Nano-Based Drug Delivery of Polyphenolic Compounds for Cancer Treatment: Progress, Opportunities, and Challenges
Abstract
1. Introduction
2. Overview of Anti-Cancer Properties and Involved Mechanisms of Polyphenolic Compounds
2.1. Effects on Inhibiting Cell Proliferation and Cell Cycle
2.2. Effects on Inducing Autophagic or Apoptotic Cell Death
2.3. Suppressing Cell Invasion and Metastasis
2.4. Other Involved Mechanisms
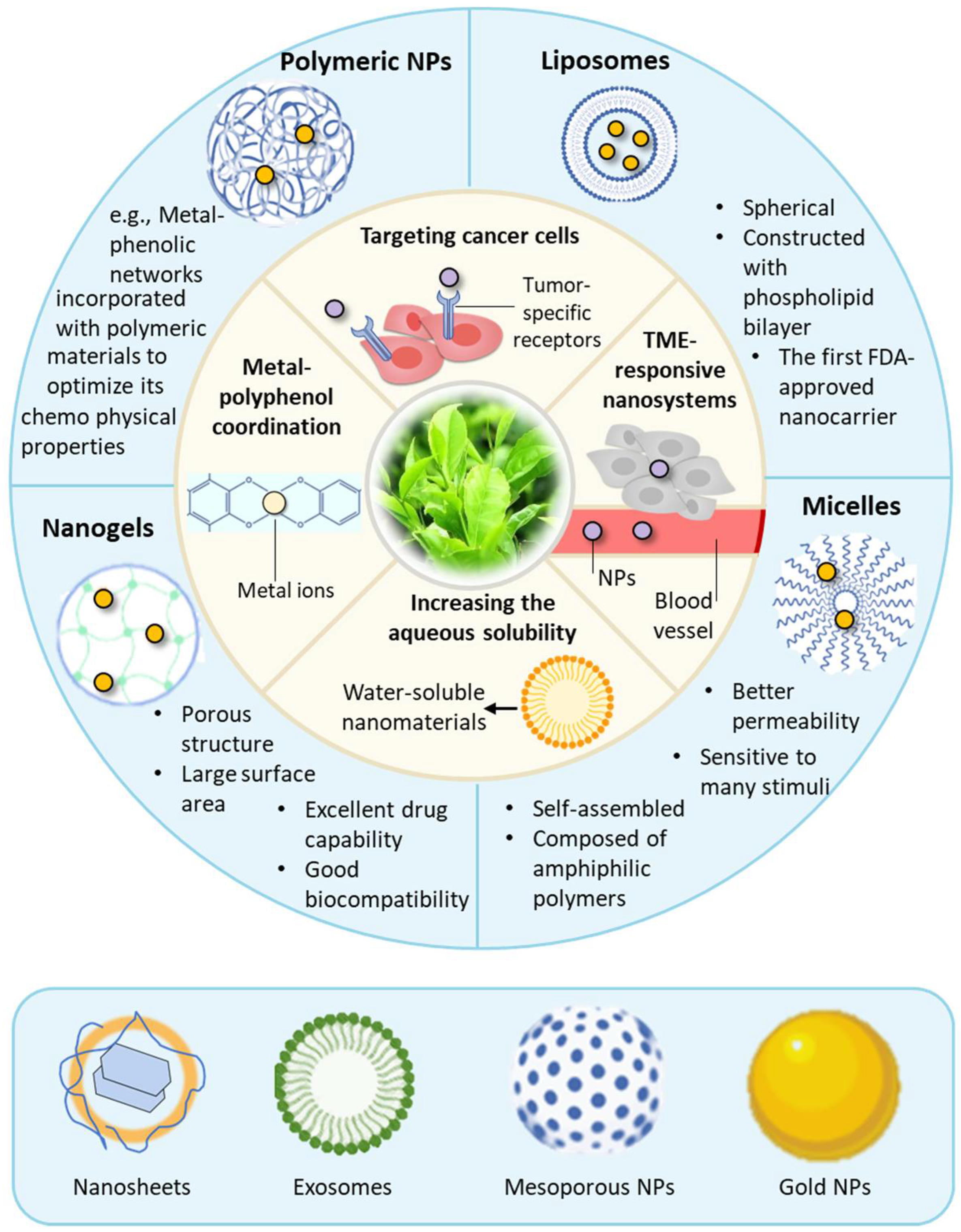
3. Advantages of Nano-Based Delivery Systems for Polyphenolic Compounds in Cancer Therapy
3.1. Increasing the Aqueous Solubility via Nanomaterials
3.2. Enhancing the Targeting Ability of Polyphenolic Compounds
3.3. Taking Advantage of the Structural Properties of Polyphenolic Compounds
4. Progress in Nanocarrier-Mediated Delivery of Polyphenolic Compounds in Cancer Therapy
4.1. Liposome-Mediated Delivery of Polyphenolic Compounds
4.2. Micelles as Nanocarriers for Drug Delivery
4.3. Drug Delivery Mediated by Nanogels
4.4. Other Nano-Based Drug Delivery Systems
5. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Garavand, F.; Jalai-Jivan, M.; Assadpour, E.; Jafari, S.M. Encapsulation of phenolic compounds within nano/microemulsion systems: A review. Food Chem. 2021, 364, 130376. [Google Scholar] [CrossRef] [PubMed]
- Beya, M.M.; Netzel, M.E.; Sultanbawa, Y.; Smyth, H.; Hoffman, L.C. Plant-Based Phenolic Molecules as Natural Preservatives in Comminuted Meats: A Review. Antioxidants 2021, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.L.; Joharian, M.; Razavi, S.A.A.; Morsali, A.; Wu, D.Z.; Azhdari Tehrani, A.; Wang, J.; Junk, P.C.; Guo, Z.F. Phenolic nitroaromatics detection by fluorinated metal-organic frameworks: Barrier elimination for selective sensing of specific group of nitroaromatics. J. Hazard. Mater. 2021, 406, 124501. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Mithul Aravind, S.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef]
- Sieminska-Kuczer, A.; Szymanska-Chargot, M.; Zdunek, A. Recent advances in interactions between polyphenols and plant cell wall polysaccharides as studied using an adsorption technique. Food Chem. 2022, 373 Pt B, 131487. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.; Ying, D.; Adhikari, B.; Fang, Z. Fermentation transforms the phenolic profiles and bioactivities of plant-based foods. Biotechnol. Adv. 2021, 49, 107763. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Chen, X.; Lei, Z.; Cao, J.; Zhang, W.; Wu, R.; Cao, F.; Guo, Q.; Wang, J. Traditional uses, phytochemistry, pharmacology and current uses of underutilized Xanthoceras sorbifolium bunge: A review. J. Ethnopharm. 2022, 283, 114747. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, J.; Zhang, Q.; Qin, X.; Li, Z.; Sun, G.; Jin, S. The Traditional Uses, Phytochemistry and Pharmacology of Sarcandra glabra (Thunb.) Nakai, a Chinese Herb With Potential for Development: Review. Front. Pharm. 2021, 12, 652926. [Google Scholar] [CrossRef]
- Baron, G.; Altomare, A.; Mol, M.; Garcia, J.L.; Correa, C.; Raucci, A.; Mancinelli, L.; Mazzotta, S.; Fumagalli, L.; Trunfio, G.; et al. Analytical Profile and Antioxidant and Anti-Inflammatory Activities of the Enriched Polyphenol Fractions Isolated from Bergamot Fruit and Leave. Antioxidants 2021, 10, 141. [Google Scholar] [CrossRef]
- Messaoudene, M.; Pidgeon, R.; Richard, C.; Ponce, M.; Diop, K.; Benlaifaoui, M.; Nolin-Lapalme, A.; Cauchois, F.; Malo, J.; Belkaid, W.; et al. A Natural Polyphenol Exerts Antitumor Activity and Circumvents Anti-PD-1 Resistance through Effects on the Gut Microbiota. Cancer Discov. 2022, 12, 1070–1087. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Rafiei, H.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. Anti-tumor activity of resveratrol against gastric cancer: A review of recent advances with an emphasis on molecular pathways. Cancer Cell Int. 2021, 21, 66. [Google Scholar] [CrossRef]
- Bracci, L.; Fabbri, A.; Del Corno, M.; Conti, L. Dietary Polyphenols: Promising Adjuvants for Colorectal Cancer Therapies. Cancers 2021, 13, 4499. [Google Scholar] [CrossRef]
- Hazafa, A.; Rehman, K.U.; Jahan, N.; Jabeen, Z. The Role of Polyphenol (Flavonoids) Compounds in the Treatment of Cancer Cells. Nutr. Cancer 2020, 72, 386–397. [Google Scholar] [CrossRef]
- Rahaiee, S.; Assadpour, E.; Faridi Esfanjani, A.; Silva, A.S.; Jafari, S.M. Application of nano/microencapsulated phenolic compounds against cancer. Adv. Colloid Interface Sci. 2020, 279, 102153. [Google Scholar] [CrossRef]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Wozniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Annunziata, G.; Jimenez-Garcia, M.; Capo, X.; Moranta, D.; Arnone, A.; Tenore, G.C.; Sureda, A.; Tejada, S. Microencapsulation as a tool to counteract the typical low bioavailability of polyphenols in the management of diabetes. Food Chem. Toxicol. 2020, 139, 111248. [Google Scholar] [CrossRef]
- Lang, T.; Liu, Y.; Zheng, Z.; Ran, W.; Zhai, Y.; Yin, Q.; Zhang, P.; Li, Y. Cocktail Strategy Based on Spatio-Temporally Controlled Nano Device Improves Therapy of Breast Cancer. Adv. Mater. 2019, 31, e1806202. [Google Scholar] [CrossRef]
- Rajitha, B.; Malla, R.R.; Vadde, R.; Kasa, P.; Prasad, G.L.V.; Farran, B.; Kumari, S.; Pavitra, E.; Kamal, M.A.; Raju, G.S.R.; et al. Horizons of nanotechnology applications in female specific cancers. Semin. Cancer Biol. 2021, 69, 376–390. [Google Scholar] [CrossRef]
- Oroojalian, F.; Charbgoo, F.; Hashemi, M.; Amani, A.; Yazdian-Robati, R.; Mokhtarzadeh, A.; Ramezani, M.; Hamblin, M.R. Recent advances in nanotechnology-based drug delivery systems for the kidney. J. Control. Release 2020, 321, 442–462. [Google Scholar] [CrossRef]
