Rosiglitazone Does Not Show Major Hidden Cardiotoxicity in Models of Ischemia/Reperfusion but Abolishes Ischemic Preconditioning-Induced Antiarrhythmic Effects in Rats In Vivo
Abstract
1. Introduction
2. Results
2.1. In Vivo Model of I/R Injury with IPC
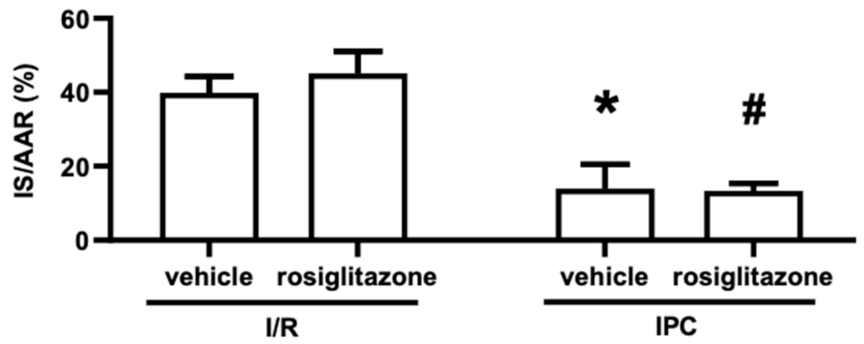
2.1.1. Chronic Rosiglitazone Treatment Did Not Influence Infarct Size or Interfere with the Infarct-Size-Limiting Effects of Ischemic Preconditioning
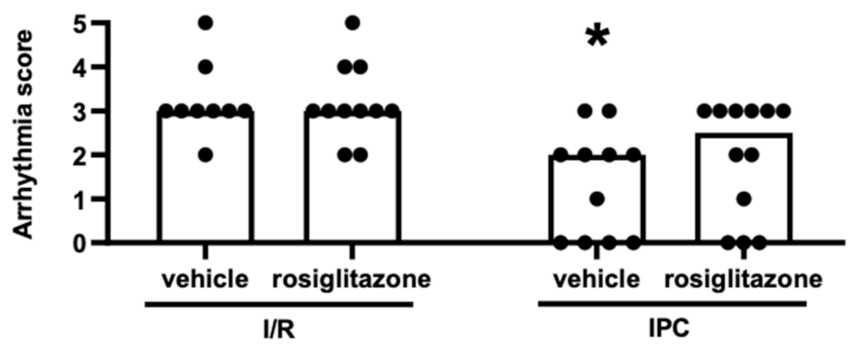
2.1.2. Chronic Rosiglitazone Treatment Abolished the Antiarrhythmic Effect of IPC
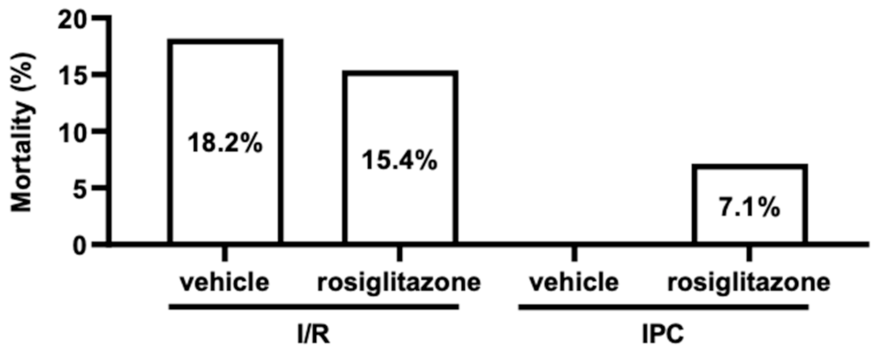
2.1.3. Chronic Rosiglitazone Treatment Did Not Affect Acute Mortality during Ischemia/Reperfusion Injury
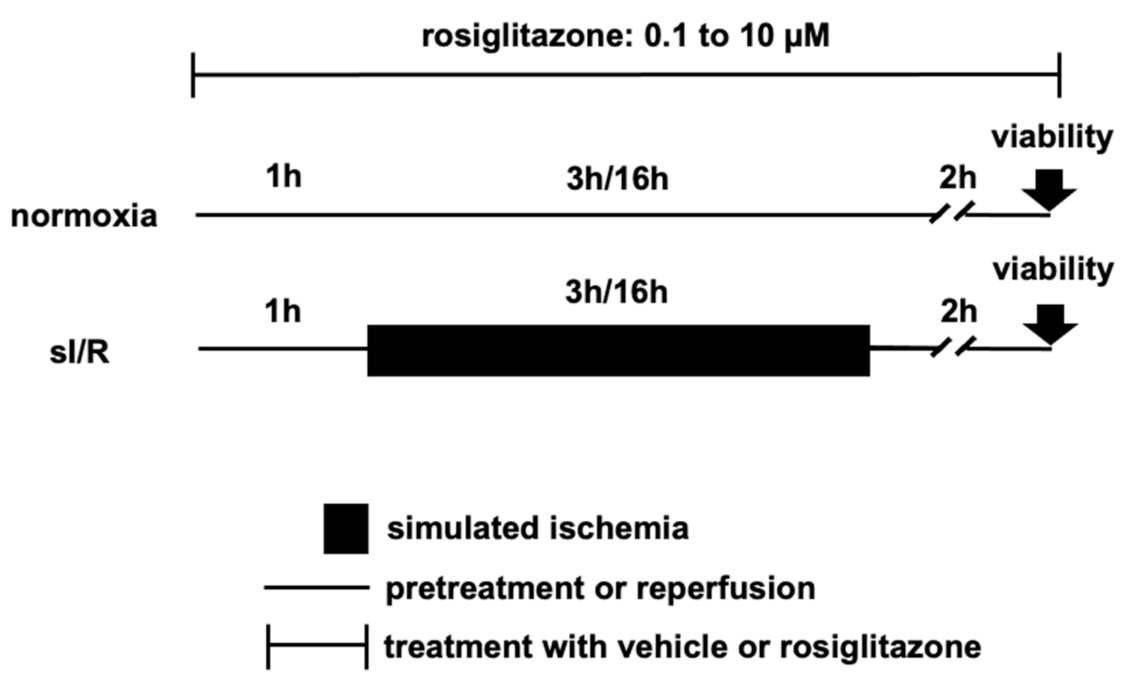
2.2. In Vitro Model of Simulated Ischemia/Reperfusion Injury
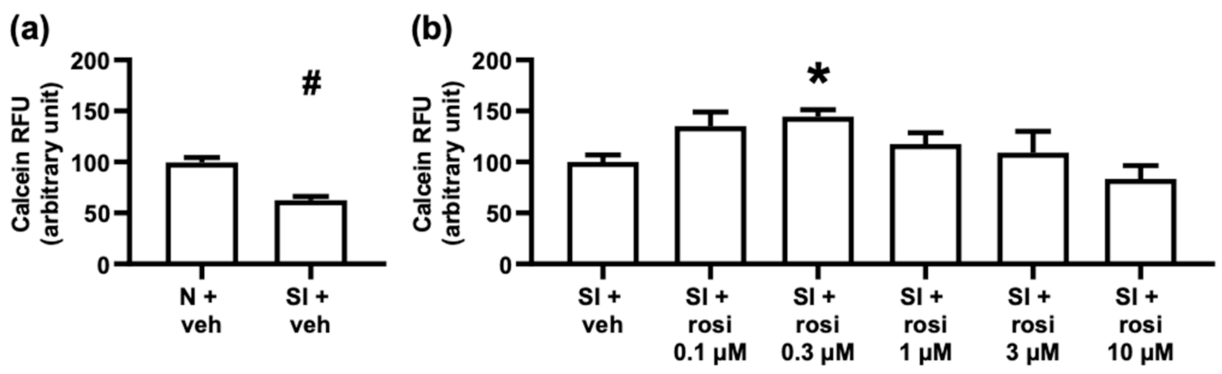
2.2.1. Rosiglitazone Treatment Increased Cell Survival of Adult Rat Cardiomyocytes with 0.3 μM in Simulated Ischemia/Reperfusion Injury
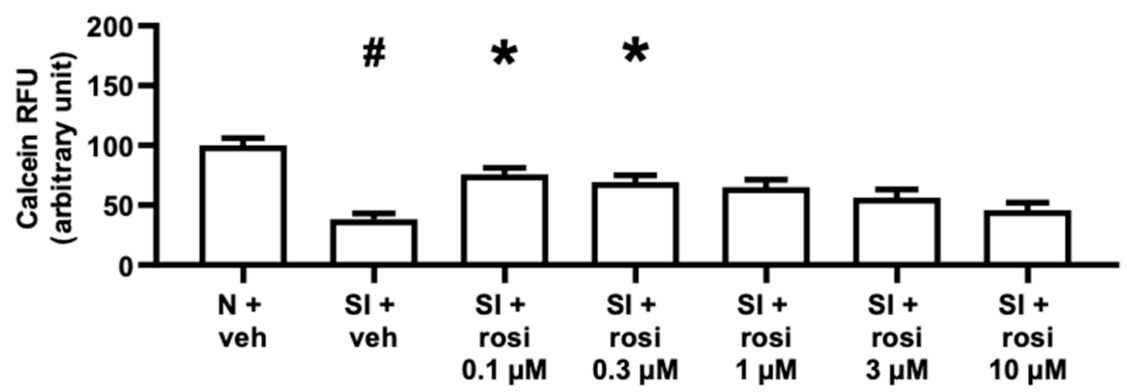
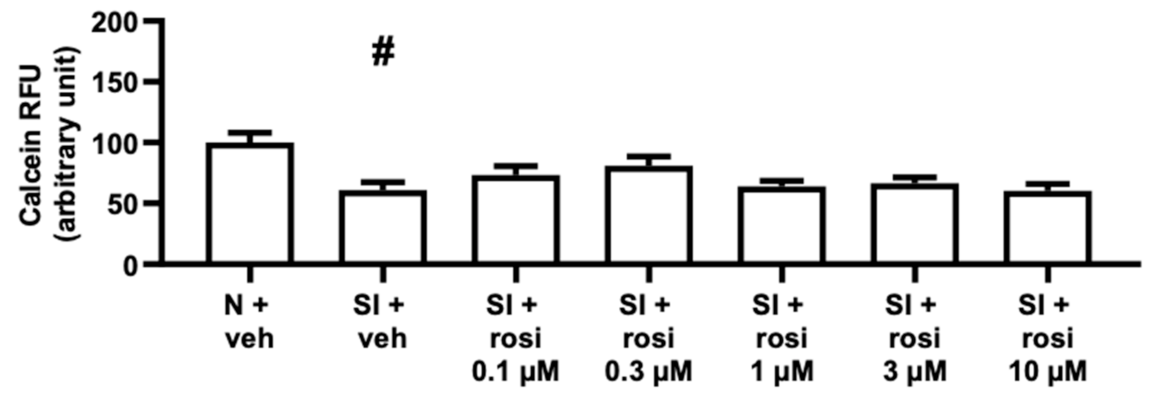
2.2.2. Rosiglitazone Treatment Increased the Viability of AC16 Cells with 0.1 and 0.3 μM Concentrations but Not of Differentiated AC16 Cells
3. Discussion
4. Materials and Methods
4.1. Ethical Considerations
4.2. Sources of Chemicals
4.3. In Vivo Ischemia/Reperfusion Injury Study
4.3.1. Animal Handling and Surgery Protocol
