Multitargeted Herbal Prescription So Shiho Tang: A Scoping Review on Biomarkers for the Evaluation of Therapeutic Effects
Abstract
1. Introduction
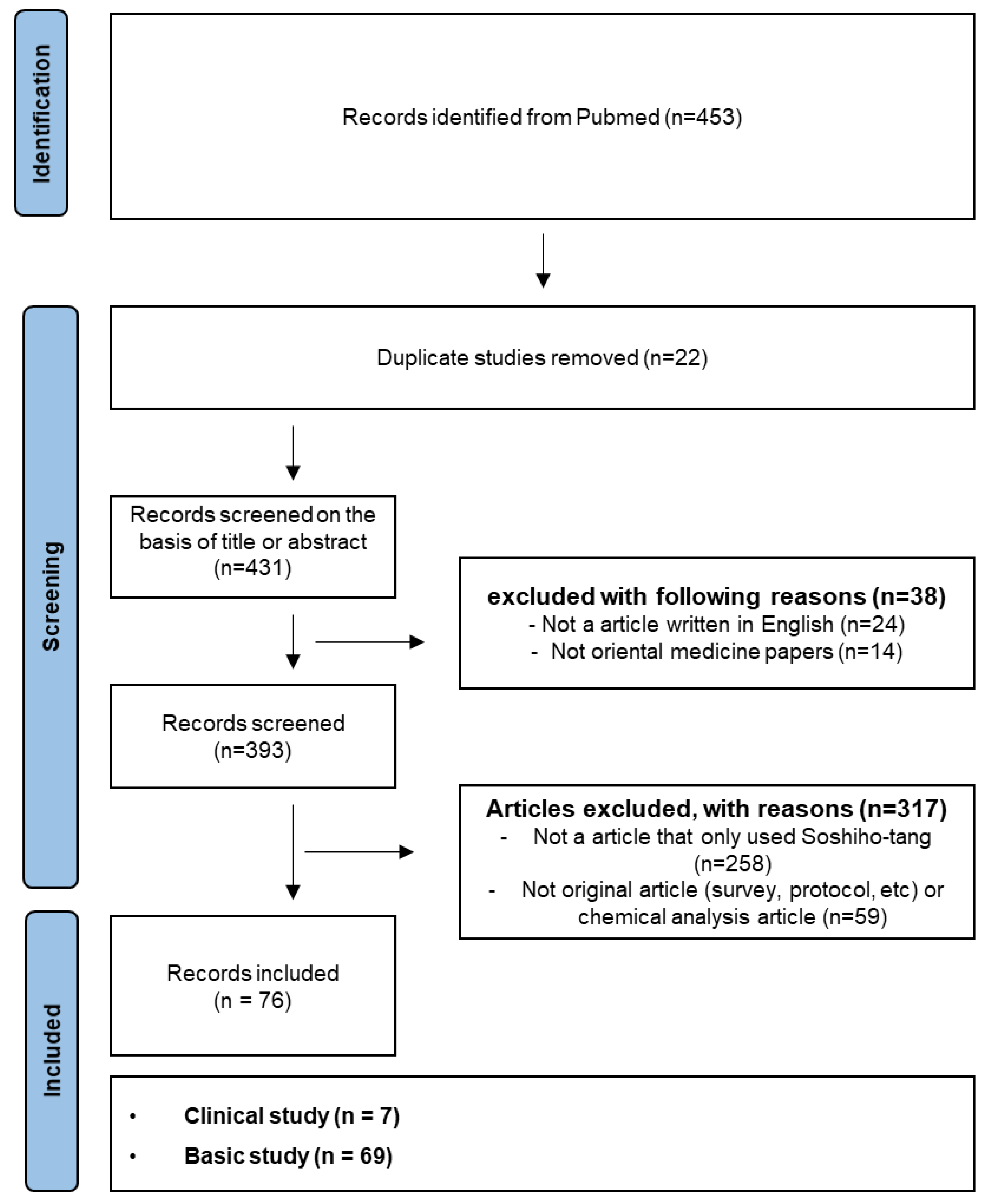
2. Literature Research Strategy
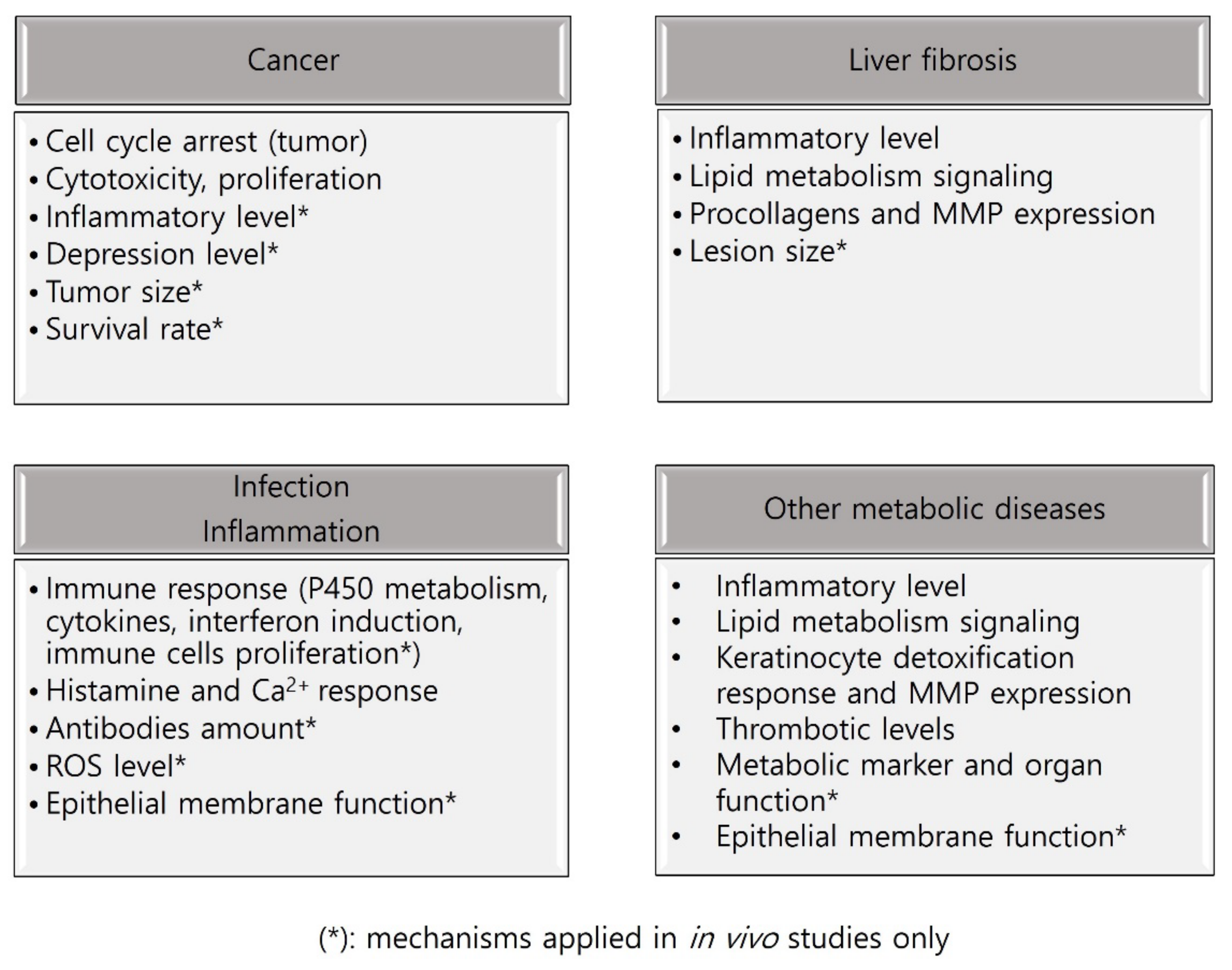
3. Results
3.1. In Vitro Studies on SSHT
| Study Design | Cell Type | Inducer | Extract Type | Treatment (* Effective Concentrations) | Positive Control | Biomarker & Outcome | Authors | Country |
|---|---|---|---|---|---|---|---|---|
| Antitumor effect | Mel-ret cell | Aq | 400 *, 2000 µg/mL | Fas-L, cdk4, cdk6, pRb | Liu et al. (1998) [36] | Japan | ||
| Antitumor effect (Proliferation) | HCC cell line (KIM-1), cholangiocarcinoma cell line (KMC-1) | Aq | 400, 1000 *, 2000 *, 10,000 µg/mL | Morphological change, DNA synthesis | Yano et al. (1994) [33] | Japan | ||
| Antitumor effect (Cytotoxic effect) | PLC, JH, HuH-7, HepG2, HepG3, H69, H69/CDDP, SBC-4, SBC-5, PC-10, ABC-1, LCD, LCT-8 | Aq | 0.5, 1, 1.5, 2.0, 2.5 mg/mL | Cell viability | Mizushima et al. (1995) [38] | Japan | ||
| Immune response (Induction of cytokines) | Peripheral blood mononuclear cell (PBMC) | Aq | 3.1 *, 12.5 *, 50 *, 200 * µg/mL | TNF-α, G-CSF | Yamashiki et al. (1996) [37] | Japan | ||
| Anticancer effect (Proliferation) | Ovarian cancer cell (KF-1, MN-1, A2780, KF-r, MN-r, A2780cp) | Aq | 25, 50, 100, 200, 500, 1000 *, 5000 * µg/mL | Annexin V-FITC | Zhu et al. (2005) [34] | Japan | ||
| Antitumor effect (Apoptosis) | Human hepatoma cell line (Huh7) | Aq | 0.5 *, 1.0 *, 1.5 * mg/mL | Bcl-2, Bax, cyclin-D1, CDK4 | Zhao et al. (2017) [35] | China | ||
| Anticancer effect (cancer comorbid with depression) | HCT116 & Lovo | Aq | 10, 20, 40, 80, 180, 320 μg/mL | Cell viability | Shao et al. (2021) [39] | China | ||
| Immune response (Induction of interferon) | Spleen cell | IFN-a/β | Aq | 7.5, 15, 30, 60, 120, 250, 500 μg/mL | IFN | Kawakita et al. (1990) [40] | Japan | |
| Immune response (Inductions of cytokines) | Peripheral blood mononuclear cells (PBMNC) | Aq | 200 µg/mL | IL-1β, TNF-α, G-CSF | Yamashiki et al. (1996) [43] | Japan | ||
| Immune response | Peripheral blood mononuclear cells & polymorphonuclear cells (PMNC) | Aq | 25, 50 *, 100 * μg/mL | PGE2, LTB4, superoxide | Miyamoto et al. (1996) [46] | USA | ||
| Immune response | Mast cell | DNP-As | Aq | 0.01, 0.1, 1 *, 10 * mg/mL | Histamine release, Ca2+ response | Matsumoto et al. (1998) [45] | Japan | |
| Immune response (Cytokine mRNA expression) | Human peripheral lymphocyte | LPS | Aq | 200 μg/mL | IL-12, IL-1β, IL-10, TNF-α, G-CSF, IFN-γ | Huang et al. (2001) [44] | Japan | |
| Immune response (Proliferation of T cell subsets) | Hepatic mononuclear cells, splenocyte | Anti-CD3 mAb | Aq | 62.5 *, 125 *, 250 * µg/mL | CD4/CD8 | Ohtake et al. (2005) [41] | Japan | |
| Immune response | Splenocyte, CD4 T cell | Aq | 10 *, 25 *, 50 *, 100 * μg/mL | IL-4, IFN-γ | Kang et al. (2009) [42] | Korea | ||
| Immune response | J774A.1 cell | LPS | Aq | 10 *, 20 *, 50 * µg/mL | Endotoxin-induced cytotoxicity (TNF-α) | Sakaguchi et al. (2004) [47] | Japan | |
| Anti-inflammatory effects | HGFs | Aq | 0.1, 0.3 *, 1 * mg/mL | PGE2, COX-2 | Ara et al. (2008) [48] | Japan | ||
| Anti-inflammatory effects | RAW 264.7 | LPS | Aq | 10 *, 50 *, 100 * μg/mL | Dexamethasone | TNF-α, IL-6, Nitric Oxide, NF-κB, IκBα, ERK, p38, JNK | Oh et al. (2013) [49] | Korea |
| Anti-inflammatory effects | RAW 264.7 | LPS | Aq | 1, 10, 100, 250 *, 500 *, 1000 * μg/mL | Dexamethasone | IL-6, TNF-α, IFN-γ | Choi et al. (2021) [50] | Korea |
| Anti-inflammatory effects (Acute pancreatitis) | AR42J cell | LPS | Aq | 12.5, 25 *, 50 *, 100 * μM | IL-6, IL-1β, TNF-α, MAPK3, TP53 | Zhan et al. (2021) [51] | China | |
| Immune response (Chronic HBV infection) | PBMC | Aq | 50 *, 100 *, 300 * μg/mL | IFN-γ, anti-HBe & anti-HBc production | Kakumu et al. (1990) [52] | Japan | ||
| Liver diseases (Chronic active hepatitis B and C) | Peripheral blood mononuclear cells, polymorphonuclear cells (PMNC) | Aq | 100 μg/mL | IL-10 | Yamashiki et al. (1997) [53] | Japan | ||
| Liver diseases (Chronic hepatitis B) | HepG2.2.15 cell | Aq | 10 *, 20 *%(vol/vol) | JAK2, STAT3, HBsAg | Chen et al. (2017) [54] | China | ||
| Liver diseases | Hepatocyte | Aq | 3 *, 30 *, 300 * μg/mL | iNOS, nitric oxide | Hattori et al. (1995) [55] | Japan | ||
| Lipid metabolism | Hepatocyte | 20% lipid emulsion | Aq | 50, 100 *, 200 *, 400 *, 800 μg/mL | G6PD, ME, FAS, ATGL, LPL, PPARα | Zou et al. (2019) [56] | China | |
| Liver fibrosis | Hepatic stellate cell (HSCs) | Aq | 10, 100, 250, 500 *, 1000 * μg/mL | Type I and type III procollagen | Kayano et al. (1998) [57] | Japan | ||
| Liver fibrosis | Rat hepatic stellate cells (HSCs) | Aq | 10, 100 *, 500 *, 1000 * µg/mL | MMP-13, MMP-2, TIMP-1, TIMP-2 | Sakaida et al. (2004) [58] | Japan | ||
