The Role of Zinc and NMDA Receptors in Autism Spectrum Disorders
Abstract
1. Introduction
1.1. NMDAR Subunit Composition and Functional Consequences
1.2. NMDARs and Synaptic Plasticity
1.3. Zinc and NMDARs
2. Pathological Effects of ASDs on NMDARs
2.1. Role of SHANKs with NMDARs in ASDs
2.2. SHANK-Independent Regulation of NMDARs in ASDs
2.3. NMDARs in Non-Genetic ASD Models
3. Effects of Zinc on NMDARs in ASDs
4. Therapeutic Potential of Zinc in Humans
5. Limitations and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohn, A.R.; Gainetdinov, R.R.; Caron, M.G.; Koller, B.H. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell 1999, 98, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.P.; Shimizu, E.; Dube, G.R.; Rampon, C.; Kerchner, G.A.; Zhuo, M.; Liu, G.; Tsien, J.Z. Genetic enhancement of learning and memory in mice. Nature 1999, 401, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Dingledine, R.; Borges, K.; Bowie, D.; Traynelis, S.F. The glutamate receptor ion channels. Pharmacol. Rev. 1999, 51, 7–61. [Google Scholar] [PubMed]
- Collingridge, G.L.; Bliss, T.V.P. NMDA Receptors—Their Role in Long-Term Potentiation. Trends Neurosci. 1987, 10, 288–293. [Google Scholar] [CrossRef]
- Burnashev, N.; Schoepfer, R.; Monyer, H.; Ruppersberg, J.P.; Gunther, W.; Seeburg, P.H.; Sakmann, B. Control by Asparagine Residues of Calcium Permeability and Magnesium Blockade in the NMDA Receptor. Science 1992, 257, 1415–1419. [Google Scholar] [CrossRef]
- Benveniste, M.; Mayer, M.L. Kinetic-Analysis of Antagonist Action at N-Methyl-D-Aspartic Acid Receptors—2 Binding-Sites Each for Glutamate and Glycine. Biophys. J. 1991, 59, 560–573. [Google Scholar] [CrossRef]
- Kuryatov, A.; Laube, B.; Betz, H.; Kuhse, J. Mutational Analysis of the Glycine-Binding Site of the NMDA Receptor—Structural Similarity with Bacterial Amino Acid-Binding Proteins. Neuron 1994, 12, 1291–1300. [Google Scholar] [CrossRef]
- Anson, L.C.; Chen, P.E.; Wyllie, D.J.A.; Colquhoun, D.; Schoepfer, R. Identification of amino acid residues of the NR2A subunit that control glutamate potency in recombinant NR1/NR2A NMDA receptors. J. Neurosci. 1998, 18, 581–589. [Google Scholar] [CrossRef]
- Mayer, M.L.; Westbrook, G.L.; Guthrie, P.B. Voltage-Dependent Block by Mg-2+ of NMDA Responses in Spinal-Cord Neurons. Nature 1984, 309, 261–263. [Google Scholar] [CrossRef]
- Lester, R.A.J.; Clements, J.D.; Westbrook, G.L.; Jahr, C.E. Channel Kinetics Determine the Time Course of NMDA Receptor-Mediated Synaptic Currents. Nature 1990, 346, 565–567. [Google Scholar] [CrossRef]
- Dzubay, J.A.; Jahr, C.E. Kinetics of NMDA channel opening. J. Neurosci. 1996, 16, 4129–4134. [Google Scholar] [CrossRef]
- Moriyoshi, K.; Masu, M.; Ishii, T.; Shigemoto, R.; Mizuno, N.; Nakanishi, S. Molecular-Cloning and Characterization of the Rat NMDA Receptor. Nature 1991, 354, 31–37. [Google Scholar] [CrossRef]
- Meguro, H.; Mori, H.; Araki, K.; Kushiya, E.; Kutsuwada, T.; Yamazaki, M.; Kumanishi, T.; Arakawa, M.; Sakimura, K.; Mishina, M. Functional-Characterization of a Heteromeric NMDA Receptor Channel Expressed from Cloned cDNAs. Nature 1992, 357, 70–74. [Google Scholar] [CrossRef]
- Ciabarra, A.M.; Sullivan, J.M.; Gahn, L.G.; Pecht, G.; Heinemann, S.; Sevarino, K.A. Cloning and Characterization of Chi-1—A Developmentally-Regulated Member of a Novel Class of the Ionotropic Glutamate-Receptor Family. J. Neurosci. 1995, 15, 6498–6508. [Google Scholar] [CrossRef]
- Paoletti, P. Molecular basis of NMDA receptor functional diversity. Eur. J. Neurosci. 2011, 33, 1351–1365. [Google Scholar] [CrossRef]
- Monyer, H.; Sprengel, R.; Schoepfer, R.; Herb, A.; Higuchi, M.; Lomeli, H.; Burnashev, N.; Sakmann, B.; Seeburg, P.H. Heteromeric NMDA Receptors—Molecular and Functional Distinction of Subtypes. Science 1992, 256, 1217–1221. [Google Scholar] [CrossRef]
- Traynelis, S.F.; Wollmuth, L.P.; McBain, C.J.; Menniti, F.S.; Vance, K.M.; Ogden, K.K.; Hansen, K.B.; Yuan, H.J.; Myers, S.J.; Dingledine, R. Glutamate Receptor Ion Channels: Structure, Regulation, and Function. Pharmacol. Rev. 2010, 62, 405–496. [Google Scholar] [CrossRef]
- Retchless, B.S.; Gao, W.; Johnson, J.W. A single GluN2 subunit residue controls NMDA receptor channel properties via intersubunit interaction. Nat. Neurosci. 2012, 15, 406–413. [Google Scholar] [CrossRef]
- Laurie, D.J.; Seeburg, P.H. Ligand Affinities at Recombinant N-Methyl-D-Aspartate Receptors Depend on Subunit Composition. Eur. J. Pharm. Mol. Pharmacol. 1994, 268, 335–345. [Google Scholar] [CrossRef]
- Watanabe, M.; Inoue, Y.; Sakimura, K.; Mishina, M. Developmental-Changes in Distribution of NMDA Receptor Channel Subunit Messenger-Rnas. Neuroreport 1992, 3, 1138–1140. [Google Scholar] [CrossRef]
- Akazawa, C.; Shigemoto, R.; Bessho, Y.; Nakanishi, S.; Mizuno, N. Differential Expression of 5 N-Methyl-D-Aspartate Receptor Subunit Messenger-RNAs in the Cerebellum of Developing-Rats and Adult-Rats. J. Comp. Neurol. 1994, 347, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Monyer, H.; Burnashev, N.; Laurie, D.J.; Sakmann, B.; Seeburg, P.H. Developmental and Regional Expression in the Rat-Brain and Functional-Properties of 4 NMDA Receptors. Neuron 1994, 12, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Henson, M.A.; Roberts, A.C.; Perez-Otano, I.; Philpot, B.D. Influence of the NR3A subunit on NMDA receptor functions. Prog. Neurobiol. 2010, 91, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Pachernegg, S.; Strutz-Seebohm, N.; Hollmann, M. GluN3 subunit-containing NMDA receptors: Not just one-trick ponies. Trends Neurosci. 2012, 35, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Crair, M.C.; Malenka, R.C. A critical period for long-term potentiation at thalamocortical synapses. Nature 1995, 375, 325–328. [Google Scholar] [CrossRef]
- Hardingham, G.E.; Bading, H. Synaptic versus extrasynaptic NMDA receptor signalling: Implications for neurodegenerative disorders. Nat. Rev. Neurosci. 2010, 11, 682–696. [Google Scholar] [CrossRef]
- Gladding, C.M.; Raymond, L.A. Mechanisms underlying NMDA receptor synaptic/extrasynaptic distribution and function. Mol. Cell. Neurosci. 2011, 48, 308–320. [Google Scholar] [CrossRef]
- Lopez de Armentia, M.; Sah, P. Development and Subunit Composition of Synaptic NMDA Receptors in the Amygdala: NR2B Synapses in the Adult Central Amygdala. J. Neurosci. 2003, 23, 6876–6883. [Google Scholar] [CrossRef]
- Harris, A.Z.; Pettit, D.L. Extrasynaptic and synaptic and uniform pools in rat hi NMDA receptors form stable ppocampal slices. J. Physiol.-Lond. 2007, 584, 509–519. [Google Scholar] [CrossRef]
- Tovar, K.R.; Westbrook, G.L. Mobile NMDA receptors at hippocampal synapses. Neuron 2002, 34, 253–264. [Google Scholar] [CrossRef]
- Groc, L.; Choquet, D. AMPA and NMDA glutamate receptor trafficking: Multiple roads for reaching and leaving the synapse. Cell Tissue Res. 2006, 326, 423–438. [Google Scholar] [CrossRef]
- Erreger, K.; Dravid, S.M.; Banke, T.G.; Wyllie, D.J.; Traynelis, S.F. Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. J. Physiol. 2005, 563, 345–358. [Google Scholar] [CrossRef]
- Vicini, S.; Wang, J.F.; Li, J.H.; Zhu, W.J.; Wang, Y.H.; Luo, J.H.; Wolfe, B.B.; Grayson, D.R. Functional and pharmacological differences between recombinant N-methyl-D-aspartate receptors. J. Neurophysiol. 1998, 79, 555–566. [Google Scholar] [CrossRef]
- Paoletti, P.; Bellone, C.; Zhou, Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013, 14, 383–400. [Google Scholar] [CrossRef]
- Lavezzari, G.; McCallum, J.; Lee, R.; Roche, K.W. Differential binding of the AP-2 adaptor complex and PSD-95 to the C-terminus of the NMDA receptor subunit NR2B regulates surface expression. Neuropharmacology 2003, 45, 729–737. [Google Scholar] [CrossRef]
- Sanz-Clemente, A.; Nicoll, R.A.; Roche, K.W. Diversity in NMDA Receptor Composition: Many Regulators, Many Consequences. Neuroscientist 2013, 19, 62–75. [Google Scholar] [CrossRef]
- Malenka, R.C.; Nicoll, R.A. Long-term potentiation—A decade of progress? Science 1999, 285, 1870–1874. [Google Scholar] [CrossRef]
- Bliss, T.V.; Lomo, T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 1973, 232, 331–356. [Google Scholar] [CrossRef]
- Dudek, S.M.; Bear, M.F. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc. Natl. Acad. Sci. USA 1992, 89, 4363–4367. [Google Scholar] [CrossRef]
- Isaac, J.T.; Nicoll, R.A.; Malenka, R.C. Evidence for silent synapses: Implications for the expression of LTP. Neuron 1995, 15, 427–434. [Google Scholar] [CrossRef]
- Liao, D.; Hessler, N.A.; Malinow, R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature 1995, 375, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, J.M.; Madison, D.V. The grass roots of synapse suppression. Neuron 2001, 29, 567–570. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Allison, D.W.; Gelfand, V.I.; Spector, I.; Craig, A.M. Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: Differential attachment of NMDA versus AMPA receptors. J. Neurosci. 1998, 18, 2423–2436. [Google Scholar] [CrossRef] [PubMed]
- Lissin, D.V.; Gomperts, S.N.; Carroll, R.C.; Christine, C.W.; Kalman, D.; Kitamura, M.; Hardy, S.; Nicoll, R.A.; Malenka, R.C.; von Zastrow, M. Activity differentially regulates the surface expression of synaptic AMPA and NMDA glutamate receptors. Proc. Natl. Acad. Sci. USA 1998, 95, 7097–7102. [Google Scholar] [CrossRef] [PubMed]
- Carroll, R.C.; Lissin, D.V.; von Zastrow, M.; Nicoll, R.A.; Malenka, R.C. Rapid redistribution of glutamate receptors contributes to long-term depression in hippocampal cultures. Nat. Neurosci. 1999, 2, 454–460. [Google Scholar] [CrossRef]
- Clark, K.A.; Collingridge, G.L. Synaptic potentiation of dual-component excitatory postsynaptic currents in the rat hippocampus. J. Physiol. 1995, 482 Pt 1, 39–52. [Google Scholar] [CrossRef]
- Kullmann, D.M. Amplitude fluctuations of dual-component EPSCs in hippocampal pyramidal cells: Implications for long-term potentiation. Neuron 1994, 12, 1111–1120. [Google Scholar] [CrossRef]
- Marino, M.J.; Rouse, S.T.; Levey, A.I.; Potter, L.T.; Conn, P.J. Activation of the genetically defined m1 muscarinic receptor potentiates N-methyl-D-aspartate (NMDA) receptor currents in hippocampal pyramidal cells. Proc. Natl. Acad. Sci. USA 1998, 95, 11465–11470. [Google Scholar] [CrossRef]
- Tyszkiewicz, J.P.; Gu, Z.; Wang, X.; Cai, X.; Yan, Z. Group II metabotropic glutamate receptors enhance NMDA receptor currents via a protein kinase C-dependent mechanism in pyramidal neurones of rat prefrontal cortex. J. Physiol. 2004, 554 Pt 3, 765–777. [Google Scholar] [CrossRef]
- Chen, L.; Huang, L.Y. Protein kinase C reduces Mg2+ block of NMDA-receptor channels as a mechanism of modulation. Nature 1992, 356, 521–523. [Google Scholar] [CrossRef]
- Xiong, Z.G.; Raouf, R.; Lu, W.Y.; Wang, L.Y.; Orser, B.A.; Dudek, E.M.; Browning, M.D.; MacDonald, J.F. Regulation of N-methyl-D-aspartate receptor function by constitutively active protein kinase C. Mol. Pharmacol. 1998, 54, 1055–1063. [Google Scholar] [CrossRef]
- Lan, J.Y.; Skeberdis, V.A.; Jover, T.; Grooms, S.Y.; Lin, Y.; Araneda, R.C.; Zheng, X.; Bennett, M.V.; Zukin, R.S. Protein kinase C modulates NMDA receptor trafficking and gating. Nat. Neurosci. 2001, 4, 382–390. [Google Scholar] [CrossRef]
- Yong, X.L.H.; Zhang, L.; Yang, L.; Chen, X.; Tan, J.Z.A.; Yu, X.; Chandra, M.; Livingstone, E.; Widagdo, J.; Vieira, M.M.; et al. Regulation of NMDA receptor trafficking and gating by activity-dependent CaMKIIα phosphorylation of the GluN2A subunit. Cell Rep. 2021, 36, 109338. [Google Scholar] [CrossRef]
- Montgomery, J.M.; Madison, D.V. State-dependent heterogeneity in synaptic depression between pyramidal cell pairs. Neuron 2002, 33, 765–777. [Google Scholar] [CrossRef]