- Mi, P.; Cabral, H.; Kataoka, K. Ligand-Installed Nanocarriers toward Precision Therapy. Adv. Mater. 2020, 32, e1902604. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Huang, S.; Zhang, Y.; Guo, Y.; Zhang, S.; Guan, J.; Meng, M.; Liu, L.; Wang, C.; Yu, D.; et al. miR-7/TGF-beta2 axis sustains acidic tumor microenvironment-induced lung cancer metastasis. Acta Pharm. Sin. B 2022, 12, 821–837. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Yang, F.; Shao, C.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 2019, 18, 157. [Google Scholar] [CrossRef] [PubMed]
- Di, Z.; Zhao, J.; Chu, H.; Xue, W.; Zhao, Y.; Li, L. An Acidic-Microenvironment-Driven DNA Nanomachine Enables Specific ATP Imaging in the Extracellular Milieu of Tumor. Adv. Mater. 2019, 31, e1901885. [Google Scholar] [CrossRef]
- Phung, C.D.; Tran, T.H.; Pham, L.M.; Nguyen, H.T.; Jeong, J.H.; Yong, C.S.; Kim, J.O. Current developments in nanotechnology for improved cancer treatment, focusing on tumor hypoxia. J. Control. Release 2020, 324, 413–429. [Google Scholar] [CrossRef]
- Liu, J.; Liew, S.S.; Wang, J.; Pu, K. Bioinspired and Biomimetic Delivery Platforms for Cancer Vaccines. Adv. Mater. 2022, 34, e2103790. [Google Scholar] [CrossRef]
- Niu, W.; Xiao, Q.; Wang, X.; Zhu, J.; Li, J.; Liang, X.; Peng, Y.; Wu, C.; Lu, R.; Pan, Y.; et al. A Biomimetic Drug Delivery System by Integrating Grapefruit Extracellular Vesicles and Doxorubicin-Loaded Heparin-Based Nanoparticles for Glioma Therapy. Nano Lett. 2021, 21, 1484–1492. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, H.; Han, L.; Qiang, Z.; Zhang, X.; Gao, W.; Zhao, K.; Song, Y. Synergistic combination therapy of lung cancer using paclitaxel- and triptolide-coloaded lipid-polymer hybrid nanoparticles. Drug Des. Dev. Ther. 2018, 12, 3199–3209. [Google Scholar] [CrossRef]
- Zhao, M.D.; Li, J.Q.; Chen, F.Y.; Dong, W.; Wen, L.J.; Fei, W.D.; Zhang, X.; Yang, P.L.; Zhang, X.M.; Zheng, C.H. Co-Delivery of Curcumin and Paclitaxel by “Core-Shell” Targeting Amphiphilic Copolymer to Reverse Resistance in the Treatment of Ovarian Cancer. Int. J. Nanomed. 2019, 14, 9453–9467. [Google Scholar] [CrossRef]
- Tauber, A.L.; Schweiker, S.S.; Levonis, S.M. From tea to treatment; epigallocatechin gallate and its potential involvement in minimizing the metabolic changes in cancer. Nutr. Res. 2020, 74, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Shi, W.; Guo, H.; Long, W.; Wang, Y.; Qi, J.; Liu, J.; Xu, Y. The Inhibitory Effect of (-)-Epigallocatechin-3-Gallate on Breast Cancer Progression via Reducing SCUBE2 Methylation and DNMT Activity. Molecules 2019, 24, 2899. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.W.; Yan, F.; Zhong, X.; Mazumder, P.B.; Xu-Monette, Z.Y.; Zou, D.; Young, K.H.; Ramos, K.S.; Li, Y. Regulation of p53-targeting microRNAs by polycyclic aromatic hydrocarbons: Implications in the etiology of multiple myeloma. Mol. Carcinog. 2015, 54, 1060–1069. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, Q. Roles of the Polyphenol-Gut Microbiota Interaction in Alleviating Colitis and Preventing Colitis-Associated Colorectal Cancer. Adv. Nutr. 2021, 12, 546–565. [Google Scholar] [CrossRef]
- Liu, G.; Shi, A.; Wang, N.; Li, M.; He, X.; Yin, C.; Tu, Q.; Shen, X.; Tao, Y.; Wang, Q.; et al. Polyphenolic Proanthocyanidin-B2 suppresses proliferation of liver cancer cells and hepatocellular carcinogenesis through directly binding and inhibiting AKT activity. Redox Biol. 2020, 37, 101701. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wu, L.; Ji, J.; Chen, K.; Yu, Q.; Zhang, J.; Chen, J.; Mao, Y.; Wang, F.; Dai, W.; et al. PKM2 is the target of proanthocyanidin B2 during the inhibition of hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2019, 38, 204. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Chang, H.; Peng, X.; Bai, Q.; Yi, L.; Zhou, Y.; Zhu, J.; Mi, M. Resveratrol inhibits breast cancer stem-like cells and induces autophagy via suppressing Wnt/beta-catenin signaling pathway. PLoS ONE 2014, 9, e102535. [Google Scholar]
- Buhrmann, C.; Yazdi, M.; Popper, B.; Shayan, P.; Goel, A.; Aggarwal, B.B.; Shakibaei, M. Evidence that TNF-beta induces proliferation in colorectal cancer cells and resveratrol can down-modulate it. Exp. Biol. Med. 2019, 244, 2413. [Google Scholar] [CrossRef]
- Izquierdo-Torres, E.; Hernandez-Oliveras, A.; Meneses-Morales, I.; Rodriguez, G.; Fuentes-Garcia, G.; Zarain-Herzberg, A. Resveratrol up-regulates ATP2A3 gene expression in breast cancer cell lines through epigenetic mechanisms. Int. J. Biochem. Cell Biol. 2019, 113, 37–47. [Google Scholar] [CrossRef]
- Kotha, A.; Sekharam, M.; Cilenti, L.; Siddiquee, K.; Khaled, A.; Zervos, A.S.; Carter, B.; Turkson, J.; Jove, R. Resveratrol inhibits Src and Stat3 signaling and induces the apoptosis of malignant cells containing activated Stat3 protein. Mol. Cancer Ther. 2006, 5, 621–629. [Google Scholar] [CrossRef]
- Li, J.; Fan, Y.; Zhang, Y.; Liu, Y.; Yu, Y.; Ma, M. Resveratrol Induces Autophagy and Apoptosis in Non-Small-Cell Lung Cancer Cells by Activating the NGFR-AMPK-mTOR Pathway. Nutrients 2022, 14, 2413. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Liu, X.; Han, Z.; Zhou, L.; Sui, H.; Yan, L.; Jiang, H.; Ren, J.; Cai, J.; Li, Q. Resveratrol suppresses epithelial-to-mesenchymal transition in colorectal cancer through TGF-beta1/Smads signaling pathway mediated Snail/E-cadherin expression. BMC Cancer 2015, 15, 97. [Google Scholar] [CrossRef]
- Ghasemi, F.; Shafiee, M.; Banikazemi, Z.; Pourhanifeh, M.H.; Khanbabaei, H.; Shamshirian, A.; Amiri Moghadam, S.; ArefNezhad, R.; Sahebkar, A.; Avan, A.; et al. Curcumin inhibits NF-kB and Wnt/beta-catenin pathways in cervical cancer cells. Pathol. Res. Pract. 2019, 215, 152556. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhou, B.H.; Qiu, X.; Wang, H.S.; Zhang, F.; Fang, R.; Wang, X.F.; Cai, S.H.; Du, J.; Bu, X.Z. T63, a new 4-arylidene curcumin analogue, induces cell cycle arrest and apoptosis through activation of the reactive oxygen species-FOXO3a pathway in lung cancer cells. Free Radic. Biol. Med. 2012, 53, 2204–2217. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Du, Q.; Zhang, Z.; Ma, J.; Han, L.; Wang, Y.; Yang, L.; Tao, N.; Qin, Z. Curcumin promotes cancer-associated fibroblasts apoptosis via ROS-mediated endoplasmic reticulum stress. Arch. Biochem. Biophys. 2020, 694, 108613. [Google Scholar] [CrossRef]