| Aging (Autocrine growth of human keratinocyte) | Normal human keratinocyte | Aq | 100 *, 500 * µg/mL | IL-1α | Matsumoto et al. (1997) [59] | Japan | ||
| Atopic dermatitis (AD) symptoms | HaCaT cell | TNF-α/IFN-γ | Aq | 10 *, 20, 50 *, 100 *, 500 μg/mL | ICAM-1, HO-1, NF-κB, Nrf2 | Lee et al. (2019) [21] | Korea | |
| Aging (UVB-induced skin damage and photoaging) | HaCaT cell | UVB irradiation | Aq | 10 *, 50 *, 100 * µg/mL | MMP-1, MMP-9 | Im et al. (2020) [60] | Korea | |
| Mitogenic activity | Spleen cell | LPS | 0.1, 1 *, 10 *, 100 * µg/mL | Mitogenic response ([3H] thymidine uptake) | Hiroko et al. (1987) [61] | Japan | ||
| Antiviral activity | Human neonatal foreskin fibroblast cell line (CCFS-1/KMC) | Aq | 25 *, 50 *, 100 *, 200 * µg/mL | Ribavirin | IFN-α, IFN-β, CVB1 | Cheng et al. (2006) [62] | Taiwan | |
| Anti-inflammatory effects (Calprotectin expression) | Human oral epithelial cell (TR146) | Aq | 10, 25 *, 50 *, 100, 250 µg/mL | S100A8, S100A9, Calprotectin, ADM, AZU1, CAMP, CST3, DEFB1, DEFB4, DEFB103A, LCN2, IL-1α, IL-6, TNF-α G-CSF, MUC5B, IL-1R1 | Hiroshima et al. (2010) [63] | Japan | ||
| Antithrombotic effect | Platelet | Collagen, thrombin, AA, ADP | Aq | 200 *, 400 *, 800 * μg/mL | ASA | Serotonin, TXB2 | Lee et al. (2013) [64] | Korea |
| Antiobesity effect | 3T3-L1 | Aq | 50 *, 100 *, 200 * µg/mL | GW9662 | PPAR-γ and C/EBP-α, FAS, perilipin, FABP4, triglyceride, leptin | Yoo et al. (2016) [65] | Korea | |
| Immune response (mRNA & microRNA expression) | Mouse primary hepatocyte | Aq | 500 µg/mL | P450 metabolism, cell cycle pathway, PPAR pathway, MAPK pathway | Song et al. (2014) [66] | Korea |
3.2. In Vivo Studies on SSHT
| Target Study | Animal (Sex, Age, Body Weight) | Inducer | Type of Extracts | Administration (Frequency/Period) | Experimental Group | Positive Control | Biomarker & Outcome | Authors | Country | |
|---|---|---|---|---|---|---|---|---|---|---|
| Lethality | Anti-lethality | ddY mice (male, 18–20 g) | rhTNF | Aq | 500 * mg/kg/day (Oral, 1/day, day 2 & 6 of induced, 72 * h) | 4 groups | Survival rate * | Sakaguchi S. et al. (1991) [74] | Japan | |
| Cancer | Antitumor | ddY mice | Ehrlich tumors | Aq | 1600 * mg/kg (Oral-drinking, 2 weeks) | 10 groups | Survival rate *, TNF *, tumor weight | Haranaka K. et.al. (1985) [68] | Japan | |
| Cancer | Antitumor activity, shock symptoms | ddY mice (male, 18–20 g) | LPS | Aq | 500 * mg/kg/day (Oral, 1/day, 5 day) | 4 groups | NO2, Fibrinogen *, Glycogen * | Sakaguchi S. et al. (1996) [70] | Japan | |
| Cancer | Carcinoma | BALB/c mice (female) | Aq | 2.5 * g/kg/day (Drink, 30 day) | 5 groups | IL-2 | IL-6 *, tumor weight * | Huang et al. (1997) [69] | Japan | |
| Fibrosis | Hepatic foci | Sprague Dawley rats (male) | N-nitroso morpholine | Aq | 0.5% *, 1% chow (Oral, 8 weeks) | 3 groups | GGT *, GST-P * lesion staining, T lymphocyte* | Tatsuta et al. (1991) [71] | Japan | |
| Fibrosis | Liver fibrosis | Wistar rats (male, 140–150 g) | Choline-deficient-amino acid-defined diet | Aq | 1% * Chow (Oral, 16 weeks) | 5 groups | Choline-supplemented-amino acid-defined diet | Hydroxyproline *, hyaluronic acid *, ALT, AST, COL3A1 *, myofibroblast-like cells *, GST-P lesion * | Isao et al. (1998) [72] | Japan |
| Fibrosis | Liver fibrosis | BALB/c mice (female, ~20 g) | S. japonicum | Aq | XCH-L: 5 * mL/kg/day, XCH-M: 15 * mL/kg/day, XCH-H: 30 * mL/kg/day (Oral, 16 weeks) | 6 groups | Serum ALT *, AST *, ALP *, HA * & PIIINP *, ALB * & GLOB *, TGF-β1 *, Hsp47 *, α-SMA *, Col1A1 *, Col3A1 * | Huang et al. (2020) [73] | China | |
| Inflammation | P. aeruginosa infection | ICR and C3H/He mice (female) | P. aeruginosa | Aq | 100 mg/kg (I.P., pretreat 6 h * or 4 days *) | 3 groups | Leukocytes * | Kawakita et al. (1987) [77] | Japan | |
| Inflammation | Peritoneal macrophage | C3H/HeJ & (BALB/c × DBA/2)F~(CDF) mice (female) | Aq | 3 * mg, 5 mg/mouse/day (I.P., 1 time, 4 days) | 3 groups | Acid phosphatase & N-acetyl-/3-D-glucosaminidase | Kumazawa. et al. (1988) [79] | Japan | ||
| Inflammation | Immune response | ICR mice (male), CBA mice (female) | Aq | 1.2 g/kg/day (Oral, 1, 2, 3 *, 5 * day)—0.24, 0.6 *, 1.2 * g/kg/day (Oral, 3 days) | 4 groups | PGE2 *, antigens, ARA, membrane fluidity * | Nagatsu et al. (1989) [81] | Japan | ||
| Inflammation | Colony-stimulating factors | C3H/He mice, (female) | Carrageenan | Aq | 100 * mg/kg (I.P., serum I.V.) | 3 groups | GM-CSF | Yonekura et al. (1990) [80] | Japan | |
| Inflammation | Ovalbumin-induced inflammation | BALB/c (OVA)-induced mice (female) | Aq | 100 *, 200 * mg/kg/day (Oral, 1/day, 18–23 days) | 5 groups | Montelukast | Th2-type cytokines *, eotaxin, (HO)-1 * | Jeon et al. (2015) [85] | Korea | |
