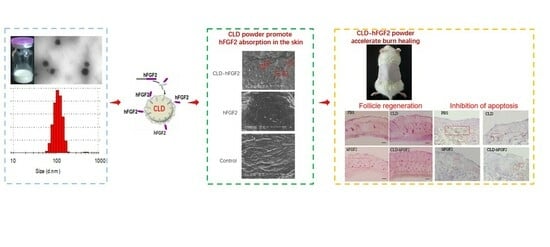
Freeze-Dried Camelina Lipid Droplets Loaded with Human Basic Fibroblast Growth Factor-2 Formulation for Transdermal Delivery: Breaking through the Cuticle Barrier to Accelerate Deep Second-Degree Burn Healing
Abstract
:1. Introduction
2. Results
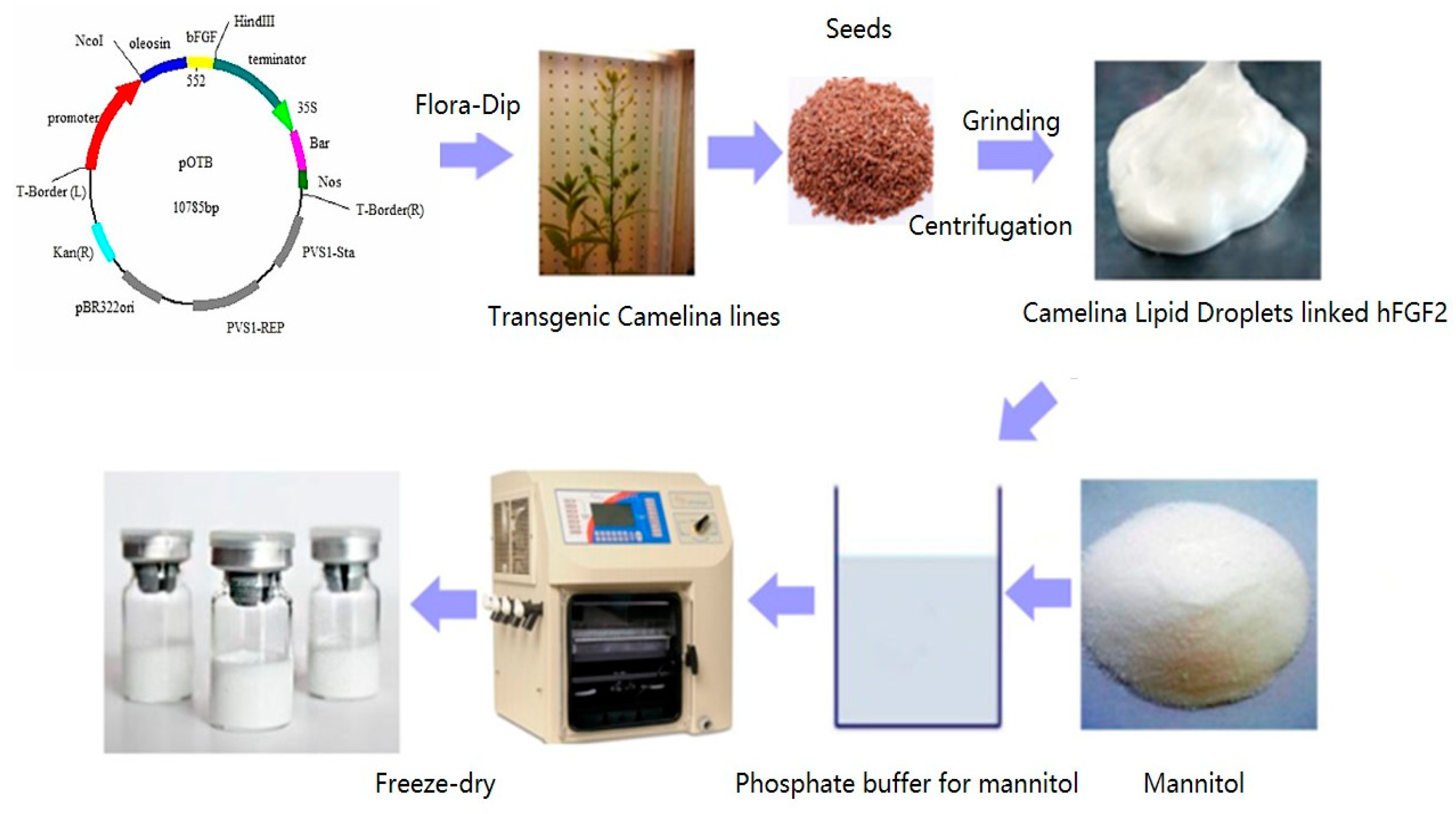
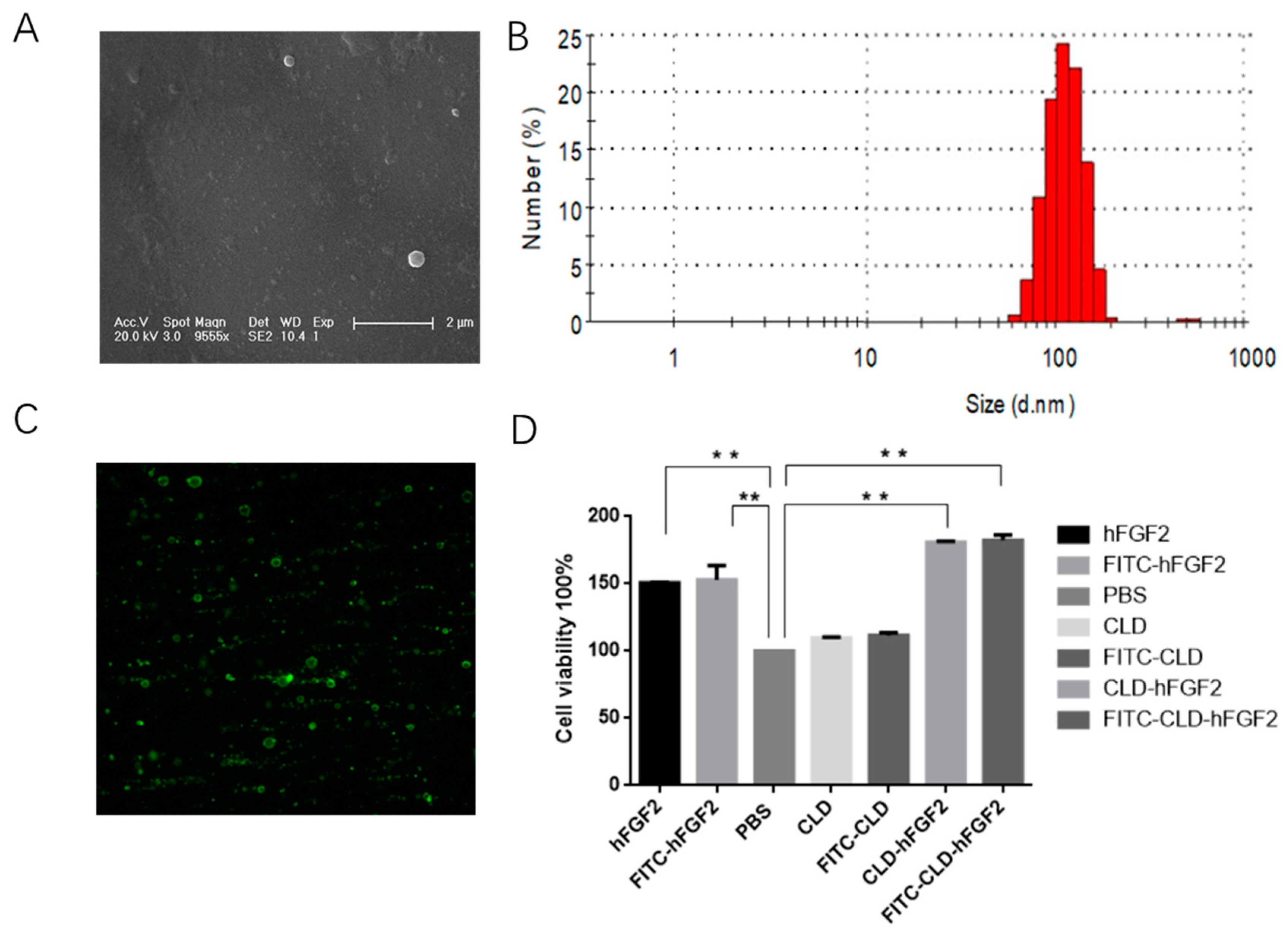
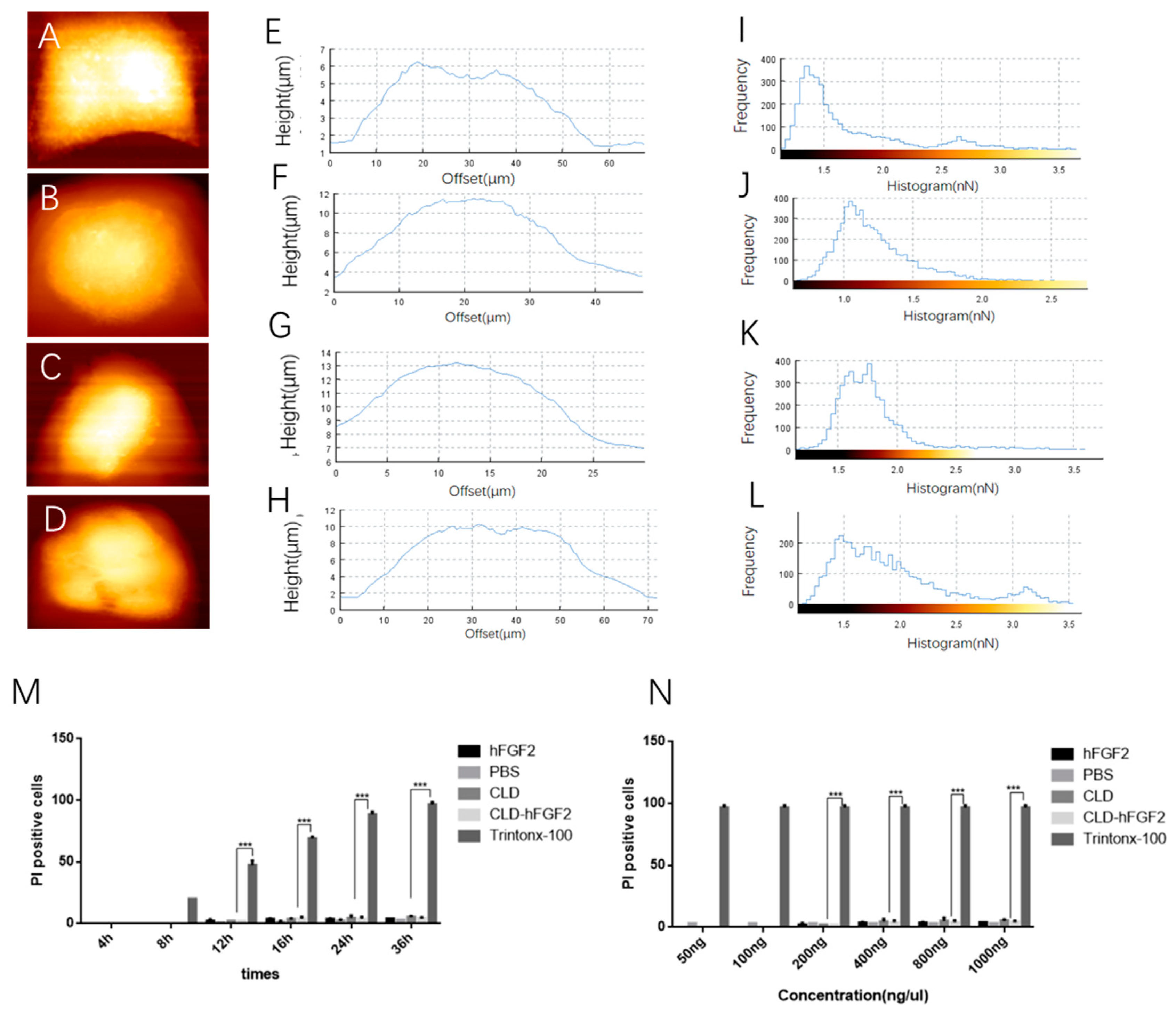
2.1. Characterization of CLD-hFGF2 Freeze-Dried Powder
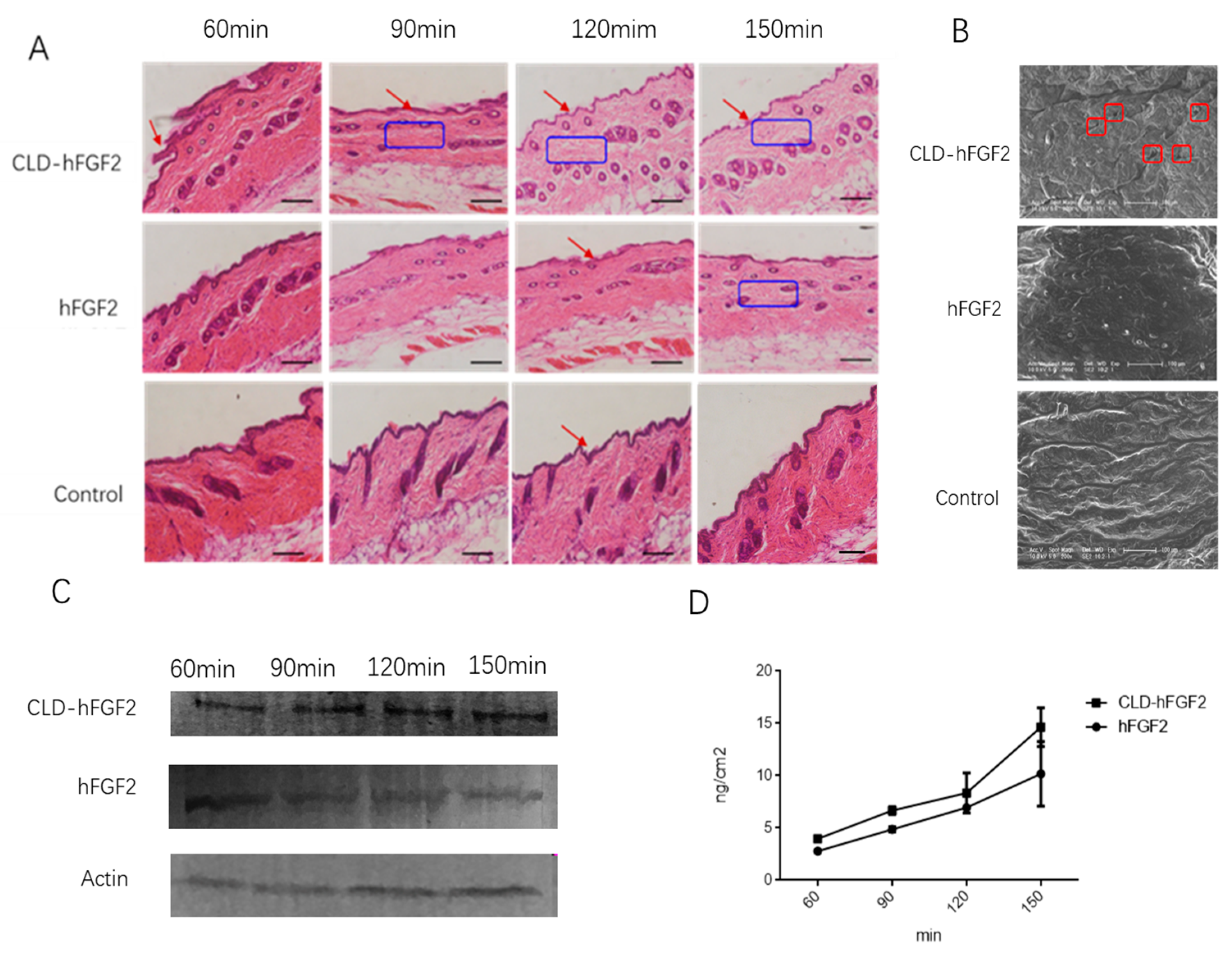
2.2. In Vitro Percutaneous Permeation for CLD-hFGF2 Freeze-Dried Powder
2.3. CLD-hFGF2 Freeze-Dried Powder Changes the Structure of the Stratum Corneum
2.4. CLD-hFGF2 Freeze-Dried Powder Changes HaCat Cell Morphology
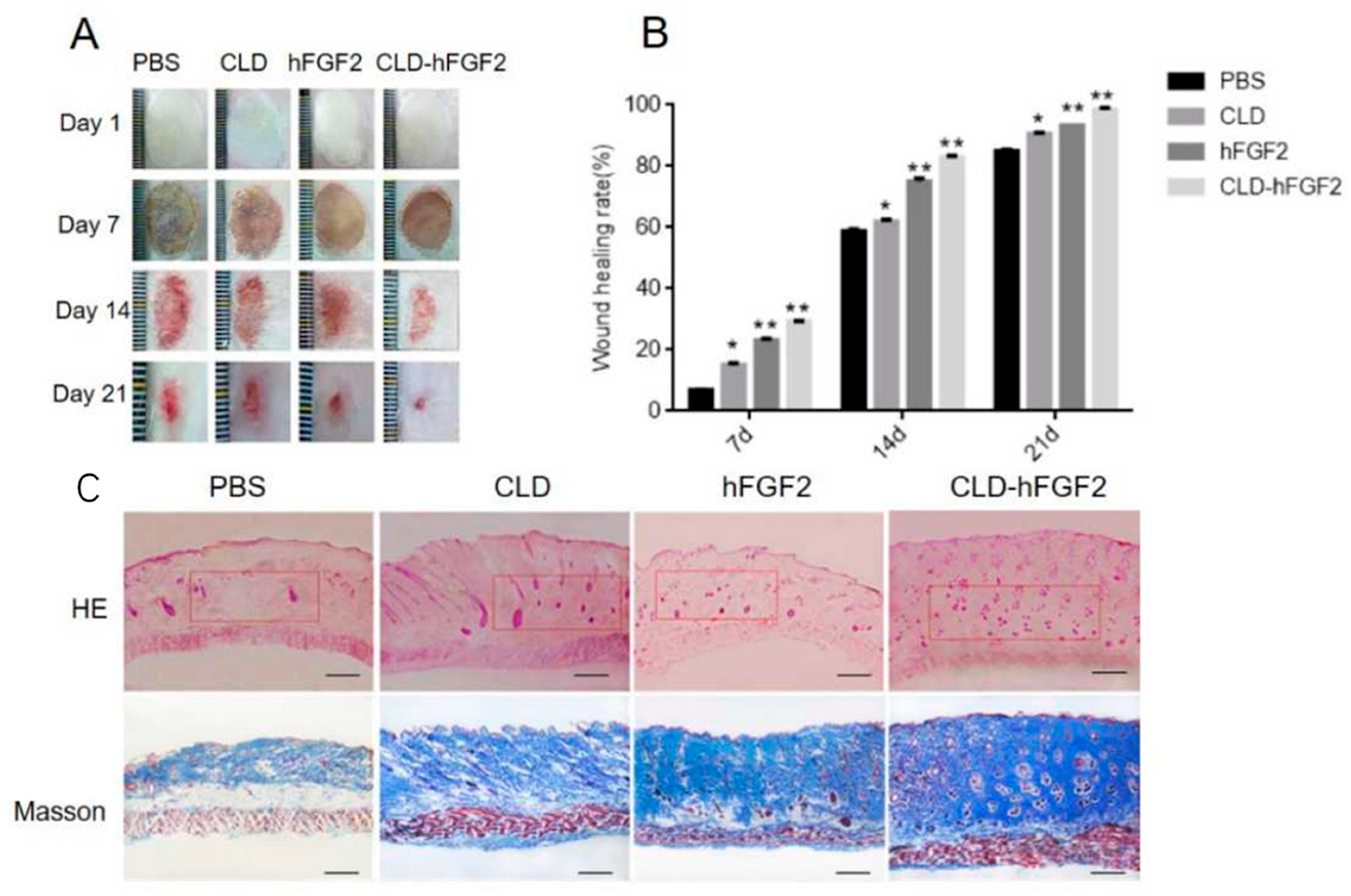
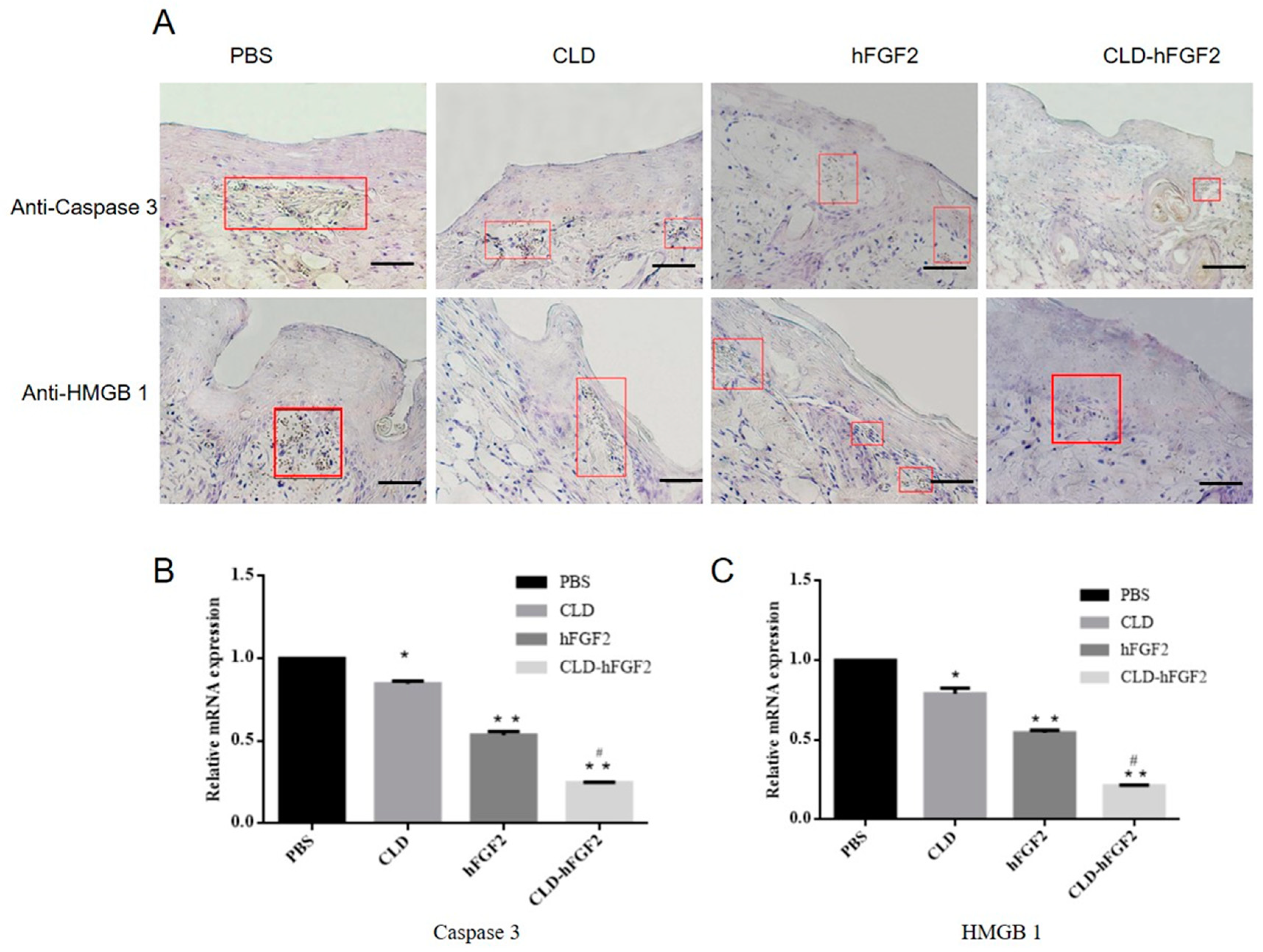
2.5. CLD-hFGF2 Freeze-Dried Powder Enhances Deep Second-Degree Burn Wound Closure in Rats
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation and Characterization of CLD-hFGF2 Freeze-Dried Powder
4.3. Permeation Assessment of CLD-hFGF2 Freeze-Dried Powder In Vitro
4.4. Histopathological Analysis of the Skin Cuticle Structure
4.5. Analysis of Skin Cuticle Thermotropic Properties with Differential Scanning Calorimetry (DSC)
4.6. Analysis of the Skin Cuticle Components Using Fourier Transform Infrared Spectroscopy (FTIR)
4.7. Atomic Force Microscope Assay
4.8. Deep Second-Degree Burn Wound Model and Treatment
4.9. Quantitative Real-Time PCR
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Homaeigohar, S.; Boccaccini, A.R. Antibacterial Biohybrid Nanofibers for Wound Dressings. Acta Biomater. 2020, 107, 25–49. [Google Scholar] [CrossRef]
- Kaur, R.; Sharma, N.; Tikoo, K.; Sinha, V.R. Development of mirtazapine loaded solid lipid nanoparticles for topical delivery: Optimization, characterization and cytotoxicity evaluation. Int. J. Pharm. 2020, 586, 119439. [Google Scholar] [CrossRef]
