Advances in Chitosan-Based Smart Hydrogels for Colorectal Cancer Treatment
Abstract
1. Introduction
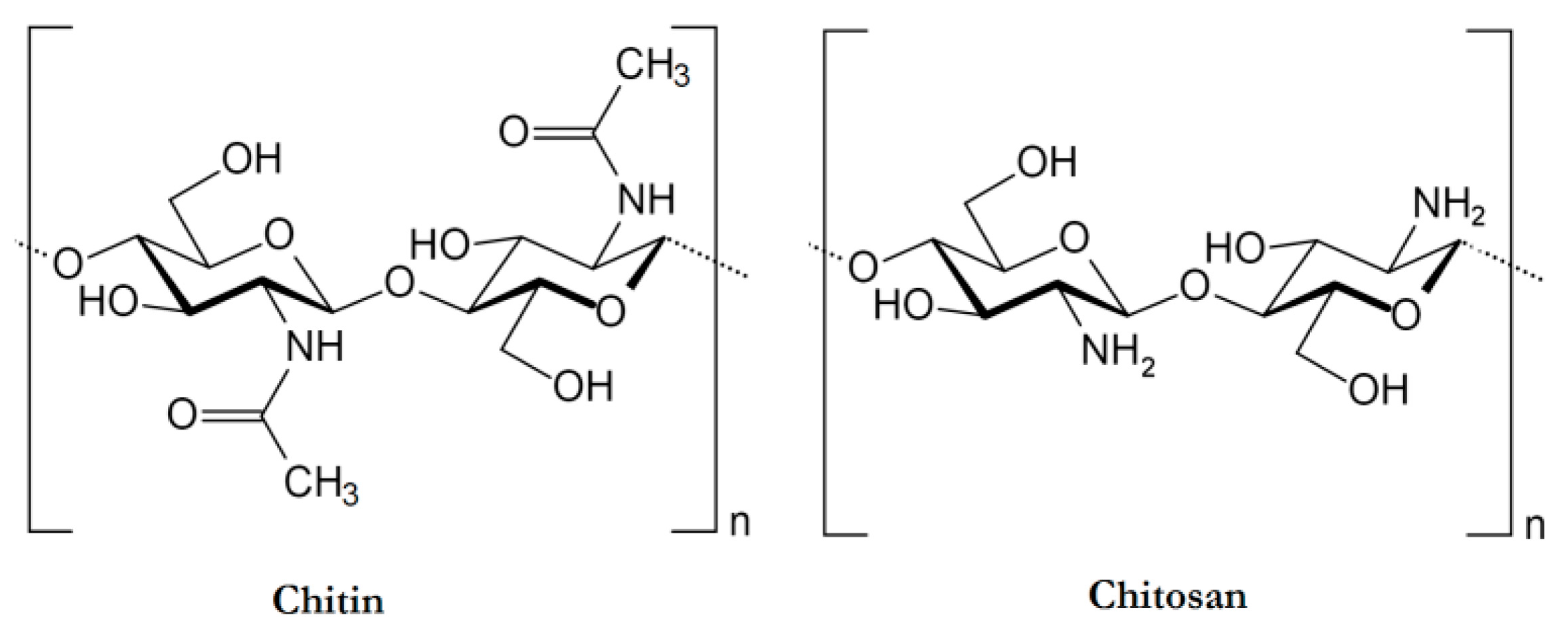
2. Physicochemical and Biological Properties of Chitosan
3. Preparation of Chitosan-Based Hydrogels
3.1. Chemical Cross-Linking
| Chemical Cross-Linking | Characteristics of the Hydrogels | Ref. |
|---|---|---|
| Schiff base reaction | High stability Self-adapting ability pH/swelling dependence | [61,67] |
| Diels–Alder reaction | Injectability Self-healing High mechanical qualities | [68] |
| Michael addition reaction | Good mechanical characteristics Structural stability Good in vivo degradability Thermal stability | [69] |
| Thiol-ene click chemistry | Improved mechanical strength Surface roughness Biocompatibility pH-sensitive, CS-based hydrogels | [70] |
| Photopolymerization | Biocompatibility (enhance cell adherence, proliferation, and differentiation) Biodegradability Wound healing Mechanically resilient Elastic hydrogel Controlled drug delivery | [71,72,73] |
| Graft copolymerization | Highly elastic hydrogels Bioscaffolds | [74] |
3.2. Physical Cross-Linking
| Physical Cross-Linking | Characteristics of the Hydrogels | Ref. |
|---|---|---|
| Ionic interaction | Stable network structure Enhanced mechanical strength and stability Control over pore morphology and surface properties of the hydrogel Low cytotoxicity | [75] |
| Hydrogen bonding interaction | Unique shapes and mechanical properties, including elasticity and the ability to bear pressure-induced deformation pH-sensitive, temperature-sensitive, and dual-responsiveness CS-based hydrogels | [76] |
| Hydrophobic interaction | Induce structural modifications, including changes in porosity and surface area | [77] |
| Electrostatic interaction | Decreased degree of swelling Increased viscoelasticity Injectable hydrogel | [78] |
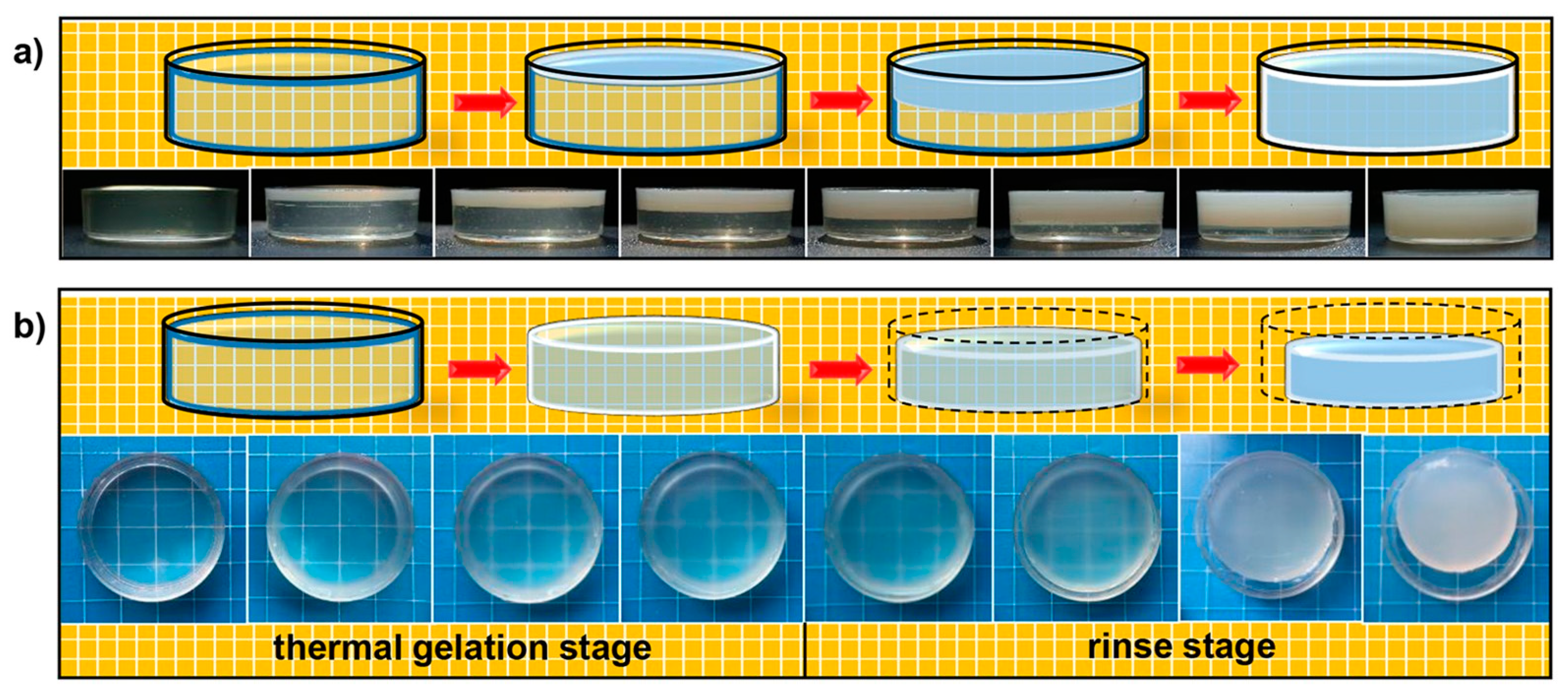
3.2.1. Cross-Linking in Different pH
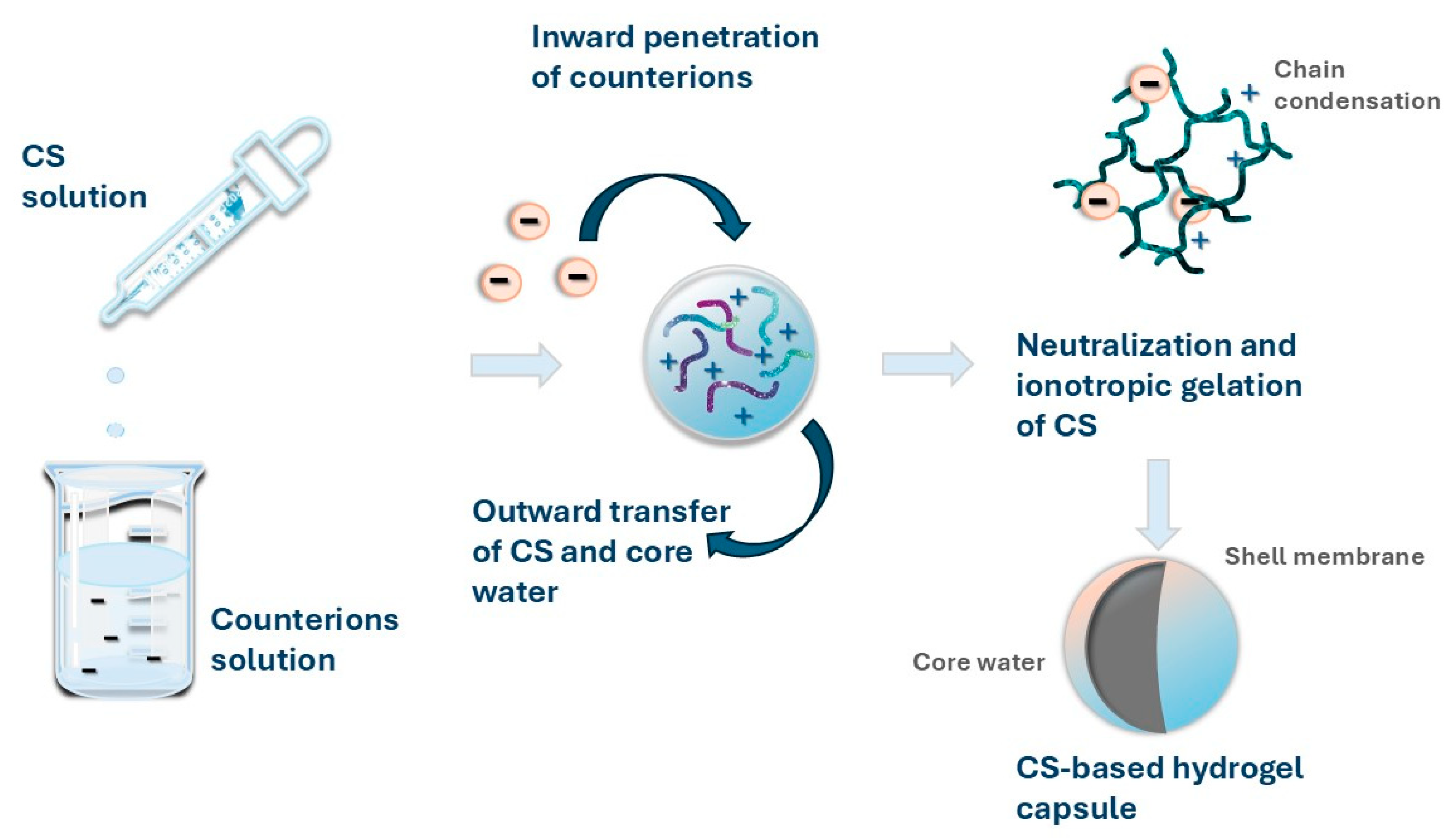
3.2.2. Ionotropic Gelation of Chitosan
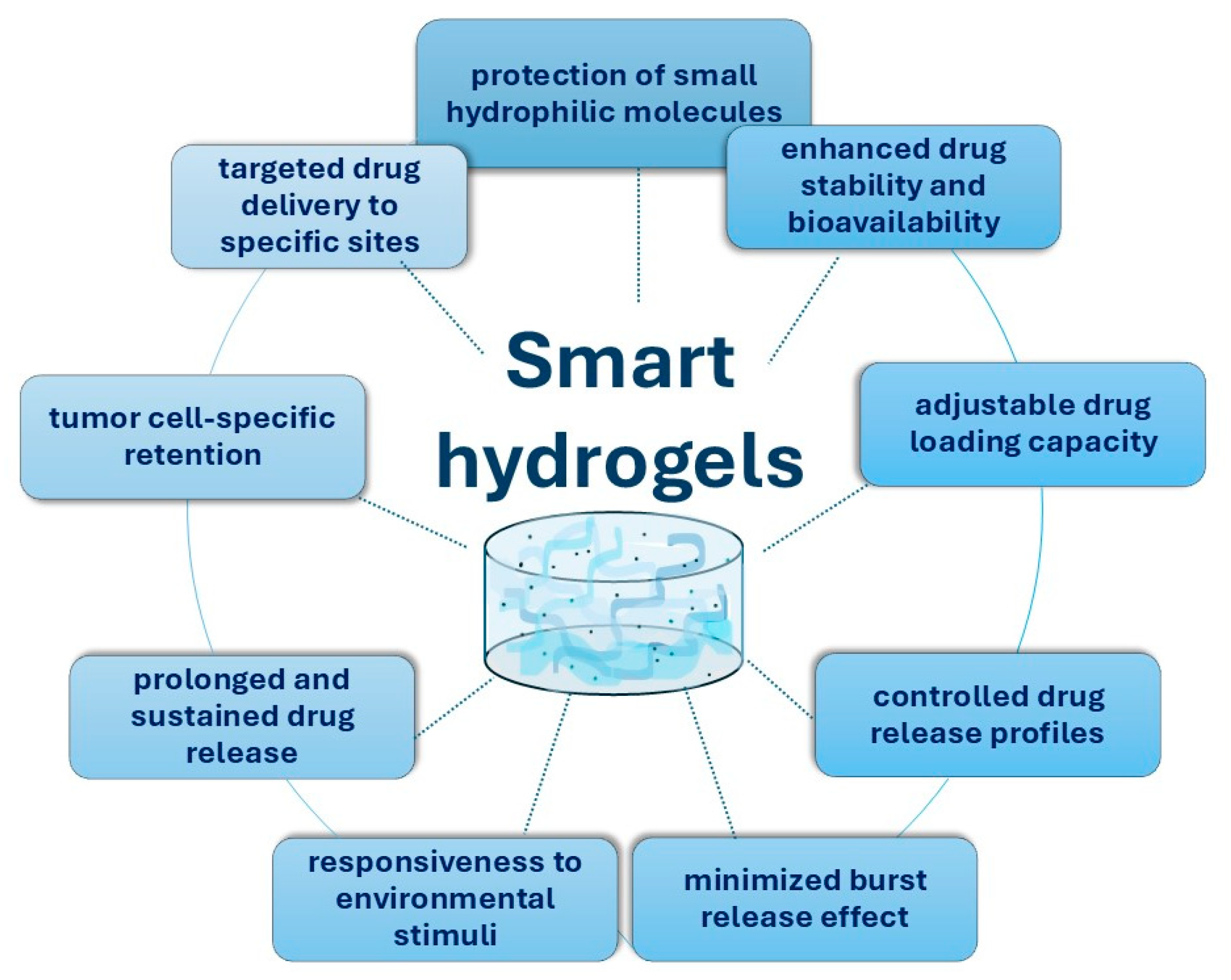
4. Smart Chitosan-Based Hydrogels for the Treatment of Colorectal Cancer
4.1. In Situ-Forming Chitosan-Based Hydrogels for Colorectal Cancer Therapy
4.2. Stimuli-Responsive Multi-Drug Chitosan-Based Hydrogels
4.3. Nanocomposite Hydrogels
5. Clinical Challenges and Limitations
6. Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-ASA | 5-aminosalicylic acid |
| 5-FU | 5-fluorouracil |
| AA | Acrylic acid |
| AMPS | 2-acrylamido-2-methylpropane sulfonic acid |
| ASA | Acetylsalicylic acid |
| CMC | Carboxymethyl cellulose |
| CMCS | Carboxymethyl chitosan |
| CRC | Colorectal cancer |
| CS | Chitosan |
| CSDAGG | Chitosan-dialdehyde guar gum hydrogels |
| CUR | Curcumin |
| DD | Degree of deacetylation |
| DDP | Cisplatin |
| DDSs | Drug delivery systems |
| DOX | Doxorubicin |
| E | Vitamin E |
| FTIR | Fourier Transform Infrared Spectroscopy |
| GG | Guar gum |
| GRAS | Generally Recognized as Safe |
| HA | Hyaluronic acid |
| HACPN | Poly(N-isopropylacrylamide)-based hydrogel |
| IMT | Imatinib |
| MTX | Methotrexate |
| NMR | Nuclear Magnetic Resonance |
| NPs | Nanoparticles |
| OD | Oxidized dextran |
| ONB | Ortho-nitro benzyl |
| PAA | Poly (acrylic acid) |
| PEG | Poly(ethylene glycol) |
| PNIPAM | Poly N-isopropyl acrylamide |
| PTT | Photothermal therapy |
| PVA | Poly(vinyl alcohol) |
| SCF | Simulated colonic fluid |
| SGF | Simulated gastric fluid |
| SIF | Simulated intestinal fluid |
| TA | Tannic acid |
| TJs | Tight junctions |
| Tzs | Tetrazines |
| β-GP | β-glycerophosphate |
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Akinkuotu, A.C.; Maduekwe, U.N.; Hayes-Jordan, A. Surgical outcomes and survival rates of colon cancer in children and young adults. Am. J. Surg. 2021, 221, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Duong, H.Q. The molecular characteristics of colorectal cancer: Implications for diagnosis and therapy. Oncol. Lett. 2018, 16, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Van Der Flier, L.G.; Clevers, H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 2009, 71, 241–260. [Google Scholar] [CrossRef]
- Ahmad, R.; Singh, J.K.; Wunnava, A.; Al-Obeed, O.; Abdulla, M.; Srivastava, S.K. Emerging trends in colorectal cancer: Dysregulated signaling pathways. Int. J. Mol. Med. 2021, 47, 14. [Google Scholar] [CrossRef]
- Burnett-Hartman, A.N.; Newcomb, P.A.; Potter, J.D. Infectious agents and colorectal cancer: A review of Helicobacter pylori, Streptococcus bovis, JC virus, and human papillomavirus. Cancer Epidemiol. Biomark. Prev. 2008, 17, 2970–2979. [Google Scholar] [CrossRef]
- Wong, S.H.; Yu, J. Gut microbiota in colorectal cancer: Mechanisms of action and clinical applications. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 690–704. [Google Scholar] [CrossRef]
- Hussain, M.; Waqas, O.; Hassan, U.; Loya, A.; Akhtar, N.; Mushtaq, S.; Yusuf, M.A.; Syed, A.A. Right-sided and left-sided colon cancers are two distinct disease entities: An analysis of 200 cases in Pakistan. Asian Pac. J. Cancer Prev. 2016, 17, 2545–2548. [Google Scholar]
- Sears, C.L.; Garrett, W.S. Microbes, microbiota, and colon cancer. Cell Host Microbe 2014, 15, 317–328. [Google Scholar] [CrossRef]
- Zhong, M.; Xiong, Y.; Ye, Z.; Zhao, J.; Zhong, L.; Liu, Y.; Zhu, Y.; Tian, L.; Qiu, X.; Hong, X. Microbial community profiling distinguishes left-sided and right-sided colon cancer. Front. Cell. Infect. Microbiol. 2020, 10, 498502. [Google Scholar] [CrossRef]
- Roeder, F.; Meldolesi, E.; Gerum, S.; Valentini, V.; Rödel, C. Recent advances in (chemo-) radiation therapy for rectal cancer: A comprehensive review. Radiat. Oncol. 2020, 15, 262. [Google Scholar] [CrossRef] [PubMed]