| Inflammation | Cachexia-related symptoms | BALB/c CT-26-bearing mice | Aq | 50 * and 100 * mg/kg/day (Oral, 1/day, 17 days) | 4 groups | IL-6, TNF-α, IL-1, IFN-γ | Kim et.al. (2016) [86] | Korea | ||
| Inflammation | Peyer’s patches (IgA production) | C3H/He mice (female) | LPS | Aq | 200, 500, 1000 *, 2000 * mg/kg/day (Oral, 1/day, 2 day) | 5 groups | SRBC-IgA & -HRBC IgA | Tauchi et al. (1993) [91] | Japan | |
| Inflammation | Drug-drug interaction (CYPs) | Sprague Dawley Rats (male, 200–240 g) | Aq | Low dose, 1.7 * g/kg/day Medium dose, 3.4 * g/kg/day High dose, 6.8 * g/kg/day (Oral, 1/day, 3 & 6 days) | Control groups Treatment groups | CYP inducer (Rifampicin) | P450s (Cyp1a2, Cyp3a1, Cyp2d6, Cyp1b1) | Li et al. (2021) [89] | China | |
| Inflammation | Endotoxemia | ddY mice (male, 18–20 g) | Endotoxin | Aq | 500 * mg/kg/day (Oral, 1/day, 5 days) | 4 groups | Lipid peroxide *, Xanthine oxidase *, SOD *, GPx *, α-Tocophero *, nonprotein SH *, acid phosphatase *, LDH * | Sakaguchi. et al. (1993) [84] | Japan | |
| Inflammation | Granuloma | Wistar rats (male, ~200 g) | Carrageenin cotton pellet | Aq | 450 * mg/kg/day (Oral, 1/day/8 days) | 4 groups | Indomethacin | Granuloma weight *, acid-soluble glycoprotein *, sialic acid | Yoshida et al. (1993) [87] | Japan |
| Inflammation | NK activities | C3H/He mice (female) | Aq | 500, 1000 * mg/kg (Oral 12 h * & 24 h) | 3 groups | GM1, CD3, CD4, CD8, NK cell * | Kaneko et al. (1994) [82] | Japan | ||
| Inflammation | Granuloma | Wistar rats (male) | Carrageenin cotton pellet | Aq | 450 * mg/kg/day (Oral, 1/day/8 days) | 4 groups | Indomethacin | Vit E *, cholesterol, phospholipid, lipid peroxide *, granuloma weight * | Yoshida et al. (1994) [88] | Japan |
| Inflammation | Endotoxemia | ddY mice (male, 18–20 g) | Endotoxin | Aq | 500 * mg/kg/day (Oral, 1/day, 5 days) | 4 groups | [Ca2+] *, Mg2+, Ca2+-ATPase *, respiratory control index * | Sakaguchi et al. (1994) [84] | Japan | |
| Inflammation | Macrophage function | Sprague Dawley rats (male, 150–155 g) | Gum arabic | Aq | 1 g/kg/day (Oral, 3 weeks, 22 *–28 days) | 4 groups | Superoxide anions | Fujiwara et al. (1995) [92] | Japan | |
| Inflammation | NK activities | C3H/He mice (female) | Aq | 1000 mg/kg, 1–12 * fraction (Oral, 1 time, 12 h) | 12 groups | NK activities * | Yamaoka et al. (1995) [83] | Japan | ||
| Inflammation | B-cell maturation | C3H/He mice (female) | TNP-LPS (TI-1), TNP-Ficol (TI-2), SRCB (TD) | Aq | 250 mg/kg (I.P., 4, 7, 10 *, 14 * days) | Control group Treatment group | Plaque-forming cells (PFC), IgM+ IgD+ * B cell, Thy1 antibody | Kawakita et al. (1987) [78] | Japan | |
| Metabolic disorder | D-galactosamine -induced liver injury | ICR mice (male, 18–22 g) | Aq | Low dose 0.02 * g/kg/day Medium dose 1 * g/kg/day High dose 5 * g/kg/day (Oral, 1/day, 14 days) | 6 groups | Biphenyl dicarboxylate | IL-6 *, TNF-α *, Fas*, Fas-L *, Bcl-2 *, Bax * | Zhou et al. (2012) [93] | China | |
| Metabolic disorder | Perimenopausal disorder | Kunming Mice (female, 18–22 g) | Aq | 2.3, 7 *, 21 * g/kg/day (Oral, 1/day, 8 weeks) | 8 Groups | E2 & fluoxetine | HPA/HPO axis, Erβ, TPH2 | Zhang et al. (2020) [94] | China | |
| Metabolic disorder | Age-induced amnesia | Fischer F254 rats (male) | Aq | 120 * mg/kg/day (Oral, 1/day, 72–110 * week-old) | 4 groups | α-Tocopherol nicotinate | PAR failure * | Amagaya et al. (1990) [95] | Japan | |
| Metabolic disorder | Liver injury | Wistar rats (male, 150–160 g) | D-galactosamine | Aq | 1 * g/kg (Oral/I.P., 1 time, 24 h *) | 8 groups | Albumin *, total protein *, 5′-nucleosidase *, glucose 6-phosphatase *, serum TG | Ohta et al. (1997) [96] | Japan | |
| Metabolic disorder | Chronic pancreatitis | Wistar rats (male, 170–190 g) | Dibutyltin dichloride | Aq | 10 * g/kg/day (Oral, 1/day, 28 days) | 3 groups | Exocrine pancreatic function (PABA *), TGF-β1 *, TGFβRII *, Smad3 *, Smad7 | Zhang et al. (2013) [97] | China | |
| Metabolic disorder | Anti-hyperlipidemia & antiatherosclerosis | Hypercholesterolemic C57BL/6J mice (male, 20–24 g) | Aq | 1.2 * g/kg/day (Oral, 1/day, 2–4 * weeks) | 4 groups | Cholesterol *, T cell *, cholesterol oleate *, HDL, LDL, Acyl-CoA *, ACAT, NCEase * | Shen et al. (1996) [98] | Japan | ||
| Metabolic disorder | Hypercholesterolemia | ICR mice (male) | Aq | 1.2 g/kg/day (Oral, 1/day, 2 weeks) | 4 groups | PGE2 *, IL-1, NO *, LPC | Inoue et al. (1996) [90] | Japan | ||
| Metabolic disorder | Hypercholesterolemia | New Zealand White rabbits (male, 1.5–2 g) | LPS | Aq | 3% chow (Oral, 20 * weeks) | 3 groups | Monocyte *, cholesterol, LDL | Shen et al. (1996) [99] | Japan | |
