Manipulated Oral and Rectal Drugs in a Paediatric Swedish University Hospital, a Registry-Based Study Comparing Two Study-Years, Ten Years Apart
Abstract
1. Introduction
Aim
2. Results
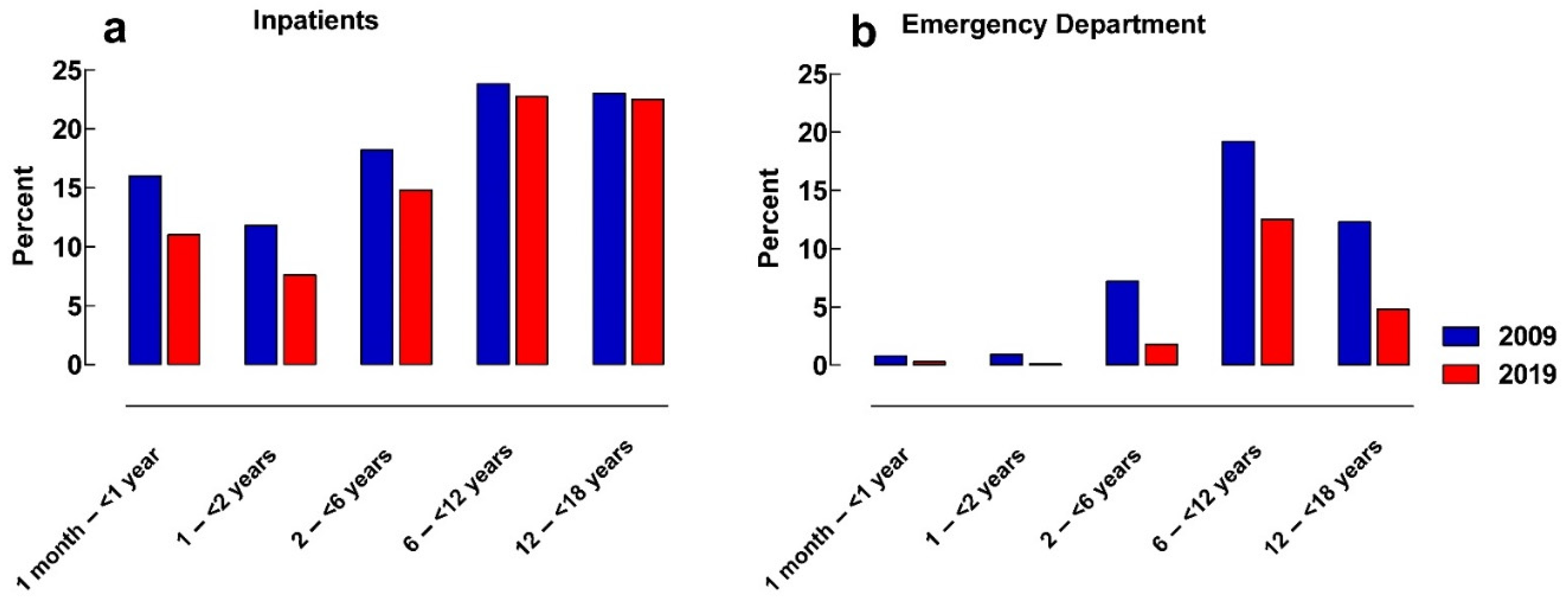
2.1. Manipulation of Oral Medicines
2.2. Manipulation of Rectal Medicines
3. Materials and Methods
4. Discussion
4.1. EU Paediatric Regulation
4.2. Inpatient Setting versus Emergency Department
4.3. Manipulation of Oral Solid Dosage Forms
4.4. Manipulations of Rectal Solid Dosage Forms
4.5. Strengths and Weaknesses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALB | Astrid Lindgren Children’s Hospital, Karolinska University Hospital |
| ATC | Anatomical Therapeutic Chemical Classification System |
| EMA/EMEA | European Medicines Agency (EMEA former abbreviation, in use until 2009) |
| SmPC | Summary of Product Characteristics |
| Definitions | |
| Liquid dosage form | Oral or rectal medication intended to be administered in the liquid form such as drops, mixtures, or suspensions and that can be administered in individual doses without manipulation. |
| Solid dosage form | Oral or rectal medication intended to be administered as a whole dosage form such as tablets, capsules, or suppositories. In this study, dispersible and chewable tablets as well as dose sachets and unit enemas are included in this definition. |
References
- Richey, R.H.; Craig, J.V.; Shah, U.U.; Nunn, A.J.; Turner, M.A.; Barker, C.E.; Ford, J.L.; Peak, M. MODRIC—Manipulation of drugs in children. Int. J. Pharm. 2013, 457, 339–341. [Google Scholar] [CrossRef] [PubMed]
- AHCHR. MODRIC Manipulation of Drugs Required in Children. Available online: https://alderhey.nhs.uk/application/files/5014/9935/2778/MODRIC-GUIDELINES.pdf (accessed on 1 May 2022).