- Chibaudel, B.; Tournigand, C.; Bonnetain, F.; Richa, H.; Benetkiewicz, M.; André, T.; de Gramont, A. Therapeutic strategy in unresectable metastatic colorectal cancer: An updated review. Ther. Adv. Med. Oncol. 2015, 7, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Zhao, J.; Feng, S.-S. Targeted co-delivery of docetaxel, cisplatin and herceptin by vitamin E TPGS-cisplatin prodrug nanoparticles for multimodality treatment of cancer. J. Control. Release 2013, 169, 185–192. [Google Scholar] [CrossRef]
- Dai, L.; Liu, J.; Luo, Z.; Li, M.; Cai, K. Tumor therapy: Targeted drug delivery systems. J. Mater. Chem. B 2016, 4, 6758–6772. [Google Scholar] [CrossRef]
- Pacheco, C.; Baiao, A.; Ding, T.; Cui, W.; Sarmento, B. Recent advances in long-acting drug delivery systems for anticancer drug. Adv. Drug Deliv. Rev. 2023, 194, 114724. [Google Scholar] [CrossRef]
- Kesharwani, P.; Bisht, A.; Alexander, A.; Dave, V.; Sharma, S. Biomedical applications of hydrogels in drug delivery system: An update. J. Drug Deliv. Sci. Technol. 2021, 66, 102914. [Google Scholar] [CrossRef]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and applications in biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Wu, Q.; Hu, Y.; Yu, B.; Hu, H.; Xu, F.-J. Polysaccharide-based tumor microenvironment-responsive drug delivery systems for cancer therapy. J. Control. Release 2023, 362, 19–43. [Google Scholar] [CrossRef]
- Kurakula, M.; Gorityala, S.; Moharir, K. Recent trends in design and evaluation of chitosan-based colon targeted drug delivery systems: Update 2020. J. Drug Deliv. Sci. Technol. 2021, 64, 102579. [Google Scholar] [CrossRef]
- Sanford, P.; Hutchings, G. Chitosan-A Natural, Cationic Biopolymer: Commercial Application in Industrial Polysaccharides; Elsevier: Amsterdam, The Netherlands, 1987. [Google Scholar]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhuang, S. Chitosan-based materials: Preparation, modification and application. J. Clean. Prod. 2022, 355, 131825. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An overview of its properties and applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, production and commercial applications of fungal chitosan: A review. J. Bioresour. Bioprod. 2022, 7, 85–98. [Google Scholar] [CrossRef]
- Abourehab, M.A.; Pramanik, S.; Abdelgawad, M.A.; Abualsoud, B.M.; Kadi, A.; Ansari, M.J.; Deepak, A. Recent advances of chitosan formulations in biomedical applications. Int. J. Mol. Sci. 2022, 23, 10975. [Google Scholar] [CrossRef]
- Desai, R.; Pachpore, R.; Patil, A.; Jain, R.; Dandekar, P. Review of the structure of chitosan in the context of other sugar-based polymers. In Chitosan for Biomaterials III: Structure-Property Relationships; Springer: Cham, Switzerland, 2021; pp. 23–74. [Google Scholar]
- Feng, C.; Li, J.; Kong, M.; Liu, Y.; Cheng, X.J.; Li, Y.; Park, H.J.; Chen, X.G. Surface charge effect on mucoadhesion of chitosan based nanogels for local anti-colorectal cancer drug delivery. Colloids Surf. B Biointerfaces 2015, 128, 439–447. [Google Scholar] [CrossRef]
- Smith, J.; Wood, E.; Dornish, M. Effect of chitosan on epithelial cell tight junctions. Pharm. Res. 2004, 21, 43–49. [Google Scholar] [CrossRef]
- Tallian, C.; Tegl, G.; Quadlbauer, L.; Vielnascher, R.; Weinberger, S.; Cremers, R.; Pellis, A.; Salari, J.W.; Guebitz, G.M. Lysozyme-responsive spray-dried chitosan particles for early detection of wound infection. ACS Appl. Bio Mater. 2019, 2, 1331–1339. [Google Scholar] [CrossRef]
- Ilium, L. Chitosan and its use as a pharmaceutical excipient. Pharm. Res. 1998, 15, 1326–1331. [Google Scholar] [CrossRef]
- Singla, A.; Chawla, M. Chitosan: Some pharmaceutical and biological aspects-an update. J. Pharm. Pharmacol. 2001, 53, 1047–1067. [Google Scholar] [CrossRef]
- Kas, H.S. Chitosan: Properties, preparations and application to microparticulate systems. J. Microencapsul. 1997, 14, 689–711. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganathan, R.; Edison, T.N.J.I.; LewisOscar, F.; Kumar, P.; Shanmugam, S.; Pugazhendhi, A. Chitosan nanopolymers: An overview of drug delivery against cancer. Int. J. Biol. Macromol. 2019, 130, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Machado, D.I.; López-Cervantes, J.; Escárcega-Galaz, A.A.; Campas-Baypoli, O.N.; Martínez-Ibarra, D.M.; Rascón-León, S. Measurement of the degree of deacetylation in chitosan films by FTIR, 1H NMR and UV spectrophotometry. MethodsX 2024, 12, 102583. [Google Scholar] [CrossRef]
- Devlieghere, F.; Vermeulen, A.; Debevere, J. Chitosan: Antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microbiol. 2004, 21, 703–714. [Google Scholar] [CrossRef]
- Roller, S.; Covill, N. The antifungal properties of chitosan in laboratory media and apple juice. Int. J. Food Microbiol. 1999, 47, 67–77. [Google Scholar] [CrossRef]
- Papineau, A.M.; Hoover, D.G.; Knorr, D.; Farkas, D.F. Antimicrobial effect of water-soluble chitosans with high hydrostatic pressure. Food Biotechnol. 1991, 5, 45–57. [Google Scholar] [CrossRef]
- Kumar, M.R.; Muzzarelli, R.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef] [PubMed]
- Sogias, I.A.; Khutoryanskiy, V.V.; Williams, A.C. Exploring the factors affecting the solubility of chitosan in water. Macromol. Chem. Phys. 2010, 211, 426–433. [Google Scholar] [CrossRef]
- Errington, N.; Harding, S.; Vårum, K.; Illum, L. Hydrodynamic characterization of chitosans varying in degree of acetylation. Int. J. Biol. Macromol. 1993, 15, 113–117. [Google Scholar] [CrossRef]
- Qin, C.; Li, H.; Xiao, Q.; Liu, Y.; Zhu, J.; Du, Y. Water-solubility of chitosan and its antimicrobial activity. Carbohydr. Polym. 2006, 63, 367–374. [Google Scholar] [CrossRef]
- Hejazi, R.; Amiji, M. Chitosan-based gastrointestinal delivery systems. J. Control. Release 2003, 89, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-s.; Chiang, C.-h.; Hong, P.-d.; Yeh, M.-k. Influence of charge on FITC-BSA-loaded chondroitin sulfate-chitosan nanoparticles upon cell uptake in human Caco-2 cell monolayers. Int. J. Nanomed. 2012, 7, 4861–4872. [Google Scholar]