| Metabolic disorder | Tolbutamide bioavailability | Sprague Dawley rats (male, 302–376) | Aq | 500 * mg/kg/day (Oral, 1/day, 6 days) | Single (2 groups) Multiple (2 groups) | Absorption rate & bioavailability of Tolbutamide | Nishimura et al. (1999) [100] | Japan | ||
| Metabolic disorder | Radical scavenging | Wistar rats (male) | Aq | 500 * mg/kg (Oral, 1 time, plasma collection at 1, 2, 4, 6, 10, 12 *, 24 *h) | 3 groups | α-Tocopherol & ascorbic acid | O2- *, -OH *, DPPH *, | Egashira et al. (1999) [101] | Japan | |
| Metabolic disorder | Gastric function | Sprague Dawley rat (male, 215–347 g) | Aq | 250 *, 750 * mg/kg (Oral, 1 time, 20 & 40 min) | Control group Treatment group | Gastric emptying rate (GER) | Nishimura et al. (2001) [102] | Japan | ||
| Metabolic disorder | Hepatic microvascular dysfunction | Wistar rats (male, 200–250 g) | Gut ischemia/reperfusion | Aq | 1 * g/kg/day (I.G., 1/day, 7 days) | 5 groups | Leukocytes * (pericentral * & midzonal region), NPS *, TNF-α *, ALT | Horie et al. (2001) [103] | Japan | |
| Metabolic disorder | Tolbutamide permeability | Ex vivo, Sprague Dawley rats (male) | Aq | 50 * mg/kg (I.P., 10–60 min) | Control group Treatment group | Epithelial membrane permeability of Tolbutamide | Nishimura et al. (2010 [104]) | Japan | ||
3.3. Clinical Studies on SSHT
| Target Disease | Study Design (Sample Size, n) | Type of Preparation Form | Extraction Method | Dose | Duration (Frequency) | Control | Outcomes | Results | Authors | Country |
|---|---|---|---|---|---|---|---|---|---|---|
| Gastrointestinal disorders of AD patients | RCT (60: I 30, C 30) | NA | NA | NA | 6 weeks (3/days) | PLA | (1) SCORAD index (2) Amount and frequency of ointment application for AD (3) Dermatology quality of life index (4) Safety evaluation | (1) (2) not statistically significant (3) reduced (4) normal range | Lee et al. (2021) [25] | Korea |
| Depressive symptoms of cancer patients | RCT (72: I 36, C 36) | Granule | Aq | 9.5 g/pack | 6 weeks (2/days) | PLA | (1) Depressive scales (SDS) (2) Circulating cytokines assay (3) Gut microbial composition | (1) p < 0.05 (2) p < 0.05 (TNF-α, IL-6) (3) not significant | Shao et al. (2021) [39] | China |
| Cirrhosis | RCT (260: I 130, C 130) | NA | NA | 7.5 g/day | 60 months (1/days) | PLA | (1) Cumulative incidence of HCC (2) Survival rate | (1) inhibitory effect (2) high survival rate | Oka et al. (1995) [105] | Japan |
| Chronic active hepatitis | RCT (222: I, C) | Granule | NA | 5.4 g/day | 24 weeks (3/days) | PLA | (1) ALT (2) AST (3) HBeAg | (1) (2) decreased blood levels (3) decreased HBeAg (increased anti-HBe antibody) | Hirayama et al. (1989) [106] | Japan |
| Hepatitis C | Case report (24) | Granule | Aq | 2.5 g/pack | 12 months (3/days) | - | (1) Liver function (2) HCV viral load (3) Liver biopsy histology | (1) improved (2) mixed (3) improved | Deng et al. (2011) [107] | USA |
| Adverse effect | Case report (1) | NA | Aq | NA | 1.5 month (2/days) | - | (1) ALT, AST (2) Liver biopsy | (1) increased (2) revealed lesion | Hsu et al. (2006) [109] | Taiwan |
| Adverse effect | Case report (4) | Granule | NA | 7.5 g/day | 6–7 weeks (1/days) | - | (1) ALT, AST, ALP, γGT, bilirubin (2) Liver histology | (1) increased (2) revealed lesion | Itoh et al. (1995) [108] | Japan |
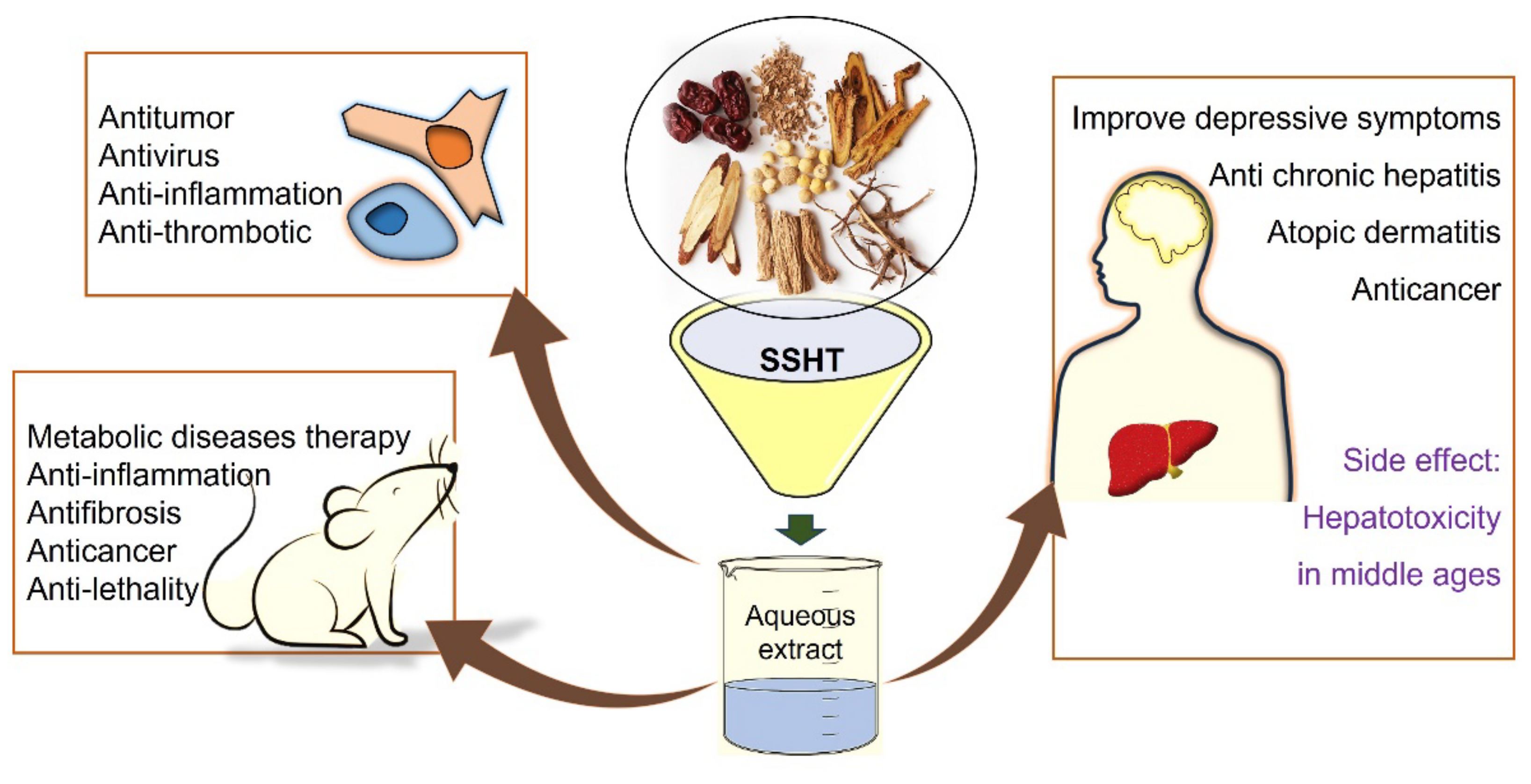
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Kominsky, D.J.; Campbell, E.L.; Colgan, S.P. Metabolic shifts in immunity and inflammation. J. Immunol. 2010, 184, 4062–4068. [Google Scholar] [CrossRef] [PubMed]
- Pålsson-McDermott, E.M.; O’Neill, L.A. Targeting immunometabolism as an anti-inflammatory strategy. Cell Res. 2020, 30, 300–314. [Google Scholar] [CrossRef]
- Bashir Dar, K.; Hussain Bhat, A.; Amin, S.; Masood, A.; Afzal Zargar, M.; Ahmad Ganie, S. Inflammation: A multidimensional insight on natural anti-inflammatory therapeutic compounds. Curr. Med. Chem. 2016, 23, 3775–3800. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ballantyne, C.M. Metabolic inflammation and insulin resistance in obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef] [PubMed]
- Ghasemian, M.; Owlia, S.; Owlia, M.B. Review of anti-inflammatory herbal medicines. Adv. Pharmacol. Pharm. Sci. 2016, 2016, 9130979. [Google Scholar] [CrossRef]
- Yatoo, M.; Gopalakrishnan, A.; Saxena, A.; Parray, O.R.; Tufani, N.A.; Chakraborty, S.; Tiwari, R.; Dhama, K.; Iqbal, H. Anti-inflammatory drugs and herbs with special emphasis on herbal medicines for countering inflammatory diseases and disorders-a review. Recent Pat. Inflamm. Allergy Drug Discov. 2018, 12, 39–58. [Google Scholar] [CrossRef]
- Food and Drug Administration. Finding and Learning about Side Effects (Adverse Reactions). 2021. Available online: https://www.fda.gov/drugs/information-consumers-and-patients-drugs/finding-and-learning-about-side-effects-adverse-reactions (accessed on 25 September 2023).
