Targeting Annexin A1 as a Druggable Player to Enhance the Anti-Tumor Role of Honokiol in Colon Cancer through Autophagic Pathway
Abstract
1. Introduction
2. Results
2.1. ANXA1 Is Overexpressed in Colon Cancer Tissues Related to a Significant Negative Correlation with the Poor Prognosis of Colon Cancer
2.2. ANXA1 Promotes the Hyperproliferation of Colon Cancer Cells
2.3. ANXA1 Regulates the Cell Cycle of Colon Cancer
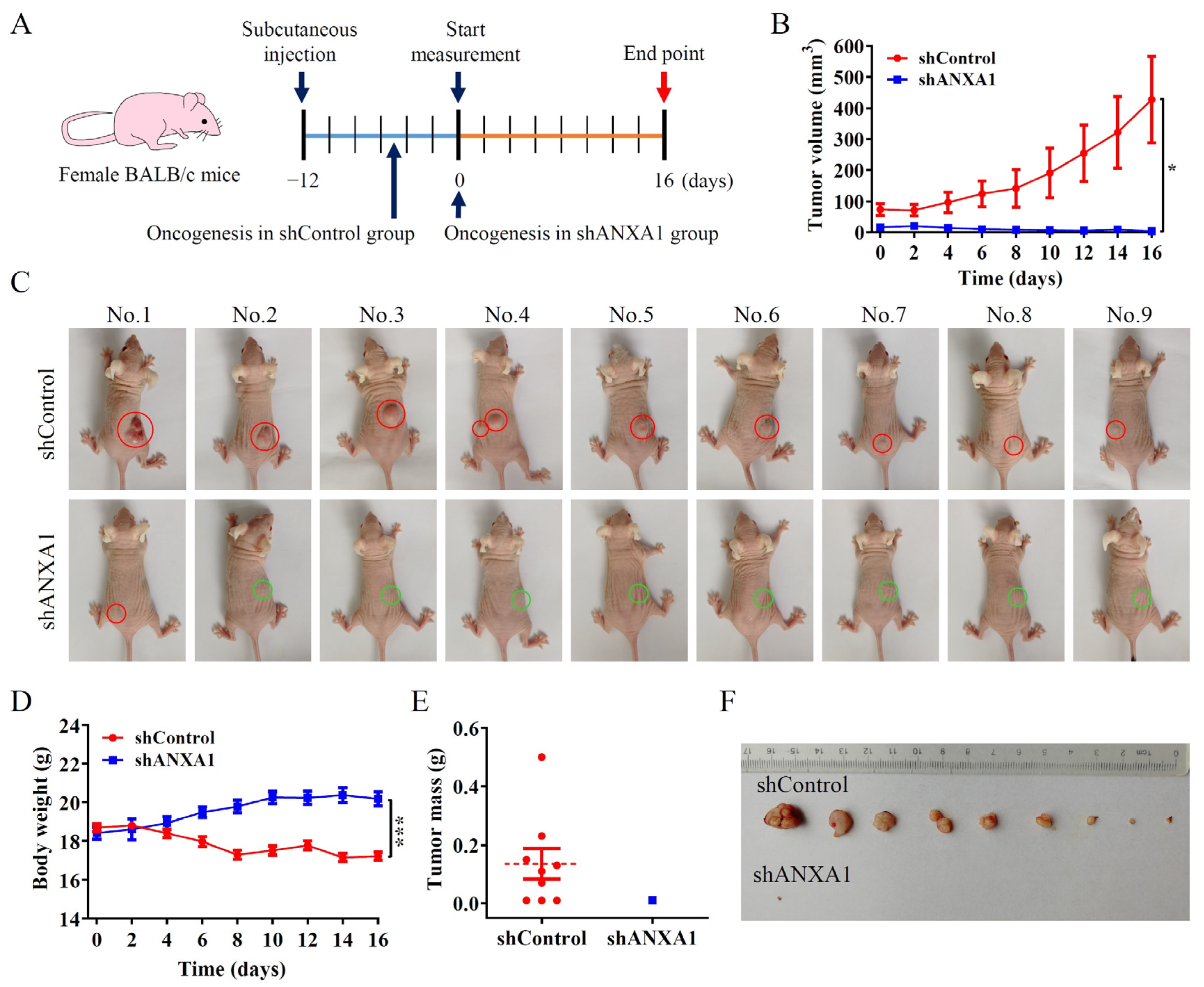
2.4. ANXA1 Promotes Colon Tumor Growth In Vivo
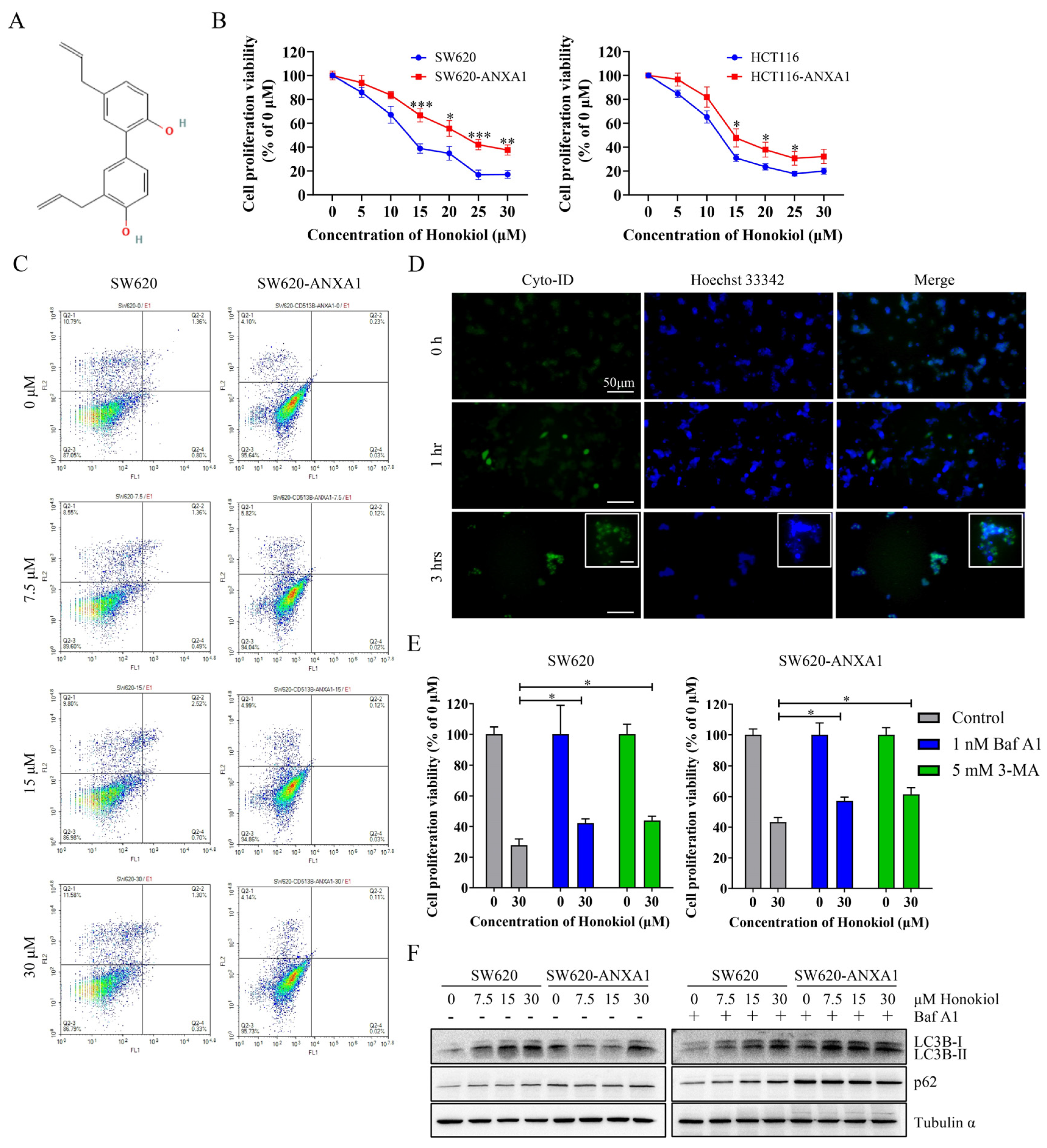
2.5. ANXA1 Antagonizes Autophagy Induced by Honokiol in Colon Cancer Cells
2.6. ANXA1 Antagonizes Autophagy Induced by Honokiol via Stabilizing mtROS in Colon Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Data Mining
4.2. Cell Culture and Drug Treatment
4.3. Plasmid Construction and Cell Screening
4.4. Cell Proliferation Ability Assay
4.5. Autophagy Detection by Fluorescence Microscopy
4.6. Flow Cytometry
4.7. Immunoblotting
4.8. Clinical Sample and Animal Experiments
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Vishwanatha, J.K.; Davis, R.G.; Rubinstein, I.; Floreani, A. Annexin I degradation in bronchoalveolar lavage fluids from healthy smokers: A possible mechanism of inflammation. Clin. Cancer Res. 1998, 4, 2559–2564. [Google Scholar] [PubMed]
- Shao, G.; Zhou, H.; Zhang, Q.; Jin, Y.; Fu, C. Advancements of Annexin A1 in inflammation and tumorigenesis. Onco Targets Ther. 2019, 12, 3245–3254. [Google Scholar] [CrossRef] [PubMed]
- Vecchi, L.; Araujo, T.G.; Azevedo, F.; Mota, S.T.S.; Avila, V.M.R.; Ribeiro, M.A.; Goulart, L.R. Phospholipase A(2) Drives Tumorigenesis and Cancer Aggressiveness through Its Interaction with Annexin A1. Cells 2021, 10, 1472. [Google Scholar] [CrossRef]
- Feng, J.; Lu, S.S.; Xiao, T.; Huang, W.; Yi, H.; Zhu, W.; Fan, S.; Feng, X.P.; Li, J.Y.; Yu, Z.Z.; et al. ANXA1 Binds and Stabilizes EphA2 to Promote Nasopharyngeal Carcinoma Growth and Metastasis. Cancer Res. 2020, 80, 4386–4398. [Google Scholar] [CrossRef]
- Babbin, B.A.; Lee, W.Y.; Parkos, C.A.; Winfree, L.M.; Akyildiz, A.; Perretti, M.; Nusrat, A. Annexin I regulates SKCO-15 cell invasion by signaling through formyl peptide receptors. J. Biol. Chem. 2006, 281, 19588–19599. [Google Scholar] [CrossRef]
- Wei, L.; Li, L.; Liu, L.; Yu, R.; Li, X.; Luo, Z. Knockdown of Annexin-A1 Inhibits Growth, Migration and Invasion of Glioma Cells by Suppressing the PI3K/Akt Signaling Pathway. ASN Neuro 2021, 13, 17590914211001218. [Google Scholar] [CrossRef]
