Radiometals in Imaging and Therapy: Highlighting Two Decades of Research
Abstract
1. Introduction
2. Diagnostic Radionuclides
- (i)
- PET measures the energy produced by the two gamma photons (511 keV) that result from annihilation of the positron emitted from the PET radionuclide with atomic electron [11]. The emitted gamma photons are detected with γ-cameras, also called scintillation detectors, which produce reconstructed three-dimensional images depicting the spatial distribution of radiotracers [11]. The common examples of PET probes includes [18F]FDG, [13N]NH3, [68Ga]Ga-PSMA, and [18F]NaF. Preclinical animal PET and clinical PET scanners offer spatial resolution of 1–2 mm and 6–10 mm, respectively, with high sensitivity of 10−11–10−12 mol/L. This level of sensitivity is sufficient to detect biological changes in an organ or tissue to identify the onset of a disease before anatomical changes occur [12].
- (ii)
- SPECT measures the single gamma photons emitted directly from γ-emitting radionuclides called SPECT radiopharmaceuticals. The conventional clinical SPECT scanners have lower sensitivity (10−10–10−11 mol/L) and lower spatial resolution (7–15 mm) compared to PET scanners due to the limited performance of collimators [12,13,14]. Despite this, SPECT is the most routinely used nuclear imaging procedure in the clinic and is less expensive compared to PET. The most common SPECT isotopes are 111In, 99mTc, 123/131/125I, and 67Ga.
3. Therapeutic Radionuclides
- (i)
- Beta minus emitters can be either of a high energy (90Y, Eβ−max = 2.28 MeV,) or a low energy (177Lu, Eβ−max = 496 keV), with tissue penetration ranges between 12 mm and 1.5 mm, respectively [18]. Given the long penetration depth of 0.2–12 mm and the moderate linear energy transfer (LET) radiation of ~0.2 keV/µm, β− emitters are more suited to treating large-sized tumors (>0.5 cm), and they are considered the current gold standard in targeted radionuclide therapy [19,20].
- (ii)
- Alpha emitters emit α particles with high LET energies of 50–230 keV/µm and shorter penetration depths of 50–100 µm (i.e., 5–10 cell diameters) [21]. Alpha radionuclide-based targeted therapy is called targeted alpha therapy (TAT), and it is well suited for the treatment of hematological disease, small tumors, metastasis, and isolated cancer cells. Alpha emitters are perceived as a better therapeutic alternative to beta emitters due to their high LET and short tissue penetration range.
- (iii)
- Meitner−Auger electrons are low-energy electrons that can penetrate up to the subcellular nanometer range (<0.5 µm), resulting in a high LET of 4–26 keV/µm [22]. Given the low tissue penetration range and high LET in an extremely small area, MAE emitters could be highly valuable for treating metastatic cancers if delivered selectively within the nucleus of the cancer cells [22]. Figure 1 explains the difference between LET, pathlength (penetration range), and the usefulness of α and β− radionuclide therapies.
4. Positron Emitters
4.1. Radioisotopes of Copper
4.1.1. General Information
4.1.2. Growth and Advancement of Radiopharmaceuticals Labeled with Copper-Radioisotopes in Clinical Practice
4.1.3. Production and Availability
4.2. Radioisotopes of Gallium
4.2.1. General Information
4.2.2. Clinical Practice
4.2.3. Production and Availability
| Radiopharmaceuticals | Biological Target | NCT Number ^ | Disease |
|---|---|---|---|
| [68Ga]Ga-PSMA-11 | PSMA | NCT03207139 (Phase II; completed) NCT03982407 (Early Phase I; completed) | Latent prostate cancer [98], hepatocellular carcinoma [99] |
| [68Ga]Ga-NGUL/[177Lu]Lu-DGUL | PSMA | NCT05547061 (Phase I/II; ongoing) | Metastatic castration-resistant prostate cancer [100] |
| [68Ga]Ga-DOTA-TATE vs. [68Ga]Ga-DOTA-TOC | SSTR2 | NCT04298541 (Phase II; ongoing) | Meningioma [101] |
| [68Ga]Ga-DOTA-TOC | SSTR | NCT02441062 (Phase II; completed) | Neuroendocrine tumors [102] |
| [68Ga]Ga-FAPi-46 | FAPI | NCT04457258 (Early Phase I; ongoing) | Sarcoma, recurrent or metastatic, sarcoma [103] |
4.3. Radioisotopes of Zirconium
4.3.1. General Information
4.3.2. Clinical Practice
4.3.3. Production and Availability
| Radiopharmaceuticals | Targets | NCT Number ^ | Disease |
|---|---|---|---|
| [89Zr]Zr-Df-hJ591 | PSMA | NCT01543659 (Phase I/II; ongoing) | Prostate cancer [137] |
| [89Zr]Zr-Df-IAB2M | PSMA | NCT02349022 (Phase II; completed) | Prostate cancer [138] |
| [89Zr]Zr-Df-IAB22M2C | CD8+ Tlymphocytes | NCT05013099 (Phase IIb; ongoing) NCT03107663 Phase I; completed) NCT03802123 (Phase II; completed) | Melanoma [139] Renal cell carcinoma [140] Metastatic solid tumors [141] |
| [89Zr]Zr-daratumumab | CD38 | NCT03665155 (Phase II; completed) | Multiple myeloma [142] |
| [89Zr]Zr-trastuzumab | HER2+ | NCT01420146 (Phase I; completed) | Breast neoplasm [143] |
| [89Zr]Zr-ss-pertuzumab | HER2- | NCT04692831 (Phase I; ongoing) | Breast carcinoma [144] |
| [89Zr]Zr-bevacizumab | VEGF | NCT01894451 (Early Phase I; completed) | Inflammatory breast carcinoma [114] |
| [89Zr]Zr-panitumumab | EGFR | NCT03733210 (Phase I; completed) | Carcinoma of head and neck [145] |
| [89Zr]Zr-cetuximab | EGFR | NCT00691548 (Phase I; completed) | Stage IV cancer [146] |
| [89Zr]Zr-girentuximab | Carbonic anhydrase | NCT03849118 (Phase III; completed) | Renal cell carcinoma [147] |
| [89Zr]Zr-durvalumab | PDL-1 | NCT03853187(Phase II; completed) | Non-small cell lung cancer [148] |
| [89Zr]-DFO-atezolizumab | PDL-1 | NCT04006522 (Phase II; ongoing) | Renal cell carcinoma [149] |
4.4. Radioisotopes of Scandium
4.4.1. General Information
4.4.2. Current Clinical Application of Scandium-44
4.4.3. Production and Availability
4.5. Radioisotopes of Terbium
4.5.1. General Information
4.5.2. Preclinical and Clinical Applications
- (i)
- 149Terbium: 149Tb represents one of the powerful candidates for TAT, which emits short penetrating (~25 µm range) α particles (Eα = 3.97 MeV; Iα = 16.7%) compared to currently employed α emitters [168]. Because 149Tb also decays by positrons (β+) and γ-radiations, 149Tb-labeled radiopharmaceuticals could also be useful for PET and SPECT imaging [168].
- (ii)
- 152Terbium: 152Tb is a diagnostic radionuclide that decays via positron emission (Eβ+avg = 1142 keV) and multiple gamma radiations, which could lead to high radiation exposure [170]. The relatively long half-life of 152Tb (t1/2 = 17.5 h) allows it to be useful in dosimetry estimation. In fact, 152Tb is an exact diagnostic match for 149Tb and 161Tb, as well as other clinically useful therapeutic radionuclides, like 177Lu, due to their similarities in coordination chemistry and pharmacokinetics.
- (iii)
- 155Terbium: 155Tb is a suitable SPECT isotope, a promising alternative to the 111In isotope, and it could be useful for dosimetry estimation of β− emitters, like 177Lu, 90Y, and 166Ho [173].
- (iv)
- 161Terbium: 161Tb decays by low-energy (Eβ−avg = 154 keV) (β−) emission, having a short tissue penetration (0.29 mm) range and a long half-life (t1/2) of 6.8 d [174]. The decay characteristics and half-life of 161Tb are like 177Lu (Eβ−avg = 134 keV, t1/2 = 6.7d) [174], although 161Tb also emits a substantial number of auger electrons, which could be advantageous for therapeutic applications. However, the clinical superiority of 161Tb over 77Lu is yet to be established [175,176,177]. In addition to radionuclide therapy, 161Tb also emits gamma photons enabling SPECT imaging [174]. Recently, Baum et al. demonstrated the first-in-human SPECT imaging using [161Tb]Tb-DOTATOC in patients with paraganglioma and NETs and showed high-quality images and visualization of hepatic metastasis as well as multiple osteoblastic skeletal metastasis in patients [178].
4.5.3. Production and Availability
4.6. Radioisotopes of Zinc
4.6.1. General Information
4.6.2. Clinical Applications
4.6.3. Production and Availability
5. SPECT Probes
5.1. Technetium-99m
5.1.1. General Information
5.1.2. Clinical Applications
5.1.3. Production and Availability
5.2. Indium-111
5.2.1. General Information
5.2.2. Clinical Practice
5.2.3. Production and Availability
| Radiopharmaceuticals | Targets | NCT Number ^ | Disease |
|---|---|---|---|
| [111In]In-CP04 | CCK2R/gastrin | NCT03246659 (Phase I; completed) | Thyroid carcinoma [217] |
| [111In]In-Ch806 | gp140, IL-13RA2 | NCT00291447 (Phase I; completed) | Neoplasm [218] |
| [111In]In-capromab pendetide (ProstaScint®) | PSMA | NCT00992745 (Phase I; completed) | Prostate cancer [219] |
| [111In]In-PSMA (I&T) | PSMA | NCT04300673 (Phase I ongoing) | Prostate cancer [220] |
| [111In]In-DOTA-Girentuximab | Carbonic anhydrase-IX | NCT02497599 (Phase I; status unknown) | Renal cell carcinoma [221] |
| [111In]In-labeled leukocytes | Leukocytes | NCT00026897 (Phase II; completed) | Neoplasm [222] |
6. Beta Minus Emitter
6.1. Yttrium-90
6.1.1. General Information
6.1.2. Clinical Application of 90Y
6.1.3. Production and Availability
| Radiopharmaceuticals | Target | NCT Number ^ | Disease |
|---|---|---|---|
| [90Y]Y-cG250 | - | NCT00199875 (Phase I; completed) | Renal and kidney cancer [243] |
| [90Y]Y-hM5A | CEA | NCT00645060 (Phase I; completed) NCT01205022 (Phase I; completed) | Unspecified adult solid tumor [244] Colon and rectal cancer [245] |
| [90Y]Y-hPAM4 | MUC1 | NCT00603863 (Phase I/II; completed) | Pancreatic [246] |
| [90Y]Y-DOTATOC | SSTR | NCT05568017 (Phase II; ongoing) | Pancreatic neuroendocrine tumor [247] |
| [90Y]Y-edotreotide | SSTR | NCT00006368 (Phase I; completed) | Brain, breast, and lung cancer, lymphoma, melanoma, neoplastic syndrome [248] |
| [90Y]Y-resin microspheres (SIR-spheres®) | - | NCT01482442 (Phase III; completed) | Liver carcinoma [249] |
| [90Y]Y- Ibritumomab Tiuxetan (ZevalinTM) | CD20 + B cells | NCT01446562 (Phase II; completed) | Follicular lymphoma [250] |
6.2. Radioisotopes of Rhenium
6.2.1. General Information
6.2.2. Clinical Applications of Rhenium Radioisotopes
6.2.3. Production and Availability
| Radiopharmaceuticals | Targets | NCT Number ^ | Disease |
|---|---|---|---|
| [188Re]Re-HEDP vs. [223Ra]RaCl2 | Bone metastasis | NCT03458559 (Phase III; ongoing) | Prostate cancer metastatic to bone [262] |
| [186Re]Re-labeled bivatuzumab | VEGF-A | NCT02204046 (Phase I; completed), NCT02204059 (Phase I; completed), NCT02204033 (Phase I; completed) | Adenocarcinoma [263] Non-small cell lung carcinoma [264] Head and neck neoplasm [265] |
| Rhenium-SCT® | Skin lesions | NCT05135052 (Phase not applicable; ongoing) | Non-melanoma skin cancer [258] |
| [186Re]Re-nanoliposome | - | NCT01906385 (Phase I/II; ongoing) | Glioma [266] |
6.3. Holomium-166
6.4. Lutetium-177
6.4.1. General Information
6.4.2. Clinical Applications
6.4.3. Production and Availability
7. Alpha-Particle-Emitting Radiopharmaceuticals
7.1. Radioisotopes of Bismuth
7.1.1. General Information
7.1.2. Clinical Applications of 213Bismuth
| Isotope | Half-Life (t1/2) | Decay Characteristics | Parent Nuclides and Their Daughter Nuclides | Energies (MeV) | Eγ; keV (Intensity%) | |
|---|---|---|---|---|---|---|
| Eα (MeV) | Eβ− (MeV) | |||||
| 212Bi | 61 min | β− = 64% α = 36% | 212Bi 212Po 208Tl 208Pb (stable) | 212Bi-6.1 212Po-8.8 | 212Po-0.769 208Tl-0.557 | 212Bi-727.3 (6.6) 208Tl-277.4 (6.3), 510.8 (22.6), 583.2 (84.5), 763 (1.8), 860.6 (12.4), 2614.5 (99.2) |
| 213Bi | 45.6 min | β− = 98% α = 2% | 213Bi 213Po 209Tl 209Pb 209Bi (essentially stable) | 5.9 (213Bi) 8.4 (213Po) | 213Bi-1400 209Tl-2000 209Pb-600 | 213Bi-440 (25.9) |
7.1.3. Production and Availability
| Radiopharmaceuticals | Targets | NCT Number ^ | Disease |
|---|---|---|---|
| [213Bi]Bi-M195 | CD33 | NCT00014495 (Phase I/II completed) | Leukemia, myelodysplastic syndromes [299] |
7.2. Actinium-225
7.2.1. General Information
7.2.2. Clinical Applications of Actinium-225
7.2.3. Production and Availability
7.3. Radioisotopes of Lead
7.3.1. General Information
7.3.2. Clinical Practice
7.3.3. Production and Availability
7.4. Radioisotopes of Radium
7.4.1. General Information
| Isotope | Half-Life (t1/2) | Decay Characteristics | Parent and Daughter Nuclides | Energy (MeV) | Eγ; keV (Intensity%) | |
|---|---|---|---|---|---|---|
| Eα max | Eβ-max | |||||
| 223Ra | 11.4 d | α = 100% | 223Ra 219Rn 215Po 211Pb 211Bi 211Po 207Tl 207Pb (stable) | 223Ra-5.78 219Rn-6.88 215Po-7.53 211Bi-6.68 211Po-7.59 | 211Pb-0.45 211Bi-0.01 207Tl-0.49 | 144.27 (3.36) 154.2 (5.84) 323.8 (4.06) 328.2 (2.85) |
| 224Ra | 3.6d | α = 100% | 224Ra 220Rn 216Po 212Pb 212Bi 212Po 208Tl 208Pb (stable) | 224Ra-5.7 220Rn-6.3 216Po-6.8 212Bi-6.1 212Po-8.8 | 212Pb-0.1 212Bi-0.8 208Tl-0.6 | 241(4.1%) |
7.4.2. Clinical Practice
7.4.3. Production and Availability
7.5. Thorium-227
7.5.1. General Information
7.5.2. Clinical Practice
7.5.3. Production and Availability
| Radiopharmaceuticals | Targets | NCT Number ^ | Disease |
|---|---|---|---|
| [227Th]Th-anti PSMA (BAY2315497) | PSMA | NCT03724747 (Phase I; ongoing) | Metastatic castration-resistant prostate cancer [355] |
| [227Th]Th-anti Mesothelin (BAY2287411) | Mesothelin | NCT03507452 (Phase I; completed) | Advanced recurrent serous ovarian, malignant peritoneal mesothelioma, pancreatic adenocarcinoma [358] |
| [227Th]Th-trastuzumab (BAY2701439) | HER2+ | NCT04147819 (Phase I; ongoing) | Cancer with HER2 + expression [359] |
| [227Th]Th-epratuzumab (BAY1862864) | CD22 | NCT02581878 (Phase I; completed) | Non-Hodgkin lymphoma [360] |
7.6. Radioisotopes of Astatine
7.6.1. General Information
7.6.2. Clinical Practice
7.6.3. Production and Availability
| Radiopharmaceuticals | Targets | NCT Number ^ | Disease |
|---|---|---|---|
| Sodium Astatide ([211At]NaAt) | - | NCT05275946 (Phase I; ongoing) | Thyroid cancer [367] |
| [211At]At- 81C6 | Glial fibrillary acidic protein | NCT00003461 (Phase I/II; completed) | Metastatic cancer, brain and central nervous system tumors, neuroblastoma [368] |
| [211At]At- bc8-b10 | CD45 | NCT04083183 (Phase I/II; ongoing) NCT03670966 (Phase I/II; ongoing) | Non-malignant neoplasm [369] Acute lymphoblastic leukemia in remission [370] |
| [211At]At-OKT-B10 | CD3 | NCT04466475 (Phase I; ongoing) | Plasma cell myeloma [371] |
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ab | = | Antibody |
| Ac | = | Actinium |
| AML | = | Acute myeloid leukemia |
| At | = | Astatine |
| Bi | = | Bismuth |
| CD8 | = | Cluster of differentiation 8 |
| CD38 | = | Cluster of differentiation 38 |
| CD20 | = | Cluster of differentiation 20 |
| CEA | = | Carcinoembryonic antigen |
| CERN | = | European Council for Nuclear Research |
| Cu | = | Copper |
| DaRT | = | Diffusing alpha-emitters radiation therapy |
| EC | = | Electron capture |
| EGFR | = | Epidermal growth factor receptor |
| FAPI | = | Fibroblast activation protein inhibitor |
| FDA | = | Food and Drug Administration |
| Ga | = | Gallium |
| GBM | = | Glioblastoma Multiforme |
| Ge | = | Germanium |
| GMP | = | Good manufacturing practice |
| HER2 | = | Human epidermal growth factor 2 |
| HOPO | = | 2-hydroxypyridone-N-oxide |
| IART® | = | Intra-operative avidination for radionuclide therapy |
| ISOLDE | = | Isotope separation on-line |
| LET | = | Linear energy transfer |
| mAb | = | Monoclonal antibody |
| MAE | = | Meitner–Auger electrons |
| mCRPC | = | Metastatic castrate-resistant prostate cancer |
| MUC1 | = | Mucin-1 |
| NCT | = | National clinical trial |
| NET | = | Neuroendocrine tumor |
| Pb | = | Lead |
| PCa | = | Prostate cancer |
| PDL-1 | = | Programmed cell death ligand-1 |
| PET | = | Positron emission tomography |
| PRRT | = | Peptide receptor radionuclide therapy |
| PSA | = | Prostate-specific antigen |
| PSMA | = | Prostate-specific membrane antigen |
| Ra | = | Radium |
| Re | = | Rhenium |
| RIT | = | Radioimmunotherapy |
| Sc | = | Scandium |
| SPECT | = | Single photon emission computed tomography |
| SSTR2 | = | Somatostatin-targeting receptor 2 |
| SUV | = | Standardized uptake value |
| TAT | = | Targeted alpha therapy |
| Tb | = | Terbium |
| Tc | = | Technetium |
| Th | = | Thorium |
| Tl | = | Thallium |
| TRT | = | Targeted radionuclide therapy |
| TTC | = | Targeted thorium conjugates |
| U | = | Uranium |
| VEGF | = | Vascular endothelial growth factor |
| Y | = | Yttrium |
| Zn | = | Zinc |
| Zr | = | Zirconium |
References
- Gianfaldoni, S.; Gianfaldoni, R.; Wollina, U.; Lotti, J.; Tchernev, G.; Lotti, T. An Overview on Radiotherapy: From Its History to Its Current Applications in Dermatology. Open Access Maced. J. Med. Sci. 2017, 5, 521–525. [Google Scholar] [CrossRef]
- Lederman, M. The early history of radiotherapy: 1895–1939. Int. J. Radiat. Oncol. Biol. Phys. 1981, 7, 639–648. [Google Scholar] [CrossRef]
- Pusey, W.A. Roentgen rays in the treatment of skin diseases and for the removal of hair. J. Cutan. Dis. Incl. Syph. 1900, 18, 302–318. [Google Scholar] [CrossRef]
- Chen, H.H.W.; Kuo, M.T. Improving radiotherapy in cancer treatment: Promises and challenges. Oncotarget 2017, 8, 62742–62758. [Google Scholar] [CrossRef]
- Jones, P.M. Treatment of lupus by x-rays. Phila. Med. J. 1900, 5, 63–64. [Google Scholar]
- Majeed, H.; Gupta, V. Adverse Effects of Radiation Therapy; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Kerr, C.P.; Grudzinski, J.J.; Nguyen, T.P.; Hernandez, R.; Weichert, J.P.; Morris, Z.S. Developments in Combining Targeted Radionuclide Therapies and Immunotherapies for Cancer Treatment. Pharmaceutics 2023, 15, 128. [Google Scholar] [CrossRef]
- Rafael, M.S.; Cohen-Gogo, S.; Irwin, M.S.; Vali, R.; Shammas, A.; Morgenstern, D.A. Theranostics in Neuroblastoma. PET Clin. 2021, 16, 419–427. [Google Scholar] [CrossRef]
- Cimini, A.; Ricci, M.; Chiaravalloti, A.; Filippi, L.; Schillaci, O. Theragnostic Aspects and Radioimmunotherapy in Pediatric Tumors. Int. J. Mol. Sci. 2020, 21, 3849. [Google Scholar] [CrossRef]
- Elisa Crestoni, M. Radiopharmaceuticals for diagnosis and therapy. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Schmitz, R.E.; Alessio, A.M.; Kinahan, P. The Physics of PET/CT Scanners; University of Washington: Washington, DC, USA, 2013. [Google Scholar]
- Chen, I.Y.; Wu, J.C. Cardiovascular molecular imaging: Focus on clinical translation. Circulation 2011, 123, 425–443. [Google Scholar] [CrossRef]
- Van Audenhaege, K.; Van Holen, R.; Vandenberghe, S.; Vanhove, C.; Metzler, S.D.; Moore, S.C. Review of SPECT collimator selection, optimization, and fabrication for clinical and preclinical imaging. Med. Phys. 2015, 42, 4796–4813. [Google Scholar] [CrossRef]
- Rahmim, A.; Zaidi, H. PET versus SPECT: Strengths, limitations, and challenges. Nucl. Med. Commun. 2008, 29, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, M.; Pretorius, P.H. SPECT/CT: An update on technological developments and clinical applications. Br. J. Radiol. 2018, 91, 20160402. [Google Scholar] [CrossRef]
- Single Photon Emission Computed Tomography (SPECT). Available online: https://cmgi.ucdavis.edu/services/single-photon-emission-computed-tomography-spect/ (accessed on 15 August 2023).