- Goldman, J.L.; Jackson, M.A. Tip of the iceberg: Understanding the unintended consequences of antibiotics. Pediatrics 2015, 136, e492–e493. [Google Scholar] [CrossRef]
- Poluzzi, E.; Raschi, E.; Godman, B.; Koci, A.; Moretti, U.; Kalaba, M.; Wettermark, B.; Sturkenboom, M.; de Ponti, F. Pro-arrhythmic potential of oral antihistamines (H1). PLoS ONE 2015, 10, e0119551. [Google Scholar] [CrossRef]
- Zeynettin, K.; Tuncez, A. Adverse Cardiac Effects of Decongestants Agents. Eur. J. Gen. Med. 2013, 10, 32–35. [Google Scholar] [CrossRef]
- Shao, I.-H.; Wu, C.-C.; Tseng, H.-J.; Lee, T.-J.; Lin, Y.-H.; Tam, Y.-Y. Voiding dysfunction in patients with nasal congestion treated with pseudoephedrine: A prospective study. Drug Des. Dev. Ther. 2016, 10, 2333–2339. [Google Scholar] [CrossRef][Green Version]
- Lu, C.Y.; Zhang, F.; Lakoma, M.D.; Butler, M.G.; Fung, V.; Larkin, E.K.; Kharbanda, E.O.; Vollmer, W.M.; Lieu, T.; Soumerai, S.B. Asthma treatments and mental health visits after a Food and Drug Administration label change for leukotriene inhibitors. Clin. Ther. 2015, 37, 1280–1291. [Google Scholar] [CrossRef] [PubMed]
- Kochetkov, P.; Svistushkin, V.; Shchennikova, E. Intranasal glucocorticosteroids for the complex treatment of patients with chronic diseases of the nose and paranasal sinuses. Meditsinskiy Sovet. 2020, 6, 66–70. [Google Scholar] [CrossRef]
- Benzie, I.F.; Wachtel-Galor, S. Herbal Medicine: Biomolecular and Clinical Aspects; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- El Mostafa, S.B.; Maamri, A. Herbal Medicine in Chronic Diseases Treatment: Determinants, Benefits and Risks. In Disease Prevention and Health Promotion in Developing Countries; Springer Nature: Berlin/Heidelberg, Germany, 2020; Volume 85. [Google Scholar] [CrossRef]
- Kang, S.-J.; Jo, E.-H.; Yang, G.-J.; Shim, Y.-H.; Hong, J.-E.; Park, M.-C. Efficacy and safety of Soshiho-tang in patients with atopic dermatitis and gastrointestinal disorders: Study protocol for a double-blind, randomized, and placebo-controlled clinical trial. Medicine 2019, 98, e15479. [Google Scholar] [CrossRef] [PubMed]
- Yamashiki, M.; Nishimura, A.; Suzuki, H.; Sakaguchi, S.; Kosaka, Y. Effects of the Japanese herbal medicine “Sho-Saiko-To” (TJ-9) on in vitro interleukin-10 production by peripheral blood mononuclear cells of patients with chronic hepatitis C. Hepatology 1997, 25, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Tao, W.; Zheng, C.; Shar, P.A.; Huang, C.; Fu, Y.; Wang, Y. Systems pharmacology-based approach for dissecting the addition and subtraction theory of traditional Chinese medicine: An example using Xiao-Chaihu-Decoction and Da-Chaihu-Decoction. Comput. Biol. Med. 2014, 53, 19–29. [Google Scholar] [CrossRef]
- Lee, J.-H.; Jo, E.H.; Lee, B.; Noh, H.M.; Park, S.; Lee, Y.-M.; Kim, D.-K.; Park, M.C. Soshiho-Tang, a traditional herbal medicine, alleviates atopic dermatitis symptoms via regulation of inflammatory mediators. Front. Pharmacol. 2019, 10, 742. [Google Scholar] [CrossRef]
- Shin, I.S.; Lee, M.Y.; Kim, Y.; Seo, C.S.; Kim, J.H.; Shin, H.K. Subacute toxicity and stability of Soshiho-tang, a traditional herbal formula, in Sprague–Dawley rats. BMC Complement. Altern. Med. 2012, 12, 266. [Google Scholar] [CrossRef]
- Lee, M.-Y.; Seo, C.-S.; Shin, I.-S.; Kim, Y.-B.; Kim, J.-H.; Shin, H.-K. Evaluation of oral subchronic toxicity of soshiho-tang water extract: The traditional herbal formula in rats. Evid. Based Complement. Altern. Med. 2013, 2013, 590181. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, S.; Lee, M.-Y.; Shin, H.-K. Therapeutic effect of Soshiho-tang, a traditional herbal formula, on liver fibrosis or cirrhosis in animal models: A systematic review and meta-analysis. J. Ethnopharmacol. 2014, 154, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jo, E.H.; youn Jung, J.; Kang, S.J.; Yang, G.J.; Shim, Y.H.; Park, M.C. Efficacy and safety of Soshiho-tang in atopic dermatitis patients with gastrointestinal disorders: A double-blinded, randomized, and placebo-controlled clinical trial. J. Ethnopharmacol. 2021, 274, 114006. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.C.H. Herbal Monograph for Xiao Chai Hu Tang. July 2007. Available online: https://acupuncturetoday.com/article/31544-herbal-monograph-for-xiao-chai-hu-tang (accessed on 25 September 2023).
