Nitrostilbenes: Synthesis and Biological Evaluation as Potential Anti-Influenza Virus Agents
Abstract
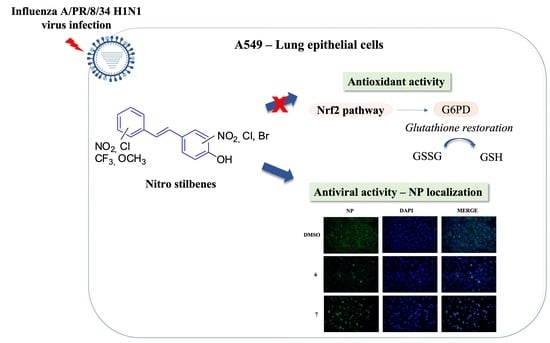
:1. Introduction
2. Results
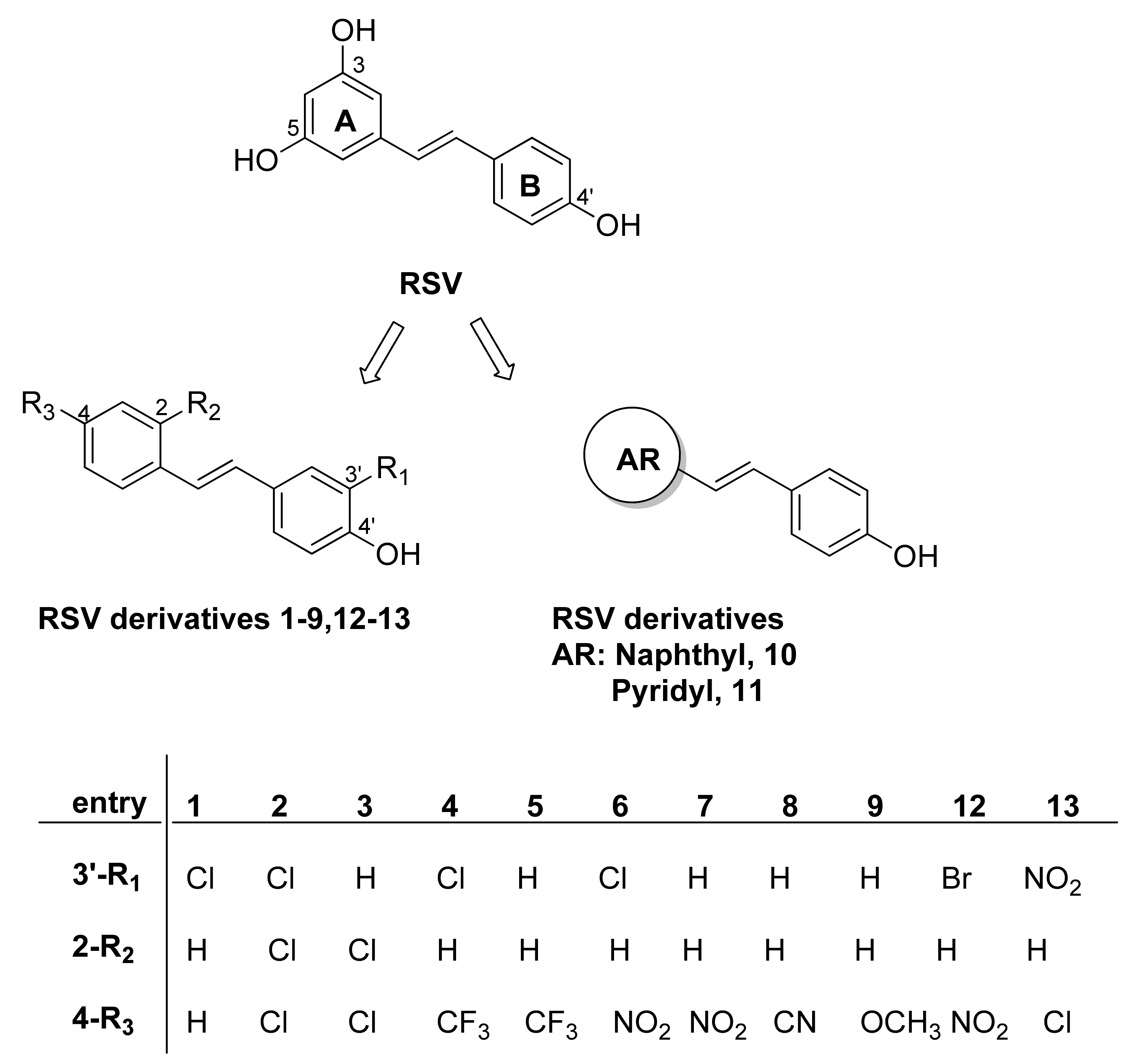
2.1. Chemistry
2.2. Antiviral Activity of RSV Derivatives against Influenza A Virus Infection
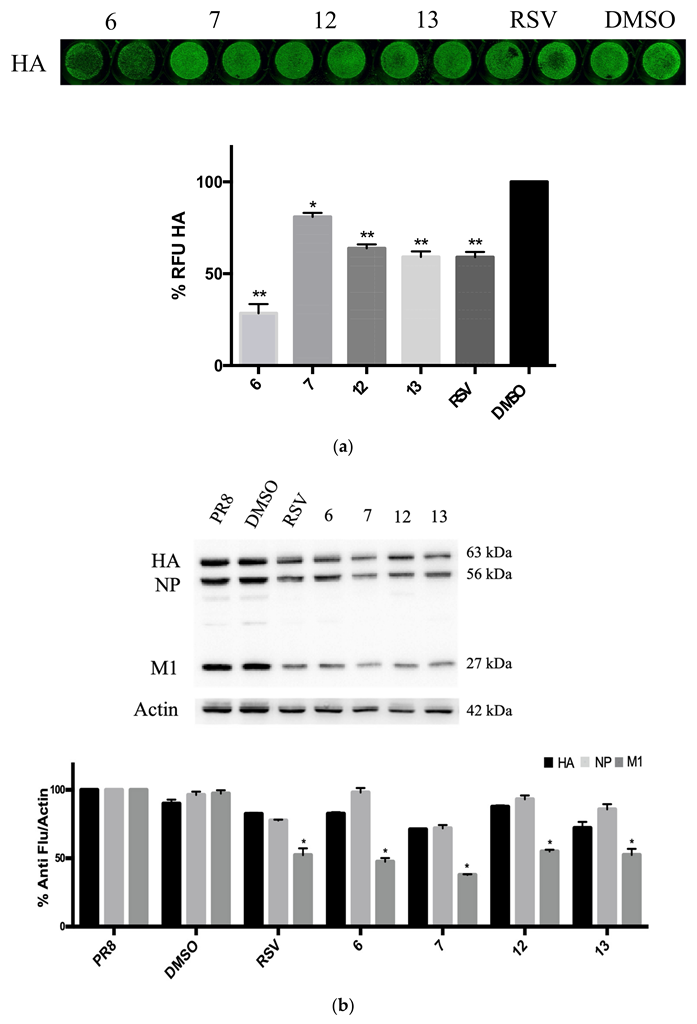
2.3. RSV Derivatives 6, 7, 12, and 13 Strongly Reduce Viral Protein Synthesis and Viral Particle Production
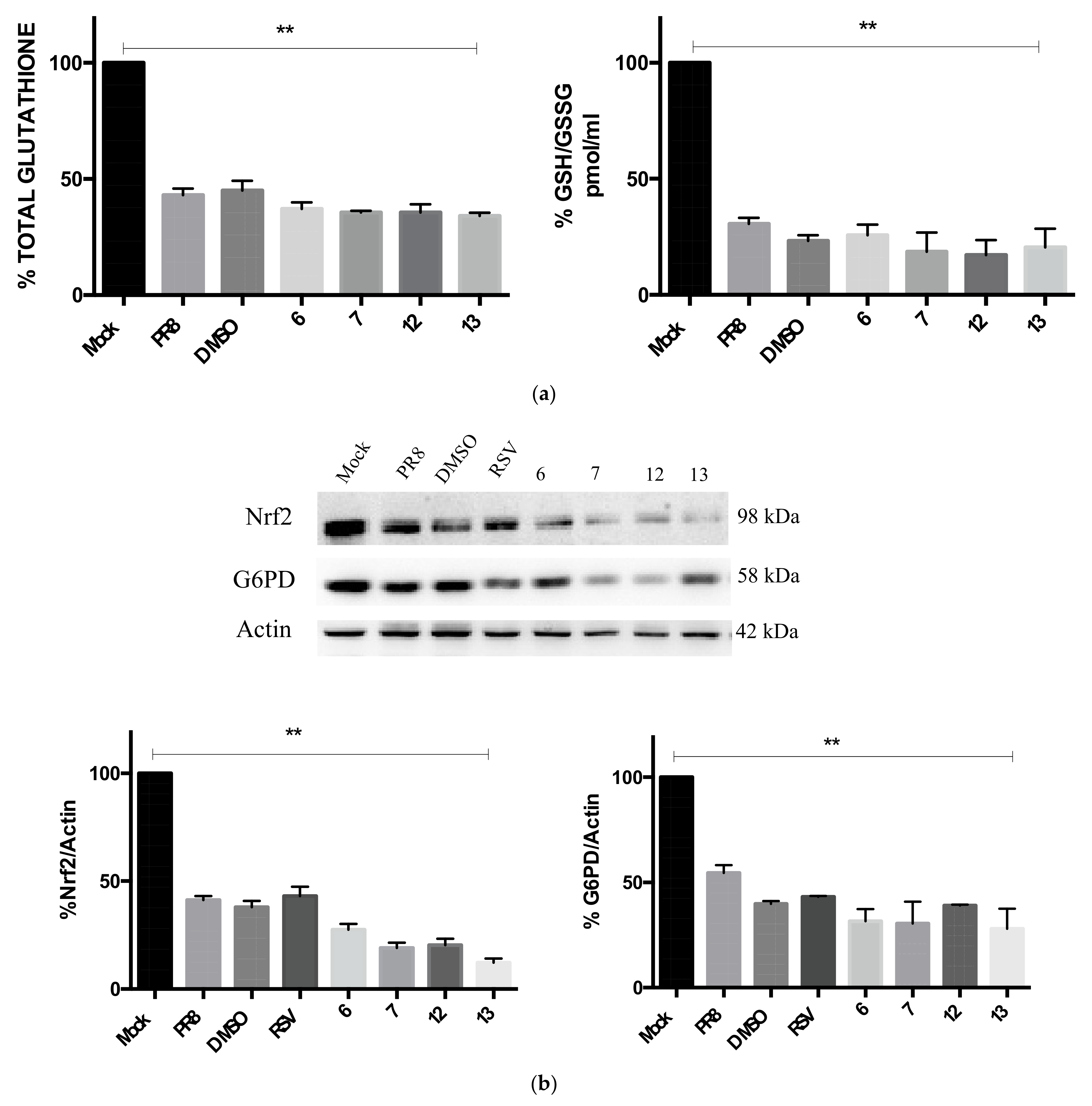
2.4. Compounds 6, 7, 12, and 13 Do Not Rescue the GSH-Mediated Antioxidant Response
2.5. Compounds 6 and 7 Impair Viral Replication by Blocking the Viral NP Protein in the Nuclear Compartment
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.1.1. General Procedure for the Preparation of Phenols 12–13
(E)-2-Bromo-4-(4-Nitrostyryl)Phenol, 12
(E)-4-(4-Chlorostyryl)-2-Nitrophenol, 13
4.2. Biology
4.2.1. Cell Cultures and Virus Production
4.2.2. Cytotoxicity Assay
4.2.3. Cell Treatment
4.2.4. Hemagglutination (HAU) Assay
4.2.5. In-Cell Western Assay
4.2.6. Western Blot Analysis
4.2.7. Glutathione Assay
4.2.8. Immunofluorescence Analysis
4.2.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Frémont, L. Biological effects of resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Mollica, A.; Scioli, G.; Della Valle, A.; Cichelli, A.; Novellino, E.; Bauer, M.; Kamysz, W.; Llorent-Martínez, E.J.; Fernández-de Córdova, M.L.; Castillo-López, R.; et al. Phenolic analysis and in vitro biological activity of red wine, pomace and grape seeds oil derived from vitis vinifera L. CV. Montepulciano d’Abruzzo. Antioxidants 2021, 10, 1704. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.X.; Li, C.X.; Kakar, M.U.; Khan, M.S.; Wu, P.F.; Amir, R.M.; Dai, D.F.; Naveed, M.; Li, Q.Y.; Saeed, M.; et al. Resveratrol (RV): A pharmacological review and call for further research. Biomed. Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, R.; Ali Ebrahimzadeh, M. Resveratrol–A comprehensive review of recent advances in anticancer drug design and development. Eur. J. Med. Chem. 2020, 200, 112356. [Google Scholar] [CrossRef] [PubMed]