- Kotze, A.; Luessen, H.; De Boer, A.; Verhoef, J.; Junginger, H. Chitosan for enhanced intestinal permeability: Prospects for derivatives soluble in neutral and basic environments. Eur. J. Pharm. Sci. 1999, 7, 145–151. [Google Scholar] [CrossRef]
- Kotzé, A.F.; Thanou, M.M.; Lueßen, H.L.; Verhoef, J.C.; Junginger, H.E. Effect of the degree of quaternization of N-trimethyl chitosan chloride on the permeability of intestinal epithelial cells (Caco-2). Eur. J. Pharm. Biopharm. 1999, 47, 269–274. [Google Scholar] [CrossRef]
- Schipper, N.G.; Olsson, S.; Hoogstraate, J.A.; deBoer, A.G.; Vårum, K.M.; Artursson, P. Chitosans as absorption enhancers for poorly absorbable drugs 2: Mechanism of absorption enhancement. Pharm. Res. 1997, 14, 923–929. [Google Scholar] [CrossRef]
- Rosenthal, R.; Günzel, D.; Finger, C.; Krug, S.M.; Richter, J.F.; Schulzke, J.-D.; Fromm, M.; Amasheh, S. The effect of chitosan on transcellular and paracellular mechanisms in the intestinal epithelial barrier. Biomaterials 2012, 33, 2791–2800. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ling, Z.; Wang, X.; Zong, S.; Yang, J.; Zhang, Q.; Zhang, J.; Li, X. The beneficial mechanism of chitosan and chitooligosaccharides in the intestine on different health status. J. Funct. Foods 2022, 97, 105232. [Google Scholar] [CrossRef]
- Wu, M.; Li, J.; An, Y.; Li, P.; Xiong, W.; Li, J.; Yan, D.; Wang, M.; Zhong, G. Chitooligosaccharides Prevents the Development of Colitis-Associated Colorectal Cancer by Modulating the Intestinal Microbiota and Mycobiota. Front. Microbiol. 2019, 10, 2101. [Google Scholar] [CrossRef]
- Calinescu, C.; Mateescu, M.A. Carboxymethyl high amylose starch: Chitosan self-stabilized matrix for probiotic colon delivery. Eur. J. Pharm. Biopharm. 2008, 70, 582–589. [Google Scholar] [CrossRef]
- Bhumkar, D.R.; Pokharkar, V.B. Studies on effect of pH on cross-linking of chitosan with sodium tripolyphosphate: A technical note. Aaps Pharmscitech 2006, 7, E138–E143. [Google Scholar] [CrossRef]
- Hong, F.; Qiu, P.; Wang, Y.; Ren, P.; Liu, J.; Zhao, J.; Gou, D. Chitosan-based hydrogels: From preparation to applications, a review. Food Chem. X 2023, 21, 101095. [Google Scholar] [CrossRef] [PubMed]
- Dragan, E.S.; Dinu, M.V. Advances in porous chitosan-based composite hydrogels: Synthesis and applications. React. Funct. Polym. 2020, 146, 104372. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.; Felt, O.; Gurny, R. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 35–52. [Google Scholar] [CrossRef]
- Jin, S.; Liu, M.; Chen, S.; Gao, C. A drug-loaded gel based on polyelectrolyte complexes of poly (acrylic acid) with poly (vinylpyrrolidone) and chitosan. Mater. Chem. Phys. 2010, 123, 463–470. [Google Scholar] [CrossRef]
- Tian, B.; Hua, S.; Tian, Y.; Liu, J. Chemical and physical chitosan hydrogels as prospective carriers for drug delivery: A review. J. Mater. Chem. B 2020, 8, 10050–10064. [Google Scholar] [CrossRef]
- Zhang, Y.; Guan, Y.; Zhou, S. Single component chitosan hydrogel microcapsule from a layer-by-layer approach. Biomacromolecules 2005, 6, 2365–2369. [Google Scholar] [CrossRef]
- McClements, D.J. Designing biopolymer microgels to encapsulate, protect and deliver bioactive components: Physicochemical aspects. Adv. Colloid Interface Sci. 2017, 240, 31–59. [Google Scholar] [CrossRef]
- Delmar, K.; Bianco-Peled, H. The dramatic effect of small pH changes on the properties of chitosan hydrogels crosslinked with genipin. Carbohydr. Polym. 2015, 127, 28–37. [Google Scholar] [CrossRef]
- Silva, S.S.; Motta, A.; Rodrigues, M.T.; Pinheiro, A.F.; Gomes, M.E.; Mano, J.F.; Reis, R.L.; Migliaresi, C. Novel genipin-cross-linked chitosan/silk fibroin sponges for cartilage engineering strategies. Biomacromolecules 2008, 9, 2764–2774. [Google Scholar] [CrossRef]
- Sharmin, N.; Rosnes, J.T.; Prabhu, L.; Böcker, U.; Sivertsvik, M. Effect of citric acid cross linking on the mechanical, rheological and barrier properties of chitosan. Molecules 2022, 27, 5118. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, H.; Li, R.; Xing, Y. Preparation and adsorption properties of citrate-crosslinked chitosan salt microspheres by microwave assisted method. Int. J. Biol. Macromol. 2020, 152, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.-T.; Liu, J.-H. Fabrication and characterization of poly (vinyl alcohol)/chitosan hydrogel thin films via UV irradiation. Eur. Polym. J. 2013, 49, 4201–4211. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Wang, J. Photo-crosslinkable hydrogel and its biological applications. Chin. Chem. Lett. 2021, 32, 1603–1614. [Google Scholar] [CrossRef]
- Hyun, H.; Park, M.H.; Lim, W.; Kim, S.Y.; Jo, D.; Jung, J.S.; Jo, G.; Um, S.; Lee, D.-W.; Yang, D.H. Injectable visible light-cured glycol chitosan hydrogels with controlled release of anticancer drugs for local cancer therapy in vivo: A feasible study. Artif. Cells Nanomed. Biotechnol. 2018, 46, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Tatarusanu, S.-M.; Sava, A.; Profire, B.-S.; Pinteala, T.; Jitareanu, A.; Iacob, A.-T.; Lupascu, F.; Simionescu, N.; Rosca, I.; Profire, L. New Smart Bioactive and Biomimetic Chitosan-Based Hydrogels for Wounds Care Management. Pharmaceutics 2023, 15, 975. [Google Scholar] [CrossRef]
- Guaresti, O.; García–Astrain, C.; Aguirresarobe, R.; Eceiza, A.; Gabilondo, N. Synthesis of stimuli–responsive chitosan–based hydrogels by Diels–Alder cross–linkingclick reaction as potential carriers for drug administration. Carbohydr. Polym. 2018, 183, 278–286. [Google Scholar] [CrossRef]
- Guaresti, O.; Basasoro, S.; González, K.; Eceiza, A.; Gabilondo, N. In situ cross–linked chitosan hydrogels via Michael addition reaction based on water–soluble thiol–maleimide precursors. Eur. Polym. J. 2019, 119, 376–384. [Google Scholar] [CrossRef]
- Li, R.; Liu, Q.; Wu, H.; Wang, K.; Li, L.; Zhou, C.; Ao, N. Preparation and characterization of in-situ formable liposome/chitosan composite hydrogels. Mater. Lett. 2018, 220, 289–292. [Google Scholar] [CrossRef]