- Watkinson, A.C.; Kearney, M.C.; Quinn, H.L.; Courtenay, A.J.; Donnelly, R.F. Future of the transdermal drug delivery market have we barely touched the surface. Expert Opin. Drug Deliv. 2016, 13, 523–532. [Google Scholar] [CrossRef]
- Karande, P.; Jain, A.; Mitragotri, S. Insights into synergistic interactions in binary mixtures of chemical permeation enhancers for transdermal drug delivery. J. Control Release 2006, 115, 85–93. [Google Scholar] [CrossRef]
- Milewski, M.; Yerramreddy, T.R.; Ghosh, P.; Crooks, P.A.; Stinchcomb, A. In vitro permeation of a pegylated naltrexone prodrug across microneedle-treated skin. J. Control Release 2010, 146, 37–44. [Google Scholar] [CrossRef]
- Wu, X.M.; Todo, H.; Sugibayashi, K. Enhancement of skin permeation of high molecular compounds by a combination of microneedle pretreatment and iontophoresis. J. Control Release 2007, 118, 189–195. [Google Scholar] [CrossRef]
- Polat, B.E.; Hart, D.; Langer, R.; Blankschtein, D. Ultrasound-mediated transdermal drug delivery: Mechanisms, scope, and emerging trends. J. Control Release 2011, 152, 330–348. [Google Scholar] [CrossRef]
- Sammeta, S.M.; Vaka, S.R.; Murthy, S. Transcutaneous electroporation mediated delivery of doxepin-HPCD complex: A sustained release approach for treatment of postherpetic neuralgia. J. Control Release 2010, 142, 361–367. [Google Scholar] [CrossRef]
- Cevc, G.; Vierl, U. Nanotechnology and the transdermal route: A state of the art review and critical appraisal. J. Control Release 2010, 141, 277–299. [Google Scholar] [CrossRef]
- Gao, H.; Wang, F.; Hu, X.; Li, Y.; Zhang, Y.; Carther, K.F.I.; Wang, B.; Min, F.; Wang, X.; Wu, H.; et al. Cameline Lipid Droplets as skin delivery system promotes wound repair by enhancing the absorption of hFGF2. Int. J. Pharm. 2021, 598, 120327. [Google Scholar] [CrossRef]