- Almatroodi, S.A.; Alsahli, M.; Aljohani, A.; Alhumaydhi, F.A.; Babiker, A.Y.; Khan, A.A.; Rahmani, A.H. Potential therapeutic targets of resveratrol, a plant polyphenol, and its role in the therapy of various types of cancer. Molecules 2022, 21, 2665. [Google Scholar] [CrossRef]
- Bal, N.B.; Bostanci, A.; Sadi, G.; Donmez, M.O.; Uludag, M.O.; Demirel-Yilmaz, E. Resveratrol and regular exercise may attenuate hypertension-induced cardiac dysfunction through modulation of cellular stress responses. Life Sci. 2022, 296, 120424. [Google Scholar] [CrossRef]
- Reinisalo, M.; Kårlund, A.; Koskela, A.; Kaarniranta, K.; Karjalainen, R.O. Polyphenol stilbenes: Molecular mechanisms of defence against oxidative stress and aging-related diseases. Oxid. Med. Cell. Longev. 2015, 2015, 340520. [Google Scholar] [CrossRef]
- Banez, M.J.; Geluz, M.I.; Chandra, A.; Hamdan, T.; Biswas, O.S.; Bryan, N.S.; Von Schwarz, E.R. A systemic review on the antioxidant and anti-inflammatory effects of resveratrol, curcumin, and dietary nitric oxide supplementation on human cardiovascular health. Nutr. Res. 2020, 78, 11–26. [Google Scholar] [CrossRef]
- Abbas, S.R.; Khan, R.T.; Shafique, S.; Mumtaz, S.; Khan, A.A.; Khan, A.M.; Hassan, Z.; Hussain, S.A.; Abbas, S.; Abbas, M.R.; et al. Study of resveratrol against bone loss by using in-silico and in-vitro methods. Braz. J. Biol. 2021, 83, e248024. [Google Scholar] [CrossRef]
- Kupisiewicz, K.; Boissy, P.; Abdallah, B.M.; Dagnaes Hansen, F.; Erben, R.G.; Savouret, G.-F.; Søe, K.; Andersen, T.L.; Plesner, T.; Delaisse, J.-M. Potential of Resveratrol Analogues as Antagonists of Osteoclasts and Promoters of Osteoblasts. Calcif. Tissue Int. 2010, 87, 437–449. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, M.G.; Schimith, L.E.; André-Miral, C.; Muccillo-Baisch, A.L.; Arbo, B.D.; Hort, M.A. Neuroprotective effects of resveratrol in in vivo and in vitro experimental models of Parkinson’s disease: A systematic review. Neurotox. Res. 2022, 40, 319–345. [Google Scholar] [CrossRef] [PubMed]
- Palamara, A.T.; Nencioni, L.; Aquilano, K.; De Chiara, G.; Hernandez, L.; Cozzolino, F.; Ciriolo, M.R.; Garaci, E. Inhibition of influenza A virus replication by resveratrol. J. Infect. Dis. 2005, 191, 1719–1729. [Google Scholar] [CrossRef] [PubMed]