- Tsuda, Y.; Ishihara, M.; Amako, M.; Arino, H.; Hattori, H.; Kanatani, Y.; Yura, H.; Nemoto, K. Photocrosslinkable chitosan hydrogel can prevent bone formation in both rat skull and fibula bone defects. Artif. Organs 2009, 33, 74–77. [Google Scholar] [CrossRef]
- Pei, M.; Mao, J.; Xu, W.; Zhou, Y.; Xiao, P. Photocrosslinkable chitosan hydrogels and their biomedical applications. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1862–1871. [Google Scholar] [CrossRef]
- Cho, I.S.; Cho, M.O.; Li, Z.; Nurunnabi, M.; Park, S.Y.; Kang, S.-W.; Huh, K.M. Synthesis and characterization of a new photo-crosslinkable glycol chitosan thermogel for biomedical applications. Carbohydr. Polym. 2016, 144, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lv, Y.; Liu, M.; Wang, X.; Mi, Y.; Wang, Q. Nanoinitiator for enzymatic anaerobic polymerization and graft enhancement of gelatin–PAAM hydrogel. J. Mater. Chem. B 2018, 6, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, K.; Dhamodharan, R. Advances in chitosan-based hydrogels: Evolution from covalently crosslinked systems to ionotropically crosslinked superabsorbents. React. Funct. Polym. 2020, 149, 104517. [Google Scholar] [CrossRef]
- Tian, B.; Liu, J. Smart stimuli-responsive chitosan hydrogel for drug delivery: A review. Int. J. Biol. Macromol. 2023, 235, 123902. [Google Scholar] [CrossRef]
- He, Q.; Huang, D.; Yang, J.; Huang, Y.; Wang, S. Dual cross-link networks to preserve physical interactions induced by soaking methods: Developing a strong and biocompatible protein-based hydrogel. ACS Appl. Bio Mater. 2019, 2, 3352–3361. [Google Scholar] [CrossRef]
- Papagiannopoulos, A.; Nikolakis, S.-P.; Pamvouxoglou, A.; Koutsopoulou, E. Physicochemical properties of electrostatically crosslinked carrageenan/chitosan hydrogels and carrageenan/chitosan/Laponite nanocomposite hydrogels. Int. J. Biol. Macromol. 2023, 225, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Wang, Z.; Hu, Q. Difference between chitosan hydrogels via alkaline and acidic solvent systems. Sci. Rep. 2016, 6, 36053. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Nie, J.; Qin, W.; Hu, Q.; Tang, B.Z. Gelation process visualized by aggregation-induced emission fluorogens. Nat. Commun. 2016, 7, 12033. [Google Scholar] [CrossRef]
- Reig-Vano, B.; Tylkowski, B.; Montané, X.; Giamberini, M. Alginate-based hydrogels for cancer therapy and research. Int. J. Biol. Macromol. 2021, 170, 424–436. [Google Scholar] [CrossRef]
- Fernández-Quiroz, D.; Loya-Duarte, J.; Silva-Campa, E.; Argüelles-Monal, W.; Sarabia-Sainz, A.í.; Lucero-Acuña, A.; del Castillo-Castro, T.; San Román, J.; Lizardi-Mendoza, J.; Burgara-Estrella, A.J. Temperature stimuli-responsive nanoparticles from chitosan-graft-poly (N-vinylcaprolactam) as a drug delivery system. J. Appl. Polym. Sci. 2019, 136, 47831. [Google Scholar] [CrossRef]
- Calvo, P.; Remunan-Lopez, C.; Vila-Jato, J.L.; Alonso, M. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Bugnicourt, L.; Ladavière, C. Interests of chitosan nanoparticles ionically cross-linked with tripolyphosphate for biomedical applications. Prog. Polym. Sci. 2016, 60, 1–17. [Google Scholar] [CrossRef]
- Angelova, N.; Hunkeler, D. Permeability and stability of chitosan-based capsules: Effect of preparation. Int. J. Pharm. 2002, 242, 229–232. [Google Scholar] [CrossRef]
- Peniche, H.; Peniche, C. Chitosan nanoparticles: A contribution to nanomedicine. Polym. Int. 2011, 60, 883–889. [Google Scholar] [CrossRef]
- Wang, Q.-S.; Wang, G.-F.; Zhou, J.; Gao, L.-N.; Cui, Y.-L. Colon targeted oral drug delivery system based on alginate-chitosan microspheres loaded with icariin in the treatment of ulcerative colitis. Int. J. Pharm. 2016, 515, 176–185. [Google Scholar] [CrossRef]
- Balima, M.; Morfin, I.; Sudre, G.; Montembault, A. Stretchable hydrogels of chitosan/hyaluronic acid induced by polyelectrolyte complexation around neutral pH. Carbohydr. Polym. 2024, 339, 122265. [Google Scholar] [CrossRef] [PubMed]
- Oyarzun-Ampuero, F.; Brea, J.; Loza, M.; Torres, D.; Alonso, M. Chitosan–hyaluronic acid nanoparticles loaded with heparin for the treatment of asthma. Int. J. Pharm. 2009, 381, 122–129. [Google Scholar] [CrossRef]
- Denuziere, A.; Ferrier, D.; Domard, A. Chitosan-chondroitin sulfate and chitosan-hyaluronate polyelectrolyte complexes. Physico-chemical aspects. Carbohydr. Polym. 1996, 29, 317–323. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Doroudian, M.; Ahadpour, A.; Azari, S. Injectable chitosan/κ-carrageenan hydrogel designed with au nanoparticles: A conductive scaffold for tissue engineering demands. Int. J. Biol. Macromol. 2019, 126, 310–317. [Google Scholar] [CrossRef]
- Sinha, V.; Singla, A.K.; Wadhawan, S.; Kaushik, R.; Kumria, R.; Bansal, K.; Dhawan, S. Chitosan microspheres as a potential carrier for drugs. Int. J. Pharm. 2004, 274, 1–33. [Google Scholar] [CrossRef]
- Bezerra, M.C.; Duarte, G.A.; Talabi, S.I.; Lucas, A.A. Microstructure and properties of thermomechanically processed chitosan citrate-based materials. Carbohydr. Polym. 2022, 278, 118984. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Y.; Jiang, L.J.; Cao, P.P.; Li, J.B.; Chen, X.G. Glycerophosphate-based chitosan thermosensitive hydrogels and their biomedical applications. Carbohydr. Polym. 2015, 117, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Ding, J.; Zhang, Y.; Huang, B.; Song, Z.; Meng, X.; Ma, X.; Gong, X.; Huang, Z.; Ma, S. Components, mechanisms and applications of stimuli-responsive polymer gels. Eur. Polym. J. 2022, 177, 111473. [Google Scholar] [CrossRef]
- Wei, M.; Gao, Y.; Li, X.; Serpe, M.J. Stimuli-responsive polymers and their applications. Polym. Chem. 2017, 8, 127–143. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, S.; Sharma, P.; Kumar, B.; Kumar, A. Single-, Dual-, and Multi-Stimuli-Responsive Nanogels for Biomedical Applications. Gels 2024, 10, 61. [Google Scholar] [CrossRef]
