Achillea fragrantissima (Forssk.) Sch.Bip Flower Dichloromethane Extract Exerts Anti-Proliferative and Pro-Apoptotic Properties in Human Triple-Negative Breast Cancer (MDA-MB-231) Cells: In Vitro and In Silico Studies
Abstract
:1. Introduction
2. Results
2.1. Screening of the Anti-Proliferative Potential of A. fragrantissima Extracts Derived from Flowers, Leaves, and Roots against TNBC Cells
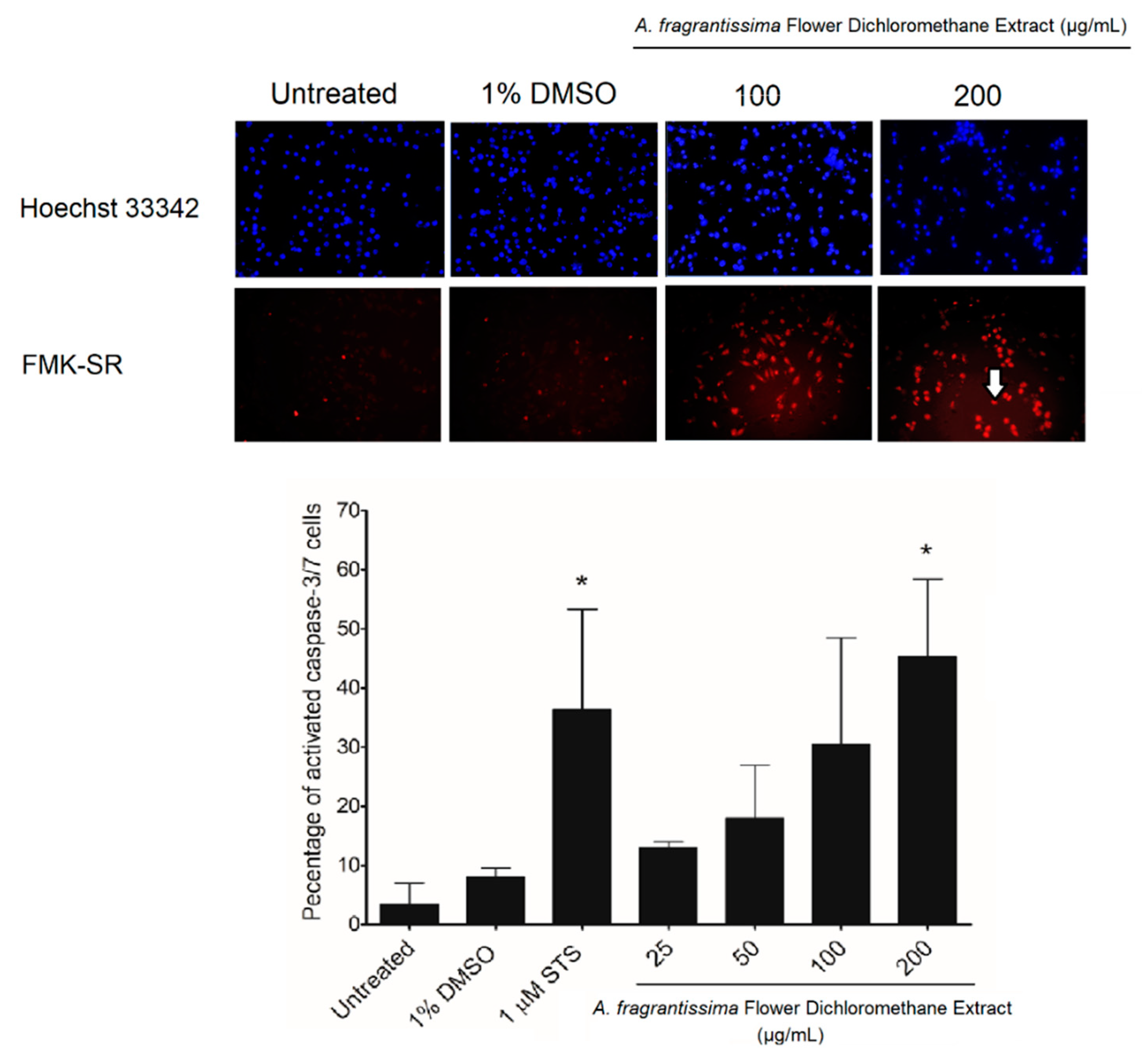
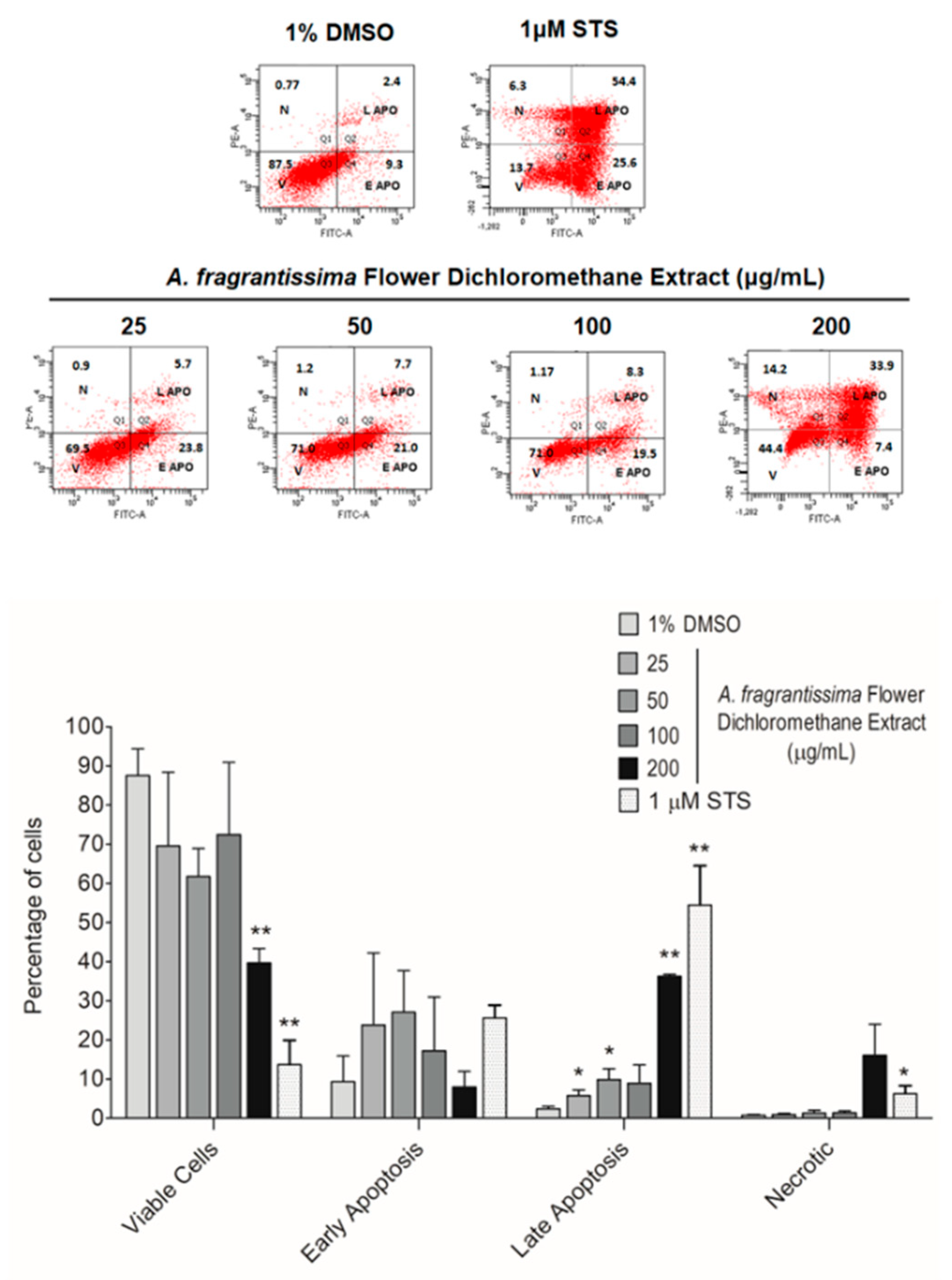
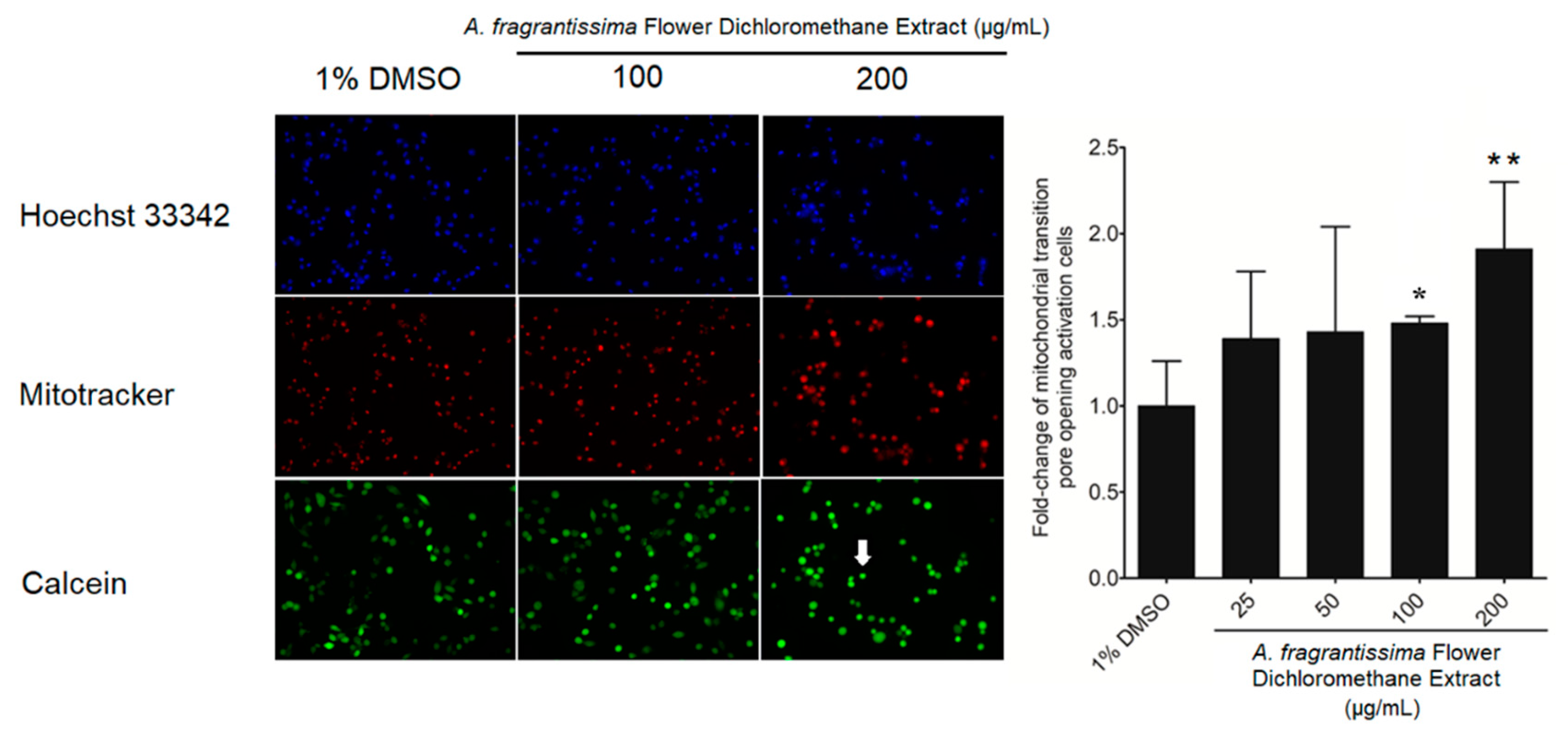
2.2. Induction of Apoptosis in MDA-MB-231 Cells by A. fragrantissima Flower Dichloromethane Extract
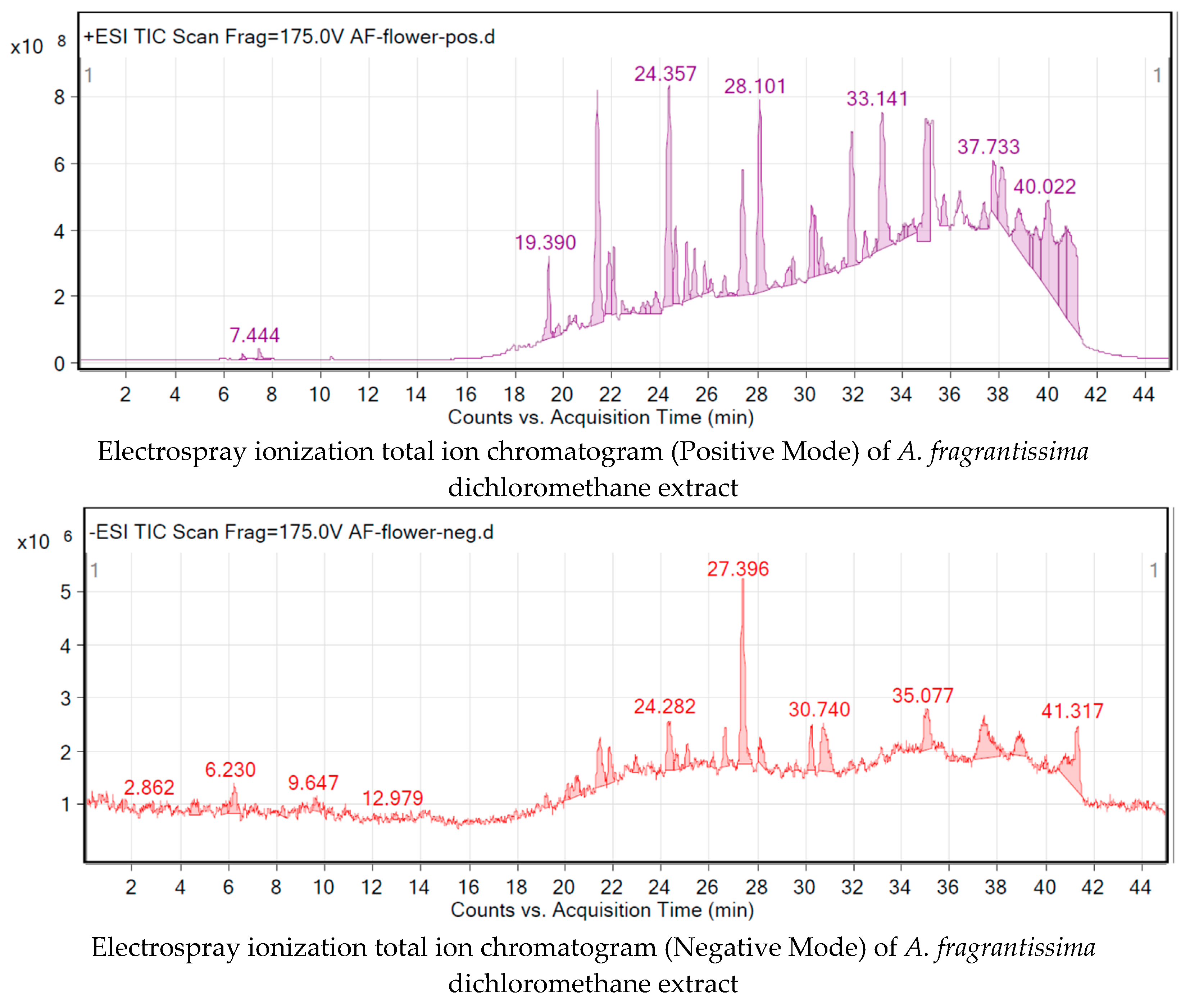
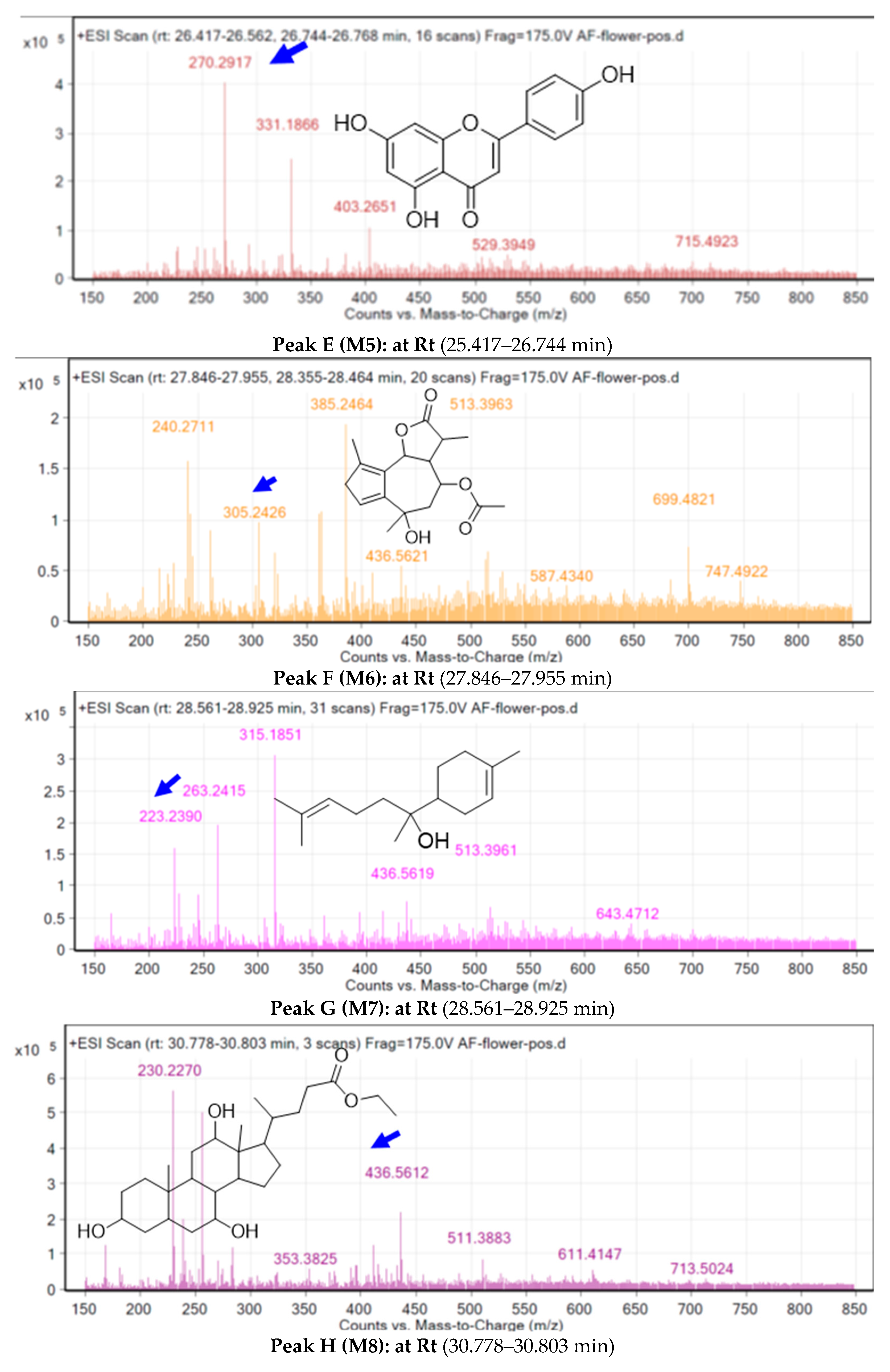
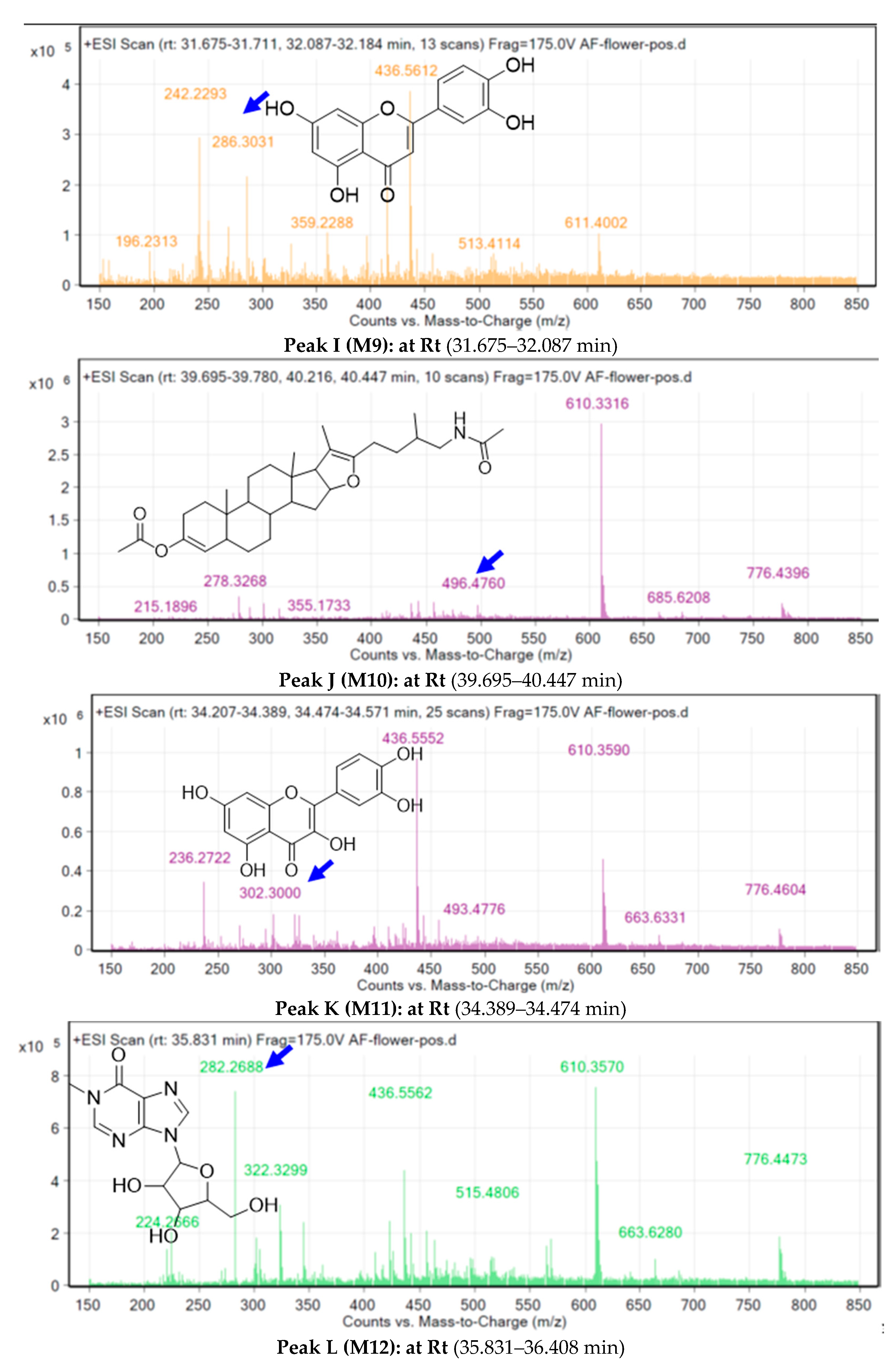
2.3. Metabolite Identification Using LC-QTOF
2.4. PASS Online Anticancer Predictions
2.5. Molecular Target Predictions
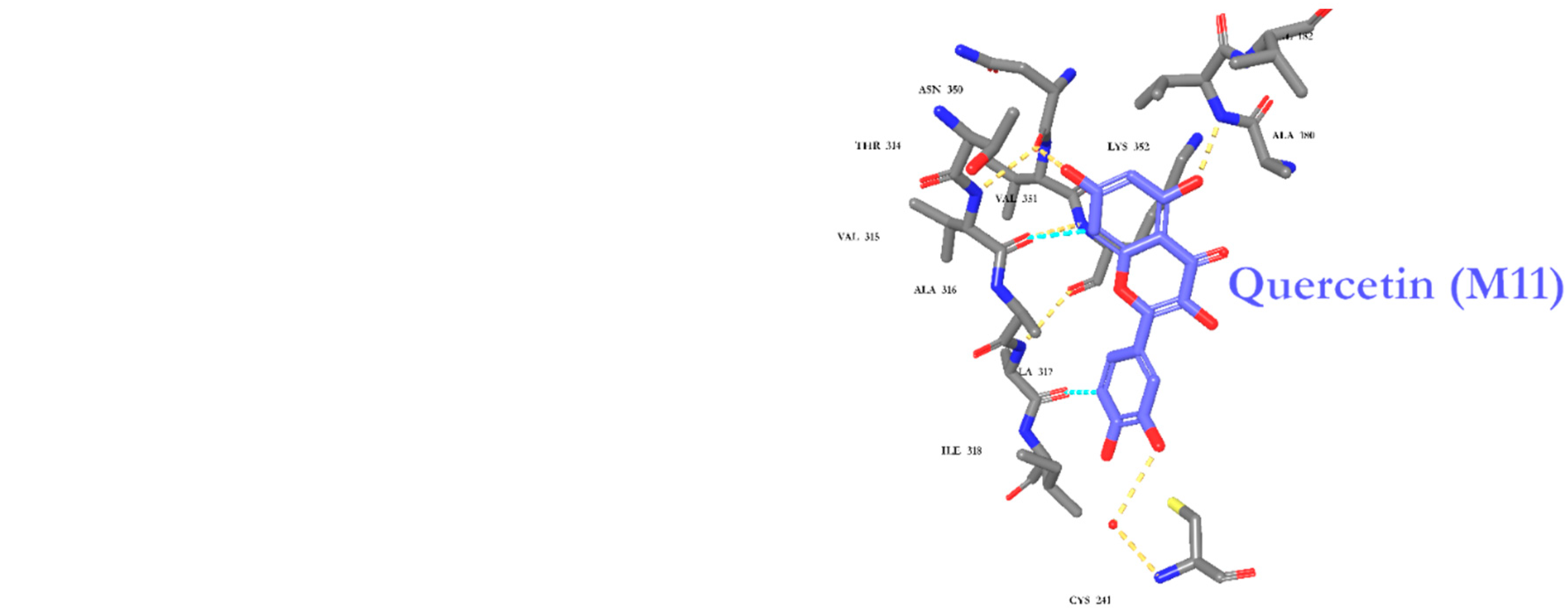
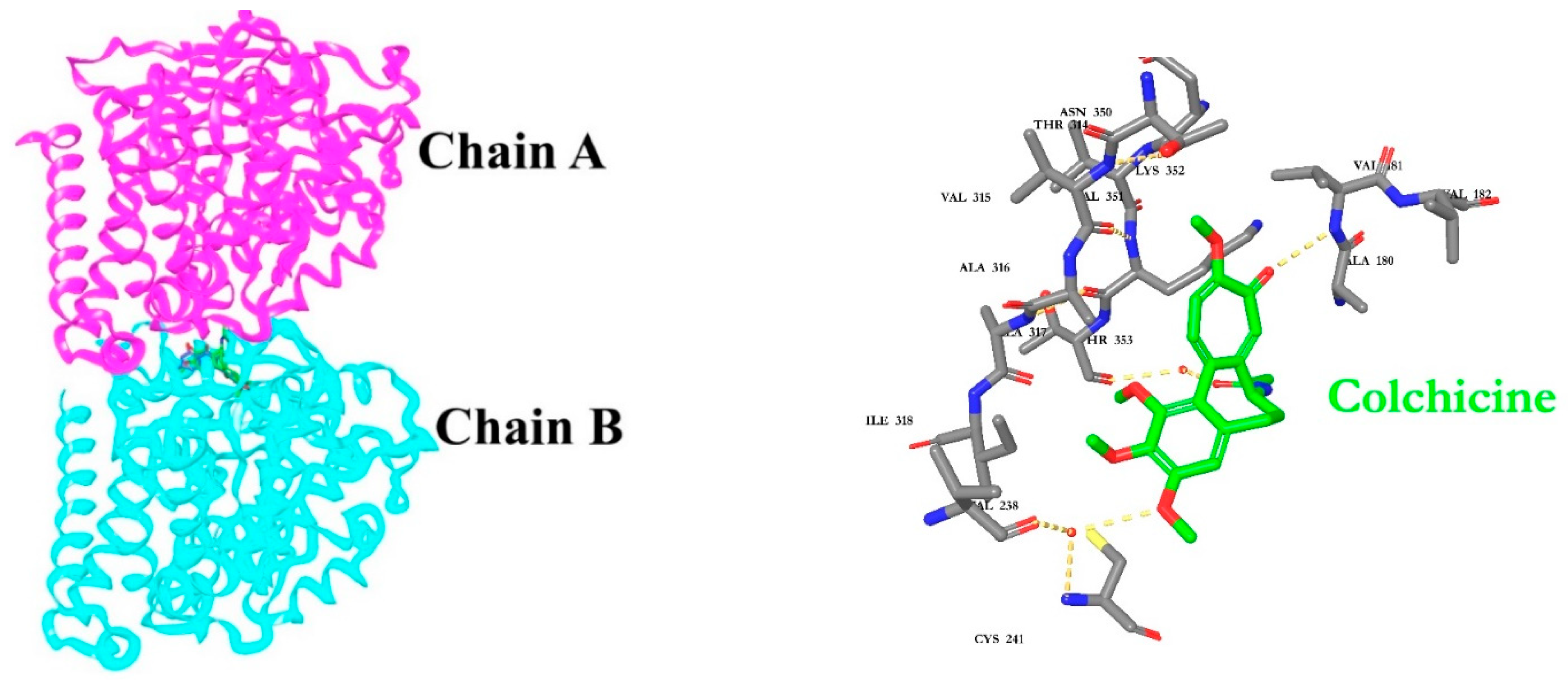
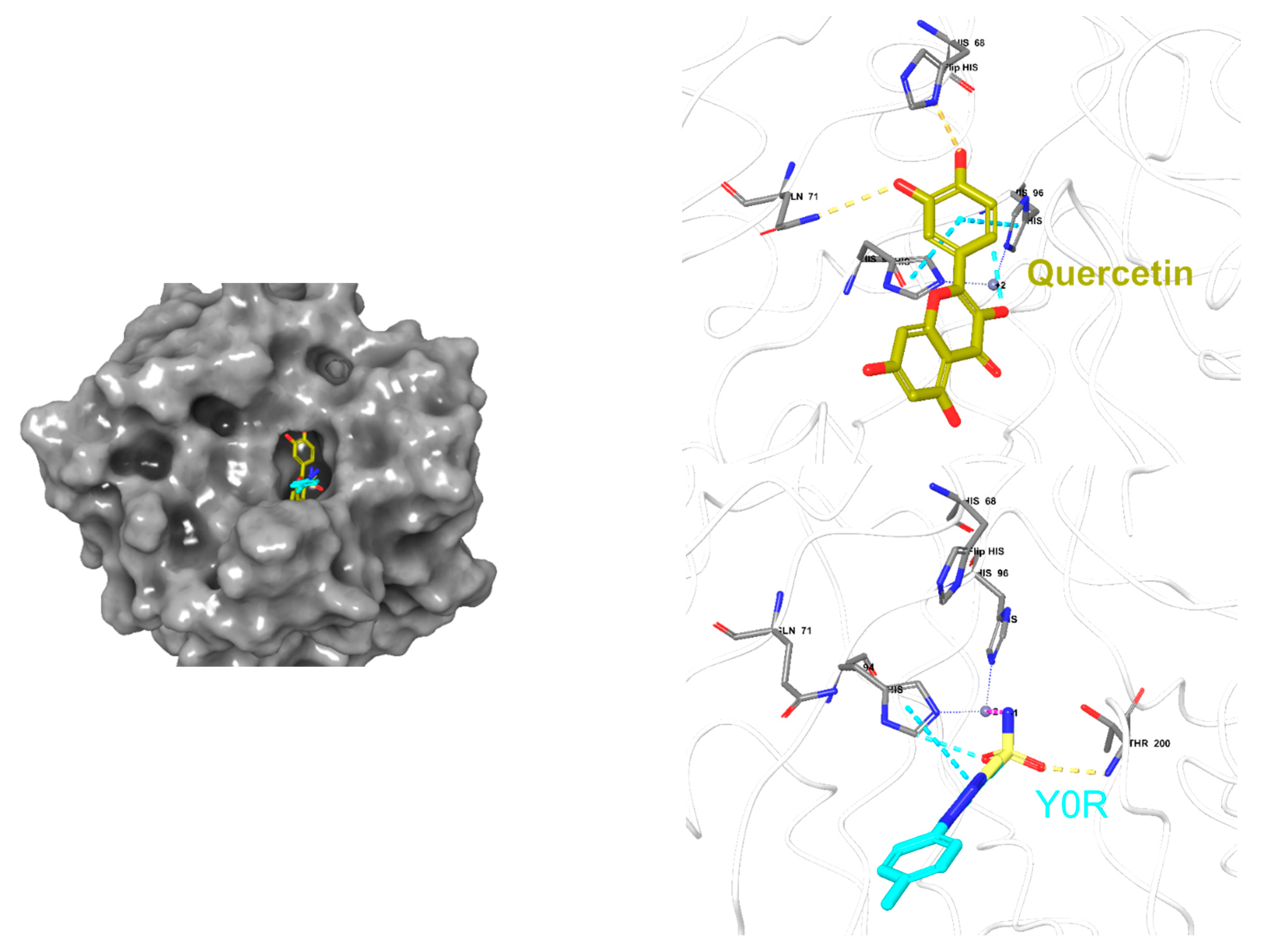
2.6. Molecular Docking
2.7. Absorption, Distribution, Metabolism, and Excretion (ADME) Predictions
2.8. Cytochrome P450 (CYP) Enzyme Inhibition Profiling
2.9. Organ and Endpoint Toxicity Predictions
3. Discussions
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Collection and Authentication of A. fragrantissima
4.3. Extraction of A. fragrantissima Plant Parts and Endotoxin Removal
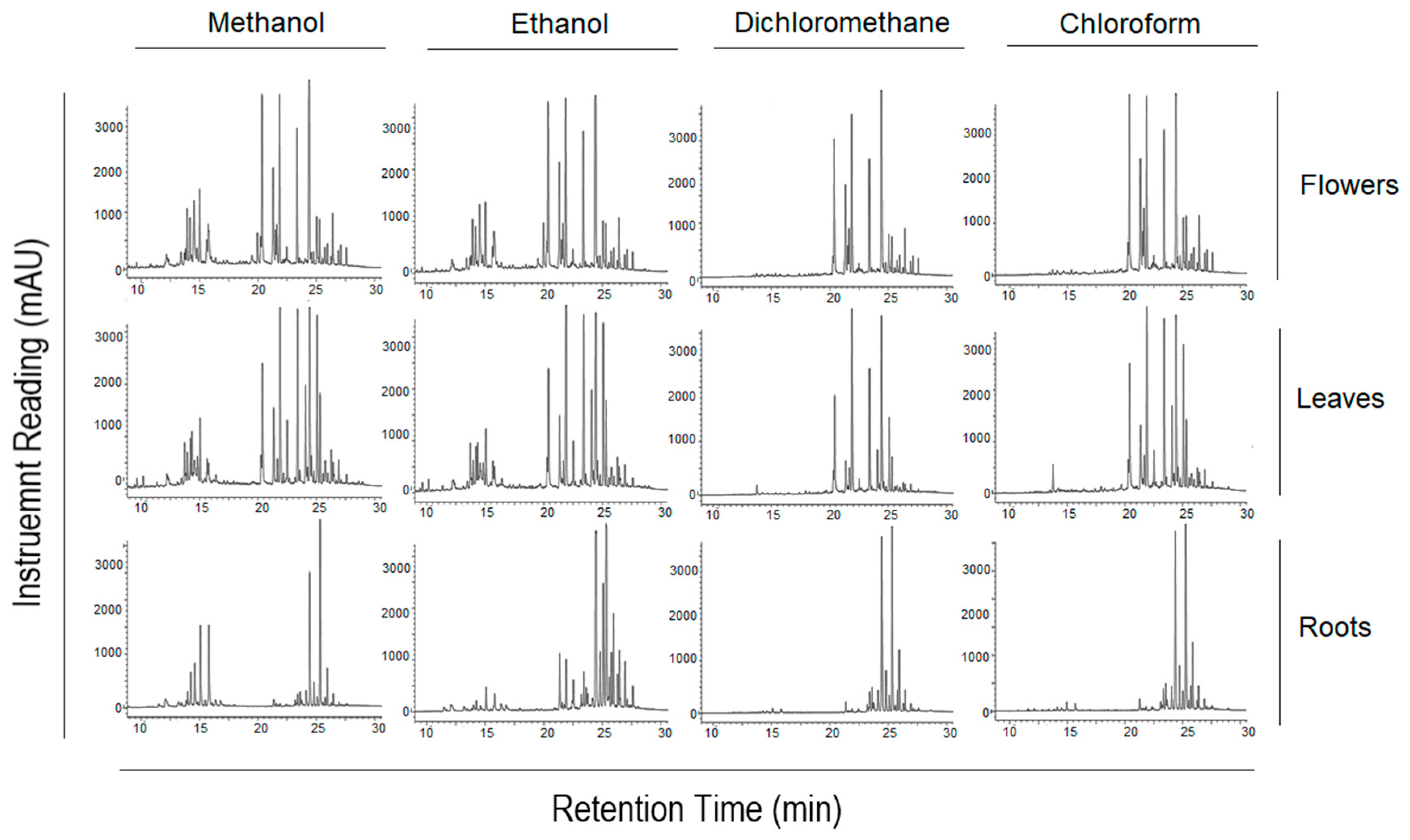
4.4. Chromatographic Fingerprinting of A. fragrantissima Extracts
4.5. TNBC MDA-MB-231 Cell Culture and Treatment
4.6. Determination of MDA-MB-231 Cell Viability Using the CellTiter-Glo™ Assay
4.7. Evaluation of the Hallmarks of Apoptosis
4.8. Metabolite Identification Using ESI-LC-QTOF
4.9. Anticancer Activity Predictions
4.10. Molecular Target Predictions
4.11. Molecular Docking
4.12. ADME Properties Predictions
4.13. CYP Enzyme Inhibition Predictions
4.14. Organ Toxicity and Safety Predictions
4.15. Data and Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADME | absorption, distribution, metabolism, and excretion |
| BBB | blood–brain barrier |
| BD | Becton Dickinson |
| CA IX | carbonic anhydrase IX |
| CADD | computer-aided drug discovery |
| CoCl2 | cobalt chloride |
| CO2 | carbon dioxide |
| CYP | cytochrome P450 |
| DNA | deoxyribonucleic acid |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | dimethyl sulfoxide |
| EC50 | half-maximal effective concentration |
| EU | endotoxin unit |
| FACS | fluorescence-activated cell sorting |
| FBS | fetal bovine serum |
| FITC | fluorescein isothiocyanate |
| FLICA | fluorochrome-labeled inhibitors of caspases |
| FMK | fluoromethyl ketone |
| GI | gastrointestinal |
| GPCR | G protein-coupled receptor |
| HPLC-UVD | high-performance liquid chromatography and ultra-violet detector |
| IC50 | half-maximal inhibitory concentration |
| log10 | common logarithm (base 10) |
| PASS | prediction of activity spectra for substances |
| PBS | phosphate-buffered saline |
| PDB ID | Protein data bank identifier |
| PE | phycoerythrin |
| PI | propidium iodide |
| QTOF | quadrupole time-of-flight |
| ROF | rule of five |
| SD | standard deviation |
| THM | traditional herbal medicine |
| TNBC | triple-negative breast cancer |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Keck, J.M.; Parmar, S.; Patterson, J.; Labrie, M.; Creason, A.L.; Johnson, B.E.; Downey, M.; Thomas, G.; Beadling, C.; et al. Characterizing advanced breast cancer heterogeneity and treatment resistance through serial biopsies and comprehensive analytics. NPJ Precis. Oncol. 2021, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Jitariu, A.-A.; Cîmpean, A.M.; Ribatti, D.; Raica, M. Triple negative breast cancer: The kiss of death. Oncotarget 2017, 8, 46652–46662. [Google Scholar] [CrossRef] [PubMed]