- Aziz, M.A.; Sarwar, M.S.; Akter, T.; Uddin, M.S.; Xun, S.; Zhu, Y.; Islam, M.S.; Hongjie, Z. Polyphenolic molecules targeting STAT3 pathway for the treatment of cancer. Life Sci. 2021, 268, 118999. [Google Scholar] [CrossRef]
- Romagnolo, D.F.; Donovan, M.G.; Papoutsis, A.J.; Doetschman, T.C.; Selmin, O.I. Genistein Prevents BRCA1 CpG Methylation and Proliferation in Human Breast Cancer Cells with Activated Aromatic Hydrocarbon Receptor. Curr. Dev. Nutr. 2017, 1, e000562. [Google Scholar] [CrossRef]
- Sun, Y.S.; Zhao, Z.; Zhu, H.P. Hispolon inhibits TPA-induced invasion by reducing MMP-9 expression through the NF-kappaB signaling pathway in MDA-MB-231 human breast cancer cells. Oncol. Lett. 2015, 10, 536–542. [Google Scholar] [CrossRef]
- Selvakumar, P.; Badgeley, A.; Murphy, P.; Anwar, H.; Sharma, U.; Lawrence, K.; Lakshmikuttyamma, A. Flavonoids and Other Polyphenols Act as Epigenetic Modifiers in Breast Cancer. Nutrients 2020, 12, 761. [Google Scholar] [CrossRef]
- Wang, K.; Liu, R.; Li, J.; Mao, J.; Lei, Y.; Wu, J.; Zeng, J.; Zhang, T.; Wu, H.; Chen, L.; et al. Quercetin induces protective autophagy in gastric cancer cells: Involvement of Akt-mTOR- and hypoxia-induced factor 1alpha-mediated signaling. Autophagy 2011, 7, 966–978. [Google Scholar] [CrossRef]
- Tungsukruthai, S.; Reamtong, O.; Roytrakul, S.; Sukrong, S.; Vinayanwattikun, C.; Chanvorachote, P. Targeting AKT/mTOR and Bcl-2 for Autophagic and Apoptosis Cell Death in Lung Cancer: Novel Activity of a Polyphenol Compound. Antioxidants 2021, 10, 534. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, H.; Fratantonio, D.; Hasan, M.M.; Sharifi, S.; Fathi, N.; Ullah, H.; Rastrelli, L. Apoptosis induced by luteolin in breast cancer: Mechanistic and therapeutic perspectives. Phytomedicine 2019, 59, 152883. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Y.; Wang, Y.; Zhang, H.; Wang, X.; Tang, H.; Huang, H.; Zhou, Z.; Chen, B.; Sun, L. Agrimoniin sensitizes pancreatic cancer to apoptosis through ROS-mediated energy metabolism dysfunction. Phytomedicine 2022, 96, 153807. [Google Scholar] [CrossRef]
- Huang, C.-C.; Hung, C.-H.; Chen, C.-C.; Kao, S.-H.; Wang, C.-J. Hibiscus sabdariffa polyphenol-enriched extract inhibits colon carcinoma metastasis associating with FAK and CD44/c-MET signaling. J. Funct. Foods 2018, 48, 542–550. [Google Scholar] [CrossRef]
- Hsieh, Y.S.; Chu, S.C.; Huang, S.C.; Kao, S.H.; Lin, M.S.; Chen, P.N. Gossypol Reduces Metastasis and Epithelial-Mesenchymal Transition by Targeting Protease in Human Cervical Cancer. Am. J. Chin. Med. 2021, 49, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Ali, S.; Ashraf, G.M.; Bilgrami, A.L.; Yadav, D.K.; Hassan, M.I. Epigallocatechin 3-gallate: From green tea to cancer therapeutics. Food Chem. 2022, 379, 132135. [Google Scholar] [CrossRef]
- Ferrari, E.; Bettuzzi, S.; Naponelli, V. The Potential of Epigallocatechin Gallate (EGCG) in Targeting Autophagy for Cancer Treatment: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 6075. [Google Scholar] [CrossRef] [PubMed]
- Sinha, D.; Sarkar, N.; Biswas, J.; Bishayee, A. Resveratrol for breast cancer prevention and therapy: Preclinical evidence and molecular mechanisms. Semin. Cancer Biol. 2016, 40–41, 209–232. [Google Scholar] [CrossRef]
- Mahjoob, M.; Stochaj, U. Curcumin nanoformulations to combat aging-related diseases. Ageing Res. Rev. 2021, 69, 101364. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S.; Acharya, A.; Ray, R.S.; Agrawal, R.; Raghuwanshi, R.; Jain, P. Cellular and molecular mechanisms of curcumin in prevention and treatment of disease. Crit. Rev. Food Sci. Nutr. 2020, 60, 887–939. [Google Scholar] [CrossRef]
- Al Saqr, A.; Majrashi, M.; Alrbyawi, H.; Govindarajulu, M.; Fujihashi, A.; Gottumukkala, S.; Poudel, I.; Arnold, R.D.; Babu, R.J.; Dhanasekaran, M. Elucidating the anti-melanoma effect and mechanisms of Hispolon. Life Sci. 2020, 256, 117702. [Google Scholar] [CrossRef]
- Sarfraz, A.; Rasul, A.; Sarfraz, I.; Shah, M.A.; Hussain, G.; Shafiq, N.; Masood, M.; Adem, S.; Sarker, S.D.; Li, X. Hispolon: A natural polyphenol and emerging cancer killer by multiple cellular signaling pathways. Environ. Res. 2020, 190, 110017. [Google Scholar] [CrossRef]
- Liao, K.F.; Chiu, T.L.; Chang, S.F.; Wang, M.J.; Chiu, S.C. Hispolon Induces Apoptosis, Suppresses Migration and Invasion of Glioblastoma Cells and Inhibits GBM Xenograft Tumor Growth in vivo. Molecules 2021, 26, 4497. [Google Scholar] [CrossRef] [PubMed]
- Sefton, P. Testing for BRCA1/2 Mutations. JAMA 2017, 318, 2054. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, R.R.; Santucci-Pereira, J.; Wang, Y.V.; Liu, J.; Nguyen, T.D.; Wang, J.; Jenkins, S.; Russo, J.; Huang, T.H.; Jin, V.X.; et al. DNA Methylation Targets Influenced by Bisphenol A and/or Genistein Are Associated with Survival Outcomes in Breast Cancer Patients. Genes 2017, 8, 4497. [Google Scholar] [CrossRef]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef]
- Papp, B.; Launay, S.; Gelebart, P.; Arbabian, A.; Enyedi, A.; Brouland, J.P.; Carosella, E.D.; Adle-Biassette, H. Endoplasmic Reticulum Calcium Pumps and Tumor Cell Differentiation. Int. J. Mol. Sci. 2020, 21, 3351. [Google Scholar] [CrossRef]
- Hayakawa, S.; Ohishi, T.; Miyoshi, N.; Oishi, Y.; Nakamura, Y.; Isemura, M. Anti-Cancer Effects of Green Tea Epigallocatchin-3-Gallate and Coffee Chlorogenic Acid. Molecules 2020, 25, 4553. [Google Scholar] [CrossRef]
