An Acid-Sensitive Bone Targeting Delivery System Carrying Acacetin Prevents Osteoporosis in Ovariectomized Mice
Abstract
1. Introduction
2. Results
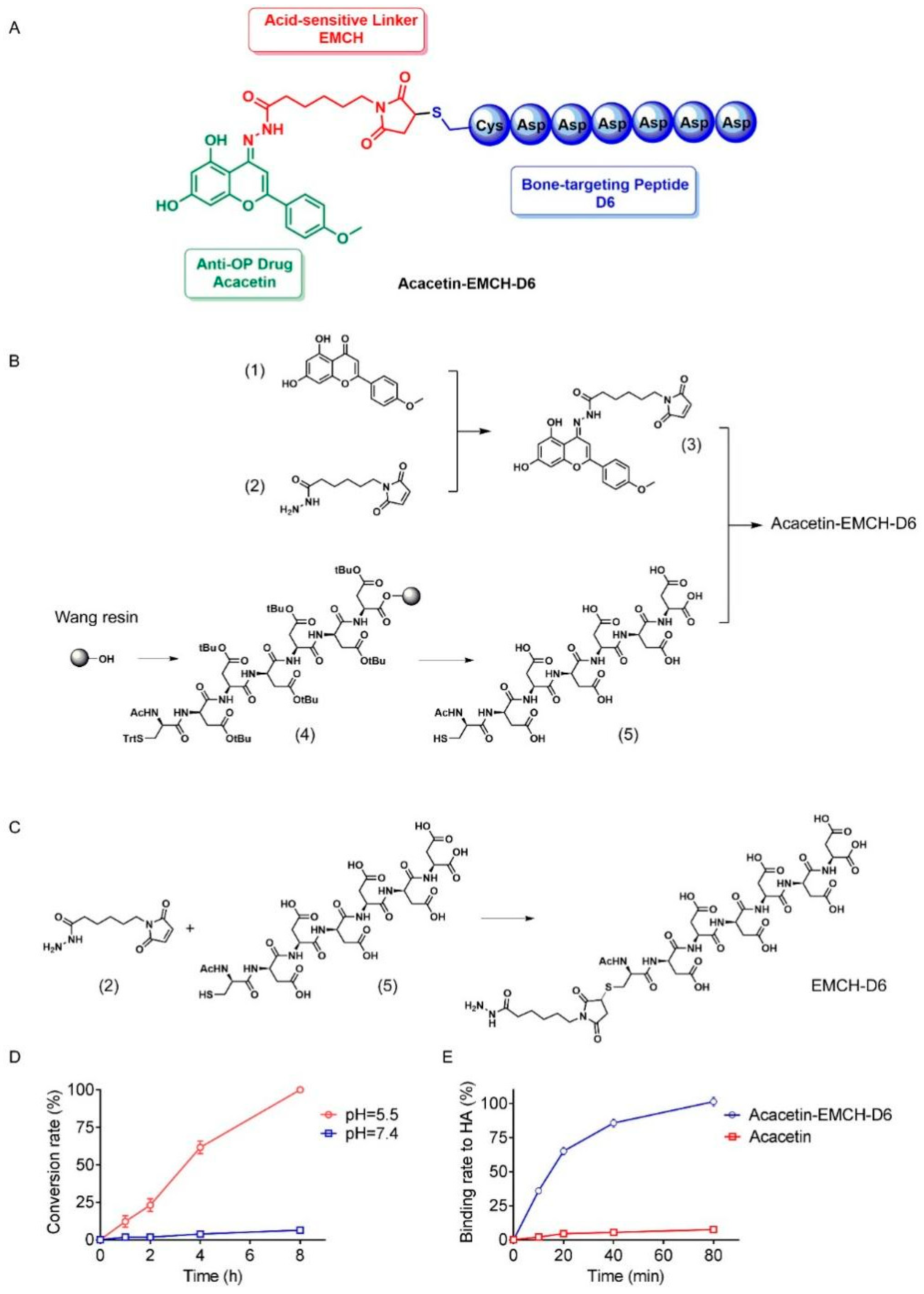
2.1. Design and Chemical Synthesis of Acacetin-EMCH-D6
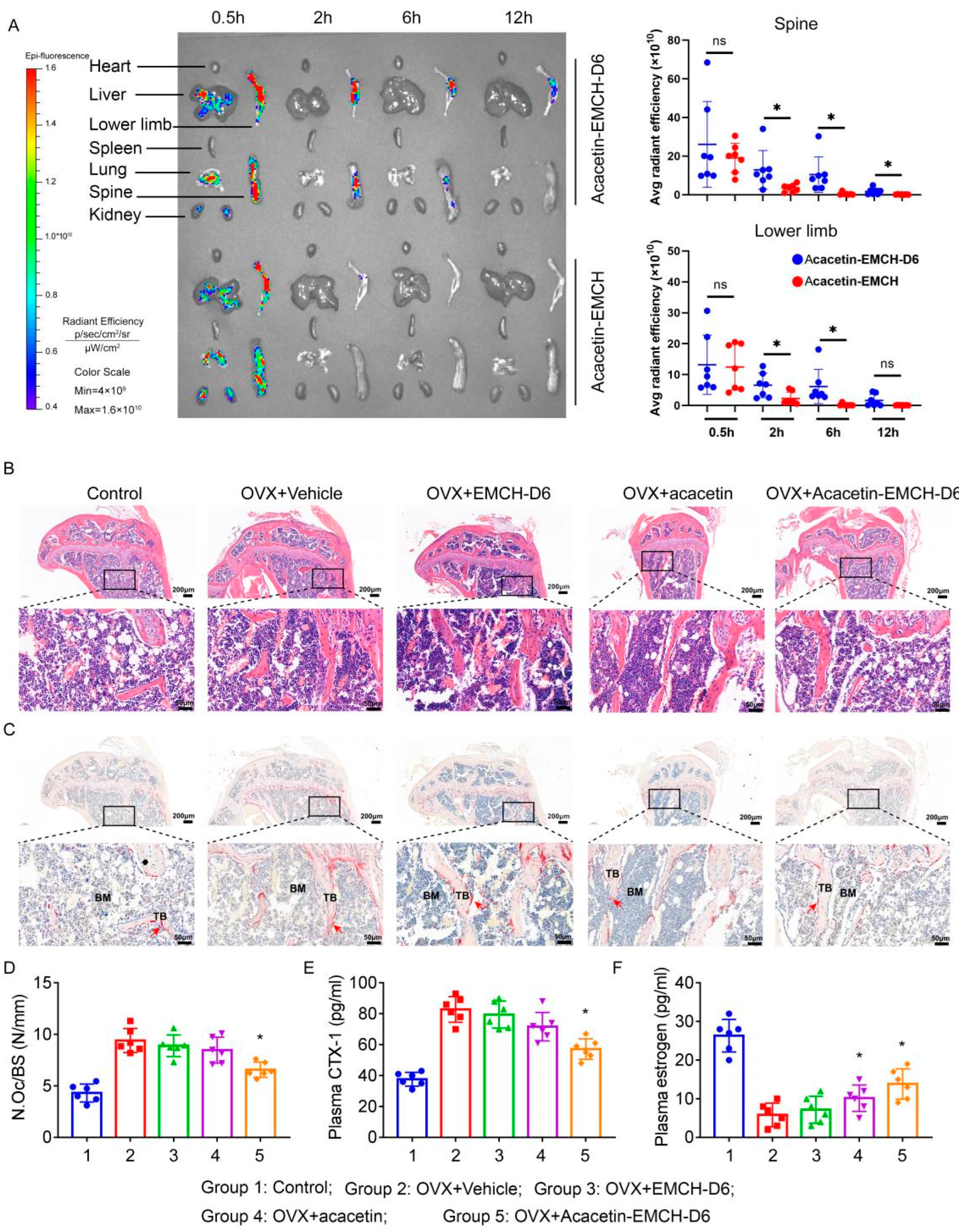
2.2. Drug Release Properties and Hydroxyapatite Affinity of Acacetin-EMCH-D6
2.3. Acacetin Inhibited Osteoclast Differentiation and Bone Resorption
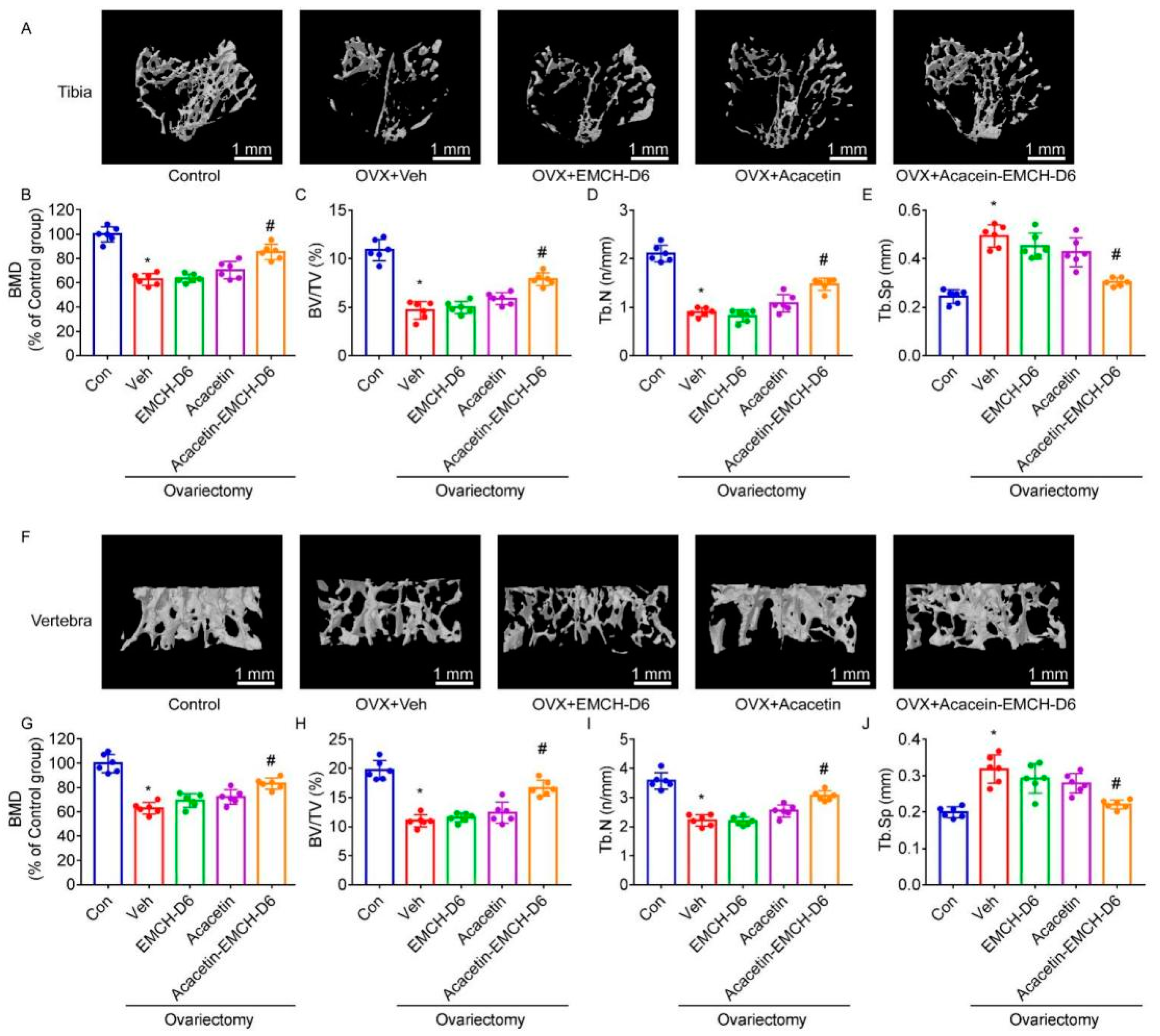
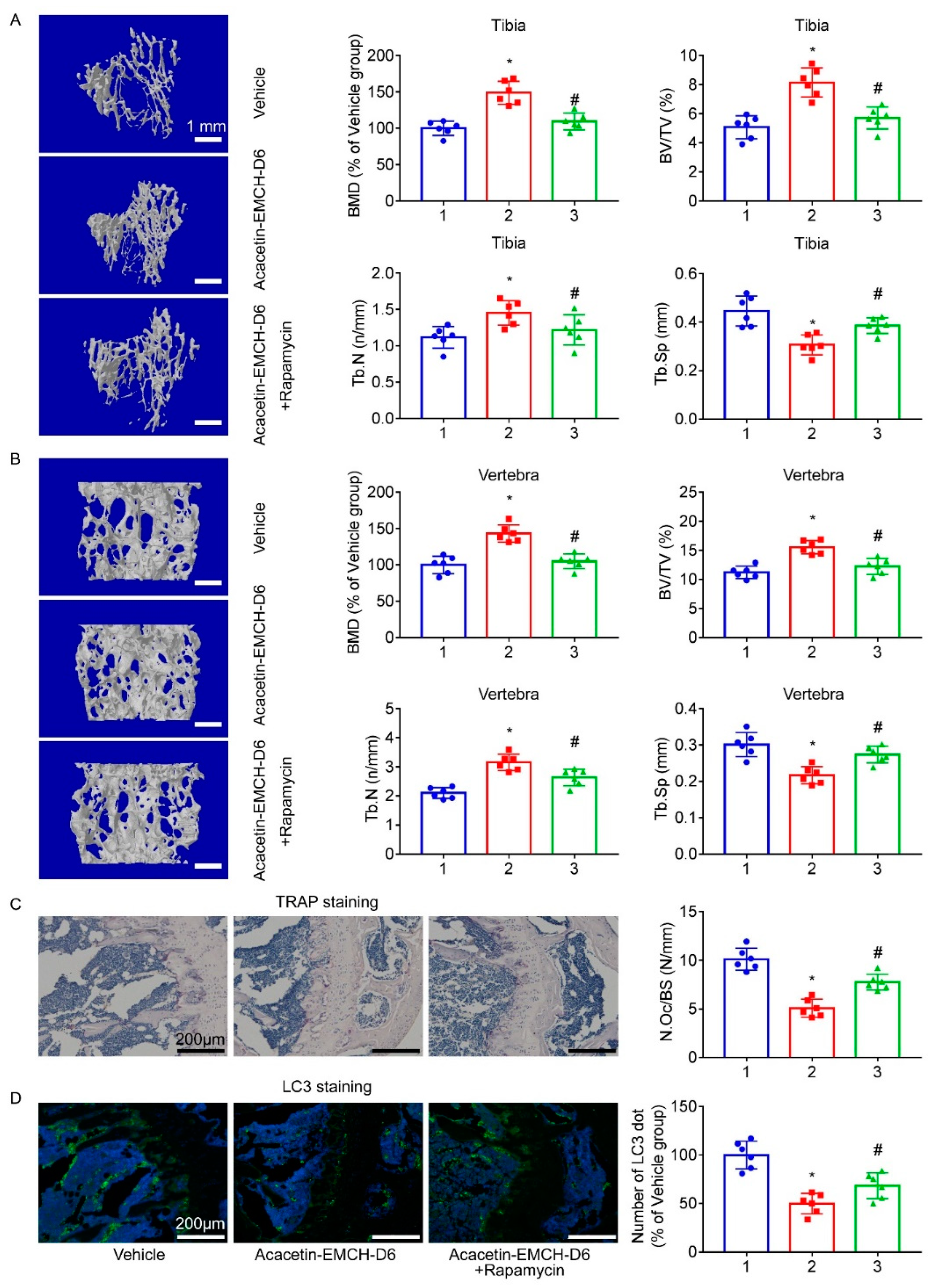
2.4. Acacetin-EMCH-D6 Treatment Inhibited OVX-Induced Bone Loss in Mice
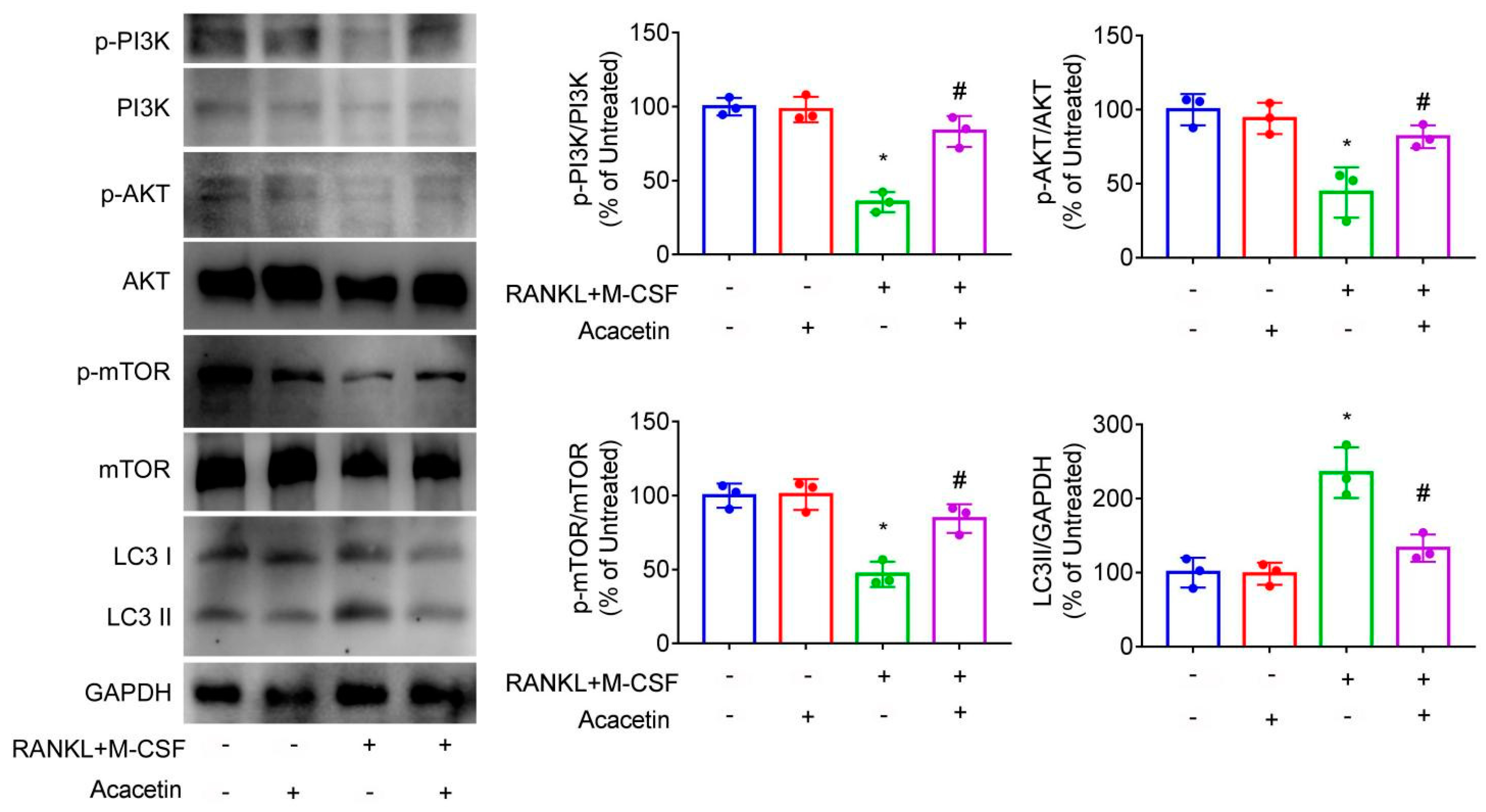
2.5. Acacetin Inhibited Autophagy in Osteoclast and Rapamycin Partly Abolished the Beneficial Effect of Acacetin on Osteoclast Differentiation and Bone Resorption
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Acacetin-EMCH-D6
4.3. pH-Dependent Drug Release Study
4.4. Hydroxyapatite (HA) Binding Assay
4.5. Bone Marrow-Derived Macrophages (BMMs)
4.6. Cytotoxicity Assay
4.7. Tartrate-Resistant Acid Phosphatase (TRAP) Staining
4.8. Bone Resorption Assay
4.9. Real-Time Quantitative PCR (RT-PCR)
4.10. Immunoblotting Assays
4.11. Animals
4.12. Construction of the OVX Mouse Model and Drug Treatment
4.13. Micro-Computed Tomography (μ. CT)
4.14. Histological Analysis
4.15. Biophotonic Imaging Analysis
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, J.P. Long-Term Treatment of Postmenopausal Osteoporosis. Endocrinol. Metab. 