- Heynen, A.J.; Quinlan, E.M.; Bae, D.C.; Bear, M.F. Bidirectional, activity-dependent regulation of glutamate receptors in the adult hippocampus in vivo. Neuron 2000, 28, 527–536. [Google Scholar] [CrossRef]
- Snyder, E.M.; Philpot, B.D.; Huber, K.M.; Dong, X.; Fallon, J.R.; Bear, M.F. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat. Neurosci. 2001, 4, 1079–1085. [Google Scholar] [CrossRef]
- Roche, K.W.; Standley, S.; McCallum, J.; Dune Ly, C.; Ehlers, M.D.; Wenthold, R.J. Molecular determinants of NMDA receptor internalization. Nat. Neurosci. 2001, 4, 794–802. [Google Scholar] [CrossRef]
- Vissel, B.; Krupp, J.J.; Heinemann, S.F.; Westbrook, G.L. A use-dependent tyrosine dephosphorylation of NMDA receptors is independent of ion flux. Nat. Neurosci. 2001, 4, 587–596. [Google Scholar] [CrossRef]
- Nong, Y.; Huang, Y.Q.; Ju, W.; Kalia, L.V.; Ahmadian, G.; Wang, Y.T.; Salter, M.W. Glycine binding primes NMDA receptor internalization. Nature 2003, 422, 302–307. [Google Scholar] [CrossRef]
- Montgomery, J.M.; Madison, D.V. Discrete synaptic states define a major mechanism of synapse plasticity. Trends Neurosci. 2004, 27, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Assaf, S.Y.; Chung, S.H. Release of Endogenous Zn-2+ from Brain-Tissue during Activity. Nature 1984, 308, 734–736. [Google Scholar] [CrossRef] [PubMed]
- Howell, G.A.; Welch, M.G.; Frederickson, C.J. Stimulation-Induced Uptake and Release of Zinc in Hippocampal Slices. Nature 1984, 308, 736–738. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.L.; Vyklicky, L. The Action of Zinc on Synaptic Transmission and Neuronal Excitability in Cultures of Mouse Hippocampus. J. Physiol.-Lond. 1989, 415, 351–365. [Google Scholar] [CrossRef]
- Smart, T.G.; Xie, X.M.; Krishek, B.J. Modulation of Inhibitory and Excitatory Amino-Acid Receptor-Ion Channels by Zinc. Prog. Neurobiol. 1994, 42, 393–441. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, G.L.; Mayer, M.L. Micromolar Concentrations of Zn-2+ Antagonize NMDA and Gaba Responses of Hippocampal-Neurons. Nature 1987, 328, 640–643. [Google Scholar] [CrossRef]
- Paoletti, P.; Vergnano, A.; Barbour, B.; Casado, M.J.N. Zinc at glutamatergic synapses. Neuroscience 2009, 158, 126–136. [Google Scholar] [CrossRef]
- Palmiter, R.D.; Cole, T.B.; Quaife, C.J.; Findley, S.D. ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc. Natl. Acad. Sci. USA 1996, 93, 14934–14939. [Google Scholar] [CrossRef]
- Wenzel, H.J.; Cole, T.B.; Born, D.E.; Schwartzkroin, P.A.; Palmiter, R.D. Ultrastructural localization of zinc transporter-3 (ZnT-3) to synaptic vesicle membranes within mossy fiber boutons in the hippocampus of mouse and monkey. Proc. Natl. Acad. Sci. USA 1997, 94, 12676–12681. [Google Scholar] [CrossRef]
- Vergnano, A.M.; Rebola, N.; Savtchenko, L.P.; Pinheiro, P.S.; Casado, M.; Kieffer, B.L.; Rusakov, D.A.; Mulle, C.; Paoletti, P.J.N. Zinc dynamics and action at excitatory synapses. Neuron 2014, 82, 1101–1114. [Google Scholar] [CrossRef]
- Williams, K.J. Separating dual effects of zinc at recombinant N-methyl-D-aspartate receptors. Neurosci. Lett. 1996, 215, 9–12. [Google Scholar] [CrossRef]
- Paoletti, P.; Ascher, P.; Neyton, J. High-affinity zinc inhibition of NMDA NR1–NR2A receptors. J. Neurosci. 1997, 17, 5711–5725. [Google Scholar] [CrossRef]
- Choi, Y.-B.; Lipton, S.A. Identification and mechanism of action of two histidine residues underlying high-affinity Zn2+ inhibition of the NMDA receptor. Neuron 1999, 23, 171–180. [Google Scholar] [CrossRef]
- Karakas, E.; Simorowski, N.; Furukawa, H.J. Structure of the zinc-bound amino-terminal domain of the NMDA receptor NR2B subunit. EMBO J. 2009, 28, 3910–3920. [Google Scholar] [CrossRef]
- Rachline, J.; Perin-Dureau, F.; Le Goff, A.; Neyton, J.; Paoletti, P.J. The micromolar zinc-binding domain on the NMDA receptor subunit NR2B. J. Neurosci. 2005, 25, 308–317. [Google Scholar] [CrossRef]
- Traynelis, S.F.; Burgess, M.F.; Zheng, F.; Lyuboslavsky, P.; Powers, J.L. Control of voltage-independent zinc inhibition of NMDA receptors by the NR1 subunit. J. Neurosci. 1998, 18, 6163–6175. [Google Scholar] [CrossRef]
- Chen, N.; Moshaver, A.; Raymond, L.A.J.M. Differential sensitivity of RecombinantN-Methyl-d-Aspartate Receptor subtypes to zinc inhibition. Mol. Pharmacol. 1997, 51, 1015–1023. [Google Scholar] [CrossRef]
- Lu, Q.; Haragopal, H.; Slepchenko, K.G.; Stork, C.; Li, Y.V. Pathophysiology; pharmacology, Intracellular zinc distribution in mitochondria, ER and the Golgi apparatus. Int. J. Physiol. Pathophysiol. Pharmacol. 2016, 8, 35. [Google Scholar]
- Li, Y.; Hough, C.J.; Frederickson, C.J.; Sarvey, J.M. Induction of mossy fiber -> CA3 long-term potentiation requires translocation of synaptically released Zn2+. J. Neurosci. 2001, 21, 8015–8025. [Google Scholar] [CrossRef]
- Lu, Y.M.; Taverna, F.A.; Tu, R.; Ackerley, C.A.; Wang, Y.T.; Roder, J. Endogenous Zn2+ is required for the induction of long-term potentiation at rat hippocampal mossy fiber-CA3 synapses. Synapse 2000, 38, 187–197. [Google Scholar] [CrossRef]
- Pan, E.H.; Zhang, X.A.; Huang, Z.; Krezel, A.; Zhao, M.; Tinberg, C.E.; Lippard, S.J.; McNamara, J.O. Vesicular Zinc Promotes Presynaptic and Inhibits Postsynaptic Long-Term Potentiation of Mossy Fiber-CA3 Synapse. Neuron 2011, 71, 1116–1126. [Google Scholar] [CrossRef]
- Izumi, Y.; Auberson, Y.P.; Zorumski, C.F. Zinc modulates bidirectional hippocampal plasticity by effects on NMDA receptors. J. Neurosci. 2006, 26, 7181–7188. [Google Scholar] [CrossRef] [PubMed]
- Lorca, R.A.; Rozas, C.; Loyola, S.; Moreira-Ramos, S.; Zeise, M.L.; Kirkwood, A.; Huidobro-Toro, J.P.; Morales, B. Zinc enhances long-term potentiation through P2X receptor modulation in the hippocampal CA1 region. Eur. J. Neurosci. 2011, 33, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Iwaki, H.; Ando, M.; Itagaki, K.; Suzuki, M.; Oku, N. Zinc differentially acts on components of long-term potentiation at hippocampal CA1 synapses. Brain Res. 2010, 1323, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Itagaki, K.; Ando, M.; Oku, N. Involvement of N-methyl-D-aspartate receptor subunits in zinc-mediated modification of CA1 long-term potentiation in the developing hippocampus. J. Neurosci. Res. 2012, 90, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Z.; Pan, E.; Xiong, Z.Q.; McNamara, J.O. Zinc-mediated transactivation of TrkB potentiates the hippocampal mossy Fiber-CA3 pyramid synapse. Neuron 2008, 57, 546–558. [Google Scholar] [CrossRef]
- Inoue, K.; Branigan, D.; Xiong, Z.G. Zinc-induced Neurotoxicity Mediated by Transient Receptor Potential Melastatin 7 Channels. J. Biol. Chem. 2010, 285, 7430–7439. [Google Scholar] [CrossRef]
- Kim, T.Y.; Hwang, J.J.; Yun, S.H.; Jung, M.W.; Koh, J.Y. Augmentation by zinc of NMDA receptor-mediated synaptic responses in CA1 of rat hippocampal slices: Mediation by Src family tyrosine kinases. Synapse 2002, 46, 49–56. [Google Scholar] [CrossRef]
- Manzerra, P.; Behrens, M.M.; Canzoniero, L.M.T.; Wang, X.Q.; Heidinger, V.; Ichinose, T.; Yu, S.P.; Choi, D.W. Zinc induces a Src family kinase-mediated up-regulation of NMDA receptor activity and excitotoxicity. Proc. Natl. Acad. Sci. USA 2001, 98, 11055–11061. [Google Scholar] [CrossRef]
- Grabrucker, A.M.; Knight, M.J.; Proepper, C.; Bockmann, J.; Joubert, M.; Rowan, M.; Nienhaus, G.U.; Garner, C.C.; Bowie, J.U.; Kreutz, M.R.; et al. Concerted action of zinc and ProSAP/Shank in synaptogenesis and synapse maturation. EMBO J. 2011, 30, 569–581. [Google Scholar] [CrossRef]
- Ebadi, M.; Murrin, L.C.; Pfeiffer, R.F. Hippocampal Zinc Thionein and Pyridoxal-Phosphate Modulate Synaptic Functions. Ann. N. Y. Acad. Sci 1990, 585, 189–201. [Google Scholar] [CrossRef]
- Grabrucker, S.; Jannetti, L.; Eckert, M.; Gaub, S.; Chhabra, R.; Pfaender, S.; Mangus, K.; Reddy, P.P.; Rankovic, V.; Schmeisser, M.J.; et al. Zinc deficiency dysregulates the synaptic ProSAP/Shank scaffold and might contribute to autism spectrum disorders. Brain 2014, 137, 137–152. [Google Scholar] [CrossRef]
- Masters, B.A.; Quaife, C.J.; Erickson, J.C.; Kelly, E.J.; Froelick, G.J.; Zambrowicz, B.P.; Brinster, R.L.; Palmiter, R.D. Metallothionein-Iii Is Expressed in Neurons That Sequester Zinc in Synaptic Vesicles. J. Neurosci. 1994, 14, 5844–5857. [Google Scholar] [CrossRef]
- Boeckers, T.M.; Winter, C.; Smalla, K.H.; Kreutz, M.R.; Bockmann, J.; Seidenbecher, C.; Garner, C.C.; Gundelfinger, E.D. Proline-rich synapse-associated proteins ProSAP1 and ProSAP2 interact with synaptic proteins of the SAPAP/GKAP family. Biochem. Bioph. Res. Co. 1999, 264, 247–252. [Google Scholar] [CrossRef]
- Naisbitt, S.; Kim, E.; Tu, J.C.; Xiao, B.; Sala, C.; Valtschanoff, J.; Weinberg, R.J.; Worley, P.F.; Sheng, M. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron 1999, 23, 569–582. [Google Scholar] [CrossRef]
- Boeckers, T.M.; Bockmann, J.; Kreutz, M.R.; Gundelfinger, E.D. ProSAP/Shank proteins—A family of higher order organizing molecules of the postsynaptic density with an emerging role in human neurological disease. J. Neurochem. 2002, 81, 903–910. [Google Scholar] [CrossRef]
- Kreienkamp, H.-J. Scaffolding Proteins at the Postsynaptic Density: Shank as the Architectural Framework. In Protein-Protein Interactions as New Drug Targets; Klussmann, E., Scott, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 365–380. [Google Scholar]
- Dong, H.; O’Brien, R.J.; Fung, E.T.; Lanahan, A.A.; Worley, P.F.; Huganir, R.L. GRIP: A synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature 2000, 386, 279–284. [Google Scholar] [CrossRef]
- Sheng, M.; Kim, E. The Shank family of scaffold proteins. J. Cell Sci. 2000, 113 Pt 11, 1851–1856. [Google Scholar] [CrossRef]
- Ha, H.T.T.; Leal-Ortiz, S.; Lalwani, K.; Kiyonaka, S.; Hamachi, I.; Mysore, S.P.; Montgomery, J.M.; Garner, C.C.; Huguenard, J.R.; Kim, S.A. Shank and Zinc Mediate an AMPA Receptor Subunit Switch in Developing Neurons. Front. Mol. Neurosci. 2018, 11, 405. [Google Scholar] [CrossRef]
- Grabrucker, A.M.; Schmeisser, M.J.; Schoen, M.; Boeckers, T.M. Postsynaptic ProSAP/Shank scaffolds in the cross-hair of synaptopathies. Trends Cell Biol. 2011, 21, 594–603. [Google Scholar] [CrossRef]
- Sala, C.; Piëch, V.; Wilson, N.R.; Passafaro, M.; Liu, G.; Sheng, M. Regulation of dendritic spine morphology and synaptic function by Shank and Homer. Neuron 2001, 31, 115–130. [Google Scholar] [CrossRef]
- Romorini, S.; Piccoli, G.; Jiang, M.; Grossano, P.; Tonna, N.; Passafaro, M.; Zhang, M.; Sala, C. A functional role of postsynaptic density-95-guanylate kinase-associated protein complex in regulating Shank assembly and stability to synapses. J. Neurosci. 2004, 24, 9391–9404. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shin, S.M.; Zhang, N.; Hansen, J.; Gerges, N.Z.; Pak, D.T.; Sheng, M.; Lee, S.H. GKAP orchestrates activity-dependent postsynaptic protein remodeling and homeostatic scaling. Nat. Neurosci. 2012, 15, 1655–1666. [Google Scholar] [CrossRef] [PubMed]
- Baron, M.K.; Boeckers, T.M.; Vaida, B.; Faham, S.; Gingery, M.; Sawaya, M.R.; Salyer, D.; Gundelfinger, E.D.; Bowie, J.U. An architectural framework that may lie at the core of the postsynaptic density. Science 2006, 311, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Arons, M.H.; Lee, K.; Thynne, C.J.; Kim, S.A.; Schob, C.; Kindler, S.; Montgomery, J.M.; Garner, C.C. Shank3 Is Part of a Zinc-Sensitive Signaling System That Regulates Excitatory Synaptic Strength. J. Neurosci. 2016, 36, 9124–9134. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; McCoy, P.A.; Rodriguiz, R.M.; Pan, Y.Z.; Je, H.S.; Roberts, A.C.; Kim, C.J.; Berrios, J.; Colvin, J.S.; Bousquet-Moore, D.; et al. Synaptic dysfunction and abnormal behaviors in mice lacking major isoforms of Shank3. Hum. Mol. Genet. 2011, 20, 3093–3108. [Google Scholar] [CrossRef]