- Xiong, W.; Zhang, B.; Zhu, L.; Yi, L.; Jin, X. RRM2 Regulates Sensitivity to Sunitinib and PD-1 Blockade in Renal Cancer by Stabilizing ANXA1 and Activating the AKT Pathway. Adv. Sci. 2021, 8, e2100881. [Google Scholar] [CrossRef]
- Foo, S.L.; Yap, G.; Cui, J.; Lim, L.H.K. Annexin-A1—A Blessing or a Curse in Cancer? Trends Mol. Med. 2019, 25, 315–327. [Google Scholar] [CrossRef]
- Oshi, M.; Tokumaru, Y.; Mukhopadhyay, S.; Yan, L.; Matsuyama, R.; Endo, I.; Takabe, K. Annexin A1 Expression Is Associated with Epithelial-Mesenchymal Transition (EMT), Cell Proliferation, Prognosis, and Drug Response in Pancreatic Cancer. Cells 2021, 10, 653. [Google Scholar] [CrossRef] [PubMed]
- Delorme, S.; Privat, M.; Sonnier, N.; Rouanet, J.; Witkowski, T.; Mishellany, F.; Radosevic-Robin, N.; Juban, G.; Molnar, I.; Quintana, M.; et al. New insight into the role of ANXA1 in melanoma progression: Involvement of stromal expression in dissemination. Am. J. Cancer Res. 2021, 11, 1600–1615. [Google Scholar] [PubMed]
- Tu, Y.; Johnstone, C.N.; Stewart, A.G. Annexin A1 influences in breast cancer: Controversies on contributions to tumour, host and immunoediting processes. Pharmacol. Res. 2017, 119, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, Q.W.; Ye, Y.; Lin, L.G. Honokiol: A naturally occurring lignan with pleiotropic bioactivities. Chin. J. Nat. Med. 2021, 19, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Patel, S.; Imran, M.; Maalik, A.; Arshad, M.; Saeed, F.; Mabkhot, Y.; Al-Showiman, S.; Ahmad, N.; Elsharkawy, E. Honokiol: An anticancer lignan. Biomed. Pharmacother. 2018, 107, 555–562. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Hrncic, M.K.; Skerget, M.; Knez, Z.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Ong, C.P.; Lee, W.L.; Tang, Y.Q.; Yap, W.H. Honokiol: A Review of Its Anticancer Potential and Mechanisms. Cancers 2019, 12, 48. [Google Scholar] [CrossRef]
- Fried, L.E.; Arbiser, J.L. Honokiol, a multifunctional antiangiogenic and antitumor agent. Antioxid. Redox Signal. 2009, 11, 1139–1148. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, S.; Gao, W.; Feng, J.; Zhao, G. Honokiol induces endoplasmic reticulum stress-mediated apoptosis in human lung cancer cells. Life Sci. 2019, 221, 204–211. [Google Scholar] [CrossRef]