2021, 36, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.R.; Wardell, S.E.; McDonnell, D.P. The molecular mechanisms underlying the pharmacological actions of estrogens, SERMs and oxysterols: Implications for the treatment and prevention of osteoporosis. Bone 2013, 53, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Kulkoyluoglu-Cotul, E.; Arca, A.; Madak-Erdogan, Z. Crosstalk between Estrogen Signaling and Breast Cancer Metabolism. Trends Endocrinol. Metab. 2019, 30, 25–38. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Yang, H.; Zhang, Q.; Liu, C.; Zhao, J.; Zhang, L.; Chen, B. Natural products for treatment of osteoporosis: The effects and mechanisms on promoting osteoblast-mediated bone formation. Life Sci. 2016, 147, 46–58. [Google Scholar] [CrossRef]
- Chang, W.; Wu, Q.Q.; Xiao, Y.; Jiang, X.H.; Yuan, Y.; Zeng, X.F.; Tang, Q.Z. Acacetin protects against cardiac remodeling after myocardial infarction by mediating MAPK and PI3K/Akt signal pathway. J. Pharmacol. Sci. 2017, 135, 156–163. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, P.; Meena, A.; Luqman, S. Acacetin, a flavone with diverse therapeutic potential in cancer, inflammation, infections and other metabolic disorders. Food Chem. Toxicol. 2020, 145, 111708. [Google Scholar] [CrossRef]
- Wu, C.; Yan, J.; Li, W. Acacetin as a Potential Protective Compound against Cardiovascular Diseases. Evid Based Complement. Altern. Med. 2022, 2022, 6265198. [Google Scholar] [CrossRef]
- Jin, M.; Nie, J.; Zhu, J.; Li, J.; Fang, T.; Xu, J.; Jiang, X.; Chen, Z.; Li, J.; Wu, F. Acacetin inhibits RANKL-induced osteoclastogenesis and LPS-induced bone loss by modulating NFATc1 transcription. Biochem. Biophys. Res. Commun. 2021, 583, 146–153. [Google Scholar] [CrossRef]