- Elias, D.; Blot, F.; El Otmany, A.; Antoun, S.; Lasser, P.; Boige, V.; Rougier, P.; Ducreux, M. Curative treatment of peritoneal carcinomatosis arising from colorectal cancer by complete resection and intraperitoneal chemotherapy. Cancer 2001, 92, 71–76. [Google Scholar] [CrossRef]
- Verwaal, V.J.; Bruin, S.; Boot, H.; Van Slooten, G.; Van Tinteren, H. 8-year follow-up of randomized trial: Cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann. Surg. Oncol. 2008, 15, 2426–2432. [Google Scholar] [CrossRef]
- Levine, E.A.; Stewart IV, J.H.; Shen, P.; Russell, G.B.; Loggie, B.L.; Votanopoulos, K.I. Intraperitoneal chemotherapy for peritoneal surface malignancy: Experience with 1,000 patients. J. Am. Coll. Surg. 2014, 218, 573–585. [Google Scholar] [CrossRef]
- Garg, A.; Agrawal, R.; Chauhan, C.S.; Deshmukh, R. In-situ gel: A smart carrier for drug delivery. Int. J. Pharm. 2024, 652, 123819. [Google Scholar] [CrossRef]
- Ruel-Gariepy, E.; Leroux, J.-C. In situ-forming hydrogels—Review of temperature-sensitive systems. Eur. J. Pharm. Biopharm. 2004, 58, 409–426. [Google Scholar] [CrossRef]
- Gao, Y.; Deng, A.; Wu, X.; Sun, C.; Qi, C. Injectable multi-responsive hydrogels cross-linked by responsive macromolecular micelles. React. Funct. Polym. 2021, 161, 104866. [Google Scholar] [CrossRef]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef] [PubMed]
- Ruel-Gariepy, E.; Chenite, A.; Chaput, C.; Guirguis, S.; Leroux, J.-C. Characterization of thermosensitive chitosan gels for the sustained delivery of drugs. Int. J. Pharm. 2000, 203, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, S.M.; Williams, N.L.; Yakubu, R.; Levine, D.A.; Chi, D.S.; Sabbatini, P.J.; Aghajanian, C.A.; Barakat, R.R.; Abu-Rustum, N.R. Incidence of intestinal obstruction following intraperitoneal chemotherapy for ovarian tubal and peritoneal malignancies. Gynecol. Oncol. 2009, 113, 228–232. [Google Scholar] [CrossRef]
- Zan, J.; Chen, H.; Jiang, G.; Lin, Y.; Ding, F. Preparation and properties of crosslinked chitosan thermosensitive hydrogel for injectable drug delivery systems. J. Appl. Polym. Sci. 2006, 101, 1892–1898. [Google Scholar] [CrossRef]
- Chenite, A.; Buschmann, M.; Wang, D.; Chaput, C.; Kandani, N. Rheological characterisation of thermogelling chitosan/glycerol-phosphate solutions. Carbohydr. Polym. 2001, 46, 39–47. [Google Scholar] [CrossRef]
- Assaad, E.; Maire, M.; Lerouge, S. Injectable thermosensitive chitosan hydrogels with controlled gelation kinetics and enhanced mechanical resistance. Carbohydr. Polym. 2015, 130, 87–96. [Google Scholar] [CrossRef]
- Goldberg, R.N.; Kishore, N.; Lennen, R.M. Thermodynamic quantities for the ionization reactions of buffers. J. Phys. Chem. Ref. Data 2002, 31, 231–370. [Google Scholar] [CrossRef]
- Chenite, A.; Chaput, C.; Wang, D.; Combes, C.; Buschmann, M.D.; Hoemann, C.; Leroux, J.; Atkinson, B.; Binette, F.; Selmani, A. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials 2000, 21, 2155–2161. [Google Scholar] [CrossRef] [PubMed]
- Lavertu, M.; Filion, D.; Buschmann, M.D. Heat-induced transfer of protons from chitosan to glycerol phosphate produces chitosan precipitation and gelation. Biomacromolecules 2008, 9, 640–650. [Google Scholar] [CrossRef]
- Cho, J.; Heuzey, M.-C.; Bégin, A.; Carreau, P.J. Physical gelation of chitosan in the presence of β-glycerophosphate: The effect of temperature. Biomacromolecules 2005, 6, 3267–3275. [Google Scholar] [CrossRef] [PubMed]
- Rezakhani, L.; Alizadeh, M.; Alizadeh, A. A three dimensional in vivo model of breast cancer using a thermosensitive chitosan-based hydrogel and 4 T1 cell line in Balb/c. J. Biomed. Mater. Res. Part A 2021, 109, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Taherian, A.R.; Lacasse, P.; Bisakowski, B.; Pelletier, M.; Lanctôt, S.; Fustier, P. Rheological and thermogelling properties of commercials chitosan/β-glycerophosphate: Retention of hydrogel in water, milk and UF-milk. Food Hydrocoll. 2017, 63, 635–645. [Google Scholar] [CrossRef]
- Deng, A.; Kang, X.; Zhang, J.; Yang, Y.; Yang, S. Enhanced gelation of chitosan/β-sodium glycerophosphate thermosensitive hydrogel with sodium bicarbonate and biocompatibility evaluated. Mater. Sci. Eng. C 2017, 78, 1147–1154. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Adoungotchodo, A.; Demarquette, N.R.; Lerouge, S. FRESH bioprinting of biodegradable chitosan thermosensitive hydrogels. Bioprinting 2022, 27, e00209. [Google Scholar] [CrossRef]
- Yun, Q.; Wang, S.S.; Xu, S.; Yang, J.P.; Fan, J.; Yang, L.L.; Chen, Y.; Fu, S.Z.; Wu, J.B. Use of 5-Fluorouracil Loaded Micelles and Cisplatin in Thermosensitive Chitosan Hydrogel as an Efficient Therapy against Colorectal Peritoneal Carcinomatosis. Macromol. Biosci. 2017, 17, 1600262. [Google Scholar] [CrossRef]
- Chen, C.-H.; Kuo, C.-Y.; Chen, S.-H.; Mao, S.-H.; Chang, C.-Y.; Shalumon, K.; Chen, J.-P. Thermosensitive injectable hydrogel for simultaneous intraperitoneal delivery of doxorubicin and prevention of peritoneal adhesion. Int. J. Mol. Sci. 2018, 19, 1373. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, W.; Zhao, J.; Wu, C.; Ye, C.; Huang, M.; Wang, S. Preparation of injectable temperature-sensitive chitosan-based hydrogel for combined hyperthermia and chemotherapy of colon cancer. Carbohydr. Polym. 2019, 222, 115039. [Google Scholar] [CrossRef]
- Songkroh, T.; Xie, H.; Yu, W.; Liu, X.; Sun, G.; Xu, X.; Ma, X. Injectable in situ forming chitosan-based hydrogels for curcumin delivery. Macromol. Res. 2015, 23, 53–59. [Google Scholar] [CrossRef]
- Dalei, G.; Das, S.; Jena, S.R.; Jena, D.; Nayak, J.; Samanta, L. In situ crosslinked dialdehyde guar gum-chitosan Schiff-base hydrogels for dual drug release in colorectal cancer therapy. Chem. Eng. Sci. 2023, 269, 118482. [Google Scholar] [CrossRef]