4.3.2. Infarct Size Measurement
4.3.3. Arrhythmia Analysis
4.3.4. Mortality Analysis
4.4. In Vitro Simulated Ischemia/Reperfusion Injury Study
4.4.1. ARCM Isolation Protocol
4.4.2. Human Cardiac Cell Line AC16 Maintenance and Differentiation
4.4.3. Simulated Ischemia/Reperfusion and Normoxia Conditions
4.4.4. Viability Assay
4.5. Literature Search Methodology
4.6. Statistical Analysis
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Onakpoya, I.J.; Heneghan, C.J.; Aronson, J.K. Post-marketing withdrawal of 462 medicinal products because of adverse drug reactions: A systematic review of the world literature. BMC Med. 2016, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Onakpoya, I.J.; Heneghan, C.J.; Aronson, J.K. Worldwide withdrawal of medicinal products because of adverse drug reactions: A systematic review and analysis. Crit. Rev. Toxicol. 2016, 46, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Ferdinandy, P.; Baczkó, I.; Bencsik, P.; Giricz, Z.; Görbe, A.; Pacher, P.; Varga, Z.V.; Varró, A.; Schulz, R. Definition of hidden drug cardiotoxicity: Paradigm change in cardiac safety testing and its clinical implications. Eur. Heart J. 2019, 40, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Ferdinandy, P.; Hausenloy, D.J.; Heusch, G.; Baxter, G.F.; Schulz, R. Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol. Rev. 2014, 66, 1142–1174. [Google Scholar] [CrossRef]