- Maida, I.; Zanna, P.; Guida, S.; Ferretta, A.; Cocco, T.; Palese, L.L.; Londei, P.; Benelli, D.; Azzariti, A.; Tommasi, S.; et al. Translational control mechanisms in cutaneous malignant melanoma: The role of eIF2alpha. J. Transl. Med. 2019, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Ghaddar, N.; Wang, S.; Woodvine, B.; Krishnamoorthy, J.; van Hoef, V.; Darini, C.; Kazimierczak, U.; Ah-Son, N.; Popper, H.; Johnson, M.; et al. The integrated stress response is tumorigenic and constitutes a therapeutic liability in KRAS-driven lung cancer. Nat. Commun. 2021, 12, 4651. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ge, Y.; Dong, J.; Wang, H.; Zhao, T.; Wang, X.; Liu, J.; Gao, S.; Shi, L.; Yang, S.; et al. BZW1 Facilitates Glycolysis and Promotes Tumor Growth in Pancreatic Ductal Adenocarcinoma Through Potentiating eIF2alpha Phosphorylation. Gastroenterology 2022, 162, 1256–1271.e14. [Google Scholar] [CrossRef]
- Ren, M.; Zhou, X.; Gu, M.; Jiao, W.; Yu, M.; Wang, Y.; Liu, S.; Yang, J.; Ji, F. Resveratrol synergizes with cisplatin in antineoplastic effects against AGS gastric cancer cells by inducing endoplasmic reticulum stressmediated apoptosis and G2/M phase arrest. Oncol. Rep. 2020, 44, 1605–1615. [Google Scholar]
- Fang, S.; Sun, S.; Cai, H.; Zou, X.; Wang, S.; Hao, X.; Wan, X.; Tian, J.; Li, Z.; He, Z.; et al. IRGM/Irgm1 facilitates macrophage apoptosis through ROS generation and MAPK signal transduction: Irgm1(+/−) mice display increases atherosclerotic plaque stability. Theranostics 2021, 11, 9358–9375. [Google Scholar] [CrossRef]
- Fedotcheva, T.A.; Sheichenko, O.P.; Fedotcheva, N.I. New Properties and Mitochondrial Targets of Polyphenol Agrimoniin as a Natural Anticancer and Preventive Agent. Pharmaceutics 2021, 13, 2089. [Google Scholar] [CrossRef]
- Habrowska-Gorczynska, D.E.; Koziel, M.J.; Kowalska, K.; Piastowska-Ciesielska, A.W. FOXO3a and Its Regulators in Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 12530. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, J.; Fu, H.Q.; Zhang, X.; Pan, Y.L. FOXO3a inhibits the EMT and metastasis of breast cancer by regulating TWIST-1 mediated miR-10b/CADM2 axis. Transl. Oncol. 2021, 14, 101096. [Google Scholar] [CrossRef]
- Wan Mohd Tajuddin, W.N.B.; Lajis, N.H.; Abas, F.; Othman, I.; Naidu, R. Mechanistic Understanding of Curcumin’s Therapeutic Effects in Lung Cancer. Nutrients 2019, 11, 2989. [Google Scholar] [CrossRef]
- Ren, B.; Kwah, M.X.; Liu, C.; Ma, Z.; Shanmugam, M.K.; Ding, L.; Xiang, X.; Ho, P.C.; Wang, L.; Ong, P.S.; et al. Resveratrol for cancer therapy: Challenges and future perspectives. Cancer Lett. 2021, 515, 63–72. [Google Scholar] [CrossRef]
- Farha, A.K.; Gan, R.Y.; Li, H.B.; Wu, D.T.; Atanasov, A.G.; Gul, K.; Zhang, J.R.; Yang, Q.Q.; Corke, H. The anticancer potential of the dietary polyphenol rutin: Current status, challenges, and perspectives. Crit. Rev. Food Sci. Nutr. 2022, 62, 832–859. [Google Scholar] [CrossRef]
- Chen, X.; Yu, M.; Xu, W.; Zou, L.; Ye, J.; Liu, Y.; Xiao, Y.; Luo, J. Rutin inhibited the advanced glycation end products-stimulated inflammatory response and extra-cellular matrix degeneration via targeting TRAF-6 and BCL-2 proteins in mouse model of osteoarthritis. Aging 2021, 13, 22134–22147. [Google Scholar] [CrossRef]
- Lodillinsky, C.; Fuhrmann, L.; Irondelle, M.; Pylypenko, O.; Li, X.Y.; Bonsang-Kitzis, H.; Reyal, F.; Vacher, S.; Calmel, C.; De Wever, O.; et al. Metastasis-suppressor NME1 controls the invasive switch of breast cancer by regulating MT1-MMP surface clearance. Oncogene 2021, 40, 4019–4032. [Google Scholar] [CrossRef]
- Sajadimajd, S.; Bahramsoltani, R.; Iranpanah, A.; Kumar Patra, J.; Das, G.; Gouda, S.; Rahimi, R.; Rezaeiamiri, E.; Cao, H.; Giampieri, F.; et al. Advances on Natural Polyphenols as Anticancer Agents for Skin Cancer. Pharmacol. Res. 2020, 151, 104584. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Zhao, L.; Zhang, Y. Structure, properties of gossypol and its derivatives-from physiological activities to drug discovery and drug design. Nat. Prod. Rep. 2022, 39, 1282–1304. [Google Scholar] [CrossRef]
- Costa, A.; Kieffer, Y.; Scholer-Dahirel, A.; Pelon, F.; Bourachot, B.; Cardon, M.; Sirven, P.; Magagna, I.; Fuhrmann, L.; Bernard, C.; et al. Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer Cell 2018, 33, 463–479e10. [Google Scholar] [CrossRef]
- Fidelis, M.; do Carmo, M.A.V.; da Cruz, T.M.; Azevedo, L.; Myoda, T.; Miranda Furtado, M.; Boscacci Marques, M.; Sant’Ana, A.S.; Ines Genovese, M.; Young Oh, W.; et al. Camu-camu seed (Myrciaria dubia)—From side stream to anantioxidant, antihyperglycemic, antiproliferative, antimicrobial, antihemolytic, anti-inflammatory, and antihypertensive ingredient. Food Chem. 2020, 310, 125909. [Google Scholar] [CrossRef]
- Mori, N.; Murphy, N.; Sawada, N.; Achaintre, D.; Yamaji, T.; Scalbert, A.; Iwasaki, M.; Inoue, M.; Gunter, M.J.; Tsugane, S. Prediagnostic plasma polyphenol concentrations and colon cancer risk: The JPHC nested case-control study. Clin. Nutr. 2022, 41, 1950–1960. [Google Scholar] [CrossRef]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, J.; Luo, Z.; Shi, Q.; Liu, G.; Wu, F.; Wang, Z.; Huang, Y.; Zhou, D. Engineering Endogenous Tumor-Associated Macrophage-Targeted Biomimetic Nano-RBC to Reprogram Tumor Immunosuppressive Microenvironment for Enhanced Chemo-Immunotherapy. Adv. Mater. 2021, 33, e2103497. [Google Scholar] [CrossRef]
- Guo, J.; Yu, Z.; Das, M.; Huang, L. Nano Codelivery of Oxaliplatin and Folinic Acid Achieves Synergistic Chemo-Immunotherapy with 5-Fluorouracil for Colorectal Cancer and Liver Metastasis. ACS Nano 2020, 14, 5075–5089. [Google Scholar] [CrossRef]
- Xu, J.; Lv, J.; Zhuang, Q.; Yang, Z.; Cao, Z.; Xu, L.; Pei, P.; Wang, C.; Wu, H.; Dong, Z.; et al. A general strategy towards personalized nanovaccines based on fluoropolymers for post-surgical cancer immunotherapy. Nat. Nanotechnol. 2020, 15, 1043–1052. [Google Scholar] [CrossRef]
- Xin, Y.; Huang, M.; Guo, W.W.; Huang, Q.; Zhang, L.Z.; Jiang, G. Nano-based delivery of RNAi in cancer therapy. Mol. Cancer 2017, 16, 134. [Google Scholar] [CrossRef]