- Kouser, M.; Speed, H.E.; Dewey, C.M.; Reimers, J.M.; Widman, A.J.; Gupta, N.; Liu, S.N.; Jaramillo, T.C.; Bangash, M.; Xiao, B.; et al. Loss of Predominant Shank3 Isoforms Results in Hippocampus-Dependent Impairments in Behavior and Synaptic Transmission. J. Neurosci. 2013, 33, 18448–18468. [Google Scholar] [CrossRef]
- Speed, H.E.; Kouser, M.; Xuan, Z.; Reimers, J.M.; Ochoa, C.F.; Gupta, N.; Liu, S.N.; Powell, C.M. Autism-Associated Insertion Mutation (InsG) of Shank3 Exon 21 Causes Impaired Synaptic Transmission and Behavioral Deficits. J. Neurosci. 2015, 35, 9648–9665. [Google Scholar] [CrossRef]
- Bozdagi, O.; Wang, X.B.; Nikitczuk, J.S.; Anderson, T.R.; Bloss, E.B.; Radice, G.L.; Zhou, Q.A.; Benson, D.L.; Huntley, G.W. Persistence of Coordinated Long-Term Potentiation and Dendritic Spine Enlargement at Mature Hippocampal CA1 Synapses Requires N-Cadherin. J. Neurosci. 2010, 30, 9984–9989. [Google Scholar] [CrossRef]
- Herron, C.E.; Lester, R.A.J.; Coan, E.J.; Collingridge, G.L. Frequency-Dependent Involvement of NMDA Receptors in the Hippocampus—A Novel Synaptic Mechanism. Nature 1986, 322, 265–268. [Google Scholar] [CrossRef]
- Wigstrom, H.; Gustafsson, B. Postsynaptic Control of Hippocampal Long-Term Potentiation. J. Physiol.-Paris 1986, 81, 228–236. [Google Scholar]
- Durand, C.M.; Perroy, J.; Loll, F.; Perrais, D.; Fagni, L.; Bourgeron, T.; Montcouquiol, M.; Sans, N. SHANK3 mutations identified in autism lead to modification of dendritic spine morphology via an actin-dependent mechanism. Mol. Psychiatr. 2012, 17, 71–84. [Google Scholar] [CrossRef]
- Duffney, L.J.; Zhong, P.; Wei, J.; Matas, E.; Cheng, J.; Qin, L.Y.; Ma, K.J.; Dietz, D.M.; Kajiwara, Y.; Buxbaum, J.D.; et al. Autism-like Deficits in Shank3-Deficient Mice Are Rescued by Targeting Actin Regulators. Cell Rep. 2015, 11, 1400–1413. [Google Scholar] [CrossRef]
- Shi, S.H.; Hayashi, Y.; Petralia, R.S.; Zaman, S.H.; Wenthold, R.J.; Svoboda, K.; Malinow, R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science 1999, 284, 1811–1816. [Google Scholar] [CrossRef]
- Oh, M.C.; Derkach, V.A.; Guire, E.S.; Soderling, T.R. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J. Biol. Chem. 2006, 281, 752–758. [Google Scholar] [CrossRef]
- Jaskolski, F.; Henley, J.M. Activity-dependent recruitment of AMPA receptors to the postsynaptic compartment by facilitated diffusion in the plasma membrane. Commun. Integr. Biol. 2009, 2, 474–476. [Google Scholar] [CrossRef]
- Makino, H.; Malinow, R. AMPA Receptor Incorporation into Synapses during LTP: The Role of Lateral Movement and Exocytosis. Neuron 2009, 64, 381–390. [Google Scholar] [CrossRef]
- Jurado, S.; Goswami, D.; Zhang, Y.S.; Molina, A.J.M.; Sudhof, T.C.; Malenka, R.C. LTP Requires a Unique Postsynaptic SNARE Fusion Machinery. Neuron 2013, 77, 542–558. [Google Scholar] [CrossRef]
- Zheng, N.; Jeyifous, O.; Munro, C.; Montgomery, J.M.; Green, W.N. Synaptic activity regulates AMPA receptor trafficking through different recycling pathways. Elife 2015, 4, e06878. [Google Scholar] [CrossRef]
- Guha, M.J. Diagnostic and statistical manual of mental disorders: DSM-5. Ref. Rev. 2014, 28, 36–37. [Google Scholar]
- Lee, E.J.; Choi, S.Y.; Kim, E. NMDA receptor dysfunction in autism spectrum disorders. Curr. Opin. Pharmacol. 2015, 20, 8–13. [Google Scholar] [CrossRef]
- Nisar, S.; Bhat, A.A.; Masoodi, T.; Hashem, S.; Akhtar, S.; Ali, T.A.; Amjad, S.; Chawla, S.; Bagga, P.; Frenneaux, M.P.; et al. Genetics of glutamate and its receptors in autism spectrum disorder. Mol. Psychiatr. 2022, 27, 2380–2392. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.M.; Jeong, J.; Roche, K.W. The role of NMDA receptor and neuroligin rare variants in synaptic dysfunction underlying neurodevelopmental disorders. Curr. Opin. Neurobiol. 2021, 69, 93–104. [Google Scholar] [CrossRef] [PubMed]
- XiangWei, W.; Jiang, Y.; Yuan, H. De Novo Mutations and Rare Variants Occurring in NMDA Receptors. Curr. Opin. Physiol. 2018, 2, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Iossifov, I.; O’Roak, B.J.; Sanders, S.J.; Ronemus, M.; Krumm, N.; Levy, D.; Stessman, H.A.; Witherspoon, K.T.; Vives, L.; Patterson, K.E.; et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature 2014, 515, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Lemke, J.R.; Geider, K.; Helbig, K.L.; Heyne, H.O.; Schutz, H.; Hentschel, J.; Courage, C.; Depienne, C.; Nava, C.; Heron, D.; et al. Delineating the GRIN1 phenotypic spectrum: A distinct genetic NMDA receptor encephalopathy. Neurology 2016, 86, 2171–2178. [Google Scholar] [CrossRef]
- Rossi, M.; Chatron, N.; Labalme, A.; Ville, D.; Carneiro, M.; Edery, P.; des Portes, V.; Lemke, J.R.; Sanlaville, D.; Lesca, G. Novel homozygous missense variant of GRIN1 in two sibs with intellectual disability and autistic features without epilepsy. Eur. J. Hum. Genet. 2017, 25, 376–380. [Google Scholar] [CrossRef][Green Version]
- Bosch, D.G.; Boonstra, F.N.; de Leeuw, N.; Pfundt, R.; Nillesen, W.M.; de Ligt, J.; Gilissen, C.; Jhangiani, S.; Lupski, J.R.; Cremers, F.P.; et al. Novel genetic causes for cerebral visual impairment. Eur. J. Hum. Genet. 2016, 24, 660–665. [Google Scholar] [CrossRef]
- Bramswig, N.C.; Ludecke, H.J.; Alanay, Y.; Albrecht, B.; Barthelmie, A.; Boduroglu, K.; Braunholz, D.; Caliebe, A.; Chrzanowska, K.H.; Czeschik, J.C.; et al. Exome sequencing unravels unexpected differential diagnoses in individuals with the tentative diagnosis of Coffin-Siris and Nicolaides-Baraitser syndromes. Hum. Genet. 2015, 134, 553–568. [Google Scholar] [CrossRef]
- Conroy, J.; McGettigan, P.A.; McCreary, D.; Shah, N.; Collins, K.; Parry-Fielder, B.; Moran, M.; Hanrahan, D.; Deonna, T.W.; Korff, C.M.; et al. Towards the identification of a genetic basis for Landau-Kleffner syndrome. Epilepsia 2014, 55, 858–865. [Google Scholar] [CrossRef]
- Lesca, G.; Rudolf, G.; Bruneau, N.; Lozovaya, N.; Labalme, A.; Boutry-Kryza, N.; Salmi, M.; Tsintsadze, T.; Addis, L.; Motte, J.; et al. GRIN2A mutations in acquired epileptic aphasia and related childhood focal epilepsies and encephalopathies with speech and language dysfunction. Nat. Genet. 2013, 45, 1061–1066. [Google Scholar] [CrossRef]
- Tarabeux, J.; Kebir, O.; Gauthier, J.; Hamdan, F.F.; Xiong, L.; Piton, A.; Spiegelman, D.; Henrion, E.; Millet, B.; S2D team; et al. Rare mutations in N-methyl-D-aspartate glutamate receptors in autism spectrum disorders and schizophrenia. Transl. Psychiatry 2011, 1, e55. [Google Scholar] [CrossRef]
- Stessman, H.A.; Xiong, B.; Coe, B.P.; Wang, T.; Hoekzema, K.; Fenckova, M.; Kvarnung, M.; Gerdts, J.; Trinh, S.; Cosemans, N.J. Targeted sequencing identifies 91 neurodevelopmental-disorder risk genes with autism and developmental-disability biases. Nat. Genet. 2017, 49, 515–526. [Google Scholar] [CrossRef]
- Endele, S.; Rosenberger, G.; Geider, K.; Popp, B.; Tamer, C.; Stefanova, I.; Milh, M.; Kortum, F.; Fritsch, A.; Pientka, F.K.; et al. Mutations in GRIN2A and GRIN2B encoding regulatory subunits of NMDA receptors cause variable neurodevelopmental phenotypes. Nat. Genet. 2010, 42, 1021–1026. [Google Scholar] [CrossRef]
- Griswold, A.J.; Ma, D.; Cukier, H.N.; Nations, L.D.; Schmidt, M.A.; Chung, R.H.; Jaworski, J.M.; Salyakina, D.; Konidari, I.; Whitehead, P.L.; et al. Evaluation of copy number variations reveals novel candidate genes in autism spectrum disorder-associated pathways. Hum. Mol. Genet. 2012, 21, 3513–3523. [Google Scholar] [CrossRef]
- Kenny, E.M.; Cormican, P.; Furlong, S.; Heron, E.; Kenny, G.; Fahey, C.; Kelleher, E.; Ennis, S.; Tropea, D.; Anney, R.; et al. Excess of rare novel loss-of-function variants in synaptic genes in schizophrenia and autism spectrum disorders. Mol. Psychiatry 2014, 19, 872–879. [Google Scholar] [CrossRef]
- O’Roak, B.J.; Deriziotis, P.; Lee, C.; Vives, L.; Schwartz, J.J.; Girirajan, S.; Karakoc, E.; Mackenzie, A.P.; Ng, S.B.; Baker, C.; et al. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat. Genet. 2011, 43, 585–589. [Google Scholar] [CrossRef]
- O’Roak, B.J.; Stessman, H.A.; Boyle, E.A.; Witherspoon, K.T.; Martin, B.; Lee, C.; Vives, L.; Baker, C.; Hiatt, J.B.; Nickerson, D.A.; et al. Recurrent de novo mutations implicate novel genes underlying simplex autism risk. Nat. Commun. 2014, 5, 5595. [Google Scholar] [CrossRef]
- O’Roak, B.J.; Vives, L.; Girirajan, S.; Karakoc, E.; Krumm, N.; Coe, B.P.; Levy, R.; Ko, A.; Lee, C.; Smith, J.D.; et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 2012, 485, 246–250. [Google Scholar] [CrossRef]
- Takasaki, Y.; Koide, T.; Wang, C.; Kimura, H.; Xing, J.; Kushima, I.; Ishizuka, K.; Mori, D.; Sekiguchi, M.; Ikeda, M.; et al. Mutation screening of GRIN2B in schizophrenia and autism spectrum disorder in a Japanese population. Sci. Rep. 2016, 6, 33311. [Google Scholar] [CrossRef]
- Talkowski, M.E.; Rosenfeld, J.A.; Blumenthal, I.; Pillalamarri, V.; Chiang, C.; Heilbut, A.; Ernst, C.; Hanscom, C.; Rossin, E.; Lindgren, A.M.; et al. Sequencing chromosomal abnormalities reveals neurodevelopmental loci that confer risk across diagnostic boundaries. Cell 2012, 149, 525–537. [Google Scholar] [CrossRef]
- Firth, H.V.; Richards, S.M.; Bevan, A.P.; Clayton, S.; Corpas, M.; Rajan, D.; Van Vooren, S.; Moreau, Y.; Pettett, R.M.; Carter, N.P. DECIPHER: Database of Chromosomal Imbalance and Phenotype in Humans Using Ensembl Resources. Am. J. Hum. Genet. 2009, 84, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Swanger, S.A.; Chen, W.; Wells, G.; Burger, P.B.; Tankovic, A.; Bhattacharya, S.; Strong, K.L.; Hu, C.; Kusumoto, H.; Zhang, J.J. Mechanistic insight into NMDA receptor dysregulation by rare variants in the GluN2A and GluN2B agonist binding domains. Am. J. Hum. Genet. 2016, 99, 1261–1280. [Google Scholar] [CrossRef] [PubMed]
- Fedele, L.; Newcombe, J.; Topf, M.; Gibb, A.; Harvey, R.J.; Smart, T.G. Disease-associated missense mutations in GluN2B subunit alter NMDA receptor ligand binding and ion channel properties. Nat. Commun. 2018, 9, 957. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhou, L.; Yuan, H.; Vieira, M.; Sanz-Clemente, A.; Badger, J.D., 2nd; Lu, W.; Traynelis, S.F.; Roche, K.W. A Rare Variant Identified Within the GluN2B C-Terminus in a Patient with Autism Affects NMDA Receptor Surface Expression and Spine Density. J. Neurosci. 2017, 37, 4093–4102. [Google Scholar] [CrossRef] [PubMed]
- Platzer, K.; Yuan, H.; Schutz, H.; Winschel, A.; Chen, W.; Hu, C.; Kusumoto, H.; Heyne, H.O.; Helbig, K.L.; Tang, S.; et al. GRIN2B encephalopathy: Novel findings on phenotype, variant clustering, functional consequences and treatment aspects. J. Med. Genet. 2017, 54, 460–470. [Google Scholar] [CrossRef]
- Sceniak, M.P.; Fedder, K.N.; Wang, Q.; Droubi, S.; Babcock, K.; Patwardhan, S.; Wright-Zornes, J.; Pham, L.; Sabo, S.L. An autism-associated mutation in GluN2B prevents NMDA receptor trafficking and interferes with dendrite growth. J. Cell Sci. 2019, 132, jcs232892. [Google Scholar] [CrossRef]
- Vyklicky, V.; Krausova, B.; Cerny, J.; Ladislav, M.; Smejkalova, T.; Kysilov, B.; Korinek, M.; Danacikova, S.; Horak, M.; Chodounska, H.; et al. Surface Expression, Function, and Pharmacology of Disease-Associated Mutations in the Membrane Domain of the Human GluN2B Subunit. Front. Mol. Neurosci. 2018, 11, 110. [Google Scholar] [CrossRef]
- Gandal, M.J.; Anderson, R.L.; Billingslea, E.N.; Carlson, G.C.; Roberts, T.P.; Siegel, S.J. Mice with reduced NMDA receptor expression: More consistent with autism than schizophrenia? Genes Brain Behav. 2012, 11, 740–750. [Google Scholar] [CrossRef]
