Changes in Pharmacokinetics and Pharmacodynamics of Losartan in Experimental Diseased Rats Treated with Curcuma longa and Lepidium sativum
Abstract
1. Introduction
2. Materials and Methods
2.1. Induction of Hypertension in Rats
2.2. Pharmacodynamics of Losartan in Hypertensive Rats Treated with CUR and LS
2.3. Pharmacokinetics of Losartan in Hypertensive Rats Treated with CUR and LS
2.4. Statistical Analysis
3. Results and Discussion
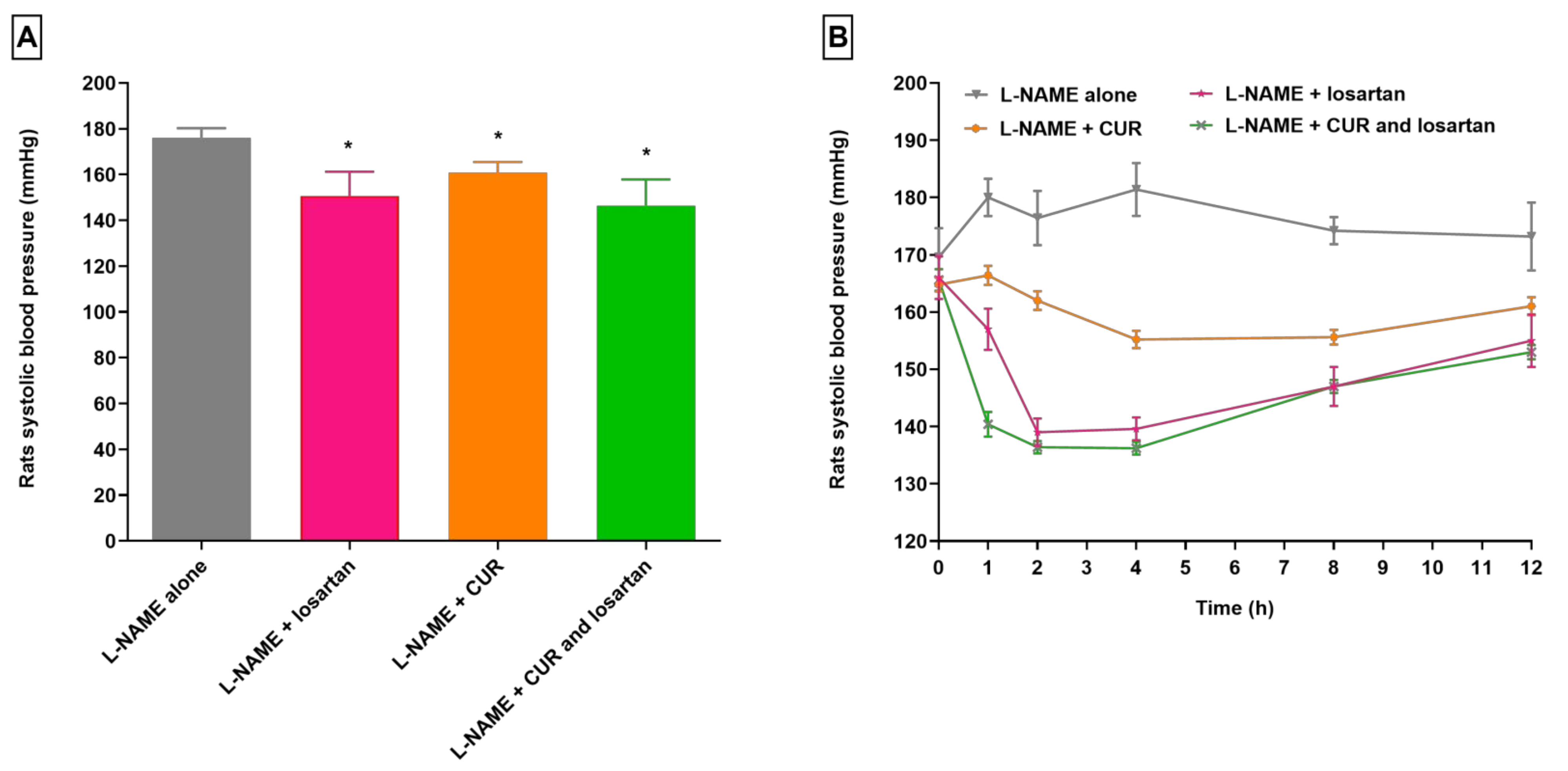
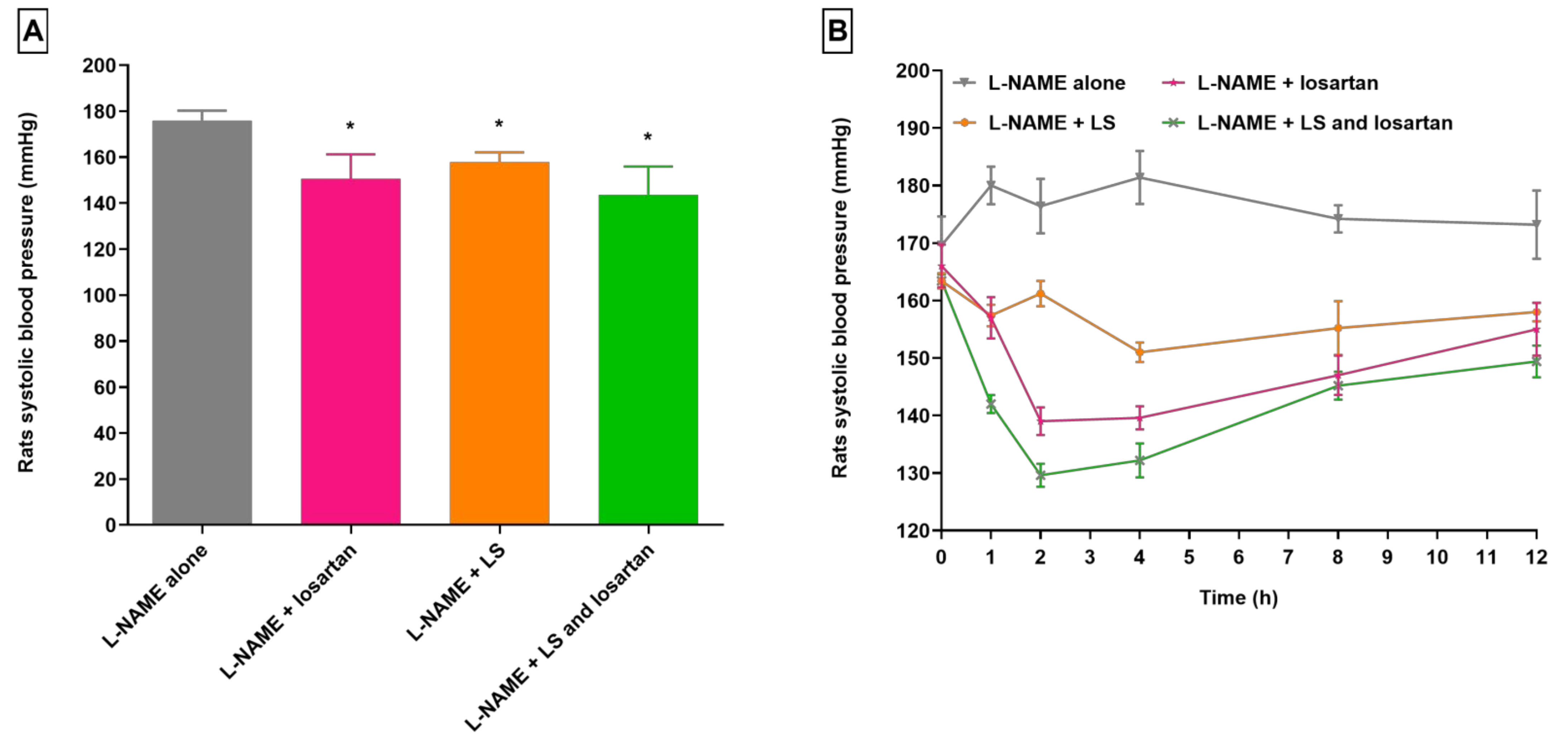
3.1. Changes in Losartan Pharmacodynamics in Hypertensive Rats Treated with CUR and LS
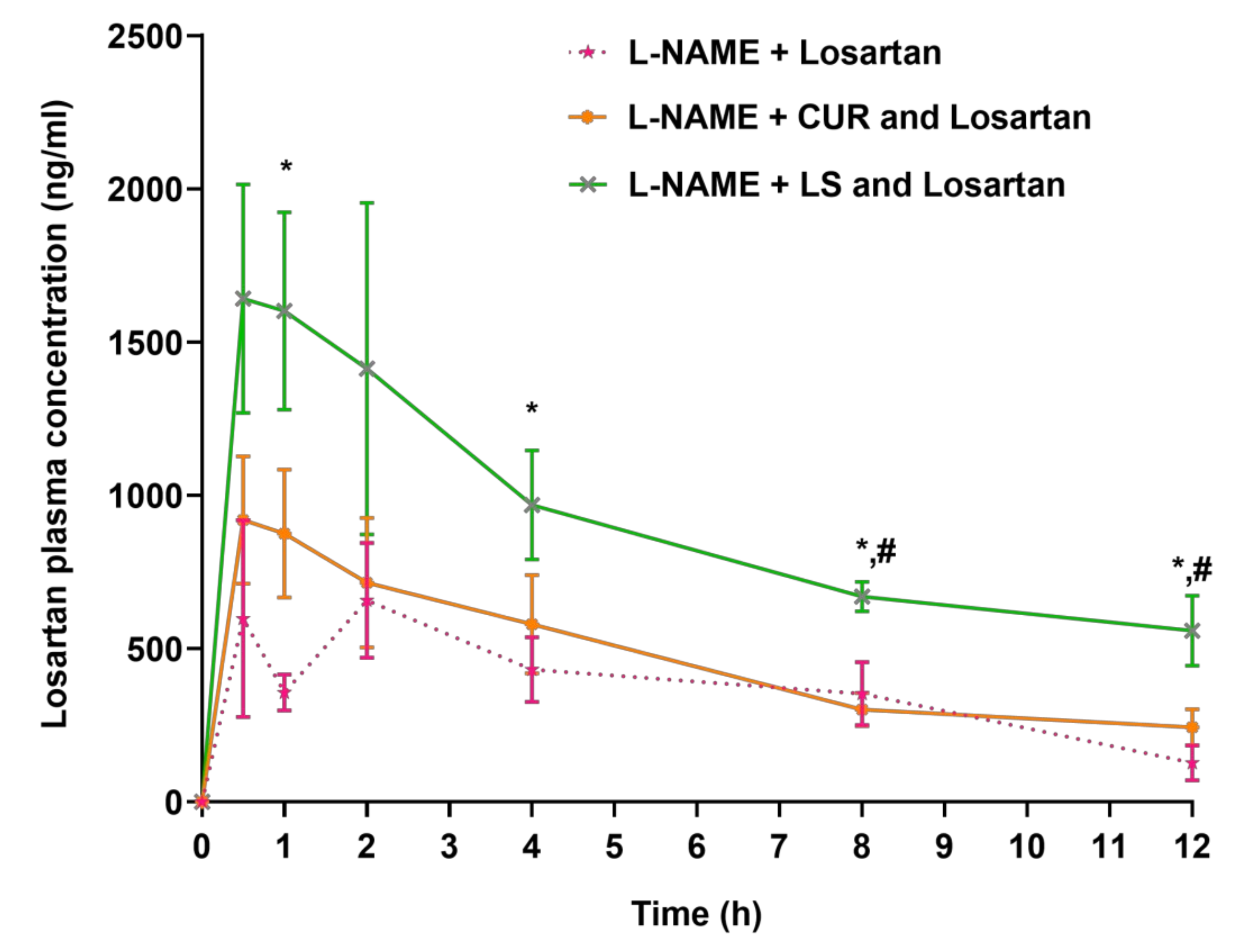
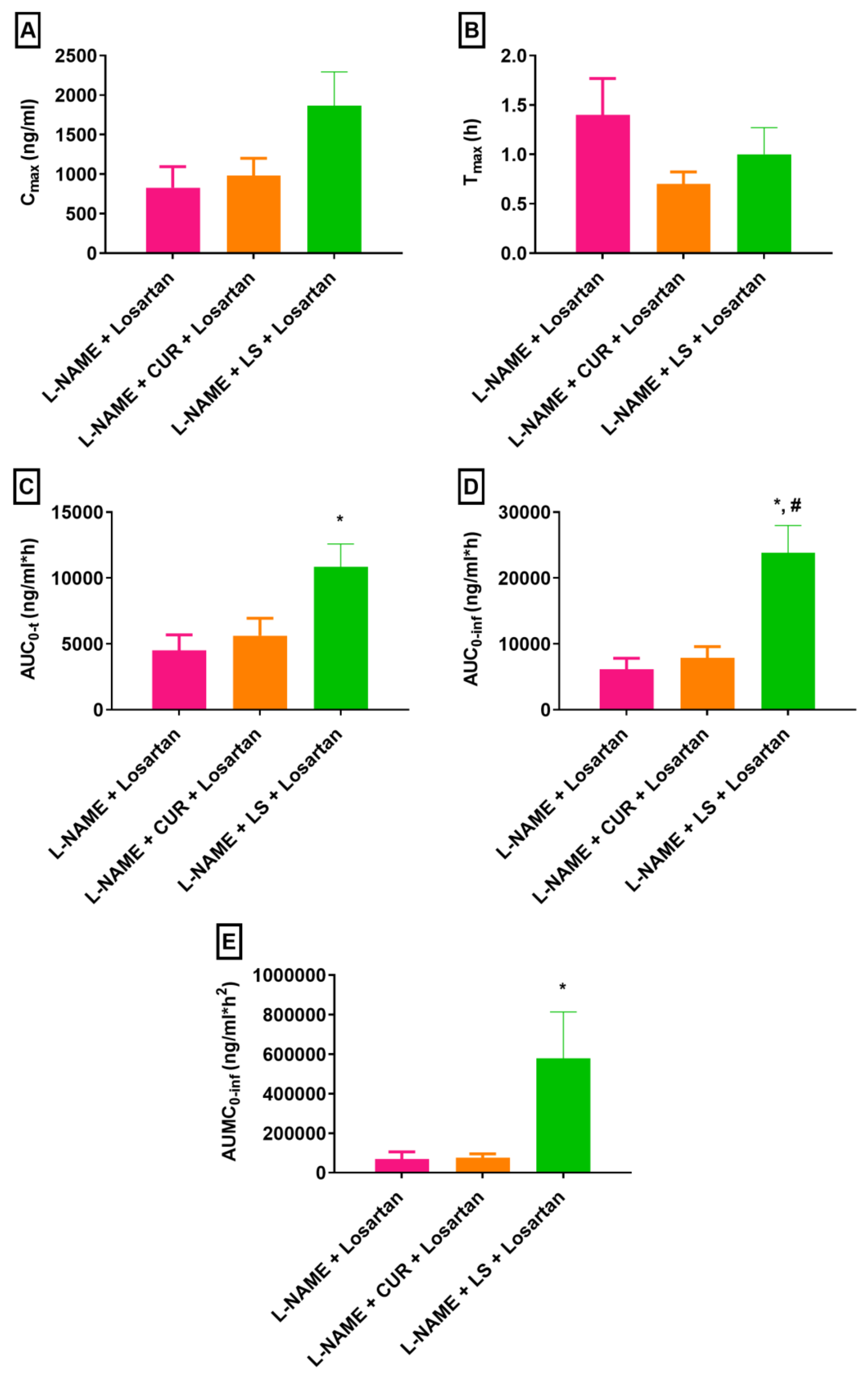
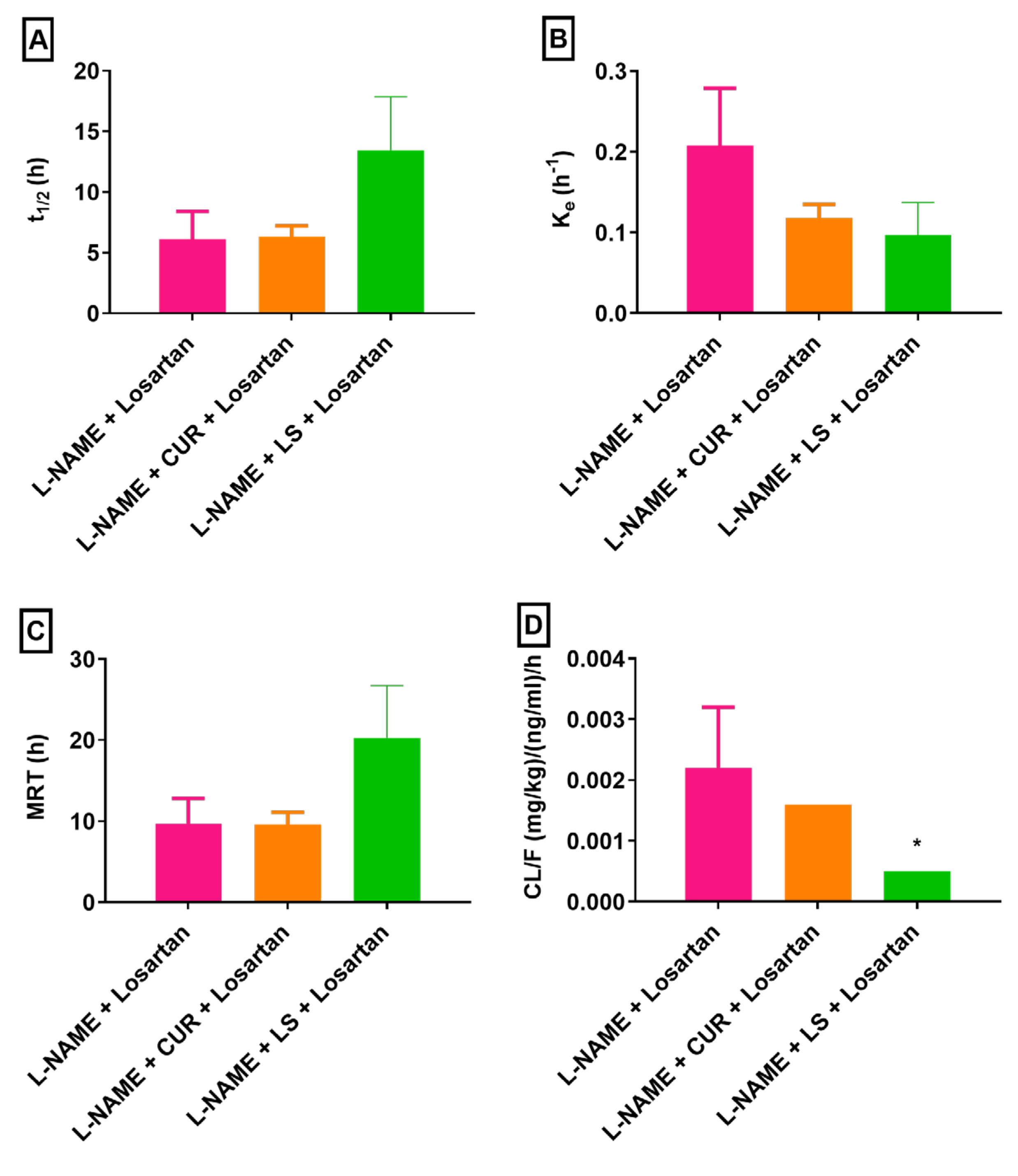
3.2. Changes in Losartan Pharmacokinetics in Hypertensive Rats Treated with CUR and LS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hussain, S.A.; Panjagari, N.R.; Singh, R.R.; Patil, G.R. Potential herbs and herbal nutraceuticals: Food applications and their interactions with food components. Crit. Rev. Food Sci. Nutr. 2015, 55, 94–122. [Google Scholar] [CrossRef] [PubMed]
- Ioannides, C. Pharmacokinetic interactions between herbal remedies and medicinal drugs. Xenobiotica 2002, 32, 451–478. [Google Scholar] [CrossRef] [PubMed]
- Malongane, F.; McGaw, L.J.; Mudau, F.N. The synergistic potential of various teas, herbs and therapeutic drugs in health improvement: A review. J. Sci. Food Agric. 2017, 97, 4679–4689. [Google Scholar] [CrossRef] [PubMed]
- Jokar, N.K.; Noorhosseini, S.A.; Allahyari, M.S.; Damalas, C.A. Consumers’ acceptance of medicinal herbs: An application of the technology acceptance model (TAM). J. Ethnopharmacol. 2017, 207, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Song, X.; Zhu, M.; Zhang, J. Study on the pharmacokinetics drug-drug interaction potential of Glycyrrhiza uralensis, a traditional Chinese medicine, with lidocaine in rats. Phytother. Res. 2009, 23, 603–607. [Google Scholar] [CrossRef]
- Vora, C.K.; Mansoor, G.A. Herbs and alternative therapies: Relevance to hypertension and cardiovascular diseases. Curr. Hypertens. Rep. 2005, 7, 275–280. [Google Scholar] [CrossRef]
- Lee, K.H. Research and future trends in the pharmaceutical development of medicinal herbs from Chinese medicine. Public Health Nutr. 2000, 3, 515–522. [Google Scholar] [CrossRef]
- Fugh-Berman, A. Herb-drug interactions. Lancet 2000, 355, 134–138. [Google Scholar] [CrossRef]