- Chue, B.M.; La Course, B.D. Case report of long-term survival with metastatic triple-negative breast carcinoma: Treatment possibilities for metastatic disease. Medicine 2019, 98, e15302. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yu, D.; Kwon, Y.; Lee, K.S.; Sim, S.H.; Kong, S.-Y.; Lee, E.S.; Park, I.H.; Park, C. Genomic characteristics of triple-negative breast cancer nominate molecular subtypes that predict chemotherapy response. Mol. Cancer Res. 2020, 18, 253–263. [Google Scholar] [CrossRef]
- Bianchini, G.; De Angelis, C.; Licata, L.; Gianni, L. Treatment landscape of triple-negative breast cancer—Expanded options, evolving needs. Nat. Rev. Clin. Oncol. 2022, 19, 91–113. [Google Scholar] [CrossRef]
- Keene, K.S.; King, T.; Hwang, E.S.; Peng, B.; McGuire, K.P.; Tapia, C.; Zhang, H.; Bae, S.; Nakhlis, F.; Klauber-Demore, N.; et al. Molecular determinants of post-mastectomy breast cancer recurrence. NPJ Breast Cancer 2018, 4, 34. [Google Scholar] [CrossRef]
- Mitra, S.; Dash, R. Natural products for the management and prevention of breast cancer. Evid. Based Complement. Altern. Med. 2018, 2018, 8324696. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, B. High-throughput screening (HTS) of natural products with triple-negative breast cancer (TNBC) organoids. J. Clin. Oncol. 2019, 37, e12558. [Google Scholar] [CrossRef]
- Zhai, Z.; Zheng, Y.; Yao, J.; Liu, Y.; Ruan, J.; Deng, Y.; Zhou, L.; Zhao, P.; Yang, S.; Hu, J.; et al. Evaluation of adjuvant treatments for T1 N0 M0 triple-negative breast cancer. JAMA Netw. Open 2020, 3, e2021881. [Google Scholar] [CrossRef]
- Abad, M.N.; Calabuig-Fariñas, S.; de Mena, M.L.; de Bremond, M.J.G.S.; González, C.G.; Martínez, S.T.; García-García, J.; González-Cruz, V.I.; Herrero, C.C. Update on systemic treatment in early triple negative breast cancer. Ther. Adv. Med. Oncol. 2021, 13, 1758835920986749. [Google Scholar]
- Millimouno, F.M.; Dong, J.; Yang, L.; Li, J.; Li, X. Targeting apoptosis pathways in cancer and perspectives with natural compounds from mother nature. Cancer Prev. Res. 2014, 7, 1081–1107. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Vong, C.T.; Chen, H.; Gao, Y.; Lyu, P.; Qiu, L.; Zhao, M.; Liu, Q.; Cheng, Z.; Zou, J.; et al. Naturally occurring anti-cancer compounds: Shining from Chinese herbal medicine. Chin. Med. 2019, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, G.C.; Schaan, A.P.; Cabral, G.F.; Santana-Da-Silva, M.N.; Pinto, P.; Vidal, A.F.; Ribeiro-Dos-Santos, A. A cell’s fate: An overview of the molecular biology and genetics of apoptosis. Int. J. Mol. Sci. 2019, 20, 4133. [Google Scholar] [CrossRef] [PubMed]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef]
- Jiang, M.; Qi, L.; Li, L.; Li, Y. The caspase-3/GSDME signal pathway as a switch between apoptosis and pyroptosis in cancer. Cell Death Discov. 2020, 6, 112. [Google Scholar] [CrossRef]
- Saeidnia, S.; Gohari, A.; Mokhber-Dezfuli, N.; Kiuchi, F. A review on phytochemistry and medicinal properties of the genus Achillea. Daru 2011, 19, 173–186. [Google Scholar]
- Patocka, J.; Navratilova, Z. Achillea fragrantissima: Pharmacology review. Clin. Oncol. 2019, 4, 1601. [Google Scholar]
- Mansi, I.; Ali, N.A.A.; Mhaidat, N.M.; Hussain, K.; Al-Kaf, A.G.; Anwar, S.; Setzer, W.N. Chemical composition and biological activity of the essential oil isolated from the leaves of Achillea fragrantissima growing wild in Yemen. Pharmacogn. J. 2019, 11, 1077–1081. [Google Scholar] [CrossRef]