- Richey, R.H.; Shah, U.U.; Peak, M.; Craig, J.V.; Ford, J.L.; Barker, C.E.; Nunn, A.J.; Turner, M.A. Manipulation of drugs to achieve the required dose is intrinsic to paediatric practice but is not supported by guidelines or evidence. BMC Pediatr. 2013, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Nunn, A.; Richey, R.; Shah, U.; Barker, C.; Craig, J.; Peak, M.; Ford, J.; Turner, M. Estimating the requirement for manipulation of medicines to provide accurate doses for children. Eur. J. Hosp. Pharm. Sci. 2013, 20, 3–7. [Google Scholar] [CrossRef]
- Zahn, J.; Hoerning, A.; Trollmann, R.; Rascher, W.; Neubert, A. Manipulation of Medicinal Products for Oral Administration to Paediatric Patients at a German University Hospital: An Observational Study. Pharmaceutics 2020, 12, 583. [Google Scholar] [CrossRef] [PubMed]
- van der Vossen, A.C.; Al-Hassany, L.; Buljac, S.; Brugma, J.D.; Vulto, A.G.; Hanff, L.M. Manipulation of oral medication for children by parents and nurses occurs frequently and is often not supported by instructions. Acta Paediatr. 2019, 108, 1475–1481. [Google Scholar] [CrossRef]
- Tayal, H.; Roy, V.; Singhal, S.; Dubey, A.P. Pediatric prescribing in tertiary care teaching hospital of Delhi (India): Fragmenting medicines for use. Eur. J. Pediatr. 2020, 179, 1435–1443. [Google Scholar] [CrossRef]
- Bjerknes, K.; Bøyum, S.; Kristensen, S.; Brustugun, J.; Wang, S. Manipulating tablets and capsules given to hospitalised children in Norway is common practice. Acta Paediatr. 2017, 106, 503–508. [Google Scholar] [CrossRef]
- Kader, R.; Liminga, G.; Ljungman, G.; Paulsson, M. Manipulations of Oral Medications in Paediatric Neurology and Oncology Care at a Swedish University Hospital: Health Professionals’ Attitudes and Sources of Information. Pharmaceutics 2021, 13, 1676. [Google Scholar] [CrossRef]
- Spishock, S.; Meyers, R.; Robinson, C.A.; Shah, P.; Siu, A.; Sturgill, M.; Kimler, K. Observational Study of Drug Formulation Manipulation in Pediatric Versus Adult Inpatients. J. Patient Saf. 2021, 17, e10–e14. [Google Scholar] [CrossRef]
- Breitkreutz, J.; Boos, J. Paediatric and geriatric drug delivery. Expert Opin. Drug Deliv. 2007, 4, 37–45. [Google Scholar] [CrossRef]
- Kirkevold, O.; Engedal, K. What is the matter with crushing pills and opening capsules? Int. J. Nurs. Pract. 2010, 16, 81–85. [Google Scholar] [CrossRef]
- Biron, C.; Licznar, P.; Hansel, S.; Schved, J.F. Oral anticoagulant drugs: Do not cut tablets in quarters. Thromb. Haemost. 1999, 82, 1201. [Google Scholar]
- Duncan, M.C.; Castle, S.S.; Streetman, D.S. Effect of tablet splitting on serum cholesterol concentrations. Ann. Pharmacother. 2002, 36, 205–209. [Google Scholar] [CrossRef]
- Gee, M.; Hasson, N.K.; Hahn, T.; Ryono, R. Effects of a tablet-splitting program in patients taking HMG-CoA reductase inhibitors: Analysis of clinical effects, patient satisfaction, compliance, and cost avoidance. J. Manag. Care Pharm. JMCP 2002, 8, 453–458. [Google Scholar] [CrossRef][Green Version]
