/IL-10 Ratio in Macrophages Infected with Antimony-Resistant Leishmania Donovani and Clears Organ Parasites in Experimental Infection. J. Immunol. 2014, 193, 4083–4094. [Google Scholar] [CrossRef]
- Xia, Z.; DePierre, J.W.; Nässberger, L. Tricyclic Antidepressants Inhibit IL-6, IL-1β and TNF-α Release in Human Blood Monocytes and IL-2 and Interferon-γ in T Cells. Immunopharmacology 1996, 34, 27–37. [Google Scholar] [CrossRef]
- Kast, R.E. Anti- and pro-Inflammatory Considerations in Antidepressant Use during Medical Illness: Bupropion Lowers and Mirtazapine Increases Circulating Tumor Necrosis Factor-Alpha Levels. Gen. Hosp. Psychiatry 2003, 25, 495–496. [Google Scholar] [CrossRef]
- Brustolim, D.; Ribeiro-dos-Santos, R.; Kast, R.E.; Altschuler, E.L.; Soares, M.B.P. A New Chapter Opens in Anti-Inflammatory Treatments:The Antidepressant Bupropion Lowers Production of Tumor Necrosis Factor-Alpha and Interferon-Gamma in Mice. Int. Immunopharmacol. 2006, 6, 903–907. [Google Scholar] [CrossRef]
- Idova, G.V.; Cheĭdo, M.A. Stimulation of the Immune Response during Blockade of Serotonin Receptors by Cyproheptadine. Biull. Eksp. Biol. Med. 1987, 103, 440–442. [Google Scholar] [CrossRef] [PubMed]
- Nordlind, K.; Sundström, E.; Bondesson, L. Inhibiting Effects of Serotonin Antagonists on the Proliferation of Mercuric Chloride Stimulated Human Peripheral Blood T Lymphocytes. Int. Arch. Allergy Immunol. 1992, 97, 105–108. [Google Scholar] [CrossRef]
- Šmejkal-Jagar, L.; Boranić, M. Serotonin, Serotoninergic Agents and Their Antagonists Suppress Humoral Immune Reaction in Vitro. Res. Exp. Med. 1994, 194, 297–304. [Google Scholar] [CrossRef]
- Young, M.R.I.; Matthewst, J.P. Serotonin Regulation of T-Cell Subpopulations and of Macrophage Accessory Function. Immunology 1995, 84, 148. [Google Scholar]
- Kubera, M.; Holan, V.; Mathison, R.; Maes, M. The Effect of Repeated Amitriptyline and Desipramine Administration on Cytokine Release in C57BL/6 Mice. Psychoneuroendocrinology 2000, 25, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Criado, S.; Muñoz-Bellido, J.L.; García-Rodríguez, J.A. In Vitro Activity of Nonsteroidal Anti-Inflammatory Agents, Phenotiazines, and Antidepressants against Brucella Species. Eur. J. Clin. Microbiol. Infect. Dis. 1996, 15, 418–420. [Google Scholar] [CrossRef] [PubMed]
- Dean, A.S.; Crump, L.; Greter, H.; Hattendorf, J.; Schelling, E.; Zinsstag, J. Clinical Manifestations of Human Brucellosis: A Systematic Review and Meta-Analysis. PLoS Negl. Trop. Dis. 2012, 6, e1929. [Google Scholar] [CrossRef]
- McDermott, J.; Grace, D.; Zinsstag, J. Economics of Brucellosis Impact and Control in Low-Income Countries. Rev. Sci. Tech. 2013, 32, 249–261. [Google Scholar] [CrossRef]
- Ariza, J.; Bosilkovski, M.; Cascio, A.; Colmenero, J.D.; Corbel, M.J.; Falagas, M.E.; Memish, Z.A.; Roushan, M.R.H.; Rubinstein, E.; Sipsas, N.V.; et al. Perspectives for the Treatment of Brucellosis in the 21st Century: The Ioannina Recommendations. PLoS Med. 2007, 4, e317. [Google Scholar] [CrossRef]
- Brebner, K.; Hayley, S.; Zacharko, R.; Merali, Z.; Anisman, H. Synergistic Effects of Interleukin-1, Interleukin-6, and Tumor Necrosis Factor-: Central Monoamine, Corticosterone, and Behavioral Variations. Neuropsychopharmacology 2000, 22, 566–580. [Google Scholar] [CrossRef]
- Glowinski, J.; Axelrod, J. Inhibition of Uptake of Tritiated-Noradrenaline in the Intact Rat Brain by Imipramine and Structurally Related Compounds. Nature 1964, 204, 1318–1319. [Google Scholar] [CrossRef] [PubMed]