- Aiello, P.; Consalvi, S.; Poce, G.; Raguzzini, A.; Toti, E.; Palmery, M.; Biava, M.; Bernardi, M.; Kamal, M.A.; Perry, G.; et al. Dietary flavonoids: Nano delivery and nanoparticles for cancer therapy. Semin. Cancer Biol. 2021, 69, 150–165. [Google Scholar] [CrossRef]
- Shi, H.; Li, X.Y.; Chen, Y.; Zhang, X.; Wu, Y.; Wang, Z.X.; Chen, P.H.; Dai, H.Q.; Feng, J.; Chatterjee, S.; et al. Quercetin Induces Apoptosis via Downregulation of Vascular Endothelial Growth Factor/Akt Signaling Pathway in Acute Myeloid Leukemia Cells. Front. Pharmacol. 2020, 11, 534171. [Google Scholar] [CrossRef]
- Erdogan, S.; Turkekul, K.; Dibirdik, I.; Doganlar, O.; Doganlar, Z.B.; Bilir, A.; Oktem, G. Midkine downregulation increases the efficacy of quercetin on prostate cancer stem cell survival and migration through PI3K/AKT and MAPK/ERK pathway. Biomed. Pharm. 2018, 107, 793–805. [Google Scholar] [CrossRef]
- Zang, X.; Cheng, M.; Zhang, X.; Chen, X. Quercetin nanoformulations: A promising strategy for tumor therapy. Food Funct. 2021, 12, 6664–6681. [Google Scholar] [CrossRef]
- Sun, D.; Zou, Y.; Song, L.; Han, S.; Yang, H.; Chu, D.; Dai, Y.; Ma, J.; O’Driscoll, C.M.; Yu, Z.; et al. A cyclodextrin-based nanoformulation achieves co-delivery of ginsenoside Rg3 and quercetin for chemo-immunotherapy in colorectal cancer. Acta Pharm. Sin. B 2022, 12, 378–393. [Google Scholar] [CrossRef]
- So, M.; Kimura, Y.; Yamaguchi, K.; Sugiki, T.; Fujiwara, T.; Aguirre, C.; Ikenaka, K.; Mochizuki, H.; Kawata, Y.; Goto, Y. Polyphenol-solubility alters amyloid fibril formation of alpha-synuclein. Protein Sci. 2021, 30, 1701–1713. [Google Scholar] [CrossRef]
- Kim, T.W.; Lee, S.Y.; Kim, M.; Cheon, C.; Ko, S.G. Kaempferol induces autophagic cell death via IRE1-JNK-CHOP pathway and inhibition of G9a in gastric cancer cells. Cell Death. Dis. 2018, 9, 875. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, S.; Song, H.; Yu, T.; Zheng, X.; Chu, Q. CaCO3 nanoparticles incorporated with KAE to enable amplified calcium overload cancer therapy. Biomaterials 2021, 277, 121080. [Google Scholar] [CrossRef]
- Andrade, F.O.; Liu, F.; Zhang, X.; Rosim, M.P.; Dani, C.; Cruz, I.; Wang, T.T.Y.; Helferich, W.; Li, R.W.; Hilakivi-Clarke, L. Genistein Reduces the Risk of Local Mammary Cancer Recurrence and Ameliorates Alterations in the Gut Microbiota in the Offspring of Obese Dams. Nutrients 2021, 13, 201. [Google Scholar] [CrossRef]
- Tang, H.; Wang, S.; Li, X.; Zou, T.; Huang, X.; Zhang, W.; Chen, Y.; Yang, C.; Pan, Q.; Liu, H.F. Prospects of and limitations to the clinical applications of genistein. Discov. Med. 2019, 27, 177–188. [Google Scholar]
- Vodnik, V.V.; Mojic, M.; Stamenovic, U.; Otonicar, M.; Ajdzanovic, V.; Maksimovic-Ivanic, D.; Mijatovic, S.; Markovic, M.M.; Barudzija, T.; Filipovic, B.; et al. Development of genistein-loaded gold nanoparticles and their antitumor potential against prostate cancer cell lines. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 124, 112078. [Google Scholar] [CrossRef]
- Lin, M.; Yao, W.; Xiao, Y.; Dong, Z.; Huang, W.; Zhang, F.; Zhou, X.; Liang, M. Resveratrol-modified mesoporous silica nanoparticle for tumor-targeted therapy of gastric cancer. Bioengineered 2021, 12, 6343–6353. [Google Scholar] [CrossRef]
- Nagesh, P.K.B.; Chowdhury, P.; Hatami, E.; Kumari, S.; Kashyap, V.K.; Tripathi, M.K.; Wagh, S.; Meibohm, B.; Chauhan, S.C.; Jaggi, M.; et al. Cross-Linked Polyphenol-Based Drug Nano-Self-Assemblies Engineered to Blockade Prostate Cancer Senescence. ACS Appl. Mater. Interfaces 2019, 11, 38537–38554. [Google Scholar] [CrossRef]
- Shehata, M.G.; Abu-Serie, M.M.; Abd El-Aziz, N.M.; El-Sohaimy, S.A. Nutritional, phytochemical, and in vitro anticancer potential of sugar apple (Annona squamosa) fruits. Sci. Rep. 2021, 11, 6224. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hematol. Oncol. 2018, 11, 64. [Google Scholar] [CrossRef]
- Lang, J.; Zhao, X.; Wang, X.; Zhao, Y.; Li, Y.; Zhao, R.; Cheng, K.; Li, Y.; Han, X.; Zheng, X.; et al. Targeted Co-delivery of the Iron Chelator Deferoxamine and a HIF1alpha Inhibitor Impairs Pancreatic Tumor Growth. ACS Nano 2019, 13, 2176–2189. [Google Scholar]
- Belfiore, L.; Saunders, D.N.; Ranson, M.; Thurecht, K.J.; Storm, G.; Vine, K.L. Towards clinical translation of ligand-functionalized liposomes in targeted cancer therapy: Challenges and opportunities. J. Control. Release 2018, 277, 1–13. [Google Scholar] [CrossRef]
- Cheng, H.W.; Chiang, C.S.; Ho, H.Y.; Chou, S.H.; Lai, Y.H.; Shyu, W.C.; Chen, S.Y. Dextran-modified Quercetin-Cu(II)/hyaluronic acid nanomedicine with natural poly(ADP-ribose) polymerase inhibitor and dual targeting for programmed synthetic lethal therapy in triple-negative breast cancer. J. Control. Release 2021, 329, 136–147. [Google Scholar] [CrossRef]
- Seok, H.Y.; Sanoj Rejinold, N.; Lekshmi, K.M.; Cherukula, K.; Park, I.K.; Kim, Y.C. CD44 targeting biocompatible and biodegradable hyaluronic acid cross-linked zein nanogels for curcumin delivery to cancer cells: In vitro and in vivo evaluation. J. Control. Release 2018, 280, 20–30. [Google Scholar] [CrossRef]
- Hu, K.; Miao, L.; Goodwin, T.J.; Li, J.; Liu, Q.; Huang, L. Quercetin Remodels the Tumor Microenvironment to Improve the Permeation, Retention, and Antitumor Effects of Nanoparticles. ACS Nano 2017, 11, 4916–4925. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chen, T.; Zhu, D.; Huang, Q. Enhanced Tumor Targeting and Radiotherapy by Quercetin Loaded Biomimetic Nanoparticles. Front. Chem. 2020, 8, 225. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Deng, W.W.; Zan, M.; Rao, L.; Yu, G.T.; Zhu, D.M.; Wu, W.T.; Chen, B.; Ji, L.W.; Chen, L.; et al. Cancer Cell Membrane Camouflaged Nanoparticles to Realize Starvation Therapy Together with Checkpoint Blockades for Enhancing Cancer Therapy. ACS Nano 2019, 13, 2849–2857. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, H.; Ren, J.; Lu, G.; Cao, Y.; Xu, Z.; Kang, Y.; Xue, P. Acidic TME-Responsive Nano-Bi2 Se3 @MnCaP as a NIR-II-Triggered Free Radical Generator for Hypoxia-Irrelevant Phototherapy with High Specificity and Immunogenicity. Small 2022, 18, e2104302. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, H.; Liu, B.; Liu, Z. Tumor microenvironment-responsive dynamic inorganic nanoassemblies for cancer imaging and treatment. Adv. Drug. Deliv. Rev. 2021, 179, 114004. [Google Scholar] [CrossRef]