- Mattio, L.M.; Catinella, G.; Pinto, A.; Dallavalle, S. Natural and nature-inspired stilbenoids as antiviral agents. Eur. J. Med. Chem. 2020, 202, 112541. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.S.L.; Tan, L.T.-H.; Chan, K.-G.; Yap, W.H.; Pusparajah, P.; Chuah, L.-H.; Ming, L.C.; Khan, T.M.; Lee, L.H.; Goh, B.H. Resveratrol-potential antibacterial agent against foodborne pathogens. Front. Pharmacol. 2018, 9, 102–118. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.; Ingmer, H. Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Abba, Y.; Hassim, H.; Hamzah, H.; Noordin, M.M. Antiviral activity of resveratrol against human and animal viruses. Adv. Virol. 2015, 2015, 184241. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Cantò, C. The molecular targets of resveratrol. Biochim. Biophys. Acta 2015, 1852, 1114–1123. [Google Scholar] [CrossRef]
- Jayaprakash, J.S.; Gowda, D.V.; Kulkarni, P.K. Therapeutic application of Resveratrol in human diseases. Int. J. Res. Pharm. Sci. 2020, 11, 1447–1456. [Google Scholar] [CrossRef]
- Espinoza, J.L.; Takami, A.; Trung, L.Q.; Kato, S.; Nakao, S. Resveratrol prevents EBV transformation and inhibits the outgrowth of EBV-immortalized human B cells. PLoS ONE 2012, 7, e51306. [Google Scholar] [CrossRef]
- Docherty, J.J.; Fu, M.M.; Stiffler, B.S.; Limperos, R.J.; Pokabla, C.M.; DeLucia, A.L. Resveratrol inhibition of herpes simplex virus replication. Antivir. Res. 1999, 43, 145–155. [Google Scholar] [CrossRef]
- Xie, X.H.; Zang, N.; Li, S.M.; Wang, L.J.; Deng, Y.; He, Y.; Yang, X.Q.; Liu, E.M. Resveratrol inhibits respiratory syncytial virus-induced IL-6 production, decreases viral replication, and downregulates TRIF expression in airway epithelial cells. Inflammation 2012, 35, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.N.; Trinité, B.; Levy, D.N. Potent Inhibition of HIV-1 Replication in Resting CD4 T Cells by Resveratrol and Pterostilbene. Antimicrob. Agents Chemother. 2017, 61, e00408-17. [Google Scholar] [CrossRef] [PubMed]