- Jung, J.; Park, J.; Choi, J.-Y.; Lee, J.A. Soshiho-tang for treating common cold in children younger than 12 years: A systematic review and meta-analysis of randomized controlled trials. Medicine 2018, 97, e13045. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.U.; Ryu, J.Y.; Lee, J.O.; Lee, S.Y. A systems approach to traditional oriental medicine. Nat. Biotechnol. 2015, 33, 264–268. [Google Scholar] [CrossRef]
- Song, B.-K.; Won, J.-H.; Kim, S. Historical medical value of Donguibogam. J. Pharmacopunct. 2016, 19, 16–20. [Google Scholar] [CrossRef]
- Pan, Z.; Han, M.; Zhang, Y.; Liu, T.; Zhou, L.; Tan, D.; Wang, Q.; Liu, Z.; Fu, Y. Characteristics of Xiao Chai Hu decoction based on randomized controlled trials: A bibliometric analysis. J. Tradit. Chin. Med. Sci. 2023, 10, 100–105. [Google Scholar] [CrossRef]
- Yu, H.; Chen, J.; Xu, X.; Li, Y.; Zhao, H.; Fang, Y.; Li, X.; Zhou, W.; Wang, W.; Wang, Y. A systematic prediction of multiple drug-target interactions from chemical, genomic, and pharmacological data. PLoS ONE 2012, 7, e37608. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Li, Y.; Li, X.; Tao, W.; Li, B.; Wang, Y.; Yang, L. Systems pharmacology uncovers Janus functions of botanical drugs: Activation of host defense system and inhibition of influenza virus replication. Integr. Biol. 2013, 5, 351–371. [Google Scholar] [CrossRef]
- Yano, H.; Mizoguchi, A.; Fukuda, K.; Haramaki, M.; Ogasawara, S.; Momosaki, S.; Kojiro, M. The herbal medicine sho-saiko-to inhibits proliferation of cancer cell lines by inducing apoptosis and arrest at the G0/G1 phase. Cancer Res. 1994, 54, 448–454. [Google Scholar]
- Zhu, K.; Fukasawa, I.; Furuno, M.; Inaba, F.; Yamazaki, T.; Kamemori, T.; Kousaka, N.; Ota, Y.; Hayashi, M.; Maehama, T. Inhibitory effects of herbal drugs on the growth of human ovarian cancer cell lines through the induction of apoptosis. Gynecol. Oncol. 2005, 97, 405–409. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, L.; Zhang, Y.; Wan, Y.; Hong, Z. The herbal mixture Xiao-Chai-Hu Tang (XCHT) induces apoptosis of human hepatocellular carcinoma Huh7 cells in vitro and in vivo. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 231–241. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, W.; Kato, M.; Akhand, A.A.; Hayakawa, A.; Takemura, M.; Yoshida, S.; Suzuki, H.; Nakashima, I. The herbal medicine sho-saiko-to inhibits the growth of malignant melanoma cells by upregulating Fas-mediated apoptosis and arresting cell cycle through downregulation of cyclin dependent kinases. Int. J. Oncol. 1998, 12, 1321–1327. [Google Scholar] [CrossRef]
- Yamashiki, M.; Nishimura, A.; Nomoto, M.; Suzuki, H.; Kosaka, Y. Herbal medicine ‘Sho-saiko-to’induces tumour necrosis factor-α and granulocyte colony-stimulating factor in vitro in peripheral blood mononuclear cells of patients with hepatocellular carcinoma. J. Gastroenterol. Hepatol. 1996, 11, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, Y.; Kashii, T.; Tokimitsu, Y.; Kobayashi, M. Cytotoxic effect of herbal medicine sho-saiko-to on human lung-cancer cell-lines in-vitro. Oncol. Rep. 1995, 2, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Jia, R.; Zhao, L.; Zhang, Y.; Guan, Y.; Wen, H.; Liu, J.; Zhao, Y.; Feng, Y.; Zhang, Z. Xiao-Chai-Hu-Tang ameliorates tumor growth in cancer comorbid depressive symptoms via modulating gut microbiota-mediated TLR4/MyD88/NF-κB signaling pathway. Phytomedicine 2021, 88, 153606. [Google Scholar] [CrossRef] [PubMed]
- Kawakita, T.; Nakai, S.; Kumazawa, Y.; Miura, O.; Yumioka, E.; Nomoto, K. Induction of interferon after administration of a traditional Chinese medicine, xiao-chai-hu-tang (shosaiko-to). Int. J. Immunopharmacol. 1990, 12, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Ohtake, N.; Yamamoto, M.; Takeda, S.; Aburada, M.; Ishige, A.; Watanabe, K.; Inoue, M. The herbal medicine Sho-saiko-to selectively inhibits CD8+ T-cell proliferation. Eur. J. Pharmacol. 2005, 507, 301–310. [Google Scholar] [CrossRef]
- Kang, H.; Choi, T.-W.; Ahn, K.-S.; Lee, J.-Y.; Ham, I.-H.; Choi, H.-Y.; Shim, E.-S.; Sohn, N.-W. Upregulation of interferon-γ and interleukin-4, Th cell-derived cytokines by So-Shi-Ho-Tang (Sho-Saiko-To) occurs at the level of antigen presenting cells, but not CD4 T cells. J. Ethnopharmacol. 2009, 123, 6–14. [Google Scholar] [CrossRef]
- Yamashiki, M.; Nishimura, A.; Sakaguchi, S.; Suzuki, H.; Kosaka, Y. Effects of the Japanese herbal medicine ‘Sho-saiko-to’as a cytokine inducer. Environ. Toxicol. Pharmacol. 1996, 2, 301–306. [Google Scholar] [CrossRef]
- Huang, X.X.; Yamashiki, M.; Nakatani, K.; Nobori, T.; Mase, A. Semi-quantitative analysis of cytokine mRNA expression induced by the herbal medicine Sho-saiko-to (TJ-9) using a gel doc system. J. Clin. Lab. Anal. 2001, 15, 199–209. [Google Scholar] [CrossRef]
- Matsumoto, T.; Shibata, T. The ex vivo effect of the herbal medicine Sho-saiko-to on histamine release from rat mast cells. Eur. Arch. Oto-Rhino-Laryngol. 1998, 255, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Lange, M.; McKinley, G.; Stavropoulos, C.; Moriya, S.-i.; Matsumoto, H.; Inada, Y. Effects of sho-saiko-to on production of prostaglandin E2 (PGE2), leukotriene B4 (LTB4) and superoxide from peripheral monocytes and polymorphonuclear cells isolated from HIV infected individuals. Am. J. Chin. Med. 1996, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Furusawa, S.; Iizuka, Y. Preventive effects of a traditional Chinese medicine (Sho-saiko-to) on septic shock symptoms; approached from heme metabolic disorders in endotoxemia. Biol. Pharm. Bull. 2005, 28, 165–168. [Google Scholar] [CrossRef][Green Version]
- Ara, T.; Maeda, Y.; Fujinami, Y.; Imamura, Y.; Hattori, T.; Wang, P.L. Preventive effects of a Kampo medicine, Shosaikoto, on inflammatory responses in LPS-treated human gingival fibroblasts. Biol. Pharm. Bull. 2008, 31, 1141–1144. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.C.; Cho, W.K.; Jeong, Y.H.; Im, G.Y.; Lee, K.J.; Yang, H.J.; Ma, J.Y. Anti-inflammatory effect of Sosihotang via inhibition of nuclear factor-kappaB and mitogen-activated protein kinases signaling pathways in lipopolysaccharide-stimulated RAW 264.7 macrophage cells. Food Chem. Toxicol. 2013, 53, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Choi, L.Y.; Kim, M.H.; Jung, D.-H.; Yang, W.M. Anti-Inflammatory Effects of Sosiho-Tang, a Traditional Herbal Formula, on Acute Lung Injury in LPS-Sensitized Mice and-Raw 264.7 Cells. Evid. Based Complement. Altern. Med. 2021, 2021, 6641689. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Pu, J.; Hu, Y.; Xu, P.; Liang, W.; Ji, C. Uncovering the pharmacology of Xiaochaihu decoction in the treatment of acute pancreatitis based on the network pharmacology. BioMed Res. Int. 2021, 2021, 6621682. [Google Scholar] [CrossRef]
- Kakumu, S.; Yoshioka, K.; Wakita, T.; Ishikawa, T. Effects of TJ-9 Sho-saiko-to (kampo medicine) on interferon gamma and antibody production specific for hepatitis B virus antigen in patients with type B chronic hepatitis. Int. J. Immunopharmacol. 1991, 13, 141–146. [Google Scholar] [CrossRef]
- Yamashiki, M.; Nishimura, A.; Nobori, T.; Nakabayashi, S.; Takagi, T.; Inoue, K.; Ito, M.; Matsushita, K.; Ohtaki, H.; Kosaka, Y. In vitro effects of Sho-saiko-to on production of granulocyte colony-stimulating factor by mononuclear cells from patients with chronic hepatitis C. Int. J. Immunopharmacol. 1997, 19, 381–385. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Z.; Wan, S.; Huang, H.; Liang, H. Effect of modified Xiaochaihu decoction-containing serum on HepG2. 2.15 cells via the JAK2/STAT3 signaling pathway. Mol. Med. Rep. 2017, 16, 7416–7422. [Google Scholar] [CrossRef]