- Andersson, Å.C.; Lindemalm, S.; Eksborg, S. Dividing the Tablets for Children—Good or Bad? Pharm. Meth. 2016, 7, 23–27. [Google Scholar] [CrossRef]
- Cohen, C.I.; Cohen, S.I. Potential savings from splitting newer antidepressant medications. CNS Drugs 2002, 16, 353–358. [Google Scholar] [CrossRef]
- Lajoinie, A.; Henin, E.; Kassai, B.; Terry, D. Solid oral forms availability in children: A cost saving investigation. Br. J. Clin. Pharmacol. 2014, 78, 1080–1089. [Google Scholar] [CrossRef]
- Best, B.M.; Capparelli, E.V.; Diep, H.; Rossi, S.S.; Farrell, M.J.; Williams, E.; Lee, G.; van den Anker, J.N.; Rakhmanina, N. Pharmacokinetics of lopinavir/ritonavir crushed versus whole tablets in children. J. Acquir. Immune Defic. Syndr. 2011, 58, 385–391. [Google Scholar] [CrossRef]
- Brustugun, J.; Notaker, N.; Paetz, L.H.; Tho, I.; Bjerknes, K. Adjusting the dose in paediatric care: Dispersing four different aspirin tablets and taking a proportion. Eur. J. Hosp. Pharm. 2021, 28, 76–82. [Google Scholar] [CrossRef]
- Broadhurst, E.C.; Ford, J.L.; Nunn, A.; Rowe, P.H.; Roberts, M. Dose uniformity of samples prepared from dispersible aspirin tablets for paediatric use. Eur. J. Hosp. Pharm. Sci. 2008, 14, 27–31. [Google Scholar]
- Kim, T.W.; Rognerud, C.L.; Ou, C.-N. Accuracy in the Alteration of Acetaminophen Suppositories. Anesth. Analg. 2005, 100, 1303–1305. [Google Scholar] [CrossRef] [PubMed]
- EMEA. Reflection Paper: Formulations of Choice for the Paediatric Population. European Medicines Agency. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-formulations-choice-paediatric-population_en.pdf (accessed on 10 May 2022).
- van der Vossen, A.; Buljaç, S.; Akçay, K.; Brugma, J.D.; Vulto, A.; Hanff, L. Availability of age-appropriate paediatric formulations in the Netherlands: The need in daily clinical practice remains. Eur. J. Hosp. Pharm. 2021, 28, 306–312. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Regulation (EC) No 1901/2006 of the European Parliament and of the Council of 12 December 2006 on Medicinal Products for Paediatric Use and Amending Regulation (EEC) No. 1768/92, Directive 2001/20/EC, Directive 2001/83/EC, and Regulation (EC) No. 726/2004e. Available online: https://ec.europa.eu/health/medicinal-products/medicines-children_en (accessed on 28 September 2022).
- Lindell-Osuagwu, L.; Hakkarainen, M.; Sepponen, K.; Vainio, K.; Naaranlahti, T.; Kokki, H. Prescribing for off-label use and unauthorized medicines in three paediatric wards in Finland, the status before and after the European Union Paediatric Regulation. J. Clin. Pharm. Ther. 2014, 39, 144–153. [Google Scholar] [CrossRef]
- European Commission. State of Paediatric Medicines in the EU. 10 Years of the EU Paediatric Regulation; European Commission: Brussels, Belgium, 2017.