- Duan, L.; Yang, L.; Jin, J.; Yang, F.; Liu, D.; Hu, K.; Wang, Q.; Yue, Y.; Gu, N. Micro/nano-bubble-assisted ultrasound to enhance the EPR effect and potential theranostic applications. Theranostics 2020, 10, 462–483. [Google Scholar] [CrossRef]
- Gonzalez-Menendez, P.; Hevia, D.; Alonso-Arias, R.; Alvarez-Artime, A.; Rodriguez-Garcia, A.; Kinet, S.; Gonzalez-Pola, I.; Taylor, N.; Mayo, J.C.; Sainz, R.M. GLUT1 protects prostate cancer cells from glucose deprivation-induced oxidative stress. Redox Biol. 2018, 17, 112–127. [Google Scholar] [CrossRef]
- Kim, S.J.; Bae, P.K.; Chung, B.H. Self-assembled levan nanoparticles for targeted breast cancer imaging. Chem. Commun. 2015, 51, 107–110. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, H.; Yin, Y.; Sun, M.; Mao, S.; Chen, H.; Deng, Y.; Chen, S.; Li, S.; Sun, B. Engineered EGCG-Containing Biomimetic Nanoassemblies as Effective Delivery Platform for Enhanced Cancer Therapy. Adv. Sci. 2022, 9, e2105894. [Google Scholar] [CrossRef]
- Ejima, H.; Richardson, J.J.; Liang, K.; Best, J.P.; van Koeverden, M.P.; Such, G.K.; Cui, J.; Caruso, F. One-step assembly of coordination complexes for versatile film and particle engineering. Science 2013, 341, 154–157. [Google Scholar] [CrossRef]
- Zhang, Z.; Sang, W.; Xie, L.; Li, W.; Li, B.; Li, J.; Tian, H.; Yuan, Z.; Zhao, Q.; Dai, Y. Polyphenol-Based Nanomedicine Evokes Immune Activation for Combination Cancer Treatment. Angew. Chem. Int. Ed. Engl. 2021, 60, 1967–1975. [Google Scholar] [CrossRef]
- Yan, J.; Wang, G.; Xie, L.; Tian, H.; Li, J.; Li, B.; Sang, W.; Li, W.; Zhang, Z.; Dai, Y. Engineering Radiosensitizer-Based Metal-Phenolic Networks Potentiate STING Pathway Activation for Advanced Radiotherapy. Adv. Mater. 2022, 34, e2105783. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Zhang, Y.; Liu, X.; Zhou, Q.; Piao, Y.; Shao, S.; Tang, J.; Zhou, Z.; Xie, T.; Shen, Y. Natural Polyphenols-Platinum Nanocomplexes Stimulate Immune System for Combination Cancer Therapy. Nano Lett. 2022, 22, 5615–5625. [Google Scholar] [CrossRef]
- Jing, L.; Lin, J.; Yang, Y.; Tao, L.; Li, Y.; Liu, Z.; Zhao, Q.; Diao, A. Quercetin inhibiting the PD-1/PD-L1 interaction for immune-enhancing cancer chemopreventive agent. Phytother. Res. 2021, 35, 6441–6451. [Google Scholar] [CrossRef]
- Li, L.; Zhang, M.; Liu, T.; Li, J.; Sun, S.; Chen, J.; Liu, Z.; Zhang, Z.; Zhang, L. Quercetin-ferrum nanoparticles enhance photothermal therapy by modulating the tumor immunosuppressive microenvironment. Acta Biomater. 2022. [Google Scholar] [CrossRef]
- Jung, S.H.; Bulut, S.; Busca Guerzoni, L.P.B.; Gunther, D.; Braun, S.; De Laporte, L.; Pich, A. Fabrication of pH-degradable supramacromolecular microgels with tunable size and shape via droplet-based microfluidics. J. Colloid Interface Sci. 2022, 617, 409–421. [Google Scholar] [CrossRef]
- Baek, S.L.; Kim, Y.; Jang, Y.; Lee, S.M. Polyphenol-Incorporated Composite Nanogels of Multimodal Interactions for Enhanced Gel Stability and Cisplatin Delivery. ACS Macro Lett. 2022, 11, 1129–1135. [Google Scholar] [CrossRef]
- Liang, M.; Guo, M.; Saw, P.E.; Yao, Y. Fully Natural Lecithin Encapsulated Nano-Resveratrol for Anti-Cancer Therapy. Int. J. Nanomed. 2022, 17, 2069–2078. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lin, Z.; Chen, Y.; Gao, D.; Wang, P.; Lin, Y.; Wang, Y.; Wang, F.; Han, Y.; Yuan, H. Co-delivery of Docetaxel and Resveratrol by liposomes synergistically boosts antitumor efficiency against prostate cancer. Eur. J. Pharm. Sci. 2022, 174, 106199. [Google Scholar] [CrossRef] [PubMed]
- Caddeo, C.; Gabriele, M.; Nacher, A.; Fernandez-Busquets, X.; Valenti, D.; Maria Fadda, A.; Pucci, L.; Manconi, M. Resveratrol and artemisinin eudragit-coated liposomes: A strategy to tackle intestinal tumors. Int. J. Pharm. 2021, 592, 120083. [Google Scholar] [CrossRef]
- Tao, Y.; Liu, Y.; Dong, Z.; Chen, X.; Wang, Y.; Li, T.; Li, J.; Zang, S.; He, X.; Chen, D.; et al. Cellular Hypoxia Mitigation by Dandelion-like Nanoparticles for Synergistic Photodynamic Therapy of Oral Squamous Cell Carcinoma. ACS Appl. Mater. Interfaces 2022, 14, 44039–44053. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Gao, X.; Raza, F.; Zafar, H.; Huang, G.; Yang, Y.; Shi, F.; Wang, D.; He, X. Design of GSH-Responsive Curcumin Nanomicelles for Oesophageal Cancer Therapy. Pharmaceutics 2022, 14, 1802. [Google Scholar] [CrossRef]
- Eskandari, Z.; Bahadori, F.; Yenigun, V.B.; Demiray, M.; Eroglu, M.S.; Kocyigit, A.; Oner, E.T. Levan enhanced the NF-kappaB suppression activity of an oral nano PLGA-curcumin formulation in breast cancer treatment. Int. J. Biol. Macromol. 2021, 189, 223–231. [Google Scholar] [CrossRef]
- Peng, Y.; Yu, S.; Wang, Z.; Huang, P.; Wang, W.; Xing, J. Nanogels loading curcumin in situ through microemulsion photopolymerization for enhancement of antitumor effects. J. Mater. Chem. B 2022, 10, 3293–3302. [Google Scholar] [CrossRef] [PubMed]
- Abedi, F.; Davaran, S.; Hekmati, M.; Akbarzadeh, A.; Baradaran, B.; Moghaddam, S.V. An improved method in fabrication of smart dual-responsive nanogels for controlled release of doxorubicin and curcumin in HT-29 colon cancer cells. J. Nanobiotechnol. 2021, 19, 18. [Google Scholar] [CrossRef]