- Shin, W.; Kim, K.; Serraz, B.; Cho, Y.S.; Kim, D.; Kang, M.; Lee, E.J.; Lee, H.; Bae, Y.C.; Paoletti, P.; et al. Early correction of synaptic long-term depression improves abnormal anxiety-like behavior in adult GluN2B-C456Y-mutant mice. PLoS Biol. 2020, 18, e3000717. [Google Scholar] [CrossRef]
- De Rubeis, S.; He, X.; Goldberg, A.P.; Poultney, C.S.; Samocha, K.; Cicek, A.E.; Kou, Y.; Liu, L.; Fromer, M.; Walker, S.; et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 2014, 515, 209–215. [Google Scholar] [CrossRef]
- Satterstrom, F.K.; Kosmicki, J.A.; Wang, J.; Breen, M.S.; De Rubeis, S.; An, J.Y.; Peng, M.; Collins, R.; Grove, J.; Klei, L.; et al. Large-Scale Exome Sequencing Study Implicates Both Developmental and Functional Changes in the Neurobiology of Autism. Cell 2020, 180, 568–584.e23. [Google Scholar] [CrossRef]
- Berkel, S.; Marshall, C.R.; Weiss, B.; Howe, J.; Roeth, R.; Moog, U.; Endris, V.; Roberts, W.; Szatmari, P.; Pinto, D.; et al. Mutations in the SHANK2 synaptic scaffolding gene in autism spectrum disorder and mental retardation. Nat. Genet. 2010, 42, 489–491. [Google Scholar] [CrossRef]
- Durand, C.M.; Betancur, C.; Boeckers, T.M.; Bockmann, J.; Chaste, P.; Fauchereau, F.; Nygren, G.; Rastam, M.; Gillberg, I.C.; Anckarsater, H.; et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat. Genet. 2007, 39, 25–27. [Google Scholar] [CrossRef]
- Leblond, C.S.; Heinrich, J.; Delorme, R.; Proepper, C.; Betancur, C.; Huguet, G.; Konyukh, M.; Chaste, P.; Ey, E.; Rastam, M.; et al. Genetic and functional analyses of SHANK2 mutations suggest a multiple hit model of autism spectrum disorders. PLoS Genet. 2012, 8, e1002521. [Google Scholar] [CrossRef]
- Leblond, C.S.; Nava, C.; Polge, A.; Gauthier, J.; Huguet, G.; Lumbroso, S.; Giuliano, F.; Stordeur, C.; Depienne, C.; Mouzat, K.; et al. Meta-analysis of SHANK Mutations in Autism Spectrum Disorders: A gradient of severity in cognitive impairments. PLoS Genet. 2014, 10, e1004580. [Google Scholar] [CrossRef]
- Berg, E.L.; Copping, N.A.; Rivera, J.K.; Pride, M.C.; Careaga, M.; Bauman, M.D.; Berman, R.F.; Lein, P.J.; Harony-Nicolas, H.; Buxbaum, J.D.; et al. Developmental social communication deficits in the Shank3 rat model of Phelan-McDermid syndrome and autism spectrum disorder. Autism Res. 2018, 11, 587–601. [Google Scholar] [CrossRef]
- Bey, A.L.; Wang, X.; Yan, H.; Kim, N.; Passman, R.L.; Yang, Y.; Cao, X.; Towers, A.J.; Hulbert, S.W.; Duffney, L.J.; et al. Brain region-specific disruption of Shank3 in mice reveals a dissociation for cortical and striatal circuits in autism-related behaviors. Transl. Psychiatry 2018, 8, 94. [Google Scholar] [CrossRef]
- Dhamne, S.C.; Silverman, J.L.; Super, C.E.; Lammers, S.H.T.; Hameed, M.Q.; Modi, M.E.; Copping, N.A.; Pride, M.C.; Smith, D.G.; Rotenberg, A.; et al. Replicable in vivo physiological and behavioral phenotypes of the Shank3B null mutant mouse model of autism. Mol. Autism 2017, 8, 26. [Google Scholar] [CrossRef]
- Drapeau, E.; Riad, M.; Kajiwara, Y.; Buxbaum, J.D. Behavioral Phenotyping of an Improved Mouse Model of Phelan-McDermid Syndrome with a Complete Deletion of the Shank3 Gene. eNeuro 2018, 5, ENEURO.0046-18.2018. [Google Scholar] [CrossRef]
- Fourie, C.; Vyas, Y.; Lee, K.; Jung, Y.; Garner, C.C.; Montgomery, J.M. Dietary Zinc Supplementation Prevents Autism Related Behaviors and Striatal Synaptic Dysfunction in Shank3 Exon 13-16 Mutant Mice. Front. Cell. Neurosci. 2018, 12, 374. [Google Scholar] [CrossRef]
- Garrido, D.; Beretta, S.; Grabrucker, S.; Bauer, H.F.; Bayer, D.; Sala, C.; Verpelli, C.; Roselli, F.; Bockmann, J.; Proepper, C.; et al. Shank2/3 double knockout-based screening of cortical subregions links the retrosplenial area to the loss of social memory in autism spectrum disorders. Mol. Psychiatry 2022. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.; Lee, D.; Cho, Y.S.; Chung, C.; Yoo, Y.E.; Kim, J.; Lee, J.; Kim, W.; Kim, H.; Bae, Y.C.; et al. Cerebellar Shank2 Regulates Excitatory Synapse Density, Motor Coordination, and Specific Repetitive and Anxiety-Like Behaviors. J. Neurosci. 2016, 36, 12129–12143. [Google Scholar] [CrossRef]
- Han, K.A.; Yoon, T.H.; Shin, J.; Um, J.W.; Ko, J. Differentially altered social dominance- and cooperative-like behaviors in Shank2- and Shank3-mutant mice. Mol. Autism 2020, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Harony-Nicolas, H.; Kay, M.; du Hoffmann, J.; Klein, M.E.; Bozdagi-Gunal, O.; Riad, M.; Daskalakis, N.P.; Sonar, S.; Castillo, P.E.; Hof, P.R.; et al. Oxytocin improves behavioral and electrophysiological deficits in a novel Shank3-deficient rat. Elife 2017, 6, e18904. [Google Scholar] [CrossRef] [PubMed]
- Hung, A.Y.; Futai, K.; Sala, C.; Valtschanoff, J.G.; Ryu, J.; Woodworth, M.A.; Kidd, F.L.; Sung, C.C.; Miyakawa, T.; Bear, M.F.; et al. Smaller dendritic spines, weaker synaptic transmission, but enhanced spatial learning in mice lacking Shank1. J. Neurosci. 2008, 28, 1697–1708. [Google Scholar] [CrossRef]
- Jaramillo, T.C.; Speed, H.E.; Xuan, Z.; Reimers, J.M.; Escamilla, C.O.; Weaver, T.P.; Liu, S.; Filonova, I.; Powell, C.M. Novel Shank3 mutant exhibits behaviors with face validity for autism and altered striatal and hippocampal function. Autism Res. 2017, 10, 42–65. [Google Scholar] [CrossRef]
- Jaramillo, T.C.; Speed, H.E.; Xuan, Z.; Reimers, J.M.; Liu, S.; Powell, C.M. Altered Striatal Synaptic Function and Abnormal Behaviour in Shank3 Exon4-9 Deletion Mouse Model of Autism. Autism Res. 2016, 9, 350–375. [Google Scholar] [CrossRef]
- Kabitzke, P.A.; Brunner, D.; He, D.; Fazio, P.A.; Cox, K.; Sutphen, J.; Thiede, L.; Sabath, E.; Hanania, T.; Alexandrov, V.; et al. Comprehensive analysis of two Shank3 and the Cacna1c mouse models of autism spectrum disorder. Genes Brain Behav. 2018, 17, 4–22. [Google Scholar] [CrossRef]
- Ehlers, M.D. Synapse structure: Glutamate receptors connected by the shanks. Curr. Biol. 1999, 9, R848–R850. [Google Scholar] [CrossRef]
- Lee, E.J.; Lee, H.; Huang, T.N.; Chung, C.; Shin, W.; Kim, K.; Koh, J.Y.; Hsueh, Y.P.; Kim, E. Trans-synaptic zinc mobilization improves social interaction in two mouse models of autism through NMDAR activation. Nat. Commun. 2015, 6, 7168. [Google Scholar] [CrossRef]
- Lim, J.S.; Lim, M.Y.; Choi, Y.; Ko, G. Modeling environmental risk factors of autism in mice induces IBD-related gut microbial dysbiosis and hyperserotonemia. Mol. Brain 2017, 10, 14. [Google Scholar] [CrossRef]
- Matas, E.; Maisterrena, A.; Thabault, M.; Balado, E.; Francheteau, M.; Balbous, A.; Galvan, L.; Jaber, M. Major motor and gait deficits with sexual dimorphism in a Shank3 mutant mouse model. Mol. Autism 2021, 12, 2. [Google Scholar] [CrossRef]
- Mei, Y.; Monteiro, P.; Zhou, Y.; Kim, J.A.; Gao, X.; Fu, Z.; Feng, G. Adult restoration of Shank3 expression rescues selective autistic-like phenotypes. Nature 2016, 530, 481–484. [Google Scholar] [CrossRef]
- Pappas, A.L.; Bey, A.L.; Wang, X.; Rossi, M.; Kim, Y.H.; Yan, H.; Porkka, F.; Duffney, L.J.; Phillips, S.M.; Cao, X.; et al. Deficiency of Shank2 causes mania-like behavior that responds to mood stabilizers. JCI Insight 2017, 2, e92052. [Google Scholar] [CrossRef]
- Peca, J.; Feliciano, C.; Ting, J.T.; Wang, W.T.; Wells, M.F.; Venkatraman, T.N.; Lascola, C.D.; Fu, Z.Y.; Feng, G.P. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature 2011, 472, 437–442. [Google Scholar] [CrossRef]
- Peter, S.; Ten Brinke, M.M.; Stedehouder, J.; Reinelt, C.M.; Wu, B.; Zhou, H.; Zhou, K.; Boele, H.J.; Kushner, S.A.; Lee, M.G.; et al. Dysfunctional cerebellar Purkinje cells contribute to autism-like behaviour in Shank2-deficient mice. Nat. Commun. 2016, 7, 12627. [Google Scholar] [CrossRef]
- Ponzoni, L.; Sala, C.; Verpelli, C.; Sala, M.; Braida, D. Different attentional dysfunctions in eEF2K(−/−), IL1RAPL1(−/−) and SHANK3Delta11(−/−) mice. Genes Brain Behav. 2019, 18, e12563. [Google Scholar] [CrossRef]
- Rendall, A.R.; Perrino, P.A.; Buscarello, A.N.; Fitch, R.H. Shank3B mutant mice display pitch discrimination enhancements and learning deficits. Int. J. Dev. Neurosci. 2019, 72, 13–21. [Google Scholar] [CrossRef]
- Schmeisser, M.J.; Ey, E.; Wegener, S.; Bockmann, J.; Stempel, A.V.; Kuebler, A.; Janssen, A.L.; Udvardi, P.T.; Shiban, E.; Spilker, C.; et al. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature 2012, 486, 256–260. [Google Scholar] [CrossRef]
- Silverman, J.L.; Turner, S.M.; Barkan, C.L.; Tolu, S.S.; Saxena, R.; Hung, A.Y.; Sheng, M.; Crawley, J.N. Sociability and motor functions in Shank1 mutant mice. Brain Res. 2011, 1380, 120–137. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Ehlers, M.D. Modeling autism by SHANK gene mutations in mice. Neuron 2013, 78, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Vicidomini, C.; Ponzoni, L.; Lim, D.; Schmeisser, M.J.; Reim, D.; Morello, N.; Orellana, D.; Tozzi, A.; Durante, V.; Scalmani, P.; et al. Pharmacological enhancement of mGlu5 receptors rescues behavioral deficits in SHANK3 knock-out mice. Mol. Psychiatry 2017, 22, 784. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pang, K.; Han, K.; Adamski, C.J.; Wang, W.; He, L.; Lai, J.K.; Bondar, V.V.; Duman, J.G.; Richman, R.; et al. An autism-linked missense mutation in SHANK3 reveals the modularity of Shank3 function. Mol. Psychiatry 2020, 25, 2534–2555. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bey, A.L.; Katz, B.M.; Badea, A.; Kim, N.; David, L.K.; Duffney, L.J.; Kumar, S.; Mague, S.D.; Hulbert, S.W.; et al. Altered mGluR5-Homer scaffolds and corticostriatal connectivity in a Shank3 complete knockout model of autism. Nat. Commun. 2016, 7, 11459. [Google Scholar] [CrossRef] [PubMed]
- Wohr, M.; Roullet, F.I.; Hung, A.Y.; Sheng, M.; Crawley, J.N. Communication impairments in mice lacking Shank1: Reduced levels of ultrasonic vocalizations and scent marking behavior. PLoS ONE 2011, 6, e20631. [Google Scholar] [CrossRef]
- Won, H.; Lee, H.R.; Gee, H.Y.; Mah, W.; Kim, J.I.; Lee, J.; Ha, S.; Chung, C.; Jung, E.S.; Cho, Y.S.; et al. Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature 2012, 486, 261–265. [Google Scholar] [CrossRef]
- Yang, M.; Bozdagi, O.; Scattoni, M.L.; Wohr, M.; Roullet, F.I.; Katz, A.M.; Abrams, D.N.; Kalikhman, D.; Simon, H.; Woldeyohannes, L.; et al. Reduced excitatory neurotransmission and mild autism-relevant phenotypes in adolescent Shank3 null mutant mice. J. Neurosci. 2012, 32, 6525–6541. [Google Scholar] [CrossRef]
- Yoo, T.; Cho, H.; Lee, J.; Park, H.; Yoo, Y.E.; Yang, E.; Kim, J.Y.; Kim, H.; Kim, E. GABA Neuronal Deletion of Shank3 Exons 14-16 in Mice Suppresses Striatal Excitatory Synaptic Input and Induces Social and Locomotor Abnormalities. Front. Cell. Neurosci. 2018, 12, 341. [Google Scholar] [CrossRef]
- Yoo, T.; Cho, H.; Park, H.; Lee, J.; Kim, E. Shank3 Exons 14-16 Deletion in Glutamatergic Neurons Leads to Social and Repetitive Behavioral Deficits Associated With Increased Cortical Layer 2/3 Neuronal Excitability. Front. Cell. Neurosci. 2019, 13, 458. [Google Scholar] [CrossRef]
- Yoo, Y.E.; Yoo, T.; Lee, S.; Lee, J.; Kim, D.; Han, H.M.; Bae, Y.C.; Kim, E. Shank3 Mice Carrying the Human Q321R Mutation Display Enhanced Self-Grooming, Abnormal Electroencephalogram Patterns, and Suppressed Neuronal Excitability and Seizure Susceptibility. Front. Mol. Neurosci. 2019, 12, 155. [Google Scholar] [CrossRef]
- Zhou, Y.; Kaiser, T.; Monteiro, P.; Zhang, X.; Van der Goes, M.S.; Wang, D.; Barak, B.; Zeng, M.; Li, C.; Lu, C.; et al. Mice with Shank3 Mutations Associated with ASD and Schizophrenia Display Both Shared and Distinct Defects. Neuron 2016, 89, 147–162. [Google Scholar] [CrossRef]