- Loaiza-Cano, V.; Monsalve-Escudero, L.M.; Filho, C.D.S.M.B.; Martinez-Gutierrez, M.; Sousa, D.P. Antiviral role of phenolic compounds against dengue virus: A review. Biomolecules 2021, 11, 11. [Google Scholar] [CrossRef]
- Fioravanti, R.; Celestino, I.; Costi, R.; Cuzzucoli Crucitti, G.; Pescatori, L.; Mattiello, L.; Novellino, E.; Checconi, P.; Palamara, A.T.; Nencioni, L.; et al. Effects of polyphenol compounds on influenza A virus replication and definition of their mechanism of action. Bioorg. Med. Chem. 2012, 20, 5046–5052. [Google Scholar] [CrossRef] [PubMed]
- Al Adem, K.; Shanti, A.; Stefanini, C.; Lee, S. Inhibition of SARS-CoV-2 entry into host cells using small molecules. Pharmaceuticals 2020, 13, 447. [Google Scholar] [CrossRef]
- Filardo, S.; Di Pietro, M.; Mastromarino, P.; Sessa, R. Therapeutic potential of resveratrol against emerging respiratory viral infections. Pharmacol. Ther. 2020, 214, 107613. [Google Scholar] [CrossRef]
- Pasquereau, S.; Nehme, Z.; Haidar Ahmad, S.; Daouad, F.; Van Assche, J.; Wallet, C.; Schwartz, C.; Rohr, O.; Morot-Bizot, S.; Herbein, G. Resveratrol inhibits HCoV-229E and SARS-CoV-2 Coronavirus Replication in vitro. Viruses 2021, 23, 354. [Google Scholar] [CrossRef]
- De Angelis, M.; Della-Morte, D.; Buttinelli, G.; Di Martino, A.; Pacifici, F.; Checconi, P.; Ambrosio, L.; Stefanelli, P.; Palamara, A.T.; Garaci, E.; et al. Protective Role of Combined Polyphenols and Micronutrients against Influenza A Virus and SARS-CoV-2 Infection In Vitro. Biomedicines 2021, 19, 1721. [Google Scholar] [CrossRef]
- Pei, W.; Shengmin, S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. BioFactors 2018, 44, 16–25. [Google Scholar] [CrossRef]
- Giacomini, E.; Rupiani, S.; Guidotti, L.; Recanatini, M.; Roberti, M. The use of stilbene scaffold in medicinal chemistry and multiTarget drug design. Curr. Med. Chem. 2016, 23, 2439–2489. [Google Scholar] [CrossRef]
- Khatoon, S.; Kalam, N.; Shaikh, M.F.; Hasnain, M.S.; Hafiz, A.K.; Ansari, M.T. Nanoencapsulation of polyphenols as drugs and supplements for enhancing therapeutic profile-a review. Curr. Mol. Pharmacol. 2022, 15, 77–107. [Google Scholar] [CrossRef] [PubMed]
