Retrospective Analysis of a Real-Life Use of Tixagevimab–Cilgavimab plus SARS-CoV-2 Antivirals for Treatment of COVID-19
Abstract
:1. Introduction
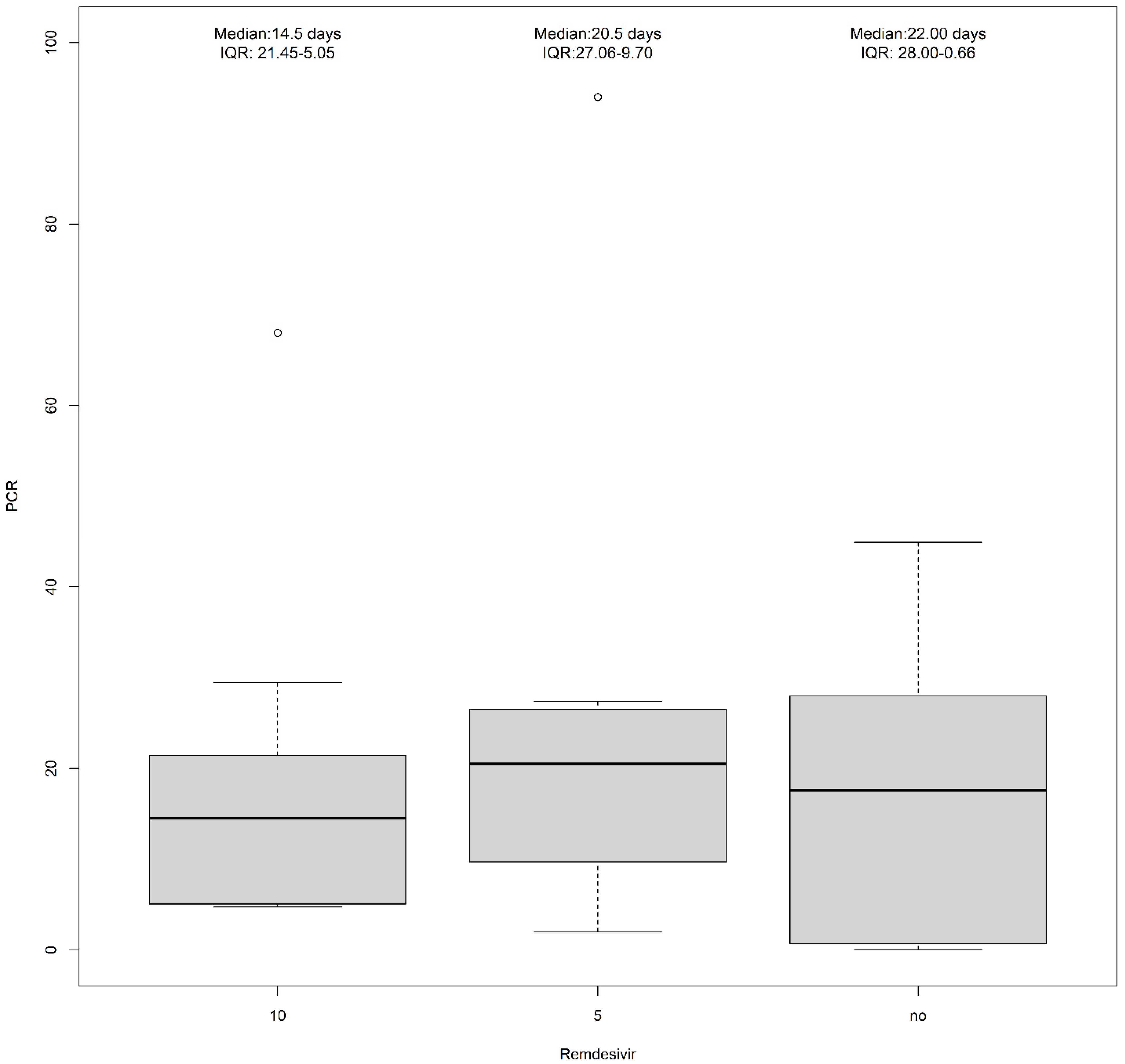
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hammond, J.; Leister-Tebbe, H.; Gardner, A.; Abreu, P.; Bao, W.; Wisemandle, W.; Baniecki, M.; Hendrick, V.M.; Damle, B.; Simón-Campos, A.; et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with COVID-19. N. Engl. J. Med. 2022, 386, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- UK NERVTAG: Antiviral Drug Resistance and the Use of Directly Acting Antiviral Drugs (DAAs) for COVID-19, 8 December 2021—GOV.UK. Available online: https://www.gov.uk/government/publications/nervtag-antiviral-drug-resistance-and-the-use-of-directly-acting-antiviral-drugs-daas-for-covid-19-8-december-2021/nervtag-antiviral-drug-resistance-and-the-use-of-directly-acting-antiviral-drugs-daas-for-covid-19-8-december-2021 (accessed on 3 May 2023).