- Zhou, Y.; Sharma, J.; Ke, Q.; Landman, R.; Yuan, J.; Chen, H.; Hayden, D.S.; Fisher, J.W., III; Jiang, M.; Menegas, W.; et al. Atypical behaviour and connectivity in SHANK3-mutant macaques. Nature 2019, 570, 326–331. [Google Scholar] [CrossRef]
- Mossa, A.; Pagano, J.; Ponzoni, L.; Tozzi, A.; Vezzoli, E.; Sciaccaluga, M.; Costa, C.; Beretta, S.; Francolini, M.; Sala, M.J. Developmental impaired Akt signaling in the Shank1 and Shank3 double knock-out mice. Mol. Psychiatry 2021, 26, 1928–1944. [Google Scholar] [CrossRef]
- Vyas, Y.; Cheyne, J.E.; Lee, K.; Jung, Y.; Cheung, P.Y.; Montgomery, J.M. Shankopathies in the Developing Brain in Autism Spectrum Disorders. Front. Neurosci. 2021, 15, 775431. [Google Scholar] [CrossRef]
- Bozdagi, O.; Sakurai, T.; Papapetrou, D.; Wang, X.; Dickstein, D.L.; Takahashi, N.; Kajiwara, Y.; Yang, M.; Katz, A.M.; Scattoni, M.L.; et al. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol. Autism 2010, 1, 15. [Google Scholar] [CrossRef]
- Wegener, S.; Buschler, A.; Stempel, A.V.; Kang, S.J.; Lim, C.S.; Kaang, B.K.; Shoichet, S.A.; Manahan-Vaughan, D.; Schmitz, D. Defective Synapse Maturation and Enhanced Synaptic Plasticity in Shank2 Deltaex7(−/−) Mice. eNeuro 2018, 5, ENEURO.0398-17.2018. [Google Scholar] [CrossRef]
- Delling, J.P.; Boeckers, T.M. Comparison of SHANK3 deficiency in animal models: Phenotypes, treatment strategies, and translational implications. J. Neurodev. Disord. 2021, 13, 55. [Google Scholar] [CrossRef]
- Reim, D.; Distler, U.; Halbedl, S.; Verpelli, C.; Sala, C.; Bockmann, J.; Tenzer, S.; Boeckers, T.M.; Schmeisser, M.J. Proteomic Analysis of Post-synaptic Density Fractions from Shank3 Mutant Mice Reveals Brain Region Specific Changes Relevant to Autism Spectrum Disorder. Front. Mol. Neurosci. 2017, 10, 26. [Google Scholar] [CrossRef]
- Song, T.J.; Lan, X.Y.; Wei, M.P.; Zhai, F.J.; Boeckers, T.M.; Wang, J.N.; Yuan, S.; Jin, M.Y.; Xie, Y.F.; Dang, W.W.; et al. Altered Behaviors and Impaired Synaptic Function in a Novel Rat Model With a Complete Shank3 Deletion. Front. Cell. Neurosci. 2019, 13, 111. [Google Scholar] [CrossRef]
- Modi, M.E.; Brooks, J.M.; Guilmette, E.R.; Beyna, M.; Graf, R.; Reim, D.; Schmeisser, M.J.; Boeckers, T.M.; O’Donnell, P.; Buhl, D.L. Hyperactivity and Hypermotivation Associated With Increased Striatal mGluR1 Signaling in a Shank2 Rat Model of Autism. Front. Mol. Neurosci. 2018, 11, 107. [Google Scholar] [CrossRef]
- Bockers, T.M.; Segger-Junius, M.; Iglauer, P.; Bockmann, J.; Gundelfinger, E.D.; Kreutz, M.R.; Richter, D.; Kindler, S.; Kreienkamp, H.J. Differential expression and dendritic transcript localization of Shank family members: Identification of a dendritic targeting element in the 3’ untranslated region of Shank1 mRNA. Mol. Cell. Neurosci. 2004, 26, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, Q.; Bey, A.L.; Lee, Y.; Jiang, Y.H. Transcriptional and functional complexity of Shank3 provides a molecular framework to understand the phenotypic heterogeneity of SHANK3 causing autism and Shank3 mutant mice. Mol. Autism 2014, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Boeckers, T.M.; Liedtke, T.; Spilker, C.; Dresbach, T.; Bockmann, J.; Kreutz, M.R.; Gundelfinger, E.D. C-terminal synaptic targeting elements for postsynaptic density proteins ProSAP1/Shank2 and ProSAP2/Shank3. J. Neurochem. 2005, 92, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Redman, P.; Ghose, D.; Hwang, H.; Liu, Y.; Ren, X.; Ding, L.J.; Liu, M.; Jones, K.J.; Xu, W. Shank Proteins Differentially Regulate Synaptic Transmission. eNeuro 2017, 4, ENEURO.0163-15.2017. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Kim, J.; Chung, C.; Ha, S.; Lee, S.; Lee, E.; Yoo, Y.E.; Kim, W.; Shin, W.; Kim, E. Cell-Type-Specific Shank2 Deletion in Mice Leads to Differential Synaptic and Behavioral Phenotypes. J. Neurosci. 2018, 38, 4076–4092. [Google Scholar] [CrossRef]
- Chung, C.; Shin, W.; Kim, E. Early and Late Corrections in Mouse Models of Autism Spectrum Disorder. Biol. Psychiatry 2022, 91, 934–944. [Google Scholar] [CrossRef]
- Chung, C.; Ha, S.; Kang, H.; Lee, J.; Um, S.M.; Yan, H.; Yoo, Y.E.; Yoo, T.; Jung, H.; Lee, D.; et al. Early Correction of N-Methyl-D-Aspartate Receptor Function Improves Autistic-like Social Behaviors in Adult Shank2(−/−) Mice. Biol. Psychiatry 2019, 85, 534–543. [Google Scholar] [CrossRef]
- Peixoto, R.T.; Chantranupong, L.; Hakim, R.; Levasseur, J.; Wang, W.; Merchant, T.; Gorman, K.; Budnik, B.; Sabatini, B.L. Abnormal Striatal Development Underlies the Early Onset of Behavioral Deficits in Shank3B(−/−) Mice. Cell Rep. 2019, 29, 2016–2027.e4. [Google Scholar] [CrossRef]
- Peixoto, R.T.; Wang, W.; Croney, D.M.; Kozorovitskiy, Y.; Sabatini, B.L. Early hyperactivity and precocious maturation of corticostriatal circuits in Shank3B(−/−) mice. Nat. Neurosci. 2016, 19, 716–724. [Google Scholar] [CrossRef]
- Vyas, Y.; Lee, K.; Jung, Y.; Montgomery, J.M. Influence of maternal zinc supplementation on the development of autism-associated behavioural and synaptic deficits in offspringShank3-knockout mice. Mol. Brain 2020, 13, 110. [Google Scholar] [CrossRef]
- Kang, J.; Park, H.; Kim, E. IRSp53/BAIAP2 in dendritic spine development, NMDA receptor regulation, and psychiatric disorders. Neuropharmacology 2016, 100, 27–39. [Google Scholar] [CrossRef]
- Celestino-Soper, P.B.; Shaw, C.A.; Sanders, S.J.; Li, J.; Murtha, M.T.; Ercan-Sencicek, A.G.; Davis, L.; Thomson, S.; Gambin, T.; Chinault, A.C.; et al. Use of array CGH to detect exonic copy number variants throughout the genome in autism families detects a novel deletion in TMLHE. Hum. Mol. Genet. 2011, 20, 4360–4370. [Google Scholar] [CrossRef]
- Levy, D.; Ronemus, M.; Yamrom, B.; Lee, Y.H.; Leotta, A.; Kendall, J.; Marks, S.; Lakshmi, B.; Pai, D.; Ye, K.; et al. Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron 2011, 70, 886–897. [Google Scholar] [CrossRef]
- Toma, C.; Hervas, A.; Balmana, N.; Vilella, E.; Aguilera, F.; Cusco, I.; del Campo, M.; Caballero, R.; De Diego-Otero, Y.; Ribases, M.; et al. Association study of six candidate genes asymmetrically expressed in the two cerebral hemispheres suggests the involvement of BAIAP2 in autism. J. Psychiatr. Res. 2011, 45, 280–282. [Google Scholar] [CrossRef]
- Chuang, H.C.; Huang, T.N.; Hsueh, Y.P. T-Brain-1--A Potential Master Regulator in Autism Spectrum Disorders. Autism Res. 2015, 8, 412–426. [Google Scholar] [CrossRef]
- Kim, M.H.; Choi, J.; Yang, J.; Chung, W.; Kim, J.H.; Paik, S.K.; Kim, K.; Han, S.; Won, H.; Bae, Y.S.; et al. Enhanced NMDA receptor-mediated synaptic transmission, enhanced long-term potentiation, and impaired learning and memory in mice lacking IRSp53. J. Neurosci. 2009, 29, 1586–1595. [Google Scholar] [CrossRef]
- Wang, C.Y.; Chang, K.; Petralia, R.S.; Wang, Y.X.; Seabold, G.K.; Wenthold, R.J. A novel family of adhesion-like molecules that interacts with the NMDA receptor. J. Neurosci. 2006, 26, 2174–2183. [Google Scholar] [CrossRef]
- Li, Y.; Kim, R.; Cho, Y.S.; Song, W.S.; Kim, D.; Kim, K.; Roh, J.D.; Chung, C.; Park, H.; Yang, E.; et al. Lrfn2-Mutant Mice Display Suppressed Synaptic Plasticity and Inhibitory Synapse Development and Abnormal Social Communication and Startle Response. J. Neurosci. 2018, 38, 5872–5887. [Google Scholar] [CrossRef]
- Betancur, C.; Sakurai, T.; Buxbaum, J.D. The emerging role of synaptic cell-adhesion pathways in the pathogenesis of autism spectrum disorders. Trends Neurosci. 2009, 32, 402–412. [Google Scholar] [CrossRef]
- Matsukawa, H.; Akiyoshi-Nishimura, S.; Zhang, Q.; Lujan, R.; Yamaguchi, K.; Goto, H.; Yaguchi, K.; Hashikawa, T.; Sano, C.; Shigemoto, R.; et al. Netrin-G/NGL complexes encode functional synaptic diversification. J. Neurosci. 2014, 34, 15779–15792. [Google Scholar] [CrossRef]
- Nakashiba, T.; Nishimura, S.; Ikeda, T.; Itohara, S. Complementary expression and neurite outgrowth activity of netrin-G subfamily members. Mech. Dev. 2002, 111, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Holt, R.; Barnby, G.; Maestrini, E.; Bacchelli, E.; Brocklebank, D.; Sousa, I.; Mulder, E.J.; Kantojarvi, K.; Jarvela, I.; Klauck, S.M.; et al. Linkage and candidate gene studies of autism spectrum disorders in European populations. Eur. J. Hum. Genet. 2010, 18, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Iossifov, I.; Ronemus, M.; Levy, D.; Wang, Z.; Hakker, I.; Rosenbaum, J.; Yamrom, B.; Lee, Y.H.; Narzisi, G.; Leotta, A.; et al. De novo gene disruptions in children on the autistic spectrum. Neuron 2012, 74, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.H.; Yuen, R.K.; Jin, X.; Wang, M.; Chen, N.; Wu, X.; Ju, J.; Mei, J.; Shi, Y.; He, M.; et al. Detection of clinically relevant genetic variants in autism spectrum disorder by whole-genome sequencing. Am. J. Hum. Genet. 2013, 93, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Um, S.M.; Ha, S.; Lee, H.; Kim, J.; Kim, K.; Shin, W.; Cho, Y.S.; Roh, J.D.; Kang, J.; Yoo, T.; et al. NGL-2 Deletion Leads to Autistic-like Behaviors Responsive to NMDAR Modulation. Cell Rep. 2018, 23, 3839–3851. [Google Scholar] [CrossRef] [PubMed]
- Gilman, S.R.; Iossifov, I.; Levy, D.; Ronemus, M.; Wigler, M.; Vitkup, D. Rare de novo variants associated with autism implicate a large functional network of genes involved in formation and function of synapses. Neuron 2011, 70, 898–907. [Google Scholar] [CrossRef]
- Jamain, S.; Quach, H.; Betancur, C.; Rastam, M.; Colineaux, C.; Gillberg, I.C.; Soderstrom, H.; Giros, B.; Leboyer, M.; Gillberg, C.; et al. Paris Autism Research International Sibpair, S. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat. Genet. 2003, 34, 27–29. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Lehr, A.W.; Roche, K.W. Neuroligins and Neurodevelopmental Disorders: X-Linked Genetics. Front. Synaptic Neurosci. 2020, 12, 33. [Google Scholar] [CrossRef]
- Sudhof, T.C. Neuroligins and neurexins link synaptic function to cognitive disease. Nature 2008, 455, 903–911. [Google Scholar] [CrossRef]
- Zoghbi, H.Y. Postnatal neurodevelopmental disorders: Meeting at the synapse? Science 2003, 302, 826–830. [Google Scholar] [CrossRef]
- Chubykin, A.A.; Atasoy, D.; Etherton, M.R.; Brose, N.; Kavalali, E.T.; Gibson, J.R.; Sudhof, T.C. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron 2007, 54, 919–931. [Google Scholar] [CrossRef]
- Song, J.Y.; Ichtchenko, K.; Sudhof, T.C.; Brose, N. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc. Natl. Acad. Sci. USA 1999, 96, 1100–1105. [Google Scholar] [CrossRef]
- Graf, E.R.; Zhang, X.; Jin, S.X.; Linhoff, M.W.; Craig, A.M. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell 2004, 119, 1013–1026. [Google Scholar] [CrossRef]
- Varoqueaux, F.; Jamain, S.; Brose, N. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur. J. Cell Biol. 2004, 83, 449–456. [Google Scholar] [CrossRef]
- Budreck, E.C.; Scheiffele, P. Neuroligin-3 is a neuronal adhesion protein at GABAergic and glutamatergic synapses. Eur. J. Neurosci. 2007, 26, 1738–1748. [Google Scholar] [CrossRef]
- Boucard, A.A.; Chubykin, A.A.; Comoletti, D.; Taylor, P.; Sudhof, T.C. A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha- and beta-neurexins. Neuron 2005, 48, 229–236. [Google Scholar] [CrossRef]
- Chih, B.; Engelman, H.; Scheiffele, P. Control of excitatory and inhibitory synapse formation by neuroligins. Science 2005, 307, 1324–1328. [Google Scholar] [CrossRef]
- Jung, S.Y.; Kim, J.; Kwon, O.B.; Jung, J.H.; An, K.; Jeong, A.Y.; Lee, C.J.; Choi, Y.B.; Bailey, C.H.; Kandel, E.R.; et al. Input-specific synaptic plasticity in the amygdala is regulated by neuroligin-1 via postsynaptic NMDA receptors. Proc. Natl. Acad. Sci. USA 2010, 107, 4710–4715. [Google Scholar] [CrossRef]