- Kim, S.I.; Kim, Y.H.; Kang, B.G.; Kang, M.K.; Lee, E.J.; Kim, D.Y.; Oh, H.; Oh, S.Y.; Na, W.; Lim, S.S.; et al. Linarin and its aglycone acacetin abrogate actin ring formation and focal contact to bone matrix of bone-resorbing osteoclasts through inhibition of αvβ3 integrin and core-linked CD44. Phytomedicine 2020, 79, 153351. [Google Scholar] [CrossRef]
- Wang, X.; Perumalsamy, H.; Kwon, H.W.; Na, Y.E.; Ahn, Y.J. Effects and possible mechanisms of action of acacetin on the behavior and eye morphology of Drosophila models of Alzheimer’s disease. Sci. Rep. 2015, 5, 16127. [Google Scholar] [CrossRef]
- Lin, X.; Xu, F.; Zhang, K.W.; Qiu, W.X.; Zhang, H.; Hao, Q.; Li, M.; Deng, X.N.; Tian, Y.; Chen, Z.H.; et al. Acacetin Prevents Bone Loss by Disrupting Osteoclast Formation and Promoting Type H Vessel Formation in Ovariectomy-Induced Osteoporosis. Front Cell Dev. Biol. 2022, 10, 796227. [Google Scholar] [CrossRef] [PubMed]
- Negishi-Koga, T.; Takayanagi, H. Ca2+-NFATc1 signaling is an essential axis of osteoclast differentiation. Immunol. Rev. 2009, 231, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Grigoriadis, A.E.; Wang, Z.Q.; Cecchini, M.G.; Hofstetter, W.; Felix, R.; Fleisch, H.A.; Wagner, E.F. c-Fos: A key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science 1994, 266, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Hochdörffer, K.; Abu Ajaj, K.; Schäfer-Obodozie, C.; Kratz, F. Development of novel bisphosphonate prodrugs of doxorubicin for targeting bone metastases that are cleaved pH dependently or by cathepsin B: Synthesis, cleavage properties, and binding properties to hydroxyapatite as well as bone matrix. J. Med. Chem. 2012, 55, 7502–7515. [Google Scholar] [CrossRef] [PubMed]