- Chavez, M.L.; Jordan, M.A.; Chavez, P.I. Evidence-based drug--herbal interactions. Life Sci. 2006, 78, 2146–2157. [Google Scholar] [CrossRef]
- Liu, M.Z.; Zhang, Y.L.; Zeng, M.Z.; He, F.Z.; Luo, Z.Y.; Luo, J.Q.; Wen, J.G.; Chen, X.P.; Zhou, H.H.; Zhang, W. Pharmacogenomics and herb-drug interactions: Merge of future and tradition. Evid. Based Complement. Alternat. Med. 2015, 2015, 321091. [Google Scholar] [CrossRef]
- Yang, C.; Kim, Y.E.; Kim, I.T.; Seo, B.N.; Park, J. Interactions between antihypertensives and herb: Review. J. Hypertens. 2016, 34, e334. [Google Scholar] [CrossRef]
- Tachjian, A.; Maria, V.; Jahangir, A. Use of herbal products and potential interactions in patients with cardiovascular diseases. J. Am. Coll. Cardiol. 2010, 55, 515–525. [Google Scholar] [CrossRef]
- Izzo, A.A.; Di Carlo, G.; Borrelli, F.; Ernst, E. Cardiovascular pharmacotherapy and herbal medicines: The risk of drug interaction. Int. J. Cardiol. 2005, 98, 1–14. [Google Scholar] [CrossRef]
- Sultana, S.; Asif, H.M. Review: Medicinal plants combating against hypertension: A green antihypertensive approach. Pak. J. Pharm. Sci. 2017, 30, 2311–2319. [Google Scholar]
- Zar, C.T.; Das, S. Potential effect of herbs on diabetic hypertension: Alternative medicine treatment modalities. Clin. Ter. 2013, 164, 529–535. [Google Scholar]
- Rouhi-Boroujeni, H.; Heidarian, E.; Deris, F.; Rafieian-Kopaei, M. Medicinal Plants with Multiple Effects on Cardiovascular Diseases: A Systematic Review. Curr. Pharm. Des. 2017, 23, 999–1015. [Google Scholar] [CrossRef]
- Mansoor, G.A. Herbs and alternative therapies in the hypertension clinic. Am. J. Hypertens. 2001, 14, 971–975. [Google Scholar] [CrossRef]
- Chrysant, S.G.; Chrysant, G.S. Herbs Used for the Treatment of Hypertension and their Mechanism of Action. Curr. Hypertens. Rep. 2017, 19, 77. [Google Scholar] [CrossRef]
- Ndu, O.O.; Nworu, C.S.; Ehiemere, C.O.; Ndukwe, N.C.; Ochiogu, I.S. Herb-drug interaction between the extract of Hibiscus sabdariffa L. and hydrochlorothiazide in experimental animals. J. Med. Food 2011, 14, 640–644. [Google Scholar] [CrossRef]
- Awang, D.V.; Fugh-Berman, A. Herbal interactions with cardiovascular drugs. J. Cardiovasc. Nurs. 2002, 16, 64–70. [Google Scholar] [CrossRef]
- Anwar, M.A.; Al Disi, S.S.; Eid, A.H. Anti-Hypertensive Herbs and Their Mechanisms of Action: Part II. Front. Pharmacol. 2016, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Pathak, L.M.; Kothiyal, P. Antihypertensive drugs interaction with herbal medicine—Review. Int. J. Pharm. Phytopharmacol. Res. 2013, 3, 139–143. [Google Scholar]