- Farouk, A.; Ali, H.; Al-Khalifa, A.R.; Mohsen, M.; Fikry, R. Comparative study for the volatile constituents and the antioxidant activity of the essential oils of dried Achillea fragrantissima cultivated in Madinah Monawara, Saudi Arabia and Egypt. Int. J. Food Prop. 2019, 22, 395–404. [Google Scholar] [CrossRef]
- Falk, A.J.; Smolenski, S.J.; Bauer, L.; Bell, C.L. Isolation and identification of three new flavones from Achillea millefolium L. J. Pharm. Sci. 1975, 64, 1838–1842. [Google Scholar] [CrossRef] [PubMed]
- Trifunović, S.; Vajs, V.; Juranić, Z.; Zizak, Z.; Tešević, V.; Macura, S.; Milosavljević, S. Cytotoxic constituents of Achillea clavennae from Montenegro. Phytochemistry 2006, 67, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Abu-Niaaj, L.F.; Abu-Zarga, M.H.; Abdalla, S.S. Isolation and inhibitory effects of eupatilin, a flavone isolated from Artemisia Monosperma Del., on rat isolated smooth muscle. Pharm. Biol. 1996, 34, 134–140. [Google Scholar] [CrossRef]
- Zonuz, N.; Akpinar, A.E.; Akpinar, N.; Dag, S.; Akpinar, M.A. Fatty acids of the seed oils of three Achillea species. Chem. Nat. Compd. 2020, 56, 115–118. [Google Scholar] [CrossRef]
- Elsharkawy, E.R.; Alghanem, S.M.; Elmorsy, E. Effect of habitat variations on the chemical composition, antioxidant, and antimicrobial activities of Achillea fragrantissima (Forssk) Sch. Bip. Biotechnol. Rep. 2020, 29, e00581. [Google Scholar] [CrossRef]
- Kiumarsi, A.; Abomahboub, R.; Rashedi, S.M.; Parvinzadeh, M. Achillea millefolium, a new source of natural dye for wool dyeing. Prog. Color. Colorants Coat. 2009, 2, 87–93. [Google Scholar]
- Cirak, C.; Radusiene, J.; Raudone, L.; Vilkickyte, G.; Seyis, F.; Marksa, M.; Ivanauskas, L.; Yayla, F. Phenolic compounds and antioxidant activity of Achillea Arabica populations. S. Afr. J. Bot. 2022, 147, 425–433. [Google Scholar] [CrossRef]
- Si, X.T.; Zhang, M.L.; Shi, Q.W.; Kiyota, H. Chemical constituents of the plants in the genus Achillea. Chem. Biodivers. 2006, 3, 1163–1180. [Google Scholar] [CrossRef]
- Ghantous, A.; Nasser, N.; Saab, I.; Darwiche, N.; Saliba, N.A. Structure-activity relationship of seco-tanapartholides isolated from Achilleafalcata for inhibition of HaCaT cell growth. Eur. J. Med. Chem. 2009, 44, 3794–3797. [Google Scholar] [CrossRef]
- Olazarán-Santibañez, F.; Rivera, G.; Vanoye-Eligio, V.; Mora-Olivo, A.; Aguirre-Guzmán, G.; Ramírez-Cabrera, M.; Arredondo-Espinoza, E. Antioxidant and antiproliferative activity of the ethanolic extract of Equisetum myriochaetum and molecular docking of its main metabolites (Apigenin, Kaempferol, and Quercetin) on β-Tubulin. Molecules 2021, 26, 443. [Google Scholar] [CrossRef]
- Gupta, K.; Panda, D. Perturbation of microtubule polymerization by quercetin through tubulin binding: A novel mechanism of its antiproliferative activity. Biochemistry 2002, 41, 13029–13038. [Google Scholar] [CrossRef] [PubMed]
- Klimaszewska-Wiśniewska, A.; Hałas-Wiśniewska, M.; Izdebska, M.; Gagat, M.; Grzanka, A.; Grzanka, D. Antiproliferative and antimetastatic action of quercetin on A549 non-small cell lung cancer cells through its effect on the cytoskeleton. Acta Histochem. 2017, 119, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Liu, Y.; Wu, Q.; Luo, L.; Cui, Y.; Wang, X.; Chen, X.; Tan, L.; Meng, X. Quercetin-modified metal–organic frameworks for dual sensitization of radiotherapy in tumor tissues by inhibiting the carbonic anhydrase IX. ACS Nano 2019, 13, 4209–4219, Correction in ACS Nano 2020, 14, 2553. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, D.; Karagoz, L.; Ekinci, D.; Senturk, M.; Supuran, C.T. Carbonic anhydrase inhibitors: In vitro inhibition of α isoforms (hCA I, hCA II, bCA III, hCA IV) by flavonoids. J. Enzym. Inhib. Med. Chem. 2013, 28, 283–288. [Google Scholar] [CrossRef]