- Qiang, W.; Zhou, T.; Lan, X.; Zhang, X.; Guo, Y.; Noman, M.; Du, L.; Zheng, J.; Li, W.; Li, H.; et al. A new nanoscale transdermal drug delivery system: Oil body-linked oleosin-hEGF improves skin regeneration to accelerate wound healing. J. Nanobiotechnology 2018, 16, 62. [Google Scholar] [CrossRef]
- Horn, P.J.; James, C.N.; Gidda, S.K.; Kilaru, A.; Dyer, J.M.; Mullen, R.T.; Ohlrogge, J.B.; Chapman, K.D. Identification of a new class of lipid droplet-associated proteins in plants. Plant Physiol. 2013, 162, 1926–1936. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mupondwa, E. Life cycle assessment of camelina oil derived biodiesel and jet fuel in the Canadian Prairies. Sci. Total Environ. 2014, 481, 17–26. [Google Scholar] [CrossRef]
- Yang, J.; Guan, L.; Guo, Y.; Du, L.; Wang, F.; Wang, Y.; Zhen, L.; Wang, Q.; Zou, D.; Chen, W.; et al. Expression of biologically recombinant human acidic fibroblast growth factor in Arabidopsis thaliana seeds via oleosin fusion technology. Gene 2015, 566, 89–94. [Google Scholar] [CrossRef]
- Shah, P.P.; Desai, P.R.; Singh, M. Effect of oleic acid modified polymeric bilayered nanoparticles on percutaneous delivery of spantide II and ketoprofen. J. Control Release 2012, 158, 336–345. [Google Scholar] [CrossRef]
- Tanojo, H.; Bos-van Geest, A.; Bouwstra, J.A.; Junginger, H.E.; Boodé, H.E. In vitro human skin barrier perturbation by oleic acid: Thermal analysis and freeze fracture electron microscopy studies. Thermochim. Acta 1997, 293, 77–85. [Google Scholar] [CrossRef]
- Mondor, M.; Hernández-Lvarez, A.J. Camelina Sativa Composition, Attributes, and Applications: A Review. Eur. J. Lipid Sci. Technol. 2021, 124, 2100035. [Google Scholar] [CrossRef]