- Iversen, L. Neurotransmitter Transporters and Their Impact on the Development of Psychopharmacology. Br. J. Pharmacol. 2006, 147, S82. [Google Scholar] [CrossRef] [PubMed]
- Klimek, V.; Stockmeier, C.; Overholser, J.; Meltzer, H.Y.; Kalka, S.; Dilley, G.; Ordway, G.A. Reduced Levels of Norepinephrine Transporters in the Locus Coeruleus in Major Depression. J. Neurosci. 1997, 17, 8451–8458. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhen, J.; Karpowich, N.K.; Goetz, R.M.; Law, C.J.; Reith, M.E.A.; Wang, D.N. LeuT-Desipramine Structure Reveals How Antidepressants Block Neurotransmitter Reuptake. Science 2007, 317, 1390–1393. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.S.; Ogbechi, J.; Clanchy, F.I.; Williams, R.O.; Stone, T.W. IDO and Kynurenine Metabolites in Peripheral and CNS Disorders. Front. Immunol. 2020, 11, 493984. [Google Scholar] [CrossRef]
- Dantzer, R. Role of the Kynurenine Metabolism Pathway in Inflammation-Induced Depression—Preclinical Approaches. Curr. Top. Behav. Neurosci. 2017, 31, 117. [Google Scholar] [CrossRef]
- Dawood, S.; Bano, S.; Badawy, A.A.B. Inflammation and Serotonin Deficiency in Major Depressive Disorder: Molecular Docking of Antidepressant and Anti-Inflammatory Drugs to Tryptophan and Indoleamine 2,3-Dioxygenases. Biosci. Rep. 2022, 42, BSR20220426. [Google Scholar] [CrossRef]
- Becerril-Villanueva, E.; Ponce-Regalado, M.D.; Pérez-Sánchez, G.; Salazar-Juárez, A.; Arreola, R.; Álvarez-Sánchez, M.E.; Juárez-Ortega, M.; Falfán-Valencia, R.; Hernández-Pando, R.; Morales-Montor, J.; et al. Chronic Infection with Mycobacterium Lepraemurium Induces Alterations in the Hippocampus Associated with Memory Loss. Sci. Rep. 2018, 8, 9063. [Google Scholar] [CrossRef]
- Lara-Espinosa, J.V.; Santana-Martínez, R.A.; Maldonado, P.D.; Zetter, M.; Becerril-Villanueva, E.; Pérez-Sánchez, G.; Pavón, L.; Mata-Espinosa, D.; Barrios-Payán, J.; López-Torres, M.O.; et al. Experimental Pulmonary Tuberculosis in the Absence of Detectable Brain Infection Induces Neuroinflammation and Behavioural Abnormalities in Male Balb/c Mice. Int. J. Mol. Sci. 2020, 21, 9483. [Google Scholar] [CrossRef]
- Ogbechi, J.; Clanchy, F.I.; Huang, Y.S.; Topping, L.M.; Stone, T.W.; Williams, R.O. IDO Activation, Inflammation and Musculoskeletal Disease. Exp. Gerontol. 2020, 131, 110820. [Google Scholar] [CrossRef]
- Michels, N.; Clarke, G.; Olavarria-Ramirez, L.; Gómez-Martínez, S.; Díaz, L.E.; Marcos, A.; Widhalm, K.; Carvalho, L.A. Psychosocial Stress and Inflammation Driving Tryptophan Breakdown in Children and Adolescents: A Cross-Sectional Analysis of Two Cohorts. Psychoneuroendocrinology 2018, 94, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Ebbinghaus, M.; Segond von Banchet, G.; Massier, J.; Gajda, M.; Bräuer, R.; Kress, M.; Schaible, H.G. Interleukin-6-Dependent Influence of Nociceptive Sensory Neurons on Antigen-Induced Arthritis. Arthritis Res. Ther. 2015, 17, 334. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Q.; Liu, Z.; Liu, Z.H.; Chen, S.P.; Li, M.; Shahveranov, A.; Ye, D.W.; Tian, Y.K. Interleukin-6: An Emerging Regulator of Pathological Pain. J. Neuroinflamm. 2016, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Tsuji, M.; Takeda, H.; Harada, Y.; Nohara, J.; Matsumiya, T.; Chiba, H. Antidepressants Enhance the Antinociceptive Effects of Carbamazepine in the Acetic Acid-Induced Writhing Test in Mice. Eur. J. Pharmacol. 2006, 550, 78–83. [Google Scholar] [CrossRef]
- Ying, G.; Karlsson, H.; DePierre, J.W.; Nässberger, L. Tricyclic Antidepressants Prevent the Differentiation of Monocytes into Macrophage-like Cells in Vitro. Cell Biol. Toxicol. 2002, 18, 425–437. [Google Scholar] [CrossRef]
- Viana Andrade-Neto, V.; Pereira, T.M.; Do Canto-Cavalheiro, M.; Caio Torres-Santos, E. Imipramine Alters the Sterol Profile in Leishmania Amazonensis and Increases Its Sensitivity to Miconazole. Parasites Vectors 2016, 9, 183. [Google Scholar] [CrossRef]
- Adams, C.M.; Goldstein, J.L.; Brown, M.S. Cholesterol-Induced Conformational Change in SCAP Enhanced by Insig Proteins and Mimicked by Cationic Amphiphiles. Proc. Natl. Acad. Sci. USA 2003, 100, 10647–10652. [Google Scholar] [CrossRef]
- Nazimek, K.; Kozlowski, M.; Bryniarski, P.; Strobel, S.; Bryk, A.; Myszka, M.; Tyszka, A.; Kuszmiersz, P.; Nowakowski, J.; Filipczak-Bryniarska, I. Repeatedly Administered Antidepressant Drugs Modulate Humoral and Cellular Immune Response in Mice through Action on Macrophages. Exp. Biol. Med. 2016, 241, 1540–1550. [Google Scholar] [CrossRef]
- Hadjimitova, V.; Traykov, T.; Goliysky, P.; Ribarov, S. Effect of Some Psychotropic Drugs on the Activated Macrophage-Induced Luminol-Dependent Chemiluminescence. Pharmacol. Toxicol. 1999, 84, 170–173. [Google Scholar] [CrossRef]
- Watarai, M.; ichi Makino, S.; Michikawa, M.; Yanagisawa, K.; Murakami, S.; Shirahata, T. Macrophage Plasma Membrane Cholesterol Contributes to Brucella Abortus Infection of Mice. Infect. Immun. 2002, 70, 4818–4825. [Google Scholar] [CrossRef]
- Underwood, K.W.; Andemariam, B.; McWilliams, G.L.; Liscum, L. Quantitative Analysis of Hydrophobic Amine Inhibition of Intracellular Cholesterol Transport. J. Lipid Res. 1996, 37, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-P.; Singla, B.; Ghoshal, P.; Faulkner, J.L.; Cherian-Shaw, M.; O’Connor, P.M.; She, J.-X.; Belin de Chantemele, E.J.; Csányi, G. Identification of Novel Macropinocytosis Inhibitors Using a Rational Screen of Food and Drug Administration-Approved Drugs. Br. J. Pharmacol. 2018, 175, 3640–3655. [Google Scholar] [CrossRef] [PubMed]
- Watarai, M.; Makino, S.I.; Fujii, Y.; Okamoto, K.; Shirahata, T. Modulation of Brucella-Induced Macropinocytosis by Lipid Rafts Mediates Intracellular Replication. Cell Microbiol. 2002, 4, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, K.; Molnar, J. Mechanism of Action of Tricyclic Drugs on Escherichia Coli and Yersinia Enterocolitica Plasmid Maintenance and Replication. Anticancer Res. 1992, 12, 2267–2272. [Google Scholar]
- Dutta, P.; Pinto, J.; Rivlin, R. Antimalarial Properties of Imipramine and Amitriptyline. J. Protozool. 1990, 37, 54–58. [Google Scholar] [CrossRef]
- Arreola, R.; Becerril-Villanueva, E.; Cruz-Fuentes, C.; Velasco-Velázquez, M.A.; Garces-Alvarez, M.E.; Hurtado-Alvarado, G.; Quintero-Fabian, S.; Pavon, L. Immunomodulatory Effects Mediated by Serotonin. J. Immunol. Res. 2015, 2015, 354957. [Google Scholar] [CrossRef]
- Stevens, M.G.; Pugh, G.W.; Tabatabai, L.B. Effects of Gamma Interferon and Indomethacin in Preventing Brucella Abortus Infections in Mice. Infect. Immun. 1992, 60, 4407–4409. [Google Scholar] [CrossRef]
- Andratsch, M.; Mair, N.; Constantin, C.E.; Scherbakov, N.; Benetti, C.; Quarta, S.; Vogl, C.; Sailer, C.A.; Üceyler, N.; Brockhaus, J.; et al. A Key Role for Gp130 Expressed on Peripheral Sensory Nerves in Pathological Pain. J. Neurosci. 2009, 29, 13473–13483. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose Translation from Animal to Human Studies Revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Ramirez, K.; Sheridan, J.F. Antidepressant Imipramine Diminishes Stress-Induced Inflammation in the Periphery and Central Nervous System and Related Anxiety- and Depressive-like Behaviors. Brain. Behav. Immun. 2016, 57, 293–303. [Google Scholar] [CrossRef]


| Groups | Post Hoc Analysis | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytokine | Cr14 | Ctr28 | Ba14 | Ba28 | Im6Ba 14 | Im28Ba 28 | ImiP6 | ImiP20 | Ctr14 vs. Ba14 | Ctr28 vs. Ba28 | Ba14 vs. Ba28 | Ba14 vs. ImBa 20 | Ba28 vs. Im20Ba28 | Im20Ba28 vs. Im6Ba 14 | Im6Ba 14 vs. Ctr14 | Im20Ba 28 vs. Ctr28 |
| IL-6 | ND | ND | 18.6 ± 1.4 | 12.4 ± 1.5 | 10.4 ± 2.9 | ND | ND | ND | -- | -- | **** | **** | -- | -- | -- | -- |
| IL-12 | ND | ND | 15.1 ± 6.7 | 10.1 ± 2.7 | 16.8 ± 4.4 | 9.8 ± 3.6 | ND | ND | -- | -- | ns | ns | ns | * | -- | -- |
| TNF-α | 13.9 ± 1.5 | 13.4 ± 1.5 | 40.8 ± 6.6 | 26.9 ± 4.7 | 29.9 ± 2.7 | 7.9 ± 11.8 | 12.5 ± 2.2 | 14.1 ± 1.9 | **** | **** | ** | ** | **** | **** | **** | **** |
| IFN-γ | ND | ND | 244.8 ± 29.6 | 64.5 ± 15.1 | 238.0 ± 29.4 | 65.9 ± 15.2 | ND | ND | -- | -- | **** | ns | ns | **** | -- | -- |
| Variable | Effects of Imipramine (6) at Day 14 of Follow Up | Effects of Imipramine (20) at Day 28 of Follow Up |
|---|---|---|
| Decrease of hopelessness (FST and TST) | ✓ | ✓ |
| Decrease of anxiety (OF) | ✓ | ± |
| Increase of serotonin in hippocampus | ✓ | ± |
| Increase of motor abilities (FGST and MBCT) | ✓ | ✓ |
| Decrease of M and DC in splenic CFU | ✓ | ✓ |
| Decrease of IL-6 serum levels | ✓ | ✓ |
| Decrease of TNF-α serum levels | ✓ | ✓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maldonado-García, J.L.; Pérez-Sánchez, G.; Becerril-Villanueva, E.; Alvarez-Herrera, S.; Pavón, L.; Sánchez-Torres, L.; Gutiérrez-Ospina, G.; Girón-Pérez, M.I.; Damian-Morales, G.; Maldonado-Tapia, J.O.; et al. Imipramine Administration in Brucella abortus 2308-Infected Mice Restores Hippocampal Serotonin Levels, Muscle Strength, and Mood, and Decreases Spleen CFU Count. Pharmaceuticals 2023, 16, 1525. https://doi.org/10.3390/ph16111525
Maldonado-García JL, Pérez-Sánchez G, Becerril-Villanueva E, Alvarez-Herrera S, Pavón L, Sánchez-Torres L, Gutiérrez-Ospina G, Girón-Pérez MI, Damian-Morales G, Maldonado-Tapia JO, et al. Imipramine Administration in Brucella abortus 2308-Infected Mice Restores Hippocampal Serotonin Levels, Muscle Strength, and Mood, and Decreases Spleen CFU Count. Pharmaceuticals. 2023; 16(11):1525. https://doi.org/10.3390/ph16111525
Chicago/Turabian StyleMaldonado-García, José Luis, Gilberto Pérez-Sánchez, Enrique Becerril-Villanueva, Samantha Alvarez-Herrera, Lenin Pavón, Luvia Sánchez-Torres, Gabriel Gutiérrez-Ospina, Manuel Iván Girón-Pérez, Gabriela Damian-Morales, Jesús Octavio Maldonado-Tapia, and et al. 2023. "Imipramine Administration in Brucella abortus 2308-Infected Mice Restores Hippocampal Serotonin Levels, Muscle Strength, and Mood, and Decreases Spleen CFU Count" Pharmaceuticals 16, no. 11: 1525. https://doi.org/10.3390/ph16111525
APA StyleMaldonado-García, J. L., Pérez-Sánchez, G., Becerril-Villanueva, E., Alvarez-Herrera, S., Pavón, L., Sánchez-Torres, L., Gutiérrez-Ospina, G., Girón-Pérez, M. I., Damian-Morales, G., Maldonado-Tapia, J. O., López-Santiago, R., & Moreno-Lafont, M. C. (2023). Imipramine Administration in Brucella abortus 2308-Infected Mice Restores Hippocampal Serotonin Levels, Muscle Strength, and Mood, and Decreases Spleen CFU Count. Pharmaceuticals, 16(11), 1525. https://doi.org/10.3390/ph16111525