- Clark, A.M. Natural products as a resource for new drugs. Pharm Res. 1996, 13, 1133–1144. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell viability assays. In Assay Guidance Manual [Internet]; Eli Lilly & Company: Bethesda, MD, USA; The National Center for Advancing Translational Sciences: Rockville, MA, USA, 2004. [Google Scholar]
- Sanders, E.J. Methods for detecting apoptotic cells in tissues. Histol. Histopathol. 1997, 12, 1169–1177. [Google Scholar]
- Grabarek, J.; Amstad, P.; Darzynkiewicz, Z. Use of fluorescently labeled caspase inhibitors as affinity labels to detect activated caspases. Hum. Cell 2002, 15, 1–12. [Google Scholar] [CrossRef]
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutellingsperger, C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods 1995, 184, 39–51. [Google Scholar] [CrossRef]
- Vermes, I.; Haanen, C.; Reutelingsperger, C. Flow cytometry of apoptotic cell death. J. Immunol. Methods 2000, 243, 167–190. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Applequist, W.L.; Moerman, D.E. Yarrow (Achillea millefolium L.): A Neglected Panacea? A Review of Ethnobotany, Bioactivity, and Biomedical Research. Econ. Bot. 2011, 65, 209–225. [Google Scholar] [CrossRef]
- Sathiyamoorthy, P.; Lugasi-Evgi, H.; Schlesinger, P.; Kedar, I.; Gopas, J.; Pollack, Y.; Golan-Goldhirsh, A. Screening for cytotoxic and antimalarial activities in desert plants of the Negev and Bedouin market plant products. Pharm. Biol. 1999, 37, 188–195. [Google Scholar] [CrossRef]
- Alenad, A.M.; Al-Jaber, N.A.; Krishnaswamy, S.; Yakout, S.M.; Al-Daghri, N.M.; Alokail, M.S. Achillea fragrantissima extract exerts its anticancer effect via induction of differentiation, cell cycle arrest and apoptosis in chronic myeloid leukemia (CML) cell line K562. J. Med. Plants Res. 2013, 7, 1561–1567. [Google Scholar]
- Bin Break, M.K.; Younes, K.M.; Elkahoui, S.; Unissa, R.; Alfahidat, S.A.; Alshawi, K.S.; Abouzied, A.S. Achillea fragrantissima (Forssk.) Sch. Bip. methanolic extract exerts potent antimicrobial activity and causes cancer cell death via induction of caspase-dependent apoptosis and S-phase arrest. Nat. Prod. Res. 2021, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Awad, B.M.; Habib, E.S.; Ibrahim, A.K.; Wanas, A.S.; Radwan, M.M.; Helal, M.A.; Ahmed, S.A.; ElSohly, M.A. Cytotoxic activity evaluation and molecular docking study of phenolic derivatives from Achillea fragrantissima (Forssk.) growing in Egypt. Med. Chem. Res. 2017, 26, 2065–2073. [Google Scholar] [CrossRef]
- Macalino, S.J.Y.; Gosu, V.; Hong, S.; Choi, S. Role of computer-aided drug design in modern drug discovery. Arch. Pharm. Res. 2015, 38, 1686–1701. [Google Scholar] [CrossRef]
- Kapetanovic, I.M. Computer-aided drug discovery and development (CADDD): In silico-chemico-biological approach. Chem. Biol. Interact. 2008, 171, 165–176. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Oosterwijk, E.; Tu, C.; Shiverick, K.T.; Silverman, D.N.; Frost, S.C. Expression and activity of carbonic anhydrase IX is associated with metabolic dysfunction in MDA-MB-231 breast cancer cells. Cancer Investig. 2009, 27, 613–623. [Google Scholar] [CrossRef]