- Dong, J.; Zost, S.J.; Greaney, A.J.; Starr, T.N.; Dingens, A.S.; Chen, E.C.; Chen, R.E.; Case, J.B.; Sutton, R.E.; Gilchuk, P.; et al. Genetic and Structural Basis for SARS-CoV-2 Variant Neutralization by a Two-Antibody Cocktail. Nat. Microbiol. 2021, 6, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Loo, Y.M.; McTamney, P.M.; Arends, R.H.; Abram, M.E.; Aksyuk, A.A.; Diallo, S.; Flores, D.J.; Kelly, E.J.; Ren, K.; Roque, R.; et al. The SARS-CoV-2 Monoclonal Antibody Combination, AZD7442, Is Protective in Nonhuman Primates and Has an Extended Half-Life in Humans. Sci. Transl. Med. 2022, 14, eabl8124. [Google Scholar] [CrossRef]
- Oganesyan, V.; Damschroder, M.M.; Woods, R.M.; Cook, K.E.; Wu, H.; Dall’Acqua, W.F. Structural Characterization of a Human Fc Fragment Engineered for Extended Serum Half-Life. Mol. Immunol. 2009, 46, 1750–1755. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.P.; Forleo-Neto, E.; Musser, B.J.; Isa, F.; Chan, K.-C.; Sarkar, N.; Bar, K.J.; Barnabas, R.V.; Barouch, D.H.; Cohen, M.S.; et al. Subcutaneous REGEN-COV Antibody Combination to Prevent COVID-19. N. Engl. J. Med. 2021, 385, 1184–1195. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Bamlanivimab and Etesevimab. Available online: Chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.fda.gov/media/145802/download (accessed on 3 May 2023).
- Montgomery, H.; Hobbs, F.D.R.; Padilla, F.; Arbetter, D.; Templeton, A.; Seegobin, S.; Kim, K.; Campos, J.A.S.; Arends, R.H.; Brodek, B.H.; et al. Efficacy and Safety of Intramuscular Administration of Tixagevimab–Cilgavimab for Early Outpatient Treatment of COVID-19 (TACKLE): A Phase 3, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Respir. Med. 2022, 10, 985–996. [Google Scholar] [CrossRef]
- Keam, S.J. Tixagevimab + Cilgavimab: First Approval. Drugs 2022, 82, 1001. [Google Scholar] [CrossRef]
- Europea Medicine Agency Evusheld: EPAR—Product Information. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/evusheld (accessed on 13 April 2023).