- Ferdinandy, P. Myocardial ischaemia/reperfusion injury and preconditioning: Effects of hypercholesterolaemia/hyperlipidaemia. Br. J. Pharmacol. 2003, 138, 283–285. [Google Scholar] [CrossRef]
- Ferdinandy, P.; Schulz, R.; Baxter, G.F. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol. Rev. 2007, 59, 418–458. [Google Scholar] [CrossRef]
- Brenner, G.B.; Makkos, A.; Nagy, C.T.; Onódi, Z.; Sayour, N.V.; Gergely, T.G.; Kiss, B.; Görbe, A.; Sághy, É.; Zádori, Z.S.; et al. Hidden Cardiotoxicity of Rofecoxib Can be Revealed in Experimental Models of Ischemia/Reperfusion. Cells 2020, 9, 551. [Google Scholar] [CrossRef]
- Kocsis, G.F.; Pipis, J.; Fekete, V.; Kovacs-Simon, A.; Odendaal, L.; Molnar, E.; Giricz, Z.; Janaky, T.; van Rooyen, J.; Csont, T.; et al. Lovastatin interferes with the infarct size-limiting effect of ischemic preconditioning and postconditioning in rat hearts. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H2406–H2409. [Google Scholar] [CrossRef]
- Food and Drug Administration, HHS. International Conference on Harmonisation; Guidance on M3(R2) Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals; availability. Notice. Fed. Regist. 2010, 75, 3471–3472. [Google Scholar]
- Guideline on Repeated Dose Toxicity. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-repeated-dose-toxicity-revision-1_en.pdf (accessed on 21 March 2022).