- Leriche, G.; Chisholm, L.; Wagner, A. Cleavable linkers in chemical biology. Bioorg. Med. Chem. 2012, 20, 571–582. [Google Scholar] [CrossRef]
- Bao, X.; Lee, S.C.; Reuss, L.; Altenberg, G.A. Change in permeant size selectivity by phosphorylation of connexin 43 gap-junctional hemichannels by PKC. Proc. Natl. Acad. Sci. USA 2007, 104, 4919–4924. [Google Scholar] [CrossRef]
- Ouertatani-Sakouhi, H.; El-Turk, F.; Fauvet, B.; Cho, M.K.; Pinar Karpinar, D.; Le Roy, D.; Dewor, M.; Roger, T.; Bernhagen, J.; Calandra, T.; et al. Identification and characterization of novel classes of macrophage migration inhibitory factor (MIF) inhibitors with distinct mechanisms of action. J. Biol. Chem. 2010, 285, 26581–26598. [Google Scholar] [CrossRef]
- Joyce, J.; Cook, J.; Chabot, D.; Hepler, R.; Shoop, W.; Xu, Q.; Stambaugh, T.; Aste-Amezaga, M.; Wang, S.; Indrawati, L.; et al. Immunogenicity and protective efficacy of Bacillus anthracis poly-gamma-D-glutamic acid capsule covalently coupled to a protein carrier using a novel triazine-based conjugation strategy. J. Biol. Chem. 2006, 281, 4831–4843. [Google Scholar] [CrossRef]
- Abu Ajaj, K.; El-Abadla, N.; Welker, P.; Azab, S.; Zeisig, R.; Fichtner, I.; Kratz, F. Comparative evaluation of the biological properties of reducible and acid-sensitive folate prodrugs of a highly potent doxorubicin derivative. Eur. J. Cancer 2012, 48, 2054–2065. [Google Scholar] [CrossRef]