- Teixeira, K.; Dos Santos, P.; Citadini-Zanette, V.; Silvia DalBó, S.; Amaral, P.A. Medicinal plants that can cause changes in blood pressure and interactions with antihypertensive agents. Am. J. Ethnomed. 2017, 4, 10.21767/2348–9502100002. [Google Scholar] [CrossRef][Green Version]
- Sica, D.A.; Gehr, T.W.; Ghosh, S. Clinical pharmacokinetics of losartan. Clin. Pharmacokinet. 2005, 44, 797–814. [Google Scholar] [CrossRef] [PubMed]
- Goa, K.L.; Wagstaff, A.J. Losartan potassium: A review of its pharmacology, clinical efficacy and tolerability in the management of hypertension. Drugs 1996, 51, 820–845. [Google Scholar] [CrossRef]
- Ahad, A.; Al-Mohizea, A.M.; Al-Jenoobi, F.I.; Aqil, M. Transdermal delivery of angiotensin II receptor blockers (ARBs), angiotensin-converting enzyme inhibitors (ACEIs) and others for management of hypertension. Drug Deliv. 2016, 23, 579–590. [Google Scholar] [CrossRef]
- Al-Majed, A.R.; Assiri, E.; Khalil, N.Y.; Abdel-Aziz, H.A. Losartan: Comprehensive Profile. Profiles Drug Subst. Excip. Relat. Methodol. 2015, 40, 159–194. [Google Scholar]
- Lo, M.W.; Goldberg, M.R.; McCrea, J.B.; Lu, H.; Furtek, C.I.; Bjornsson, T.D. Pharmacokinetics of losartan, an angiotensin II receptor antagonist, and its active metabolite EXP3174 in humans. Clin. Pharmacol. Ther. 1995, 58, 641–649. [Google Scholar] [CrossRef]
- Sachinidis, A.; Ko, Y.; Weisser, P.; Meyer zu Brickwedde, M.K.; Dusing, R.; Christian, R.; Wieczorek, A.J.; Vetter, H. EXP3174, a metabolite of losartan (MK 954, DuP 753) is more potent than losartan in blocking the angiotensin II-induced responses in vascular smooth muscle cells. J. Hypertens. 1993, 11, 155–162. [Google Scholar] [CrossRef]
- Meadowcroft, A.M.; Williamson, K.M.; Patterson, J.H.; Hinderliter, A.L.; Pieper, J.A. The effects of fluvastatin, a CYP2C9 inhibitor, on losartan pharmacokinetics in healthy volunteers. J. Clin. Pharmacol. 1999, 39, 418–424. [Google Scholar] [CrossRef]
- Yasar, U.; Tybring, G.; Hidestrand, M.; Oscarson, M.; Ingelman-Sundberg, M.; Dahl, M.L.; Eliasson, E. Role of CYP2C9 polymorphism in losartan oxidation. Drug Metab. Dispos. 2001, 29, 1051–1056. [Google Scholar]
- Flockhart, D.A.; Tanus-Santos, J.E. Implications of cytochrome P450 interactions when prescribing medication for hypertension. Arch. Intern. Med. 2002, 162, 405–412. [Google Scholar] [CrossRef]