- Hattori, Y.; Kasai, K.; Sekiguchi, Y.; Hattori, S.; Banba, N.; Shimoda, S.-I. The herbal medicine sho-saiko-to induces nitric oxide synthase in rat hepatocytes. Life Sci. 1995, 56, PL143–PL148. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Xu, M.; Chen, L.; Liu, Q.; Zhou, Y.; Sun, Z.; Ye, H.; Su, N.; Ye, C.; Wang, A. Xiaochaihu Decoction reduces hepatic steatosis and improves D-GalN/LPS-induced liver injury in hybrid grouper (Epinephelus lanceolatus♂ × Epinephelus fuscoguttatus♀). Fish Shellfish Immunol. 2019, 91, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Kayano, K.; Sakaida, I.; Uchida, K.; Okita, K. Inhibitory effects of the herbal medicine Sho-saiko-to (TJ-9) on cell proliferation and procollagen gene expressions in cultured rat hepatic stellate cells. J. Hepatol. 1998, 29, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Sakaida, I.; Hironaka, K.; Kimura, T.; Terai, S.; Yamasaki, T.; Okita, K. Herbal medicine Sho-saiko-to (TJ-9) increases expression matrix metalloproteinases (MMPs) with reduced expression of tissue inhibitor of metalloproteinases (TIMPs) in rat stellate cell. Life Sci. 2004, 74, 2251–2263. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Kato, M.; Tamada, Y.; Mori, H.; Ohashi, M. Enhancement of Interleukin-1α Mediated Autocrine Growth of Cultured Human Keratinocytes by Sho-saiko-to. Jpn. J. Pharmacol. 1997, 73, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Im, A.-R.; Ji, K.-Y.; Nam, K.-W.; Chae, S. Protective effects of Sosihotang extract against ultraviolet B-induced skin photoageing in hairless mice. J. Pharm. Pharmacol. 2020, 72, 1278–1286. [Google Scholar] [CrossRef]
- Iwama, H.; Amagaya, S.; Ogihara, Y. Effect of shosaikoto, a Japanese and Chinese traditional herbal medicinal mixture, on the mitogenic activity of lipopolysaccharide: A new pharmacological testing method. J. Ethnopharmacol. 1987, 21, 45–53. [Google Scholar] [CrossRef]
- Cheng, P.-W.; Ng, L.-T.; Lin, C.-C. Xiao chai hu tang inhibits CVB1 virus infection of CCFS-1 cells through the induction of Type I interferon expression. Int. Immunopharmacol. 2006, 6, 1003–1012. [Google Scholar] [CrossRef]
- Hiroshima, Y.; Bando, M.; Kataoka, M.; Shinohara, Y.; Herzberg, M.; Ross, K.; Inagaki, Y.; Nagata, T.; Kido, J. Shosaikoto increases calprotectin expression in human oral epithelial cells. J. Periodontal Res. 2010, 45, 79–86. [Google Scholar] [CrossRef]
- Lee, J.-J.; Kim, T.; Cho, W.-K.; Ma, J.Y. Antithrombotic and antiplatelet activities of Soshiho-tang extract. BMC Complement. Altern. Med. 2013, 13, 137. [Google Scholar] [CrossRef]
- Yoo, S.-R.; Lee, M.-y.; Kang, B.-K.; Shin, H.-K.; Jeong, S.-J. Soshiho-tang aqueous extract exerts antiobesity effects in high fat diet-fed mice and inhibits adipogenesis in 3T3-L1 adipocytes. Evid.-Based Complement. Altern. Med. 2016, 2016, 2628901. [Google Scholar] [CrossRef] [PubMed]
- Song, K.H.; Kim, Y.H.; Kim, B.-Y. Sho-saiko-to, a traditional herbal medicine, regulates gene expression and biological function by way of microRNAs in primary mouse hepatocytes. BMC Complement. Altern. Med. 2014, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Fact Sheet: Cancer. Available online: http://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 3 February 2022).
- Haranaka, K.; Satomi, N.; Sakurai, A.; Haranaka, R.; Okada, N.; Kobayashi, M. Antitumor activities and tumor necrosis factor producibility of traditional Chinese medicines and crude drugs. Cancer Immunol. Immunother. 1985, 20, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Marumo, K.; Murai, M. Antitumor effects and pharmacological interaction of xiao-chai-hu-tang (sho-saiko-to) and interleukin 2 in murine renal cell carcinoma. Keio J. Med. 1997, 46, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Furusawa, S.; Yokota, K.; Sasaki, K.-I.; Takayanagi, M.; Takayanagi, Y. Effects of antitumor activity and protection of shock symptoms by a traditional Chinese medicine (sho-saiko-to) in recombinant human tumor necrosis factor administered mice. Biol. Pharm. Bull. 1996, 19, 1474–1478. [Google Scholar] [CrossRef][Green Version]
- Tatsuta, M.; Iishi, H.; Baba, M.; Nakaizumi, A.; Uehara, H. Inhibition by xiao-chai-hu-tang (TJ-9) of development of hepatic foci induced by N-nitrosomorpholine in Sprague-Dawley rats. Jpn. J. Cancer Res. 1991, 82, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Isao, S.; Yasuhiro, M.; Shinta, A.; Koji, H.; Atsushi, I.; Kiwamu, O. Herbal medicine Sho-saiko-to (TJ-9) prevent liver fibrosis and enzyme-altered lesions in rat liver cirrhosis induced by a choline-deficient l-amino acid-defined diet. J. Hepatol. 1998, 28, 298–306. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, J.; Xu, Y.; Xiong, C.; Tong, D.; Hu, N.; Yang, H. Xiaochaihu decorction relieves liver fibrosis caused by Schistosoma japonicum infection via the HSP47/TGF-β pathway. Parasites Vectors 2020, 13, 254. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Tutumi, E.; Yokota, K.; Furusawa, S.; Sasaki, K.i.; Takayanagi, Y. Preventive Effects of a Chinese Herb Medicine (Sho-saiko-to) against Lethality after Recombinant Human Tumor Necrosis Factor Administration in Mice. Microbiol. Immunol. 1991, 35, 389–394. [Google Scholar] [CrossRef][Green Version]
- Fehervari, Z. Vaccine sex differences. Nat. Immunol. 2019, 20, 111. [Google Scholar] [CrossRef]
- Scotland, R.S.; Stables, M.J.; Madalli, S.; Watson, P.; Gilroy, D.W. Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood J. Am. Soc. Hematol. 2011, 118, 5918–5927. [Google Scholar] [CrossRef] [PubMed]
- Kawakita, T.; Yamada, A.; Mitsuyama, M.; Kumazawa, Y.; Nomoto, K. Protective effect of a traditional Chinese medicine, xiao-chai-hu-tang (Japanese name: Shosaiko-to), on Pseudomonas aeruginosa infection in mice. Immunopharmacol. Immunotoxicol. 1987, 9, 523–540. [Google Scholar] [CrossRef] [PubMed]
- Kawakita, T.; Yamada, A.; Kumazawa, Y.; Nomoto, K. Functional maturation of immature B cells accumulated in the periphery by an intraperitoneal administration of a traditional Chinese medicine, xiao-chai-hu-tang (Japanese name: Shosaiko-to). Immunopharmacol. Immunotoxicol. 1987, 9, 299–317. [Google Scholar] [CrossRef] [PubMed]
- Kumazawa, Y.; Takimoto, H.; Miura, S.-I.; Nishimura, C.; Yamada, A.; Kawakita, T.; Nomoto, K. Activation of murine peritoneal macrophages by intraperitoneal administration of a traditional Chinese herbal medicine, xiao-chai-hu-tang (Japanese name: Shosaiko-to). Int. J. Immunopharmacol. 1988, 10, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Yonekura, K.; Kawakita, T.; Mitsuyama, M.; Miura, O.; Yumioka, E.; Suzuki, A.; Nomoto, K. Induction of colony-stimulating factor (s) after administration of a traditional Chinese medicine, xiao-chai-hu-tang (Japanese name: Shosaiko-to). Immunopharmacol. Immunotoxicol. 1990, 12, 647–667. [Google Scholar] [CrossRef] [PubMed]
- Nagatsu, Y.; Inoue, M.; Ogihara, Y. Modification of macrophage functions by Shosaikoto (kampo medicine) leads to enhancement of immune response. Chem. Pharm. Bull. 1989, 37, 1540–1542. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.; Kawakita, T.; Tauchi, Y.; Saito, Y.; Suzuki, A.; Nomoto, K. Augmentation of NK activity after oral administration of a traditional Chinese medicine, xiao-chai-hu-tang (shosaiko-to). Immunopharmacol. Immunotoxicol. 1994, 16, 41–53. [Google Scholar] [CrossRef]
- Yamaoka, Y.; Kawakita, T.; Kaneko, M.; Nomoto, K. A polysaccharide fraction of shosaiko-to active in augmentation of natural killer activity by oral administration. Biol. Pharm. Bull. 1995, 18, 846–849. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Tsutsumi, E.; Yokota, K. Preventive effects of a traditional Chinese medicine (sho-saiko-to) against oxygen toxicity and membrane damage during endotoxemia. Biol. Pharm. Bull. 1993, 16, 782–786. [Google Scholar] [CrossRef]