- Moktan, S.; Ryppa, C.; Kratz, F.; Raucher, D. A thermally responsive biopolymer conjugated to an acid-sensitive derivative of paclitaxel stabilizes microtubules, arrests cell cycle, and induces apoptosis. Investig. New Drugs 2012, 30, 236–248. [Google Scholar] [CrossRef]
- Dao, K.L.; Sawant, R.R.; Hendricks, J.A.; Ronga, V.; Torchilin, V.P.; Hanson, R.N. Design, synthesis, and initial biological evaluation of a steroidal anti-estrogen-doxorubicin bioconjugate for targeting estrogen receptor-positive breast cancer cells. Bioconjug Chem. 2012, 23, 785–795. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Perrin, D.D. Binding of tetracyclines to bone. Nature 1965, 208, 787–788. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Ebetino, F.H.; Boeckman, R.K., Jr.; Srinivasan, V.; Tao, J.; Sawyer, T.K.; Li, J.; Yao, Z.; Boyce, B.F. Targeting anti-cancer agents to bone using bisphosphonates. Bone 2020, 138, 115492. [Google Scholar] [CrossRef] [PubMed]
- Caselli, G.; Mantovanini, M.; Gandolfi, C.A.; Allegretti, M.; Fiorentino, S.; Pellegrini, L.; Melillo, G.; Bertini, R.; Sabbatini, W.; Anacardio, R.; et al. Tartronates: A new generation of drugs affecting bone metabolism. J. Bone Miner. Res. 1997, 12, 972–981. [Google Scholar] [CrossRef]
- Willson, T.M.; Henke, B.R.; Momtahen, T.M.; Myers, P.L.; Sugg, E.E.; Unwalla, R.J.; Croom, D.K.; Dougherty, R.W.; Grizzle, M.K.; Johnson, M.F.; et al. 3-[2-(N-phenylacetamide)]-1,5-benzodiazepines: Orally active, binding selective CCK-A agonists. J. Med. Chem. 1996, 39, 3030–3034. [Google Scholar] [CrossRef]
