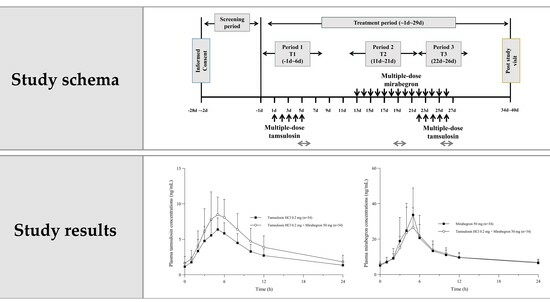
Drug–Drug Interactions between Tamsulosin and Mirabegron in Healthy Individuals Do Not Affect Pharmacokinetics and Hemodynamic Parameters Significantly
Abstract
1. Introduction
2. Results
2.1. Demographic Characteristics
2.2. Pharmacokinetic Analysis
2.3. Safety Analysis
3. Discussion
4. Materials and Methods
4.1. Participants and Study Design
4.2. Blood Sampling and Determination of Tamsulosin and Mirabegron Plasma Concentrations
4.3. Pharmacokinetic Assessment
4.4. Safety Assessment
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gormley, E.A.; Lightner, D.J.; Faraday, M.; Vasavada, S.P.; American Urological Association; Society of Urodynamics, Female Pelvic Medicine. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline amendment. J. Urol. 2015, 193, 1572–1580. [Google Scholar] [PubMed]
- Vrijens, D.; Drossaerts, J.; van Koeveringe, G.; Van Kerrebroeck, P.; van Os, J.; Leue, C. Affective symptoms and the overactive bladder—A systematic review. J. Psychosom. Res. 2015, 78, 95–108. [Google Scholar] [PubMed]
- Milsom, I.; Abrams, P.; Cardozo, L.; Roberts, R.G.; Thuroff, J.; Wein, A.J. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int. 2001, 87, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Stewart, W.F.; Van Rooyen, J.B.; Cundiff, G.W.; Abrams, P.; Herzog, A.R.; Corey, R.; Hunt, T.L.; Wein, A.J. Prevalence and burden of overactive bladder in the United States. World J. Urol. 2003, 20, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.; Parsons, M. Treatment of overactive bladder in the aging population: Focus on darifenacin. Clin. Interv. Aging 2006, 1, 309–316. [Google Scholar] [CrossRef]
- Sussman, D.O. Overactive bladder: Treatment options in primary care medicine. J. Am. Osteopath. Assoc. 2007, 107, 379–385. [Google Scholar]
- Thiagamoorthy, G.; Cardozo, L.; Srikrishna, S. Drug therapy for an overactive bladder. Womens Health 2015, 11, 445–448. [Google Scholar] [CrossRef]
- Sacco, E.; Bientinesi, R. Mirabegron: A review of recent data and its prospects in the management of overactive bladder. Ther. Adv. Urol. 2012, 4, 315–324. [Google Scholar] [CrossRef]
- Arnold, J.; McLeod, N.; Thani-Gasalam, R.; Rashid, P. Overactive bladder syndrome—Management and treatment options. Aust. Fam. Physician 2012, 41, 878–883. [Google Scholar]
- Nitti, V.W.; Khullar, V.; van Kerrebroeck, P.; Herschorn, S.; Cambronero, J.; Angulo, J.C.; Blauwet, M.B.; Dorrepaal, C.; Siddiqui, E.; Martin, N.E. Mirabegron for the treatment of overactive bladder: A prespecified pooled efficacy analysis and pooled safety analysis of three randomised, double-blind, placebo-controlled, phase III studies. Int. J. Clin. Pract. 2013, 67, 619–632. [Google Scholar] [CrossRef]