- Zanetti, F.; Alberghini, B.; Jeromela, A.M.; Grahovac, N.; Rajković, D.; Kiprovski, B.; Monti, A. Camelina, an ancient oilseed crop actively contributing to the rural renaissance in Europe. A review. Agron. Sustain. Dev. 2021, 41, 2. [Google Scholar] [CrossRef]
- Kim, N.; Li, Y.; Sun, X.S. Epoxidation of Camelina sativa oil and peel adhesion properties. Ind. Crops Prod. 2015, 64, 1–8. [Google Scholar] [CrossRef]
- Waraich, E.A.; Ahmed, Z.; Ahmad, R.; Ashraf, M.; Naeem, M.S.; Rengel, Z. Camelina sativa, a climate proof crop, has high nutritive value and multiple-uses: A review. Aust. J. Crop Sci. 2013, 7, 1551–1559. [Google Scholar]
- Righini, D.; Zanetti, F.; Martinez-Force, E.; Mandrioli, M.; Toschi, T.G.; Monti, A. Shifting sowing of camelina from spring to autumn enhances the oil quality for bio-based applications in response to temperature and seed carbon stock. Ind. Crops Prod. 2019, 137, 66–73. [Google Scholar] [CrossRef]
- Righini, D.; Zanetti, F.; Monti, A. The bio-based economy can serve as the springboard for camelina and crambe to quit the limbo. OCL 2016, 23, D504. [Google Scholar] [CrossRef]
- Zhao, Y.Z.; Tian, X.Q.; Zhang, M.; Cai, L.; Ru, A.; Shen, X.-T.; Jiang, X.; Jin, R.-R.; Zheng, L.; Hawkins, K.; et al. Functional and pathological improvements of the hearts in diabetes model by the combined therapy of bFGF-loaded nanoparticles with ultrasound-targeted microbubble destruction. J. Control Release 2014, 186, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Alemdaroğlu, C.; Degim, Z.; Celebi, N.; Şengezer, M.; Alömeroglu, M.; Nacar, A. Investigation of epidermal growth factor containing liposome formulation effects on burn wound healing. J. Biomed. Mater Res. A 2008, 85, 271–283. [Google Scholar] [CrossRef]
- Bartolini, B.; Caravà, E.; Caon, I.; Parnigoni, A. Heparan Sulfate in the Tumor Micro-environment. Adv. Exp. Med. Biol. 2020, 1245, 147–161. [Google Scholar] [PubMed]