- Pan, J.; Lee, Y.; Wang, Y.; You, M. Honokiol targets mitochondria to halt cancer progression and metastasis. Mol. Nutr. Food Res. 2016, 60, 1383–1395. [Google Scholar] [CrossRef]
- Li, Q.; Ma, Y.; Liu, X.L.; Mu, L.; He, B.C.; Wu, K.; Sun, W.J. Anti-proliferative effect of honokiol on SW620 cells through upregulating BMP7 expression via the TGF-beta1/p53 signaling pathway. Oncol. Rep. 2020, 44, 2093–2107. [Google Scholar]
- Subramaniam, D.; Ponnurangam, S.; Ramalingam, S.; Kwatra, D.; Dandawate, P.; Weir, S.; Umar, S.; Jensen, R.; Anant, S. Honokiol Affects Stem Cell Viability by Suppressing Oncogenic YAP1 Function to Inhibit Colon Tumorigenesis. Cells 2021, 10, 1607. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Chen, W.; Shen, L.; Sheng, Q.; Teng, L. Honokiol augments the anti-cancer effects of oxaliplatin in colon cancer cells. Acta Biochim. Biophys. Sin. 2013, 45, 773–779. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ponnurangam, S.; Mammen, J.M.; Ramalingam, S.; He, Z.; Zhang, Y.; Umar, S.; Subramaniam, D.; Anant, S. Honokiol in combination with radiation targets notch signaling to inhibit colon cancer stem cells. Mol. Cancer Ther. 2012, 11, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, T.; Zhu, J.; Ruan, S.; Li, R.; Guo, B.; Lin, L. Honokiol attenuates lipotoxicity in hepatocytes via activating SIRT3-AMPK mediated lipophagy. Chin. Med. 2021, 16, 115. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wang, Y.; Yao, M.; Zhang, D.; Fang, W.; Zhou, T.; Gan, D.; Yue, S.; Qian, H.; Chen, T. Honokiol inhibits the growth of SKBR3 cells. Transl. Cancer Res. 2020, 9, 7596–7604. [Google Scholar] [CrossRef]
- Qin, T.; Li, J.; Xiao, Y.; Wang, X.; Gong, M.; Wang, Q.; Zhu, Z.; Zhang, S.; Zhang, W.; Cao, F.; et al. Honokiol Suppresses Perineural Invasion of Pancreatic Cancer by Inhibiting SMAD2/3 Signaling. Front. Oncol. 2021, 11, 728583. [Google Scholar] [CrossRef]
- Lee, J.S.; Sul, J.Y.; Park, J.B.; Lee, M.S.; Cha, E.Y.; Ko, Y.B. Honokiol induces apoptosis and suppresses migration and invasion of ovarian carcinoma cells via AMPK/mTOR signaling pathway. Int. J. Mol. Med. 2019, 43, 1969–1978. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, X.; Yang, Z.; Zhao, X. Honokiol induces caspase-independent paraptosis via reactive oxygen species production that is accompanied by apoptosis in leukemia cells. Biochem. Biophys. Res. Commun. 2013, 430, 876–882. [Google Scholar] [CrossRef]
- Huang, K.; Chen, Y.; Zhang, R.; Wu, Y.; Ma, Y.; Fang, X.; Shen, S. Honokiol induces apoptosis and autophagy via the ROS/ERK1/2 signaling pathway in human osteosarcoma cells in vitro and in vivo. Cell Death Dis. 2018, 9, 157. [Google Scholar] [CrossRef]
- Lin, M.C.; Lee, Y.W.; Tseng, Y.Y.; Lin, Y.W.; Chen, J.T.; Liu, S.H.; Chen, R.M. Honokiol Induces Autophagic Apoptosis in Neuroblastoma Cells through a P53-Dependent Pathway. Am. J. Chin. Med. 2019, 47, 895–912. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.X.; Li, Y.; Liu, Z.Q.; Fan, X.X.; Duan, F.G.; Li, R.Z.; Yao, X.J.; Leung, E.L.H.; Liu, L. Honokiol Induces Apoptosis, G1 Arrest, and Autophagy in KRAS Mutant Lung Cancer Cells. Front. Pharmacol. 2017, 8, 199. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Kang, J.; Fu, C. The independence of and associations among apoptosis, autophagy, and necrosis. Signal Transduct. Target. Ther. 2018, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- van der Pas, M.H.; Meijer, S.; Hoekstra, O.S.; Riphagen, I.I.; de Vet, H.C.; Knol, D.L.; van Grieken, N.C.; Meijerink, W.J. Sentinel-lymph-node procedure in colon and rectal cancer: A systematic review and meta-analysis. Lancet Oncol. 2011, 12, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Golfinopoulos, V.; Salanti, G.; Pavlidis, N.; Ioannidis, J.P. Survival and disease-progression benefits with treatment regimens for advanced colorectal cancer: A meta-analysis. Lancet Oncol. 2007, 8, 898–911. [Google Scholar] [CrossRef]
- André, T.; Vernerey, D.; Im, S.; Bodoky, G.; Buzzoni, R.; Reingold, S.; Rivera, F.; McKendrick, J.; Scheithauer, W.; Ravit, G.; et al. Bevacizumab as adjuvant treatment of colon cancer: Updated results from the S-AVANT phase III study by the GERCOR Group. Ann. Oncol. 2020, 31, 246–256. [Google Scholar] [CrossRef]
- Banerjee, A.; Pathak, S.; Subramanium, V.D.; Dharanivasan, G.; Murugesan, R.; Verma, R.S. Strategies for targeted drug delivery in treatment of colon cancer: Current trends and future perspectives. Drug Discov. Today 2017, 22, 1224–1232. [Google Scholar] [CrossRef]
- Mao, Z.; Xiao, H.; Shen, P.; Yang, Y.; Xue, J.; Yang, Y.; Shang, Y.; Zhang, L.; Li, X.; Zhang, Y.; et al. KRAS(G12D) can be targeted by potent inhibitors via formation of salt bridge. Cell Discov. 2022, 8, 5. [Google Scholar] [CrossRef]
- Siddiqui, A.D.; Piperdi, B. KRAS mutation in colon cancer: A marker of resistance to EGFR-I therapy. Ann. Surg. Oncol. 2010, 17, 1168–1176. [Google Scholar] [CrossRef]
- Prahallad, A.; Sun, C.; Huang, S.; Di Nicolantonio, F.; Salazar, R.; Zecchin, D.; Beijersbergen, R.L.; Bardelli, A.; Bernards, R. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature 2012, 483, 100–103. [Google Scholar] [CrossRef]
- Bai, F.; Zhang, P.; Fu, Y.; Chen, H.; Zhang, M.; Huang, Q.; Li, D.; Li, B.; Wu, K. Targeting ANXA1 abrogates Treg-mediated immune suppression in triple-negative breast cancer. J. Immunother. Cancer 2020, 8, e000169. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, W.; Wang, Z.; Wang, C.; Ai, Z. Exosomal ANXA1 derived from thyroid cancer cells is associated with malignant transformation of human thyroid follicular epithelial cells by promoting cell proliferation. Int. J. Oncol. 2021, 59, 104. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Z.; Liu, Q.; Han, Y.; Zang, Y.; Zhang, H.; Du, X.; Qin, T.; Wu, Y. Honokiol Suppressed Pancreatic Cancer Progression via miR-101/Mcl-1 Axis. Cancer Manag. Res. 2020, 12, 5243–5254. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.C.; Lai, D.W.; Lan, K.H.; Shen, C.C.; Wu, S.M.; Chiu, C.S.; Wang, K.B.; Sheu, M.L. Honokiol thwarts gastric tumor growth and peritoneal dissemination by inhibiting Tpl2 in an orthotopic model. Carcinogenesis 2013, 34, 2568–2579. [Google Scholar] [CrossRef]
- Yi, X.; Lou, L.; Wang, J.; Xiong, J.; Zhou, S. Honokiol antagonizes doxorubicin resistance in human breast cancer via miR-188-5p/FBXW7/c-Myc pathway. Cancer Chemother. Pharmacol. 2021, 87, 647–656. [Google Scholar] [CrossRef]
- Wang, T.; Wang, H.; Yang, F.; Gao, K.; Luo, S.; Bai, L.; Ma, K.; Liu, M.; Wu, S.; Wang, H.; et al. Honokiol inhibits proliferation of colorectal cancer cells by targeting anoctamin 1/TMEM16A Ca(2+) -activated Cl(-) channels. Br. J. Pharmacol. 2021, 178, 4137–4154. [Google Scholar] [CrossRef]
- Guo, C.; Liu, P.; Deng, G.; Han, Y.; Chen, Y.; Cai, C.; Shen, H.; Deng, G.; Zeng, S. Honokiol induces ferroptosis in colon cancer cells by regulating GPX4 activity. Am. J. Cancer Res. 2021, 11, 3039–3054. [Google Scholar]
- Cheng, N.; Xia, T.; Han, Y.; He, Q.J.; Zhao, R.; Ma, J.R. Synergistic antitumor effects of liposomal honokiol combined with cisplatin in colon cancer models. Oncol. Lett. 2011, 2, 957–962. [Google Scholar]
- Zhu, J.F.; Huang, W.; Yi, H.M.; Xiao, T.; Li, J.Y.; Feng, J.; Lu, S.S.; Li, X.H.; Lu, R.H.; He, Q.Y.; et al. Annexin A1-suppressed autophagy promotes nasopharyngeal carcinoma cell invasion and metastasis by PI3K/AKT signaling activation. Cell Death Dis. 2018, 9, 1154. [Google Scholar] [CrossRef]
- Berns, K.; Sonnenblick, A.; Gennissen, A.; Brohée, S.; Hijmans, E.M.; Evers, B.; Fumagalli, D.; Desmedt, C.; Loibl, S.; Denkert, C.; et al. Loss of ARID1A Activates ANXA1, which Serves as a Predictive Biomarker for Trastuzumab Resistance. Clin. Cancer Res. 2016, 22, 5238–5248. [Google Scholar] [CrossRef]
- Fuhrmann, D.C.; Brüne, B. Mitochondrial composition and function under the control of hypoxia. Redox Biol. 2017, 12, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ni, Y.; Zhang, L.; Jiang, R.; Xu, J.; Yang, H.; Hu, Y.; Qiu, J.; Pu, L.; Tang, J.; et al. HIF-1alpha-induced expression of m6A reader YTHDF1 drives hypoxia-induced autophagy and malignancy of hepatocellular carcinoma by promoting ATG2A and ATG14 translation. Signal Transduct. Target. Ther. 2021, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Pillai, V.B.; Samant, S.; Sundaresan, N.R.; Raghuraman, H.; Kim, G.; Bonner, M.Y.; Arbiser, J.L.; Walker, D.I.; Jones, D.P.; Gius, D.; et al. Honokiol blocks and reverses cardiac hypertrophy in mice by activating mitochondrial Sirt3. Nat. Commun. 2015, 6, 6656. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Lee, C.F.; Huang, W.H.; Chou, T.C. Magnolol suppresses hypoxia-induced angiogenesis via inhibition of HIF-1alpha/VEGF signaling pathway in human bladder cancer cells. Biochem. Pharmacol. 2013, 85, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Lan, K.L.; Sheu, M.L.; Chen, M.Y.; Shih, Y.S.; Hsu, F.C.; Wang, H.M.; Liu, R.S.; Yen, S.H. Honokiol inhibits hypoxia-inducible factor-1 pathway. Int. J. Radiat. Biol. 2011, 87, 579–590. [Google Scholar] [CrossRef]
- Bizzarro, V.; Belvedere, R.; Migliaro, V.; Romano, E.; Parente, L.; Petrella, A. Hypoxia regulates ANXA1 expression to support prostate cancer cell invasion and aggressiveness. Cell Adhes. Migr. 2017, 11, 247–260. [Google Scholar] [CrossRef]
- Jin, H.; Shi, Y.; Lv, Y.; Yuan, S.; Ramirez, C.F.A.; Lieftink, C.; Wang, L.; Wang, S.; Wang, C.; Dias, M.H.; et al. EGFR activation limits the response of liver cancer to lenvatinib. Nature 2021, 595, 730–734. [Google Scholar] [CrossRef]
- Furlan, V.; Bren, U. Insight into Inhibitory Mechanism of PDE4D by Dietary Polyphenols Using Molecular Dynamics Simulations and Free Energy Calculations. Biomolecules 2021, 11, 479. [Google Scholar] [CrossRef]
- Furlan, V.; Konc, J.; Bren, U. Inverse Molecular Docking as a Novel Approach to Study Anticarcinogenic and Anti-Neuroinflammatory Effects of Curcumin. Molecules 2018, 23, 3351. [Google Scholar] [CrossRef]
- Kores, K.; Kolenc, Z.; Furlan, V.; Bren, U. Inverse Molecular Docking Elucidating the Anticarcinogenic Potential of the Hop Natural Product Xanthohumol and Its Metabolites. Foods 2022, 11, 1253. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, X.; Meng, Y.; Ma, J.; Zhang, Q.; Shao, G.; Wang, L.; Cheng, X.; Hong, X.; Wang, Y.; et al. A Novel Mechanism of Endoplasmic Reticulum Stress- and c-Myc-Degradation-Mediated Therapeutic Benefits of Antineurokinin-1 Receptor Drugs in Colorectal Cancer. Adv. Sci. 2021, 8, e2101936. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Liang, D.; Wang, P.; Yin, L.; Zhang, H.; Xing, C.; Huang, Z.; Wu, Y.; Li, H.; Cheng, Z.; et al. HIF-PH Encoded by EGLN1 Is a Potential Therapeutic Target for Chronic Lymphocytic Leukemia. Pharmaceuticals 2022, 15, 734. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Mahmod, A.I.; Awajan, D.; Hamed, R.A.; Al-Yasari, I.H. Immunomodulatory, Anticancer, and Antimicrobial Effects of Rice Bran Grown in Iraq: An In Vitro and In Vivo Study. Pharmaceuticals 2022, 15, 1502. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Shao, G.; Hong, X.; Shi, Y.; Zheng, Y.; Yu, Y.; Fu, C. Targeting Annexin A1 as a Druggable Player to Enhance the Anti-Tumor Role of Honokiol in Colon Cancer through Autophagic Pathway. Pharmaceuticals 2023, 16, 70. https://doi.org/10.3390/ph16010070
Wang X, Shao G, Hong X, Shi Y, Zheng Y, Yu Y, Fu C. Targeting Annexin A1 as a Druggable Player to Enhance the Anti-Tumor Role of Honokiol in Colon Cancer through Autophagic Pathway. Pharmaceuticals. 2023; 16(1):70. https://doi.org/10.3390/ph16010070
Chicago/Turabian StyleWang, Xi, Gang Shao, Xiangyu Hong, Yue Shi, Yiting Zheng, Yucheng Yu, and Caiyun Fu. 2023. "Targeting Annexin A1 as a Druggable Player to Enhance the Anti-Tumor Role of Honokiol in Colon Cancer through Autophagic Pathway" Pharmaceuticals 16, no. 1: 70. https://doi.org/10.3390/ph16010070
APA StyleWang, X., Shao, G., Hong, X., Shi, Y., Zheng, Y., Yu, Y., & Fu, C. (2023). Targeting Annexin A1 as a Druggable Player to Enhance the Anti-Tumor Role of Honokiol in Colon Cancer through Autophagic Pathway. Pharmaceuticals, 16(1), 70. https://doi.org/10.3390/ph16010070