- Abrams, P.; Andersson, K.E.; Apostolidis, A.; Birder, L.; Bliss, D.; Brubaker, L.; Cardozo, L.; Castro-Diaz, D.; O’Connell, P.R.; Cottenden, A.; et al. 6th International Consultation on Incontinence: Recommendations of the International Scientific Committee: Evaluation and treatment of urinary incontinence, pelvic organ prolapse and faecal incontinence. Neurourol. Urodyn. 2018, 37, 2271–2272. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, A.O.; Smith, M.J.; Miller, L.A.; Doyle, J.; Ariely, R. Persistence, adherence, and switch rates among extended-release and immediate-release overactive bladder medications in a regional managed care plan. J. Manag. Care Pharm. 2008, 14, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Sexton, C.C.; Notte, S.M.; Maroulis, C.; Dmochowski, R.R.; Cardozo, L.; Subramanian, D.; Coyne, K.S. Persistence and adherence in the treatment of overactive bladder syndrome with anticholinergic therapy: A systematic review of the literature. Int. J. Clin. Pract. 2011, 65, 567–585. [Google Scholar] [PubMed]
- Yeaw, J.; Benner, J.S.; Walt, J.G.; Sian, S.; Smith, D.B. Comparing adherence and persistence across 6 chronic medication classes. J. Manag. Care Pharm. 2009, 15, 728–740. [Google Scholar] [CrossRef] [PubMed]
- Chancellor, M.B.; Migliaccio-Walle, K.; Bramley, T.J.; Chaudhari, S.L.; Corbell, C.; Globe, D. Long-term patterns of use and treatment failure with anticholinergic agents for overactive bladder. Clin. Ther. 2013, 35, 1744–1751. [Google Scholar] [CrossRef] [PubMed]
- Goldman, H.B.; Oelke, M.; Kaplan, S.A.; Kitta, T.; Russell, D.; Carlsson, M.; Arumi, D.; Mangan, E.; Ntanios, F. Do patient characteristics predict which patients with overactive bladder benefit from a higher fesoterodine dose? Int. Urogynecol. J. 2019, 30, 239–344. [Google Scholar] [CrossRef]
- Wang, C.C.; Jiang, Y.H.; Kuo, H.C. Efficacy and Adherence of Flexibly Adding on a Second Antimuscarinic Agent for Patients with Refractory Overactive Bladder. Low Urin. Tract Symptoms 2017, 9, 27–32. [Google Scholar] [CrossRef]
- Starkman, J.S.; Smith, C.P.; Staskin, D.R. Surgical options for drug-refractory overactive bladder patients. Rev. Urol. 2010, 12, e97–e110. [Google Scholar]
- El-Zawahry, A.; Rizk, D.E.E. Combination pharmacotherapy for the treatment of the overactive bladder syndrome: A new solution for an old problem? Int. Urogynecol. J. 2020, 31, 855–856. [Google Scholar] [CrossRef]
- Renal & Urology News. FDA Approves Mirabegron, Solifenacin Combo for OAB. 2018. Available online: https://www.renalandurologynews.com/home/news/urology/overactive-bladder-oab/fda-approves-mirabegron-solifenacin-combo-for-oab/ (accessed on 9 May 2023).