- Stearns, R.A.; Chakravarty, P.K.; Chen, R.; Chiu, S.H. Biotransformation of losartan to its active carboxylic acid metabolite in human liver microsomes. Role of cytochrome P4502C and 3A subfamily members. Drug Metab. Dispos. 1995, 23, 207–215. [Google Scholar]
- Yun, C.H.; Lee, H.S.; Lee, H.; Rho, J.K.; Jeong, H.G.; Guengerich, F.P. Oxidation of the angiotensin II receptor antagonist losartan (DuP 753) in human liver microsomes. Role of cytochrome P4503A(4) in formation of the active metabolite EXP3174. Drug Metab. Dispos. 1995, 23, 285–289. [Google Scholar]
- Wang, R.; Zhang, H.; Wang, Y.; Yu, X.; Yuan, Y. Effects of salvianolic acid B and tanshinone IIA on the pharmacokinetics of losartan in rats by regulating the activities and expression of CYP3A4 and CYP2C9. J. Ethnopharmacol. 2016, 180, 87–96. [Google Scholar] [CrossRef]
- Li, Z.; Wang, G.; Wang, L.S.; Zhang, W.; Tan, Z.R.; Fan, L.; Chen, B.L.; Li, Q.; Liu, J.; Tu, J.H.; et al. Effects of the CYP2C9*13 allele on the pharmacokinetics of losartan in healthy male subjects. Xenobiotica 2009, 39, 788–793. [Google Scholar] [CrossRef]
- Al-Jenoobi, F.I.; Ahad, A.; Mahrous, G.M.; Al-Mohizea, A.M.; AlKharfy, K.M.; Al-Suwayeh, S.A. Effects of fenugreek, garden cress, and black seed on theophylline pharmacokinetics in beagle dogs. Pharm. Biol. 2015, 53, 296–300. [Google Scholar] [CrossRef]
- Ahmad, A.; Jan, B.L.; Raish, M.; Alkharfy, K.M.; Ahad, A.; Khan, A.; Ganaie, M.A.; Hamidaddin, M.A.A. Inhibitory effects of Lepidium sativum polysaccharide extracts on TNF-alpha production in Escherichia coli-stimulated mouse. 3 Biotech 2018, 8, 286. [Google Scholar] [CrossRef]
- Prajapati, V.D.; Maheriya, P.M.; Jani, G.K.; Patil, P.D.; Patel, B.N. Lepidium sativum Linn.: A current addition to the family of mucilage and its applications. Int. J. Biol. Macromol. 2014, 65, 72–80. [Google Scholar] [CrossRef]
- Chatoui, K.; Harhar, H.; El Kamli, T.; Tabyaoui, M. Chemical Composition and Antioxidant Capacity of Lepidium sativum Seeds from Four Regions of Morocco. Evid. Based Complement Alternat. Med. 2020, 2020, 7302727. [Google Scholar] [CrossRef]