- Li, Y.; Tu, C.; Wang, H.; Silverman, D.N.; Frost, S.C. Catalysis and pH control by membrane-associated carbonic anhydrase IX in MDA-MB-231 breast cancer cells. J. Biol. Chem. 2011, 286, 15789–15796. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, H.; Tu, C.; Shiverick, K.T.; Silverman, D.N.; Frost, S.C. Role of hypoxia and EGF on expression, activity, localization and phosphorylation of carbonic anhydrase IX in MDA-MB-231 breast cancer cells. Biochim. Biophys. Acta 2011, 1813, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, E.; Adhami, V.M.; Sechi, M.; Mukhtar, H. Dietary flavonoid fisetin binds to β-tubulin and disrupts microtubule dynamics in prostate cancer cells. Cancer Lett. 2015, 367, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, D.; Ganguli, A.; Dastidar, D.G.; Acharya, B.R.; Das, A.; Chakrabarti, G. Apigenin shows synergistic anticancer activity with curcumin by binding at different sites of tubulin. Biochimie 2013, 95, 1297–1309. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Bansal, T. Role of flavonoids in cancer prevention: Chemistry and mode of action. Eur. J. Mol. Clin. Med. 2020, 7, 3608–3625. [Google Scholar]
- Mboge, M.Y.; McKenna, R.; Frost, S.C. Advances in anti-cancer drug development targeting carbonic anhydrase IX and XII. Top. Anticancer Res. 2015, 5, 3–42. [Google Scholar] [PubMed]
- Winum, J.Y.; Scozzafava, A.; Montero, J.L.; Supuran, C.T. Inhibition of carbonic anhydrase IX: A new strategy against cancer. Anticancer Agents Med. Chem. 2009, 9, 693–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, P.C.; Winum, J.Y.; Supuran, C.T.; Dedhar, S. Recent developments in targeting carbonic anhydrase IX for cancer therapeutics. Oncotarget 2012, 3, 84–97. [Google Scholar] [CrossRef]
- Innocenti, A.; Beyza Öztürk Sarıkaya, S.; Gülçin, I.; Supuran, C.T. Carbonic anhydrase inhibitors. Inhibition of mammalian isoforms I–XIV with a series of natural product polyphenols and phenolic acids. Bioorg. Med. Chem. 2010, 18, 2159–2164. [Google Scholar] [CrossRef]
- Karioti, A.; Carta, F.; Supuran, C.T. Phenols and polyphenols as carbonic anhydrase inhibitors. Molecules 2016, 21, 1649. [Google Scholar] [CrossRef]
- Gülçin, I.; Beydemir, S. Phenolic compounds as antioxidants: Carbonic anhydrase isoenzymes inhibitors. Mini Rev. Med. Chem. 2013, 13, 408–430. [Google Scholar] [PubMed]
- Lipinski, C.A. Lead-and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Alehaideb, Z.; Alatar, G.; Nehdi, A.; Albaz, A.; Al-Eidi, H.; Almutairi, M.; Hawsa, E.; Alshuail, N.; Matou-Nasri, S. Commiphora myrrha (Nees) Engl. resin extracts induce phase-I cytochrome P450 2C8, 2C9, 2C19, and 3A4 isoenzyme expressions in human hepatocellular carcinoma (HepG2) cells. Saudi Pharm. J. 2021, 29, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Alehaideb, Z.; Sheriffdeen, M.; Law, F.C.P. Furanocoumarin bioactives in the Apiceae and Rutaceae families of plants. Can. J. Pure Appl. Sci. 2017, 11, 4157–4167. [Google Scholar]
- Rameshbabu, S.; Messaoudi, S.A.; Alehaideb, Z.I.; Ali, M.S.; Venktraman, A.; Alajmi, H.; Al-Eidi, H.; Matou-Nasri, S. Anastatica hierochuntica (L.) methanolic and aqueous extracts exert antiproliferative effects through the induction of apoptosis in MCF-7 breast cancer cells. Saudi Pharm. J. 2020, 28, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Matou-Nasri, S.; Rabhan, Z.; Al-Baijan, H.; Al-Eidi, H.; Yahya, W.B.; Abdulrahman, A.A.; Almobadel, N.; Alsubeai, M.; Ghamdi, S.A.; Alaskar, A.; et al. CD95-mediated apoptosis in Burkitt’s lymphoma B-cells is associated with Pim-1 down-regulation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Alehaideb, Z.; AlGhamdi, S.; Bin Yahya, W.; Al-Eidi, H.; Alharbi, M.; Alaujan, M.; Albaz, A.; Tukruni, M.; Nehdi, A.; Abdulla, M.-H.; et al. Anti-proliferative and pro-apoptotic effects of Calligonum comosum (L’Her.) methanolic extract in human triple-negative MDA-MB-231 breast cancer cells. J. Evid. Based Integr. Med. 2020, 25, 2515690X20978391. [Google Scholar] [CrossRef]
- Alehaideb, Z.I.; Venkatraman, A.; Kokane, M.; Mohamed, S.A.; Rameshbabu, S.; Suliman, R.S.; Alghamdi, S.S.; Al-Eidi, H.; Alghanem, B.; Abdulla, M.-H.; et al. Bursatella leachii purple ink secretion concentrate exerts cytotoxic properties against human hepatocarcinoma cell line (HepG2): In vitro and in silico studies. Molecules 2022, 27, 826. [Google Scholar] [CrossRef]
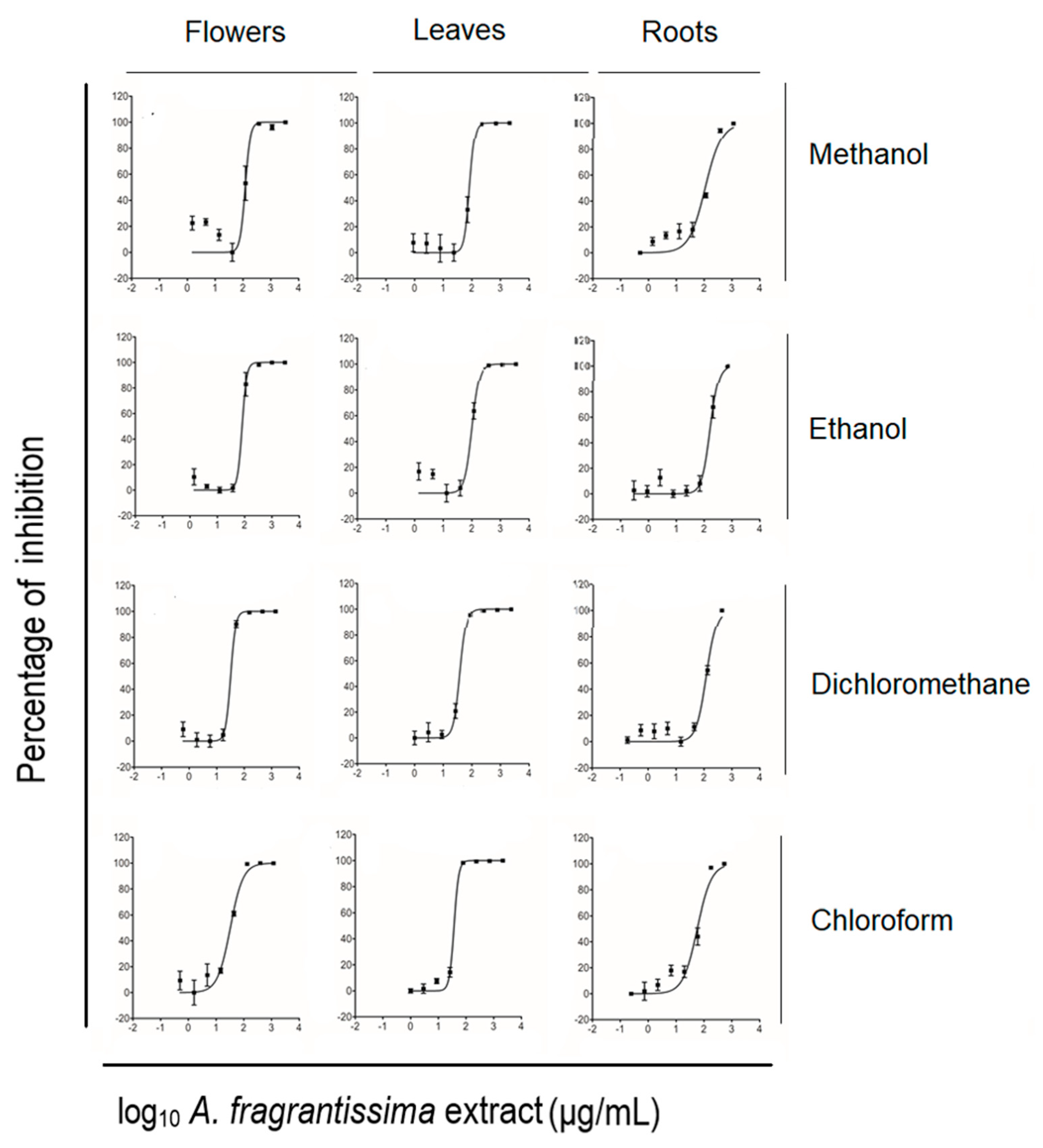


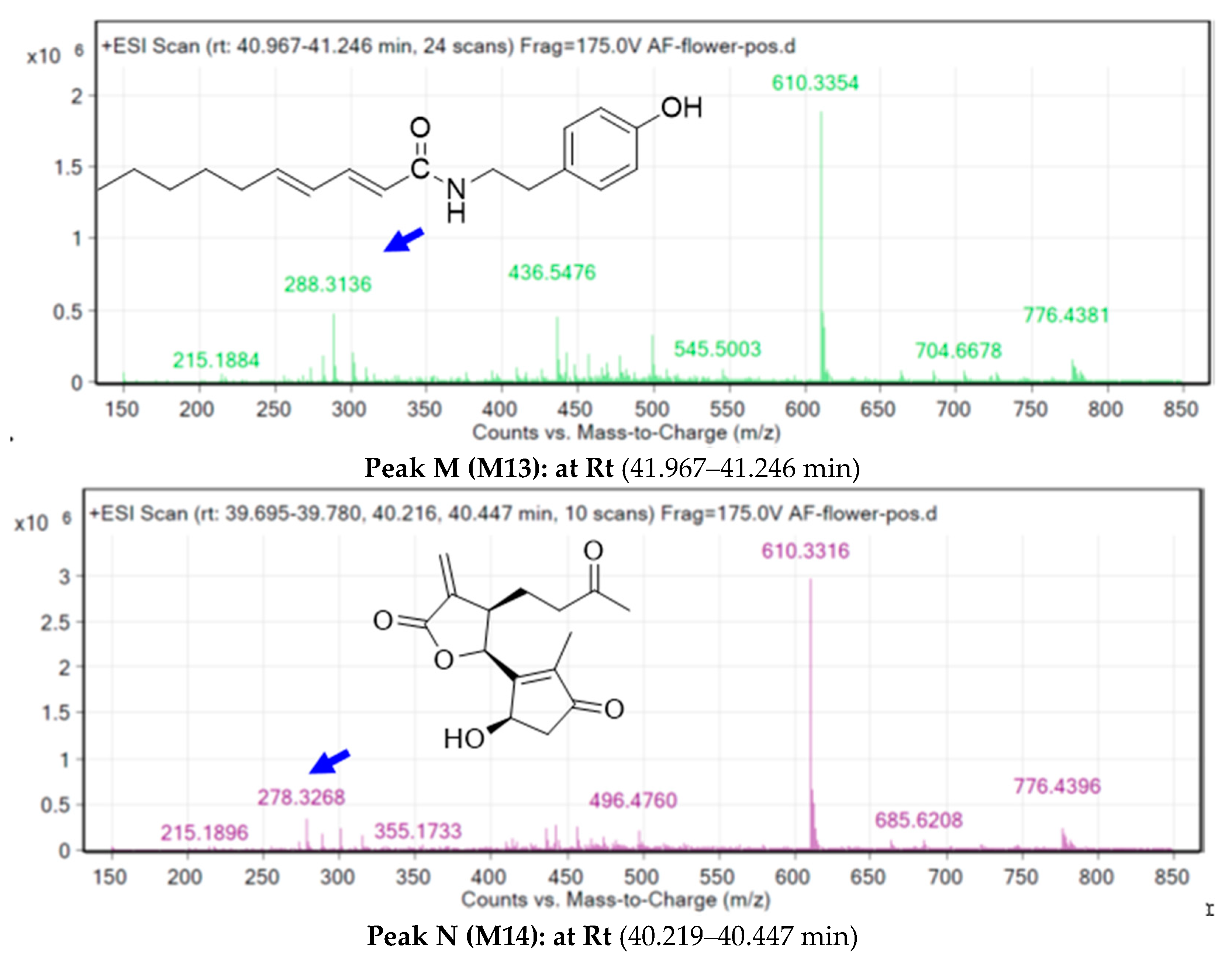
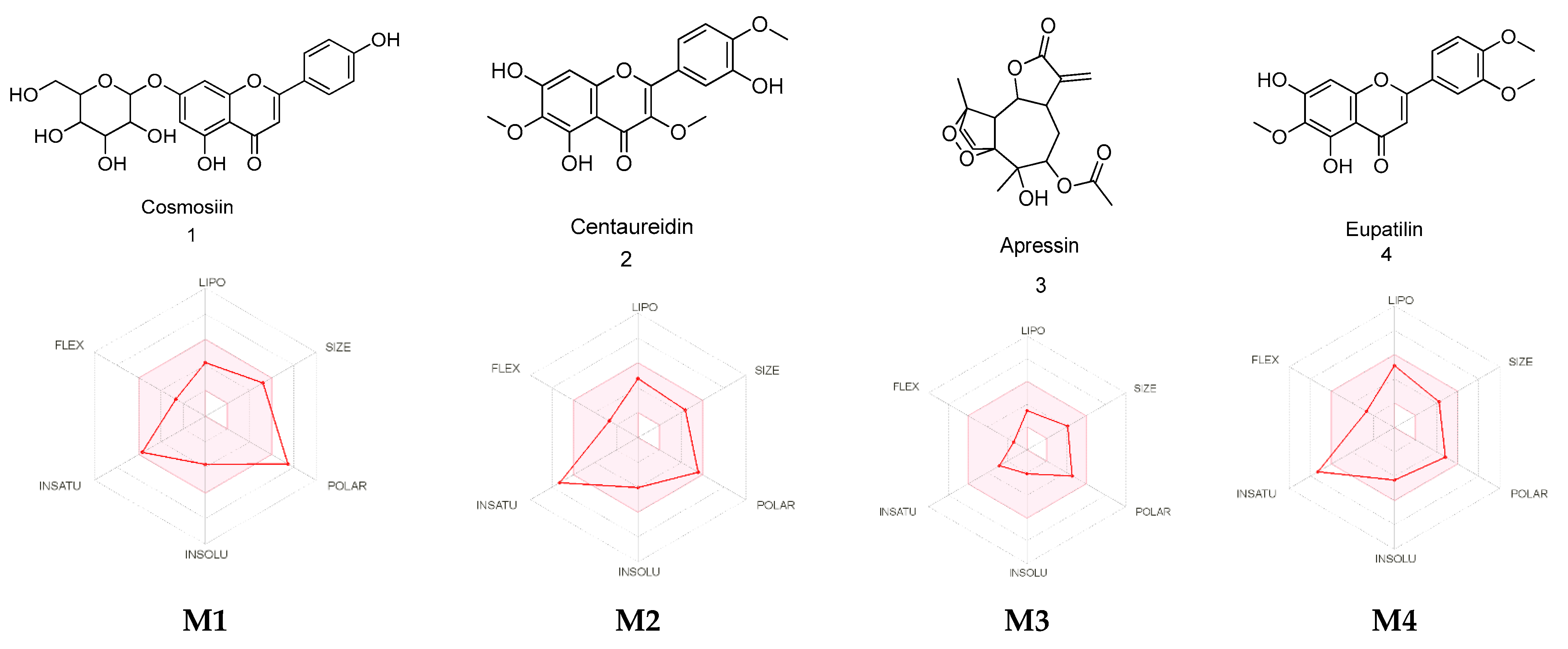
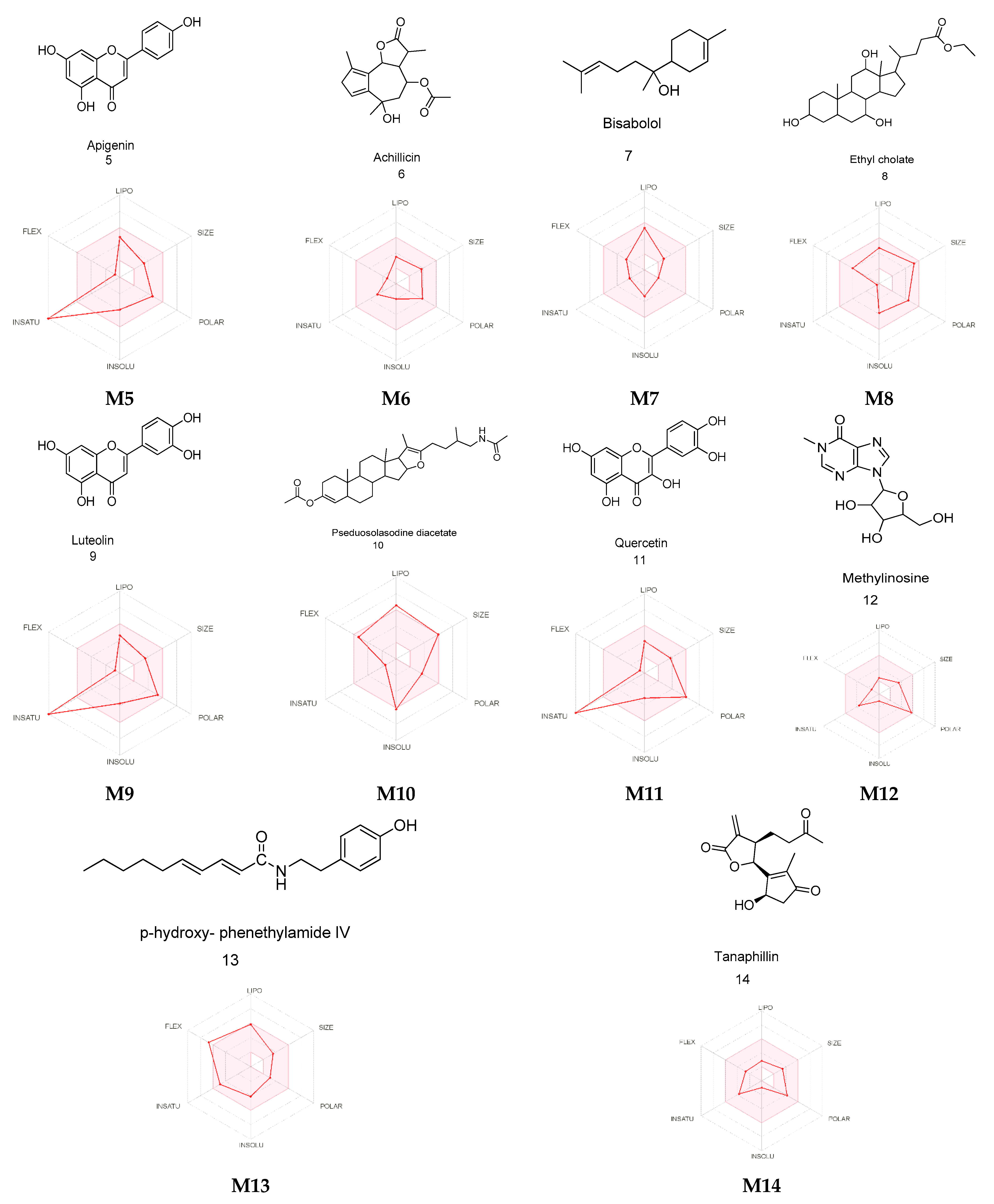
| Plant Part | Extraction Solvent | Inhibition Values (IC50) (µg/mL) |
|---|---|---|
| Mean ± SD | ||
| Flowers | ||
| Methanol | 122.97 ± 24.50 | |
| Ethanol | 66.77 ± 31.11 | |
| Dichloromethane | 32.43 ± 3.41 | |
| Chloroform | 33.13 ± 7.20 | |
| Leaves | ||
| Methanol | 82.66 ± 3.88 | |
| Ethanol | 99.71 ± 9.24 | |
| Dichloromethane | 36.67 ± 3.94 | |
| Chloroform | 36.48 ± 3.02 | |
| Roots | ||
| Methanol | 103.85 ± 27.02 | |
| Ethanol | 161.77 ± 45.59 | |
| Dichloromethane | 120.50 ± 17.15 | |
| Chloroform | 52.24 ± 11.63 |
| Anti-Carcinogenic Activity | Probability of Being Active (Pa) | Probability of Being Inactive (Pi) |
|---|---|---|
| M1 | 0.926 | 0.002 |
| M2 | 0.831 | 0.008 |
| M3 | 0.941 | 0.004 |
| M4 | 0.819 | 0.010 |
| M5 | 0.641 | 0.011 |
| M6 | 0.867 | 0.005 |
| M7 | 0.657 | 0.034 |
| M8 | 0.634 | 0.003 |
| M9 | 0.783 | 0.014 |
| M10 | 0.461 | 0.083 |
| M11 | 0.797 | 0.012 |
| M12 | 0.575 | 0.014 |
| M13 | 0.445 | 0.068 |
| M14 | 0.905 | 0.005 |
| Compound Name | Target Predictions Using Molinspiration | |
|---|---|---|
| M1 | GPCR ligand Ion channel modulator Kinase inhibitor Nuclear receptor ligand Protease inhibitor Enzyme inhibitor | 0.10 −0.01 0.14 0.31 0.02 0.43 |
| M2 | GPCR ligand Ion channel modulator Kinase inhibitor Nuclear receptor ligand Protease inhibitor Enzyme inhibitor | −0.15 −0.28 0.14 0.01 −0.35 0.13 |
| M3 | GPCR ligand Ion channel modulator Kinase inhibitor Nuclear receptor ligand Protease inhibitor Enzyme inhibitor | 0.31 0.17 −0.08 0.81 0.21 0.81 |
| M4 | GPCR ligand Ion channel modulator Kinase inhibitor Nuclear receptor ligand Protease inhibitor Enzyme inhibitor | −0.09 −0.23 0.20 0.13 −0.29 0.14 |
| M5 | GPCR ligand Ion channel modulator Kinase inhibitor Nuclear receptor ligand Protease inhibitor Enzyme inhibitor | −0.07 −0.09 0.18 0.34 −0.25 0.26 |
| M6 | GPCR ligand Ion channel modulator Kinase inhibitor Nuclear receptor ligand Protease inhibitor Enzyme inhibitor | −0.03 0.02 −0.48 0.34 0.00 0.33 |
| M7 | GPCR ligand Ion channel modulator Kinase inhibitor Nuclear receptor ligand Protease inhibitor Enzyme inhibitor | −0.06 0.26 −0.78 0.37 −0.38 0.43 |
| M8 | GPCR ligand Ion channel modulator Kinase inhibitor Nuclear receptor ligand Protease inhibitor Enzyme inhibitor | 0.17 0.21 −0.38 0.65 0.18 0.58 |
| M9 | GPCR ligand Ion channel modulator Kinase inhibitor Nuclear receptor ligand Protease inhibitor Enzyme inhibitor | −0.02 −0.07 0.26 0.39 −0.22 0.28 |
| M10 | GPCR ligand Ion channel modulator Kinase inhibitor Nuclear receptor ligand Protease inhibitor Enzyme inhibitor | −0.06 −0.07 −0.62 0.09 −0.04 0.24 |
| M11 | GPCR ligand Ion channel modulator Kinase inhibitor Nuclear receptor ligand Protease inhibitor Enzyme inhibitor | −0.06 −0.19 0.28 0.36 −0.25 0.28 |
| M12 | GPCR ligand Ion channel modulator Kinase inhibitor Nuclear receptor ligand Protease inhibitor Enzyme inhibitor | 0.78 0.17 0.27 −1.52 −0.28 0.86 |
| M13 | GPCR ligand Ion channel modulator Kinase inhibitor Nuclear receptor ligand Protease inhibitor Enzyme inhibitor | 0.27 0.10 −0.12 0.22 0.14 0.20 |
| M14 | GPCR ligand Ion channel modulator Kinase inhibitor Nuclear receptor ligand Protease inhibitor Enzyme inhibitor | 0.12 −0.04 −0.52 0.69 0.08 0.64 |
| Compound Name | Docking Score (kcal/mol) | Interactions with Amino Acid Residues |
|---|---|---|
| M1 | −9.59 | Lys254, and Val181 |
| M2 | −9.16 | Asp251, and Glu183 |
| M3 | −6.90 | Thr353, Cys241, and Val238 |
| M4 | −8.60 | Asp251, and Cys241 |
| M5 | −8.10 | Asp251, Cys241, and Asn350 |
| M6 | −7.07 | Lys254 |
| M7 | −4.75 | Cys241, and Val238 |
| M8 | - | - |
| M9 | −8.43 | Val181, Cys241 |
| M10 | −6.38 | Asp251, Cys241 |
| M11 | −9.89 | Cys241, Val181, and Asn350 |
| M12 | −7.35 | Val181, Cys241 |
| M13 | −4.23 | Val238 |
| M14 | −7.40 | Thr179, Val181 |
| Colchicine | −11.35 | Val181, Cys241, Thr353, Val238 |
| Compound Name | Docking Score (kcal/mol) | Interactions with Amino Acid Residues |
|---|---|---|
| M1 | −5.01 | Thr200, Thr201, Gln92, Leu91, and zinc coordination |
| M2 | −4.08 | Asn66, and zinc coordination |
| M3 | −4.14 | Gln92 |
| M4 | −5.25 | Asn66, Thr200, Zinc coordination |
| M5 | −5.21 | His68, Thr200, and zinc coordination |
| M6 | −4.07 | Gln92, and zinc coordination |
| M7 | −3.32 | Gln92 |
| M8 | - | - |
| M9 | −5.20 | Zinc coordination |
| M10 | - | - |
| M11 | −5.46 | His68, Thr200, His94, Zinc coordination |
| M12 | −4.52 | His68, Thr201, and zinc coordination |
| M13 | −1.30 | Gln92, His94, Thr200, and zinc coordination |
| M14 | −4.40 | Gln71, Thr201, Gln92, and zinc coordination |
| Y0R | −7.13 | Gln92, Thr200, and zinc coordination |
| Compound Name | Molecular Weight (g/mol) | Log Po/w | Log S | BBB Permeant | GI Absorption | Rule of Five (ROF) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SWISS ADME | QikProp | SWISS ADME | Qik Prop | SWISS ADME | Qik Prop | SWISS ADME | Qik Prop | SWISS ADME | Qik Prop (%) | SWISS ADME | |
| M1 | 432.38 | 432.38 | 0.05 | −0.36 | −2.69 Soluble | −3.22 | No | No | Low | 29.50 | Yes; 1 violation: NHorOH > 5 |
| M2 | 360.31 | 360.32 | 2.60 | 2.21 | −4.74 Moderately soluble | −3.92 | No | No | High | 82.42 | Yes; 0 violation |
| M3 | 336.34 | 336.34 | 0.82 | 1.66 | −1.52 Soluble | −3.466 | No | No | High | 82.33 | Yes; 0 violation |
| M4 | 344.32 | 344.32 | 2.90 | 2.79 | −5.33 Moderately soluble | −4.142 | No | No | High | 92.79 | Yes; 0 violation |
| M5 | 270.24 | 270.24 | 2.58 | 1.59 | −4.40 Moderately soluble | −3.297 | No | No | High | 73.11 | Yes; 0 violation |
| M6 | 306.35 | 306.35 | 1.90 | 2.34 | −2.27 Soluble | −3.888 | Yes | Yes | High | 92.97 | Yes; 0 violation |
| M7 | 222.37 | 222.37 | 4.23 | 4.60 | −3.00 Soluble | −4.95 | Yes | Yes | High | 100 | Yes; 0 violation |
| M8 | 436.62 | 436.63 | 3.93 | 3.80 | −3.39 Soluble | −5.759 | No | No | High | 95.21 | Yes; 0 violation |
| M9 | 286.24 | 286.24 | 2.28 | 0.91 | −3.82 Soluble | −3.026 | No | No | High | 61.10 | Yes; 0 violation |
| M10 | 497.71 | 497.71 | 6.54 | 6.40 | −6.30 Poorly soluble | −8.969 | No | No | High | 100 | Yes; 1 violation: MLOGP > 4.15 |
| M11 | 302.24 | 302.24 | 1.99 | 0.353 | −3.24 Soluble | −2.882 | No | No | High | 51.56 | Yes; 0 violation |
| M12 | 282.25 | 282.25 | −2.58 | −1.68 | 0.59 Soluble | −1.693 | No | No | Low | 49.49 | Yes; 0 violation |
| M13 | 287.40 | 287.40 | 3.74 | 4.28 | −4.81 Moderately soluble | −5.433 | Yes | No | High | 100 | Yes; 0 violation |
| M14 | 278.30 | 278.30 | 1.10 | 0.28 | −2.52 Soluble | −2.155 | No | No | High | 68.622 | Yes; 0 violation |
| Compound | CYP1A2 | CYP2C19 | CYP2C9 | CYP2D6 | CYP3A4 |
|---|---|---|---|---|---|
| M1 | No | No | No | No | No |
| M2 | Yes | No | Yes | Yes | Yes |
| M3 | No | No | No | No | No |
| M4 | Yes | No | Yes | Yes | Yes |
| M5 | Yes | No | No | Yes | Yes |
| M6 | No | No | No | No | No |
| M7 | No | No | Yes | No | No |
| M8 | No | No | No | No | No |
| M9 | Yes | No | No | Yes | Yes |
| M10 | No | No | Yes | No | No |
| M11 | Yes | No | No | Yes | Yes |
| M12 | No | No | No | No | No |
| M13 | Yes | Yes | Yes | No | Yes |
| M14 | No | No | No | No | No |
| Compound Name | Classification | ||||
|---|---|---|---|---|---|
| Organ Toxicity (% Probability) | Toxicity Endpoint (% Probability) | ||||
| Hepatotoxicity | Carcinogenicity | Immunotoxicity | Mutagenicity | Cytotoxicity | |
| M1 | 0.82 (inactive) | 0.86 (inactive) | 0.93 (inactive) | 0.59 (active) | 0.69 (inactive) |
| M2 | 0.70 (inactive) | 0.69 (inactive) | 0.97 (Active) | 0.82 (inactive) | 0.75 (inactive) |
| M3 | 0.67 (inactive) | 0.61 (inactive) | 0.99 (Active) | 0.55 (Active) | 0.58 (inactive) |
| M4 | 0.70 (inactive) | 0.69 (inactive) | 0.84 (Active) | 0.82 (inactive) | 0.75 (inactive) |
| M5 | 0.68 (inactive) | 0.62 (inactive) | 0.99 (inactive) | 0.57 (inactive) | 0.87 (inactive) |
| M6 | 0.72 (inactive) | 0.54 (Active) | 0.94 (Active) | 0.68 (inactive) | 0.77 (inactive) |
| M7 | 0.78 (inactive) | 0.70 (inactive) | 0.97 (inactive) | 0.83 (inactive) | 0.75 (inactive) |
| M8 | 0.60 (inactive) | 0.75 (inactive) | 0.57 (Active) | 0.73 (inactive) | 0.75 (inactive) |
| M9 | 0.69 (inactive) | 0.68 (Active) | 0.97 (inactive) | 0.51 (Active) | 0.99 (inactive) |
| M10 | 0.81 (inactive) | 0.52 (inactive) | 0.98 (Active) | 0.79 (inactive) | 0.75 (inactive) |
| M11 | 0.69 (inactive) | 0.68 (Active) | 0.87 (inactive) | 0.51 (Active) | 0.99 (inactive) |
| M12 | 0.68 (inactive) | 0.73 (inactive) | 0.97 (inactive) | 0.83 (inactive) | 0.62 (inactive) |
| M13 | 0.83 (inactive) | 0.62 (inactive) | 0.84 (Active) | 0.76 (inactive) | 0.83 (inactive) |
| M14 | 0.71 (inactive) | 0.63 (inactive) | 0.71 (inactive) | 0.84 (inactive) | 0.64 (inactive) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshuail, N.; Alehaideb, Z.; Alghamdi, S.; Suliman, R.; Al-Eidi, H.; Ali, R.; Barhoumi, T.; Almutairi, M.; Alwhibi, M.; Alghanem, B.; et al. Achillea fragrantissima (Forssk.) Sch.Bip Flower Dichloromethane Extract Exerts Anti-Proliferative and Pro-Apoptotic Properties in Human Triple-Negative Breast Cancer (MDA-MB-231) Cells: In Vitro and In Silico Studies. Pharmaceuticals 2022, 15, 1060. https://doi.org/10.3390/ph15091060
Alshuail N, Alehaideb Z, Alghamdi S, Suliman R, Al-Eidi H, Ali R, Barhoumi T, Almutairi M, Alwhibi M, Alghanem B, et al. Achillea fragrantissima (Forssk.) Sch.Bip Flower Dichloromethane Extract Exerts Anti-Proliferative and Pro-Apoptotic Properties in Human Triple-Negative Breast Cancer (MDA-MB-231) Cells: In Vitro and In Silico Studies. Pharmaceuticals. 2022; 15(9):1060. https://doi.org/10.3390/ph15091060
Chicago/Turabian StyleAlshuail, Nora, Zeyad Alehaideb, Sahar Alghamdi, Rasha Suliman, Hamad Al-Eidi, Rizwan Ali, Tlili Barhoumi, Mansour Almutairi, Mona Alwhibi, Bandar Alghanem, and et al. 2022. "Achillea fragrantissima (Forssk.) Sch.Bip Flower Dichloromethane Extract Exerts Anti-Proliferative and Pro-Apoptotic Properties in Human Triple-Negative Breast Cancer (MDA-MB-231) Cells: In Vitro and In Silico Studies" Pharmaceuticals 15, no. 9: 1060. https://doi.org/10.3390/ph15091060
APA StyleAlshuail, N., Alehaideb, Z., Alghamdi, S., Suliman, R., Al-Eidi, H., Ali, R., Barhoumi, T., Almutairi, M., Alwhibi, M., Alghanem, B., Alamro, A., Alghamdi, A., & Matou-Nasri, S. (2022). Achillea fragrantissima (Forssk.) Sch.Bip Flower Dichloromethane Extract Exerts Anti-Proliferative and Pro-Apoptotic Properties in Human Triple-Negative Breast Cancer (MDA-MB-231) Cells: In Vitro and In Silico Studies. Pharmaceuticals, 15(9), 1060. https://doi.org/10.3390/ph15091060