- Ginde, A.A.; Paredes, R.; Murray, T.A.; Engen, N.; Grandits, G.; Vekstein, A.; Ivey, N.; Mourad, A.; Sandkovsky, U.; Gottlieb, R.L.; et al. Tixagevimab-Cilgavimab for Treatment of Patients Hospitalised with COVID-19: A Randomised, Double-Blind, Phase 3 Trial. Lancet. Respir. Med. 2022, 10, 972–984. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, J.; Zhu, K.; Xu, C.; Wang, D.; Hou, M. The Effect of Tixagevimab-Cilgavimab on Clinical Outcomes in Patients with COVID-19: A Systematic Review with Meta-Analysis. J. Infect. 2023, 86, e15–e17. [Google Scholar] [CrossRef]
- Lafont, E.; Pere, H.; Lebeaux, D.; Cheminet, G.; Thervet, E.; Guillemain, R.; Flahault, A. Targeted SARS-CoV-2 Treatment Is Associated with Decreased Mortality in Immunocompromised Patients with COVID-19. J. Antimicrob. Chemother. 2022, 77, 2688–2692. [Google Scholar] [CrossRef]
- Dougan, M.; Nirula, A.; Azizad, M.; Mocherla, B.; Gottlieb, R.L.; Chen, P.; Hebert, C.; Perry, R.; Boscia, J.; Heller, B.; et al. Bamlanivimab plus Etesevimab in Mild or Moderate COVID-19. N. Engl. J. Med. 2021, 385, 1382–1392. [Google Scholar] [CrossRef]
- Hippisley-Cox, J.; Coupland, C.A.C.; Mehta, N.; Keogh, R.H.; Diaz-Ordaz, K.; Khunti, K.; Lyons, R.A.; Kee, F.; Sheikh, A.; Rahman, S.; et al. Risk Prediction of COVID-19 Related Death and Hospital Admission in Adults after COVID-19 Vaccination: National Prospective Cohort Study. BMJ 2021, 374, n2244. [Google Scholar] [CrossRef]
- Agrawal, U.; Katikireddi, S.V.; McCowan, C.; Mulholland, R.H.; Azcoaga-Lorenzo, A.; Amele, S.; Fagbamigbe, A.F.; Vasileiou, E.; Grange, Z.; Shi, T.; et al. COVID-19 Hospital Admissions and Deaths after BNT162b2 and ChAdOx1 NCoV-19 Vaccinations in 2·57 Million People in Scotland (EAVE II): A Prospective Cohort Study. Lancet. Respir. Med. 2021, 9, 1439–1449. [Google Scholar] [CrossRef]
- Antonelli, M.; Penfold, R.S.; Merino, J.; Sudre, C.H.; Molteni, E.; Berry, S.; Canas, L.S.; Graham, M.S.; Klaser, K.; Modat, M.; et al. Risk Factors and Disease Profile of Post-Vaccination SARS-CoV-2 Infection in UK Users of the COVID Symptom Study App: A Prospective, Community-Based, Nested, Case-Control Study. Lancet. Infect. Dis. 2022, 22, 43–55. [Google Scholar] [CrossRef]
- Kemp, S.A.; Collier, D.A.; Datir, R.P.; Ferreira, I.A.T.M.; Gayed, S.; Jahun, A.; Hosmillo, M.; Rees-Spear, C.; Mlcochova, P.; Lumb, I.U.; et al. SARS-CoV-2 Evolution during Treatment of Chronic Infection. Nature 2021, 592, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Langerbeins, P.; Hallek, M. COVID-19 in Patients with Hematologic Malignancy. Blood 2022, 140, 236–252. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, E.A.; Segal, B.H. Immune Responses to COVID-19 Vaccines in Patients with Cancer: Promising Results and a Note of Caution. Cancer Cell 2021, 39, 1045–1047. [Google Scholar] [CrossRef] [PubMed]
- Greenberger, L.M.; Saltzman, L.A.; Senefeld, J.W.; Johnson, P.W.; DeGennaro, L.J.; Nichols, G.L. Antibody Response to SARS-CoV-2 Vaccines in Patients with Hematologic Malignancies. Cancer Cell 2021, 39, 1031–1033. [Google Scholar] [CrossRef] [PubMed]
- Nkolola, J.P.; Yu, J.; Wan, H.; Chang, A.; McMahan, K.; Anioke, T.; Jacob-Dolan, C.; Powers, O.; Ye, T.; Chandrashekar, A.; et al. A Bivalent SARS-CoV-2 Monoclonal Antibody Combination Does Not Affect the Immunogenicity of a Vector-Based COVID-19 Vaccine in Macaques. Sci. Transl. Med. 2022, 14, eabo6160. [Google Scholar] [CrossRef] [PubMed]