- Jeon, W.-Y.; Shin, H.-K.; Shin, I.-S.; Kim, S.K.; Lee, M.-Y. Soshiho-tang water extract inhibits ovalbumin-induced airway inflammation via the regulation of heme oxygenase-1. BMC Complement. Altern. Med. 2015, 15, 329. [Google Scholar] [CrossRef]
- Kim, A.; Im, M.; Ma, J.Y. Sosiho-tang ameliorates cachexia-related symptoms in mice bearing colon 26 adenocarcinoma by reducing systemic inflammation and muscle loss. Oncol. Rep. 2016, 35, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Mizukawa, H.; Honmura, A.; Uchiyama, Y.; Kaku, H.; Nakajima, S.; Haruki, E. The effect of sho-saiko-to on the concentration of acid soluble glycoprotein in serum and on granuloma formation in carrageenin cotton pellet-induced granuloma rats. Am. J. Chin. Med. 1993, 21, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Mizukawa, H.; Honmura, A.; Uchiyama, Y.; Nakajima, S.; Haruki, E. The effect of sho-saiko-to on concentration of vitamin E in serum and on granuloma formation in carrageenin cotton pellet-induced granuloma rats. Am. J. Chin. Med. 1994, 22, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tang, Y.; Wei, W.; Yin, C.; Tang, F. Effects of Xiaochaihu decoction on the expression of cytochrome P450s in rats. Exp. Ther. Med. 2021, 21, 588. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Shen, Y.R.; Ogihara, Y. Restorative Effect of Shosaikoto (Kampo Medicine) on Diminution of Nitric Oxide Synthesis in Murine Peritoneal Macrophages induced by Hyperocholesterolemia. Biol. Pharm. Bull. 1996, 19, 1468–1473. [Google Scholar] [CrossRef] [PubMed]
- Tauchi, Y.; Kawakita, T.; Saito, Y.; Suzuki, A.; Yamada, A.; Yoshikai, Y.; Nomoto, K. Enhancement of immunoglobulin A production in Peyer’s patches by oral administration of a traditional Chinese medicine, xiao-chai-hu-tang (Shosaiko-to). Immunopharmacol. Immunotoxicol. 1993, 15, 251–272. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Mochida, S.; Nagoshi, S.; Iijima, O.; Matsuzaki, Y.; Takeda, S.; Aburada, M. Regulation of hepatic macrophage function by oral administration of xiao-chai-hu-tang (sho-saiko-to, TJ-9) in rats. J. Ethnopharmacol. 1995, 46, 107–114. [Google Scholar] [CrossRef]
- Zhou, Y.-X.; Qiu, Y.-Q.; Xu, L.-Q.; Guo, J.; Li, L.-J. Xiao-Chai-Hu Tang in treating model mice with D-galactosamine-induced liver injury. Afr. J. Tradit. Complement. Altern. Med. 2012, 9, 405–411. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Z.; Pan, X.; Yang, J.; Wu, C. Antidepressant-like effects of Xiaochaihutang in perimenopausal mice. J. Ethnopharmacol. 2020, 248, 112318. [Google Scholar] [CrossRef]
- Amagaya, S.; Umeda, S.H.M.; Ogihara, Y. Effects of Shosaikoto, an oriental herbal medicinal mixture, on age-induced amnesia in rats. J. Ethnopharmacol. 1990, 28, 349–356. [Google Scholar] [CrossRef]
- Ohta, Y.; Nishida, K.; Sasaki, E.; Kongo, M.; Hayashi, T.; Nagata, M.; Ishiguro, I. Comparative study of oral and parenteral administration of sho-saiko-to (xiao-chaihu-tang) extract on D-galactosamine-induced liver injury in rats. Am. J. Chin. Med. 1997, 25, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-k.; Cui, N.-q.; Zhuo, Y.-z.; Li, D.-h.; Liu, J.-h. Modified Xiaochaihu decoction (小柴胡汤) prevents the progression of chronic pancreatitis in rats possibly by inhibiting transforming growth factor-β1/Sma-and mad-related proteins signaling pathway. Chin. J. Integr. Med. 2013, 19, 935–939. [Google Scholar] [CrossRef]
- Shen, Y.R.; Inoue, M.; Nagatsu, Y.; Ogihara, Y.; Aburada, M. Anti-hyperlipidemic and anti-atherosclerotic actions of shosaikoto (kampo medicine). Biol. Pharm. Bull. 1996, 19, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.R.; Inoue, M.; Ogihara, Y. Effect of Shosaikoto (Kampo Medicine) on the Adherence of Monocytes in Hypercholesterolemic Rabbit. Biol. Pharm. Bull. 1996, 19, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, N.; Naora, K.; Hirano, H.; Iwamoto, K. A Chinese traditional medicine, sho-saiko-to (xiao-chaihu-tang), reduces the bioavailability of tolbutamide after oral administration in rats. Am. J. Chin. Med. 1999, 27, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Egashira, T.; Takayama, F.; Yamanaka, Y.; Komatsu, Y. Monitoring of radical scavenging activity of peroral administration of the Kampo medicine Sho-saiko-to in rats. Jpn. J. Pharmacol. 1999, 80, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, N.; Naora, K.; Hirano, H.; IWAMOTO, K. Effects of sho-saiko-to (xiao chai hu tang), a Chinese traditional medicine, on the gastric function and absorption of tolbutamide in rats. Yakugaku Zasshi 2001, 121, 153–159. [Google Scholar] [CrossRef][Green Version]
- Horie, Y.; Kajihara, M.; Yamagishi, Y.; Kimura, H.; Tamai, H.; Kato, S.; Ishii, H. A Japanese herbal medicine, Sho-saiko-to, prevents gut ischemia/reperfusion-induced hepatic microvascular dysfunction in rats. J. Gastroenterol. Hepatol. 2001, 16, 1260–1266. [Google Scholar] [CrossRef]
- Nishimura, N.; Uemura, T.; Iwamoto, K.; Naora, K. Change in tolbutamide permeability in rat jejunum and Caco-2 cells by Sho-saiko-to (Xiao Chai Hu Tang), a Chinese traditional medicine. J. Pharm. Pharmacol. 2010, 62, 651–657. [Google Scholar] [CrossRef]
- Oka, H.; Yamamoto, S.; Kuroki, T.; Harihara, S.; Marumo, T.; Kim, S.R.; Monna, T.; Kobayashi, K.; Tango, T. Prospective study of chemoprevention of hepatocellular carcinoma with Sho-saiko-to (TJ-9). Cancer 1995, 76, 743–749. [Google Scholar] [CrossRef]
- Hirayama, C.; Okumura, M.; Tanikawa, K.; Yano, M.; Mizuta, M.; Ogawa, N. A multicenter randomized controlled clinical trial of Shosaiko-to in chronic active hepatitis. Gastroenterol. Jpn. 1989, 24, 715–719. [Google Scholar] [CrossRef]
- Deng, G.; Kurtz, R.C.; Vickers, A.; Lau, N.; Yeung, K.S.; Shia, J.; Cassileth, B. A single arm phase II study of a Far-Eastern traditional herbal formulation (sho-sai-ko-to or xiao-chai-hu-tang) in chronic hepatitis C patients. J. Ethnopharmacol. 2011, 136, 83–87. [Google Scholar] [CrossRef]
- Itoh, S.; Marutani, K.; Nishijima, T.; Matsuo, S.; Itabashi, M. Liver injuries induced by herbal medicine, syo-saiko-to (xiao-chai-hu-tang). Dig. Dis. Sci. 1995, 40, 1845–1848. [Google Scholar] [CrossRef]
- Hsu, L.-M.; Huang, Y.-S.; Tsay, S.-H.; Chang, F.-Y.; Lee, S.-D. Acute hepatitis induced by Chinese hepatoprotective herb, xiao-chai-hu-tang. J. Chin. Med. Assoc. 2006, 69, 86–88. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, N.K.S.; Lee, J.H.; Lee, M.J.; Park, J.Y.; Kang, K.S. Multitargeted Herbal Prescription So Shiho Tang: A Scoping Review on Biomarkers for the Evaluation of Therapeutic Effects. Pharmaceuticals 2023, 16, 1371. https://doi.org/10.3390/ph16101371
Tran NKS, Lee JH, Lee MJ, Park JY, Kang KS. Multitargeted Herbal Prescription So Shiho Tang: A Scoping Review on Biomarkers for the Evaluation of Therapeutic Effects. Pharmaceuticals. 2023; 16(10):1371. https://doi.org/10.3390/ph16101371
Chicago/Turabian StyleTran, Nguyen Khoi Song, Ji Hwan Lee, Myong Jin Lee, Jun Yeon Park, and Ki Sung Kang. 2023. "Multitargeted Herbal Prescription So Shiho Tang: A Scoping Review on Biomarkers for the Evaluation of Therapeutic Effects" Pharmaceuticals 16, no. 10: 1371. https://doi.org/10.3390/ph16101371
APA StyleTran, N. K. S., Lee, J. H., Lee, M. J., Park, J. Y., & Kang, K. S. (2023). Multitargeted Herbal Prescription So Shiho Tang: A Scoping Review on Biomarkers for the Evaluation of Therapeutic Effects. Pharmaceuticals, 16(10), 1371. https://doi.org/10.3390/ph16101371