- Lu, F.M.; Yuan, Z. PET/SPECT molecular imaging in clinical neuroscience: Recent advances in the investigation of CNS diseases. Quant. Imaging Med. Surg. 2015, 5, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Bast, R.C., Jr.; Kousparou, C.A.; Epenetos, A.A.; Zalutsky, M.R.; Kreitman, R.J.; Sausville, E.A.; Frankel, A.E. Radioimmunotherapy of Cancer. Holland-Frei Cancer Medicine, 6th ed.; Decker: Hamilton, ON, Canada, 2003. Available online: https://www.ncbi.nlm.nih.gov/books/NBK13926/ (accessed on 15 August 2023).
- Sofou, S. Radionuclide carriers for targeting of cancer. Int. J. Nanomed. 2008, 3, 181–199. [Google Scholar] [CrossRef] [PubMed]
- Álvarez N., H.; Hernández-Gil, J.; Lewis, J.S. Recent Advances in Radiometals for Combined Imaging and Therapy in Cancer. ChemMedChem 2021, 16, 2909. [Google Scholar] [CrossRef]
- De Kruijff, R.M.; Wolterbeek, H.T.; Denkova, A.G. A Critical Review of Alpha Radionuclide Therapy-How to Deal with Recoiling Daughters? Pharmaceuticals 2015, 8, 321–336. [Google Scholar] [CrossRef]
- Aghevlian, S.; Boyle, A.J.; Reilly, R.M. Radioimmunotherapy of cancer with high linear energy transfer (LET) radiation delivered by radionuclides emitting α-particles or Auger electrons. Adv. Drug Deliv. Rev. 2017, 109, 102–118. [Google Scholar] [CrossRef] [PubMed]
- De Nardo, L.; Pupillo, G.; Mou, L.; Esposito, J.; Rosato, A.; Meléndez-Alafort, L. A feasibility study of the therapeutic application of a mixture of 67/64Cu radioisotopes produced by cyclotrons with proton irradiation. Med. Phys. 2022, 49, 2709–2724. [Google Scholar] [CrossRef] [PubMed]
- Mou, L.; Martini, P.; Pupillo, G.; Cieszykowska, I.; Cutler, C.S.; Mikołajczak, R. 67Cu Production Capabilities: A Mini Review. Molecules 2022, 27, 1501. [Google Scholar] [CrossRef]
- Ling, X.; Cutler, C.S.; Anderson, C.J. The radiopharmaceutical chemistry of the radioisotopes of copper. In Radiopharmaceutical Chemistry; Lewis, J.S., Windhorst, A.D., Zeglis, B.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 335–358. [Google Scholar] [CrossRef]
- Fujibayashi, Y.; Taniuchi, H.; Yonekura, Y.; Ohtani, H. Copper-62-ATSM: A new hypoxia imaging agent with high membrane permeability and low redox potential. J. Nucl. Med. 1997, 38, 1155. [Google Scholar]
- Lewis, J.S.; Laforest, R.; Dehdashti, F.; Grigsby, P.W.; Welch, M.J.; Siegel, B.A. An imaging comparison of 64Cu-ATSM and 60Cu-ATSM in cancer of the uterine cervix. J. Nucl. Med. 2008, 49, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Colombié, M.; Gouard, S.; Frindel, M.; Vidal, A.; Chérel, M.; Kraeber-Bodéré, F.; Rousseau, C.; Bourgeois, M. Focus on the Controversial Aspects of (64)Cu-ATSM in Tumoral Hypoxia Mapping by PET Imaging. Front. Med. 2015, 2, 58. [Google Scholar] [CrossRef]
- Dehdashti, F.; Grigsby, P.W.; Lewis, J.S.; Laforest, R.; Siegel, B.A.; Welch, M.J. Assessing tumor hypoxia in cervical cancer by PET with 60Cu-labeled diacetyl-bis (N4-methylthiosemicarbazone). J. Nucl. Med. 2008, 49, 201–205. [Google Scholar] [CrossRef]
- Dietz, D.W.; Dehdashti, F.; Grigsby, P.W.; Malyapa, R.S.; Myerson, R.J.; Picus, J.; Ritter, J.; Lewis, J.S.; Welch, M.J.; Siegel, B.A. Tumor hypoxia detected by positron emission tomography with 60Cu-ATSM as a predictor of response and survival in patients undergoing neoadjuvant chemoradiotherapy for rectal carcinoma: A pilot study. Dis. Colon Rectum 2008, 51, 1641–1648. [Google Scholar] [CrossRef]
- Tateishi, K.; Tateishi, U.; Sato, M.; Yamanaka, S.; Kanno, H.; Murata, H.; Inoue, T.; Kawahara, N. Application of 62Cu-diacetyl-bis (N4-methylthiosemicarbazone) PET imaging to predict highly malignant tumor grades and hypoxia-inducible factor-1α expression in patients with glioma. Am. J. Neuroradiol. 2013, 34, 92–99. [Google Scholar] [CrossRef]
- 64Cu-ATSM PET/CT in Rectum Cancer (TEP 64Cu-ATSM-Rectum). Available online: https://clinicaltrials.gov/search?term=NCT03951337 (accessed on 20 August 2023).
- Lewis, J.S.; Laforest, R.; Buettner, T.L.; Song, S.-K.; Fujibayashi, Y.; Connett, J.M.; Welch, M.J. Copper-64-diacetyl-bis(N4-methylthiosemicarbazone): An agent for radiotherapy. Proc. Natl. Acad. Sci. USA 2001, 98, 1206–1211. [Google Scholar] [CrossRef]
- Yoshii, Y.; Furukawa, T.; Yoshimoto, M.; Matsumoto, H.; Zhang, M.-R.; Inubushi, M.; Fujibayashi, Y.; Saga, T. 64Cu-ATSM internal radiotherapy to treat colon cancer acquiring resistance to antiangiogenic therapy with bevacizumab. J. Nucl. Med. 2016, 57, 471. [Google Scholar]
- Yoshii, Y.; Furukawa, T.; Kiyono, Y.; Watanabe, R.; Mori, T.; Yoshii, H.; Asai, T.; Okazawa, H.; Welch, M.J.; Fujibayashi, Y. Internal radiotherapy with copper-64-diacetyl-bis (N4-methylthiosemicarbazone) reduces CD133+ highly tumorigenic cells and metastatic ability of mouse colon carcinoma. Nucl. Med. Biol. 2011, 38, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Fujibayashi, Y.; Yoshii, Y.; Furukawa, T.; Yoshimoto, M.; Matsumoto, H.; Saga, T. Imaging and therapy against hypoxic tumors with 64Cu-ATSM. In Make Life Visible; Springer: Singapore, 2020; pp. 285–292. [Google Scholar]
- Anderson, C.J.; Connett, J.M.; Schwarz, S.W.; Rocque, P.A.; Guo, L.W.; Philpott, G.W.; Zinn, K.R.; Meares, C.F.; Welch, M.J. Copper-64-labeled antibodies for PET imaging. J. Nucl. Med. 1992, 33, 1685–1691. [Google Scholar]
- Suzuki, H.; Kise, S.; Kaizuka, Y.; Watanabe, R.; Sugawa, T.; Furukawa, T.; Fujii, H.; Uehara, T. Copper-64-Labeled Antibody Fragments for Immuno-PET/Radioimmunotherapy with Low Renal Radioactivity Levels and Amplified Tumor-Kidney Ratios. ACS Omega 2021, 6, 21556–21562. [Google Scholar] [CrossRef] [PubMed]
- Bryan, J.N.; Lewis, M.R.; Henry, C.J.; Owen, N.K.; Zhang, J.; Mohsin, H.; Jia, F.; Sivaguru, G.; Anderson, C.J. Development of a two-antibody model for the evaluation of copper-64 radioimmunotherapy. Vet. Comp. Oncol. 2004, 2, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, J.E.; Bading, J.R.; Colcher, D.M.; Conti, P.S.; Frankel, P.H.; Carroll, M.I.; Tong, S.; Poku, E.; Miles, J.K.; Shively, J.E.; et al. Functional imaging of human epidermal growth factor receptor 2-positive metastatic breast cancer using (64)Cu-DOTA-trastuzumab PET. J. Nucl. Med. 2014, 55, 23–29. [Google Scholar] [CrossRef]
- Mortimer, J.E.; Bading, J.R.; Park, J.M.; Frankel, P.H.; Carroll, M.I.; Tran, T.T.; Poku, E.K.; Rockne, R.C.; Raubitschek, A.A.; Shively, J.E.; et al. Tumor Uptake of (64)Cu-DOTA-Trastuzumab in Patients with Metastatic Breast Cancer. J. Nucl. Med. 2018, 59, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Krenning, E.P.; Kwekkeboom, D.J.; Bakker, W.H.; Breeman, W.A.P.; Kooij, P.P.M.; Oei, H.Y.; van Hagen, M.; Postema, P.T.E.; de Jong, M.; Reubi, J.C.; et al. Somatostatin receptor scintigraphy with [111In-DTPA-d-Phe1]- and [123I-Tyr3]-octreotide: The Rotterdam experience with more than 1000 patients. Eur. J. Nucl. Med. 1993, 20, 716–731. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, M.; Muehllechner, P.; Decristoforo, C.; Guggenberg, E.v. 99-mTc-EDDA/HYNIC-Tyr (3)-octreotide for staging and follow-up of patients with neuroendocrine gastro-entero-pancreatic tumors. Q. J. Nucl. Med. Mol. Imaging 2005, 49, 237–244. [Google Scholar] [PubMed]
- Hofmann, M.; Maecke, H.; Börner, A.; Weckesser, E.; Schöffski, P.; Oei, M.; Schumacher, J.; Henze, M.; Heppeler, A.; Meyer, G. Biokinetics and imaging with the somatostatin receptor PET radioligand 68Ga-DOTATOC: Preliminary data. Eur. J. Nucl. Med. 2001, 28, 1751–1757. [Google Scholar] [CrossRef]
- Pfeifer, A.; Knigge, U.; Mortensen, J.; Oturai, P.; Berthelsen, A.K.; Loft, A.; Binderup, T.; Rasmussen, P.; Elema, D.; Klausen, T.L.; et al. Clinical PET of Neuroendocrine Tumors Using 64Cu-DOTATATE: First-in-Humans Study. J. Nucl. Med. 2012, 53, 1207–1215. [Google Scholar] [CrossRef]
- Johnbeck, C.B.; Knigge, U.; Loft, A.; Berthelsen, A.K.; Mortensen, J.; Oturai, P.; Langer, S.W.; Elema, D.R.; Kjaer, A. Head-to-Head Comparison of (64)Cu-DOTATATE and 68Ga-DOTATOC PET/CT: A Prospective Study of 59 Patients with Neuroendocrine Tumors. J. Nucl. Med. 2017, 58, 451–457. [Google Scholar] [CrossRef]
- Loft, M.; Carlsen, E.A.; Johnbeck, C.B.; Johannesen, H.H.; Binderup, T.; Pfeifer, A.; Mortensen, J.; Oturai, P.; Loft, A.; Berthelsen, A.K.; et al. 64Cu-DOTATATE PET in Patients with Neuroendocrine Neoplasms: Prospective, Head-to-Head Comparison of Imaging at 1 Hour and 3 Hours After Injection. J. Nucl. Med. 2021, 62, 73–80. [Google Scholar] [CrossRef]
- FDA Approves Radioactive Diagnostic Agent Detectnet. Available online: https://www.empr.com/home/news/detectnet-approved-positron-emission-tomography-pet-neuroendocrine-tumors/#:~:text=The%20Food%20and%20Drug%20Administration (accessed on 15 August 2023).
- SARTATE Clinical Development. Available online: https://www.claritypharmaceuticals.com/pipeline/sartate/ (accessed on 15 August 2023).
- Horoszewicz, J.S.; Kawinski, E.; Murphy, G.P. Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients. Anticancer Res. 1987, 7, 927–935. [Google Scholar]
- Hosseindokht, M.; Bakherad, H.; Zare, H. Nanobodies: A tool to open new horizons in diagnosis and treatment of prostate cancer. Cancer Cell Int. 2021, 21, 580. [Google Scholar] [CrossRef] [PubMed]
- Novakova, Z.; Belousova, N.; Foss, C.A.; Havlinova, B.; Gresova, M.; Das, G.; Lisok, A.; Prada, A.; Barinkova, M.; Hubalek, M.; et al. Engineered Fragments of the PSMA-Specific 5D3 Antibody and Their Functional Characterization. Int. J. Mol. Sci. 2020, 21, 6672. [Google Scholar] [CrossRef] [PubMed]
- Grubmüller, B.; Baum, R.P.; Capasso, E.; Singh, A.; Ahmadi, Y.; Knoll, P.; Floth, A.; Righi, S.; Zandieh, S.; Meleddu, C.; et al. (64)Cu-PSMA-617 PET/CT Imaging of Prostate Adenocarcinoma: First In-Human Studies. Cancer Biother. Radiopharm. 2016, 31, 277–286. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Zechmann, C.M.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.G.; Holland-Letz, T.; Hadaschik, B.A.; Giesel, F.L.; Debus, J.; et al. Comparison of PET imaging with a 68Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 11–20. [Google Scholar] [CrossRef]
- Denardo, G.L.; Denardo, S.J.; Kukis, D.L.; O’Donnell, R.T.; Shen, S.; Goldstein, D.S.; Kroger, L.A.; Salako, Q.; Denardo, D.A.; Mirick, G.R.; et al. Maximum tolerated dose of 67Cu-2IT-BAT-LYM-1 for fractionated radioimmunotherapy of non-Hodgkin’s lymphoma: A pilot study. Anticancer Res. 1998, 18, 2779–2788. [Google Scholar] [PubMed]
- Cu64-DOTA-trastuzumab PET and Markers Predicting Response to Neoadjuvant Trastuzumab + Pertuzum in HER2+ Breast Cancer. Available online: https://clinicaltrials.gov/search?term=NCT02827877 (accessed on 20 August 2023).
- An Investigational Scan (64Cu-Labeled M5A Antibody) in Combination with SOC Chemotherapy and Radiotherapy in Locally Advanced Rectal Cancer. Available online: https://clinicaltrials.gov/search?term=NCT05245786 (accessed on 20 August 2023).
- A Diagnostic Imaging Study of 64Cu-SARTATE Using PET on Patients with Known or Suspected Neuroendocrine Tumors (DISCO). Available online: https://clinicaltrials.gov/search?term=NCT04438304 (accessed on 20 August 2023).
- Copper Cu 64 TP3805 PET in Detecting Cancer in Patients with Prostate Cancer Undergoing Surgery. Available online: https://clinicaltrials.gov/search?term=NCT02603965 (accessed on 15 August 2023).
- Evaluation of a New Radiotracer (64Cu-DOTA-AE105) for Diagnosing Aggressive Cancer with Positron Emission Tomography. Available online: https://clinicaltrials.gov/search?term=NCT02139371 (accessed on 15 August 2023).
- Positron Emission Tomography (PET) Imaging of Participants with Confirmed Prostate Cancer Using 64Cu-SAR-bisPSMA (PROPELLER) (PROPELLER). Available online: https://clinicaltrials.gov/search?term=NCT04839367 (accessed on 15 August 2023).
- 64Cu-SAR-bisPSMA for Identification of Participants with Recurrence of Prostate Cancer (COBRA) (COBRA). Available online: https://clinicaltrials.gov/search?term=NCT05249127 (accessed on 15 August 2023).
- Peptide Receptor Radionuclide Therapy Administered to Participants with Meningioma With 67Cu-SARTATE™. Available online: https://clinicaltrials.gov/search?term=NCT03936426 (accessed on 15 August 2023).
- 67Cu-SARTATE™ Peptide Receptor Radionuclide Therapy Administered to Pediatric Patients with High-Risk, Relapsed, Refractory Neuroblastoma. Available online: https://clinicaltrials.gov/search?term=NCT04023331 (accessed on 15 August 2023).
- 64Cu-SAR-bisPSMA and 67Cu-SAR-bisPSMA for Identification and Treatment of PSMA-Expressing Metastatic Castrate Resistant Prostate Cancer (SECuRE) (SECuRE). Available online: https://clinicaltrials.gov/search?term=NCT04868604 (accessed on 15 August 2023).
- McCarthy, D.W.; Shefer, R.E.; Klinkowstein, R.E.; Bass, L.A.; Margeneau, W.H.; Cutler, C.S.; Anderson, C.J.; Welch, M.J. Efficient production of high specific activity 64Cu using a biomedical cyclotron. Nucl. Med. Biol. 1997, 24, 35–43. [Google Scholar] [CrossRef]
- Szelecsényi, F.; Steyn, G.; Dolley, S.; Kovács, Z.; Vermeulen, C.; Van der Walt, T. Investigation of the 68Zn (p, 2p) 67Cu nuclear reaction: New measurements up to 40 MeV and compilation up to 100 MeV. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2009, 267, 1877–1881. [Google Scholar] [CrossRef]
- Mou, L.P.G.; Martini, P.; Pasquali, M. A Method and a Target for the Production of 67Cu 2019. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2019220224 (accessed on 15 August 2023).