- Prange, O.; Wong, T.P.; Gerrow, K.; Wang, Y.T.; El-Husseini, A. A balance between excitatory and inhibitory synapses is controlled by PSD-95 and neuroligin. Proc. Natl. Acad. Sci. USA 2004, 101, 13915–13920. [Google Scholar] [CrossRef]
- Soler-Llavina, G.J.; Fuccillo, M.V.; Ko, J.; Sudhof, T.C.; Malenka, R.C. The neurexin ligands, neuroligins and leucine-rich repeat transmembrane proteins, perform convergent and divergent synaptic functions in vivo. Proc. Natl. Acad. Sci. USA 2011, 108, 16502–16509. [Google Scholar] [CrossRef]
- Glessner, J.T.; Wang, K.; Cai, G.; Korvatska, O.; Kim, C.E.; Wood, S.; Zhang, H.; Estes, A.; Brune, C.W.; Bradfield, J.P.; et al. Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature 2009, 459, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Oliveira, G.; Coutinho, A.; Yang, C.; Feng, J.; Katz, C.; Sram, J.; Bockholt, A.; Jones, I.R.; Craddock, N.; et al. Analysis of the neuroligin 3 and 4 genes in autism and other neuropsychiatric patients. Mol. Psychiatry 2005, 10, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Ylisaukko-oja, T.; Rehnstrom, K.; Auranen, M.; Vanhala, R.; Alen, R.; Kempas, E.; Ellonen, P.; Turunen, J.A.; Makkonen, I.; Riikonen, R.; et al. Analysis of four neuroligin genes as candidates for autism. Eur. J. Hum. Genet. 2005, 13, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.; Blaiss, C.A.; Etherton, M.R.; Espinosa, F.; Tabuchi, K.; Walz, C.; Bolliger, M.F.; Sudhof, T.C.; Powell, C.M. Neuroligin-1 deletion results in impaired spatial memory and increased repetitive behavior. J. Neurosci. 2010, 30, 2115–2129. [Google Scholar] [CrossRef] [PubMed]
- Budreck, E.C.; Kwon, O.B.; Jung, J.H.; Baudouin, S.; Thommen, A.; Kim, H.S.; Fukazawa, Y.; Harada, H.; Tabuchi, K.; Shigemoto, R.; et al. Neuroligin-1 controls synaptic abundance of NMDA-type glutamate receptors through extracellular coupling. Proc. Natl. Acad. Sci. USA 2013, 110, 725–730. [Google Scholar] [CrossRef]
- Etherton, M.; Foldy, C.; Sharma, M.; Tabuchi, K.; Liu, X.; Shamloo, M.; Malenka, R.C.; Sudhof, T.C. Autism-linked neuroligin-3 R451C mutation differentially alters hippocampal and cortical synaptic function. Proc. Natl. Acad. Sci. USA 2011, 108, 13764–13769. [Google Scholar] [CrossRef]
- Alarcon, M.; Abrahams, B.S.; Stone, J.L.; Duvall, J.A.; Perederiy, J.V.; Bomar, J.M.; Sebat, J.; Wigler, M.; Martin, C.L.; Ledbetter, D.H.; et al. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am. J. Hum. Genet. 2008, 82, 150–159. [Google Scholar] [CrossRef]
- Arking, D.E.; Cutler, D.J.; Brune, C.W.; Teslovich, T.M.; West, K.; Ikeda, M.; Rea, A.; Guy, M.; Lin, S.; Cook, E.H.; et al. A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. Am. J. Hum. Genet. 2008, 82, 160–164. [Google Scholar] [CrossRef]
- Bakkaloglu, B.; O’Roak, B.J.; Louvi, A.; Gupta, A.R.; Abelson, J.F.; Morgan, T.M.; Chawarska, K.; Klin, A.; Ercan-Sencicek, A.G.; Stillman, A.A.; et al. Molecular cytogenetic analysis and resequencing of contactin associated protein-like 2 in autism spectrum disorders. Am. J. Hum. Genet. 2008, 82, 165–173. [Google Scholar] [CrossRef]
- Poliak, S.; Gollan, L.; Martinez, R.; Custer, A.; Einheber, S.; Salzer, J.L.; Trimmer, J.S.; Shrager, P.; Peles, E. Caspr2, a new member of the neurexin superfamily, is localized at the juxtaparanodes of myelinated axons and associates with K+ channels. Neuron 1999, 24, 1037–1047. [Google Scholar] [CrossRef]
- Poliak, S.; Salomon, D.; Elhanany, H.; Sabanay, H.; Kiernan, B.; Pevny, L.; Stewart, C.L.; Xu, X.; Chiu, S.Y.; Shrager, P.; et al. Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J. Cell Biol. 2003, 162, 1149–1160. [Google Scholar] [CrossRef]
- Varea, O.; Martin-de-Saavedra, M.D.; Kopeikina, K.J.; Schurmann, B.; Fleming, H.J.; Fawcett-Patel, J.M.; Bach, A.; Jang, S.; Peles, E.; Kim, E.; et al. Synaptic abnormalities and cytoplasmic glutamate receptor aggregates in contactin associated protein-like 2/Caspr2 knockout neurons. Proc. Natl. Acad. Sci. USA 2015, 112, 6176–6181. [Google Scholar] [CrossRef]
- Anderson, G.R.; Galfin, T.; Xu, W.; Aoto, J.; Malenka, R.C.; Sudhof, T.C. Candidate autism gene screen identifies critical role for cell-adhesion molecule CASPR2 in dendritic arborization and spine development. Proc. Natl. Acad. Sci. USA 2012, 109, 18120–18125. [Google Scholar] [CrossRef]
- Penagarikano, O.; Abrahams, B.S.; Herman, E.I.; Winden, K.D.; Gdalyahu, A.; Dong, H.; Sonnenblick, L.I.; Gruver, R.; Almajano, J.; Bragin, A.; et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell 2011, 147, 235–246. [Google Scholar] [CrossRef]
- Jurgensen, S.; Castillo, P.E. Selective Dysregulation of Hippocampal Inhibition in the Mouse Lacking Autism Candidate Gene CNTNAP2. J. Neurosci. 2015, 35, 14681–14687. [Google Scholar] [CrossRef]
- Chen, Y.K.; Chen, C.Y.; Hu, H.T.; Hsueh, Y.P. CTTNBP2, but not CTTNBP2NL, regulates dendritic spinogenesis and synaptic distribution of the striatin-PP2A complex. Mol. Biol. Cell 2012, 23, 4383–4392. [Google Scholar] [CrossRef]
- Hering, H.; Sheng, M. Activity-dependent redistribution and essential role of cortactin in dendritic spine morphogenesis. J. Neurosci. 2003, 23, 11759–11769. [Google Scholar] [CrossRef]
- Shih, P.Y.; Hsieh, B.Y.; Lin, M.H.; Huang, T.N.; Tsai, C.Y.; Pong, W.L.; Lee, S.P.; Hsueh, Y.P. CTTNBP2 Controls Synaptic Expression of Zinc-Related Autism-Associated Proteins and Regulates Synapse Formation and Autism-like Behaviors. Cell Rep. 2020, 31, 107700. [Google Scholar] [CrossRef]
- Ruzzo, E.K.; Perez-Cano, L.; Jung, J.Y.; Wang, L.K.; Kashef-Haghighi, D.; Hartl, C.; Singh, C.; Xu, J.; Hoekstra, J.N.; Leventhal, O.; et al. Inherited and De Novo Genetic Risk for Autism Impacts Shared Networks. Cell 2019, 178, 850–866.e26. [Google Scholar] [CrossRef]
- Sanders, S.J.; He, X.; Willsey, A.J.; Ercan-Sencicek, A.G.; Samocha, K.E.; Cicek, A.E.; Murtha, M.T.; Bal, V.H.; Bishop, S.L.; Dong, S.; et al. Insights into Autism Spectrum Disorder Genomic Architecture and Biology from 71 Risk Loci. Neuron 2015, 87, 1215–1233. [Google Scholar] [CrossRef]
- Ben-David, E.; Shifman, S.J. Combined analysis of exome sequencing points toward a major role for transcription regulation during brain development in autism. Mol. Psychiatry 2013, 18, 1054–1056. [Google Scholar] [CrossRef] [PubMed]
- de la Torre-Ubieta, L.; Won, H.; Stein, J.L.; Geschwind, D.H. Advancing the understanding of autism disease mechanisms through genetics. Nat. Med. 2016, 22, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Zhang, R.; Yao, V.; Theesfeld, C.L.; Wong, A.K.; Tadych, A.; Volfovsky, N.; Packer, A.; Lash, A.; Troyanskaya, O.G. Genome-wide prediction and functional characterization of the genetic basis of autism spectrum disorder. Nat. Neurosci. 2016, 19, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Parikshak, N.N.; Luo, R.; Zhang, A.; Won, H.; Lowe, J.K.; Chandran, V.; Horvath, S.; Geschwind, D.H. Integrative functional genomic analyses implicate specific molecular pathways and circuits in autism. Cell 2013, 155, 1008–1021. [Google Scholar] [CrossRef] [PubMed]
- Bulfone, A.; Wang, F.; Hevner, R.; Anderson, S.; Cutforth, T.; Chen, S.; Meneses, J.; Pedersen, R.; Axel, R.; Rubenstein, J.L. An olfactory sensory map develops in the absence of normal projection neurons or GABAergic interneurons. Neuron 1998, 21, 1273–1282. [Google Scholar] [CrossRef]
- Hevner, R.F.; Shi, L.; Justice, N.; Hsueh, Y.; Sheng, M.; Smiga, S.; Bulfone, A.; Goffinet, A.M.; Campagnoni, A.T.; Rubenstein, J.L. Tbr1 regulates differentiation of the preplate and layer 6. Neuron 2001, 29, 353–366. [Google Scholar] [CrossRef]
- Huang, T.N.; Chuang, H.C.; Chou, W.H.; Chen, C.Y.; Wang, H.F.; Chou, S.J.; Hsueh, Y.P. Tbr1 haploinsufficiency impairs amygdalar axonal projections and results in cognitive abnormality. Nat. Neurosci. 2014, 17, 240–247. [Google Scholar] [CrossRef]
- Huang, T.N.; Hsueh, Y.P. Brain-specific transcriptional regulator T-brain-1 controls brain wiring and neuronal activity in autism spectrum disorders. Front. Neurosci. 2015, 9, 406. [Google Scholar] [CrossRef]
- Huang, T.N.; Yen, T.L.; Qiu, L.R.; Chuang, H.C.; Lerch, J.P.; Hsueh, Y.P. Haploinsufficiency of autism causative gene Tbr1 impairs olfactory discrimination and neuronal activation of the olfactory system in mice. Mol. Autism 2019, 10, 5. [Google Scholar] [CrossRef]
- Remedios, R.; Huilgol, D.; Saha, B.; Hari, P.; Bhatnagar, L.; Kowalczyk, T.; Hevner, R.F.; Suda, Y.; Aizawa, S.; Ohshima, T.; et al. A stream of cells migrating from the caudal telencephalon reveals a link between the amygdala and neocortex. Nat. Neurosci. 2007, 10, 1141–1150. [Google Scholar] [CrossRef]
- Chuang, H.C.; Huang, T.N.; Hsueh, Y.P. Neuronal excitation upregulates Tbr1, a high-confidence risk gene of autism, mediating Grin2b expression in the adult brain. Front. Cell. Neurosci. 2014, 8, 280. [Google Scholar] [CrossRef]
- Hsueh, Y.P.; Wang, T.F.; Yang, F.C.; Sheng, M. Nuclear translocation and transcription regulation by the membrane-associated guanylate kinase CASK/LIN-2. Nature 2000, 404, 298–302. [Google Scholar] [CrossRef]
- Landrigan, P.J. What causes autism? Exploring the environmental contribution. Curr. Opin. Pediatr. 2010, 22, 219–225. [Google Scholar] [CrossRef]
- Persico, A.M.; Merelli, S.J. Environmental factors in the onset of autism spectrum disorder. Curr. Dev. Dis. Rep. 2014, 1, 8–19. [Google Scholar] [CrossRef]
- Hughes, H.K.; Mills Ko, E.; Rose, D.; Ashwood, P.J. Immune dysfunction and autoimmunity as pathological mechanisms in autism spectrum disorders. Front. Cell. Neurosci. 2018, 12, 405. [Google Scholar] [CrossRef]
- Braunschweig, D.; Krakowiak, P.; Duncanson, P.; Boyce, R.; Hansen, R.L.; Ashwood, P.; Hertz-Picciotto, I.; Pessah, I.N.; Van de Water, J. Autism-specific maternal autoantibodies recognize critical proteins in developing brain. Transl. Psychiatry 2013, 3, e277. [Google Scholar] [CrossRef]
- Hertz-Picciotto, I.; Croen, L.A.; Hansen, R.; Jones, C.R.; van de Water, J.; Pessah, I.N. The CHARGE study: An epidemiologic investigation of genetic and environmental factors contributing to autism. Environ. Health Perspect. 2006, 114, 1119–1125. [Google Scholar] [CrossRef]
- Ramirez-Celis, A.; Becker, M.; Nuno, M.; Schauer, J.; Aghaeepour, N.; Van de Water, J. Risk assessment analysis for maternal autoantibody-related autism (MAR-ASD): A subtype of autism. Mol. Psychiatry 2021, 26, 1551–1560. [Google Scholar] [CrossRef]
- Ramirez-Celis, A.; Croen, L.A.; Yoshida, C.K.; Alexeeff, S.E.; Schauer, J.; Yolken, R.H.; Ashwood, P.; Van de Water, J. Maternal autoantibody profiles as biomarkers for ASD and ASD with co-occurring intellectual disability. Mol. Psychiatry 2022, 27, 3760–3767. [Google Scholar] [CrossRef]
- Elshahawi, H.H.; Taha, G.R.A.; Azzam, H.M.E.; El Ghamry, R.H.; Abdelgawad, A.A.M.; Elshiekh, M.A. N-Methyl-d-Aspartate (NMDA) receptor antibody in relation to autism spectrum disorder (ASD): Presence and association with symptom profile. Middle East Curr. Psychiatry 2021, 28, 62. [Google Scholar] [CrossRef]
- Coutinho, E.; Jacobson, L.; Pedersen, M.G.; Benros, M.E.; Norgaard-Pedersen, B.; Mortensen, P.B.; Harrison, P.J.; Vincent, A. CASPR2 autoantibodies are raised during pregnancy in mothers of children with mental retardation and disorders of psychological development but not autism. J. Neurol. Neurosurg. Psychiatry 2017, 88, 718–721. [Google Scholar] [CrossRef] [PubMed]
- Kiani, R.; Lawden, M.; Eames, P.; Critchley, P.; Bhaumik, S.; Odedra, S.; Gumber, R. Anti-NMDA-receptor encephalitis presenting with catatonia and neuroleptic malignant syndrome in patients with intellectual disability and autism. BJPsych Bull. 2015, 39, 32–35. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kurita, D.; Wakuda, T.; Takagai, S.; Takahashi, Y.; Iwata, Y.; Suzuki, K.; Mori, N. Deterioration of clinical features of a patient with autism spectrum disorder after anti-N-methyl-D-aspartate receptor encephalitis. Psychiatry Clin. Neurosci. 2015, 69, 507. [Google Scholar] [CrossRef] [PubMed]