- Yongvongsoontorn, N.; Chung, J.E.; Gao, S.J.; Bae, K.H.; Yamashita, A.; Tan, M.H.; Ying, J.Y.; Kurisawa, M. Carrier-Enhanced Anticancer Efficacy of Sunitinib-Loaded Green Tea-Based Micellar Nanocomplex beyond Tumor-Targeted Delivery. ACS Nano 2019, 13, 7591–7602. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, G.; Wang, B.; Cao, G.; Li, D.; Wang, Y.; Zhang, Y.; Geng, J.; Li, H.; Li, Y. Reinforcing the Combinational Immuno-Oncotherapy of Switching “Cold” Tumor to “Hot” by Responsive Penetrating Nanogels. ACS Appl. Mater. Interfaces 2021, 13, 36824–36838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, G.; Ouyang, Z.; Yang, R.; Gao, Y.; Cao, X.; Banyai, I.; Shi, X.; Guo, R. Intelligent design of iron-doped LDH nanosheets for cooperative chemo-chemodynamic therapy of tumors. Biomater. Sci. 2022, 10, 2029–2039. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, J.; Xiu, J.; Zhao, X.; Yang, C.; Li, D.; Li, K.; Hu, H.; Qiao, M.; Chen, D.; et al. A phenolic based tumor-permeated nano-framework for immunogenic cell death induction combined with PD-L1 immune checkpoint blockade. Biomater. Sci. 2022, 10, 3808–3822. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, J.; Wu, P.; Zou, J.; Le, J.; Lin, J.; Li, C.; Luo, B.; Zhang, Y.; Huang, R.; et al. A smart dual-drug nanosystem based on co-assembly of plant and food-derived natural products for synergistic HCC immunotherapy. Acta. Pharm. Sin. B 2021, 11, 246–257. [Google Scholar] [CrossRef]
- Chen, Q.; Li, Q.; Liang, Y.; Zu, M.; Chen, N.; Canup, B.S.B.; Luo, L.; Wang, C.; Zeng, L.; Xiao, B. Natural exosome-like nanovesicles from edible tea flowers suppress metastatic breast cancer via ROS generation and microbiota modulation. Acta. Pharm. Sin. B 2022, 12, 907–923. [Google Scholar] [CrossRef]
- Crommelin, D.J.A.; van Hoogevest, P.; Storm, G. The role of liposomes in clinical nanomedicine development. What Now? Now What? J. Control. Release 2020, 318, 256–263. [Google Scholar] [CrossRef]
- Kao, Y.S.; Chen, C.W.; Wei, J.C. Helicobacter pylori infection and risk of gastric cancer. Lancet Public Health 2022, 7, e302. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Wu, Y.; Song, Z.; Li, S.; Du, M.; Deng, J.; Xu, Q.; Deng, L.; Bahlol, H.S.; Han, H. Tea Polyphenol Liposomes Overcome Gastric Mucus to Treat Helicobacter Pylori Infection and Enhance the Intestinal Microenvironment. ACS Appl. Mater. Interfaces 2022, 14, 13001–13012. [Google Scholar] [CrossRef] [PubMed]
- Piwowarczyk, L.; Kucinska, M.; Tomczak, S.; Mlynarczyk, D.T.; Piskorz, J.; Goslinski, T.; Murias, M.; Jelinska, A. Liposomal Nanoformulation as a Carrier for Curcumin and pEGCG-Study on Stability and Anticancer Potential. Nanomaterials 2022, 12, 1274. [Google Scholar] [CrossRef]
- Zoulikha, M.; Xiao, Q.; Boafo, G.F.; Sallam, M.A.; Chen, Z.; He, W. Pulmonary delivery of siRNA against acute lung injury/acute respiratory distress syndrome. Acta. Pharm. Sin. B 2022, 12, 600–620. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantu, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Ghosh, B.; Biswas, S. Polymeric micelles in cancer therapy: State of the art. J. Control. Release 2021, 332, 127–147. [Google Scholar] [CrossRef]
- Wan, Y.; Fu, L.H.; Li, C.; Lin, J.; Huang, P. Conquering the Hypoxia Limitation for Photodynamic Therapy. Adv. Mater. 2021, 33, e2103978. [Google Scholar] [CrossRef]
- Wang, H.; Gao, L.; Fan, T.; Zhang, C.; Zhang, B.; Al-Hartomy, O.A.; Al-Ghamdi, A.; Wageh, S.; Qiu, M.; Zhang, H. Strategic Design of Intelligent-Responsive Nanogel Carriers for Cancer Therapy. ACS Appl. Mater. Interfaces 2021, 13, 54621–54647. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Shi, L.; Gao, X.; Ma, Y.; Yang, J.; Zhang, Z.; Qian, J.; Zhu, X. A Paclitaxel-Based Mucoadhesive Nanogel with Multivalent Interactions for Cervical Cancer Therapy. Small 2019, 15, e1903208. [Google Scholar] [CrossRef]
- Fu, S.; Li, G.; Zang, W.; Zhou, X.; Shi, K.; Zhai, Y. Pure drug nano-assemblies: A facile carrier-free nanoplatform for efficient cancer therapy. Acta. Pharm. Sin. B 2022, 12, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Ding, L.; Yao, H.; Chen, Y.; Shi, J. A Metal-Organic Framework (MOF) Fenton Nanoagent-Enabled Nanocatalytic Cancer Therapy in Synergy with Autophagy Inhibition. Adv. Mater. 2020, 32, e1907152. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Ning, X.; Wang, M.; Yu, A.; Wang, Y. A multifunctional nano-delivery system enhances the chemo-co-phototherapy of tumor multidrug resistance via mitochondrial-targeting and inhibiting P-glycoprotein-mediated efflux. J. Mater. Chem. B 2021, 9, 9174–9182. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shi, W.; Zeng, D.; Huang, Q.; Xie, J.; Wen, H.; Li, J.; Yu, X.; Qin, L.; Zhou, Y. pH-activated, mitochondria-targeted, and redox-responsive delivery of paclitaxel nanomicelles to overcome drug resistance and suppress metastasis in lung cancer. J. Nanobiotechnol. 2021, 19, 152. [Google Scholar] [CrossRef]
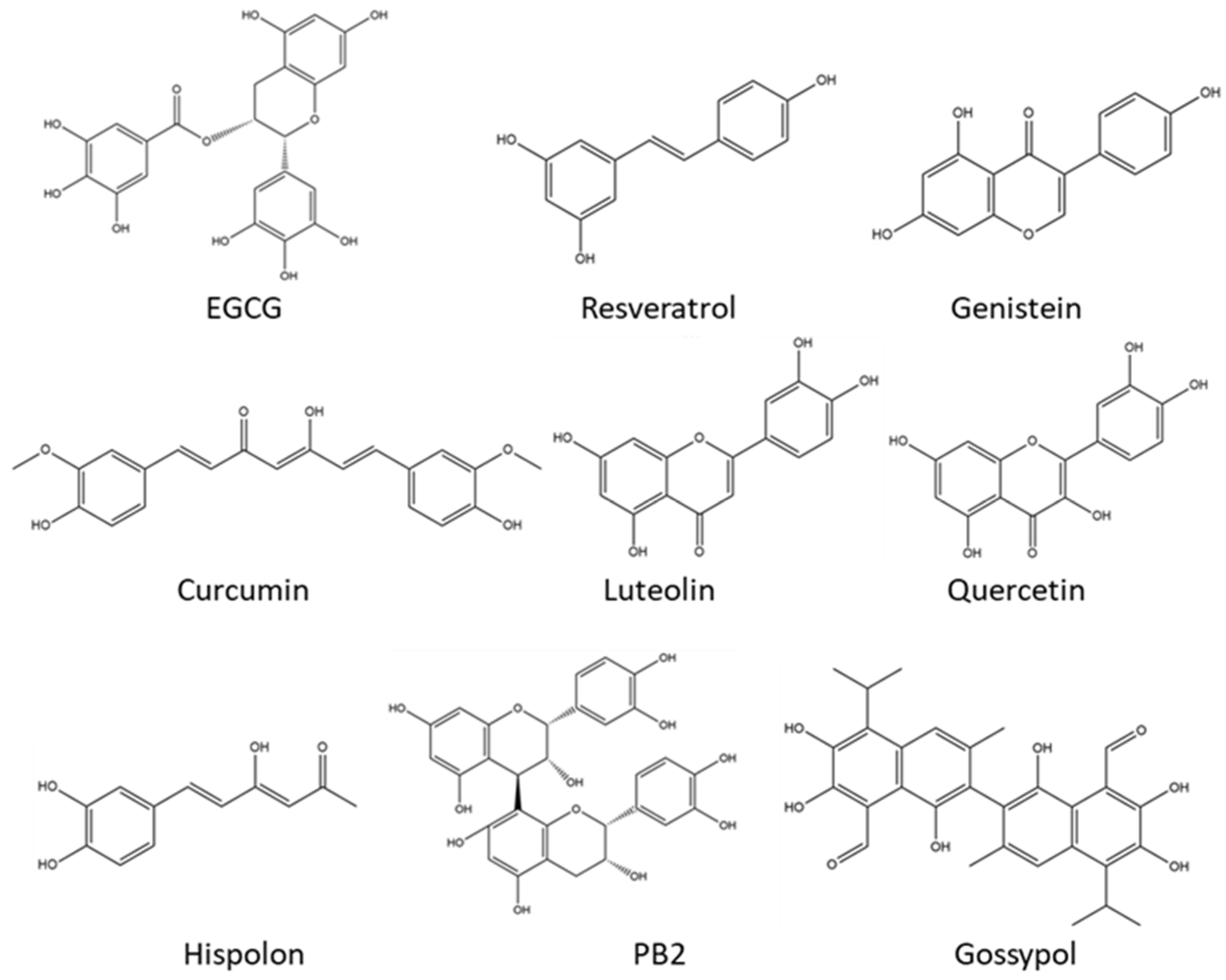
| Name | Source | Tumor Model | Mechanisms | Ref. |
|---|---|---|---|---|
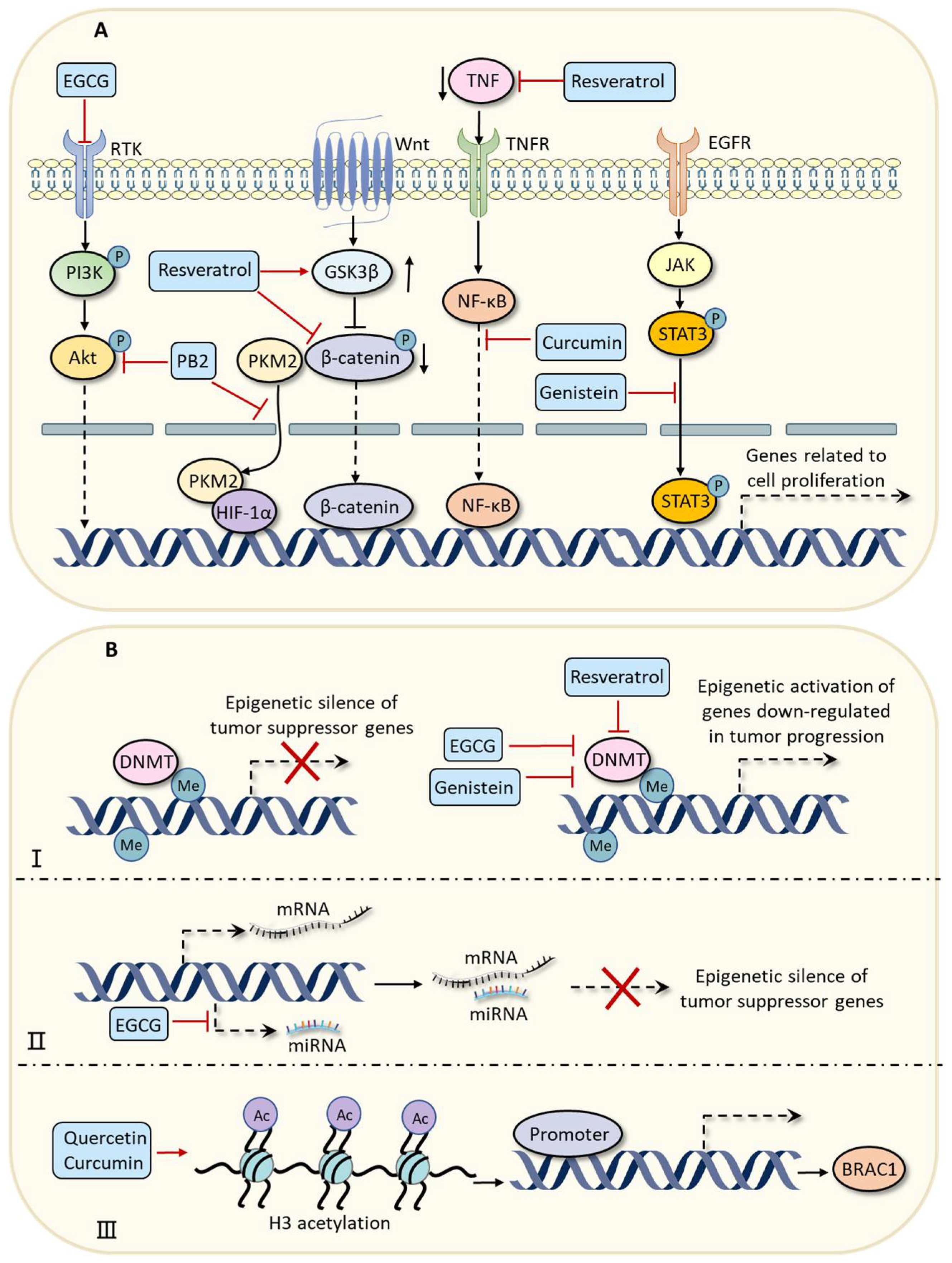
| EGCG | Green tea, etc. | Prostate cancer | Targeting the Akt/PI3K pathway to inhibit cell proliferation. | [31] |
| Breast cancer | Suppressing the activities of DNMT to promote the expressions of tumor suppressor genes. | [32] | ||
| Myeloma | Downregulating the expressions of miR-25, miR-92, miR-141, and miR-200a to activate the expression of tumor suppressor gene p53. | [33] | ||
| Colorectal cancer | Modulating gut microbial composition. | [34] | ||
| PB2 | Grape seeds, peanut skin, etc. | Liver cancer; hepatocellular cancer | Targeting the Akt/PI3K pathway to inhibit cell proliferation. | [35] |
| Hepatocellular cancer | Targeting the PKM2/HIF-1α signaling pathway to trigger apoptosis and inhibit cell proliferation. | [36] | ||
| Resveratrol | Grapes, berries, soybeans, etc. | Breast cancer | Targeting the Wnt/β-catenin signaling pathway to inhibit cell proliferation. | [37] |