- Hsu, F.C.; Weeks, C.E.; Selph, S.S.; Blazina, I.; Holmes, R.S.; McDonagh, M.S. Updating the evidence on drugs to treat overactive bladder: A systematic review. Int. Urogynecol. J. 2019, 30, 1603–1617. [Google Scholar] [CrossRef]
- Kelleher, C.; Hakimi, Z.; Zur, R.; Siddiqui, E.; Maman, K.; Aballéa, S.; Nazir, J.; Chapple, C. Efficacy and Tolerability of Mirabegron Compared with Antimuscarinic Monotherapy or Combination Therapies for Overactive Bladder: A Systematic Review and Network Meta-analysis. Eur. Urol. 2018, 74, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Staskin, D.; Herschorn, S.; Fialkov, J.; Tu, L.M.; Walsh, T.; Schermer, C.R. A prospective, double-blind, randomized, two-period crossover, multicenter study to evaluate tolerability and patient preference between mirabegron and tolterodine in patients with overactive bladder (PREFER study). Int. Urogynecol. J. 2018, 29, 273–2283. [Google Scholar] [CrossRef] [PubMed]
- Lepor, H. Alpha blockers for the treatment of benign prostatic hyperplasia. Rev. Urol. 2007, 9, 181–190. [Google Scholar] [CrossRef]
- Trevisani, M.; Campi, B.; Gatti, R.; André, E.; Materazzi, S.; Nicoletti, P.; Gazzieri, D.; Geppetti, P. The influence of alpha1-adrenoreceptors on neuropeptide release from primary sensory neurons of the lower urinary tract. Eur. Urol. 2007, 52, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, O.; Yusup, A.; Oyama, N.; Aoki, Y.; Miwa, Y.; Akino, H. Improvement in bladder storage function by tamsulosin depends on suppression of C-fiber urethral afferent activity in rats. J. Urol. 2007, 177, 771–775. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ichihara, K.; Masumori, N.; Fukuta, F.; Tsukamoto, T.; Iwasawa, A.; Tanaka, Y. A randomized controlled study of the efficacy of tamsulosin monotherapy and its combination with mirabegron for overactive bladder induced by benign prostatic obstruction. J. Urol. 2015, 193, 921–926. [Google Scholar] [CrossRef]
- Wada, N.; Iuchi, H.; Kita, M.; Hashizume, K.; Matsumoto, S.; Kakizaki, H. Urodynamic Efficacy and Safety of Mirabegron Add-on Treatment with Tamsulosin for Japanese Male Patients with Overactive Bladder. Low Urin. Tract Symptoms 2016, 8, 171–176. [Google Scholar] [CrossRef]
- Nomura, Y.; Iitsuka, H.; Toyoshima, J.; Kuroishi, K.; Hatta, T.; Kaibara, A.; Katashima, M.; Moy, S.; Sawamoto, T. Pharmacokinetic drug interaction study between overactive bladder drugs mirabegron and tolterodine in Japanese healthy postmenopausal females. Drug Metab. Pharmacokinet. 2016, 31, 411–416. [Google Scholar] [CrossRef]
- Krauwinkel, W.; Dickinson, J.; Schaddelee, M.; Meijer, J.; Tretter, R.; van de Wetering, J.; Strabach, G.; van Gelderen, M. The effect of mirabegron, a potent and selective beta3-adrenoceptor agonist, on the pharmacokinetics of CYP2D6 substrates desipramine and metoprolol. Eur. J. Drug Metab. Pharmacokinet. 2014, 39, 43–52. [Google Scholar] [CrossRef]
- Franco-Salinas, G.; de la Rosette, J.J.; Michel, M.C. Pharmacokinetics and pharmacodynamics of tamsulosin in its modified-release and oral controlled absorption system formulations. Clin. Pharmacokinet. 2010, 49, 177–188. [Google Scholar]
- Food and Drug Administration. Clinical Drug Interaction Studies—Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions Guidance for Industry. 2020. Available online: https://www.fda.gov/media/134581/download (accessed on 7 May 2023).
- Food and Drug Administration. Highlights of Prescribing Information, Flomax (Tamsulosin Hydrochloride) Capsules. In Initial U.S. Approval.; 1997. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020579s026lbl.pdf (accessed on 21 November 2009).
- van Gelderen, M.; Tretter, R.; Meijer, J.; Dorrepaal, C.; Gangaram-Panday, S.; Brooks, A.; Krauwinkel, W.; Dickinson, J. Absence of clinically relevant cardiovascular interaction upon add-on of mirabegron or tamsulosin to an established tamsulosin or mirabegron treatment in healthy middle-aged to elderly men. Int. J. Clin. Pharmacol. Ther. 2014, 52, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Highlights of Prescribing Information, Myrbetriq (Mirabegron Extended-Release Tablets) for Oral Use. In Initial U.S. Approval.; 2012. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/202611s011lbl.pdf (accessed on 27 April 2018).