- Bar, D.Z.; Atkatsh, K.; Tavarez, U.; Erdos, M.R.; Gruenbaum, Y.; Collins, F.S. Biotinylation by Antibody Recognition—A Method for Proximity Labeling. Nat. Methods 2017, 15, 127–133. [Google Scholar] [CrossRef]
- Conte, W.L.; Golzarri-Arroyo, L. Tixagevimab and Cilgavimab (Evusheld) Boosts Antibody Levels to SARS-CoV-2 in Patients with Multiple Sclerosis on b-Cell Depleters. Mult. Scler. Relat. Disord. 2022, 63, 103905. [Google Scholar] [CrossRef] [PubMed]
- Soeroto, A.Y.; Yanto, T.A.; Kurniawan, A.; Hariyanto, T.I. Efficacy and Safety of Tixagevimab-Cilgavimab as Pre-Exposure Prophylaxis for COVID-19: A Systematic Review and Meta-Analysis. Rev. Med. Virol. 2023, 33, e2420. [Google Scholar] [CrossRef] [PubMed]
- Suribhatla, R.; Starkey, T.; Ionescu, M.C.; Pagliuca, A.; Richter, A.; Lee, L.Y.W. Systematic Review and Meta-Analysis of the Clinical Effectiveness of Tixagevimab/Cilgavimab for Prophylaxis of COVID-19 in Immunocompromised Patients. Br. J. Haematol. 2023, 201, 813–823. [Google Scholar] [CrossRef]
- Levin, M.J.; Ustianowski, A.; Thomas, S.; Templeton, A.; Yuan, Y.; Seegobin, S.; Houlihan, C.F.; Menendez-Perez, I.; Pollett, S.; Arends, R.H.; et al. AZD7442 (Tixagevimab/Cilgavimab) for Post-Exposure Prophylaxis of Symptomatic Coronavirus Disease 2019. Clin. Infect. Dis. 2023, 76, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Iketani, S.; Liu, L.; Guo, Y.; Liu, L.; Chan, J.F.W.; Huang, Y.; Wang, M.; Luo, Y.; Yu, J.; Chu, H.; et al. Antibody Evasion Properties of SARS-CoV-2 Omicron Sublineages. Nature 2022, 604, 553–556. [Google Scholar] [CrossRef]
- VanBlargan, L.A.; Errico, J.M.; Halfmann, P.J.; Zost, S.J.; Crowe, J.E.; Purcell, L.A.; Kawaoka, Y.; Corti, D.; Fremont, D.H.; Diamond, M.S. An Infectious SARS-CoV-2 B.1.1.529 Omicron Virus Escapes Neutralization by Therapeutic Monoclonal Antibodies. Nat. Med. 2022, 28, 490–495. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, L.; Misasi, J.; Pegu, A.; Zhang, Y.; Harris, D.R.; Olia, A.S.; Talana, C.A.; Yang, E.S.; Chen, M.; et al. Structural Basis for Potent Antibody Neutralization of SARS-CoV-2 Variants Including B.1.1.529. Science 2022, 376, eabn8897. [Google Scholar] [CrossRef]
- Tuekprakhon, A.; Nutalai, R.; Dijokaite-Guraliuc, A.; Zhou, D.; Ginn, H.M.; Selvaraj, M.; Liu, C.; Mentzer, A.J.; Supasa, P.; Duyvesteyn, H.M.E.; et al. Antibody Escape of SARS-CoV-2 Omicron BA.4 and BA.5 from Vaccine and BA.1 Serum. Cell 2022, 185, 2422–2433.e13. [Google Scholar] [CrossRef]
- Das, N.C.; Chakraborty, P.; Bayry, J.; Mukherjee, S. In Silico Analyses on the Comparative Potential of Therapeutic Human Monoclonal Antibodies Against Newly Emerged SARS-CoV-2 Variants Bearing Mutant Spike Protein. Front. Immunol. 2022, 12, 782506. [Google Scholar] [CrossRef]
- Das, N.C.; Chakraborty, P.; Bayry, J.; Mukherjee, S. Comparative Binding Ability of Human Monoclonal Antibodies against Omi. cron Variants of SARS-CoV-2: An In Silico Investigation. Antibodies 2023, 12, 17. [Google Scholar] [CrossRef]
- Perrella, A.; Bisogno, M.; D’Argenzio, A.; Trama, U.; Coscioni, E.; Orlando, V. Risk of SARS-CoV-2 Infection Breakthrough among the Non-Vaccinated and Vaccinated Population in Italy: A Real-World Evidence Study Based on Big Data. Healthcare 2022, 10, 1085. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ecdc.europa.eu/en/news-events/epidemiological-update-covid-19-transmission-eueea-sars-cov-2-variants-and-public (accessed on 1 August 2023).