- Wahedi, H.M.; Ahmad, S.; Abbasi, S.W. Stilbene-based natural compounds as promising drug candidates against COVID-19. J. Biomol. Struct. Dyn. 2021, 39, 3225–3234. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, A.N.; Gomes, B.A.; Moraes, W.M., Jr.; Borges, R.S. A theoretical antioxidant pharmacophore for resveratrol. Eur. J. Med. Chem. 2009, 44, 1644–1649. [Google Scholar] [CrossRef]
- Al-Mamary, M.A.; Moussa, Z. Antioxidant Activity: The Presence and Impact of Hydroxyl Groups in Small Molecules of Natural and Synthetic Origin. In Antioxidants-Benefits, Sources, Mechanisms of Action; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- De Filippis, B.; De Lellis, L.; Florio, R.; Ammazzalorso, A.; Amoia, P.; Fantacuzzi, M.; Giampietro, L.; Maccallini, C.; Amoroso, R.; Veschi, S.; et al. Synthesis and cytotoxic effects on pancreatic cancer cells of resveratrol analogs. Med. Chem. Res. 2019, 28, 984–991. [Google Scholar] [CrossRef]
- Fantacuzzi, M.; Gallorini, M.; Gambacorta, N.; Ammazzalorso, A.; Aturki, Z.; Balaha, M.; Carradori, S.; Giampietro, L.; Maccallini, C.; Cataldi, A.; et al. Design, synthesis and biological evaluation of aromatase inhibitors based on sulfonates and sulfonamides of resveratrol. Pharmaceuticals 2021, 14, 984. [Google Scholar] [CrossRef]
- Di Fermo, P.; Di Lodovico, S.; Amoroso, R.; De Filippis, B.; D’Ercole, S.; Di Campli, E.; Cellini, L.; Di Giulio, M. Searching for new tools to counteract the Helicobacter pylori resistance: The positive action of resveratrol derivatives. Antibiotics 2020, 9, 891. [Google Scholar] [CrossRef]
- di Filippo, E.S.; Giampietro, L.; De Filippis, B.; Balaha, M.; Ferrone, V.; Locatelli, M.; Pietrangelo, T.; Tartaglia, A.; Amoroso, A.; Fulle, S. Synthesis and biological evaluation of halogenated E-stilbenols as promising antiaging agents. Molecules. 2020, 25, 5770. [Google Scholar] [CrossRef]
- Orgován, G.; Gonda, I.; Noszál, B. Biorelevant physicochemical profiling of (E)-and (Z)-resveratrol determined from isomeric mixtures. J. Pharm. Biomed. Anal. 2017, 138, 322–329. [Google Scholar] [CrossRef]
- Noriega, S.; Cardoso-Ortiz, J.; López-Luna, A.; Cuevas-Flores, M.D.R.; Flores De La Torre, J.A. The Diverse Biological Activity of Recently Synthesized Nitro Compounds. Pharmaceuticals 2022, 15, 717. [Google Scholar] [CrossRef]