- Kakizaki, H.; Lee, K.S.; Yamamoto, O.; Jong, J.J.; Katou, D.; Sumarsono, B.; Uno, S.; Yamaguchi, O. Mirabegron Add-on Therapy to Tamsulosin for the Treatment of Overactive Bladder in Men with Lower Urinary Tract Symptoms: A Randomized, Placebo-controlled Study (MATCH). Eur. Urol. Focus 2020, 6, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.C.; Korstanje, C.; Krauwinkel, W.; Kuipers, M. The pharmacokinetic profile of tamsulosin oral controlled absorption system (OCAS((R))). Eur. Urol. Suppl. 2005, 4, 15–24. [Google Scholar] [CrossRef]
- Park, S.-I.; Rhee, S.-J.; Jang, I.-J.; Yu, K.-S.; Yim, S.-V.; Kim, B.-H. Bioequivalence of the pharmacokinetics between two formulations of 0.2 mg tamsulosin hydrochloride in healthy subjects. Transl. Clin. Pharmacol. 2015, 23, 21–25. [Google Scholar] [CrossRef]
- Food and Drug Administration. Bioanalytical Method Validation Guidance for Industry Food and Drug Administration. 2018. Available online: https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf (accessed on 24 May 2018).
- Ministry of Food and Drug Safety. Guidelines of Bioequivalence Studies of Medical Products Guideline on Bioanalytical Method Validation. 2013. Available online: https://www.mfds.go.kr/brd/m_1060/view.do?seq=13054&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=79 (accessed on 2 June 2017).