- Mikuš, P.; Melník, M.; Forgácsová, A.; Krajčiová, D.; Havránek, E. Gallium compounds in nuclear medicine and oncology. Main Group Met. Chem. 2014, 37, 53–65. [Google Scholar] [CrossRef]
- Amor-Coarasa, A.; Kelly, J.M.; Ponnala, S.; Nikolopoulou, A.; Williams, C., Jr.; Babich, J.W. 66Ga: A Novelty or a Valuable Preclinical Screening Tool for the Design of Targeted Radiopharmaceuticals? Molecules 2018, 23, 2575. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.L.; Sabanathan, D.; Aslani, A.; Campbell, D.H.; Walsh, B.J.; Lengkeek, N.A. RetroSPECT: Gallium-67 as a Long-Lived Imaging Agent for Theranostics. Asia Ocean J. Nucl. Med. Biol. 2021, 9, 1. [Google Scholar] [CrossRef]
- Rosenthall, L.; Kloiber, R.; Damtew, B.; Al-Majid, H. Sequential use of radiophosphate and radiogallium imaging in the differential diagnosis of bone, joint and soft tissue infection: Quantitative analysis. Diagn. Imaging 1982, 51, 249–258. [Google Scholar]
- Hetherington, V.J. Technetium and combined gallium and technetium scans in the neurotrophic foot. J. Am. Podiatry Assoc. 1982, 72, 458–463. [Google Scholar] [CrossRef]
- Jhanwar, Y.S.; Straus, D.J. The Role of PET in Lymphoma. J. Nucl. Med. 2006, 47, 1326–1334. [Google Scholar]
- Klech, H.; Köhn, H.; Huppmann, M.; Pohl, W. Thoracic imaging with gallium-67. Eur. J. Nucl. Med. 1987, 13 (Suppl. S1), S24–S36. [Google Scholar] [CrossRef]
- Fosburg, R.G.; Hopkins, G.B.; Kan, M.K. Evaluation of the mediastinum by gallium-67 scintigraphy in lung cancer. J. Thorac. Cardiovasc. Surg. 1979, 77, 76–82. [Google Scholar] [CrossRef]
- Graham, F.; Lord, M.; Froment, D.; Cardinal, H.; Bollée, G. The use of gallium-67 scintigraphy in the diagnosis of acute interstitial nephritis. Clin. Kidney J. 2015, 9, 76–81. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Alternative Radionuclide Production with a Cyclotron; International Atomic Energy Agency: Vienna, Austria, 2021. [Google Scholar]
- Banerjee, S.R.; Pomper, M.G. Clinical applications of Gallium-68. Appl. Radiat. Isot. 2013, 76, 2–13. [Google Scholar] [CrossRef]
- Fani, M.; André, J.P.; Maecke, H.R. 68Ga-PET: A powerful generator-based alternative to cyclotron-based PET radiopharmaceuticals. Contrast Media Mol. Imaging 2008, 3, 67–77. [Google Scholar] [CrossRef]
- Zhernosekov, K.P.; Filosofov, D.V.; Baum, R.P.; Aschoff, P.; Bihl, H.; Razbash, A.A.; Jahn, M.; Jennewein, M.; Rösch, F. Processing of generator-produced 68Ga for medical application. J. Nucl. Med. 2007, 48, 1741–1748. [Google Scholar] [CrossRef]
- Yang, J.; Kan, Y.; Ge, B.H.; Yuan, L.; Li, C.; Zhao, W. Diagnostic role of Gallium-68 DOTATOC and Gallium-68 DOTATATE PET in patients with neuroendocrine tumors: A meta-analysis. Acta Radiol. 2014, 55, 389–398. [Google Scholar] [CrossRef]
- Ambrosini, V.; Campana, D.; Bodei, L.; Nanni, C.; Castellucci, P.; Allegri, V.; Montini, G.C.; Tomassetti, P.; Paganelli, G.; Fanti, S. 68Ga-DOTANOC PET/CT clinical impact in patients with neuroendocrine tumors. J. Nucl. Med. 2010, 51, 669–673. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Verma, P.; Malhotra, G.; Goel, A.; Rakshit, S.; Chandak, A.; Chedda, R.; Banerjee, S.; Asopa, R.V. Differential Uptake of 68Ga-PSMA-HBED-CC (PSMA-11) in Low-Grade Versus High-Grade Gliomas in Treatment-Naive Patients. Clin. Nucl. Med. 2019, 44, e318–e322. [Google Scholar] [CrossRef] [PubMed]
- Lenzo, N.P.; Meyrick, D.; Turner, J.H. Review of Gallium-68 PSMA PET/CT Imaging in the Management of Prostate Cancer. Diagnostics 2018, 8, 16. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, M.; Zang, J.; Jiang, Y.; Chen, X.; Zhu, Z.; Chen, X. A pilot study of 68 Ga-PSMA-617 PET/CT imaging and 177Lu-EB-PSMA-617 radioligand therapy in patients with adenoid cystic carcinoma. EJNMMI Res. 2022, 12, 52. [Google Scholar] [CrossRef] [PubMed]
- Maffey-Steffan, J.; Scarpa, L.; Svirydenka, A.; Nilica, B.; Mair, C.; Buxbaum, S.; Bektic, J.; von Guggenberg, E.; Uprimny, C.; Horninger, W.; et al. The 68Ga/177Lu-theragnostic concept in PSMA-targeting of metastatic castration-resistant prostate cancer: Impact of post-therapeutic whole-body scintigraphy in the follow-up. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 695–712. [Google Scholar] [CrossRef]
- Ling, S.W.; de Blois, E.; Hooijman, E.; van der Veldt, A.; Brabander, T. Advances in 177Lu-PSMA and 225Ac-PSMA Radionuclide Therapy for Metastatic Castration-Resistant Prostate Cancer. Pharmaceutics 2022, 14, 2166. [Google Scholar] [CrossRef]
- Nelson, B.J.B.; Andersson, J.D.; Wuest, F.; Spreckelmeyer, S. Good practices for 68Ga radiopharmaceutical production. EJNMMI Radiopharm. Chem. 2022, 7, 27. [Google Scholar] [CrossRef]
- Lepareur, N. Cold Kit Labeling: The Future of 68Ga Radiopharmaceuticals? Front. Med. 2022, 9, 812050. [Google Scholar] [CrossRef]
- Pandey, M.K.; Byrne, J.F.; Jiang, H.; Packard, A.B.; DeGrado, T.R. Cyclotron production of 68Ga via the 68Zn(p,n)68Ga reaction in aqueous solution. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 303–310. [Google Scholar]
- Pandey, M.K.; Byrne, J.F.; Schlasner, K.N.; Schmit, N.R.; DeGrado, T.R. Cyclotron production of 68Ga in a liquid target: Effects of solution composition and irradiation parameters. Nucl. Med. Biol. 2019, 74, 49–55. [Google Scholar] [CrossRef]
- Pandey, M.K.; DeGrado, T.R. Cyclotron Production of PET Radiometals in Liquid Targets: Aspects and Prospects. Curr. Radiopharm. 2021, 14, 325–339. [Google Scholar] [CrossRef]
- Mueller, D.; Schlitter, M.; Senftleben, S.; Proehl, M.; Fuchs, A.; Leshch, Y. First Results of Cyclotron Produced 68Ga Using Solid Targets. J. Nucl. Med. 2019, 60, 1163. [Google Scholar]
- Lin, M.; Mukhopadhyay, U.; Waligorski, G.; Balatoni, J.; Gonzalez-Lepera, C. Production of Curie quantities of 68Ga with a medical cyclotron via the 68Zn(p,n)68Ga reaction. J. Nucl. Med. 2017, 58, 336. [Google Scholar] [CrossRef] [PubMed]
- Alnahwi, A.H.; Tremblay, S.; Ait-Mohand, S.; Beaudoin, J.F.; Guérin, B. Automated radiosynthesis of 68Ga for large-scale routine production using 68Zn pressed target. Appl. Radiat. Isot. 2020, 156, 109014. [Google Scholar] [CrossRef] [PubMed]
- Andrade Martins, P.d.; Osso, J.A. Thermal diffusion of 67Ga from irradiated Zn targets. Appl. Radiat. Isot. 2013, 82, 279–282. [Google Scholar] [CrossRef]
- Ga-68 PSMA Ligand: A Radiopharmaceutical for Localization of Prostate Cancer. Available online: https://clinicaltrials.gov/search?term=NCT03207139 (accessed on 15 August 2023).
- 68Ga PSMA PET/MRI for Hepatocellular Carcinoma. Available online: https://clinicaltrials.gov/search?term=NCT03982407 (accessed on 15 August 2023).
- A Phase 1/2 Clinical Trial to Evaluate the Safety, Tolerability, Dosimetry, and Anti-tumor Activity of Ga-68-NGUL/Lu-177-DGUL in Patients with Metastatic Castration-resistant Prostate Cancer (mCRPC) Refractory to Standard Therapy. Available online: https://clinicaltrials.gov/search?term=NCT05547061 (accessed on 15 August 2023).
- Direct Comparison of Ga-68-DOTATATE and Ga-68-DOTATOC. Available online: https://clinicaltrials.gov/search?term=NCT04298541 (accessed on 15 August 2023).
- Impact of Ga-68 DOTATOC PET-CT Imaging in Management of Neuroendocrine Tumors. Available online: https://clinicaltrials.gov/search?term=NCT02441062 (accessed on 15 August 2023).
- 68Ga-FAPi-46 PET/CT Scan in Imaging Patients with Sarcoma. Available online: https://clinicaltrials.gov/search?term=NCT04457258 (accessed on 15 August 2023).
- Hegi-Johnson, F.; Rudd, S.; Hicks, R.J.; De Ruysscher, D.; Trapani, J.A.; John, T.; Donnelly, P.; Blyth, B.; Hanna, G.; Everitt, S.; et al. Imaging immunity in patients with cancer using positron emission tomography. NPJ Precis. Oncol. 2022, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Deri, M.A.; Zeglis, B.M.; Francesconi, L.C.; Lewis, J.S. PET imaging with ⁸⁹Zr: From radiochemistry to the clinic. Nucl Med Biol 2013, 40, 3–14. [Google Scholar] [CrossRef]
- Börjesson, P.K.; Jauw, Y.W.; Boellaard, R.; de Bree, R.; Comans, E.F.; Roos, J.C.; Castelijns, J.A.; Vosjan, M.J.; Kummer, J.A.; Leemans, C.R.; et al. Performance of immuno-positron emission tomography with zirconium-89-labeled chimeric monoclonal antibody U36 in the detection of lymph node metastases in head and neck cancer patients. Clin. Cancer Res. 2006, 12, 2133–2140. [Google Scholar] [CrossRef]
- Mullard, A. FDA approves 100th monoclonal antibody product. Nat. Rev. Drug Discov. 2021, 20, 491–495. [Google Scholar] [CrossRef]
- Jauw, Y.W.; Menke-van der Houven van Oordt, C.W.; Hoekstra, O.S.; Hendrikse, N.H.; Vugts, D.J.; Zijlstra, J.M.; Huisman, M.C.; van Dongen, G.A. Immuno-Positron Emission Tomography with Zirconium-89-Labeled Monoclonal Antibodies in Oncology: What Can We Learn from Initial Clinical Trials? Front. Pharmacol. 2016, 7, 131. [Google Scholar] [CrossRef]
- Dijkers, E.C.; Oude Munnink, T.H.; Kosterink, J.G.; Brouwers, A.H.; Jager, P.L.; de Jong, J.R.; van Dongen, G.A.; Schröder, C.P.; Lub-de Hooge, M.N.; de Vries, E.G. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin. Pharmacol. Ther. 2010, 87, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Ulaner, G.A.; Lyashchenko, S.K.; Riedl, C.; Ruan, S.; Zanzonico, P.B.; Lake, D.; Jhaveri, K.; Zeglis, B.; Lewis, J.S.; O’Donoghue, J.A. First-in-Human Human Epidermal Growth Factor Receptor 2-Targeted Imaging Using 89Zr-Pertuzumab PET/CT: Dosimetry and Clinical Application in Patients with Breast Cancer. J. Nucl. Med. 2018, 59, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Bahce, I.; Huisman, M.C.; Verwer, E.E.; Ooijevaar, R.; Boutkourt, F.; Vugts, D.J.; van Dongen, G.A.M.S.; Boellaard, R.; Smit, E.F. Pilot study of 89Zr-bevacizumab positron emission tomography in patients with advanced non-small cell lung cancer. EJNMMI Res. 2014, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- Jauw, Y.W.; Zijlstra, J.M.; de Jong, D.; Vugts, D.J.; Zweegman, S.; Hoekstra, O.S.; van Dongen, G.A.; Huisman, M.C. Performance of 89Zr-Labeled-Rituximab-PET as an Imaging Biomarker to Assess CD20 Targeting: A Pilot Study in Patients with Relapsed/Refractory Diffuse Large B Cell Lymphoma. PLoS ONE 2017, 12, e0169828. [Google Scholar] [CrossRef] [PubMed]
- Menke-van der Houven van Oordt, C.W.; Gootjes, E.C.; Huisman, M.C.; Vugts, D.J.; Roth, C.; Luik, A.M.; Mulder, E.R.; Schuit, R.C.; Boellaard, R.; Hoekstra, O.S.; et al. 89Zr-cetuximab PET imaging in patients with advanced colorectal cancer. Oncotarget 2015, 6, 30384–30393. [Google Scholar] [CrossRef]
- Pilot Study of Zirconium-89 Bevacizumab Positron Emission Tomography for Imaging Angiogenesis in Patients with Inflammatory Breast Carcinoma Receiving Preoperative Chemotherapy. Available online: https://clinicaltrials.gov/search?term=NCT01894451 (accessed on 15 August 2023).
- Chames, P.; Van Regenmortel, M.; Weiss, E.; Baty, D. Therapeutic antibodies: Successes, limitations and hopes for the future. Br. J. Pharmacol. 2009, 157, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Vlachostergios, P.J.; Niaz, M.J.; Thomas, C.; Christos, P.J.; Osborne, J.R.; Margolis, D.J.A.; Khani, F.; Bander, N.H.; Scherr, D.S.; Tagawa, S.T. Pilot study of the diagnostic utility of 89 Zr-df-IAB2M and 68 Ga-PSMA-11 PET imaging and multiparametric MRI in localized prostate cancer. Prostate 2022, 82, 483–492. [Google Scholar] [CrossRef]
- Matsuda, M.; Ishikawa, E.; Yamamoto, T.; Hatano, K.; Joraku, A.; Iizumi, Y.; Masuda, Y.; Nishiyama, H.; Matsumura, A. Potential use of prostate specific membrane antigen (PSMA) for detecting the tumor neovasculature of brain tumors by PET imaging with 89Zr-Df-IAB2M anti-PSMA minibody. J. Neurooncol. 2018, 138, 581–589. [Google Scholar] [CrossRef]
- Burley, T.A.; Da Pieve, C.; Martins, C.D.; Ciobota, D.M.; Allott, L.; Oyen, W.J.G.; Harrington, K.J.; Smith, G.; Kramer-Marek, G. Affibody-Based PET Imaging to Guide EGFR-Targeted Cancer Therapy in Head and Neck Squamous Cell Cancer Models. J. Nucl. Med. 2019, 60, 353–361. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, L.; Pan, D.; Yan, J.; Wang, X.; Yang, R.; Li, M.; Liu, Y.; Yang, M. Synthesis of a novel 89Zr-labeled HER2 affibody and its application study in tumor PET imaging. EJNMMI Res. 2020, 10, 58. [Google Scholar] [CrossRef]
- Weist, M.R.; Starr, R.; Aguilar, B.; Chea, J.; Miles, J.K.; Poku, E.; Gerdts, E.; Yang, X.; Priceman, S.J.; Forman, S.J.; et al. PET of Adoptively Transferred Chimeric Antigen Receptor T Cells with 89Zr-Oxine. J. Nucl. Med. 2018, 59, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Yusufi, N.; Mall, S.; Bianchi, H.O.; Steiger, K.; Reder, S.; Klar, R.; Audehm, S.; Mustafa, M.; Nekolla, S.; Peschel, C.; et al. In-depth Characterization of a TCR-specific Tracer for Sensitive Detection of Tumor-directed Transgenic T Cells by Immuno-PET. Theranostics 2017, 7, 2402–2416. [Google Scholar] [CrossRef]
- Bansal, A.; Pandey, M.K.; Demirhan, Y.E.; Nesbitt, J.J.; Crespo-Diaz, R.J.; Terzic, A.; Behfar, A.; DeGrado, T.R. Novel 89Zr cell labeling approach for PET-based cell trafficking studies. EJNMMI Res. 2015, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Brahmbhatt, A.; Nieves Torres, E.; Thielen, B.; McCall, D.L.; Engel, S.; Bansal, A.; Pandey, M.K.; Dietz, A.B.; Leof, E.B.; et al. Tracking and Therapeutic Value of Human Adipose Tissue-derived Mesenchymal Stem Cell Transplantation in Reducing Venous Neointimal Hyperplasia Associated with Arteriovenous Fistula. Radiology 2016, 279, 513–522. [Google Scholar] [CrossRef]
- Bansal, A.; Pandey, M.K.; Yamada, S.; Goyal, R.; Schmit, N.R.; Jeon, R.; Nesbitt, J.J.; Witt, T.A.; Singh, R.D.; Gunderson, T.M.; et al. [89Zr]Zr-DBN labeled cardiopoietic stem cells proficient for heart failure. Nucl. Med. Biol. 2020, 90, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Sharma, S.; Klasen, B.; Rösch, F.; Pandey, M.K. Evaluation of different 89Zr-labeled synthons for direct labeling and tracking of white blood cells and stem cells in healthy athymic mice. Sci. Rep. 2022, 12, 15646. [Google Scholar] [CrossRef]
- Pandey, M.K.; Bansal, A.; Ellinghuysen, J.R.; Vail, D.J.; Berg, H.M.; DeGrado, T.R. A new solid target design for the production of 89Zr and radiosynthesis of high molar activity [89Zr]Zr-DBN. Am. J. Nucl. Med. Mol. Imaging 2022, 12, 15–24. [Google Scholar] [PubMed]
- Holland, J.P.; Sheh, Y.; Lewis, J.S. Standardized methods for the production of high specific-activity zirconium-89. Nucl. Med. Biol. 2009, 36, 729–739. [Google Scholar] [CrossRef]
- Pandey, M.; Engelbrecht, H.; Byrne, J.; Packard, A.; Degrado, T. Production of 89Zr via the 89Y(p,n)89Zr reaction in aqueous solution: Effect of solution composition on in-target chemistry. Nucl. Med. Biol. 2014, 41, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Kasbollah, A.; Eu, P.; Cowell, S.; Deb, P. Review on Production of 89Zr in a Medical Cyclotron for PET Radiopharmaceuticals. J. Nucl. Med. Technol. 2013, 41, 35–41. [Google Scholar] [CrossRef]
- Queern, S.L.; Aweda, T.A.; Massicano, A.V.F.; Clanton, N.A.; El Sayed, R.; Sader, J.A.; Zyuzin, A.; Lapi, S.E. Production of Zr-89 using sputtered yttrium coin targets 89Zr using sputtered yttrium coin targets. Nucl. Med. Biol. 2017, 50, 11–16. [Google Scholar] [CrossRef]
- Larenkov, A.; Bubenschikov, V.; Makichyan, A.; Zhukova, M.; Krasnoperova, A.; Kodina, G. Preparation of Zirconium-89 Solutions for Radiopharmaceutical Purposes: Interrelation Between Formulation, Radiochemical Purity, Stability and Biodistribution. Molecules 2019, 24, 1534. [Google Scholar] [CrossRef] [PubMed]
- Alnahwi, A.H.; Tremblay, S.; Guérin, B. Comparative Study with 89Y-foil and 89Y-pressed Targets for the Production of 89Zr. Appl. Sci. 2018, 8, 1579. [Google Scholar] [CrossRef]
- Wooten, A.L.; Madrid, E.; Schweitzer, G.D.; Lawrence, L.A.; Mebrahtu, E.; Lewis, B.C.; Lapi, S.E. Routine Production of 89Zr Using an Automated Module. Appl. Sci. 2013, 3, 593–613. [Google Scholar] [CrossRef]
- Pandey, M.K.; Bansal, A.; Engelbrecht, H.P.; Byrne, J.F.; Packard, A.B.; DeGrado, T.R. Improved production and processing of ⁸⁹Zr using a solution target. Nucl. Med. Biol. 2016, 43, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Lapi, S.E. Production of Positron Emitting Radiometals: Cu-64, Y-86, Zr-89; Final Report; Washington University: St. Louis, MO, USA, 2014. [Google Scholar] [CrossRef]
- Severin, G.W.; Engle, J.W.; Barnhart, T.E.; Nickles, R.J. 89Zr radiochemistry for positron emission tomography. Med. Chem. 2011, 7, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Molecular Correlates of Positron Emission Tomography (PET) with 89Zr-DFO-huJ591 in Metastatic Prostate Cancer. Available online: https://clinicaltrials.gov/search?term=NCT01543659 (accessed on 15 August 2023).