| A (N = 27) | B (N = 15) | Overall (N = 42) | |
|---|---|---|---|
| Age | |||
| Mean (SD) | 66.8 (18.2) | 69.9 (10.1) | 68.0 (15.6) |
| Median [Min, Max] | 71.0 [35.0, 98.0] | 73.0 [49.0, 88.0] | 71.0 [35.0, 98.0] |
| Missing | 2 (7.4%) | 0 (0%) | 2 (4.8%) |
| Gender | |||
| F | 15 (55.6%) | 6 (40.0%) | 21 (50.0%) |
| M | 12 (44.4%) | 9 (60.0%) | 21 (50.0%) |
| CRP | |||
| Mean (SD) | 16.3 (12.6) | 25.3 (30.9) | 19.3 (20.5) |
| Median [Min, Max] | 16.1 [0.0200, 44.9] | 13.2 [4.70, 94.0] | 14.8 [0.0200, 94.0] |
| Missing | 7 (25.9%) | 5 (33.3%) | 12 (28.6%) |
| IL6 | |||
| Mean (SD) | 176 (509) | 36.6 (28.6) | 116 (387) |
| Median [Min, Max] | 19.0 [3.20, 2030] | 27.7 [3.10, 96.3] | 22.9 [3.10, 2030] |
| Missing | 11 (40.7%) | 3 (20.0%) | 14 (33.3%) |
| D-Dimer | |||
| Mean (SD) | 1880 (1910) | 521 (477) | 1400 (1680) |
| Median [Min, Max] | 1030 [220, 6890] | 290 [103, 1470] | 776 [103, 6890] |
| Missing | 5 (18.5%) | 3 (20.0%) | 8 (19.0%) |
| Fibrinogen | |||
| Mean (SD) | 539 (254) | 493 (107) | 527 (221) |
| Median [Min, Max] | 554 [179, 1140] | 451 [387, 666] | 519 [179, 1140] |
| Missing | 14 (51.9%) | 10 (66.7%) | 24 (57.1%) |
| Procalcitonin | |||
| Mean (SD) | 2.57 (5.82) | 0.788 (2.54) | 1.92 (4.91) |
| Median [Min, Max] | 0.940 [0.0200, 26.6] | 0.0500 [0.0200, 8.86] | 0.140 [0.0200, 26.6] |
| Missing | 6 (22.2%) | 3 (20.0%) | 9 (21.4%) |
| IgA | |||
| Mean (SD) | 247 (124) | 124 (135) | 196 (140) |
| Median [Min, Max] | 235 [35.0, 519] | 78.5 [11.0, 495] | 156 [11.0, 519] |
| Missing | 10 (37.0%) | 3 (20.0%) | 13 (31.0%) |
| IgM | |||
| Mean (SD) | 125 (133) | 29.4 (11.5) | 95.5 (119) |
| Median [Min, Max] | 73.0 [29.0, 580] | 25.5 [21.0, 53.0] | 62.5 [21.0, 580] |
| Missing | 9 (33.3%) | 7 (46.7%) | 16 (38.1%) |
| IgG | |||
| Mean (SD) | 953 (413) | 631 (315) | 822 (404) |
| Median [Min, Max] | 991 [245, 1780] | 662 [149, 1290] | 771 [149, 1780] |
| Missing | 8 (29.6%) | 2 (13.3%) | 10 (23.8%) |
| Antiviral therapy | |||
| Remdesivir (10 mg) | 4 (14.8%) | 9 (60.0%) | 13 (31.0%) |
| Remdesivir (5 mg) | 7 (25.9%) | 3 (20.0%) | 10 (23.8%) |
| No treatment | 11 (40.7%) | 1 (6.7%) | 12 (28.6%) |
| Molnupiravir | 1 (3.7%) | 0 (0%) | 1 (2.4%) |
| Missing | 4 (14.8%) | 2 (13.3%) | 6 (14.3%) |
| COVID-19 vaccine | |||
| Not vaccinated | 12 (44.4%) | 4 (26.7%) | 16 (38.1%) |
| Two doses | 5 (18.5%) | 1 (3.7%) | 6 (14.3%) |
| Three doses | 8 (29.6%) | 10 (66.7%) | 18 (42.8%) |
| Four doses | 2 (7.4%) | - | 2 (4.8%) |
| Diseases | Group A | Group B |
|---|---|---|
| Cardiovascular disorders (n = 8) | ||
| Hypertensive cardiopathy | 3 | - |
| Atrial fibrillation | 2 | - |
| Arterial hypertension | 1 | - |
| Ischemic cardiopathy | 1 | - |
| Stroke | 1 | - |
| Degenerative diseases (n = 7) | ||
| Wagner syndrome | 1 | - |
| Alzheimer’s disease | 4 | - |
| Multiple sclerosis | 1 | - |
| Creutzfeldt-Jakob disease | 1 | - |
| Solid tumors (n = 5) | ||
| Lung carcinoma | 4 | - |
| Breast carcinoma | 1 | - |
| Infections (n = 4) | ||
| Cirrhosis HBV related | 1 | - |
| Cryptococcal meningitis | 1 | - |
| HIV | 2 | - |