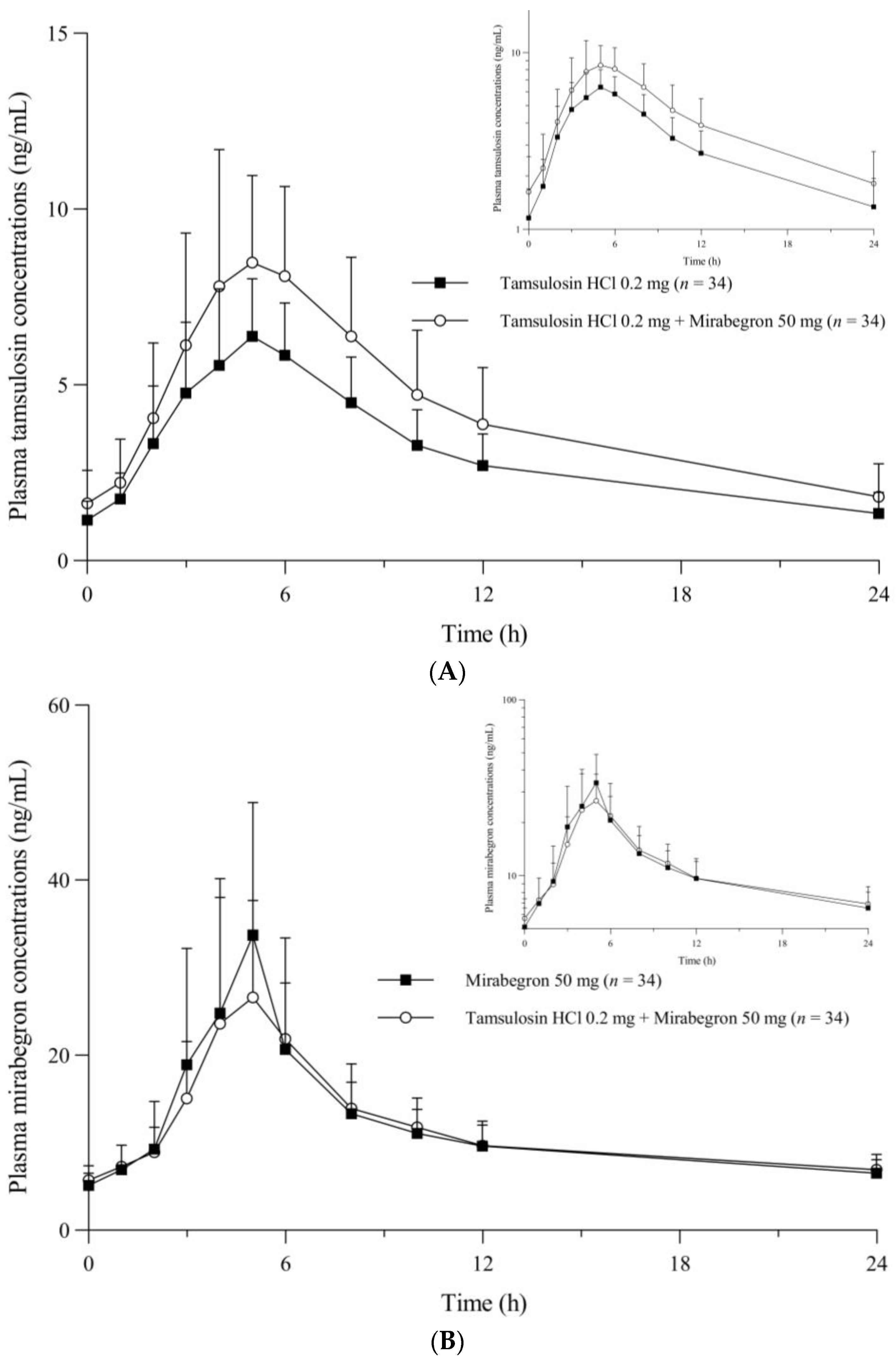
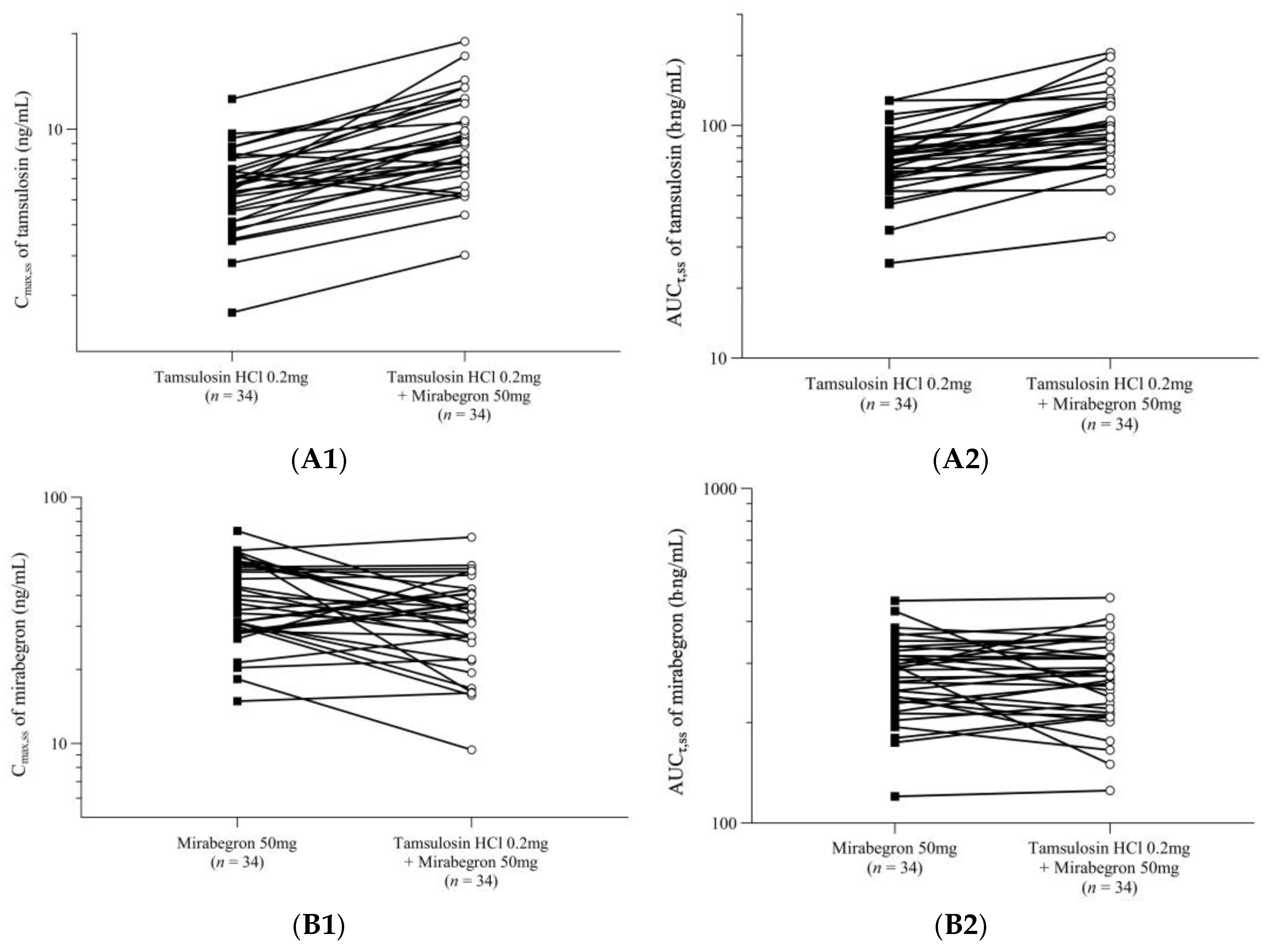

| Treatment | Cmax,ss (ng/mL) | AUCτ,ss (h·ng/mL) | Tmax,ss (h) | t1/2 (h) | Cmin,ss (ng/mL) | CLss/F (L/h) | Vdss/F (L) |
|---|---|---|---|---|---|---|---|
| Tamsulosin | |||||||
| Tamsulosin alone | 6.70 (1.89) | 73.63 (22.54) | 5.00 (3.00–6.00) | 10.98 (2.6) | 1.15 (0.53) | 2.77 (1.09) | 42.02 (12.75) |
| Tamsulosin + Mirabegron | 9.53 (3.31) | 101.96 (38.36) | 5.00 (3.00–6.00) | 10.20 (2.11) | 1.59 (0.93) | 2.06 (0.86) | 28.85 (8.2) |
| GMR (90% CI) | 1.4018 (1.3127–1.4969) | 1.3609 (1.2786–1.4486) | |||||
| Intra-participant CV (%) | 16.1 | 15.29 | |||||
| p-values (1) | 0.0001 | 0.0005 | 0.4517 | 0.1769 | 0.0214 | 0.0039 | <0.0001 |
| Mirabegron | |||||||
| Mirabegron alone | 39.50 (14.37) | 282.75 (73.91) | 5.00 (2.00–6.00) | 19.72 (3.24) | 5.11 (1.44) | 190.65 (59.36) | 5440.18 (1900.02) |
| Tamsulosin + Mirabegron | 33.48 (12.92) | 277.63 (78.61) | 4.53 (3.00–6.00) | 19.81 (5.4) | 5.70 (1.67) | 196.40 (63.77) | 5696.03 (2781.96) |
| GMR (90% CI) | 0.8367 (0.7439–0.9410) | 0.9761 (0.9189–1.0369) | |||||
| Intra-participant CV (%) | 29.23 | 14.8 | |||||
| p-values | 0.0739 | 0.7829 | 0.308 | 0.9333 | 0.1192 | 0.7016 | 0.6593 |
| TEAE | Tamsulosin Alone (T1) (n = 36) | Mirabegron Alone (T2) (n = 35) | Tamsulosin + Mirabegron (T3) (n = 34) | Total (n = 36) |