- Comparison of PET With [89Zr]-Df-IAb2M and [111In]-Capromab Pendetide in the Detection of Prostate Cancer Pre-Prostatectomy. Available online: https://clinicaltrials.gov/search?term=NCT02349022 (accessed on 15 August 2023).
- Study of Zirconium Zr 89 Crefmirlimab Berdoxam PET/CT in Subjects with Advanced or Metastatic Malignancies. Available online: https://clinicaltrials.gov/search?term=NCT05013099 (accessed on 15 August 2023).
- ⁸⁹Zr-Df-IAB22M2C PET/CT in Patients with Selected Solid Malignancies or Hodgkin’s Lymphoma. Available online: https://clinicaltrials.gov/search?term=NCT03107663 (accessed on 15 August 2023).
- ⁸⁹Zr-Df-IAB22M2C (CD8 PET Tracer) for PET/CT in Patients with Metastatic Solid Tumors. Available online: https://clinicaltrials.gov/search?term=NCT03802123 (accessed on 15 August 2023).
- First-in- Human Imaging of Multiple Myeloma Using 89Zr-DFO-daratumumab, a CD38-targeting Monoclonal Antibody. Available online: https://clinicaltrials.gov/search?term=NCT03665155 (accessed on 15 August 2023).
- Pilot Imaging Study With 89Zr-Trastuzumab in HER2-positive Metastatic Breast Cancer Patients. Available online: https://clinicaltrials.gov/search?term=NCT01420146 (accessed on 15 August 2023).
- Testing a New Imaging Agent to Identify Cancer. Available online: https://clinicaltrials.gov/search?term=NCT04692831 (accessed on 15 August 2023).
- Panitumumab-IRDye800 and 89Zr-Panitumumab in Identifying Metastatic Lymph Nodes in Patients with Squamous Cell Head and Neck Cancer. Available online: https://clinicaltrials.gov/search?term=NCT03733210%20 (accessed on 15 August 2023).
- Non-invasive Imaging of Cetuximab-Zr. 89 Uptake Wit PET: A Phase I Trial in Stage IV Cancer Patients. Available online: https://clinicaltrials.gov/search?term=NCT00691548 (accessed on 15 August 2023).
- 89Zr-TLX250 for PET/CT Imaging of ccRCC—ZIRCON Study. Available online: https://clinicaltrials.gov/search?term=NCT03849118 (accessed on 15 August 2023).
- Imaging Tumor-infiltrating T-cells in Non-small Cell Lung Cancer. Available online: https://clinicaltrials.gov/search?term=NCT03853187 (accessed on 15 August 2023).
- 89Zr-DFO-Atezolizumab ImmunoPET/CT in Patients with Locally Advanced or Metastatic Renal Cell Carcinoma. Available online: https://clinicaltrials.gov/search?term=NCT04006522 (accessed on 15 August 2023).
- Müller, C.; Domnanich, K.A.; Umbricht, C.A.; van der Meulen, N.P. Scandium and terbium radionuclides for radiotheranostics: Current state of development towards clinical application. Br. J. Radiol. 2018, 91, 20180074. [Google Scholar] [CrossRef]
- Rosar, F.; Buchholz, H.-G.; Michels, S.; Roesch, F.; Piel, M.; Miederer, M.; Reuss, S.; Schreckenberger, M. A performance comparison of Sc-44 to Ga-68 using preclinical PET scanners. J. Nucl. Med. 2018, 59, 1776. [Google Scholar]
- Ferguson, S.; Jans, H.S.; Wuest, M.; Riauka, T.; Wuest, F. Comparison of scandium-44 g with other PET radionuclides in pre-clinical PET phantom imaging. EJNMMI Phys. 2019, 6, 23. [Google Scholar] [CrossRef]
- Singh, A.; van der Meulen, N.P.; Müller, C.; Klette, I.; Kulkarni, H.R.; Türler, A.; Schibli, R.; Baum, R.P. First-in-Human PET/CT Imaging of Metastatic Neuroendocrine Neoplasms with Cyclotron-Produced 44Sc-DOTATOC: A Proof-of-Concept Study. Cancer Biother. Radiopharm. 2017, 32, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Eppard, E.; de la Fuente, A.; Benešová, M.; Khawar, A.; Bundschuh, R.A.; Gärtner, F.C.; Kreppel, B.; Kopka, K.; Essler, M.; Rösch, F. Clinical Translation and First In-Human Use of [44Sc]Sc-PSMA-617 for PET Imaging of Metastasized Castrate-Resistant Prostate Cancer. Theranostics 2017, 7, 4359–4369. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Bunka, M.; Haller, S.; Köster, U.; Groehn, V.; Bernhardt, P.; van der Meulen, N.; Türler, A.; Schibli, R. Promising prospects for 44Sc-/47Sc-based theragnostics: Application of 47Sc for radionuclide tumor therapy in mice. J. Nucl. Med. 2014, 55, 1658–1664. [Google Scholar] [CrossRef]
- Siwowska, K.; Guzik, P.; Domnanich, K.A.; Monné Rodríguez, J.M.; Bernhardt, P.; Ponsard, B.; Hasler, R.; Borgna, F.; Schibli, R.; Köster, U.; et al. Therapeutic Potential of 47Sc in Comparison to 177Lu and 90Y: Preclinical Investigations. Pharmaceutics 2019, 11, 424. [Google Scholar] [CrossRef] [PubMed]
- Filosofov, D.V.; Loktionova, N.S.; Rösch, F. A 44Ti/44Sc radionuclide generator for potential application of 44Sc-based PET-radiopharmaceuticals. rca Radiochim. Acta 2010, 98, 149–156. [Google Scholar] [CrossRef]
- Van der Meulen, N.P.; Bunka, M.; Domnanich, K.A.; Müller, C.; Haller, S.; Vermeulen, C.; Türler, A.; Schibli, R. Cyclotron production of 44Sc: From bench to bedside. Nucl. Med. Biol. 2015, 42, 745–751. [Google Scholar] [CrossRef]
- Szkliniarz, K.; Sitarz, M.; Walczak, R.; Jastrzębski, J.; Bilewicz, A.; Choiński, J.; Jakubowski, A.; Majkowska, A.; Stolarz, A.; Trzcińska, A.; et al. Production of medical Sc radioisotopes with an alpha particle beam. Appl. Radiat. Isot. 2016, 118, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Domnanich, K.A.; Eichler, R.; Müller, C.; Jordi, S.; Yakusheva, V.; Braccini, S.; Behe, M.; Schibli, R.; Türler, A.; van der Meulen, N.P. Production and separation of 43Sc for radiopharmaceutical purposes. EJNMMI Radiopharm. Chem. 2017, 2, 14. [Google Scholar] [CrossRef]
- Wibowo, F.A.; Kambali, I. Calculated cyclotron-based scandium-47 production yields via 48Ca(p, 2n)47Sc nuclear reaction. J. Phys. Conf. Ser. 2021, 1816, 012099. [Google Scholar] [CrossRef]
- Gizawy, M.A.; Mohamed, N.M.A.; Aydia, M.I.; Soliman, M.A.; Shamsel-Din, H.A. Feasibility study on production of Sc-47 from neutron irradiated Ca target for cancer theranostics applications. Radiochim. Acta 2020, 108, 207–215. [Google Scholar] [CrossRef]
- Rotsch, D.A.; Brown, M.A.; Nolen, J.A.; Brossard, T.; Henning, W.F.; Chemerisov, S.D.; Gromov, R.G.; Greene, J. Electron linear accelerator production and purification of scandium-47 from titanium dioxide targets. Appl. Radiat. Isot. 2018, 131, 77–82. [Google Scholar] [CrossRef]
- Mamtimin, M.; Harmon, F.; Starovoitova, V.N. Sc-47 production from titanium targets using electron linacs. Appl. Radiat. Isot. 2015, 102, 1–4. [Google Scholar] [CrossRef]
- Terbium: A New ‘Swiss Army Knife’ for Nuclear Medicine. Available online: https://cerncourier.com/a/terbium-a-new-swiss-army-knife-for-nuclear-medicine/ (accessed on 15 August 2023).
- Naskar, N.; Lahiri, S. Theranostic Terbium Radioisotopes: Challenges in Production for Clinical Application. Front. Med. 2021, 8, 675014. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Zhernosekov, K.; Köster, U.; Johnston, K.; Dorrer, H.; Hohn, A.; van der Walt, N.T.; Türler, A.; Schibli, R. A unique matched quadruplet of terbium radioisotopes for PET and SPECT and for α- and β- radionuclide therapy: An in vivo proof-of-concept study with a new receptor-targeted folate derivative. J. Nucl. Med. 2012, 53, 1951–1959. [Google Scholar] [CrossRef]
- Müller, C.; Vermeulen, C.; Köster, U.; Johnston, K.; Türler, A.; Schibli, R.; van der Meulen, N.P. Alpha-PET with terbium-149: Evidence and perspectives for radiotheragnostics. EJNMMI Radiopharm. Chem. 2016, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Beyer, G.J.; Miederer, M.; Vranjes-Durić, S.; Comor, J.J.; Künzi, G.; Hartley, O.; Senekowitsch-Schmidtke, R.; Soloviev, D.; Buchegger, F. Targeted alpha therapy in vivo: Direct evidence for single cancer cell kill using 149Tb-rituximab. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 547–554. [Google Scholar] [CrossRef]
- Baum, R.P.; Singh, A.; Benešová, M.; Vermeulen, C.; Gnesin, S.; Köster, U.; Johnston, K.; Müller, D.; Senftleben, S.; Kulkarni, H.R.; et al. Clinical evaluation of the radiolanthanide terbium-152: First-in-human PET/CT with 152Tb-DOTATOC. Dalton Trans. 2017, 46, 14638–14646. [Google Scholar] [CrossRef] [PubMed]
- Beyer, G.J.; Čomor, J.J.; Daković, M.; Soloviev, D.; Tamburella, C.; Hagebø, E.; Allan, B.; Dmitriev, S.N.; Zaitseva, N.G.; Collaboration, I. Production routes of the alpha emitting 149Tb for medical application. Radiochim. Acta 2002, 90, 247–252. [Google Scholar] [CrossRef]
- Umbricht, C.A.; Köster, U.; Bernhardt, P.; Gracheva, N.; Johnston, K.; Schibli, R.; van der Meulen, N.P.; Müller, C. Alpha-PET for Prostate Cancer: Preclinical investigation using 149Tb-PSMA-617. Sci. Rep. 2019, 9, 17800. [Google Scholar] [CrossRef]
- Müller, C.; Fischer, E.; Behe, M.; Köster, U.; Dorrer, H.; Reber, J.; Haller, S.; Cohrs, S.; Blanc, A.; Grünberg, J.; et al. Future prospects for SPECT imaging using the radiolanthanide terbium-155—Production and preclinical evaluation in tumor-bearing mice. Nucl. Med. Biol. 2014, 41 (Suppl. S1), e58–e65. [Google Scholar] [CrossRef]
- Haller, S.; Pellegrini, G.; Vermeulen, C.; van der Meulen, N.P.; Köster, U.; Bernhardt, P.; Schibli, R.; Müller, C. Contribution of Auger/conversion electrons to renal side effects after radionuclide therapy: Preclinical comparison of 161Tb-folate and 177Lu-folate. EJNMMI Res. 2016, 6, 13. [Google Scholar] [CrossRef]
- Grünberg, J.; Lindenblatt, D.; Dorrer, H.; Cohrs, S.; Zhernosekov, K.; Köster, U.; Türler, A.; Fischer, E.; Schibli, R. Anti-L1CAM radioimmunotherapy is more effective with the radiolanthanide terbium-161 compared to lutetium-177 in an ovarian cancer model. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1907–1915. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Reber, J.; Haller, S.; Dorrer, H.; Bernhardt, P.; Zhernosekov, K.; Türler, A.; Schibli, R. Direct in vitro and in vivo comparison of 161Tb and 177Lu using a tumour-targeting folate conjugate. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 476–485. [Google Scholar] [CrossRef]
- Hindié, E.; Zanotti-Fregonara, P.; Quinto, M.A.; Morgat, C.; Champion, C. Dose Deposits from 90Y, 177Lu, 111In, and 161Tb in Micrometastases of Various Sizes: Implications for Radiopharmaceutical Therapy. J. Nucl. Med. 2016, 57, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.P.; Singh, A.; Kulkarni, H.R.; Bernhardt, P.; Rydén, T.; Schuchardt, C.; Gracheva, N.; Grundler, P.V.; Köster, U.; Müller, D.; et al. First-in-Humans Application of 161Tb: A Feasibility Study Using 161Tb-DOTATOC. J. Nucl. Med. 2021, 62, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Combined Beta- Plus Auger Electron Therapy Using a Novel Somatostatin Receptor Subtype 2 Antagonist Labelled with Terbium-161 (161Tb-DOTA-LM3). Available online: https://clinicaltrials.gov/search?term=NCT05359146 (accessed on 15 August 2023).
- Evaluation of Radioligand Treatment in Men with Metastatic Castration-Resistant Prostate Cancer With [161Tb]Tb-PSMA-I&T. Available online: https://clinicaltrials.gov/search?term=NCT05521412 (accessed on 15 August 2023).
- Lehenberger, S.; Barkhausen, C.; Cohrs, S.; Fischer, E.; Grünberg, J.; Hohn, A.; Köster, U.; Schibli, R.; Türler, A.; Zhernosekov, K. The low-energy β(-) and electron emitter 161Tb as an alternative to 177Lu for targeted radionuclide therapy. Nucl. Med. Biol. 2011, 38, 917–924. [Google Scholar] [CrossRef]
- Firth, G.; Yu, Z.; Bartnicka, J.J.; Parker, D.; Kim, J.; Sunassee, K.; Greenwood, H.E.; Al-Salamee, F.; Jauregui-Osoro, M.; Di Pietro, A.; et al. Imaging zinc trafficking in vivo by positron emission tomography with zinc-62. Metallomics 2022, 14, mfac076. [Google Scholar] [CrossRef]
- Fujibayashi, Y.; Saji, H.; Kawai, K.; Unuma, Y.; Miyata, S.; Okuno, T.; Hosotani, R.; Inoue, K.; Adachi, H.; Horiuchi, K.; et al. A radiopharmaceutical for pancreatic exocrine functional diagnosis: 62Zn-EDDA metabolism in pancreas. Int. J. Nucl. Med. Biol. 1986, 12, 447–451. [Google Scholar] [CrossRef] [PubMed]
- DeGrado, T.R.; Pandey, M.K.; Byrne, J.F.; Engelbrecht, H.P.; Jiang, H.; Packard, A.B.; Thomas, K.A.; Jacobson, M.S.; Curran, G.L.; Lowe, V.J. Preparation and preliminary evaluation of 63Zn-zinc citrate as a novel PET imaging biomarker for zinc. J. Nucl. Med. 2014, 55, 1348–1354. [Google Scholar] [CrossRef]
- DeGrado, T.R.; Kemp, B.J.; Pandey, M.K.; Jiang, H.; Gunderson, T.M.; Linscheid, L.R.; Woodwick, A.R.; McConnell, D.M.; Fletcher, J.G.; Johnson, G.B.; et al. First PET Imaging Studies With 63Zn-Zinc Citrate in Healthy Human Participants and Patients with Alzheimer Disease. Mol. Imaging 2016, 15, 1536012116673793. [Google Scholar] [CrossRef]
- World Nuclear Association. Radioisotopes in Medicine. Available online: http://www.world-nuclear.org/information-library/non-power-nuclear-applications/radioisotopes-research/radioisotopes-in-medicine.aspx (accessed on 15 August 2023).
- Boschi, A.; Uccelli, L.; Marvelli, L.; Cittanti, C.; Giganti, M.; Martini, P. Technetium-99m Radiopharmaceuticals for Ideal Myocardial Perfusion Imaging: Lost and Found Opportunities. Molecules 2022, 27, 1188. [Google Scholar] [CrossRef]
- Duatti, A. Review on 99mTc radiopharmaceuticals with emphasis on new advancements. Nucl. Med. Biol. 2021, 92, 202–216. [Google Scholar] [CrossRef]
- Boschi, A.; Uccelli, L.; Martini, P. A Picture of Modern Tc-99m Radiopharmaceuticals: Production, Chemistry, and Applications in Molecular Imaging. Appl. Sci. 2019, 9, 2526. [Google Scholar] [CrossRef]
- Artiko, V.; Sobic-Saranovic, D.; Pavlovic, S.; Petrovic, M.; Zuvela, M.; Antic, A.; Matic, S.; Odalovic, S.; Petrovic, N.; Milovanovic, A.; et al. The clinical value of scintigraphy of neuroendocrine tumors using 9mTc-HYNIC-TOC. J. BUON 2012, 17, 537–542. [Google Scholar] [PubMed]
- Fallahi, B.; Manafi-Farid, R.; Eftekhari, M.; Fard-Esfahani, A.; Emami-Ardekani, A.; Geramifar, P.; Akhlaghi, M.; Hashemi Taheri, A.P.; Beiki, D. Diagnostic efficiency of 68Ga-DOTATATE PET/CT as compared to 99mTc-Octreotide SPECT/CT and conventional morphologic modalities in neuroendocrine tumors. Asia Ocean J. Nucl. Med. Biol. 2019, 7, 129–140. [Google Scholar] [CrossRef]
- Slomka, P.; Hung, G.U.; Germano, G.; Berman, D.S. Novel SPECT Technologies and Approaches in Cardiac Imaging. Cardiovasc. Innov. Appl. 2016, 2, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Vallabhajosula, S.; Nikolopoulou, A.; Babich, J.W.; Osborne, J.R.; Tagawa, S.T.; Lipai, I.; Solnes, L.; Maresca, K.P.; Armor, T.; Joyal, J.L.; et al. 99mTc-Labeled Small-Molecule Inhibitors of Prostate-Specific Membrane Antigen: Pharmacokinetics and Biodistribution Studies in Healthy Subjects and Patients with Metastatic Prostate Cancer. J. Nucl. Med. 2014, 55, 1791–1798. [Google Scholar] [CrossRef]
- Goffin, K.E.; Joniau, S.; Tenke, P.; Slawin, K.; Klein, E.A.; Stambler, N.; Strack, T.; Babich, J.; Armor, T.; Wong, V. Phase 2 Study of 99mTc-Trofolastat SPECT/CT to Identify and Localize Prostate Cancer in Intermediate- and High-Risk Patients Undergoing Radical Prostatectomy and Extended Pelvic LN Dissection. J. Nucl. Med. 2017, 58, 1408–1413. [Google Scholar] [CrossRef]
- Robu, S.; Schottelius, M.; Eiber, M.; Maurer, T.; Gschwend, J.; Schwaiger, M.; Wester, H.-J. Preclinical Evaluation and First Patient Application of 99mTc-PSMA-I&S for SPECT Imaging and Radioguided Surgery in Prostate Cancer. J. Nucl. Med. 2017, 58, 235–242. [Google Scholar] [CrossRef]
- Evaluation of Tc 99m Tilmanocept Localization in Primary Cutaneous Kaposi’s Sarcoma and Lymphatic Drainage by SPECT/CT. Available online: https://clinicaltrials.gov/search?term=NCT02201420 (accessed on 15 August 2023).