- EMA. 10-Year Report to the European Commission. General Report on the Experience Acquired as a Result of the Application of the Paediatric Regulation; European Medicines Agency and Paediatric Committee: London, UK, 2017.
- Lepola, P.; Wang, S.; Tötterman, A.M.; Gullberg, N.; Harboe, K.M.; Kimland, E. Does the EU’s Paediatric Regulation work for new medicines for children in Denmark, Finland, Norway and Sweden? A cross-sectional study. BMJ Paediatr. Open 2020, 4, e000880. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, Y.; Pan, P.; Hong, C.; Fang, L. Estimated Manipulation of Tablets and Capsules to Meet Dose Requirements for Chinese Children: A Cross-Sectional Study. Front. Pediatr. 2021, 9, 747499. [Google Scholar] [CrossRef] [PubMed]
- Nahata, M.C. Lack of pediatric drug formulations. Pediatrics 1999, 104, 607–609. [Google Scholar] [CrossRef]
- Kimland, E.; Odlind, V. Off-label drug use in pediatric patients. Clin. Pharmacol. Ther. 2012, 91, 796–801. [Google Scholar] [CrossRef]
- Thabet, Y.; Slavkova, M.; Breitkreutz, J. 10 years EU regulation of pediatric medicines—Impact on cardiovascular drug formulations. Expert Opin. Drug Deliv. 2018, 15, 261–270. [Google Scholar] [CrossRef]
- Jannin, V.; Lemagnen, G.; Gueroult, P.; Larrouture, D.; Tuleu, C. Rectal route in the 21st Century to treat children. Adv. Drug Deliv. Rev. 2014, 73, 34–49. [Google Scholar] [CrossRef]
- Lajoinie, A.; Henin, E.; Nguyen, K.A.; Malik, S.; Mimouni, Y.; Sapori, J.M.; Breant, V.; Cochat, P.; Kassai, B. Oral drug dosage forms administered to hospitalized children: Analysis of 117,665 oral administrations in a French paediatric hospital over a 1-year period. Int. J. Pharm. 2016, 500, 336–344. [Google Scholar] [CrossRef]




| ORAL ADMINISTRATIONS | RECTAL ADMINISTRATIONS | |||||||
|---|---|---|---|---|---|---|---|---|
| Inpatient Units | Emergency Department | Inpatient Units | Emergency Department | |||||
| 2009 | 2019 | 2009 | 2019 | 2009 | 2019 | 2009 | 2019 | |
| Total number of patients | 4905 | 4718 | 5260 | 15,038 | 2355 | 1240 | 3883 | 5902 |
| 1 month–<1 year, n (%) | 856 (17.5) | 876 (18.6) | 370 (7.0) | 1547 (10.3) | 706 (30.0) | 480 (38.7) | 986 (25.4) | 2065 (35.0) |
| 1–<2 years, n (%) | 661 (13.5) | 489 (10.4) | 535 (10.2) | 2015 (13.4) | 544 (23.1) | 242 (19.5) | 1044 (26.9) | 1548 (26.2) |
| 2–<6 years, n (%) | 1045 (21.3) | 1097 (23.3) | 1446 (27.5) | 4384 (29.2) | 650 (27.6) | 303 (24.4) | 1164 (30.0) | 1624 (27.5) |
| 6–<12 years, n (%) | 1122 (22.9) | 1259 (26.7) | 1629 (31.0) | 4471 (29.7) | 289 (12.3) | 134 (10.8) | 500 (12.9) | 528 (8.9) |
| 12–<18 years, n (%) | 1221 (24.9) | 997 (21.1) | 1280 (24.3) | 2621 (17.4) | 166 (7.0) | 81 (6.5) | 189 (4.9) | 137 (2.3) |