- Van Rooijen, G.J.H.; Moloney, M.M. Plant seed oil-bodies as carriers for foreign proteins. Bio-Technology 1995, 13, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Deckers, H.; Moloney, M.M.; Baum, A. The case for recombinant production of pharmaceutical proteins in plants. Annu. Rep. Med. Chem. 1999, 34, 237–245. [Google Scholar]
- Markley, N.; Nykiforuk, C.; Boothe, J.; Moloney, M. Producing proteins using transgenic oilbody oleosin technology. BioPharm. Int. 2006, 19, 34–47. [Google Scholar]
- Boothe, J.; Nykiforuk, C.; Shen, Y.; Zaplachinski, S.; Szarka, S.; Kuhlman, P.; Murray, E.; Morck, D.; Moloney, M.M. Seed-based expression systems for plant molecular farming. Plant Biotechnol. J. 2010, 8, 588–606. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, T.; Chen, F.; Wei, W.; Liu, C.; He, S.; Li, X. Electrospun Fibers with Plasmid hFGF2 Polyplex Loadings Promote Skin Wound Healing in Diabetic Rats. Mol. Pharm. 2012, 9, 48–58. [Google Scholar] [CrossRef]
- Cotte, M.; Dumas, P.; Besnard, M.; Tchoreloff, P.; Walter, P. Synchrotron FT-IR microscopic study of chemical enhancers in transdermal drug delivery: Example of fatty acids. J. Control Release 2004, 97, 269–281. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, S.K.; Handa, V.; Kathuria, H. Oleic Acid Nanovesicles of Minoxidil for Enhanced Follicular Delivery. Medicines 2018, 3, 103. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Yang, M.; Tang, X.; Wang, T.; Yang, D.; Zhai, G.; Liu, J. Lipid nanoparticles loading triptolide for transdermal delivery: Mechanisms of penetration enhancement and transport properties. J. Nanobiotechnology 2018, 16, 68. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, F.; Baboota, S.; Ahuja, A.; Ali, J.; Shafiq, S. Skin permeation mechanism and bioavailability enhancement of celecoxib from transdermally applied nanoemulsion. J. Nanobiotechnology 2008, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Poljšak, N.; Kreft, S.; Kočevar Glavač, N. Vegetable butters and oils in skin wound healing: Scientific evidence for new opportunities in dermatology. Phytother. Res. 2020, 34, 254–269. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Gu, Y.; Yang, D.; Tang, X.; Liu, J. Development of triptolide-nanoemulsion gels for percutaneous administration: Physicochemical, transport, pharmacokinetic and pharmacodynamic characteristics. J. Nanobiotechnology 2017, 15, 88. [Google Scholar] [CrossRef]
- Maione, F.; Russo, R.; Khan, H.; Mascolo, N. Medicinal plants with anti-inflammatory activities. Nat. Prod. Res. 2016, 30, 1343–1352. [Google Scholar] [CrossRef]
- Chu, C.; Nyam, K. Kenaf (Hibiscus cannabinus L.) seed oil: Application as cosmetic product ingredients. Ind. Crops Prod. 2020, 156, 112871. [Google Scholar] [CrossRef]
- Saribekova, D.; Kunik, O.; Harhaun, R.; Saleba, L.; Cavallaro, G. The use of silicones as extractants of biologically active substances from vegetable raw materials. Appl. Sci. 2021, 11, 10625. [Google Scholar] [CrossRef]
- Mahbub, K.; Octaviani, I.; Astuti, I.; Sisunandar, S.; Dhiani, B. Oil from kopyor coconut (Cocos nucifera var. Kopyor) for cosmetic application. Ind. Crops Prod. 2022, 186, 115221. [Google Scholar] [CrossRef]
- Sala, M.; Diab, R.; Elaissari, A.; Fessi, H. Lipid nanocarriers as skin drug delivery systems: Properties, mechanisms of skin interactions and medical applications. Int. J. Pharm. 2018, 535, 1–17. [Google Scholar] [CrossRef]
- Kunik, O.; Saribekova, D.; Lazzara, G.; Cavallaro, G. Emulsions based on fatty acid from vegetable oils for cosmetics. Ind. Crops Prod. 2022, 189, 115776. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhao, L.; Wang, Z.; Zhai, G. RETRACTED ARTICLE: Preparation and characterization of novel lipid nanocapsules of ropivacaine for transdermal delivery. Drug Deliv. 2014, 23, 619–628. [Google Scholar] [CrossRef]