- Liang, Z.; Gao, J.; Yin, Z.-Z.; Li, J.; Cai, W.; Kong, Y. A sequential delivery system based on MoS2 nanoflower doped chitosan/oxidized dextran hydrogels for colon cancer treatment. Int. J. Biol. Macromol. 2023, 233, 123616. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Anwar, N. Highly porous pH-responsive carboxymethyl chitosan-grafted-poly (acrylic acid) based smart hydrogels for 5-fluorouracil controlled delivery and colon targeting. Int. J. Polym. Sci. 2019, 2019, 6579239. [Google Scholar] [CrossRef]
- Ghobashy, M.M.; Elbarbary, A.M.; Hegazy, D.E. Gamma radiation synthesis of a novel amphiphilic terpolymer hydrogel pH-responsive based chitosan for colon cancer drug delivery. Carbohydr. Polym. 2021, 263, 117975. [Google Scholar] [CrossRef]
- Zarbab, A.; Sajjad, A.; Rasul, A.; Jabeen, F.; Iqbal, M.J. Synthesis and characterization of Guar gum based biopolymeric hydrogels as carrier materials for controlled delivery of methotrexate to treat colon cancer. Saudi J. Biol. Sci. 2023, 30, 103731. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Sun, M.; Fan, Z.; Du, J.-Z. Intestine enzyme-responsive polysaccharide-based hydrogel to open epithelial tight junctions for oral delivery of imatinib against colon cancer. Chin. J. Polym. Sci. 2022, 40, 1154–1164. [Google Scholar] [CrossRef]
- Hoang, H.T.; Jo, S.-H.; Phan, Q.-T.; Park, H.; Park, S.-H.; Oh, C.-W.; Lim, K.T. Dual pH-/thermo-responsive chitosan-based hydrogels prepared using” click” chemistry for colon-targeted drug delivery applications. Carbohydr. Polym. 2021, 260, 117812. [Google Scholar] [CrossRef]
- Nisar, S.; Pandit, A.H.; Wang, L.-F.; Rattan, S. Strategy to design a smart photocleavable and pH sensitive chitosan based hydrogel through a novel crosslinker: A potential vehicle for controlled drug delivery. RSC Adv. 2020, 10, 14694–14704. [Google Scholar] [CrossRef]
- Chen, J.-P.; Cheng, T.-H. Preparation and evaluation of thermo-reversible copolymer hydrogels containing chitosan and hyaluronic acid as injectable cell carriers. Polymer 2009, 50, 107–116. [Google Scholar] [CrossRef]
- Hua, S. Advances in oral drug delivery for regional targeting in the gastrointestinal tract-influence of physiological, pathophysiological and pharmaceutical factors. Front. Pharmacol. 2020, 11, 524. [Google Scholar] [CrossRef]
- Ding, H.; Tan, P.; Fu, S.; Tian, X.; Zhang, H.; Ma, X.; Gu, Z.; Luo, K. Preparation and application of pH-responsive drug delivery systems. J. Control. Release 2022, 348, 206–238. [Google Scholar] [CrossRef]
- Tan, J.P.; Goh, C.H.; Tam, K.C. Comparative drug release studies of two cationic drugs from pH-responsive nanogels. Eur. J. Pharm. Sci. 2007, 32, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, R.; Kunz, W.; Osswald, H.; Ritter, M.; Port, R. The effect of methotrexate pretreatment on 5-fluorouracil kinetics in sarcoma 180 in vivo. Eur. J. Cancer Clin. Oncol. 1985, 21, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Hu, Y.; Zhao, Y.; Tang, K.; Zhang, Z.; Liu, Z.; Wang, Y.; Guo, H.; Miao, Y.; Du, H. Nanomaterials for photothermal cancer therapy. RSC Adv. 2023, 13, 14443–14460. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Xia, G.; Yang, N.; Yuan, L.; Li, J.; Wang, Q.; Li, D.; Ding, L.; Fan, Z.; Li, J. Noble Metal Nanoparticle-Based Photothermal Therapy: Development and Application in Effective Cancer Therapy. Int. J. Mol. Sci. 2024, 25, 5632. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Peppas, N.A.; Khademhosseini, A. Nanocomposite hydrogels for biomedical applications. Biotechnol. Bioeng. 2014, 111, 441–453. [Google Scholar] [CrossRef]
- Cheng, X.; Xie, Q.; Sun, Y. Advances in nanomaterial-based targeted drug delivery systems. Front. Bioeng. Biotechnol. 2023, 11, 1177151. [Google Scholar] [CrossRef]
- Zhu, A.; Yuan, L.; Liao, T. Suspension of Fe3O4 nanoparticles stabilized by chitosan and o-carboxymethylchitosan. Int. J. Pharm. 2008, 350, 361–368. [Google Scholar] [CrossRef]
- Zhu, N.; Ji, H.; Yu, P.; Niu, J.; Farooq, M.; Akram, M.W.; Udego, I.; Li, H.; Niu, X. Surface modification of magnetic iron oxide nanoparticles. Nanomaterials 2018, 8, 810. [Google Scholar] [CrossRef]
- Thanou, M.; Verhoef, J.; Junginger, H. Oral drug absorption enhancement by chitosan and its derivatives. Adv. Drug Deliv. Rev. 2001, 52, 117–126. [Google Scholar] [CrossRef]
- Fang, N.; Chan, V.; Mao, H.-Q.; Leong, K.W. Interactions of phospholipid bilayer with chitosan: Effect of molecular weight and pH. Biomacromolecules 2001, 2, 1161–1168. [Google Scholar] [CrossRef]
- Bowman, K.; Leong, K.W. Chitosan nanoparticles for oral drug and gene delivery. Int. J. Nanomed. 2006, 1, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Vllasaliu, D.; Exposito-Harris, R.; Heras, A.; Casettari, L.; Garnett, M.; Illum, L.; Stolnik, S. Tight junction modulation by chitosan nanoparticles: Comparison with chitosan solution. Int. J. Pharm. 2010, 400, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Kudsiova, L.; Lawrence, M. A comparison of the effect of chitosan and chitosan-coated vesicles on monolayer integrity and permeability across Caco-2 and 16HBE14o-cells. J. Pharm. Sci. 2008, 97, 3998–4010. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, C.; Omer, A.; Lu, W.; Zhang, S.; Jiang, X.; Wu, H.; Yu, D.; Ouyang, X.-k. pH-sensitive ZnO/carboxymethyl cellulose/chitosan bio-nanocomposite beads for colon-specific release of 5-fluorouracil. Int. J. Biol. Macromol. 2019, 128, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Dalei, G.; Jena, D.; Das, B.R.; Das, S. Bio-valorization of Tagetes floral waste extract in fabrication of self-healing Schiff-base nanocomposite hydrogels for colon cancer remedy. Environ. Sci. Pollut. Res. 2024, 31, 4330–4347. [Google Scholar] [CrossRef]
- Amini-Fazl, M.S.; Mohammadi, R.; Kheiri, K. 5-Fluorouracil loaded chitosan/polyacrylic acid/Fe3O4 magnetic nanocomposite hydrogel as a potential anticancer drug delivery system. Int. J. Biol. Macromol. 2019, 132, 506–513. [Google Scholar] [CrossRef]