| Autoimmune disorders (n = 3) | ||
| Autoimmune gastritis | 1 | - |
| Rheumatoid arthritis | 1 | - |
| Magic Syndrome | 1 | - |
| Other (n = 5) | ||
| Iatrogenic marrow aplasia | 1 | - |
| Chronic kidney disease | 4 | - |
| Oncohematological diseases (n = 15) | ||
| Chronic lymphocytic leukemia | - | 4 |
| Non-Hodgkin lymphoma | - | 11 |
| Group A |
| Patient 2: GGO + consolidation |
| Patient 3: 7/20 + acinetobacter multi-drug-resistant |
| Patient 4: 15/20 |
| Patient 7: 5/20 |
| Patient 14: GGO |
| Patient 15: GGO + effusion |
| Patient 16: 9/20 |
| Patient 18: GGO + thickening |
| Patient 19: GGO + effusion |
| Patient 20: Not available |
| Patient 21: GGO + thickening |
| Patient 23: no pneumonia |
| Patient 24: GGO |
| Patient 27: GGO + thickening |
| Patient 28: GGO |
| Patient 29: 13/20 |
| Patient 30: Not available |
| Patient 31: 7/20 |
| Patient 32: GGO + thickening |
| Patient 33: GGO |
| Patient 34: no pneumonia |
| Patient 35: Not available |
| Patients 36: Not available |
| Patient 37: GGO + thickening + effusion |
| Patient 38: Not available |
| Patient 39: GGO + thickening + effusion |
| Patient 40: GGO + thickening |
| Group B |
| Patient 1: GGO + Legionella infection |
| Patient 5: 13/20 |
| Patient 6: areoles |
| Patient 8: Not available |
| Patient 9: GGO |
| Patient 10: 18/25 |
| Patient 11: 12/20 |
| Patient 12: GGO |
| Patient 13: GGO + consolidation + effusion |
| Patient 17: GGO + thickening |
| Patient 22: cerebral edema, no pneumonia |
| Patient 25: GGO |
| Patient 26: GGO + consolidation |
| Patient 41: 4/20 + thickening |
| Patient 42: bilateral GGO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capoluongo, N.; Mascolo, A.; Bernardi, F.F.; Sarno, M.; Mattera, V.; di Flumeri, G.; Pustorino, B.; Spaterella, M.; Trama, U.; Capuano, A.; et al. Retrospective Analysis of a Real-Life Use of Tixagevimab–Cilgavimab plus SARS-CoV-2 Antivirals for Treatment of COVID-19. Pharmaceuticals 2023, 16, 1493. https://doi.org/10.3390/ph16101493
Capoluongo N, Mascolo A, Bernardi FF, Sarno M, Mattera V, di Flumeri G, Pustorino B, Spaterella M, Trama U, Capuano A, et al. Retrospective Analysis of a Real-Life Use of Tixagevimab–Cilgavimab plus SARS-CoV-2 Antivirals for Treatment of COVID-19. Pharmaceuticals. 2023; 16(10):1493. https://doi.org/10.3390/ph16101493
Chicago/Turabian StyleCapoluongo, Nicolina, Annamaria Mascolo, Francesca Futura Bernardi, Marina Sarno, Valentina Mattera, Giusy di Flumeri, Bruno Pustorino, Micaela Spaterella, Ugo Trama, Annalisa Capuano, and et al. 2023. "Retrospective Analysis of a Real-Life Use of Tixagevimab–Cilgavimab plus SARS-CoV-2 Antivirals for Treatment of COVID-19" Pharmaceuticals 16, no. 10: 1493. https://doi.org/10.3390/ph16101493
APA StyleCapoluongo, N., Mascolo, A., Bernardi, F. F., Sarno, M., Mattera, V., di Flumeri, G., Pustorino, B., Spaterella, M., Trama, U., Capuano, A., & Perrella, A. (2023). Retrospective Analysis of a Real-Life Use of Tixagevimab–Cilgavimab plus SARS-CoV-2 Antivirals for Treatment of COVID-19. Pharmaceuticals, 16(10), 1493. https://doi.org/10.3390/ph16101493