- Safety and Efficacy of Folatescan (Technetium TC 99M EC20) in Patients with Suspected Ovarian Carcinoma or Recurrent Endometrial Carcinoma. Available online: https://clinicaltrials.gov/search?term=NCT01689714%20 (accessed on 15 August 2023).
- Technical Performance Assessment of Tc-99m Tetrofosmin for Differentiation of Recurrence vs. Radiation Necrosis in Patients with Glioma. Available online: https://clinicaltrials.gov/search?term=NCT02971319 (accessed on 15 August 2023).
- Comparative Performance of Molecular Breast Imaging (MBI) to Magnetic Resonance Imaging (MRI) of the Breast in Identifying and Excluding Breast Carcinoma in Women at High Risk for Breast Cancer. Available online: https://clinicaltrials.gov/search?term=NCT05042687 (accessed on 15 August 2023).
- Validation of 99mTc- EDDA–HYNIC -TOC Kits for Diagnosis of Neuroendocrine Tumors. Available online: https://clinicaltrials.gov/search?term=NCT02691078%20 (accessed on 15 August 2023).
- Study to Evaluate 99mTc-MIP-1404 SPECT/CT Imaging in Men with Biopsy Proven Low-Grade Prostate Cancer. Available online: https://clinicaltrials.gov/search?term=NCT02615067%20 (accessed on 15 August 2023).
- A Study of 99mTc-MIP-1404 and 99mTc-MIP-1405 in Patients with Metastatic Prostate Adenocarcinoma and Healthy Volunteers. Available online: https://clinicaltrials.gov/search?term=NCT01261754%20 (accessed on 15 August 2023).
- Imaging Guided Surgery to Improve the Detection of Lymph Node Metastases in Prostate Cancer Patients. Available online: https://clinicaltrials.gov/search?term=NCT04832958%20 (accessed on 15 August 2023).
- Predictive Value of 99mTc- Albumin Spheres Before 90Y- SIR Therapy. Available online: https://clinicaltrials.gov/search?term=NCT01186263 (accessed on 15 August 2023).
- Jang, J.; Yamamoto, M.; Uesaka, M. Design of an X -band electron linear accelerator dedicated to decentralized 99Mo/99mTc supply: From beam energy selection to yield estimation. Phys. Rev. Accel. Beams 2017, 20, 104701. [Google Scholar] [CrossRef]
- Rovais, M.R.; Aardaneh, K.; Aslani, G.; Rahiminejad, A.; Yousefi, K.; Boulouri, F. Assessment of the direct cyclotron production of 99mTc: An approach to crisis management of 99mTc shortage. Appl. Radiat. Isot. 2016, 112, 55–61. [Google Scholar] [CrossRef]
- Dewulf, J.; Adhikari, K.; Vangestel, C.; Wyngaert, T.V.D.; Elvas, F. Development of Antibody Immuno-PET/SPECT Radiopharmaceuticals for Imaging of Oncological Disorders—An Update. Cancers 2020, 12, 1868. [Google Scholar] [CrossRef] [PubMed]
- Lubberink, M.; Tolmachev, V.; Widström, C.; Bruskin, A.; Lundqvist, H.; Westlin, J.E. 110mIn-DTPA-D-Phe1-octreotide for imaging of neuroendocrine tumors with PET. J. Nucl. Med. 2002, 43, 1391–1397. [Google Scholar] [PubMed]
- Salmanoglu, E.; Kim, S.; Thakur, M.L. Currently Available Radiopharmaceuticals for Imaging Infection and the Holy Grail. Semin. Nucl. Med. 2018, 48, 86–99. [Google Scholar] [CrossRef]
- Gawne, P.J.; Man, F.; Blower, P.J.; de Rosales, T.M.R. Direct Cell Radiolabeling for in Vivo Cell Tracking with PET and SPECT Imaging. Chem. Rev. 2022, 122, 10266–10318. [Google Scholar] [CrossRef]
- Lewis, S.S.; Cox, G.M.; Stout, J.E. Clinical utility of indium 111-labeled white blood cell scintigraphy for evaluation of suspected infection. Open Forum Infect. Dis. 2014, 1, ofu089. [Google Scholar] [CrossRef]
- Han, M.; Partin, A.W. Current Clinical Applications of the In-capromab Pendetide Scan (ProstaScint(R) Scan, Cyt-356). Rev. Urol. 2001, 3, 165–171. [Google Scholar]
- Morris, M.J.; Divgi, C.R.; Pandit-Taskar, N.; Batraki, M.; Warren, N.; Nacca, A.; Smith-Jones, P.; Schwartz, L.; Kelly, W.K.; Slovin, S.; et al. Pilot trial of unlabeled and indium-111-labeled anti-prostate-specific membrane antigen antibody J591 for castrate metastatic prostate cancer. Clin. Cancer Res. 2005, 11, 7454–7461. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vallabhajosula, S.; Kuji, I.; Hamacher, K.A.; Konishi, S.; Kostakoglu, L.; Kothari, P.A.; Milowski, M.I.; Nanus, D.M.; Bander, N.H.; Goldsmith, S.J. Pharmacokinetics and biodistribution of 111In- and 177Lu-labeled J591 antibody specific for prostate-specific membrane antigen: Prediction of 90Y-J591 radiation dosimetry based on 111In or 177Lu? J. Nucl. Med. 2005, 46, 634–641. [Google Scholar]
- Hekman, M.C.; Rijpkema, M.; Muselaers, C.H.; Oosterwijk, E.; Hulsbergen-Van de Kaa, C.A.; Boerman, O.C.; Oyen, W.J.; Langenhuijsen, J.F.; Mulders, P.F. Tumor-targeted Dual-modality Imaging to Improve Intraoperative Visualization of Clear Cell Renal Cell Carcinoma: A First in Man Study. Theranostics 2018, 8, 2161–2170. [Google Scholar] [CrossRef]
- Gao, J.; Liao, Z.; Liu, W.; Hu, Y.; Ma, H.; Xia, L.; Li, F.; Lan, T.; Yang, Y.; Yang, J.; et al. Simple and efficient method for producing high radionuclidic purity 111In using enriched 112Cd target. Appl. Radiat. Isot. 2021, 176, 109828. [Google Scholar] [CrossRef] [PubMed]
- Radiolabelled CCK-2/Gastrin Receptor Analogue for Personalized Theranostic Strategy in Advanced MTC. Available online: https://clinicaltrials.gov/search?term=NCT03246659 (accessed on 15 August 2023).
- 111In-ch806 in Patients with Advanced Tumours Expressing the 806 Antigen. Available online: https://clinicaltrials.gov/search?term=NCT00291447 (accessed on 15 August 2023).
- A Phase I Pilot Study Comparing 123I MIP 1072 Versus 111In Capromab Pendetide in Subjects with Metastatic Prostate Cancer. Available online: https://clinicaltrials.gov/search?term=NCT00992745 (accessed on 15 August 2023).
- Radio Guided Lymph Node Dissection in Oligometastatic Prost ate Cancer Patients. Available online: https://clinicaltrials.gov/search?term=NCT04300673 (accessed on 15 August 2023).
- Intraoperative Dual-modality Imaging in Renal Cell Carcinoma. Available online: https://clinicaltrials.gov/search?term=NCT02497599 (accessed on 15 August 2023).
- Trafficking of Indium-III-Labeled Cultured Immune Cells in Patients Undergoing Immunotherapy for Advanced Cancer. Available online: https://clinicaltrials.gov/search?term=NCT00026897 (accessed on 15 August 2023).
- Jødal, L. Beta emitters and radiation protection. Acta Oncol. 2009, 48, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Sugyo, A.; Tsuji, A.B.; Sudo, H.; Okada, M.; Koizumi, M.; Satoh, H.; Kurosawa, G.; Kurosawa, Y.; Saga, T. Evaluation of Efficacy of Radioimmunotherapy with 90Y-Labeled Fully Human Anti-Transferrin Receptor Monoclonal Antibody in Pancreatic Cancer Mouse Models. PLoS ONE 2015, 10, e0123761. [Google Scholar] [CrossRef]
- Kletting, P.; Kull, T.; Maaß, C.; Malik, N.; Luster, M.; Beer, A.J.; Glatting, G. Optimized Peptide Amount and Activity for 90Y-Labeled DOTATATE Therapy. J. Nucl. Med. 2016, 57, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Tong, A.K.; Kao, Y.H.; Too, C.W.; Chin, K.F.; Ng, D.C.; Chow, P.K. Yttrium-90 hepatic radioembolization: Clinical review and current techniques in interventional radiology and personalized dosimetry. Br. J. Radiol. 2016, 89, 20150943. [Google Scholar] [CrossRef]
- Ahmadzadehfar, H.; Biersack, H.J.; Ezziddin, S. Radioembolization of liver tumors with yttrium-90 microspheres. Semin. Nucl. Med. 2010, 40, 105–121. [Google Scholar] [CrossRef]
- Aranda, E.; Aparicio, J.; Bilbao, J.I.; García-Alfonso, P.; Maurel, J.; Rodríguez, J.; Sangro, B.; Vieitez, J.M.; Feliu, J. Recommendations for SIR-Spheres Y-90 resin microspheres in chemotherapy-refractory/intolerant colorectal liver metastases. Future Oncol. 2017, 13, 2065–2082. [Google Scholar] [CrossRef] [PubMed]
- Sposito, C.; Mazzaferro, V. The SIRveNIB and SARAH trials, radioembolization vs. sorafenib in advanced HCC patients: Reasons for a failure, and perspectives for the future. Hepatobiliary Surg. Nutr. 2018, 7, 487–489. [Google Scholar] [CrossRef]
- Vilgrain, V.; Pereira, H.; Assenat, E.; Guiu, B.; Ilonca, A.D.; Pageaux, G.-P.; Sibert, A.; Bouattour, M.; Lebtahi, R.; Allaham, W.; et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): An open-label randomised controlled phase 3 trial. Lancet Oncol. 2017, 18, 1624–1636. [Google Scholar] [CrossRef]
- Alsultan, A.A.; Braat, A.; Smits, M.L.J.; Barentsz, M.W.; Bastiaannet, R.; Bruijnen, R.C.G.; de Keizer, B.; de Jong, H.; Lam, M.; Maccauro, M.; et al. Current Status and Future Direction of Hepatic Radioembolisation. Clin. Oncol. 2021, 33, 106–116. [Google Scholar] [CrossRef]
- Garin, E.; Tselikas, L.; Guiu, B.; Chalaye, J.; Edeline, J.; de Baere, T.; Assenat, E.; Tacher, V.; Robert, C.; Terroir-Cassou-Mounat, M.; et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): A randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 17–29. [Google Scholar] [CrossRef]
- Wiseman, G.A.; Witzig, T.E. Yttrium-90 (90Y) ibritumomab tiuxetan (Zevalin) induces long-term durable responses in patients with relapsed or refractory B-Cell non-Hodgkin’s lymphoma. Cancer Biother. Radiopharm. 2005, 20, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Sachpekidis, C.; Jackson, D.B.; Soldatos, T.G. Radioimmunotherapy in Non-Hodgkin’s Lymphoma: Retrospective Adverse Event Profiling of Zevalin and Bexxar. Pharmaceuticals 2019, 12, 141. [Google Scholar] [CrossRef]
- Salles, G.; Barrett, M.; Foà, R.; Maurer, J.; O’Brien, S.; Valente, N.; Wenger, M.; Maloney, D.G. Rituximab in B-Cell Hematologic Malignancies: A Review of 20 Years of Clinical Experience. Adv. Ther. 2017, 34, 2232–2273. [Google Scholar] [CrossRef] [PubMed]
- Juweid, M.E. Radioimmunotherapy of B-Cell Non-Hodgkin’s Lymphoma: From Clinical Trials to Clinical Practice. J. Nucl. Med. 2002, 43, 1507–1529. [Google Scholar] [PubMed]
- Houghton, J.L.; Membreno, R.; Abdel-Atti, D.; Cunanan, K.M.; Carlin, S.; Scholz, W.W.; Zanzonico, P.B.; Lewis, J.S.; Zeglis, B.M. Establishment of the In Vivo Efficacy of Pretargeted Radioimmunotherapy Utilizing Inverse Electron Demand Diels-Alder Click Chemistry. Mol. Cancer Ther. 2017, 16, 124–133. [Google Scholar] [CrossRef]
- Paganelli, G.; Grana, C.; Chinol, M.; Cremonesi, M.; De Cicco, C.; De Braud, F.; Robertson, C.; Zurrida, S.; Casadio, C.; Zoboli, S.; et al. Antibody-guided three-step therapy for high grade glioma with yttrium-90 biotin. Eur. J. Nucl. Med. 1999, 26, 348–357. [Google Scholar] [CrossRef]
- Knox, S.J.; Goris, M.L.; Tempero, M.; Weiden, P.L.; Gentner, L.; Breitz, H.; Adams, G.P.; Axworthy, D.; Gaffigan, S.; Bryan, K.; et al. Phase II trial of yttrium-90-DOTA-biotin pretargeted by NR-LU-10 antibody/streptavidin in patients with metastatic colon cancer. Clin. Cancer Res. 2000, 6, 406–414. [Google Scholar]
- Jallinoja, V.I.J.; Houghton, J.L. Current Landscape in Clinical Pretargeted Radioimmunoimaging and Therapy. J. Nucl. Med. 2021, 62, 1200–1206. [Google Scholar] [CrossRef]
- Paganelli, G.; De Cicco, C.; Ferrari, M.E.; McVie, G.; Pagani, G.; Leonardi, M.C.; Cremonesi, M.; Ferrari, A.; Pacifici, M.; Di Dia, A.; et al. IART (Intra-Operative Avidination for Radionuclide Therapy) for accelerated radiotherapy in breast cancer patients. Technical aspects and preliminary results of a phase II study with 90Y-labelled biotin. Ecancermedicalscience 2010, 4, 166. [Google Scholar] [CrossRef]
- Chinol, M.; Hnatowich, D.J. Generator-produced yttrium-90 for radioimmunotherapy. J. Nucl. Med. 1987, 28, 1465–1470. [Google Scholar]
- Treatment of Patients with Advanced Renal Cancer with a Radiolabeled Antibody, Yttrium-90 Conjugated Chimeric G250. Available online: https://clinicaltrials.gov/search?term=NCT00199875 (accessed on 15 August 2023).
- Yttrium Y 90 DOTA Anti-CEA Monoclonal Antibody M5A in Treating Patients with Advanced Solid Tumors. Available online: https://clinicaltrials.gov/search?term=NCT00645060 (accessed on 15 August 2023).
- Radiolabeled Monoclonal Antibody Therapy, Combination Chemotherapy, and Bevacizumab in Treating Patients with Metastatic Colorectal Cancer. Available online: https://clinicaltrials.gov/search?term=NCT01205022 (accessed on 15 August 2023).
- Safety and Efficacy Study of Different Doses of 90Y-hPAM4 Combined with Gemcitabine in Pancreatic Cancer. Available online: https://clinicaltrials.gov/search?term=NCT00603863 (accessed on 15 August 2023).
- Neoadjuvant PRRT With Y-90-DOTATOC in pNET. Available online: https://clinicaltrials.gov/search?term=NCT05568017 (accessed on 15 August 2023).
- Yttrium Y 90 SMT 487 in Treating Patients with Refractory or Recurrent Cancer. Available online: https://clinicaltrials.gov/search?term=NCT00006368 (accessed on 15 August 2023).
- SorAfenib Versus Radioembolization in Advanced Hepatocellular Carcinoma. Available online: https://clinicaltrials.gov/search?term=NCT01482442%20 (accessed on 15 August 2023).
- Y90 Ibritumomab Tiuxetan Post R-CHOP Chemotherapy for Advanced Stage Follicular Lymphoma. Available online: https://clinicaltrials.gov/search?term=NCT01446562 (accessed on 15 August 2023).
- Uccelli, L.; Martini, P.; Urso, L.; Ghirardi, T.; Marvelli, L.; Cittanti, C.; Carnevale, A.; Giganti, M.; Bartolomei, M.; Boschi, A. Rhenium Radioisotopes for Medicine, a Focus on Production and Applications. Molecules 2022, 27, 5283. [Google Scholar] [CrossRef] [PubMed]
- Lepareur, N.; Lacœuille, F.; Bouvry, C.; Hindré, F.; Garcion, E.; Chérel, M.; Noiret, N.; Garin, E.; Knapp, F.F.R. Rhenium-188 Labeled Radiopharmaceuticals: Current Clinical Applications in Oncology and Promising Perspectives. Front. Med. 2019, 6, 132. [Google Scholar] [CrossRef]
- Maxon, H.R., 3rd; Schroder, L.E.; Washburn, L.C.; Thomas, S.R.; Samaratunga, R.C.; Biniakiewicz, D.; Moulton, J.S.; Cummings, D.; Ehrhardt, G.J.; Morris, V. Rhenium-188(Sn)HEDP for treatment of osseous metastases. J. Nucl. Med. 1998, 39, 659–663. [Google Scholar]
- De Decker, M.; Bacher, K.; Thierens, H.; Slegers, G.; Dierckx, R.A.; De Vos, F. In vitro and in vivo evaluation of direct rhenium-188-labeled anti-CD52 monoclonal antibody alemtuzumab for radioimmunotherapy of B-cell chronic lymphocytic leukemia. Nucl. Med. Biol. 2008, 35, 599–604. [Google Scholar] [CrossRef]
- Torres-García, E.; Ferro-Flores, G.; Arteaga de Murphy, C.; Correa-González, L.; Pichardo-Romero, P.A. Biokinetics and dosimetry of 188Re-anti-CD20 in patients with non-Hodgkin’s lymphoma: Preliminary experience. Arch. Med. Res. 2008, 39, 100–109. [Google Scholar] [CrossRef]
- Juweid, M.; Sharkey, R.M.; Swayne, L.C.; Griffiths, G.L.; Dunn, R.; Goldenberg, D.M. Pharmacokinetics, dosimetry and toxicity of rhenium-188-labeled anti-carcinoembryonic antigen monoclonal antibody, MN-14, in gastrointestinal cancer. J. Nucl. Med. 1998, 39, 34–42. [Google Scholar] [PubMed]
- Börjesson, P.K.; Postema, E.J.; Roos, J.C.; Colnot, D.R.; Marres, H.A.; van Schie, M.H.; Stehle, G.; de Bree, R.; Snow, G.B.; Oyen, W.J.; et al. Phase I therapy study with 186Re-labeled humanized monoclonal antibody BIWA 4 (bivatuzumab) in patients with head and neck squamous cell carcinoma. Clin. Cancer Res. 2003, 9, 3961s–3972s. [Google Scholar]
- Rhenium-Skin Cancer Therapy (SCT) for the Treatment of Non-Melanoma Skin Cancer. Available online: https://clinicaltrials.gov/search?term=NCT05135052 (accessed on 15 August 2023).
- Castellucci, P.; Savoia, F.; Farina, A.; Lima, G.M.; Patrizi, A.; Baraldi, C.; Zagni, F.; Vichi, S.; Pettinato, C.; Morganti, A.G.; et al. High dose brachytherapy with non sealed 188Re (rhenium) resin in patients with non-melanoma skin cancers (NMSCs): Single center preliminary results. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Argyrou, M.; Valassi, A.; Andreou, M.; Lyra, M. Rhenium-188 production in hospitals, by w-188/re-188 generator, for easy use in radionuclide therapy. Int. J. Mol. Imaging 2013, 2013, 290750. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, G.J.; Ketring, A.R.; Ayers, L.M. Reactor-produced radionuclides at the University of Missouri Research Reactor. Appl. Radiat. Isot. 1998, 49, 295–297. [Google Scholar] [CrossRef]
- Rhenium-188-HEDP vs. Radium-223-chloride in Patients with Advanced Prostate Cancer Refractory to Hormonal Therapy. Available online: https://clinicaltrials.gov/search?term=NCT03458559 (accessed on 15 August 2023).