- El-Maadawy, M.W.; Mohamed, R.R.; Hanna, D.H. Preparation of carrageenan/chitosan-based (N, N, N-trimeth (yl chitosan chloride) silver nanocomposites as pH sensitive carrier for effective controlled curcumin delivery in cancer cells. OpenNano 2022, 7, 100050. [Google Scholar] [CrossRef]
- Dhanavel, S.; Revathy, T.; Sivaranjani, T.; Sivakumar, K.; Palani, P.; Narayanan, V.; Stephen, A. 5-Fluorouracil and curcumin co-encapsulated chitosan/reduced graphene oxide nanocomposites against human colon cancer cell lines. Polym. Bull. 2020, 77, 213–233. [Google Scholar] [CrossRef]
- Sabra, R.; Billa, N.; Roberts, C.J. An augmented delivery of the anticancer agent, curcumin, to the colon. React. Funct. Polym. 2018, 123, 54–60. [Google Scholar] [CrossRef]
- Nag, S.; Das Saha, K. Chitosan-decorated PLGA-NPs loaded with tannic acid/vitamin E mitigate colon cancer via the NF-κB/β-Cat/EMT pathway. ACS Omega 2021, 6, 28752–28769. [Google Scholar] [CrossRef]
- Alsadooni, J.F.K.; Haghi, M.; Barzegar, A.; Feizi, M.A.H. The effect of chitosan hydrogel containing gold nanoparticle complex with paclitaxel on colon cancer cell line. Int. J. Biol. Macromol. 2023, 247, 125612. [Google Scholar] [CrossRef] [PubMed]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Kantak, M.N.; Bharate, S.S. Analysis of clinical trials on biomaterial and therapeutic applications of chitosan: A review. Carbohydr. Polym. 2022, 278, 118999. [Google Scholar] [CrossRef] [PubMed]

| Hydrogel System | Therapeutic Agent | Route of Administration | In Vitro Studies | In Vivo Studies | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| Drug Release Study | Cytotoxicity | Cell Line | Animal Model | PD | Cell Line | ||||
| CS/GP | 5-FU, DDP | intraperitoneal injection | Sustained manner over an extended period, cumulative release rate of DDP is higher than that of 5-FU. | IC50 = 3.43 μg mL−1 (5-FU micelles after 48 h); IC50 = 6.48 μg mL−1 (5-FU after 48 h). | CT26 | CRPC mouse model BALB/c female mice | CS hydrogel drug suppressed the growth of implanted tumor (10.33 ± 2.66, 0.49 ± 0.11 g) compared with NS group (53.83 ± 9.99, 2.31 ± 0.38 g, p < 0.001) and impaired tumor metastasis, as well as prolonged survival time of the tumor-bearing mice. | CT26 | [118] |
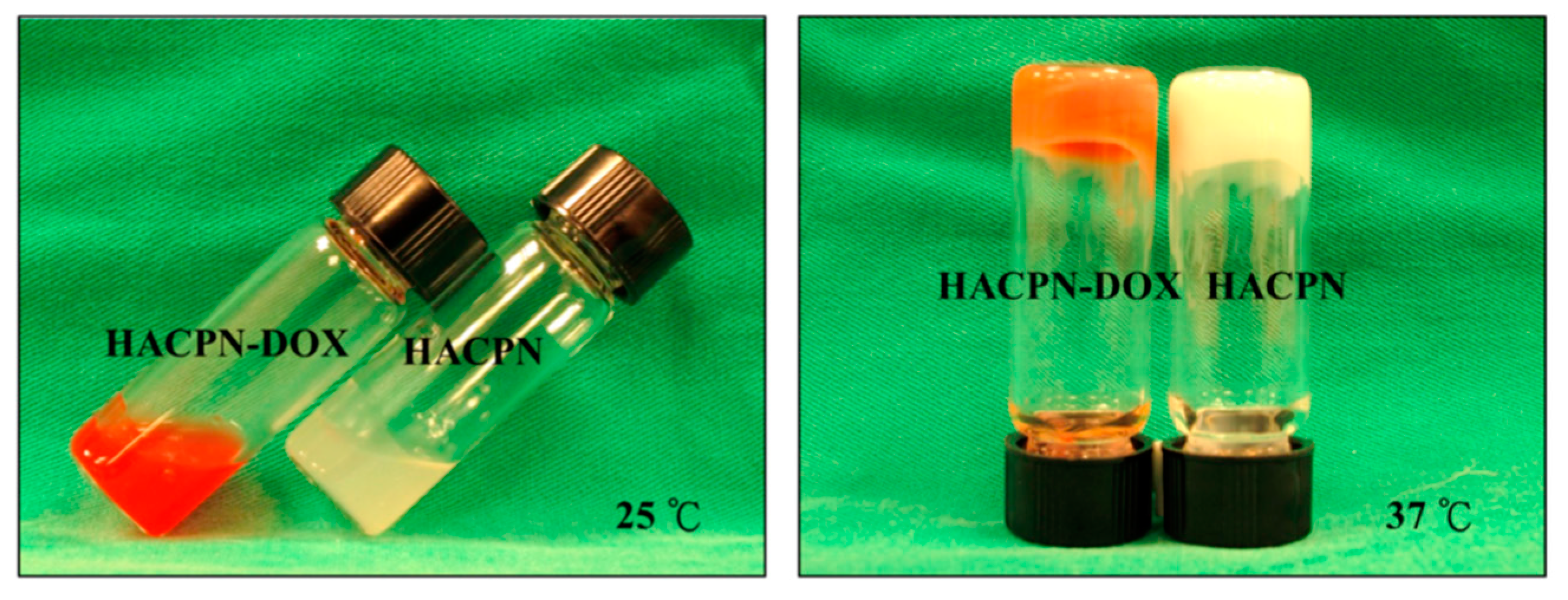
| HACPN (hyaluronic acid-g-chitosan-g-PNIPAM) | DOX | intraperitoneal injection | At pH 7.4, 40% of the drug was released within 8 h (burst release),sustained release of DOX was observed thereafter with 80% of the drug released in 12 days. | Cell viability in the HACPN-DOX group was significantly reduced, reaching 48% and 2% of the control group after 24 and 48 h, respectively. | CT26 | BALB/c mice | Suppressed tumor growth and inhibited tumor angiogenesis. | CT26 | [119] |
| CS/MoS2/Bi2S3-PEG | DOX | injection | 4.5% (at 37 °C and pH = 7.4), 20.3% (at 37 °C and pH = 5.4) and 16.5% (at 50 °C and pH = 5.4) | L929 cells viability treated with CS/MoS2/Bi2S3-PEG/DOX: 47.7% (higher compared to DOX alone 36.53%). HT29 cells viability treated with CS/MoS2/Bi2S3-PEG/DOX decreased to 57.12% ± 3.87% When irradiated with 1064 nm laser, CS/MoS2/Bi2S3-PEG/DOX, CS/MoS2/Bi2S3-PEG +NIR and CS/MoS2/Bi2S3-PEG/DOX+NIR cultured HT29 tumor cell viability declined to 55.13% ± 3.77%, 36.03 ± 3.29%, and 12.02% ± 0.41%, respectively confirming the combined tumor therapy efficiency. | HT29 L929 | HT29 xenografted tumor bearing mice | The volume of the tumor was significantly reduced or even disappeared under the function of combined photothermal and chemotherapy after 14 days’ feeding. | HT29 | [120] |
| CS/genipin/sodium salts | CUR | injection | The initial burst releases at the first 24 h were observed in all gel samples and followed by the sustained release of c.a. 1.0% to 1.8% over day 2 to 7. | Cell viability in 3T3 mouse fibroblast cell lines was above 80%, indicating high cell survival and minimal cytotoxicity under the experimental conditions. | 3T3 | Sprague-Dawley rat | - | - | [121] |
| CSDAGG | CUR, ASA | oral | A minute amount of CUR and ASA was released during the initial 2 h in the SGF (pH 1.2). In the SIF (pH 7.4), the release of ASA and CUR was 50% and 25%, respectively. In colonic fluid (pH 6.5), the cumulative release of ASA and CUR was approximately 90% and 42%, respectively, at 24 h. | At an equivalent drug concentration, the dual drug-loaded hydrogel exhibited higher cellular cytotoxicity compared to the other samples (pristine CUR, CUR-loaded hydrogel, and ASA-loaded hydrogel). | HT29 | - | - | [122] | |
| CS/OD/MTX/TFPM | 5-FU, MTX | oral | Cumulative release of MTX was determined to be 10.99%, 27.53%, 21.47% and 86.51% at pH 1.2, 5.0, 6.8 and 7.4, respectively. The cumulative release of 5-FU at pH 7.4 (maximum of release of MTX) remarkably increased to 89.78% under NIR irradiation. | Cell viability < 10% after treatment with 512 μg/mL CS/OD/MTX/TFPM. The cell viability decreased to 4.9% under NIR irradiation for 30 min. | HT-29 | - | - | - | [123] |
| CMCS/AA | 5-FU | oral | at pH 1.2: 21.37–27.76%; at pH 6.5: 61.79–77.69%; at pH 7.5, 77.08–88.89% of the drug was released within 12 h | 5-FU had dose-dependent cytotoxic potential and the % cell viability decreased with increasing dose per well; 5-FU retained its cytotoxic potential after loading into the hydrogel matrix; no detectable cytotoxicity on Vero cells. | HeLa, Vero cells | - | - | - | [124] |
| CS/AA/AMPS | 5-FU | oral | release of 5-FU after 30 min at pH 1 and 7 was 1.55% and 25.3%, respectively; 96% after 7 h at pH 7. | - | - | - | - | - | [125] |
| GG/PVA/CS | MTX | injection | 50% drug release was observed in the first 5 h and a sustained drug release of 96% in 7.25 h. | IC50 = 11.7 µg/mL at GG/PVA/CS +MTX concentration of 2.34 µg/200 mL | HCT-116 | - | - | - | [126] |