- Food and Drug Administration, HHS. International Conference on Harmonisation; guidance on E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs; availability. Notice. Fed. Regist. 2005, 70, 61134–61135. [Google Scholar]
- Food and Drug Administration, HHS. International Conference on Harmonisation; guidance on S7B Nonclinical Evaluation of the Potential for Delayed Ventricular Repolarization (QT Interval Prolongation) by Human Pharmaceuticals; availability. Notice. Fed. Regist. 2005, 70, 61133–61134. [Google Scholar]
- Young, P.W.; Buckle, D.R.; Cantello, B.C.; Chapman, H.; Clapham, J.C.; Coyle, P.J.; Haigh, D.; Hindley, R.M.; Holder, J.C.; Kallender, H.; et al. Identification of high-affinity binding sites for the insulin sensitizer rosiglitazone (BRL-49653) in rodent and human adipocytes using a radioiodinated ligand for peroxisomal proliferator-activated receptor gamma. J. Pharmacol. Exp. Ther. 1998, 284, 751–759. [Google Scholar] [PubMed]
- Assessment Report for AVANDIA. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/avandia (accessed on 12 May 2022).
- FDA Drug Safety Communication: FDA Requires Removal of Some Prescribing and Dispensing Restrictions for Rosiglitazone-Containing Diabetes Medicines. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-requires-removal-some-prescribing-and-dispensing-restrictions (accessed on 12 May 2022).
- Mahaffey, K.W.; Hafley, G.; Dickerson, S.; Burns, S.; Tourt-Uhlig, S.; White, J.; Newby, L.K.; Komajda, M.; McMurray, J.; Bigelow, R.; et al. Results of a reevaluation of cardiovascular outcomes in the RECORD trial. Am. Heart J. 2013, 166, 240–249 e241. [Google Scholar] [CrossRef] [PubMed]
- Florez, H.; Reaven, P.D.; Bahn, G.; Moritz, T.; Warren, S.; Marks, J.; Reda, D.; Duckworth, W.; Abraira, C.; Hayward, R.; et al. Rosiglitazone treatment and cardiovascular disease in the Veterans Affairs Diabetes Trial. Diabetes Obes. Metab. 2015, 17, 949–955. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, D.; Xu, W.; Shao, S.; Yu, X. Effect and cardiovascular safety of adding rosiglitazone to insulin therapy in type 2 diabetes: A meta-analysis. J. Diabetes Investig. 2015, 6, 78–86. [Google Scholar] [CrossRef]
- Nissen, S.E.; Wolski, K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N. Engl. J. Med. 2007, 356, 2457–2471. [Google Scholar] [CrossRef]
- Singh, S.; Loke, Y.K.; Furberg, C.D. Long-term risk of cardiovascular events with rosiglitazone: A meta-analysis. JAMA 2007, 298, 1189–1195. [Google Scholar] [CrossRef]
- Zhu, J.; Yu, X.; Zheng, Y.; Li, J.; Wang, Y.; Lin, Y.; He, Z.; Zhao, W.; Chen, C.; Qiu, K.; et al. Association of glucose-lowering medications with cardiovascular outcomes: An umbrella review and evidence map. Lancet Diabetes Endocrinol. 2020, 8, 192–205. [Google Scholar] [CrossRef]
- Zhang, X.J.; Xiong, Z.B.; Tang, A.L.; Ma, H.; Ma, Y.D.; Wu, J.G.; Dong, Y.G. Rosiglitazone-induced myocardial protection against ischaemia-reperfusion injury is mediated via a phosphatidylinositol 3-kinase/Akt-dependent pathway. Clin. Exp. Pharmacol. Physiol. 2010, 37, 156–161. [Google Scholar] [CrossRef]
- Yue, T.L.; Bao, W.; Gu, J.L.; Cui, J.; Tao, L.; Ma, X.L.; Ohlstein, E.H.; Jucker, B.M. Rosiglitazone treatment in Zucker diabetic Fatty rats is associated with ameliorated cardiac insulin resistance and protection from ischemia/reperfusion-induced myocardial injury. Diabetes 2005, 54, 554–562. [Google Scholar] [CrossRef]
- Yue, T.L.; Chen, J.; Bao, W.; Narayanan, P.K.; Bril, A.; Jiang, W.; Lysko, P.G.; Gu, J.L.; Boyce, R.; Zimmerman, D.M.; et al. In vivo myocardial protection from ischemia/reperfusion injury by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Circulation 2001, 104, 2588–2594. [Google Scholar] [CrossRef] [PubMed]