- Jezuita, A.; Ejsmont, K.; Szatylowicz, H. Substituent effects of nitro group in cyclic compounds. Struct. Chem. 2021, 32, 179–203. [Google Scholar] [CrossRef]
- Ulomskiy, E.N.; Ivanova, A.V.; Gorbunov, E.B.; Esaulkova, I.L.; Slita, A.V.; Sinegubova, E.O.; Voinkov, E.K.; Drokin, R.A.; Butorin, I.I.; Gazizullina, E.R.; et al. Synthesis and biological evaluation of 6-nitro-1,2,4-triazoloazines containing polyphenol fragments possessing antioxidant and antiviral activity. Bioorg. Med. Chem. Lett. 2020, 30, 127216. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhan, P.; Naesens, L.; Vanderlinden, E.; Liu, A.; Du, G.; De Clercq, E.; Liu, X. Synthesis and preliminary biological evaluation of 5-substituted-2-(4-substituted phenyl)-1,3-benzoxazoles as a novel class of influenza virus A inhibitors. Chem. Biol. Drug Des. 2012, 79, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Bastrakov, M.; Starosotnikov, A. Recent Progress in the Synthesis of Drugs and Bioactive Molecules Incorporating Nitro(het)arene Core. Pharmaceuticals 2022, 15, 705. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Chen, Y.; Seth, S.; Furukawa, S.; Compans, R.W.; Jones, D.P. Inhibition of influenza infection by glutathione. Free. Radic. Biol. Med. 2003, 34, 928–936. [Google Scholar] [CrossRef]
- De Angelis, M.; Amatore, D.; Checconi, P.; Zevini, A.; Fraternale, A.; Magnani, M.; Hiscott, J.; De Chiara, G.; Palamara, A.T.; Nencioni, L. Influenza Virus Down-Modulates G6PD Expression and Activity to Induce Oxidative Stress and Promote its Replication. Front. Cell. Infect. Microbiol. 2022, 11, 804976. [Google Scholar] [CrossRef]
- Nencioni, L.; De Chiara, G.; Sgarbanti, R.; Amatore, D.; Aquilano, K.; Marcocci, M.E.; Serafino, A.; Torcia, M.; Cozzolino, F.; Ciriolo, M.R.; et al. Bcl-2 expression and p38MAPK activity in cells infected with influenza A virus: Impact on virally induced apoptosis and viral replication. J. Biol. Chem. 2009, 284, 16004–16015. [Google Scholar] [CrossRef]