| Male patients, % | 56 | 56 | 58 | 55 | 55 | 57 | 57 | 55 |
| Number of administrations | 117,023 | 128,638 | 6680 | 24,013 | 12,449 | 5315 | 4639 | 9979 |
| Number of administrations/patients | 24 | 27 | 1.3 | 1.6 | 5.3 | 4.3 | 1.2 | 1.7 |
| Patients with solid manipulated medicines, n (%) | 953 (19) | 805 (17) | 581 (11) | 767 (5) | 509 (22) | 122 (10) | 1362 (35) | 438 (7) |
| Manipulations including halves 0.5; 1.5, etc., % | 64 | 72 | 97 | 97 | NA | NA | NA | NA |
| Solid dosage forms prescribed as mL, % | 15 | 8 | 0.2 | 0.2 | NA | NA | NA | NA |
| Substance | All Oral Administrations, n | Solid Manipulated, n | % Manipulated * | p-Value | % of All Solid Manipulations | ||||
|---|---|---|---|---|---|---|---|---|---|
| 2009 | 2019 | 2009 | 2019 | 2009 | 2019 | 2009 | 2019 | ||
| Paracetamol | 14,751 | 18,794 | 1025 | 951 | 7 | 5 | <0.0001 | 9 | 11 |
| Clonidine | 9 848 | 12,287 | 568 | 323 | 6 | 3 | <0.0001 | 5 | 4 |
| Prednisolone | 2963 | 2221 | 697 | 696 | 24 | 31 | <0.0001 | 6 | 8 |
| Spironolactone | 1719 | 1073 | 672 | 230 | 39 | 21 | <0.0001 | 6 | 3 |
| Sildenafil | 1645 | 483 | 1175 | 0 | 71 | 0 | <0.0001 | 10 | 0 |
| Baclofen | 1555 | 1105 | 483 | 670 | 31 | 61 | <0.0001 | 4 | 8 |
| Esomeprazole | 1109 | 2530 | 584 | 655 | 53 | 26 | <0.0001 | 5 | 8 |
| Hydrochlorotiazide | 639 | 16 | 572 | 15 | 90 | 94 | 1.000 | 5 | 0.2 |
| Clobazam | 523 | 954 | 265 | 726 | 51 | 76 | <0.0001 | 2 | 8 |
| Dexamethasone | 494 | 1772 | 137 | 352 | 27 | 20 | 0.0002 | 1 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andersson, Å.C.; Eksborg, S.; Förberg, U.; Nydert, P.; Lindemalm, S. Manipulated Oral and Rectal Drugs in a Paediatric Swedish University Hospital, a Registry-Based Study Comparing Two Study-Years, Ten Years Apart. Pharmaceuticals 2023, 16, 8. https://doi.org/10.3390/ph16010008
Andersson ÅC, Eksborg S, Förberg U, Nydert P, Lindemalm S. Manipulated Oral and Rectal Drugs in a Paediatric Swedish University Hospital, a Registry-Based Study Comparing Two Study-Years, Ten Years Apart. Pharmaceuticals. 2023; 16(1):8. https://doi.org/10.3390/ph16010008
Chicago/Turabian StyleAndersson, Åsa C., Staffan Eksborg, Ulrika Förberg, Per Nydert, and Synnöve Lindemalm. 2023. "Manipulated Oral and Rectal Drugs in a Paediatric Swedish University Hospital, a Registry-Based Study Comparing Two Study-Years, Ten Years Apart" Pharmaceuticals 16, no. 1: 8. https://doi.org/10.3390/ph16010008
APA StyleAndersson, Å. C., Eksborg, S., Förberg, U., Nydert, P., & Lindemalm, S. (2023). Manipulated Oral and Rectal Drugs in a Paediatric Swedish University Hospital, a Registry-Based Study Comparing Two Study-Years, Ten Years Apart. Pharmaceuticals, 16(1), 8. https://doi.org/10.3390/ph16010008