| Colorectal cancer | Targeting the TNF-β/NF-κB signaling pathway to inhibit cell proliferation. | [38] | ||
| Breast cancer | Suppressing the activity of DNMT to enhance the expression of ATP2A3. | [39] | ||
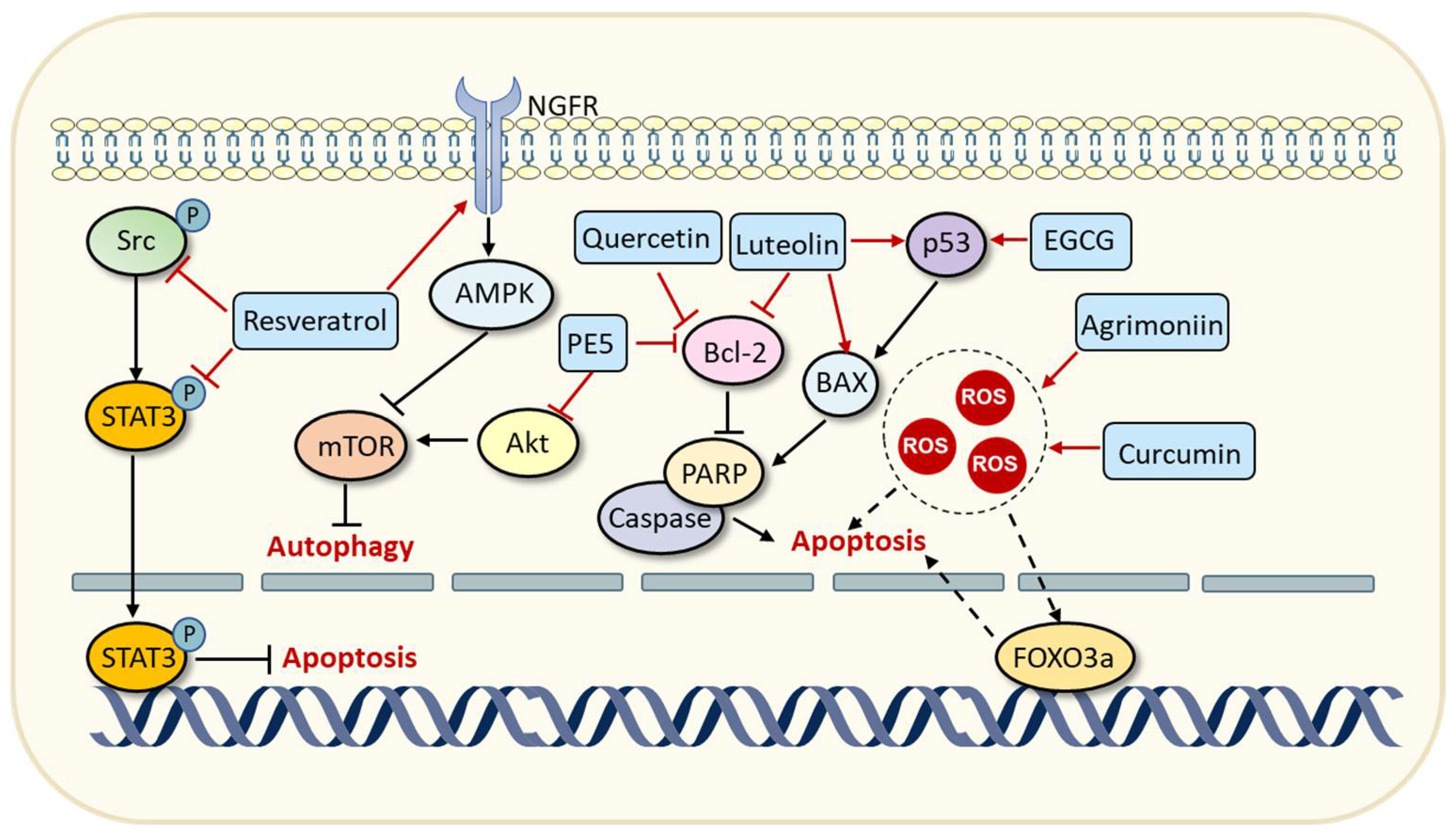
| Breast cancer; pancreatic cancer; prostate cancer | Inducing apoptosis of cancer cells by suppressing the phosphorylation of the Src-STAT3 signaling pathway. | [40] | ||
| Non-small-cell lung cancer | Modulating the AMPK/mTOR signaling pathway to trigger autophagy. | [41] | ||
| Colorectal cancer | Preventing EMT by inhibiting the TGF-β/Smad signaling pathway. | [42] | ||
| Curcumin | Curcuma longa (turmeric) | Cervical cancer | Targeting the NF-κB signaling pathway to inhibit cell proliferation. | [43] |
| Lung cancer | Enhancing ROS generation and FOXO3a expression, thereby triggering apoptosis. | [44] | ||
| Prostate cancer | Inducing the apoptosis of CAFs to prevent the growth and metastasis of tumors. | [45] | ||
| Genistein | Legumes and dentate plants | Esophageal carcinoma | Targeting the JAK/STAT3 signaling pathway to inhibit cell proliferation. | [46] |
| Breast cancer | Decreasing the CpG methylation in the promoters of BRAC1. | [47] | ||
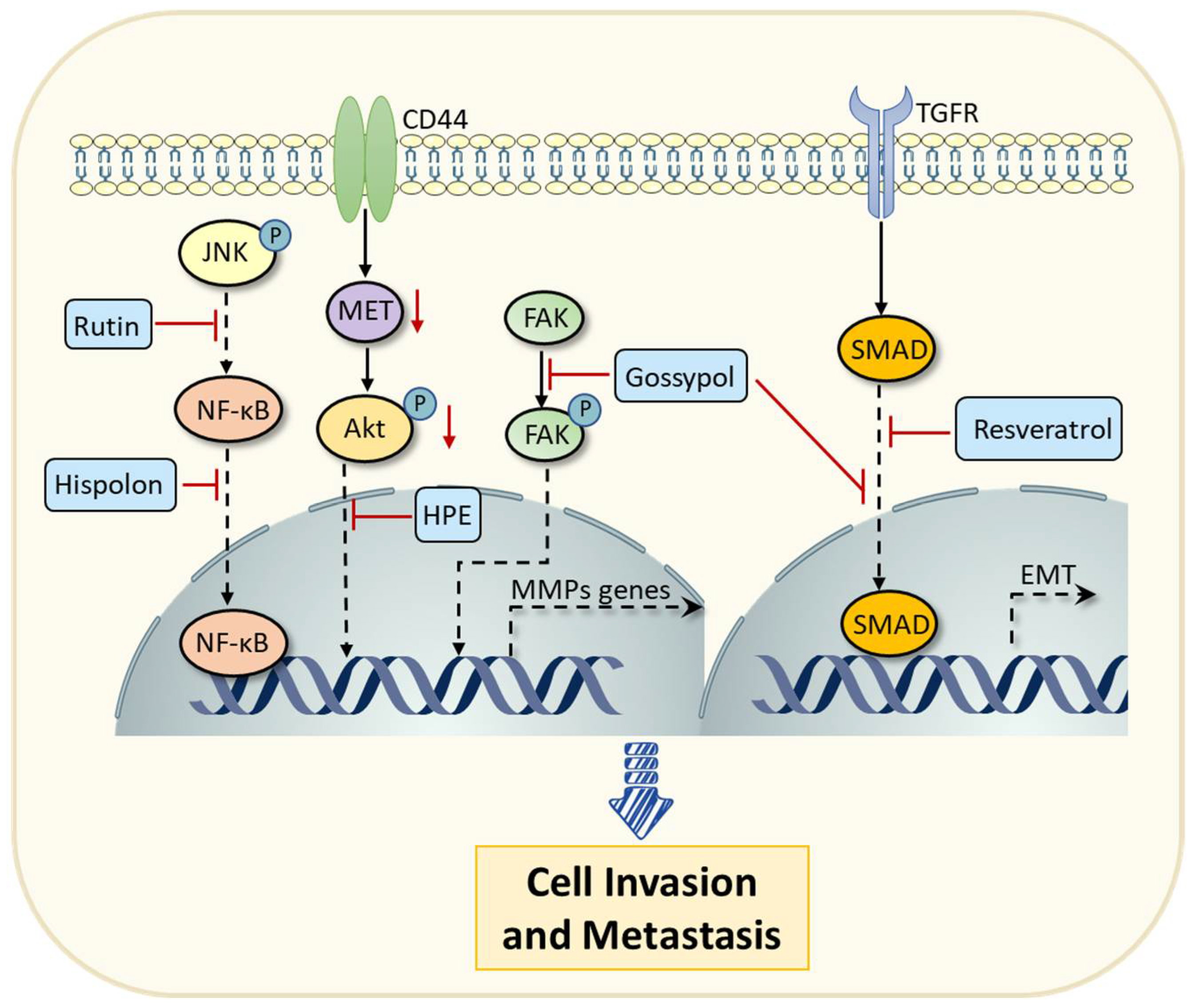
| Hispolon | Traditional medicinal mushroom phellinus linteus | Breast cancer | Inhibiting the NF-κB signaling pathway and suppressing the expression of MMP-9 to prevent cell invasion. | [48] |
| Quercetin | Green tea, onion, etc. | Breast cancer | Increasing the acetylation of histone H3K9 in the promoter of BRCA1 (combination effects with curcumin). | [49] |
| Gastric cancer | Decreasing the expression of Bcl-2 and triggering apoptosis. | [50] | ||
| PE5 | Roots of Phragmipedium species | Lung cancer | Targeting Akt/mTOR and Bcl-2 signaling pathways to trigger autophagy and apoptosis. | [51] |
| Luteolin | Celery; chrysanthemum flowers | Breast cancer | Increasing the expressions of p53 and BAX while decreasing the level of Bcl-2, thereby triggering apoptosis. | [52] |
| Agrimoniin | Agrimonia pilosa ledeb | Pancreatic cancer | Increasing intracellular ROS levels and triggering apoptosis. | [53] |
| HPE | Hibiscus sabdariffa | Colon carcinoma | Inhibiting CD44/c-MET signaling pathway to decrease the expression of MMPs, thereby preventing tumor metastasis. | [54] |
| Gossypol | Cottonseed, etc. | Cervical cancer | Inhibiting the FAK signaling pathway and decreasing the expression of MMPs, thereby preventing tumor metastasis. | [55] |
| Cervical cancer | Reversing the EMT mediated by TGF-β. | [55] | ||
| Castalagin | Camu-camu (Myrciaria dubia) | Non-small-cell lung cancer | Improving the infiltration of CD8+ T cells and enhancing the efficacy of anti-PD-1 therapy by modulating gut microbiota. | [12] |
| Nanocarriers/Nanosystem | Natural Products (Therapeutic Agents) | Tumor Model | Therapy Strategies | Ref. |
|---|---|---|---|---|
| Cyclodextrin-based nanoformulation | Quercetin (ginsenoside Rg3) | Colorectal cancer | Chemotherapy; immunotherapy | [96] |
| Quercetin–ferrum NPs | Quercetin | Melanoma | Photothermal therapy; immunotherapy | [125] |
| CaCO3 NPs | Kaempferol | Lung cancer | Chemotherapy | [99] |
| Gold NPs | Genistein | Prostate cancer | Chemotherapy | [102] |
| Mesoporous silica NPs | Resveratrol | Gastric cancer | Chemotherapy | [103] |
| Lecithin | Resveratrol | Breast cancer | Chemotherapy | [128] |
| Liposomes | Resveratrol (docetaxel) | Prostate cancer | Chemotherapy | [129] |
| Eudragit-coated liposomes | Resveratrol (artemisinin) | Intestinal tumors | Chemotherapy | [130] |
| Micelles conjugated on hyaluronic nanogel | Resveratrol (Ce6) | Oral squamous cell carcinoma | Chemotherapy; photodynamic therapy | [131] |
| Micelles | Curcumin | Esophageal cancer | Chemotherapy | [132] |
| Micelles | Curcumin | Breast cancer | Chemotherapy | [133] |
| Nanogels | Curcumin | Liver cancer | Chemotherapy | [134] |
| Nanogels | Curcumin | Colon cancer | Chemotherapy | [110] |
| Nanogels | Curcumin (doxorubicin) | Colon cancer | Chemotherapy | [135] |
| Metal–phenolic network | Gossypol (Ce6) | Breast cancer | Chemotherapy; immunotherapy; photodynamic therapy | [121] |
| Metal–phenolic network | Tannic acid (oxaliplatin) | Colon cancer | Chemotherapy; immunotherapy | [123] |
| Nanogels | Tannic acid (cisplatin) | / | / | [127] |
| Nanoassembly | EGCG (siPD-L1) | Liver cancer | Immunotherapy | [119] |
| Micellar nanocomplex | EGCG (sunitinib) | Kidney cancer | Chemotherapy | [136] |
| Nanogels | EGCG (resiquimod) | Melanoma | Immunotherapy | [137] |
| Iron-doped LDH Nanosheets | EGCG | Melanoma | Chemotherapy; chemodynamic therapy | [138] |
| Platinum NPs | EGCG | Breast cancer | Immunotherapy | [139] |
| Nanoassembly | EGCG (ursolic acid) | Hepatocellular carcinoma | Immunotherapy | [140] |
| Exosome-like natural nanovesicles from tea flowers | EGCG, ECG, etc. | Breast cancer | Chemotherapy | [141] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, W.; Zhou, L.; Li, L.; Zhou, P.; Shen, Z. Nano-Based Drug Delivery of Polyphenolic Compounds for Cancer Treatment: Progress, Opportunities, and Challenges. Pharmaceuticals 2023, 16, 101. https://doi.org/10.3390/ph16010101
Jia W, Zhou L, Li L, Zhou P, Shen Z. Nano-Based Drug Delivery of Polyphenolic Compounds for Cancer Treatment: Progress, Opportunities, and Challenges. Pharmaceuticals. 2023; 16(1):101. https://doi.org/10.3390/ph16010101
Chicago/Turabian StyleJia, Wenhui, Li Zhou, Lei Li, Ping Zhou, and Zhisen Shen. 2023. "Nano-Based Drug Delivery of Polyphenolic Compounds for Cancer Treatment: Progress, Opportunities, and Challenges" Pharmaceuticals 16, no. 1: 101. https://doi.org/10.3390/ph16010101
APA StyleJia, W., Zhou, L., Li, L., Zhou, P., & Shen, Z. (2023). Nano-Based Drug Delivery of Polyphenolic Compounds for Cancer Treatment: Progress, Opportunities, and Challenges. Pharmaceuticals, 16(1), 101. https://doi.org/10.3390/ph16010101