- Molavi, B.; Chen, J.; Mehta, J.L. Cardioprotective effects of rosiglitazone are associated with selective overexpression of type 2 angiotensin receptors and inhibition of p42/44 MAPK. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H687–H693. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Potenza, M.A.; Gagliardi, S.; De Benedictis, L.; Zigrino, A.; Tiravanti, E.; Colantuono, G.; Federici, A.; Lorusso, L.; Benagiano, V.; Quon, M.J.; et al. Treatment of spontaneously hypertensive rats with rosiglitazone ameliorates cardiovascular pathophysiology via antioxidant mechanisms in the vasculature. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E685–E694. [Google Scholar] [CrossRef] [PubMed]
- Palee, S.; Weerateerangkul, P.; Chinda, K.; Chattipakorn, S.C.; Chattipakorn, N. Mechanisms responsible for beneficial and adverse effects of rosiglitazone in a rat model of acute cardiac ischaemia-reperfusion. Exp. Physiol. 2013, 98, 1028–1037. [Google Scholar] [CrossRef]
- Wayman, N.S.; Hattori, Y.; McDonald, M.C.; Mota-Filipe, H.; Cuzzocrea, S.; Pisano, B.; Chatterjee, P.K.; Thiemermann, C. Ligands of the peroxisome proliferator-activated receptors (PPAR-gamma and PPAR-alpha) reduce myocardial infarct size. FASEB J. 2002, 16, 1027–1040. [Google Scholar] [CrossRef]
- Abe, M.; Takiguchi, Y.; Ichimaru, S.; Kaji, S.; Tsuchiya, K.; Wada, K. Different effect of acute treatment with rosiglitazone on rat myocardial ischemia/reperfusion injury by administration method. Eur. J. Pharmacol. 2008, 589, 215–219. [Google Scholar] [CrossRef]
- Gao, W.; Jusko, W.J. Modeling disease progression and rosiglitazone intervention in type 2 diabetic Goto-Kakizaki rats. J. Pharmacol. Exp. Ther. 2012, 341, 617–625. [Google Scholar] [CrossRef]
- Giri, P.; Delvadia, P.; Ladani, M.K.; Prajapati, N.; Gupta, L.; Patel, N.; Joshi, V.; Giri, S.; Jain, M.R.; Srinivas, N.R.; et al. Lack of inhibition of CYP2C8 by saroglitazar magnesium: In vivo assessment using montelukast, rosiglitazone, pioglitazone, repaglinide and paclitaxel as victim drugs in Wistar rats. Eur. J. Pharm. Sci. 2019, 130, 107–113. [Google Scholar] [CrossRef]
- Hruska, M.W.; Frye, R.F. Simplified method for determination of rosiglitazone in human plasma. J. Chromatogr. B Analyt. Technol. Biomed Life Sci. 2004, 803, 317–320. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, Z.; Shen, M.; Ma, A. Simultaneous determination and pharmacokinetic study of metformin and rosiglitazone in human plasma by HPLC-ESI-MS. J. Chromatogr. Sci. 2011, 49, 94–100. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, K.A.; Shin, J.G.; Lee, K.Y. Effect of ketoconazole on the pharmacokinetics of rosiglitazone in healthy subjects. Br. J. Clin. Pharmacol. 2004, 58, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Kim, K.A.; Kang, M.H.; Kim, S.L.; Shin, J.G. Effect of rifampin on the pharmacokinetics of rosiglitazone in healthy subjects. Clin. Pharmacol. Ther. 2004, 75, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Naik, H.; Wu, J.T.; Palmer, R.; McLean, L. The effects of febuxostat on the pharmacokinetic parameters of rosiglitazone, a CYP2C8 substrate. Br. J. Clin. Pharmacol. 2012, 74, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.A.; Park, J.Y. Simple and extractionless high-performance liquid chromatographic determination of rosiglitazone in human plasma and application to pharmacokinetics in humans. Biomed. Chromatogr. 2004, 18, 613–615. [Google Scholar] [CrossRef]
- Miller, A.K.; Inglis, A.M.; Culkin, K.T.; Jorkasky, D.K.; Freed, M.I. The effect of acarbose on the pharmacokinetics of rosiglitazone. Eur. J. Clin. Pharmacol. 2001, 57, 105–109. [Google Scholar] [CrossRef]
- Hu, L.D.; Xing, Q.B.; Shang, C.; Liu, W.; Liu, C.; Luo, Z.L.; Xu, H.X. Preparation of rosiglitazone maleate sustained-release floating microspheres for improved bioavailability. Pharmazie 2010, 65, 477–480. [Google Scholar]