- Biodistribution Study With 186 Re-labelled Humanised Monoclonal Antibody BIWA 4 in Patients with Non-small Cell Lung Cancer. Available online: https://clinicaltrials.gov/search?term=NCT02204059%20 (accessed on 15 August 2023).
- Biodistribution Study With 186 Re-Labelled Humanised Monoclonal Antibody BIWA 4 in Patients with Adenocarcinoma of the Breast. Available online: https://clinicaltrials.gov/search?term=NCT02204046 (accessed on 15 August 2023).
- Dose Escalation Study with 99mTC—Or 186 Re-labelled Humanised Monoclonal Antibody (hMAb) BIWA 4 in Patients with Head and Neck Cancer. Available online: https://clinicaltrials.gov/search?term=NCT02204033 (accessed on 15 August 2023).
- Maximum Tolerated Dose, Safety, and Efficacy of Rhenium Nanoliposomes in Recurrent Glioma (ReSPECT). Available online: https://clinicaltrials.gov/search?term=NCT01906385 (accessed on 15 August 2023).
- Vosoughi, S.; Shirvani-Arani, S.; Bahrami-Samani, A.; Salek, N.; Jalilian, A.R. Production of no-carrier-added Ho-166 for targeted therapy purposes. Iran. J. Nucl. Med. 2017, 25, 15–20. [Google Scholar]
- Stella, M.; Braat, A.; van Rooij, R.; de Jong, H.; Lam, M. Holmium-166 Radioembolization: Current Status and Future Prospective. Cardiovasc. Intervent. Radiol. 2022, 45, 1634–1645. [Google Scholar] [CrossRef] [PubMed]
- Mumper, R.J.; Ryo, U.Y.; Jay, M. Neutron-activated holmium-166-poly (L-lactic acid) microspheres: A potential agent for the internal radiation therapy of hepatic tumors. J. Nucl. Med. 1991, 32, 2139–2143. [Google Scholar]
- Klaassen, N.J.M.; Arntz, M.J.; Gil Arranja, A.; Roosen, J.; Nijsen, J.F.W. The various therapeutic applications of the medical isotope holmium-166: A narrative review. EJNMMI Radiopharm. Chem. 2019, 4, 19. [Google Scholar] [CrossRef]
- Dash, A.; Pillai, M.R.; Knapp, F.F., Jr. Production of 177Lu for Targeted Radionuclide Therapy: Available Options. Nucl. Med. Mol. Imaging 2015, 49, 85–107. [Google Scholar] [CrossRef]
- Kim, K.; Kim, S.J. Lu-177-Based Peptide Receptor Radionuclide Therapy for Advanced Neuroendocrine Tumors. Nucl. Med. Mol. Imaging 2018, 52, 208–215. [Google Scholar] [CrossRef]
- Yadav, M.P.; Ballal, S.; Sahoo, R.K.; Dwivedi, S.N.; Bal, C. Radioligand Therapy With 177Lu-PSMA for Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. Am. J. Roentgenol. 2019, 213, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Weineisen, M.; Schottelius, M.; Simecek, J.; Baum, R.P.; Yildiz, A.; Beykan, S.; Kulkarni, H.R.; Lassmann, M.; Klette, I.; Eiber, M.; et al. 68Ga- and 177Lu-Labeled PSMA I&T: Optimization of a PSMA-Targeted Theranostic Concept and First Proof-of-Concept Human Studies. J. Nucl. Med. 2015, 56, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Caplin, M.E.; Kunz, P.L.; Ruszniewski, P.B.; Bodei, L.; Hendifar, A.E.; Mittra, E.; Wolin, E.M.; Yao, J.C.; Pavel, M.E.; et al. Final overall survival in the phase 3 NETTER-1 study of lutetium-177-DOTATATE in patients with midgut neuroendocrine tumors. J. Clin. Oncol. 2021, 39, 4112. [Google Scholar] [CrossRef]
- Cankaya, A.; Balzer, M.; Amthauer, H.; Brenner, W.; Spreckelmeyer, S. Optimization of 177Lu-labelling of DOTA-TOC, PSMA-I&T and FAPI-46 for clinical application. EJNMMI Radiopharm. Chem. 2023, 8, 10. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.; Moon, E.S.; Kumari, S.; Roesch, F.; Tripathi, M.; Tupalli, A.; Bal, C. First clinical experience and initial outcomes of 177Lu-DOTAGA.(SA.FAPi2 therapy in patients with end-stage radioiodine-refractory differentiated thyroid cancer: A Salvage treatment option. J. Nucl. Med. 2021, 62, 1701. [Google Scholar]
- Study of 177Lu-PSMA-617 In Metastatic Castrate-Resistant Prostate Cancer (VISION). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03511664 (accessed on 17 August 2023).
- Combination of Radium-223 and Lutetium-177 PSMA-I&T in Men with Metastatic Castration-Resistant Prostate Cancer (AlphaBet). Available online: https://clinicaltrials.gov/study/NCT05383079?term=Lu177&page=4&rank=37 (accessed on 17 August 2023).
- Study of Cabozantinib with Lu-177 in Patients with Somatostatin Receptor 2 Positive Neuroendocrine Tumors. Available online: https://clinicaltrials.gov/study/NCT05249114?term=Lu177&rank=3 (accessed on 17 August 2023).
- Capecitabine ON Temozolomide Radionuclide Therapy Octreotate Lutetium-177 NeuroEndocrine Tumours Study (CONTROL NETS). Available online: https://clinicaltrials.gov/study/NCT02358356?term=Lu177&page=5&rank=42 (accessed on 17 August 2023).
- Lu-177-DOTATATE (Lutathera) in Therapy of Inoperable Pheochromocytoma/Paraganglioma. Available online: https://clinicaltrials.gov/study/NCT03206060?term=Lu177&page=2&rank=12 (accessed on 17 September 2023).
- Lutetium 177Lu-Edotreotide Versus Best Standard of Care in Well-Differentiated Aggressive Grade-2 and Grade-3 GastroEnteroPancreatic NeuroEndocrine Tumors (GEP-NETs)—COMPOSE (COMPOSE). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04919226 (accessed on 17 September 2023).
- Clinical Application of Lutetium [177Lu]-Catalase in Tumor Radionuclide Therapy. Available online: https://clinicaltrials.gov/study/NCT05985278?term=Lu177&rank=5 (accessed on 17 September 2023).
- Diagnosis of Metastatic Tumors on 68Ga-FAPI PET-CT and Radioligand Therapy With 177Lu-EB-FAPI. Available online: https://clinicaltrials.gov/study/NCT05400967?term=Lu177&rank=6 (accessed on 17 September 2023).
- Phase II Study of Lutetium-177 Labeled Girentuximab in Patients with Advanced Renal Cancer. Available online: https://clinicaltrials.gov/study/NCT02002312?term=Lu177&rank=8 (accessed on 17 September 2023).
- Chakravarty, R.; Chakraborty, S. A review of advances in the last decade on targeted cancer therapy using 177Lu: Focusing on 177Lu produced by the direct neutron activation route. Am. J. Nucl. Med. Mol. Imaging 2021, 11, 443–475. [Google Scholar]
- Eychenne, R.; Chérel, M.; Haddad, F.; Guérard, F.; Gestin, J.F. Overview of the Most Promising Radionuclides for Targeted Alpha Therapy: The “Hopeful Eight”. Pharmaceutics 2021, 13, 906. [Google Scholar] [CrossRef] [PubMed]
- Jurcic, J.G.; Larson, S.M.; Sgouros, G.; McDevitt, M.R.; Finn, R.D.; Divgi, C.R.; Ballangrud, A.M.; Hamacher, K.A.; Ma, D.; Humm, J.L.; et al. Targeted alpha particle immunotherapy for myeloid leukemia. Blood 2002, 100, 1233–1239. [Google Scholar] [CrossRef]
- Rosenblat, T.L.; McDevitt, M.R.; Mulford, D.A.; Pandit-Taskar, N.; Divgi, C.R.; Panageas, K.S.; Heaney, M.L.; Chanel, S.; Morgenstern, A.; Sgouros, G.; et al. Sequential cytarabine and alpha-particle immunotherapy with bismuth-213-lintuzumab (HuM195) for acute myeloid leukemia. Clin. Cancer Res. 2010, 16, 5303–5311. [Google Scholar] [CrossRef]
- Bruchertseifer, F.; Kellerbauer, A.; Malmbeck, R.; Morgenstern, A. Targeted alpha therapy with bismuth-213 and actinium-225: Meeting future demand. J. Labelled Comp. Radiopharm. 2019, 62, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Ahenkorah, S.; Cassells, I.; Deroose, C.M.; Cardinaels, T.; Burgoyne, A.R.; Bormans, G.; Ooms, M.; Cleeren, F. Bismuth-213 for Targeted Radionuclide Therapy: From Atom to Bedside. Pharmaceutics 2021, 13, 599. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Bruchertseifer, F.; Mier, W.; Apostolidis, C.; Boll, R.; Murphy, K.; Haberkorn, U.; Morgenstern, A. ²¹³Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: A first-in-human experience. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2106–2119. [Google Scholar] [CrossRef]
- Sathekge, M.; Knoesen, O.; Meckel, M.; Modiselle, M.; Vorster, M.; Marx, S. 213Bi-PSMA-617 targeted alpha-radionuclide therapy in metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1099–1100. [Google Scholar] [CrossRef]
- Kvassheim, M.; Revheim, M.-E.R.; Stokke, C. Quantitative SPECT/CT imaging of lead-212: A phantom study. EJNMMI Phys. 2022, 9, 52. [Google Scholar] [CrossRef]
- Robertson, A.K.H.; Ramogida, C.F.; Rodríguez-Rodríguez, C.; Blinder, S.; Kunz, P.; Sossi, V.; Schaffer, P. Multi-isotope SPECT imaging of the 225Ac decay chain: Feasibility studies. Phys. Med. Biol. 2017, 62, 4406–4420. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, A.; Bruchertseifer, F.; Apostolidis, C. Bismuth-213 and actinium-225—Generator performance and evolving therapeutic applications of two generator-derived alpha-emitting radioisotopes. Curr. Radiopharm. 2012, 5, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Chemotherapy and Monoclonal Antibody Therapy in Treating Patients with Advanced Myeloid Cancer. Available online: https://clinicaltrials.gov/search?term=NCT00014495 (accessed on 15 August 2023).
- Nelson, B.J.B.; Andersson, J.D.; Wuest, F. Targeted Alpha Therapy: Progress in Radionuclide Production, Radiochemistry, and Applications. Pharmaceutics 2020, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Jurcic, J.G.; Rosenblat, T.L.; McDevitt, M.R.; Pandit-Taskar, N.; Carrasquillo, J.A.; Chanel, S.M.; Ryan, C.; Frattini, M.G.; Cicic, D.; Larson, S.M.; et al. Phase I trial of the targeted alpha-particle nano-generator actinium-225 (225Ac-lintuzumab) (anti-CD33; HuM195) in acute myeloid leukemia (AML). J. Clin. Oncol. 2011, 29, 6516. [Google Scholar] [CrossRef]
- Juergens, R.A.; Zukotynski, K.A.; Juneau, D.; Krnezich, L.; Simms, R.; Forbes, J.; Burak, E.S.; Valliant, J.; Stafford, L.; Armor, T.; et al. A phase I study of [225Ac]-FPI-1434 radioimmunotherapy in patients with IGF-1R expressing solid tumors. J. Clin. Oncol. 2019, 37, TPS3152. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Bronzel, M.; Apostolidis, C.; Weichert, W.; Haberkorn, U.; Giesel, F.L.; Morgenstern, A. Targeted α-Therapy of Metastatic Castration-Resistant Prostate Cancer with 225Ac-PSMA-617: Dosimetry Estimate and Empiric Dose Finding. J. Nucl. Med. 2017, 58, 1624–1631. [Google Scholar] [CrossRef]
- Venetoclax and Lintuzumab-Ac225 in AML Patients. Available online: https://clinicaltrials.gov/search?term=NCT03867682%20 (accessed on 15 August 2023).
- Radioimmunotherapy (111Indium/225Actinium-DOTA-daratumumab) for the Treatment of Relapsed/Refractory Multiple Myeloma. Available online: https://clinicaltrials.gov/search?term=NCT05363111%20 (accessed on 15 August 2023).
- A Phase 1/2 Study of [225Ac]-FPI-1434 Injection. Available online: https://clinicaltrials.gov/search?term=NCT03746431%20 (accessed on 15 August 2023).
- Actinium 225 Labeled Anti-CEA Antibody (Ac225-DOTA-M5A) for the Treatment of CEA Producing Advanced or Metastatic Cancers. Available online: https://clinicaltrials.gov/search?term=NCT05204147%20 (accessed on 15 August 2023).
- Study of 225Ac-PSMA-617 in Men with PSMA-positive Prostate Cancer. Available online: https://clinicaltrials.gov/search?term=NCT04597411 (accessed on 15 August 2023).
- Phase I Trial of 225Ac-J591 in Patients With mCRPC. Available online: https://clinicaltrials.gov/search?term=NCT03276572%20 (accessed on 15 August 2023).
- Weidner, J.W.; Mashnik, S.G.; John, K.D.; Ballard, B.; Birnbaum, E.R.; Bitteker, L.J.; Couture, A.; Fassbender, M.E.; Goff, G.S.; Gritzo, R.; et al. 225Ac and 223Ra production via 800 MeV proton irradiation of natural thorium targets. Appl. Radiat. Isot. 2012, 70, 2590–2595. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, X.; Quinn, T.P.; Lee, D.; Liu, D.; Kunkel, F.; Zimmerman, B.E.; McAlister, D.; Olewein, K.; Menda, Y.; et al. Automated cassette-based production of high specific activity [203/212Pb]peptide-based theranostic radiopharmaceuticals for image-guided radionuclide therapy for cancer. Appl. Radiat. Isot. 2017, 127, 52–60. [Google Scholar] [CrossRef]
- McNeil, B.L.; Robertson, A.K.H.; Fu, W.; Yang, H.; Hoehr, C.; Ramogida, C.F.; Schaffer, P. Production, purification, and radiolabeling of the 203Pb/212Pb theranostic pair. EJNMMI Radiopharm. Chem. 2021, 6, 6. [Google Scholar] [CrossRef]
- Meredith, R.F.; Torgue, J.; Azure, M.T.; Shen, S.; Saddekni, S.; Banaga, E.; Carlise, R.; Bunch, P.; Yoder, D.; Alvarez, R. Pharmacokinetics and imaging of 212Pb-TCMC-trastuzumab after intraperitoneal administration in ovarian cancer patients. Cancer Biother. Radiopharm. 2014, 29, 12–17. [Google Scholar] [CrossRef]
- Meredith, R.; Torgue, J.; Shen, S.; Fisher, D.R.; Banaga, E.; Bunch, P.; Morgan, D.; Fan, J.; Straughn, J.M., Jr. Dose escalation and dosimetry of first-in-human α radioimmunotherapy with 212Pb-TCMC-trastuzumab. J. Nucl. Med. 2014, 55, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Meredith, R.F.; Torgue, J.J.; Rozgaja, T.A.; Banaga, E.P.; Bunch, P.W.; Alvarez, R.D.; Straughn, J.M., Jr.; Dobelbower, M.C.; Lowy, A.M. Safety and Outcome Measures of First-in-Human Intraperitoneal α Radioimmunotherapy With 212Pb-TCMC-Trastuzumab. Am. J. Clin. Oncol. 2018, 41, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Delpassand, E.S.; Tworowska, I.; Esfandiari, R.; Torgue, J.; Hurt, J.; Shafie, A.; Núñez, R. Targeted α-Emitter Therapy with 212Pb-DOTAMTATE for the Treatment of Metastatic SSTR-Expressing Neuroendocrine Tumors: First-in-Humans Dose-Escalation Clinical Trial. J. Nucl. Med. 2022, 63, 1326–1333. [Google Scholar] [CrossRef]
- Targeted Alpha-emitter Therapy of PRRT Naive Neuroendocrine Tumor Patients. Available online: https://clinicaltrials.gov/search?term=NCT05153772 (accessed on 15 August 2023).
- Safety Study of ²¹²Pb-TCMC-Trastuzumab Radio Immunotherapy. Available online: https://clinicaltrials.gov/search?term=NCT01384253 (accessed on 15 August 2023).
- Kokov, K.V.; Egorova, B.V.; German, M.N.; Klabukov, I.D.; Krasheninnikov, M.E.; Larkin-Kondrov, A.A.; Makoveeva, K.A.; Ovchinnikov, M.V.; Sidorova, M.V.; Chuvilin, D.Y. 212Pb: Production Approaches and Targeted Therapy Applications. Pharmaceutics 2022, 14, 189. [Google Scholar] [CrossRef]
- Sartor, O.; Sharma, D. Radium and other alpha emitters in prostate cancer. Transl. Androl. Urol. 2018, 7, 436–444. [Google Scholar] [CrossRef]
- Flux, G.D. Imaging and dosimetry for radium-223: The potential for personalized treatment. Br. J. Radiol. 2017, 90, 20160748. [Google Scholar] [CrossRef]
- Akabani, G.; Kennel, S.J.; Zalutsky, M.R. Microdosimetric Analysis of α-Particle-Emitting Targeted Radiotherapeutics Using Histological Images. J. Nucl. Med. 2003, 44, 792–805. [Google Scholar] [PubMed]
- Sgouros, G.; Hobbs, R.F. Dosimetry for radiopharmaceutical therapy. Semin. Nucl. Med. 2014, 44, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Pacilio, M.; Ventroni, G.; De Vincentis, G.; Cassano, B.; Pellegrini, R.; Di Castro, E.; Frantellizzi, V.; Follacchio, G.A.; Garkavaya, T.; Lorenzon, L.; et al. Dosimetry of bone metastases in targeted radionuclide therapy with alpha-emitting 223Ra-dichloride. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 21–33. [Google Scholar] [CrossRef]
- Gott, M.; Steinbach, J.; Mamat, C. The Radiochemical and Radiopharmaceutical Applications of Radium. Open Chem. 2016, 14, 118–129. [Google Scholar] [CrossRef]
- Jadvar, H.; Quinn, D.I. Targeted α-particle therapy of bone metastases in prostate cancer. Clin. Nucl. Med. 2013, 38, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.Y.; Chung, K.H.; Lim, J.M.; Kim, C.J.; Jang, M.; Kang, M.J.; Park, S.T.; Woo, Z.; Koo, B.; Seo, B. Feasibility about the direct measurement of 226 Ra using the gamma-ray spectrometry. J. Nucl. Fuel Cycle Waste Technol. 2014, 12, 97–105. [Google Scholar] [CrossRef]
- Westrøm, S.; Generalov, R.; Bønsdorff, T.B.; Larsen, R.H. Preparation of 212Pb-labeled monoclonal antibody using a novel 224Ra-based generator solution. Nucl. Med. Biol. 2017, 51, 1–9. [Google Scholar] [CrossRef]
- Lassmann, M.; Eberlein, U. Comparing absorbed doses and radiation risk of the α-emitting bone-seekers [223Ra]RaCl(2) and [224Ra]RaCl(2). Front. Med. 2022, 9, 1057373. [Google Scholar] [CrossRef]
- Juzeniene, A.; Bernoulli, J.; Suominen, M.; Halleen, J.; Larsen, R.H. Antitumor Activity of Novel Bone-seeking, α-emitting 224Ra-solution in a Breast Cancer Skeletal Metastases Model. Anticancer. Res. 2018, 38, 1947–1955. [Google Scholar] [PubMed]
- Wick, R.R.; Atkinson, M.J.; Nekolla, E.A. Incidence of leukaemia and other malignant diseases following injections of the short-lived α-emitter 224Ra into man. Radiat. Environ. Biophys. 2009, 48, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Arazi, L.; Cooks, T.; Schmidt, M.; Keisari, Y.; Kelson, I. Treatment of solid tumors by interstitial release of recoiling short-lived alpha emitters. Phys. Med. Biol. 2007, 52, 5025. [Google Scholar] [CrossRef] [PubMed]
- Popovtzer, A.; Rosenfeld, E.; Mizrachi, A.; Bellia, S.R.; Ben-Hur, R.; Feliciani, G.; Sarnelli, A.; Arazi, L.; Deutsch, L.; Kelson, I.; et al. Initial Safety and Tumor Control Results From a “First-in-Human” Multicenter Prospective Trial Evaluating a Novel Alpha-Emitting Radionuclide for the Treatment of Locally Advanced Recurrent Squamous Cell Carcinomas of the Skin and Head and Neck. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 571–578. [Google Scholar] [CrossRef]
- Nilsson, S.; Larsen, R.H.; Fosså, S.D.; Balteskard, L.; Borch, K.W.; Westlin, J.E.; Salberg, G.; Bruland, O.S. First clinical experience with alpha-emitting radium-223 in the treatment of skeletal metastases. Clin. Cancer Res. 2005, 11, 4451–4459. [Google Scholar] [CrossRef] [PubMed]
- Florimonte, L.; Dellavedova, L.; Maffioli, L.S. Radium-223 dichloride in clinical practice: A review. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1896–1909. [Google Scholar] [CrossRef]
- Vuong, W.; Sartor, O.; Pal, S.K. Radium-223 in metastatic castration resistant prostate cancer. Asian J. Androl. 2014, 16, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Phase I Dose Escalation of Monthly Intravenous Ra-223 Dichloride in Osteosarcoma. Available online: https://clinicaltrials.gov/search?term=NCT01833520 (accessed on 15 August 2023).