- Ahmad, A.; Nabi, R.; Mishra, A.; Ahmad, I.Z. A Panoramic Review on Lepidium sativum L. Bioactives as Prospective Therapeutics. Drug Res. 2021, 71, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Bin Jardan, Y.A.; Ahad, A.; Raish, M.; Alam, M.A.; Al-Mohizea, A.M.; Al-Jenoobi, F.I. Effects of garden cress, fenugreek and black seed on the pharmacodynamics of metoprolol: An herb-drug interaction study in rats with hypertension. Pharm. Biol. 2021, 59, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, F.Y.; Aleanizy, F.S.; Mahmoud, A.Z.; Farshori, N.N.; Alfaraj, R.; Al-Sheddi, E.S.; Alsarra, I.A. Chemical composition and antimicrobial, antioxidant, and anti-inflammatory activities of Lepidium sativum seed oil. Saudi J. Biol. Sci. 2019, 26, 1089–1092. [Google Scholar] [CrossRef] [PubMed]
- Attia, E.S.; Amer, A.H.; Hasanein, M.A. The hypoglycemic and antioxidant activities of garden cress (Lepidium sativum L.) seed on alloxan-induced diabetic male rats. Nat. Prod. Res. 2019, 33, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Jouad, H.; Haloui, M.; Rhiouani, H.; El Hilaly, J.; Eddouks, M. Ethnobotanical survey of medicinal plants used for the treatment of diabetes, cardiac and renal diseases in the North centre region of Morocco (Fez-Boulemane). J. Ethnopharmacol. 2001, 77, 175–182. [Google Scholar] [CrossRef]
- Balgoon, M.J. Assessment of the Protective Effect of Lepidium sativum against Aluminum-Induced Liver and Kidney Effects in Albino Rat. Biomed. Res. Int. 2019, 2019, 4516730. [Google Scholar] [CrossRef]
- Al-Mohizea, A.M.; Ahad, A.; El-Maghraby, G.M.; Al-Jenoobi, F.I.; AlKharfy, K.M.; Al-Suwayeh, S.A. Effects of Nigella sativa, Lepidium sativum and Trigonella foenum-graecum on sildenafil disposition in beagle dogs. Eur. J. Drug Metab. Pharmacokinet. 2015, 40, 219–224. [Google Scholar] [CrossRef]
- Alkharfy, K.M.; Al-Jenoobi, F.I.; Al-Mohizea, A.M.; Al-Suwayeh, S.A.; Khan, R.M.; Ahmad, A. Effects of Lepidium sativum, Nigella sativa and Trigonella foenum-graceum on phenytoin pharmacokinetics in beagle dogs. Phytother. Res. 2013, 27, 1800–1804. [Google Scholar] [CrossRef]
- Alkharfy, K.M.; Al-Jenoobi, F.I.; Alam, M.A.; Al-Mohizea, A.M.; Al-Suwayeh, S.A.; Korashy, H.M.; Khan, R.M.; Muzaffar, I. Lepidium sativum but not Nigella sativa affects carbamazepine disposition in an animal model. Drug Metab. Lett. 2013, 7, 47–51. [Google Scholar] [CrossRef]
- Karlowicz-Bodalska, K.; Han, S.; Freier, J.; Smolenski, M.; Bodalska, A. Curcuma Longa as Medicinal Herb in the Treatment of Diabet- Ic Complications. Acta Pol. Pharm. 2017, 74, 605–610. [Google Scholar]
- Jurenka, J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. 2009, 14, 141–153. [Google Scholar]
- Vaughn, A.R.; Branum, A.; Sivamani, R.K. Effects of Turmeric (Curcuma longa) on Skin Health: A Systematic Review of the Clinical Evidence. Phytother. Res. 2016, 30, 1243–1264. [Google Scholar] [CrossRef]
- Ayati, Z.; Ramezani, M.; Amiri, M.S.; Moghadam, A.T.; Rahimi, H.; Abdollahzade, A.; Sahebkar, A.; Emami, S.A. Ethnobotany, Phytochemistry and Traditional Uses of Curcuma spp. and Pharmacological Profile of Two Important Species (C. longa and C. zedoaria): A Review. Curr. Pharm. Des. 2019, 25, 871–935. [Google Scholar] [CrossRef]
- Akbik, D.; Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Curcumin as a wound healing agent. Life Sci. 2014, 116, 1–7. [Google Scholar] [CrossRef]
- Vatsavai, L.K.; Kilari, E.K. Influence of curcumin on the pharmacodynamics and pharmacokinetics of gliclazide in animal models. J. Exp. Pharmacol. 2016, 8, 69–76. [Google Scholar] [CrossRef]
- Liu, A.C.; Zhao, L.X.; Xing, J.; Liu, T.; Du, F.Y.; Lou, H.X. Pre-treatment with curcumin enhances plasma concentrations of losartan and its metabolite EXP3174 in rats. Biol. Pharm. Bull. 2012, 35, 145–150. [Google Scholar] [CrossRef]
- Zhang, W.; Tan, T.M.; Lim, L.Y. Impact of curcumin-induced changes in P-glycoprotein and CYP3A expression on the pharmacokinetics of peroral celiprolol and midazolam in rats. Drug Metab. Dispos. 2007, 35, 110–115. [Google Scholar] [CrossRef]
- Sung, J.H.; Jo, Y.S.; Kim, S.J.; Ryu, J.S.; Kim, M.C.; Ko, H.J.; Sim, S.S. Effect of Lutein on L-NAME-Induced Hypertensive Rats. Korean J. Physiol. Pharmacol. 2013, 17, 339–345. [Google Scholar] [CrossRef]
- Adaramoye, O.A.; Nwosu, I.O.; Farombi, E.O. Sub-acute effect of N(G)-nitro-l-arginine methyl-ester (L-NAME) on biochemical indices in rats: Protective effects of Kolaviron and extract of Curcuma longa L. Pharmacogn. Res. 2012, 4, 127–133. [Google Scholar] [CrossRef]
- Ahad, A.; Aqil, M.; Kohli, K.; Sultana, Y.; Mujeeb, M. Nano vesicular lipid carriers of angiotensin II receptor blocker: Anti-hypertensive and skin toxicity study in focus. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1002–1007. [Google Scholar] [CrossRef]
- Ahad, A.; Al-Saleh, A.A.; Al-Mohizea, A.M.; Al-Jenoobi, F.I.; Raish, M.; Yassin, A.E.B.; Alam, M.A. Pharmacodynamic study of eprosartan mesylate-loaded transfersomes Carbopol((R)) gel under Dermaroller((R)) on rats with methyl prednisolone acetate-induced hypertension. Biomed. Pharmacother. 2017, 89, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A.; Raish, M.; Al-Jenoobi, F.I.; Al-Mohizea, A.M. Sorbitane Monostearate and Cholesterol based Niosomes for Oral Delivery of Telmisartan. Curr. Drug Deliv. 2018, 15, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A.; Raish, M.; Bin Jardan, Y.A.; Alam, M.A.; Al-Mohizea, A.M.; Al-Jenoobi, F.I. Effect of Hibiscus sabdariffa and Zingiber officinale on the antihypertensive activity and pharmacokinetic of losartan in hypertensive rats. Xenobiotica 2020, 50, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A.; Raish, M.; Bin Jardan, Y.A.; Alam, M.A.; Al-Mohizea, A.M.; Al-Jenoobi, F.I. Potential pharmacodynamic and pharmacokinetic interactions of Nigella Sativa and Trigonella Foenum-graecum with losartan in L-NAME induced hypertensive rats. Saudi J. Biol. Sci. 2020, 27, 2544–2550. [Google Scholar] [CrossRef] [PubMed]
- Raish, M.; Ahmad, A.; Alkharfy, K.M.; Ahamad, S.R.; Mohsin, K.; Al-Jenoobi, F.I.; Al-Mohizea, A.M.; Ansari, M.A. Hepatoprotective activity of Lepidium sativum seeds against D-galactosamine/lipopolysaccharide induced hepatotoxicity in animal model. BMC Complement. Altern. Med. 2016, 16, 501. [Google Scholar] [CrossRef] [PubMed]
- Al-Jenoobi, F.I.; Korashy, H.M.; Ahad, A.; Raish, M.; Al-Mohizea, A.M.; Alam, M.A.; Al-Suwayeh, S.A.; Alkharfy, K.M. Potential inhibitory effect of herbal medicines on rat hepatic cytochrome P450 2D gene expression and metabolic activity. Pharmazie 2014, 69, 799–803. [Google Scholar]
- Nahar, S.; Akhter, Q.S. Effect of Curcuma longa (Turmeric) on serum creatine kinase-MB and troponin I in isoproterenol induced myocardial infarction in wistar albino rats. J. Bangladesh Soc. Physiol. 2018, 13, 47–53. [Google Scholar] [CrossRef]
- Olatunde, A.; Joel, E.B.; Tijjani, H.; Obidola, S.M.; Luka, C.D. Anti-diabetic activity of aqueous extract of curcuma longa (linn) rhizome in normal and alloxan-induced diabetic rats. Researcher 2014, 6, 58–65. [Google Scholar]
- Rai, P.K.; Jaiswal, D.; Mehta, S.; Rai, D.K.; Sharma, B.; Watal, G. Effect of curcuma longa freeze dried rhizome powder with milk in stz induced diabetic rats. Indian J. Clin. Biochem. 2010, 25, 175–181. [Google Scholar] [CrossRef][Green Version]
- Alam, M.A.; Abou Obaid, N.I.; Ibrahim, M.A.; Raish, M.; Al-Jenoobi, F.I. A Validated Ultra-Performance Liquid Chromatography Tandem Triple Quadrupole Mass Spectrometric Method for Fast Determination of Losartan in Rabbit Plasma. J. Chromatogr. Sci. 2019, 57, 323–330. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, W.; Huang, C.K.; Wang, L.; Xia, M.M.; Cui, X.; Hu, G.X.; Wang, Z.S. Inhibitory effects of curcumin on activity of cytochrome P450 2C9 enzyme in human and 2C11 in rat liver microsomes. Drug Dev. Ind. Pharm. 2015, 41, 613–616. [Google Scholar] [CrossRef]