- Pleschka, S.; Wolff, T.; Ehrhardt, C.; Hobom, G.; Planz, O.; Rapp, U.R.; Ludwig, S. Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK signalling cascade. Nat. Cell Biol. 2001, 3, 301–305. [Google Scholar] [CrossRef]
- Zheng, W.; Li, J.; Wang, S.; Cao, S.; Jiang, J.; Chen, C.; Ding, C.; Qin, C.; Ye, X.; Gao, G.F.; et al. Phosphorylation controls the nuclear-cytoplasmic shuttling of influenza A virus nucleoprotein. J. Virol. 2015, 89, 5822–5834. [Google Scholar] [CrossRef]
- Fang, W.Y.; Ravindar, L.; Rakesh, K.P.; Manukumar, H.M.; Shantharam, C.S.; Alharbi, N.S.; Qin, H.L. Synthetic approaches and pharmaceutical applications of chloro-containing molecules for drug discovery: A critical review. Eur. J. Med. Chem. 2019, 173, 117–153. [Google Scholar] [CrossRef]
- Benedetto Tiz, D.; Bagnoli, L.; Rosati, O.; Marini, F.; Sancineto, L.; Santi, C. New halogen-containing drugs approved by FDA in 2021: An overview on their syntheses and pharmaceutical use. Molecules 2022, 27, 1643. [Google Scholar] [CrossRef]
- Zini, R.; Morin, C.; Bertelli, A.; Bertelli, A.A.; Tillement, J.P. Effects of resveratrol on the rat brain respiratory chain. Drugs Under Exp. Clin. Res. 1999, 25, 87–97. [Google Scholar]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, K.; Ikeda, K.; Nishikata, T.; Yamori, Y. Phytoestrogens attenuate oxidative DNA damage in vascular smooth muscle cells from stroke-prone spontaneously hypertensive rats. J. Hypertens. 2000, 18, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Gupta, Y.K. Chronic treatment with trans resveratrol prevents intracerebroventricular streptozotocin induced cognitive impairment and oxidative stress in rats. Life Sci. 2002, 71, 2489–2498. [Google Scholar] [CrossRef]
- Burkitt, M.J.; Duncan, J. Effectsoftrans-resveratroloncopper-dependent hydroxyl-radical formation and DNA damage: Evidence for hydroxyl- radical scavenging and a novel, glutathione-sparing mechanism of action. Arch. Biochem. Biophys. 2000, 381, 253–263. [Google Scholar] [CrossRef]
- Martins, L.A.; Coelho, B.P.; Behr, G.; Pettenuzzo, L.F.; Souza, I.C.; Moreira, J.C.; Borojevic, R.; Gottfried, C.; Guma, F.C. Resveratrol induces pro-oxidant effects and time-dependent resistance to cytotoxicity in activated hepatic stellate cells. Cell Biochem. Biophys. 2014, 68, 247–257. [Google Scholar] [CrossRef]