|---|---|---|---|---|
| Subjects with at least one TEAE | 5 (13.89) [5] | 5 (14.29) [8] | 5 (14.71) [6] | 10 (27.79) [19] |
| p = 0.9952 (1) | ||||
| Otitis media | 1 (2.94) [1] | 1 (2.78) [1] | ||
| Alanine aminotransferase increased | 3 (8.33) [3] | 2 (5.71) [2] | 3 (8.33) [5] | |
| Aspartate aminotransferase increased | 1 (2.86) [1] | 1 (2.78) [1] | ||
| Blood creatine phosphokinase increased | 1 (2.78) [1] | 3 (8.57) [3] | 3 (8.33) [4] | |
| Hemoglobin increased | 1 (2.86) [1] | 1 (2.78) [1] | ||
| Dizziness | 1 (2.86) [1] | 1 (2.94) [1] | 2 (5.56) [2] | |
| Headache | 1 (2.78) [1] | 2 (5.88) [2] | 3 (8.33) [3] | |
| Retrograde ejaculation | 2 (5.88) [2] | 2 (5.56) [2] |
| Vital Sign | Timepoint of Measurement (1) | ||||
|---|---|---|---|---|---|
| Screening (n = 36) | Tamsulosin Alone (T1) (n = 35) | Mirabegron Alone (T2) (n = 34) | Tamsulosin + Mirabegron (T3) (n = 34) | p-Value (2) | |
| SBP (mmHg) | 128.97 (12.04) | 119.23 (8.92) | 120.56 (9.56) | 117.18 (8.72) | 0.3058 |
| DBP (mmHg) | 78.78 (8.25) | 76.26 (9.71) | 73.76 (6.77) | 75.29 (8.61) | 0.4708 |
| HR (bpm) | 77.08 (9.47) | 64.97 (9.28) | 67.97 (8.82) | 67.00 (8.32) | 0.5490 |
| Change from the Screening in each treatment | |||||
| SBP (mmHg) | - | −10.57 (12.99) | −9.06 (11.22) | −12.44 (12.24) | 0.5120 |