- He, L.; Wickremasingha, P.; Lee, J.; Tao, B.; Mendell-Harary, J.; Walker, J.; Wight, D. Lack of effect of colesevelam HCl on the single-dose pharmacokinetics of aspirin, atenolol, enalapril, phenytoin, rosiglitazone, and sitagliptin. Diabetes Res. Clin. Pract. 2014, 104, 401–409. [Google Scholar] [CrossRef]
- Kumar, J.N.; Devi, P.; Narasu, L.; Mullangi, R. Effect of ciprofloxacin and ibuprofen on the in vitro metabolism of rosiglitazone and oral pharmacokinetics of rosiglitazone in healthy human volunteers. Eur. J. Drug Metab. Pharmacokinet. 2008, 33, 237–242. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, K.B.; Um, S.Y.; Oh, Y.; Chung, M.W.; Oh, H.Y.; Choi, K.H. Changes in the Pharmacokinetics of Rosiglitazone, a CYP2C8 Substrate, When Co-Administered with Amlodipine in Rats. Biomol. Ther. 2009, 17, 299–304. [Google Scholar] [CrossRef]
- Zhou, X.; Rougee, L.R.; Bedwell, D.W.; Cramer, J.W.; Mohutsky, M.A.; Calvert, N.A.; Moulton, R.D.; Cassidy, K.C.; Yumibe, N.P.; Adams, L.A.; et al. Difference in the Pharmacokinetics and Hepatic Metabolism of Antidiabetic Drugs in Zucker Diabetic Fatty and Sprague-Dawley Rats. Drug Metab. Dispos. 2016, 44, 1184–1192. [Google Scholar] [CrossRef]
- Gao, X.Q.; Li, H.W.; Ling, X.; Qiu, Y.H.; Gao, Y.; Zhang, Y. Effect of rosiglitazone on rabbit model of myocardial ischemia-reperfusion injury. Asian Pac. J. Trop. Med. 2013, 6, 228–231. [Google Scholar] [CrossRef]
- Sarraf, M.; Lu, L.; Ye, S.; Reiter, M.J.; Greyson, C.R.; Schwartz, G.G. Thiazolidinedione drugs promote onset, alter characteristics, and increase mortality of ischemic ventricular fibrillation in pigs. Cardiovasc. Drugs Ther. 2012, 26, 195–204. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hueb, W.; Uchida, A.H.; Gersh, B.J.; Betti, R.T.; Lopes, N.; Moffa, P.J.; Ferreira, B.M.; Ramires, J.A.; Wajchenberg, B.L. Effect of a hypoglycemic agent on ischemic preconditioning in patients with type 2 diabetes and stable angina pectoris. Coron. Artery Dis. 2007, 18, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Rahmi, R.M.; Uchida, A.H.; Rezende, P.C.; Lima, E.G.; Garzillo, C.L.; Favarato, D.; Strunz, C.M.; Takiuti, M.; Girardi, P.; Hueb, W.; et al. Effect of hypoglycemic agents on ischemic preconditioning in patients with type 2 diabetes and symptomatic coronary artery disease. Diabetes Care 2013, 36, 1654–1659. [Google Scholar] [CrossRef] [PubMed]
- Palee, S.; Weerateerangkul, P.; Surinkeaw, S.; Chattipakorn, S.; Chattipakorn, N. Effect of rosiglitazone on cardiac electrophysiology, infarct size and mitochondrial function in ischaemia and reperfusion of swine and rat heart. Exp. Physiol. 2011, 96, 778–789. [Google Scholar] [CrossRef]
- Lu, L.; Reiter, M.J.; Xu, Y.; Chicco, A.; Greyson, C.R.; Schwartz, G.G. Thiazolidinedione drugs block cardiac KATP channels and may increase propensity for ischaemic ventricular fibrillation in pigs. Diabetologia 2008, 51, 675–685. [Google Scholar] [CrossRef]
- Lee, T.I.; Chen, Y.C.; Kao, Y.H.; Hsiao, F.C.; Lin, Y.K.; Chen, Y.J. Rosiglitazone induces arrhythmogenesis in diabetic hypertensive rats with calcium handling alteration. Int. J. Cardiol. 2013, 165, 299–307. [Google Scholar] [CrossRef]
- Moreland-Head, L.N.; Coons, J.C.; Seybert, A.L.; Gray, M.P.; Kane-Gill, S.L. Use of Disproportionality Analysis to Identify Previously Unknown Drug-Associated Causes of Cardiac Arrhythmias Using the Food and Drug Administration Adverse Event Reporting System (FAERS) Database. J. Cardiovasc. Pharmacol. Ther. 2021, 26, 341–348. [Google Scholar] [CrossRef]