- Phase IB Trial of Radium-223 and Niraparib in Patients with Castrate Resistant Prostate Cancer (NiraRad). Available online: https://clinicaltrials.gov/search?term=NCT03076203 (accessed on 15 August 2023).
- Radium-223 Dichloride and Abiraterone Acetate Compared to Placebo and Abiraterone Acetate for Men with Cancer of the Prostate When Medical or Surgical Castration Does Not Work and When the Cancer Has Spread to the Bone, Has Not Been Treated with Chemotherapy and Is Causing No Or Only Mild Symptoms (ERA 223). Available online: https://clinicaltrials.gov/search?term=NCT02043678 (accessed on 15 August 2023).
- Radium Ra 223 With Enzalutamide Compared to Enzalutamide Alone in Men with Metastatic Castration Refractory Prostate Cancer. Available online: https://clinicaltrials.gov/search?term=NCT02199197%20 (accessed on 15 August 2023).
- The Combination Therapy with Ra-223 and Enzalutamide. Available online: https://clinicaltrials.gov/search?term=NCT03305224 (accessed on 15 August 2023).
- Trial of Ra-223 Dichloride in Combination with Hormonal Therapy and Denosumab in the Treatment of Patients with Hormone-Positive Bone-Dominant Metastatic Breast Cancer. Available online: https://clinicaltrials.gov/search?term=NCT02366130%20 (accessed on 15 August 2023).
- Phase Ib Study of Radium Ra 223 Dichloride in Combination with Paclitaxel in Cancer Subjects with Bone Lesions. Available online: https://clinicaltrials.gov/search?term=NCT02442063%20 (accessed on 15 August 2023).
- A Study to Test Radium-223 With Docetaxel in Patients with Prostate Cancer. Available online: https://clinicaltrials.gov/search?term=NCT03574571 (accessed on 15 August 2023).
- Radium Ra 223 Dichloride, Hormone Therapy and Stereotactic Body Radiation Therapy in Treating Patients with Metastatic Prostate Cancer. Available online: https://clinicaltrials.gov/search?term=NCT03361735 (accessed on 15 August 2023).
- Study Evaluating the Addition of Pembrolizumab to Radium-223 in mCRPC. Available online: https://clinicaltrials.gov/search?term=NCT03093428 (accessed on 15 August 2023).
- Safety and Tolerability of Atezolizumab (ATZ) in Combination with Radium-223 Dichloride (R-223-D) in Metastatic Castrate-Resistant Prostate Cancer (CRPC) Progressed Following Treatment with an Androgen Pathway Inhibitor. Available online: https://clinicaltrials.gov/search?term=NCT02814669 (accessed on 15 August 2023).
- Alpha Radiation Emitters Device for the Treatment of Advanced Pancreatic Cancer. Available online: https://clinicaltrials.gov/search?term=NCT04002479%20 (accessed on 15 August 2023).
- Alpha Radiation Emitters Device for the Treatment of Newly Diagnosed Breast Carcinoma Patients with Distant Metastases. Available online: https://clinicaltrials.gov/search?term=NCT03970967 (accessed on 15 August 2023).
- Abou, D.S.; Pickett, J.; Mattson, J.E.; Thorek, D.L.J. A Radium-223 microgenerator from cyclotron-produced trace Actinium-227. Appl. Radiat. Isot. 2017, 119, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Pruszyński, M.; Walczak, R.; Rodak, M.; Bruchertseifer, F.; Morgenstern, A.; Bilewicz, A. Radiochemical separation of 224Ra from 232U and 228Th sources for 224Ra/212Pb/212Bi generator. Appl. Radiat. Isot. 2021, 172, 109655. [Google Scholar] [CrossRef]
- Murray, I.; Rojas, B.; Gear, J.; Callister, R.; Cleton, A.; Flux, G.D. Quantitative Dual-Isotope Planar Imaging of Thorium-227 and Radium-223 Using Defined Energy Windows. Cancer Biother. Radiopharm. 2020, 35, 530–539. [Google Scholar] [CrossRef]
- Karlsson, J.; Schatz, C.A.; Wengner, A.M.; Hammer, S.; Scholz, A.; Cuthbertson, A.; Wagner, V.; Hennekes, H.; Jardine, V.; Hagemann, U.B. Targeted thorium-227 conjugates as treatment options in oncology. Front. Med. 2022, 9, 1071086. [Google Scholar] [CrossRef] [PubMed]
- Heyerdahl, H.; Abbas, N.; Sponheim, K.; Mollatt, C.; Bruland, Ø.; Dahle, J. Targeted Alpha Therapy with 227Th-trastuzumab of Intraperitoneal Ovarian Cancer in Nude Mice. Curr. Radiopharm. 2013, 6, 106–116. [Google Scholar] [CrossRef]
- Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Antitumor Activity of a Thorium-227 Labeled Antibody-Chelator Conjugate Alone and in Combination With Darolutamide, in Patients with Metastatic Castration Resistant Prostate Cancer. Available online: https://clinicaltrials.gov/search?term=NCT03724747 (accessed on 15 August 2023).
- Hammer, S.; Zitzmann-Kolbe, S.; Oden, F.; Jannsen, J.; Bickel, E.; Mueller, A.; Everz, L.; Hagemann, U.B.; Karlsson, J.; Ryan, O.B.; et al. Abstract 1141: Preclinical analysis of biodistribution and PET imaging of a zirconium-89 labeled PSMA-targeted antibody-chelator conjugate. Cancer Res. 2019, 79, 1141. [Google Scholar] [CrossRef]
- Ermolaev, S.V.; Zhuikov, B.L.; Kokhanyuk, V.M.; Matushko, V.L.; Kalmykov, S.N.; Aliev, R.A.; Tananaev, I.G.; Myasoedov, B.F. Production of actinium, thorium and radium isotopes from natural thorium irradiated with protons up to 141 MeV. Radiochim. Acta 2012, 100, 223–229. [Google Scholar] [CrossRef]
- First-in-human Study of BAY2287411 Injection, a Thorium-227 Labeled Antibody-chelator Conjugate, in Patients with Tumors Known to Express Mesothelin. Available online: https://clinicaltrials.gov/search?term=NCT03507452 (accessed on 15 August 2023).
- A First in Human Study of BAY2701439 to Look at Safety, How the Body Absorbs, Distributes and Excretes the Drug, and How Well the Drug Works in Participants with Advanced Cancer Expressing the HER2 Protein. Available online: https://clinicaltrials.gov/search?term=NCT04147819 (accessed on 15 August 2023).
- Safety and Tolerability of BAY1862864 Injection in Subjects with Relapsed or Refractory CD22-Positive Non-Hodgkin’s Lymphoma. Available online: https://clinicaltrials.gov/search?term=NCT02581878%20 (accessed on 15 August 2023).
- Lindegren, S.; Albertsson, P.; Bäck, T.; Jensen, H.; Palm, S.; Aneheim, E. Realizing Clinical Trials with Astatine-211: The Chemistry Infrastructure. Cancer Biother. Radiopharm. 2020, 35, 425–436. [Google Scholar] [CrossRef]
- Zalutsky, M.R.; Reardon, D.A.; Akabani, G.; Coleman, R.E.; Friedman, A.H.; Friedman, H.S.; McLendon, R.E.; Wong, T.Z.; Bigner, D.D. Clinical experience with alpha-particle emitting 211At: Treatment of recurrent brain tumor patients with 211At-labeled chimeric antitenascin monoclonal antibody 81C6. J. Nucl. Med. 2008, 49, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Hallqvist, A.; Bergmark, K.; Bäck, T.; Andersson, H.; Dahm-Kähler, P.; Johansson, M.; Lindegren, S.; Jensen, H.; Jacobsson, L.; Hultborn, R.; et al. Intraperitoneal α-Emitting Radioimmunotherapy with 211At in Relapsed Ovarian Cancer: Long-Term Follow-up with Individual Absorbed Dose Estimations. J. Nucl. Med. 2019, 60, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Guérard, F.; Gestin, J.F.; Brechbiel, M.W. Production of [211At]-astatinated radiopharmaceuticals and applications in targeted α-particle therapy. Cancer Biother. Radiopharm. 2013, 28, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Larsen, R.H.; Wieland, B.W.; Zalutsky, M.R. Evaluation of an internal cyclotron target for the production of 211At via the 209Bi (α,2n)211At reaction. Appl. Radiat. Isot. 1996, 47, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.R.; Yang, H.; Kunz, P.; Wilbur, D.S.; Schaffer, P.; Ruth, T.J. Development of a preclinical 211Rn/211At generator system for targeted alpha therapy research with 211At. Nucl. Med. Biol. 2017, 48, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Targeted Alpha Therapy Using Astatine (At-211) Against Differentiated Thyroid Cancer. Available online: https://clinicaltrials.gov/search?term=NCT05275946 (accessed on 15 August 2023).
- Radiolabeled Monoclonal Antibody Therapy in Treating Patients with Primary or Metastatic Brain Tumors. Available online: https://clinicaltrials.gov/search?term=NCT00003461%20 (accessed on 15 August 2023).
- Total Body Irradiation and Astatine-211-Labeled BC8-B10 Monoclonal Antibody for the Treatment of Nonmalignant Diseases. Available online: https://clinicaltrials.gov/search?term=NCT04083183 (accessed on 15 August 2023).
- 211At-BC8-B10 Followed by Donor Stem Cell Transplant in Treating Patients with Relapsed or Refractory High-Risk Acute Leukemia or Myelodysplastic Syndrome. Available online: https://clinicaltrials.gov/search?term=NCT03670966 (accessed on 15 August 2023).
- Radioimmunotherapy (211At-OKT10-B10) and Chemotherapy (Melphalan) Before Stem Cell Transplantation for the Treatment of Multiple Myeloma. Available online: https://clinicaltrials.gov/search?term=NCT04466475 (accessed on 15 August 2023).
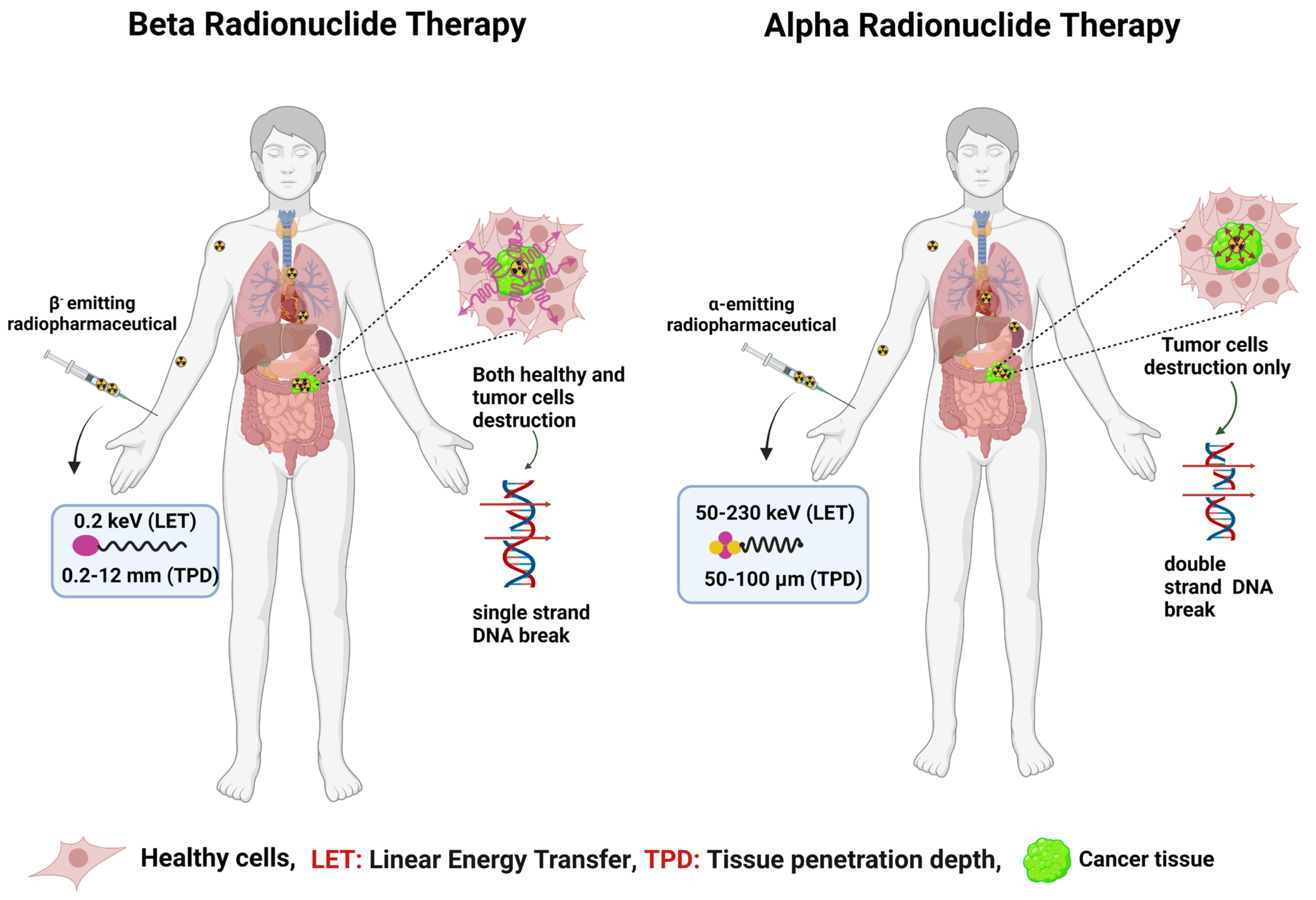
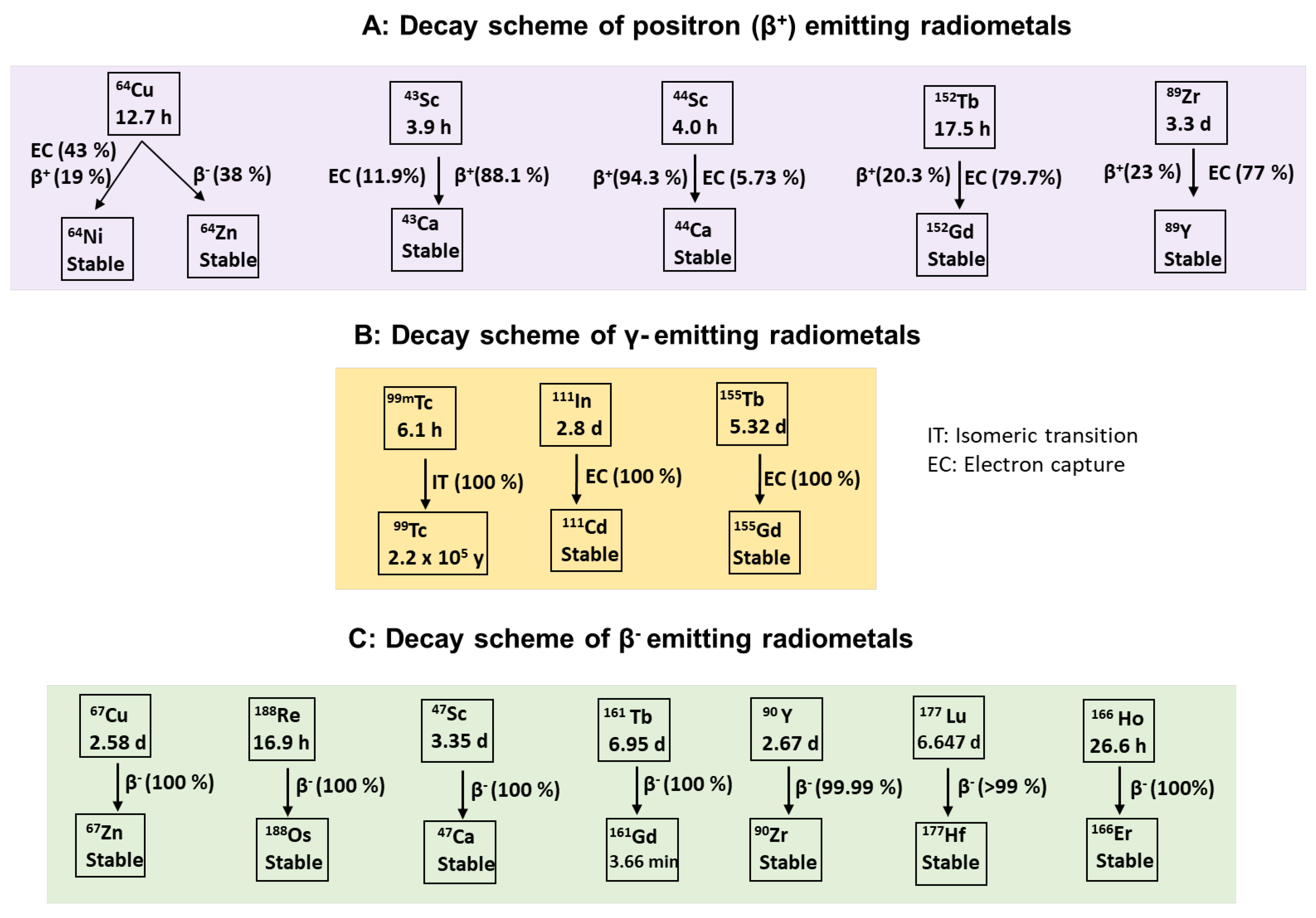
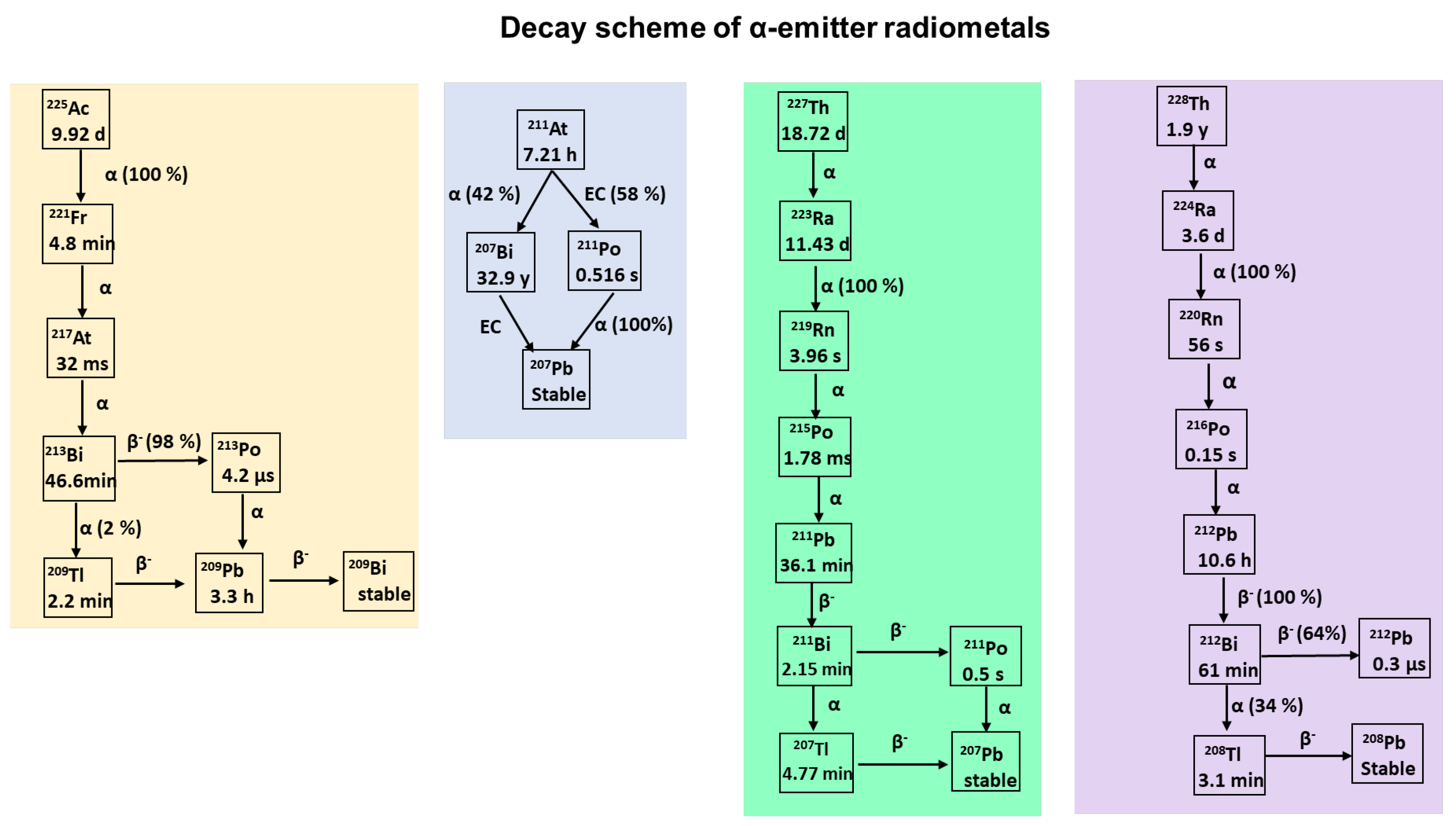
| Isotope | Half-Life (t1/2) | Decay Characteristics | Energies | Eγ; keV (Intensity %) | |
|---|---|---|---|---|---|
| Eβ+avg (keV) | Eβ−avg (keV) | ||||
| 60Cu | 23.7 min | β+ = 93% EC = 7% | 970 | - | 1332.5 (88) 1791.6 (45.4) 826.4 (21.7) |
| 61Cu | 3.33 h | β+ = 61% EC = 39% | 500 | - | 282.95 (12.7) 656 (10.4) |
| 62Cu | 9.76 min | β+ = 97% EC = 2% | 2910 | - | 511 (194) |
| 64Cu | 12.70 h | β− = 38.5% β+ = 17.6% EC = 43.9% | 278 | 191 | 1345.77 (0.475) |
| 67Cu | 61.83 h | β− = 100% | - | 141 | 184.57 (48.7) |
| Radiopharmaceuticals | Targets | NCT Number ^ | Disease [Ref.] |
|---|---|---|---|
| [64Cu]Cu-ATSM | Hypoxia-targeted | NCT03951337 (Phase II; ongoing) | Rectum cancer [32] |
| [64Cu]Cu-DOTA-trastuzumab | HER2+ | NCT02827877 (Phase II; ongoing) | Breast cancer [56] |
| [64Cu]Cu-DOTA-M5A | CEA | NCT05245786 (Early phase I; ongoing) | Rectal cancer [57] |
| [64Cu]Cu- SarTATE | SSTR | NCT04438304 (Phase II; ongoing) | Neuroendocrine tumors [58] |
| [64Cu]Cu-TP3805 | VPAC1 | NCT02603965; (Phase I; completed) | Prostate cancer [59] |
| [64Cu]Cu-DOTA-AE105 | uPAR | NCT02139371 (Early phase I; completed) | Breast, prostate, and bladder cancer [60] |
| [64Cu]Cu-SAR-bisPSMA | PSMA | NCT04839367 (Phase I; completed) NCT05249127 (Phase I/II; ongoing) | Prostate neoplasms [61] Recurrent prostate neoplasm [62] |