| DBP (mmHg) | - | −2.83 (10.34) | −5.06 (7.65) | −3.53 (10.84) | 0.6243 |
| HR (bpm) | - | −12.26 (10.84) | −12.44 (11.27) | −10.41 (10.07) | 0.6898 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, W.; Yang, A.-Y.; Yoo, H.; Kim, A. Drug–Drug Interactions between Tamsulosin and Mirabegron in Healthy Individuals Do Not Affect Pharmacokinetics and Hemodynamic Parameters Significantly. Pharmaceuticals 2023, 16, 1457. https://doi.org/10.3390/ph16101457
Shin W, Yang A-Y, Yoo H, Kim A. Drug–Drug Interactions between Tamsulosin and Mirabegron in Healthy Individuals Do Not Affect Pharmacokinetics and Hemodynamic Parameters Significantly. Pharmaceuticals. 2023; 16(10):1457. https://doi.org/10.3390/ph16101457
Chicago/Turabian StyleShin, Wonsuk, A-Young Yang, Hyounggyoon Yoo, and Anhye Kim. 2023. "Drug–Drug Interactions between Tamsulosin and Mirabegron in Healthy Individuals Do Not Affect Pharmacokinetics and Hemodynamic Parameters Significantly" Pharmaceuticals 16, no. 10: 1457. https://doi.org/10.3390/ph16101457
APA StyleShin, W., Yang, A.-Y., Yoo, H., & Kim, A. (2023). Drug–Drug Interactions between Tamsulosin and Mirabegron in Healthy Individuals Do Not Affect Pharmacokinetics and Hemodynamic Parameters Significantly. Pharmaceuticals, 16(10), 1457. https://doi.org/10.3390/ph16101457