- Kasugai, S.; Fujisawa, R.; Waki, Y.; Miyamoto, K.; Ohya, K. Selective drug delivery system to bone: Small peptide (Asp)6 conjugation. J. Bone Miner. Res. 2000, 15, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ye, X.; Cai, M.; Liu, X.; Xiao, J.; Zhang, C.; Wang, Y.; Yang, L.; Liu, J.; Li, S.; et al. Osteoblast-Targeting-Peptide Modified Nanoparticle for siRNA/microRNA Delivery. ACS Nano. 2016, 10, 5759–5768. [Google Scholar] [CrossRef]
- Nowakowski, G.S.; Dooner, M.S.; Valinski, H.M.; Mihaliak, A.M.; Quesenberry, P.J.; Becker, P.S. A specific heptapeptide from a phage display peptide library homes to bone marrow and binds to primitive hematopoietic stem cells. Stem Cells. 2004, 22, 1030–1038. [Google Scholar] [CrossRef]
- Fujisawa, R.; Tamura, M. Acidic bone matrix proteins and their roles in calcification. Front. Biosci. 2012, 17, 1891–1903. [Google Scholar] [CrossRef]
- Stone, T.A.; Deber, C.M. Therapeutic design of peptide modulators of protein-protein interactions in membranes. Biochim. Biophys. Acta Biomembr. 2017, 1859, 577–585. [Google Scholar] [CrossRef]
- Montaseri, A.; Giampietri, C.; Rossi, M.; Riccioli, A.; Del Fattore, A.; Filippini, A. The Role of Autophagy in Osteoclast Differentiation and Bone Resorption Function. Biomolecules 2020, 10, 1398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cui, Y.; Wang, L.; Han, J. Autophagy promotes osteoclast podosome disassembly and cell motility athrough the interaction of kindlin3 with LC3. Cell Signal. 2020, 67, 109505. [Google Scholar] [CrossRef] [PubMed]