- Scott, O.; Richer, L.; Forbes, K.; Sonnenberg, L.; Currie, A.; Eliyashevska, M.; Goez, H.R. Anti-N-methyl-D-aspartate (NMDA) receptor encephalitis: An unusual cause of autistic regression in a toddler. J. Child Neurol. 2014, 29, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Atladottir, H.O.; Thorsen, P.; Ostergaard, L.; Schendel, D.E.; Lemcke, S.; Abdallah, M.; Parner, E.T. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J. Autism Dev. Disord. 2010, 40, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Zerbo, O.; Qian, Y.; Yoshida, C.; Grether, J.K.; Van de Water, J.; Croen, L.A. Maternal Infection During Pregnancy and Autism Spectrum Disorders. J. Autism Dev. Disord. 2015, 45, 4015–4025. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Magnusson, C.; Gardner, R.M.; Blomström, Å.; Newschaffer, C.J.; Burstyn, I.; Karlsson, H.; Dalman, C.J.B. Maternal hospitalization with infection during pregnancy and risk of autism spectrum disorders. Brain Behav. Immun. 2015, 44, 100–105. [Google Scholar] [CrossRef]
- Carlezon, W.A., Jr.; Kim, W.; Missig, G.; Finger, B.C.; Landino, S.M.; Alexander, A.J.; Mokler, E.L.; Robbins, J.O.; Li, Y.; Bolshakov, V.Y.; et al. Maternal and early postnatal immune activation produce sex-specific effects on autism-like behaviors and neuroimmune function in mice. Sci. Rep. 2019, 9, 16928. [Google Scholar] [CrossRef]
- Choi, G.B.; Yim, Y.S.; Wong, H.; Kim, S.; Kim, H.; Kim, S.V.; Hoeffer, C.A.; Littman, D.R.; Huh, J.R. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 2016, 351, 933–939. [Google Scholar] [CrossRef]
- de Cossío, L.F.; Guzmán, A.; Van Der Veldt, S.; Luheshi, G.N. Prenatal infection leads to ASD-like behavior and altered synaptic pruning in the mouse offspring. Brain Behav. Immun. 2017, 63, 88–98. [Google Scholar] [CrossRef]
- Hsueh, P.T.; Wang, H.H.; Liu, C.L.; Ni, W.F.; Chen, Y.L.; Liu, J.K. Expression of cerebral serotonin related to anxiety-like behaviors in C57BL/6 offspring induced by repeated subcutaneous prenatal exposure to low-dose lipopolysaccharide. PLoS ONE 2017, 12, e0179970. [Google Scholar] [CrossRef]
- Kalish, B.T.; Kim, E.; Finander, B.; Duffy, E.E.; Kim, H.; Gilman, C.K.; Yim, Y.S.; Tong, L.; Kaufman, R.J.; Griffith, E.C.; et al. Maternal immune activation in mice disrupts proteostasis in the fetal brain. Nat. Neurosci. 2021, 24, 204–213. [Google Scholar] [CrossRef]
- Kwon, H.K.; Choi, G.B.; Huh, J.R. Maternal inflammation and its ramifications on fetal neurodevelopment. Trends Immunol. 2022, 43, 230–244. [Google Scholar] [CrossRef]
- Schwartzer, J.J.; Careaga, M.; Onore, C.E.; Rushakoff, J.A.; Berman, R.F.; Ashwood, P. Maternal immune activation and strain specific interactions in the development of autism-like behaviors in mice. Transl. Psychiatry 2013, 3, e240. [Google Scholar] [CrossRef]
- Smith, S.E.; Li, J.; Garbett, K.; Mirnics, K.; Patterson, P.H. Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci. 2007, 27, 10695–10702. [Google Scholar] [CrossRef]
- Kirsten, T.B.; Bernardi, M.M.J.B. Prenatal lipopolysaccharide induces hypothalamic dopaminergic hypoactivity and autistic-like behaviors: Repetitive self-grooming and stereotypies. Behav Brain Res. 2017, 331, 25–29. [Google Scholar] [CrossRef]
- Xiao, L.; Yan, J.; Feng, D.; Ye, S.; Yang, T.; Wei, H.; Li, T.; Sun, W.; Chen, J. Critical Role of TLR4 on the Microglia Activation Induced by Maternal LPS Exposure Leading to ASD-Like Behavior of Offspring. Front. Cell Dev. Biol. 2021, 9, 634837. [Google Scholar] [CrossRef]
- Forrest, C.M.; Khalil, O.S.; Pisar, M.; Smith, R.A.; Darlington, L.G.; Stone, T.W. Prenatal activation of Toll-like receptors-3 by administration of the viral mimetic poly(I:C) changes synaptic proteins, N-methyl-D-aspartate receptors and neurogenesis markers in offspring. Mol. Brain 2012, 5, 22. [Google Scholar] [CrossRef]
- Su, Y.; Lian, J.; Hodgson, J.; Zhang, W.; Deng, C. Prenatal Poly I:C Challenge Affects Behaviors and Neurotransmission via Elevated Neuroinflammation Responses in Female Juvenile Rats. Int. J. Neuropsychopharmacol. 2022, 25, 160–171. [Google Scholar] [CrossRef]
- Croen, L.A.; Grether, J.K.; Yoshida, C.K.; Odouli, R.; Hendrick, V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch. Gen. Psychiatry 2011, 68, 1104–1112. [Google Scholar] [CrossRef]
- Gardener, H.; Spiegelman, D.; Buka, S.L. Prenatal risk factors for autism: Comprehensive meta-analysis. Br. J. Psychiatry 2009, 195, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Rasalam, A.D.; Hailey, H.; Williams, J.H.; Moore, S.J.; Turnpenny, P.D.; Lloyd, D.J.; Dean, J.C. Characteristics of fetal anticonvulsant syndrome associated autistic disorder. Dev. Med. Child Neurol. 2005, 47, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Christianson, A.L.; Chesler, N.; Kromberg, J.G. Fetal valproate syndrome: Clinical and neuro-developmental features in two sibling pairs. Dev. Med. Child Neurol. 1994, 36, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.J.; Turnpenny, P.; Quinn, A.; Glover, S.; Lloyd, D.J.; Montgomery, T.; Dean, J.C. A clinical study of 57 children with fetal anticonvulsant syndromes. J. Med. Genet. 2000, 37, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.G.; Hersh, J.H. A male with fetal valproate syndrome and autism. Dev. Med. Child Neurol. 1997, 39, 632–634. [Google Scholar] [CrossRef]
- Chomiak, T.; Turner, N.; Hu, B.J. What we have learned about autism spectrum disorder from valproic acid. Pathol. Res. Int. 2013, 2013, 712758. [Google Scholar] [CrossRef]
- Chaliha, D.; Albrecht, M.; Vaccarezza, M.; Takechi, R.; Lam, V.; Al-Salami, H.; Mamo, J. A Systematic Review of the Valproic-Acid-Induced Rodent Model of Autism. Dev. Neurosci. 2020, 42, 12–48. [Google Scholar] [CrossRef]
- Fereshetyan, K.; Chavushyan, V.; Danielyan, M.; Yenkoyan, K. Assessment of behavioral, morphological and electrophysiological changes in prenatal and postnatal valproate induced rat models of autism spectrum disorder. Sci. Rep. 2021, 11, 23471. [Google Scholar] [CrossRef]
- Kim, J.W.; Seung, H.; Kim, K.C.; Gonzales, E.L.T.; Oh, H.A.; Yang, S.M.; Ko, M.J.; Han, S.H.; Banerjee, S.; Shin, C.Y. Agmatine rescues autistic behaviors in the valproic acid-induced animal model of autism. Neuropharmacology 2017, 113 Pt A, 71–81. [Google Scholar] [CrossRef]
- Kim, J.-W.; Seung, H.; Kwon, K.J.; Ko, M.J.; Lee, E.J.; Oh, H.A.; Choi, C.S.; Kim, K.C.; Gonzales, E.L.; You, J.S. Subchronic treatment of donepezil rescues impaired social, hyperactive, and stereotypic behavior in valproic acid-induced animal model of autism. PLoS ONE 2014, 9, e104927. [Google Scholar] [CrossRef]
- Kim, K.C.; Kim, P.; Go, H.S.; Choi, C.S.; Yang, S.-I.; Cheong, J.H.; Shin, C.Y.; Ko, K.H. The critical period of valproate exposure to induce autistic symptoms in Sprague–Dawley rats. Toxicol. Lett. 2011, 201, 137–142. [Google Scholar] [CrossRef]
- Mehta, M.V.; Gandal, M.J.; Siegel, S.J. mGluR5-antagonist mediated reversal of elevated stereotyped, repetitive behaviors in the VPA model of autism. PLoS ONE 2011, 6, e26077. [Google Scholar] [CrossRef]
- Mohammadi, F.; Rakhshan, M.; Molazem, Z.; Zareh, N.; Gillespie, M. Development of Parental Competence Scale in Parents of Children with Autism. J. Pediatr. Nurs. 2020, 50, e77–e84. [Google Scholar] [CrossRef]
- Roullet, F.I.; Wollaston, L.; Decatanzaro, D.; Foster, J.A. Behavioral and molecular changes in the mouse in response to prenatal exposure to the anti-epileptic drug valproic acid. Neuroscience 2010, 170, 514–522. [Google Scholar] [CrossRef]
- Schneider, T.; Przewlocki, R. Behavioral alterations in rats prenatally exposed to valproic acid: Animal model of autism. Neuropsychopharmacology 2005, 30, 80–89. [Google Scholar] [CrossRef]
- Schneider, T.; Roman, A.; Basta-Kaim, A.; Kubera, M.; Budziszewska, B.; Schneider, K.; Przewlocki, R. Gender-specific behavioral and immunological alterations in an animal model of autism induced by prenatal exposure to valproic acid. Psychoneuroendocrinology 2008, 33, 728–740. [Google Scholar] [CrossRef]
- Schneider, T.; Ziolkowska, B.; Gieryk, A.; Tyminska, A.; Przewlocki, R. Prenatal exposure to valproic acid disturbs the enkephalinergic system functioning, basal hedonic tone, and emotional responses in an animal model of autism. Psychopharmacology 2007, 193, 547–555. [Google Scholar] [CrossRef]
- Go, H.S.; Kim, K.C.; Choi, C.S.; Jeon, S.J.; Kwon, K.J.; Han, S.H.; Lee, J.; Cheong, J.H.; Ryu, J.H.; Kim, C.H.; et al. Prenatal exposure to valproic acid increases the neural progenitor cell pool and induces macrocephaly in rat brain via a mechanism involving the GSK-3beta/beta-catenin pathway. Neuropharmacology 2012, 63, 1028–1041. [Google Scholar] [CrossRef]
- Hao, Y.; Creson, T.; Zhang, L.; Li, P.; Du, F.; Yuan, P.; Gould, T.D.; Manji, H.K.; Chen, G. Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. J. Neurosci. 2004, 24, 6590–6599. [Google Scholar] [CrossRef]
- Foley, A.G.; Gannon, S.; Rombach-Mullan, N.; Prendergast, A.; Barry, C.; Cassidy, A.W.; Regan, C.M. Class I histone deacetylase inhibition ameliorates social cognition and cell adhesion molecule plasticity deficits in a rodent model of autism spectrum disorder. Neuropharmacology 2012, 63, 750–760. [Google Scholar] [CrossRef]
- Fukuchi, M.; Nii, T.; Ishimaru, N.; Minamino, A.; Hara, D.; Takasaki, I.; Tabuchi, A.; Tsuda, M.J. Valproic acid induces up-or down-regulation of gene expression responsible for the neuronal excitation and inhibition in rat cortical neurons through its epigenetic actions. Neurosci. Res. 2009, 65, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Tung, E.W.; Winn, L.M. Epigenetic modifications in valproic acid-induced teratogenesis. Toxicol. App. Pharmacol. 2010, 248, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, S.; Liang, M.; Marinova, Z.; Yahyavi, A.; Chuang, D.J. The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons. Mol. Psychiatry 2009, 14, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Chomiak, T.; Karnik, V.; Block, E.; Hu, B.J. Altering the trajectory of early postnatal cortical development can lead to structural and behavioural features of autism. BMC Neurosci. 2010, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, T.; Kulangara, K.; Antoniello, K.; Markram, H.J. Elevated NMDA receptor levels and enhanced postsynaptic long-term potentiation induced by prenatal exposure to valproic acid. Proc. Natl. Acad. Sci. USA 2007, 104, 13501–13506. [Google Scholar] [CrossRef]
- Rinaldi, T.; Silberberg, G.; Markram, H. Hyperconnectivity of local neocortical microcircuitry induced by prenatal exposure to valproic acid. Cereb. Cortex 2008, 18, 763–770. [Google Scholar] [CrossRef]
- Silva, G.T.; Le Be, J.V.; Riachi, I.; Rinaldi, T.; Markram, K.; Markram, H. Enhanced long-term microcircuit plasticity in the valproic Acid animal model of autism. Front. Synaptic Neurosci. 2009, 1, 1. [Google Scholar] [CrossRef]
- Banerjee, A.; García-Oscos, F.; Roychowdhury, S.; Galindo, L.C.; Hall, S.; Kilgard, M.P.; Atzori, M.J. Impairment of cortical GABAergic synaptic transmission in an environmental rat model of autism. Int. J. Neuropsychopharm. 2013, 16, 1309–1318. [Google Scholar] [CrossRef]
- Iijima, Y.; Behr, K.; Iijima, T.; Biemans, B.; Bischofberger, J.; Scheiffele, P.J. Distinct defects in synaptic differentiation of neocortical neurons in response to prenatal valproate exposure. Sci. Rep. 2016, 6, 27400. [Google Scholar] [CrossRef]
- Kolozsi, E.; Mackenzie, R.; Roullet, F.; Decatanzaro, D.; Foster, J.J.N. Prenatal exposure to valproic acid leads to reduced expression of synaptic adhesion molecule neuroligin 3 in mice. Neuroscience 2009, 163, 1201–1210. [Google Scholar] [CrossRef]