- de la Lastra, C.A.; Villegas, I. Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and clinical implications. Biochem. Soc. Trans. 2007, 35, 1156–1160. [Google Scholar] [CrossRef] [Green Version]



| A549 | |||||
|---|---|---|---|---|---|
| Compound | IC50 | CC50 | SI | ||
| (μg/mL) | (μmol/mL) | (μg/mL) | (μmol/mL) | ||
| 1 | 20.7 | 0.089 | 87.3 | 0.378 | 4.2 |
| 2 | 13.5 | 0.045 | 39.5 | 0.131 | 2.9 |
| 3 | 12.2 | 0.046 | 30.3 | 0.114 | 2.4 |
| 4 | 14.6 | 0.048 | 38.8 | 0.129 | 2.6 |
| 5 | 23.4 | 0.088 | 59 | 0.223 | 2.5 |
| 6 | 17.1 | 0.062 | 212.3 | 0.770 | 12.4 |
| 7 | 19.5 | 0.080 | 116.1 | 0.481 | 5.9 |
| 8 | 45.1 | 0.203 | 92 | 0.415 | 2 |
| 9 | 31.7 | 0.104 | 105.5 | 0.466 | 3.3 |
| 10 | 68.3 | 0.277 | 74.1 | 0.300 | 1 |
| 11 | 1239 | 6.282 | 345 | 1.749 | 2.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Angelis, M.; De Filippis, B.; Balaha, M.; Giampietro, L.; Miteva, M.T.; De Chiara, G.; Palamara, A.T.; Nencioni, L.; Mollica, A. Nitrostilbenes: Synthesis and Biological Evaluation as Potential Anti-Influenza Virus Agents. Pharmaceuticals 2022, 15, 1061. https://doi.org/10.3390/ph15091061
De Angelis M, De Filippis B, Balaha M, Giampietro L, Miteva MT, De Chiara G, Palamara AT, Nencioni L, Mollica A. Nitrostilbenes: Synthesis and Biological Evaluation as Potential Anti-Influenza Virus Agents. Pharmaceuticals. 2022; 15(9):1061. https://doi.org/10.3390/ph15091061
Chicago/Turabian StyleDe Angelis, Marta, Barbara De Filippis, Marwa Balaha, Letizia Giampietro, Mariya Timotey Miteva, Giovanna De Chiara, Anna Teresa Palamara, Lucia Nencioni, and Adriano Mollica. 2022. "Nitrostilbenes: Synthesis and Biological Evaluation as Potential Anti-Influenza Virus Agents" Pharmaceuticals 15, no. 9: 1061. https://doi.org/10.3390/ph15091061
APA StyleDe Angelis, M., De Filippis, B., Balaha, M., Giampietro, L., Miteva, M. T., De Chiara, G., Palamara, A. T., Nencioni, L., & Mollica, A. (2022). Nitrostilbenes: Synthesis and Biological Evaluation as Potential Anti-Influenza Virus Agents. Pharmaceuticals, 15(9), 1061. https://doi.org/10.3390/ph15091061