- Hasimun, P.; Sulaeman, A.; Hidayatullah, A.; Mulyani, Y. Effect of curcuma longa L. extract on noninvasive cardiovascular biomarkers in hypertension animal models. J. Appl. Pharm. Sci. 2021, 11, 085–089. [Google Scholar]
- Hasimu, P.; Mulyani, Y.; Yulianti, I. Antihypertensive activity and acute toxicity of turmeric (Curcuma longa L.) in L-NAME-induced hypertension animals. Curr. Res. Biosci. Biotechnol. 2022, 4, 246–250. [Google Scholar]
- Yao, Y.; Wang, W.; Li, M.; Ren, H.; Chen, C.; Wang, J.; Wang, W.E.; Yang, J.; Zeng, C. Curcumin Exerts its Anti-hypertensive Effect by Down-regulating the AT1 Receptor in Vascular Smooth Muscle Cells. Sci. Rep. 2016, 6, 25579. [Google Scholar] [CrossRef]
- Maghrani, M.; Zeggwagh, N.A.; Michel, J.B.; Eddouks, M. Antihypertensive effect of Lepidium sativum L. in spontaneously hypertensive rats. J. Ethnopharmacol. 2005, 100, 193–197. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahad, A.; Raish, M.; Abdelrahman, I.A.; Jardan, Y.A.B.; Alam, M.A.; Al-Mohizea, A.M.; Al-Jenoobi, F.I. Changes in Pharmacokinetics and Pharmacodynamics of Losartan in Experimental Diseased Rats Treated with Curcuma longa and Lepidium sativum. Pharmaceuticals 2023, 16, 33. https://doi.org/10.3390/ph16010033
Ahad A, Raish M, Abdelrahman IA, Jardan YAB, Alam MA, Al-Mohizea AM, Al-Jenoobi FI. Changes in Pharmacokinetics and Pharmacodynamics of Losartan in Experimental Diseased Rats Treated with Curcuma longa and Lepidium sativum. Pharmaceuticals. 2023; 16(1):33. https://doi.org/10.3390/ph16010033
Chicago/Turabian StyleAhad, Abdul, Mohammad Raish, Ibrahim Abdelsalam Abdelrahman, Yousef A. Bin Jardan, Mohd Aftab Alam, Abdullah M. Al-Mohizea, and Fahad I. Al-Jenoobi. 2023. "Changes in Pharmacokinetics and Pharmacodynamics of Losartan in Experimental Diseased Rats Treated with Curcuma longa and Lepidium sativum" Pharmaceuticals 16, no. 1: 33. https://doi.org/10.3390/ph16010033
APA StyleAhad, A., Raish, M., Abdelrahman, I. A., Jardan, Y. A. B., Alam, M. A., Al-Mohizea, A. M., & Al-Jenoobi, F. I. (2023). Changes in Pharmacokinetics and Pharmacodynamics of Losartan in Experimental Diseased Rats Treated with Curcuma longa and Lepidium sativum. Pharmaceuticals, 16(1), 33. https://doi.org/10.3390/ph16010033