- Arai, A.; Kim, S.; Goldshteyn, V.; Kim, T.; Park, N.H.; Wang, C.Y.; Kim, R.H. Beclin1 Modulates Bone Homeostasis by Regulating Osteoclast and Chondrocyte Differentiation. J. Bone Miner. Res. 2019, 34, 1753–1766. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wu, W.; Sun, X.; Zhang, P. Glucocorticoids Enhanced Osteoclast Autophagy Through the PI3K/Akt/mTOR Signaling Pathway. Calcif. Tissue Int. 2020, 107, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Lotinun, S.; Kiviranta, R.; Matsubara, T.; Alzate, J.A.; Neff, L.; Lüth, A.; Koskivirta, I.; Kleuser, B.; Vacher, J.; Vuorio, E.; et al. Osteoclast-specific cathepsin K deletion stimulates S1P-dependent bone formation. J. Clin. Invest. 2013, 123, 666–681. [Google Scholar] [CrossRef]
- Dai, Q.; Xie, F.; Han, Y.; Ma, X.; Zhou, S.; Jiang, L.; Zou, W.; Wang, J. Inactivation of Regulatory-associated Protein of mTOR (Raptor)/Mammalian Target of Rapamycin Complex 1 (mTORC1) Signaling in Osteoclasts Increases Bone Mass by Inhibiting Osteoclast Differentiation in Mice. J. Biol. Chem. 2017, 292, 196–204. [Google Scholar] [CrossRef]
- Shen, G.; Ren, H.; Shang, Q.; Zhao, W.; Zhang, Z.; Yu, X.; Tang, K.; Tang, J.; Yang, Z.; Liang, D.; et al. Foxf1 knockdown promotes BMSC osteogenesis in part by activating the Wnt/β-catenin signalling pathway and prevents ovariectomy-induced bone loss. EBioMedicine 2020, 52, 102626. [Google Scholar] [CrossRef]
- Martin, S.A.; Riordan, R.T.; Wang, R.; Yu, Z.; Aguirre-Burk, A.M.; Wong, C.P.; Olson, D.A.; Branscum, A.J.; Turner, R.T.; Iwaniec, U.T.; et al. Rapamycin impairs bone accrual in young adult mice independent of Nrf2. Exp. Gerontol. 2021, 154, 111516. [Google Scholar] [CrossRef]
- Li, X.; Yang, J.; Liu, D.; Li, J.; Niu, K.; Feng, S.; Yokota, H.; Zhang, P. Knee loading inhibits osteoclast lineage in a mouse model of osteoarthritis. Sci. Rep. 2016, 6, 24668. [Google Scholar] [CrossRef]