- Leonard, C.E.; Brensinger, C.M.; Dawwas, G.K.; Deo, R.; Bilker, W.B.; Soprano, S.E.; Dhopeshwarkar, N.; Flory, J.H.; Bloomgarden, Z.T.; Gagne, J.J.; et al. The risk of sudden cardiac arrest and ventricular arrhythmia with rosiglitazone versus pioglitazone: Real-world evidence on thiazolidinedione safety. Cardiovasc. Diabetol. 2020, 19, 25. [Google Scholar] [CrossRef]
- Tao, L.; Wang, Y.; Gao, E.; Zhang, H.; Yuan, Y.; Lau, W.B.; Chan, L.; Koch, W.J.; Ma, X.L. Adiponectin: An indispensable molecule in rosiglitazone cardioprotection following myocardial infarction. Circ. Res. 2010, 106, 409–417. [Google Scholar] [CrossRef]
- Lygate, C.A.; Hulbert, K.; Monfared, M.; Cole, M.A.; Clarke, K.; Neubauer, S. The PPARgamma-activator rosiglitazone does not alter remodeling but increases mortality in rats post-myocardial infarction. Cardiovasc. Res. 2003, 58, 632–637. [Google Scholar] [CrossRef]
- Bach, R.G.; Brooks, M.M.; Lombardero, M.; Genuth, S.; Donner, T.W.; Garber, A.; Kennedy, L.; Monrad, E.S.; Pop-Busui, R.; Kelsey, S.F.; et al. Rosiglitazone and outcomes for patients with diabetes mellitus and coronary artery disease in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Circulation 2013, 128, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Tannen, R.; Xie, D.; Wang, X.; Yu, M.; Weiner, M.G. A new “Comparative Effectiveness” assessment strategy using the THIN database: Comparison of the cardiac complications of pioglitazone and rosiglitazone. Pharmacoepidemiol. Drug Saf. 2013, 22, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Wolski, K. Rosiglitazone revisited: An updated meta-analysis of risk for myocardial infarction and cardiovascular mortality. Arch. Intern. Med. 2010, 170, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.J.; Ouellet-Hellstrom, R.; MaCurdy, T.E.; Ali, F.; Sholley, C.; Worrall, C.; Kelman, J.A. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone. JAMA 2010, 304, 411–418. [Google Scholar] [CrossRef]
- Winkelmayer, W.C.; Setoguchi, S.; Levin, R.; Solomon, D.H. Comparison of cardiovascular outcomes in elderly patients with diabetes who initiated rosiglitazone vs pioglitazone therapy. Arch. Intern. Med. 2008, 168, 2368–2375. [Google Scholar] [CrossRef]
- Tzoulaki, I.; Molokhia, M.; Curcin, V.; Little, M.P.; Millett, C.J.; Ng, A.; Hughes, R.I.; Khunti, K.; Wilkins, M.R.; Majeed, A.; et al. Risk of cardiovascular disease and all cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: Retrospective cohort study using UK general practice research database. BMJ 2009, 339, b4731. [Google Scholar] [CrossRef]
- Kilter, H.; Werner, M.; Roggia, C.; Reil, J.C.; Schafers, H.J.; Kintscher, U.; Bohm, M. The PPAR-gamma agonist rosiglitazone facilitates Akt rephosphorylation and inhibits apoptosis in cardiomyocytes during hypoxia/reoxygenation. Diabetes Obes. Metab. 2009, 11, 1060–1067. [Google Scholar] [CrossRef]
- Wang, Y.; Lau, W.B.; Gao, E.; Tao, L.; Yuan, Y.; Li, R.; Wang, X.; Koch, W.J.; Ma, X.L. Cardiomyocyte-derived adiponectin is biologically active in protecting against myocardial ischemia-reperfusion injury. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E663–E670. [Google Scholar] [CrossRef]
- Onodi, Z.; Visnovitz, T.; Kiss, B.; Hambalko, S.; Koncz, A.; Agg, B.; Varadi, B.; Toth, V.E.; Nagy, R.N.; Gergely, T.G.; et al. Systematic transcriptomic and phenotypic characterization of human and murine cardiac myocyte cell lines and primary cardiomyocytes reveals serious limitations and low resemblances to adult cardiac phenotype. J. Mol. Cell Cardiol. 2022, 165, 19–30. [Google Scholar] [CrossRef]
- Mersmann, J.; Tran, N.; Zacharowski, P.A.; Grotemeyer, D.; Zacharowski, K. Rosiglitazone is cardioprotective in a murine model of myocardial I/R. Shock 2008, 30, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Avandia Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/021071s031lbl.pdf (accessed on 12 May 2022).
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Gomori, K.; Szabados, T.; Kenyeres, E.; Pipis, J.; Foldesi, I.; Siska, A.; Dorman, G.; Ferdinandy, P.; Gorbe, A.; Bencsik, P. Cardioprotective Effect of Novel Matrix Metalloproteinase Inhibitors. Int. J. Mol. Sci. 2020, 21, 6990. [Google Scholar] [CrossRef] [PubMed]
- Kiss, K.; Csonka, C.; Paloczi, J.; Pipis, J.; Gorbe, A.; Kocsis, G.F.; Murlasits, Z.; Sarkozy, M.; Szucs, G.; Holmes, C.P.; et al. Novel, selective EPO receptor ligands lacking erythropoietic activity reduce infarct size in acute myocardial infarction in rats. Pharmacol. Res. 2016, 113, 62–70. [Google Scholar] [CrossRef]
- Curtis, M.J.; Hancox, J.C.; Farkas, A.; Wainwright, C.L.; Stables, C.L.; Saint, D.A.; Clements-Jewery, H.; Lambiase, P.D.; Billman, G.E.; Janse, M.J.; et al. The Lambeth Conventions (II): Guidelines for the study of animal and human ventricular and supraventricular arrhythmias. Pharmacol. Ther. 2013, 139, 213–248. [Google Scholar] [CrossRef]
- Curtis, M.J.; Walker, M.J. Quantification of arrhythmias using scoring systems: An examination of seven scores in an in vivo model of regional myocardial ischaemia. Cardiovasc. Res. 1988, 22, 656–665. [Google Scholar] [CrossRef]
- Makkos, A.; Szantai, A.; Paloczi, J.; Pipis, J.; Kiss, B.; Poggi, P.; Ferdinandy, P.; Chatgilialoglu, A.; Gorbe, A. A Comorbidity Model of Myocardial Ischemia/Reperfusion Injury and Hypercholesterolemia in Rat Cardiac Myocyte Cultures. Front. Physiol. 2019, 10, 1564. [Google Scholar] [CrossRef]
- Voros, I.; Saghy, E.; Pohoczky, K.; Makkos, A.; Onodi, Z.; Brenner, G.B.; Baranyai, T.; Agg, B.; Varadi, B.; Kemeny, A.; et al. Somatostatin and Its Receptors in Myocardial Ischemia/Reperfusion Injury and Cardioprotection. Front. Pharmacol. 2021, 12, 663655. [Google Scholar] [CrossRef]
- Gaspar, R.; Gomori, K.; Kiss, B.; Szantai, A.; Paloczi, J.; Varga, Z.V.; Pipis, J.; Varadi, B.; Agg, B.; Csont, T.; et al. Decorin Protects Cardiac Myocytes against Simulated Ischemia/Reperfusion Injury. Molecules 2020, 25, 3426. [Google Scholar] [CrossRef]
- Pecan, P.; Hambalko, S.; Ha, V.T.; Nagy, C.T.; Pelyhe, C.; Lainscek, D.; Kenyeres, B.; Brenner, G.B.; Gorbe, A.; Kittel, A.; et al. Calcium Ionophore-Induced Extracellular Vesicles Mediate Cytoprotection against Simulated Ischemia/Reperfusion Injury in Cardiomyocyte-Derived Cell Lines by Inducing Heme Oxygenase 1. Int. J. Mol. Sci. 2020, 21, 7687. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weber, B.Y.; Brenner, G.B.; Kiss, B.; Gergely, T.G.; Sayour, N.V.; Tian, H.; Makkos, A.; Görbe, A.; Ferdinandy, P.; Giricz, Z. Rosiglitazone Does Not Show Major Hidden Cardiotoxicity in Models of Ischemia/Reperfusion but Abolishes Ischemic Preconditioning-Induced Antiarrhythmic Effects in Rats In Vivo. Pharmaceuticals 2022, 15, 1055. https://doi.org/10.3390/ph15091055
Weber BY, Brenner GB, Kiss B, Gergely TG, Sayour NV, Tian H, Makkos A, Görbe A, Ferdinandy P, Giricz Z. Rosiglitazone Does Not Show Major Hidden Cardiotoxicity in Models of Ischemia/Reperfusion but Abolishes Ischemic Preconditioning-Induced Antiarrhythmic Effects in Rats In Vivo. