Risperidone Decreases Expression of Serotonin Receptor-2A (5-HT2A) and Serotonin Transporter (SERT) but Not Dopamine Receptors and Dopamine Transporter (DAT) in PBMCs from Patients with Schizophrenia
Abstract
1. Introduction
2. Results
2.1. Participants, Demographic Data, and Scales Scores
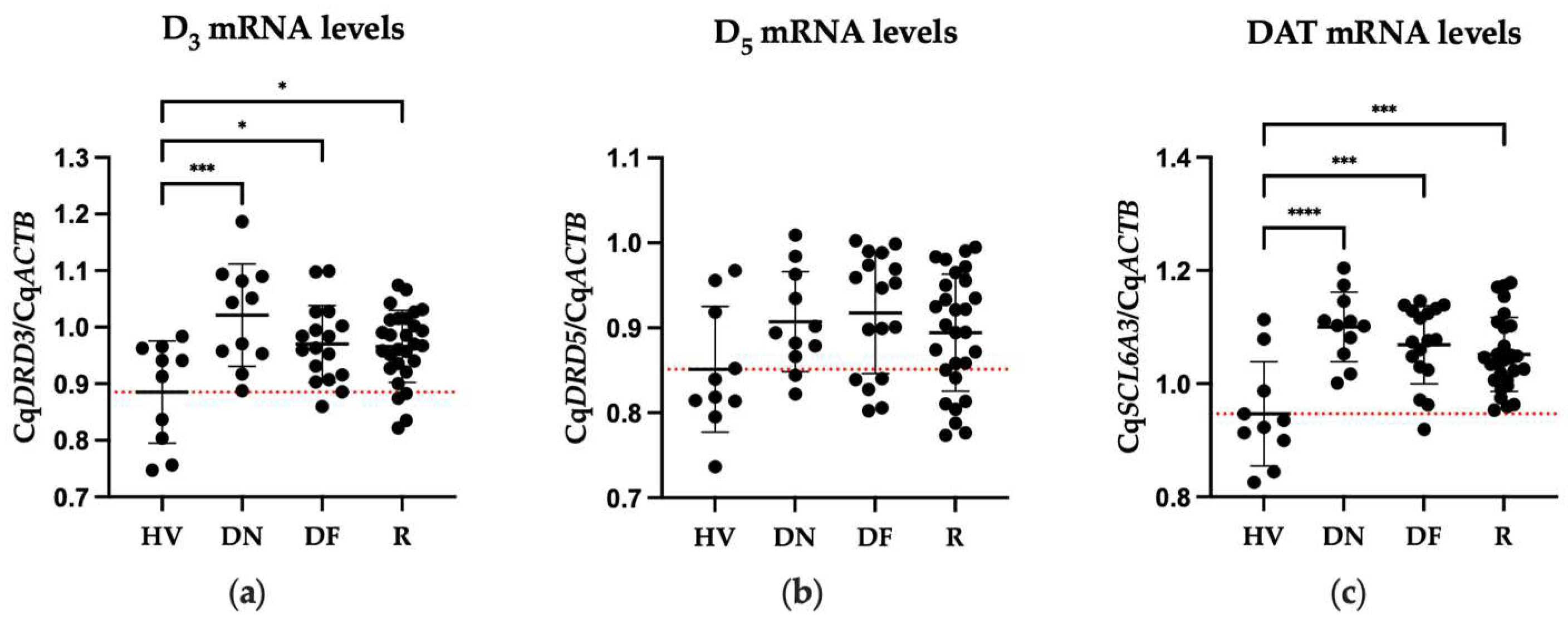
2.2. Differences in Expression of DA Receptors between Patient and Healthy Volunteers
2.3. Differences in Gene Expression of 5-HT2A and SERT of Patients and Healthy Volunteers
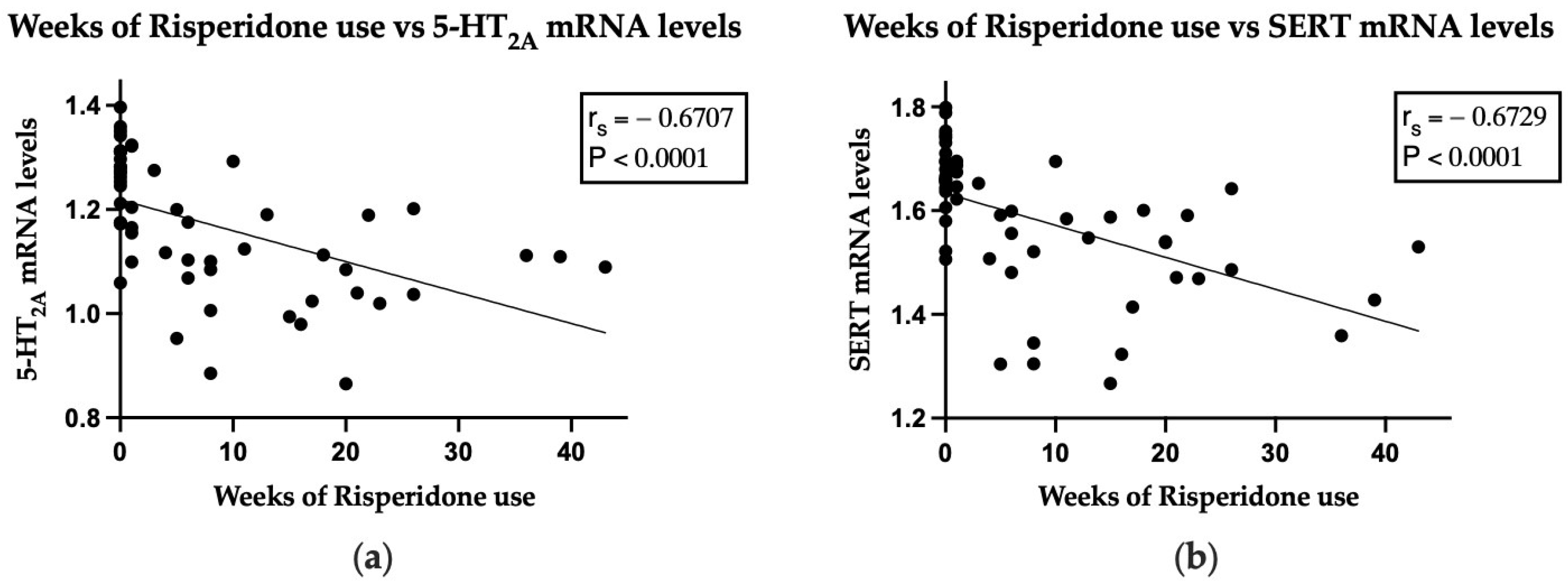
2.4. Correlation between Gene Expression and Both PANSS Total Score or Subscale Scores, and Duration of Risperidone Consumption
3. Discussion
4. Materials and Methods
4.1. Participant Information and Clinical Measures
4.2. Sample Processing
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Owen, M.J.; Sawa, A.; Mortensen, P.B. Schizophrenia. Lancet 2016, 388, 86–97. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association Publishing: Washington, DC, USA, 2022; ISBN 0890425752. [Google Scholar]
- Tandon, R.; Keshavan, M.S.; Nasrallah, H.A. Schizophrenia, “Just the Facts”: What We Know in 2008. Schizophr. Res. 2008, 100, 4–19. [Google Scholar] [CrossRef] [PubMed]
- Marcellusi, A.; Fabiano, G.; Viti, R.; Francesa Morel, P.C.; Nicolò, G.; Siracusano, A.; Mennini, F.S. Economic Burden of Schizophrenia in Italy: A Probabilistic Cost of Illness Analysis. BMJ Open 2018, 8, e018359. [Google Scholar] [CrossRef] [PubMed]
- Laruelle, M. Schizophrenia: From Dopaminergic to Glutamatergic Interventions. Curr. Opin. Pharmacol. 2014, 14, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Erritzoe, D.; Rasmussen, H.; Kristiansen, K.T.; Frokjaer, V.G.; Haugbol, S.; Pinborg, L.; Baaré, W.; Svarer, C.; Madsen, J.; Lublin, H.; et al. Cortical and Subcortical 5-HT2A Receptor Binding in Neuroleptic-Naive First-Episode Schizophrenic Patients. Neuropsychopharmacology 2008, 33, 2435–2441. [Google Scholar] [CrossRef] [PubMed]
- Okubo, Y.; Suhara, T.; Suzuki, K.; Kobayashi, K.; Inoue, O.; Terasaki, O.; Someya, Y.; Sassa, T.; Sudo, Y.; Matsushima, E.; et al. Decreased Prefrontal Dopamine D1 Receptors in Schizophrenia Revealed by PET. Nature 1997, 385, 634–636. [Google Scholar] [CrossRef] [PubMed]
- García-Bea, A.; Miranda-Azpiazu, P.; Muguruza, C.; Marmolejo-Martinez-Artesero, S.; Diez-Alarcia, R.; Gabilondo, A.M.; Callado, L.F.; Morentin, B.; González-Maeso, J.; Meana, J.J. Serotonin 5-HT2A Receptor Expression and Functionality in Postmortem Frontal Cortex of Subjects with Schizophrenia: Selective Biased Agonism via Gαi1-Proteins. Eur. Neuropsychopharmacol. 2019, 29, 1453–1463. [Google Scholar] [CrossRef]
- Stahl, S.M. Beyond the Dopamine Hypothesis of Schizophrenia to Three Neural Networks of Psychosis: Dopamine, Serotonin, and Glutamate. CNS Spectr. 2018, 23, 187–191. [Google Scholar] [CrossRef]
- Miyamoto, S.; Duncan, G.E.; Marx, C.E.; Lieberman, J.A. Treatments for Schizophrenia: A Critical Review of Pharmacology and Mechanisms of Action of Antipsychotic Drugs. Mol. Psychiatry 2005, 10, 79–104. [Google Scholar] [CrossRef]
- Ilani, T.; Strous, R.D.; Fuchs, S. Dopaminergic Regulation of Immune Cells via D3 Dopamine Receptor: A Pathway Mediated by Activated T Cells. FASEB J. 2004, 18, 1600–1602. [Google Scholar] [CrossRef]
- Arreola, R.; Becerril-Villanueva, E.; Cruz-Fuentes, C.; Velasco-Velázquez, M.A.; Garcés-Alvarez, M.E.; Hurtado-Alvarado, G.; Quintero-Fabian, S.; Pavón, L. Immunomodulatory Effects Mediated by Serotonin. J. Immunol. Res. 2015, 2015, 354957. [Google Scholar] [CrossRef] [PubMed]
- Arreola, R.; Alvarez-Herrera, S.; Pérez-Sánchez, G.; Becerril-Villanueva, E.; Cruz-Fuentes, C.; Flores-Gutierrez, E.O.; Garcés-Alvarez, M.E.; de la Cruz-Aguilera, D.L.; Medina-Rivero, E.; Hurtado-Alvarado, G.; et al. Immunomodulatory Effects Mediated by Dopamine. J. Immunol. Res. 2016, 2016, 3160486. [Google Scholar] [CrossRef] [PubMed]
- Penedo, M.A.; Rivera-Baltanás, T.; Pérez-Rodríguez, D.; Allen, J.; Borrajo, A.; Alonso-Crespo, D.; Fernández-Pereira, C.; Nieto-Araujo, M.; Ramos-García, S.; Barreiro-Villar, C.; et al. The Role of Dopamine Receptors in Lymphocytes and Their Changes in Schizophrenia. Brain Behav. Immun. Health 2021, 12, 100199. [Google Scholar] [CrossRef] [PubMed]
- Ilani, T. A Peripheral Marker for Schizophrenia: Increased Levels of D3 Dopamine Receptor MRNA in Blood Lymphocytes. Proc. Natl. Acad. Sci. USA 2001, 98, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, G.P.; Hrutkay, R.J.; Shurin, M.R.; Shurin, G.V.; Tourkova, I.L.; Vanyukov, M.M. Dopamine Receptors in Human Lymphocytes: Radioligand Binding and Quantitative RT-PCR Assays. J. Neurosci. Methods 2008, 174, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Varun, C.N.; Venkataswamy, M.M.; Ravikumar, R.; Nagaraju, R.; Debnath, M.; Varambally, S.; Venkatasubramanian, G.; Ravi, V. Th17 and MAIT Cell Mediated Inflammation in Antipsychotic Free Schizophrenia Patients. Schizophr. Res. 2019, 212, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Herrera, S.; Escamilla, R.; Medina-Contreras, O.; Saracco, R.; Flores, Y.; Hurtado-Alvarado, G.; Maldonado-García, J.L.; Becerril-Villanueva, E.; Pérez-Sánchez, G.; Pavón, L. Immunoendocrine Peripheral Effects Induced by Atypical Antipsychotics. Front. Endocrinol. 2020, 11, 195. [Google Scholar] [CrossRef]
- Orzelska-Górka, J.; Mikulska, J.; Wiszniewska, A.; Biała, G. New Atypical Antipsychotics in the Treatment of Schizophrenia and Depression. Int. J. Mol. Sci. 2022, 23, 10624. [Google Scholar] [CrossRef]
- Fabrazzo, M.; Cipolla, S.; Camerlengo, A.; Perris, F.; Catapano, F. Second-Generation Antipsychotics’ Effectiveness and Tolerability: A Review of Real-World Studies in Patients with Schizophrenia and Related Disorders. J. Clin. Med. 2022, 11, 4530. [Google Scholar] [CrossRef]
- Kwak, Y.T.; Koo, M.-S.; Choi, C.-H.; Sunwoo, I. Change of Dopamine Receptor MRNA Expression in Lymphocyte of Schizophrenic Patients. BMC Med. Genet. 2001, 2, 3. [Google Scholar] [CrossRef]
- Prieto, G.A. Abnormalities of Dopamine D3 Receptor Signaling in the Diseased Brain. J. Cent. Nerv. Syst. Dis. 2017, 9, 117957351772633. [Google Scholar] [CrossRef] [PubMed]
- Arranz, B.; Rosel, P.; San, L.; Ramírez, N.; Dueñas, R.M.; Salavert, J.; Centeno, M.; Moral, E. del Low Baseline Serotonin-2A Receptors Predict Clinical Response to Olanzapine in First-Episode Schizophrenia Patients. Psychiatry Res. 2007, 153, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-L.; Tsai, T.-C.; Lin, Y.-Y.; Tsai, Y.-M.; Wang, L.-K.; Lee, M.-C.; Tsai, F.-M. Antipsychotic Drugs Suppress the AKT/NF-ΚB Pathway and Regulate the Differentiation of T-Cell Subsets. Immunol. Lett. 2011, 140, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.N.; Barlas, C.; Saeedi, H.; Mishra, R.K. Effect of Loxapine on Peripheral Dopamine-like and Serotonin Receptors in Patients with Schizophrenia. J. Psychiatry Neurosci. 2003, 28, 39–47. [Google Scholar] [PubMed]
- Shariati, G.R.; Ahangari, G.; Hossein-nezhad, A.; Asadi, S.M.; Pooyafard, F.; Ahmadkhaniha, H.R. Expression Changes of Serotonin Receptor Gene Subtype 5HT3a in Peripheral Blood Mononuclear Cells from Schizophrenic Patients Treated with Haloperidol and Olanzapin. Iran. J. Allergy Asthma Immunol. 2009, 8, 135–139. [Google Scholar] [PubMed]
- Fernandez-Egea, E.; Vértes, P.E.; Flint, S.M.; Turner, L.; Mustafa, S.; Hatton, A.; Smith, K.G.C.; Lyons, P.A.; Bullmore, E.T. Peripheral Immune Cell Populations Associated with Cognitive Deficits and Negative Symptoms of Treatment-Resistant Schizophrenia. PLoS ONE 2016, 11, e0155631. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Kerr, J.R.; Lafuente, M.-J.; Maclean, A.; Chibalina, M.V.; Liu, B.; Burke, B.; Bevan, S.; Nasir, J. Altered Expression and Coregulation of Dopamine Signalling Genes in Schizophrenia and Bipolar Disorder. Neuropathol. Appl. Neurobiol. 2011, 37, 206–219. [Google Scholar] [CrossRef]
- Bono, F.; Mutti, V.; Fiorentini, C.; Missale, C. Dopamine D3 Receptor Heteromerization: Implications for Neuroplasticity and Neuroprotection. Biomolecules 2020, 10, 1016. [Google Scholar] [CrossRef]
- Maggio, R.; Scarselli, M.; Capannolo, M.; Millan, M.J. Novel Dimensions of D3 Receptor Function: Focus on Heterodimerisation, Transactivation and Allosteric Modulation. Eur. Neuropsychopharmacol. 2015, 25, 1470–1479. [Google Scholar] [CrossRef]
- Gurevich, E.V. Mesolimbic Dopamine D3 Receptors and Use of Antipsychotics in Patients with Schizophrenia. Arch. Gen. Psychiatry 1997, 54, 225–232. [Google Scholar] [CrossRef]
- Schmauss, C.; Haroutunian, V.; Davis, K.L.; Davidson, M. Selective Loss of Dopamine D3-Type Receptor MRNA Expression in Parietal and Motor Cortices of Patients with Chronic Schizophrenia. Proc. Natl. Acad. Sci. USA 1993, 90, 8942–8946. [Google Scholar] [CrossRef] [PubMed]
- Castello, J.; Cortés, M.; Malave, L.; Kottmann, A.; Sibley, D.R.; Friedman, E.; Rebholz, H. The Dopamine D5 Receptor Contributes to Activation of Cholinergic Interneurons during L-DOPA Induced Dyskinesia. Sci. Rep. 2020, 10, 2542. [Google Scholar] [CrossRef]
- Carr, G.V.; Maltese, F.; Sibley, D.R.; Weinberger, D.R.; Papaleo, F. The Dopamine D5 Receptor Is Involved in Working Memory. Front. Pharmacol. 2017, 8, 666. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xiao, H.; Sun, H.; Zou, L.; Zhu, L.-Q. Role of Dopamine Receptors in ADHD: A Systematic Meta-Analysis. Mol. Neurobiol. 2012, 45, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Bronzetti, E.; Mannino, F.; Mignini, F.; Morosetti, C.; Tayebati, S.K.; Amenta, F. Dopamine Receptors in Human Platelets. Naunyn Schmiedebergs Arch. Pharmacol. 2001, 363, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Sedvall, G.; Pauli, S.; Karlsson, P.; Farde, L.; Nordström, A.-L.; Nyberg, S.; Halldin, C. PET Imaging of Neuroreceptors in Schizophrenia. Eur. Neuropsychopharmacol. 1995, 5, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Bu, M.; Farrer, M.J.; Khoshbouei, H. Dynamic Control of the Dopamine Transporter in Neurotransmission and Homeostasis. NPJ Parkinsons Dis. 2021, 7, 22. [Google Scholar] [CrossRef]
- McHugh, P.C.; Buckley, D.A. The Structure and Function of the Dopamine Transporter and Its Role in CNS Diseases. Vitam. Horm. 2015, 98, 339–369. [Google Scholar] [CrossRef]
- Salatino-Oliveira, A.; Rohde, L.A.; Hutz, M.H. The Dopamine Transporter Role in Psychiatric Phenotypes. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2018, 177, 211–231. [Google Scholar] [CrossRef]
- Schmitt, G.J.E.; Meisenzahl, E.M.; Frodl, T.; La Fougère, C.; Hahn, K.; Möller, H.-J.; Dresel, S. The Striatal Dopamine Transporter in First-Episode, Drug-Naive Schizophrenic Patients: Evaluation by the New SPECT-Ligand[99mTc]TRODAT-1. J. Psychopharmacol. 2005, 19, 488–493. [Google Scholar] [CrossRef]
- Mateos, J.J.; Lomeña, F.; Parellada, E.; Mireia, F.; Fernandez-Egea, E.; Pavia, J.; Prats, A.; Pons, F.; Bernardo, M. Lower Striatal Dopamine Transporter Binding in Neuroleptic-Naive Schizophrenic Patients Is Not Related to Antipsychotic Treatment but It Suggests an Illness Trait. Psychopharmacology 2007, 191, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, H.; Pavey, G.; Dean, B. Altered Levels of Dopamine Transporter in the Frontal Pole and Dorsal Striatum in Schizophrenia. NPJ Schizophr. 2019, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Markota, M.; Sin, J.; Pantazopoulos, H.; Jonilionis, R.; Berretta, S. Reduced Dopamine Transporter Expression in the Amygdala of Subjects Diagnosed With Schizophrenia. Schizophr. Bull. 2014, 40, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Sjoholm, H.; Bratlid, T.; Sundsfjord, J. 123 I-b-CIT SPECT Demonstrates Increased Presynaptic Dopamine Transporter Binding Sites in Basal Ganglia in Vivo in Schizophrenia. Psychopharmacology 2004, 173, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Murnane, K.S. Serotonin 2A Receptors Are a Stress Response System: Implications for Post-Traumatic Stress Disorder. Behav. Pharmacol. 2019, 30, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Zięba, A.; Stępnicki, P.; Matosiuk, D.; Kaczor, A.A. Overcoming Depression with 5-HT2A Receptor Ligands. Int. J. Mol. Sci. 2021, 23, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Stackman, R.W. The Role of Serotonin 5-HT2A Receptors in Memory and Cognition. Front. Pharmacol. 2015, 6, 225. [Google Scholar] [CrossRef]
- Hurlemann, R.; Boy, C.; Meyer, P.T.; Scherk, H.; Wagner, M.; Herzog, H.; Coenen, H.H.; Vogeley, K.; Falkai, P.; Zilles, K.; et al. Decreased Prefrontal 5-HT2A Receptor Binding in Subjects at Enhanced Risk for Schizophrenia. Anat. Embryol. 2005, 210, 519–523. [Google Scholar] [CrossRef]
- Muguruza, C.; Moreno, J.L.; Umali, A.; Callado, L.F.; Meana, J.J.; González-Maeso, J. Dysregulated 5-HT2A Receptor Binding in Postmortem Frontal Cortex of Schizophrenic Subjects. Eur. Neuropsychopharmacol. 2013, 23, 852–864. [Google Scholar] [CrossRef]
- Burnet, P. 5-HT1A and 5-HT2A Receptor MRNAs and Binding Site Densities Are Differentially Altered in Schizophrenia. Neuropsychopharmacology 1996, 15, 442–455. [Google Scholar] [CrossRef]
- Matsumoto, I.; Inoue, Y.; Iwazaki, T.; Pavey, G.; Dean, B. 5-HT2A and Muscarinic Receptors in Schizophrenia: A Postmortem Study. Neurosci. Lett. 2005, 379, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Huang, X.-F.; Wang, Q.; Deng, C. Decreased Density of Serotonin 2A Receptors in the Superior Temporal Gyrus in Schizophrenia-a Postmortem Study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 867–871. [Google Scholar] [CrossRef] [PubMed]
- López-Figueroa, A.L.; Norton, C.S.; López-Figueroa, M.O.; Armellini-Dodel, D.; Burke, S.; Akil, H.; López, J.F.; Watson, S.J. Serotonin 5-HT1A, 5-HT1B, and 5-HT2A Receptor MRNA Expression in Subjects with Major Depression, Bipolar Disorder, and Schizophrenia. Biol. Psychiatry 2004, 55, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Rudnick, G.; Sandtner, W. Serotonin Transport in the 21st Century. J. Gen. Physiol. 2019, 151, 1248–1264. [Google Scholar] [CrossRef]
- Cooper, A.; Woulfe, D.; Kilic, F. Post-Translational Modifications of Serotonin Transporter. Pharmacol. Res. 2019, 140, 7–13. [Google Scholar] [CrossRef]
- Kim, J.-H.; Son, Y.-D.; Kim, J.-H.; Choi, E.-J.; Lee, S.-Y.; Lee, J.E.; Cho, Z.-H.; Kim, Y.-B. Serotonin Transporter Availability in Thalamic Subregions in Schizophrenia: A Study Using 7.0-T MRI with [11C]DASB High-Resolution PET. Psychiatry Res. Neuroimaging 2015, 231, 50–57. [Google Scholar] [CrossRef]
- Aggarwal, S.; Mortensen, O.V. Overview of Monoamine Transporters. Curr. Protoc. Pharmacol. 2017, 79, 12.16.1–12.16.17. [Google Scholar] [CrossRef]
- Joyce, J.N.; Shane, A.; Lexow, N.; Winokur, A.; Casanova, M.F.; Kleinman, J.E. Serotonin Uptake Sites and Serotonin Receptors Are Altered in the Limbic System of Schizophrenics. Neuropsychopharmacology 1993, 8, 315–336. [Google Scholar] [CrossRef]
- Hernandez, I.; Sokolov, B.P. Abnormal Expression of Serotonin Transporter MRNA in the Frontal and Temporal Cortex of Schizophrenics. Mol. Psychiatry 1997, 2, 57–64. [Google Scholar] [CrossRef]
- Lally, J.; MacCabe, J.H. Antipsychotic Medication in Schizophrenia: A Review. Br. Med. Bull. 2015, 114, 169–179. [Google Scholar] [CrossRef]
- Aringhieri, S.; Carli, M.; Kolachalam, S.; Verdesca, V.; Cini, E.; Rossi, M.; McCormick, P.J.; Corsini, G.U.; Maggio, R.; Scarselli, M. Molecular Targets of Atypical Antipsychotics: From Mechanism of Action to Clinical Differences. Pharmacol. Ther. 2018, 192, 20–41. [Google Scholar] [CrossRef] [PubMed]
- Juncal-Ruiz, M.; Riesco-Dávila, L.; Ortiz-García de la Foz, V.; Martínez-Garcia, O.; Ramírez-Bonilla, M.; Ocejo-Viñals, J.G.; Leza, J.C.; López-Hoyos, M.; Crespo-Facorro, B. Comparison of the Anti-Inflammatory Effect of Aripiprazole and Risperidone in 75 Drug-Naïve First Episode Psychosis Individuals: A 3 months Randomized Study. Schizophr. Res. 2018, 202, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Chopko, T.C.; Lindsley, C.W. Classics in Chemical Neuroscience: Risperidone. ACS Chem. Neurosci. 2018, 9, 1520–1529. [Google Scholar] [CrossRef] [PubMed]
- Escamilla-Orozco, R.I.; Becerra-Palars, C.; Armendáriz-Vázquez, Y.; Corlay-Noriega, I.S.Y.; Herrera-Estrella, M.A.; Llamas-Núñez, R.E.; Meneses-Luna, Ó.; Quijada-Gaytán, J.M.; Reyes-Madrigal, F.; Rosado-Franco, A.; et al. Tratamiento de La Esquizofrenia En México: Recomendaciones de Un Panel de Expertos. Gac. Méd. Méx. 2021, 157, S1–S12. [Google Scholar] [CrossRef]
- Franz, D.; Contreras, F.; González, H.; Prado, C.; Elgueta, D.; Figueroa, C.; Pacheco, R. Dopamine Receptors D3 and D5 Regulate CD4+T-Cell Activation and Differentiation by Modulating ERK Activation and CAMP Production. J. Neuroimmunol. 2015, 284, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, S.; Delavari, G.; Ghanbari, D.; Zaeifi, D. D3 as a Possible Marker Based on D1-D4 Dopamine Receptors Expression in Paranoid Schizophrenia Patients. J. Mol. Biomark. Diagn. 2014, 5, 1000171. [Google Scholar] [CrossRef]
- Cui, Y.; Prabhu, V.; Nguyen, T.; Yadav, B.; Chung, Y.-C. The MRNA Expression Status of Dopamine Receptor D2, Dopamine Receptor D3 and DARPP-32 in T Lymphocytes of Patients with Early Psychosis. Int. J. Mol. Sci. 2015, 16, 26677–26686. [Google Scholar] [CrossRef]
- Vogel, M.; Pfeifer, S.; Schaub, R.T.; Grabe, H.-J.; Barnow, S.; Freyberger, H.J.; Cascorbi, I. Decreased Levels of Dopamine D<Sub>3</Sub> Receptor MRNA in Schizophrenic and Bipolar Patients. Neuropsychobiology 2004, 50, 305–310. [Google Scholar] [CrossRef]
- Zhang, F.; Fan, H.; Xu, Y.; Zhang, K.; Huang, X.; Zhu, Y.; Sui, M.; Sun, G.; Feng, K.; Xu, B.; et al. Converging Evidence Implicates the Dopamine D3 Receptor Gene in Vulnerability to Schizophrenia. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2011, 156, 613–619. [Google Scholar] [CrossRef]
- Rodrigues, K.P.; Souza, P.A.; Lima, P.M.; Dutra, W.O.; Corrêa, H.; Romano-Silva, M.A. Expression of D3 and D4 Dopamine Receptors in Leukocytes Is Related to Schizophrenic Symptoms. Schizophr. Res. 2005, 80, 363–365. [Google Scholar] [CrossRef]
- Wysokiński, A.; Kozłowska, E.; Szczepocka, E.; Łucka, A.; Agier, J.; Brzezińska-Błaszczyk, E.; Sobierajska, K. Expression of Dopamine D1−4 and Serotonin 5-HT1A-3A Receptors in Blood Mononuclear Cells in Schizophrenia. Front. Psychiatry 2021, 12, 645081. [Google Scholar] [CrossRef] [PubMed]
- Ugalde, V.; Contreras, F.; Prado, C.; Chovar, O.; Espinoza, A.; Pacheco, R. Dopaminergic Signalling Limits Suppressive Activity and Gut Homing of Regulatory T Cells upon Intestinal Inflammation. Mucosal Immunol. 2021, 14, 652–666. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, X.; Li, M.; Geng, Y.; Wang, N.; Jin, Y.; Zhang, W.; Xu, K.; Wang, J.; Tao, L.; et al. Dopamine D3 Receptor Signaling Alleviates Mouse Rheumatoid Arthritis by Promoting Toll-like Receptor 4 Degradation in Mast Cells. Cell Death Dis. 2022, 13, 240. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.; Contreras, F.; González, H.; Díaz, P.; Elgueta, D.; Barrientos, M.; Herrada, A.A.; Lladser, Á.; Bernales, S.; Pacheco, R. Stimulation of Dopamine Receptor D5 Expressed on Dendritic Cells Potentiates Th17-Mediated Immunity. J. Immunol. 2012, 188, 3062–3070. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Higashi, T.; Hashimoto, K.; Takagi, R.; Tanaka, Y.; Matsushita, S. Antagonizing Dopamine D1-like Receptor Inhibits Th17 Cell Differentiation: Preventive and Therapeutic Effects on Experimental Autoimmune Encephalomyelitis. Biochem. Biophys. Res. Commun. 2008, 373, 286–291. [Google Scholar] [CrossRef]
- Nasi, G.; Ahmed, T.; Rasini, E.; Fenoglio, D.; Marino, F.; Filaci, G.; Cosentino, M. Dopamine Inhibits Human CD8+ Treg Function through D1-like Dopaminergic Receptors. J. Neuroimmunol. 2019, 332, 233–241. [Google Scholar] [CrossRef]
- Marazziti, D.; Catena Dell’Osso, M.; Baroni, S.; Masala, I.; Dell’Osso, B.; Consoli, G.; Giannaccini, G.; Betti, L.; Lucacchini, A. Alterations of the Dopamine Transporter in Resting Lymphocytes of Patients with Different Psychotic Disorders. Psychiatry Res. 2010, 175, 54–57. [Google Scholar] [CrossRef]
- Liu, L.; Yuan, G.; Cheng, Z.; Zhang, G.; Liu, X.; Zhang, H. Identification of the MRNA Expression Status of the Dopamine D2 Receptor and Dopamine Transporter in Peripheral Blood Lymphocytes of Schizophrenia Patients. PLoS ONE 2013, 8, e75259. [Google Scholar] [CrossRef]
- Gopinath, A.; Mackie, P.M.; Phan, L.T.; Mirabel, R.; Smith, A.R.; Miller, E.; Franks, S.; Syed, O.; Riaz, T.; Law, B.K.; et al. Who Knew? Dopamine Transporter Activity Is Critical in Innate and Adaptive Immune Responses. Cells 2023, 12, 269. [Google Scholar] [CrossRef]
- Chang, P.-K.; Chien, K.-Y.; Chen, J.-C. Dopamine Transporter Is Downregulated and Its Association with Chaperone Protein Hsc70 Is Enhanced by Activation of Dopamine D3 Receptor. Brain Res. Bull. 2020, 165, 263–271. [Google Scholar] [CrossRef]
- Luis-Ravelo, D.; Fumagallo-Reading, F.; Castro-Hernandez, J.; Barroso-Chinea, P.; Afonso-Oramas, D.; Febles-Casquero, A.; Cruz-Muros, I.; Salas-Hernandez, J.; Mesa-Infante, V.; Rodriguez-Nuñez, J.; et al. Prolonged Dopamine D3 Receptor Stimulation Promotes Dopamine Transporter Ubiquitination and Degradation through a PKC-Dependent Mechanism. Pharmacol. Res. 2021, 165, 105434. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim Al, M.; Abdel Gawa, M.; Abd-Elftah, G.; Abdel-Lati, M.; Mohamed Sh, R.; Salah, H.; Sayed Abde, S.; Abu-Sarea, E.Y. Toxoplasmosis in Schizophrenic Patients: Immune-Diagnosis and Serum Dopamine Level. Pak. J. Biol. Sci. 2020, 23, 1131–1137. [Google Scholar] [CrossRef]
- Kim, S.A. 5-HT1A and 5-HT2A Signaling, Desensitization, and Downregulation: Serotonergic Dysfunction and Abnormal Receptor Density in Schizophrenia and the Prodrome. Cureus 2021, 13, e15811. [Google Scholar] [CrossRef] [PubMed]
- Govitrapong, P.; Chagkutip, J.; Turakitwanakan, W.; Srikiatkhachorn, A. Platelet 5-HT2A Receptors in Schizophrenic Patients with and without Neuroleptic Treatment. Psychiatry Res. 2000, 96, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Arranz, B.; Rosel, P.; Sarró, S.; Ramirez, N.; Dueñas, R.; Cano, R.; María Sanchez, J.; San, L. Altered Platelet Serotonin 5-HT2A Receptor Density but Not Second Messenger Inositol Trisphosphate Levels in Drug-Free Schizophrenic Patients. Psychiatry Res. 2003, 118, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Hu, C.; Jiang, T.; Luo, J.; Hu, J.; Ling, S.; Liu, M.; Xing, G. Overexpression of Serotonin Receptor and Transporter MRNA in Blood Leukocytes of Antipsychotic-Free and Antipsychotic-Naïve Schizophrenic Patients: Gender Differences. Schizophr. Res. 2010, 121, 160–171. [Google Scholar] [CrossRef]
- Nau, F.; Yu, B.; Martin, D.; Nichols, C.D. Serotonin 5-HT2A Receptor Activation Blocks TNF-α Mediated Inflammation In Vivo. PLoS ONE 2013, 8, e75426. [Google Scholar] [CrossRef]
- Inoue, M.; Okazaki, T.; Kitazono, T.; Mizushima, M.; Omata, M.; Ozaki, S. Regulation of Antigen-Specific CTL and Th1 Cell Activation through 5-Hydroxytryptamine 2A Receptor. Int. Immunopharmacol. 2011, 11, 67–73. [Google Scholar] [CrossRef]
- Galling, B.; Vernon, J.A.; Pagsberg, A.K.; Wadhwa, A.; Grudnikoff, E.; Seidman, A.J.; Tsoy-Podosenin, M.; Poyurovsky, M.; Kane, J.M.; Correll, C.U. Efficacy and Safety of Antidepressant Augmentation of Continued Antipsychotic Treatment in Patients with Schizophrenia. Acta Psychiatr. Scand. 2018, 137, 187–205. [Google Scholar] [CrossRef]
- Govitrapong, P.; Mukda, S.; Turakitwanakan, W.; Dumrongphol, H.; Chindaduangratn, C.; Sanvarinda, Y. Platelet Serotonin Transporter in Schizophrenic Patients with and without Neuroleptic Treatment. Neurochem. Int. 2002, 41, 209–216. [Google Scholar] [CrossRef]
- Barkan, T.; Peled, A.; Modai, I.; Barak, P.; Weizman, A.; Rehavi, M. Serotonin Transporter Characteristics in Lymphocytes and Platelets of Male Aggressive Schizophrenia Patients Compared to Non-Aggressive Schizophrenia Patients. Eur. Neuropsychopharmacol. 2006, 16, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; He, Y.; Han, H.; Zhou, Y.; Ma, X.; Wang, D.; Zhou, J.; Ren, H.; Yuan, L.; Tang, J.; et al. COMT, 5-HTR2A, and SLC6A4 MRNA Expressions in First-Episode Antipsychotic-Naïve Schizophrenia and Association with Treatment Outcomes. Front. Psychiatry 2018, 9, 577. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Doe, J.M.; Horstmann, S.A.; Ahmad, S.; Ahmad, A.; Min, S.-J.; Reynolds, P.R.; Suram, S.; Gaydos, J.; Burnham, E.L.; et al. Neuroendocrine Signaling via the Serotonin Transporter Regulates Clearance of Apoptotic Cells. J. Biol. Chem. 2014, 289, 10466–10475. [Google Scholar] [CrossRef] [PubMed]
- Mercado, C.P.; Kilic, F. Molecular Mechanisms of SERT in Platelets: Regulation of Plasma Serotonin Levels. Mol. Interv. 2010, 10, 231–241. [Google Scholar] [CrossRef]
- D’Ascola, A.; Bruschetta, G.; Zanghì, G.; Campo, S.; Medica, P.; Campana, S.; Ferlazzo, G.; Gibbs, B.F.; Ferlazzo, A.M. Changes in Plasma 5-HT Levels and Equine Leukocyte SERT Expression in Response to Treadmill Exercise. Res. Vet. Sci. 2018, 118, 184–190. [Google Scholar] [CrossRef]
- Brenner, B.; Harney, J.T.; Ahmed, B.A.; Jeffus, B.C.; Unal, R.; Mehta, J.L.; Kilic, F. Plasma Serotonin Levels and the Platelet Serotonin Transporter. J. Neurochem. 2007, 102, 206–215. [Google Scholar] [CrossRef]
- Ertugrul, A.; Ucar, G.; Basar, K.; Demir, B.; Yabanoglu, S.; Ulug, B. Influence of Clozapine on Platelet Serotonin, Monoamine Oxidase and Plasma Serotonin Levels. Psychiatry Res. 2007, 149, 49–57. [Google Scholar] [CrossRef]
- McCutcheon, R.A.; Abi-Dargham, A.; Howes, O.D. Schizophrenia, Dopamine and the Striatum: From Biology to Symptoms. Trends Neurosci. 2019, 42, 205–220. [Google Scholar] [CrossRef]
- Lau, C.-I.; Wang, H.-C.; Hsu, J.-L.; Liu, M.-E. Does the Dopamine Hypothesis Explain Schizophrenia? Rev. Neurosci. 2013, 24, 389–400. [Google Scholar] [CrossRef]
- Kahn, R.S.; Sommer, I.E.; Murray, R.M.; Meyer-Lindenberg, A.; Weinberger, D.R.; Cannon, T.D.; O’Donovan, M.; Correll, C.U.; Kane, J.M.; van Os, J.; et al. Schizophrenia. Nat. Rev. Dis. Primers 2015, 1, 15067. [Google Scholar] [CrossRef]
- Kirkpatrick, B.; Miller, B.J. Inflammation and Schizophrenia. Schizophr. Bull. 2013, 39, 1174–1179. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.J.; Goldsmith, D.R. Inflammatory Biomarkers in Schizophrenia: Implications for Heterogeneity and Neurobiology. Biomark. Neuropsychiatry 2019, 1, 100006. [Google Scholar] [CrossRef]
- Haroon, E.; Raison, C.L.; Miller, A.H. Psychoneuroimmunology Meets Neuropsychopharmacology: Translational Implications of the Impact of Inflammation on Behavior. Neuropsychopharmacology 2012, 37, 137–162. [Google Scholar] [CrossRef] [PubMed]
- Patlola, S.R.; Donohoe, G.; McKernan, D.P. Anti-Inflammatory Effects of 2nd Generation Antipsychotics in Patients with Schizophrenia: A Systematic Review and Meta-Analysis. J. Psychiatr. Res. 2023, 160, 126–136. [Google Scholar] [CrossRef]
- Fresán, A.; De la Fuente-Sandoval, C.; Loyzaga, C.; García-Anaya, M.; Meyenberg, N.; Nicolini, H.; Apiquian, R. A Forced Five-Dimensional Factor Analysis and Concurrent Validity of the Positive and Negative Syndrome Scale in Mexican Schizophrenic Patients. Schizophr. Res. 2005, 72, 123–129. [Google Scholar] [CrossRef]
- Akoglu, H. User’s Guide to Correlation Coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]

| Parameter | HV | DN | DF | R | F or H Value | p |
|---|---|---|---|---|---|---|
| n | 10 | 11 | 17 | 28 | - | - |
| Age b | 27.30 ± 4.71 | 33.27 ± 13.86 | 38.24 ± 12.15 | 28.00 ± 8.74 | H (3) = 7.751 | 0.0514 |
| BMI a | 24.02 ± 2.58 | 26.22 ± 3.22 | 25.44 ± 3.31 | 25.73 ± 3.18 | F (3, 62) = 0.982 | 0.4069 |
| Gender female: male | 4: 6 | 4: 7 | 6: 11 | 9: 19 | - | - |
| Smoke yes: no | 1: 9 | 4: 7 | 7: 10 | 10: 18 | - | - |
| PANSS total and subscale scores | ||||||
| TOTAL a | - | 85.73 ± 20.30 | 95.47 ± 19.45 | 73.36 ± 17.46 | F (2, 53) = 7.652 | 0.0012 |
| Positive subscale a | - | 29.18 ± 6.60 | 30.94 ± 7.80 | 18.75 ±7.30 | F (2, 53) = 17.410 | <0.0001 |
| Negative subscale a | - | 21.55 ± 9.79 | 24.00 ± 6.48 | 22.04 ±7.57 | F (2, 53) = 0.452 | 0.6388 |
| Cognitive subscale a | - | 17.27 ± 4.92 | 19.94 ± 4.02 | 16.46 ± 4.49 | F (2, 53) = 0.077 | 0.9254 |
| Excitability subscale b | - | 8.09 ± 3.98 | 9.52 ± 3.31 | 6.75 ± 3.26 | H (2) = 6.791 | 0.0335 |
| Depression subscale a | - | 9.56 ± 4.56 | 11.06 ± 3.45 | 9.35 ± 3.64 | F (2, 53) = 1.112 | 0.3365 |
| Receptor or Transporter | D3 | D5 | DAT | 5-HT2A | SERT | |
|---|---|---|---|---|---|---|
| Scale/ Subscale Score | ||||||
| PANSS total | rs = −0.1501 p = 0.2785 | rs = −0.1611 p = 0.2447 | r = −0.1352 p = 0.3297 | r = 0.5801 p < 0.0001 | rs = 0.5199 p < 0.0001 | |
| Positive | r = −0.1306 p = 0.2465 | rs = −0.1258 p = 0.3647 | rs = 0.0010 p = 0.9939 | r = 0.5608 p < 0.0001 | rs = 0.5698 p < 0.0001 | |
| Negative | r = −0.0200 p = 0.8858 | rs = −0.0533 p = 0.7178 | rs = −0.1353 p = 0.3293 | r = 0.2426 p = 0.0772 | rs = −0.0409 p = 0.7689 | |
| Cognitive | r = −0.2433 p = 0.0763 | rs = −0.1106 p = 0.4258 | r = −0.1581 p = 0.2534 | r = 0.0364 p = 0.0073 | rs = 0.2578 p = 0.0598 | |
| Excitability | rs = −0.0763 p = 0.5833 | rs = −0.1037 p = 0.4554 | rs = −0.2180 p = 0.1133 | rs = 0.2346 p = 0.0878 | rs = 0.2057 p = 0.1356 | |
| Depression | rs = −0.1877 p = 0.1742 | rs = −0.1659 p = 0.2306 | rs = −0.0332 p = 0.8113 | rs = 0.1680 p = 0.2245 | rs = 0.3679 p = 0.0067 | |
| Receptor or Transporter | Family | Action Mechanism | Function | Distribution in CNS | Distribution in Leukocytes | Examples of Reported Alterations in PWS |
|---|---|---|---|---|---|---|
| D3 | D2-like subfamily [28]. | It is coupled to the Gai/o protein. It inhibits AC, blunts cAMP formation, and PKA activation [28]. | It modulates rewarding and motivating behavior, some features of cognitive functions, and locomotor activity [29]. | The ventral striatum (nucleus accumbens), thalamus, hippocampus, islands of Calleja, cerebellum, cortex, substantia nigra, and VTA [30]. | T and B lymphocytes, dendritic cells, neutrophils, monocytes, and NK cells [13]. | The increase in D3 levels in the rostral and caudal basal ganglia structures of drug-free patients. The decrease in the expression in the parietal and motor cortices of medicated chronic patients [31,32]. |
| D5 | D1-like subfamily [33]. | Coupled to Ga2/Golf protein that initiates the cAMP/PKA signaling via AC activation [33]. | Involves in working memory, visual attention, and recency memory [34]. | Cortical, subcortical, and limbic regions such as the cerebral cortex. Hippocampus, substantia nigra pars compacta, hypothalamus, striatum, nucleus accumbens, cerebellum, and olfactory tubercule [35]. | Dendritic cells, T and B lymphocytes, eosinophils, neutrophils, NK cells, and platelets [13,36]. | Reduction in D1-like receptors in PFC of drug-naïve and drug-free patients [7]. A decreased density in basal ganglia of drug-naïve patients [37]. |