- Lin, H.C.; Gean, P.W.; Wang, C.C.; Chan, Y.H.; Chen, P.S. The amygdala excitatory/inhibitory balance in a valproate-induced rat autism model. PLoS ONE 2013, 8, e55248. [Google Scholar] [CrossRef]
- Qi, C.; Chen, A.; Mao, H.; Hu, E.; Ge, J.; Ma, G.; Ren, K.; Xue, Q.; Wang, W.; Wu, S.J. Excitatory and Inhibitory Synaptic Imbalance Caused by Brain-Derived Neurotrophic Factor Deficits During Development in a Valproic Acid Mouse Model of Autism. Front. Mol. Neurosci. 2022, 15, 860275. [Google Scholar] [CrossRef]
- Walcott, E.C.; Higgins, E.A.; Desai, N.S. Synaptic and intrinsic balancing during postnatal development in rat pups exposed to valproic acid in utero. J. Neurosci. 2011, 31, 13097–13109. [Google Scholar] [CrossRef]
- Martin, H.G.; Manzoni, O.J. Late onset deficits in synaptic plasticity in the valproic acid rat model of autism. Front. Cell. Neurosci. 2014, 8, 23. [Google Scholar] [CrossRef]
- Sauer, A.K.; Hagmeyer, S.; Grabrucker, A.M. Prenatal Zinc Deficient Mice as a Model for Autism Spectrum Disorders. Int. J. Mol. Sci. 2022, 23, 6082. [Google Scholar] [CrossRef]
- Mielnik, C.A.; Binko, M.A.; Chen, Y.; Funk, A.J.; Johansson, E.M.; Intson, K.; Sivananthan, N.; Islam, R.; Milenkovic, M.; Horsfall, W.J. Consequences of NMDA receptor deficiency can be rescued in the adult brain. Mol. Psychiatry 2021, 26, 2929–2942. [Google Scholar] [CrossRef]
- Posey, D.J.; Kem, D.L.; Swiezy, N.B.; Sweeten, T.L.; Wiegand, R.E.; McDougle, C.J. A pilot study of D-cycloserine in subjects with autistic disorder. Am. J. Psychiatry 2004, 161, 2115–2117. [Google Scholar] [CrossRef]
- Urbano, M.; Okwara, L.; Manser, P.; Hartmann, K.; Herndon, A.; Deutsch, S.I. A trial of D-cycloserine to treat stereotypies in older adolescents and young adults with autism spectrum disorder. Clin. Neuropharmacol. 2014, 37, 69. [Google Scholar] [CrossRef]
- Chung, W.; Choi, S.Y.; Lee, E.; Park, H.; Kang, J.; Park, H.; Choi, Y.; Lee, D.; Park, S.-G.; Kim, R.J. Social deficits in IRSp53 mutant mice improved by NMDAR and mGluR5 suppression. Nat. Neurosci. 2015, 18, 435–443. [Google Scholar] [CrossRef]
- Qin, L.; Ma, K.; Wang, Z.-J.; Hu, Z.; Matas, E.; Wei, J.; Yan, Z.J. Social deficits in Shank3-deficient mouse models of autism are rescued by histone deacetylase (HDAC) inhibition. Nat. Neurosci. 2018, 21, 564–575. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Zhong, P.; Ma, K.; Seo, J.-S.; Yang, F.; Hu, Z.; Zhang, F.; Lin, L.; Wang, J.; Liu, T.J. Amelioration of autism-like social deficits by targeting histone methyltransferases EHMT1/2 in Shank3-deficient mice. Mol. Psychiatry 2020, 25, 2517–2533. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Qin, L.; Matas, E.; Duffney, L.J.; Liu, A.; Yan, Z.J.N. Histone deacetylase inhibitor MS-275 restores social and synaptic function in a Shank3-deficient mouse model of autism. Neuropharmacology 2018, 43, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Hagmeyer, S.; Sauer, A.K.; Grabrucker, A.M. Prospects of zinc supplementation in autism spectrum disorders and Shankopathies such as Phelan McDermid Syndrome. Front. Syn. Neurosci. 2018, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Alsufiani, H.M.; Alkhanbashi, A.S.; Laswad, N.A.B.; Bakhadher, K.K.; Alghamdi, S.A.; Tayeb, H.O.; Tarazi, F.I. Zinc deficiency and supplementation in autism spectrum disorder and Phelan-McDermid syndrome. J. Neurosci. 2022, 100, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Sensi, S.L.; Paoletti, P.; Bush, A.I.; Sekler, I.J. Zinc in the physiology and pathology of the CNS. Nat. Rev. Neurosci. 2009, 10, 780–791. [Google Scholar] [CrossRef]
- Smart, T.G.; Hosie, A.M.; Miller, P.S. Zn2+ ions: Modulators of excitatory and inhibitory synaptic activity. Neuroscientist 2004, 10, 432–442. [Google Scholar] [CrossRef]
- Babaknejad, N.; Sayehmiri, F.; Sayehmiri, K.; Mohamadkhani, A.; Bahrami, S.J. The relationship between zinc levels and autism: A systematic review and meta-analysis. Iran. J. Child Neurol. 2016, 10, 1. [Google Scholar]
- Crăciun, E.C.; Bjørklund, G.; Tinkov, A.A.; Urbina, M.A.; Skalny, A.V.; Rad, F.; Dronca, E.J. Evaluation of whole blood zinc and copper levels in children with autism spectrum disorder. Metab. Brain Dis. 2016, 31, 887–890. [Google Scholar] [CrossRef]
- Faber, S.; Zinn, G.M.; Kern Ii, J.C.; Skip Kingston, H.J.B. The plasma zinc/serum copper ratio as a biomarker in children with autism spectrum disorders. Biomarkers 2009, 14, 171–180. [Google Scholar] [CrossRef]
- Li, S.-O.; Wang, J.-L.; Bjørklund, G.; Zhao, W.-N.; Yin, C. Serum copper and zinc levels in individuals with autism spectrum disorders. Neuroreport 2014, 25, 1216–1220. [Google Scholar] [CrossRef]
- Yasuda, H.; Yoshida, K.; Yasuda, Y.; Tsutsui, T.J. Infantile zinc deficiency: Association with autism spectrum disorders. Sci. Rep. 2011, 1, 129. [Google Scholar] [CrossRef]
- Cezar, L.C.; Kirsten, T.B.; da Fonseca, C.C.N.; de Lima, A.P.N.; Bernardi, M.M.; Felicio, L.F. Zinc as a therapy in a rat model of autism prenatally induced by valproic acid. Prog. Neuropsychiatry Biol. Psychiatry 2018, 84, 173–180. [Google Scholar] [CrossRef]
- Kirsten, T.B.; Queiroz-Hazarbassanov, N.; Bernardi, M.M.; Felicio, L.F. Prenatal zinc prevents communication impairments and BDNF disturbance in a rat model of autism induced by prenatal lipopolysaccharide exposure. Life Sci. 2015, 130, 12–17. [Google Scholar] [CrossRef]
- Paudel, R.; Raj, K.; Gupta, Y.; Singh, S.J. Oxiracetam and zinc ameliorates autism-like symptoms in propionic acid model of rats. Neurotox. Res. 2020, 37, 815–826. [Google Scholar] [CrossRef]
- Coyle, P.; Tran, N.; Fung, J.N.; Summers, B.L.; Rofe, A.M. Maternal dietary zinc supplementation prevents aberrant behaviour in an object recognition task in mice offspring exposed to LPS in early pregnancy. Behav. Brain Res. 2009, 197, 210–218. [Google Scholar] [CrossRef]
- Lee, K.; Jung, Y.; Vyas, Y.; Skelton, I.; Abraham, W.C.; Hsueh, Y.-P.; Montgomery, J.M. Dietary zinc supplementation rescues fear-based learning and synaptic function in the Tbr1+/− mouse model of autism spectrum disorders. Mol. Autism 2022, 13, 13. [Google Scholar] [CrossRef]
- Carey, L.C.; Berbée, P.L.; Coyle, P.; Philcox, J.C.; Rofe, A.M. Zinc treatment prevents lipopolysaccharide-induced teratogenicity in mice. Birth Defects Res. Part A Clin. Mol. Teratol. 2003, 67, 240–245. [Google Scholar] [CrossRef]
- Daston, G.P.; Overmann, G.J.; Taubeneck, M.W.; Lehman-McKeeman, L.D.; Rogers, J.M.; Keen, C.L. The role of metallothionein induction and altered zinc status in maternally mediated developmental toxicity: Comparison of the effects of urethane and styrene in rats. Toxicol. App. Pharmacol. 1991, 110, 450–463. [Google Scholar] [CrossRef]
- Taubeneck, M.W.; Daston, G.P.; Rogers, J.M.; Gershwin, M.E.; Ansari, A.; Keen, C.L. Tumor necrosis factor-α alters maternal and embryonic zinc metabolism and is developmentally toxic in mice. J. Nutr. 1995, 125, 908–919. [Google Scholar]
- Lewis, M.; Kim, S.-J. The pathophysiology of restricted repetitive behavior. J. Neurodev. Dis. 2009, 1, 114–132. [Google Scholar] [CrossRef]
- Bareggi, S.R.; Cornelli, U.J. Clioquinol: Review of its mechanisms of action and clinical uses in neurodegenerative disorders. CNS Neurosci Ther. 2012, 18, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Kodirov, S.A.; Takizawa, S.; Joseph, J.; Kandel, E.R.; Shumyatsky, G.P.; Bolshakov, V.Y. Synaptically released zinc gates long-term potentiation in fear conditioning pathways. Proc. Natl. Acad. Sci. USA 2006, 103, 15218–15223. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, R.; Tosi, G.; Grabrucker, A.M. Emerging use of nanotechnology in the treatment of neurological disorders. Curr. Pharm. Des. 2015, 21, 3111–3130. [Google Scholar] [CrossRef] [PubMed]
- Saghazadeh, A.; Ahangari, N.; Hendi, K.; Saleh, F.; Rezaei, N.J. Status of essential elements in autism spectrum disorder: Systematic review and meta-analysis. Rev. Neurosci. 2017, 28, 783–809. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Finkelstein, J.L.; Venkatramanan, S.; Huey, S.L.; Udipi, S.A.; Ghugre, P.; Ruth, C.; Canfield, R.L.; Kurpad, A.V.; Potdar, R.D. Effect of iron and zinc-biofortified pearl millet consumption on growth and immune competence in children aged 12–18 months in India: Study protocol for a randomised controlled trial. BMJ 2017, 7, e017631. [Google Scholar] [CrossRef]
- Russo, A.; Devito, R.J. Analysis of copper and zinc plasma concentration and the efficacy of zinc therapy in individuals with asperger’s syndrome, pervasive developmental disorder not otherwise specified (PDD-NOS) and autism. Biomark. Insights 2011, 6, S7286. [Google Scholar] [CrossRef]
- Blaurock-Busch, E.; DESSOKI, H.H.; RABAH, T.J.M. Toxic metals and essential elements in hair and severity of symptoms among children with autism. Medica 2012, 7, 38. [Google Scholar]
- Fiore, M.; Barone, R.; Copat, C.; Grasso, A.; Cristaldi, A.; Rizzo, R.; Ferrante, M.J. Biology, Metal and essential element levels in hair and association with autism severity. J. Trace Elem. Med. Biol. 2020, 57, 126409. [Google Scholar] [CrossRef]
- Skalny, A.V.; Mazaletskaya, A.L.; Ajsuvakova, O.P.; Bjørklund, G.; Skalnaya, M.G.; Notova, S.V.; Chernova, L.N.; Skalny, A.A.; Burtseva, T.I.; Tinkov, A.A. Biology, Hair trace element concentrations in autism spectrum disorder (ASD) and attention deficit/hyperactivity disorder (ADHD). J. Trace Elem. Med. Biol. 2020, 61, 126539. [Google Scholar] [CrossRef]
- Meguid, N.A.; Bjørklund, G.; Gebril, O.H.; Doşa, M.D.; Anwar, M.; Elsaeid, A.; Gaber, A.; Chirumbolo, S.J. The role of zinc supplementation on the metallothionein system in children with autism spectrum disorder. Acta Neurol. Belg. 2019, 119, 577–583. [Google Scholar] [CrossRef]
- Sajedi, F.; Shahshahani, S.; Ghiasvand, H.; Mosallanezhad, Z.; Fatollahierad, S.J. Does zinc with and without iron co-supplementation have effect on motor and mental development of children? A systematic review and meta-analysis. BMC Paediatr. 2020, 20, 451. [Google Scholar] [CrossRef]
- Zahiri Sorouri, Z.; Sadeghi, H.; Pourmarzi, D.J.; Medicine, N. The effect of zinc supplementation on pregnancy outcome: A randomized controlled trial. J. Matern.-Fetal Neonat. Med. 2016, 29, 2194–2198. [Google Scholar] [CrossRef]
- Carducci, B.; Bhutta, Z.A. Care of the growth-restricted newborn. Obstet. Gynaecol. 2018, 49, 103–116. [Google Scholar] [CrossRef]
- Hamadani, J.D.; Fuchs, G.J.; Osendarp, S.J.; Huda, S.N.; Grantham-McGregor, S.M. Zinc supplementation during pregnancy and effects on mental development and behaviour of infants: A follow-up study. Lancet 2002, 360, 290–294. [Google Scholar] [CrossRef]
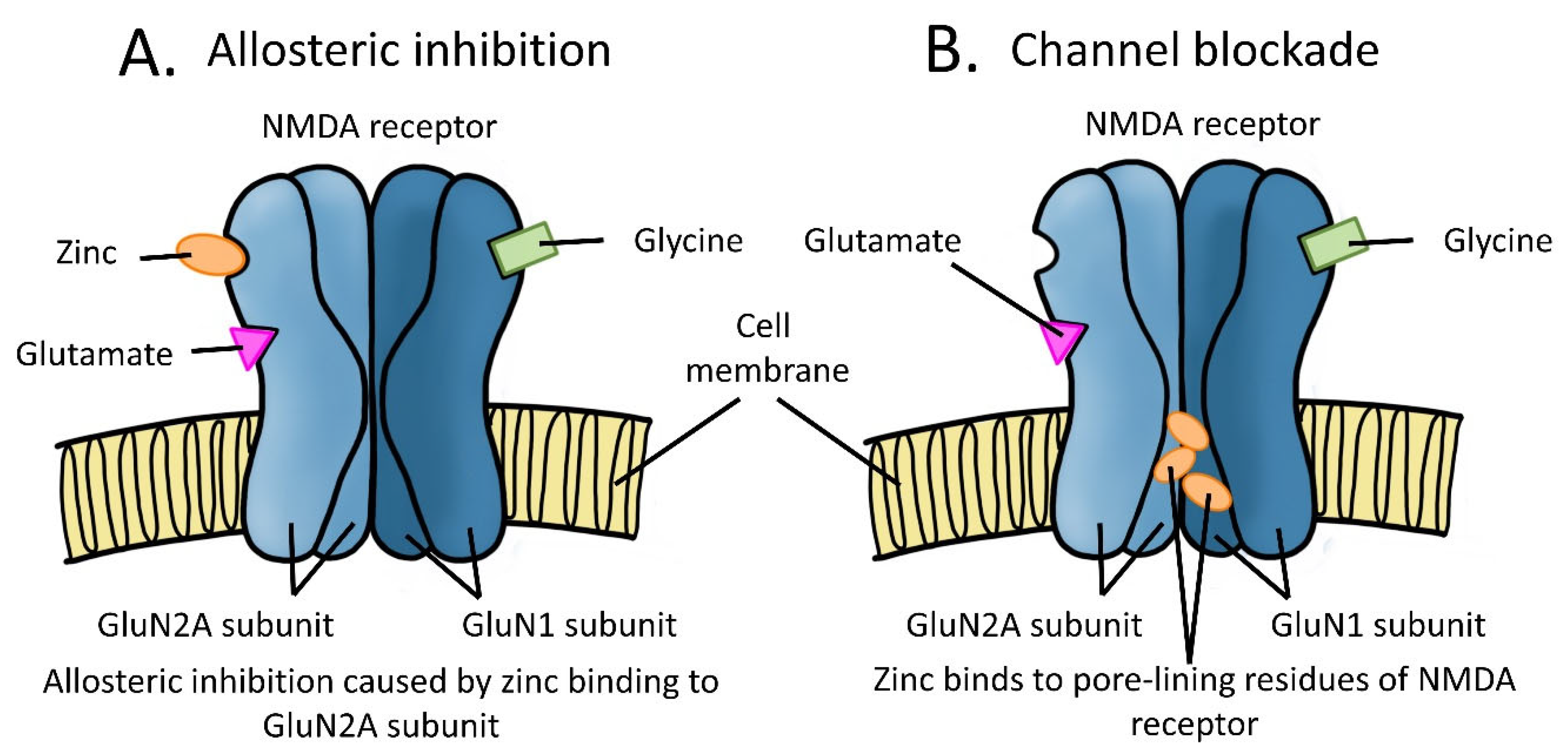
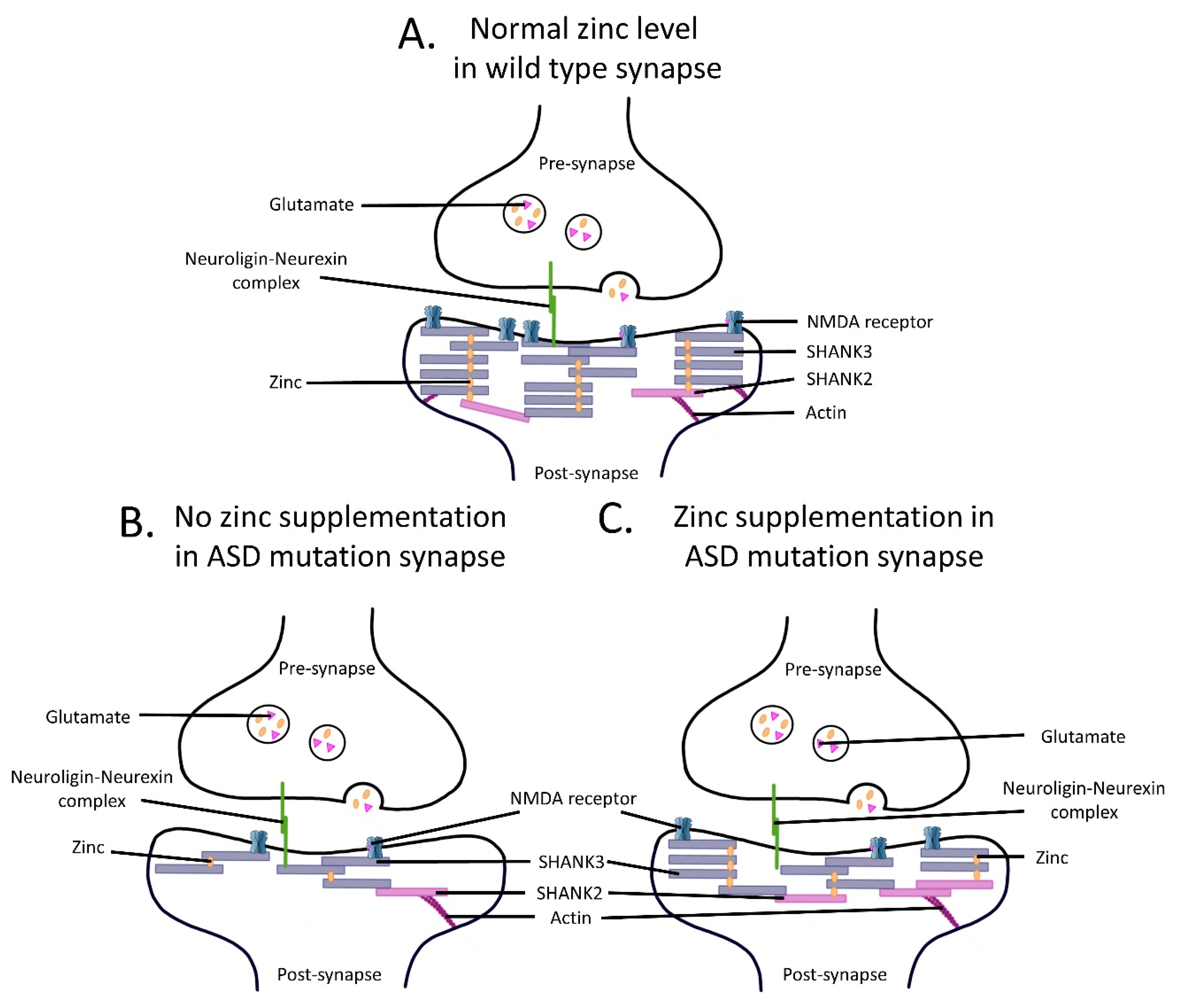
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.; Mills, Z.; Cheung, P.; Cheyne, J.E.; Montgomery, J.M. The Role of Zinc and NMDA Receptors in Autism Spectrum Disorders. Pharmaceuticals 2023, 16, 1. https://doi.org/10.3390/ph16010001
Lee K, Mills Z, Cheung P, Cheyne JE, Montgomery JM. The Role of Zinc and NMDA Receptors in Autism Spectrum Disorders. Pharmaceuticals. 2023; 16(1):1. https://doi.org/10.3390/ph16010001
Chicago/Turabian StyleLee, Kevin, Zoe Mills, Pangying Cheung, Juliette E. Cheyne, and Johanna M. Montgomery. 2023. "The Role of Zinc and NMDA Receptors in Autism Spectrum Disorders" Pharmaceuticals 16, no. 1: 1. https://doi.org/10.3390/ph16010001
APA StyleLee, K., Mills, Z., Cheung, P., Cheyne, J. E., & Montgomery, J. M. (2023). The Role of Zinc and NMDA Receptors in Autism Spectrum Disorders. Pharmaceuticals, 16(1), 1. https://doi.org/10.3390/ph16010001