| [67Cu]Cu- SarTATE | SSTR | NCT03936426 (Phase I/IIa; completed), NCT04023331 (Phase I/IIa; ongoing) | Meningioma [63] Neuroblastoma [64] |
| [64/67Cu]Cu-SAR-bisPSMA | PSMA | NCT04868604 (Phase I/IIa; ongoing) | Castration-resistant prostate cancer [65] |
| Isotope | Half-Life (t1/2) | Decay Characteristics | Energy | Eγ; keV (Intensity %) |
|---|---|---|---|---|
| Eβ+avg (keV) | ||||
| 66Ga | 9.49 h | β+ = 57% | 1750 | 1039.22 (37) |
| EC = 43% | ||||
| 67Ga | 3.26 d | EC = 100% | - | 93.31 (38.81) |
| 68Ga | 67.71 min | β+ = 88.91% | 829.5 | 1077.34 (3.22) |
| EC = 11.09% |
| Isotope | Half-Life (t1/2) | Decay Characteristics | Energies | Eγ; keV (Intensity%) | |
|---|---|---|---|---|---|
| Eβ+avg (keV) | Eβ−avg (keV) | ||||
| 43Sc | 3.9 h | β+ = 88% | 476 | - | 372(23) |
| EC = 12% | |||||
| 44Sc | 4.0 h | β+ = 94% | 632 | - | 1157(100) |
| EC = 6% | |||||
| 47Sc | 3.35 d | β− = 100% | - | 162 | 159(68) |
| Isotope | Half-Life (t1/2) | Decay Characteristics | Energy | Eγ; keV (Intensity %) | ||
|---|---|---|---|---|---|---|
| Eβ+avg (keV) | Eα avg (keV) | Eβ-avg (keV) | ||||
| 149Tb (α-therapy) | 4.12 h | α = 16.7% | 730 | 3967 | - | 165 (26), 352 (29) |
| β+ = 7.1% | 388.6 (18) | |||||
| EC = 76.2% | 652.1 (16) | |||||
| 152Tb (PET) | 17.5 h | β+ = 17% | 1080 | - | - | 344.3 (65) |
| EC = 83% | 586.3(9.4) | |||||
| 155Tb (SPECT) | 5.32 d | EC = 100% | - | - | - | 86.55 (32) |
| 105.3 (25) | ||||||
| 161Tb (β−/MAE therapy) | 6.89 d | β− = 100% | - | - | 154 | 25.65 (23) |
| 48.92 (17) | ||||||
| 74.57 (10) | ||||||
| Radiopharmaceuticals | Targets | NCT Number ^ | Disease |
|---|---|---|---|
| [161Tb]Tb-DOTA-LM3 | SSTR2 | NCT05359146 (Early phase 1; recruiting) | Neuroendocrine neoplasia or gastroenteropancreatic neuroendocrine tumor [179] |
| [161Tb]Tb-PSMA-I&T | PSMA | NCT05521412 (Phase I/II; recruiting) | Prostate cancer or metastatic castration-resistant prostate cancer [180] |
| Isotope | Half-Life (t1/2) | Decay Characteristics | Eβ+avg (keV) | Eγ; keV (Intensity%) |
|---|---|---|---|---|
| 62Zn | 9.26 h | β+ = 8.2% | 259 | 508 (15), 550 (15) |
| 600 (26) | ||||
| 63Zn | 38.47 min | β+ = 93% | 992 | 670 (8) |
| 960 (7) | ||||
| 65Zn | 243.9 d | β+ = 98% | 142.5 | 1110 (50.6) |
| Isotope | Half-Life (t1/2) | Decay Characteristics | Eγ; keV (Intensity %) |
|---|---|---|---|
| 99mTc | 6.0 h | IT = 100% | 140.51 (98.6) |
| Radiopharmaceuticals | Targets | NCT Number ^ | Disease |
|---|---|---|---|
| [99mTc]Tc-tilmanocept | Lymph node | NCT02201420 (Phase II; completed) | Kaposi’s sarcoma [196] |
| [99m Tc]Tc-EC20 | Folate | NCT01689714 (Phase II; completed) | Ovarian or recurrent endometrial carcinoma [197] |
| [99m Tc]Tc-Tetrofosmin | - | NCT02971319 (Phase II; completed) | Glioma [198] |
| [99m Tc]Tc-Sestamibi | - | NCT05042687 (Phase not applicable) | Breast cancer [199] |
| [99m Tc]Tc-HYNIC TOC EDAA | SSTR | NCT02691078 (Phase II completed) | Neuroendocrine tumors [200] |
| [99m Tc]Tc-MP-1404 | PSMA | NCT02615067 (Phase III completed) | Prostate cancer [201] |
| [99m Tc]Tc-MP-1404 [99m Tc]Tc-MP-1405 | PSMA | NCT01261754 (Phase I; completed) | Prostate cancer [202] |
| [99m Tc]Tc-PSMA I&S | PSMA | NCT04832958 (Phase II; ongoing) | Prostate cancer [203] |
| [99m Tc]Tc-labeled albumin in macroaggregates (MAA) and in microspheres (B20) | - | NCT01186263 (Phase II; completed) | Colorectal cancer, liver metastasis [204] |
| Isotope | Half-Life (t1/2) | Decay Characteristics | Eβ+avg (keV) | Eγ; keV (Intensity %) |
|---|---|---|---|---|
| 110mIn | 69.1min | β+ = 61.3% | 1011 | 657.75(97.74) |
| EC = 38% | ||||
| 111In | 2.8 d | EC = 100% | - | 245.35(94.1) |
| Isotope | Half-Life (t1/2) | Decay Characteristics | Energy | |
|---|---|---|---|---|
| Eβ−max (MeV) | Eβ−avg (MeV) | |||
| 90Y | 64.0 h | β− = 100% | 2.284 | 0.933 |
| Isotope | Half-Life (t1/2) | Decay Characteristics | Eβ−avg KeV | Eγ; keV (Intensity%) |
|---|---|---|---|---|
| 186Re | 90 h | β− = 92.59% | 346.7 | 137.15 (9.47) |
| EC = 7.41% | 106 (12.1) | |||
| 188Re | 17.0 h | β− = 100% | 763 | 155.04 (15.49) |
| 478 (1.076%) |
| Isotope | Half-Life (t1/2) | Eβ-max (keV) | Eϒ; keV (Intensity%) |
|---|---|---|---|
| 177Lu | 6.647 d | 497 (78.6%) 384 (9.1%) 176 (12.2%) | 208 (11%) 113 (6.6%) |
| Radiopharmaceuticals | Targets | NCT Number ^ | Disease [Ref.] |
|---|---|---|---|
| [177Lu]Lu-PSMA-617 (PLUVICTO ®) | PSMA | NCT03511664 (Phase III; ongoing) | Metastatic prostate cancer [279] |
| 223Ra + [177Lu]Lu-PSMA- I & T | PSMA | NCT05383079 (phase II; recruiting) | Metastatic castration-resistant prostate cancer [280] |
| Cabozantinib in Combination With [177Lu]Lu- DOTATATE (LuTATE) | SSTR2 | NCT05249114 (Phase Ib; ongoing) | Neuroendocrine tumors [281] |
| capecitabine (CAP)/temozolomide (TEM) + [177Lu]Lu- DOTATATE (LuTATE) | SSTR | NCT02358356 (phase II; completed) | Mid gut or pancreatic neuroendocrine tumors [282] |
| [177Lu]Lu- DOTATATE (Lutathera) | SSTR2 | NCT03206060 (phase II; ongoing) | Pheochromocytoma/Paraganglioma, neuroendocrine tumor [283] |
| [177Lu]Lu-Edotreotide | SSTR | NCT04919226 (phase III; ongoing) | Gastroenteropancreatic neuroendocrine tumors [284] |
| [177Lu]Lu- catalase | - | NCT05985278 (Early phase 1; ongoing) | Advanced malignant neoplasm [285] |
| [177Lu]Lu-EB-FAPI | FAP | NCT05400967 (Early phase 1; ongoing) | Metastatic tumors [286] |
| [177Lu]Lu-DOTA-girentuximab | Carbonic anhydrase IX | NCT02002312 (phase II; completed) | Metastatic clear cell renal cancer [287] |
| Isotope | Half-Life (t1/2) | Decay Characteristics | 225Ac and Daughter Nuclides | Energies (MeV) | Eγ; keV (Intensity%) | |
|---|---|---|---|---|---|---|
| Eαmax | Eβ− max | |||||
| 225Ac | 9.9 d | α = 100% | 225Ac 221Fr 217At 213Bi 213Po 209Tl 209Pb 209Bi (stable) | 225Ac-5.8 221Fr-6.3 217At-7.1 213Bi-5.9 213Po-8.4 | 213Bi-0.492 209Tl-0.178 209Pb-0.198 | 213Bi-100 (1) 221Fr-218 (11.4) 213Bi-440 (26) 209Tl-1567 (99.7) |
| Radiopharmaceuticals | Targets | NCT Number ^ | Disease |
|---|---|---|---|
| [225Ac]Ac-lintuzumab with Venetoclax | BCL-2 | NCT03867682 (Phase I/II; ongoing) | Acute and relapsed myeloid leukemia [304] |
| [225Ac]Ac-DOTA-Daratumumab | CD38 | NCT05363111 (Phase I; ongoing) | Recurrent plasma cell myeloma [305] |
| [225Ac]Ac-FPI-1434 | IGF-1R | NCT03746431 (Phase I/II; ongoing) | Advanced solid tumor, endometrial cancer, ovarian, cervical cancer [306] |
| [225Ac]Ac-DOTA-M5A | CEA | NCT05204147 (Phase I; ongoing) | Advanced and metastatic cancer [307] |
| [225Ac]Ac-PSMA-617 | PSMA | NCT04597411 (Phase I; ongoing) | Castration-resistant prostate cancer [308] |
| [225Ac]Ac-J591 | PSMA | NCT03276572 (Phase I; ongoing) | Prostate cancer [309] |
| Isotope | Half-Life (t1/2) | Decay Characteristics | Parent and Daughter Nuclides | Energies | Eγ; keV; (Intensity%) | |
|---|---|---|---|---|---|---|
| Eαmax (MeV) | Eβ- max (MeV) | |||||
| 203Pb | 51.9h | EC = 100% | 203Tl (stable) | - | - | 279 (81) |
| 212Pb | 10.6h | β− = 100% | 212Pb 212Bi 212Po 208Tl 208Pb (stable) | 212Bi-6.1 212Po-8.8 | 212Pb-0.102 212Bi-0.769 208Tl-0.557 | 212Pb-238.6 (43.6) 212Bi-727.3 (6.6) 208Tl-277.4 (6.3), 510.8 (22.6), 583.2 (84.5), 763 (1.8), 860.6 (12.4), 2614.5 (99.2) |
| Radiopharmaceuticals | Targets | NCT Number ^ | Disease |
|---|---|---|---|
| [212Pb]Pb-DOTAMTATE (Alpha MedixTM) | SSTR | NCT05153772 (Phase II; ongoing) | Neuroendocrine tumors [317] |
| [212Pb]Pb-TCMC-Trastuzumab | HER2+ | NCT01384253 (Phase I; completed) | Breast, ovarian, peritoneal, pancreatic, and stomach neoplasm [318] |
| Radiopharmaceuticals | Targets | NCT Number ^ | Disease |
|---|---|---|---|
| [223Ra]Ra-dichloride | Skeletal metastasis | NCT01833520 (Phase II; completed) | Sarcoma [337] |
| [223Ra]Ra-dichloride + Niraparib | PARP inhibitor | NCT03076203 (Phase I; completed) | Prostate cancer metastatic to bone, stage IV prostate cancer, hormone refractory prostate cancer [338] |
| [223Ra]Ra-dichloride + Abiraterone, Prednisone/Prednisolone | CYP17 inhibitor | NCT02043678 (Phase III; active) | Prostate cancer [339] |
| [223Ra]Ra-dichloride + Enzalutamide | AR inhibitor | NCT02199197 (Phase II; completed) NCT03305224 (Phase II; ongoing) | Prostate cancer, [340], bone metastatic prostate cancer [341] |
| [223Ra]Ra-dichloride + Denosumab | Cytokine RANKL | NCT02366130 (Phase II; completed) | Breast carcinoma [342] |
| [223Ra]Ra-dichloride + Paclitaxel | Tubulin | NCT02442063 (Phase I; completed) | Neoplasm, bone disease [343] |
| [223Ra]Ra-dichloride + Docetaxel | P300 | NCT03574571 (Phase III; ongoing) | Prostate cancer [344] |
| [223Ra]Ra-dichloride + Leuprolide acetate, | GnRH-receptor agonist | NCT03361735 (Phase II; ongoing) | Prostate cancer [345] |
| [223Ra]Ra-dichloride + Pembrolizumab | PDL-1 | NCT03093428 (Phase II; ongoing) | Prostate cancer [346] |
| [223Ra]Ra-dichloride + Atezolizumab | PDL-1 | NCT02814669 (Phase I; completed) | Castration-resistant prostate cancer [347] |
| Alpha-DaRT seeds (224Ra containing 316LVM tubes) | Implantation sites | NCT04002479 (Phase not applicable) NCT03970967 (Phase not applicable) | Metastatic pancreatic cancer [348] Metastatic breast cancer [349] |
| Isotope | Half-Life (t1/2) | 227Th and Daughter Nuclides | Eαmax (MeV) | ||
|---|---|---|---|---|---|
| Eαmax | Eβ−max | ||||
| 227Th | 18.7 d | α = 100% | 227Th 223Ra 219Rn 215Po 211Pb 211Bi 207Tl 211Po 207Pb (stable) | 227Th-5.9 223Ra-5.7 219Rn-6.8 215Po-7.4 211Bi-6.6 211Po-7.6 | 211Pb-0.4 211Bi-0.6 207Tl-0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, S.; Pandey, M.K. Radiometals in Imaging and Therapy: Highlighting Two Decades of Research. Pharmaceuticals 2023, 16, 1460. https://doi.org/10.3390/ph16101460
Sharma S, Pandey MK. Radiometals in Imaging and Therapy: Highlighting Two Decades of Research. Pharmaceuticals. 2023; 16(10):1460. https://doi.org/10.3390/ph16101460
Chicago/Turabian StyleSharma, Shalini, and Mukesh K. Pandey. 2023. "Radiometals in Imaging and Therapy: Highlighting Two Decades of Research" Pharmaceuticals 16, no. 10: 1460. https://doi.org/10.3390/ph16101460
APA StyleSharma, S., & Pandey, M. K. (2023). Radiometals in Imaging and Therapy: Highlighting Two Decades of Research. Pharmaceuticals, 16(10), 1460. https://doi.org/10.3390/ph16101460