| DAT | SLC6 class of NA+/Cl− dependent transporters [38]. | It reuptakes DA from the synaptic cleft back inside the presynaptic neuron for a rerelease, limiting DA effects [39]. | The principal regulator of DA neurotransmitter synaptic availability in mammals [39]. | DA neurons—high density in the striatum and nucleus accumbens-, anterior cingulate, amygdala, midbrain, and frontal areas [40]. | B and T lymphocytes, macrophages, and platelets [13]. | No DAT expression changes in the striatum of non-medicated patients [41]. A decreased DAT expression in the striatum in naïve patients [42]. A decreased expression in the striatum [43], amygdala [44], and an increased expression in basal ganglia [45] and Brodmann area 9 [43] in drug-treated patients. |
| 5-TH2A | Serotonin receptor family [46]. | It is involved in the regulation of the mood, and acts as a cognitive process modulator, such as memory and learning [47]. | It is the primary excitatory 5-HT receptor in the brain [46]. | High density: the dendrites of layer V cortical pyramidal glutamatergic cells [46]. Intermediate density: hippocampus, basal ganglia, thalamus, putamen, and nucleus accumbens [48]. Minimal density: cerebellum and brain stem [48]. | B and T lymphocytes, monocytes and macrophages, dendritic cells, eosinophils, and platelets [12]. | A decrease in 5-HT2A receptors in the caudate nucleus [6] and PFC [49] of drug-naïve patients and individuals with an elevated risk. Increased 5-HT2A binding sites in PFC in antipsychotic free patients but no in medicated patients [50]. Medicated patients with decreased density in the superior temporal cortex [51], BA9 [52], superior temporal gyrus [53], and lower mRNA expression in the hippocampus [54]. |
| SERT | SLC6 class of NA+/Cl− dependent transporters [55]. | It transports 5-HT from the synaptic cleft to the presynaptic neuron [56]. | It regulates the duration and concentration of 5-HT in the synaptic cleft via a saturable reuptake mechanism [56]. | Serotonergic neurons that project from raphe nuclei of the pons and upper brain stem to the hypothalamus, thalamus, amygdala, striatum, and cortical mantle [57,58]. | Platelets, T and B lymphocytes, dendritic cells, mast cells, and monocytes [12]. | The increase in SERT density in the caudate nucleus, nucleus accumbens, dorsal putamen [59], frontal cortex (BA9), and a decreased density in the temporal cortex (BA21) [60] in patients. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez-Herrera, S.; Rosel Vales, M.; Pérez-Sánchez, G.; Becerril-Villanueva, E.; Flores-Medina, Y.; Maldonado-García, J.L.; Saracco-Alvarez, R.; Escamilla, R.; Pavón, L. Risperidone Decreases Expression of Serotonin Receptor-2A (5-HT2A) and Serotonin Transporter (SERT) but Not Dopamine Receptors and Dopamine Transporter (DAT) in PBMCs from Patients with Schizophrenia. Pharmaceuticals 2024, 17, 167. https://doi.org/10.3390/ph17020167
Alvarez-Herrera S, Rosel Vales M, Pérez-Sánchez G, Becerril-Villanueva E, Flores-Medina Y, Maldonado-García JL, Saracco-Alvarez R, Escamilla R, Pavón L. Risperidone Decreases Expression of Serotonin Receptor-2A (5-HT2A) and Serotonin Transporter (SERT) but Not Dopamine Receptors and Dopamine Transporter (DAT) in PBMCs from Patients with Schizophrenia. Pharmaceuticals. 2024; 17(2):167. https://doi.org/10.3390/ph17020167
Chicago/Turabian StyleAlvarez-Herrera, Samantha, Mauricio Rosel Vales, Gilberto Pérez-Sánchez, Enrique Becerril-Villanueva, Yvonne Flores-Medina, José Luis Maldonado-García, Ricardo Saracco-Alvarez, Raúl Escamilla, and Lenin Pavón. 2024. "Risperidone Decreases Expression of Serotonin Receptor-2A (5-HT2A) and Serotonin Transporter (SERT) but Not Dopamine Receptors and Dopamine Transporter (DAT) in PBMCs from Patients with Schizophrenia" Pharmaceuticals 17, no. 2: 167. https://doi.org/10.3390/ph17020167
APA StyleAlvarez-Herrera, S., Rosel Vales, M., Pérez-Sánchez, G., Becerril-Villanueva, E., Flores-Medina, Y., Maldonado-García, J. L., Saracco-Alvarez, R., Escamilla, R., & Pavón, L. (2024). Risperidone Decreases Expression of Serotonin Receptor-2A (5-HT2A) and Serotonin Transporter (SERT) but Not Dopamine Receptors and Dopamine Transporter (DAT) in PBMCs from Patients with Schizophrenia. Pharmaceuticals, 17(2), 167. https://doi.org/10.3390/ph17020167