- Staiger, K.; Staiger, H.; Weigert, C.; Haas, C.; Häring, H.U.; Kellerer, M. Saturated, but not unsaturated, fatty acids induce apoptosis of human coronary artery endothelial cells via nuclear factor-kappaB activation. Diabetes 2006, 55, 3121–3126. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Yang, X.; Zhao, L.; Wang, Z.; Zhai, G. Lipid nanocapsules for transdermal delivery of ropivacaine: In vitro and in vivo evaluation. Int. J. Pharm. 2014, 471, 103–111. [Google Scholar] [CrossRef]
- Kumar, M.; Sharma, G.; Singla, D.; Singh, S.; Sahwney, S.; Chauhan, A.S.; Singh, G.; Kaur, I.P. Development of a validated UPLC method for simultaneous estimation of both free and entrapped (in solid lipid nanoparticles) all-trans retinoic acid and cholecalciferol (vitamin D3) and its pharmacokinetic applicability in rats. J. Pharm. Biomed. Anal. 2014, 91, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Puglia, C.; Offerta, A.; Tirendi, G.G.; Tarico, M.S.; Curreri, S.; Bonina, F.; Perrotta, R.E. Design of solid lipid nanoparticles for caffeine topical administration. Drug Deliv. 2016, 23, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wen, X.S.; Di, W. In vitro and in vivo evaluation of Triptolide-loaded pluronic P105 polymeric micelles. Arzneimittelforschung 2012, 62, 340–344. [Google Scholar] [CrossRef]
- Jia, Y.; Liu, J.; Xu, J. Influence of grapefruit juice on pharmacokinetics of triptolide in rat’s grapefruit juice on the effects of triptolide. Xenobiotic 2018, 48, 407–411. [Google Scholar] [CrossRef]