| MA-CMCS | IMT | oral | After 48 h in PBS (pH 7.4), the accumulated percentage of drug release for hydrogel was 55.8%. | CS-based hydrogel was non-cytotoxic and had a good biocompatibility against normal and cancer cells; IMT-loaded hydrogel displayed a dose-dependent cytotoxicity, and cell viabilities declined with the increase of drug concentration. | LS174T, L02 | Balb/c female mice | Significantly enhanced in vivo tumor inhibition (six-fold higher compared to IMT) was achieved after oral administration with IMT-loaded hydrogel. | LS174T | [127] |
| PAA/CSNb/bisTz-PNIPAM | 5-ASA | oral | The cumulative drug release was 8.5% at pH 2.2 and reached 92% at pH 7.4 within 48 h. Additionally, the cumulative drug release from the hydrogels at 25 °C was lower compared to that at 37 °C. | cell viability exceeded 70% | HFF-1 | - | - | - | [128] |
| ONB–CS | DOX | oral | Hydrogel exhibited higher drug release at pH 5.7 (71.75%) than at pH 7.4 (30.82%) after 24 h. | - | - | - | - | - | [129] |
| Nanocomposite DDSs | Therapeutic Agent | Route of Administration | In Vitro Studies | Ref. | ||
|---|---|---|---|---|---|---|
| Drug Release Study | Cytotoxicity (If Available) | Cell Line | ||||
| core–shell ZnO/CMC/CS | 5-FU | The drug accumulated release rate was <20 from ZnO/CMC/CS beads within 2 h at SGF (pH 1.2). The cumulative release reached 80% after the next 3 h at SIF (pH 6.8); at SCF (pH 7.4) for a further 3 h the release rate was still rising due to the more hydrophilic system, leading the whole state to collapse drastically. | - | - | [146] | |
| CsDAP@ZnO | 5-FU | oral | Negligible amount of 5-FU was released during the initial 2 h in SGF (pH 1.2). The release was considerably expedited from 2 to 7 h in the SIF (pH 7.4) from both the hydrogels and gradually increased in SCF (pH 6.5). | CsDAP@ZnO nanocomposite hydrogel demonstrated greater toxicity on the colon cancer cells with respect to Sap hydrogel at an equivalent concentration. | HT-29 | [147] |
| CS/PAA/Fe3O4 | 5-FU | colon and rectal | At pH 7.4 in 37 °C the release rate of 5-FU from hydrogel was decreased with the increase of cross-linker and Fe3O4 NPs. Release kinetics from nanohydrogel conformed to the Weibull model. | - | - | [148] |
| CAR/TMC-Ag | CUR | oral | Sustained drug release reached 98.9% ± 0.9 within 24 h in pH 7.4. | High cytotoxic effect with apoptotic induction against Caco-2 cells through G2/M cell cycle arrest | Caco-2 | [149] |
| CS/rGO | 5-FU CUR | - | pH 5.0 In 72 h, 90% of the release was attained in 5-FU-loaded systems showing higher release over CUR-loaded composites. | IC50 = 23.8 μg/mL for dual-drug-loaded nanocomposite; IC50 = 37.61 μg/mL for 5-FU loaded nanocomposite, IC50 = 48.12 μg/mL for CUR-loaded nanocomposite. The cell viability at 40 µg/mL for the NIH 3T3 mouse embryonic fibroblast cells was found to be 80.3%. | HT-29 NIH 3T3 | [150] |
| MCPC | CUR | oral | At pH 1.2 18% CU was released during 2h, and up to 68% release in caecal medium over 24 h. | - | [151] | |
| CS-PLGA NPs | TA/E | intraperitoneally | - | CS-PLGA NPs significantly inhibited tumor number and tumor volume and normalized colon histology in the colon cancer. | - | [152] |
| CS hydrogel-coated Au NPs | PTX | - | CS hydrogel-coated Au NPs were able to increase the expression of pro-apoptotic BAX and BAD and decrease the expression of anti-apoptotic BCL2 more than PTX alone. | LS174T | [153] | |
| CS-Based Hydrogel System | Advantages | Disadvantages |
|---|---|---|
| In situ gels | Localized therapy Controlled drug delivery Postoperative adjuvant chemotherapy Endoscopic mucosal resection technique for accurate removal of polyps and early-stage tumors Intraperitoneal chemotherapy Unique pharmacokinetics parameters High payload efficiency Eliminating the need for surgical removal Reduced peritoneal adhesion formation Antibacterial activity (efflux pump inhibition) | High intraperitoneal-to-plasma drug concentration ratio Low mechanical strength Slow gelation time Burst release Inability of CS to remain in solution at physiological pH Risk of obstructing the endoscopic needle during injection Large-scale production challenges Potential immunogenicity |
| Stimuli-responsive multi-drug hydrogels | Porous structures increase drug loading Responses to colon selectivity (pH-, enzymatic-, temperature-, redox, pressure, and mechanical stimuli) Bio-adhesiveness Enhanced drug release Antibacterial activity Targeting photodynamic and PTT therapy | Burst release in stomach when applied oral Low mechanical strength Large-scale production challenges Tendency to coagulate with protein at high pH Complex drug release control Potential immunogenicity Biodegradation rate challenges May cause localized tissue damage |
| Nanocomposite hydrogels | Mechanical strength Thermal stability Enhance drug delivery Minimize side effects Extend drug lifetime in the bloodstream Protection against acidic and enzymatic degradation in the gastrointestinal tract Reduce burst release Permeation enhancement Controlled drug release Improve drug-loading efficiency Stabilize NPs CS-based NPs and CS-coated microspheres facilitate drug transport partly through endocytosis and transcytosis Antibacterial activity | Complex manufacturing process Large-scale production challenges Batch-to-batch variability Rapid biodegradation Potential immunogenicity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piotrowska, U.; Orzechowska, K. Advances in Chitosan-Based Smart Hydrogels for Colorectal Cancer Treatment. Pharmaceuticals 2024, 17, 1260. https://doi.org/10.3390/ph17101260
Piotrowska U, Orzechowska K. Advances in Chitosan-Based Smart Hydrogels for Colorectal Cancer Treatment. Pharmaceuticals. 2024; 17(10):1260. https://doi.org/10.3390/ph17101260
Chicago/Turabian StylePiotrowska, Urszula, and Klaudia Orzechowska. 2024. "Advances in Chitosan-Based Smart Hydrogels for Colorectal Cancer Treatment" Pharmaceuticals 17, no. 10: 1260. https://doi.org/10.3390/ph17101260
APA StylePiotrowska, U., & Orzechowska, K. (2024). Advances in Chitosan-Based Smart Hydrogels for Colorectal Cancer Treatment. Pharmaceuticals, 17(10), 1260. https://doi.org/10.3390/ph17101260