| Genes | Sequence (5′–3′) | |
|---|---|---|
| NFATc1 | Sense Antisense | CCGTTGCTTCCAGAAAATAACA TGTGGGATGTGAACTCGGAA |
| CTSK | Sense Antisense | CTTCCAATACGTGCAGCAGA TCTTCAGGGCTTTCTCGTTC |
| TRAP | Sense Antisense | TCCTGGCTCAAAAAGCAGTT ACATAGCCCACACCGTTCTC |
| GAPDH | Sense Antisense | ACCCAGAAGACTGTGGATGG CACATTGGGGGTAGGAACAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Song, C.; Zhang, C.; Xing, C.; Lv, J.; Bian, H.; Lv, N.; Chen, D.; Dong, X.; Liu, M.; et al. An Acid-Sensitive Bone Targeting Delivery System Carrying Acacetin Prevents Osteoporosis in Ovariectomized Mice. Pharmaceuticals 2023, 16, 2. https://doi.org/10.3390/ph16010002
Sun X, Song C, Zhang C, Xing C, Lv J, Bian H, Lv N, Chen D, Dong X, Liu M, et al. An Acid-Sensitive Bone Targeting Delivery System Carrying Acacetin Prevents Osteoporosis in Ovariectomized Mice. Pharmaceuticals. 2023; 16(1):2. https://doi.org/10.3390/ph16010002
Chicago/Turabian StyleSun, Xiaochen, Chenyu Song, Chenxi Zhang, Chunlei Xing, Juan Lv, Huihui Bian, Nanning Lv, Dagui Chen, Xin Dong, Mingming Liu, and et al. 2023. "An Acid-Sensitive Bone Targeting Delivery System Carrying Acacetin Prevents Osteoporosis in Ovariectomized Mice" Pharmaceuticals 16, no. 1: 2. https://doi.org/10.3390/ph16010002
APA StyleSun, X., Song, C., Zhang, C., Xing, C., Lv, J., Bian, H., Lv, N., Chen, D., Dong, X., Liu, M., & Su, L. (2023). An Acid-Sensitive Bone Targeting Delivery System Carrying Acacetin Prevents Osteoporosis in Ovariectomized Mice. Pharmaceuticals, 16(1), 2. https://doi.org/10.3390/ph16010002