- Anderson, J.V.; Wittenberg, A.; Li, H.; Berti, M. High throughput phenotyping of Camelina sativa seeds for crude protein, total oil, and fatty acids profile by near infrared spectroscopy. Ind. Crops Prod. 2019, 137, 501–507. [Google Scholar] [CrossRef]
- Fang, C.L.; Al-Suwayeh, S.A.; Fang, J.Y. Nanostructured lipid carriers (NLCs) for drug delivery and targeting. Recent Pat. Nanotechnol. 2013, 7, 41–55. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Wang, X.; Wu, H.; Zhang, Y.; Zhang, W.; Wang, Z.; Liu, X.; Li, X.; Li, H. Freeze-Dried Camelina Lipid Droplets Loaded with Human Basic Fibroblast Growth Factor-2 Formulation for Transdermal Delivery: Breaking through the Cuticle Barrier to Accelerate Deep Second-Degree Burn Healing. Pharmaceuticals 2023, 16, 1492. https://doi.org/10.3390/ph16101492
Gao H, Wang X, Wu H, Zhang Y, Zhang W, Wang Z, Liu X, Li X, Li H. Freeze-Dried Camelina Lipid Droplets Loaded with Human Basic Fibroblast Growth Factor-2 Formulation for Transdermal Delivery: Breaking through the Cuticle Barrier to Accelerate Deep Second-Degree Burn Healing. Pharmaceuticals. 2023; 16(10):1492. https://doi.org/10.3390/ph16101492
Chicago/Turabian StyleGao, Hongtao, Xue Wang, Hao Wu, Yuan Zhang, Wenxiao Zhang, Zuobin Wang, Xin Liu, Xiaokun Li, and Haiyan Li. 2023. "Freeze-Dried Camelina Lipid Droplets Loaded with Human Basic Fibroblast Growth Factor-2 Formulation for Transdermal Delivery: Breaking through the Cuticle Barrier to Accelerate Deep Second-Degree Burn Healing" Pharmaceuticals 16, no. 10: 1492. https://doi.org/10.3390/ph16101492
APA StyleGao, H., Wang, X., Wu, H., Zhang, Y., Zhang, W., Wang, Z., Liu, X., Li, X., & Li, H. (2023). Freeze-Dried Camelina Lipid Droplets Loaded with Human Basic Fibroblast Growth Factor-2 Formulation for Transdermal Delivery: Breaking through the Cuticle Barrier to Accelerate Deep Second-Degree Burn Healing. Pharmaceuticals, 16(10), 1492. https://doi.org/10.3390/ph16101492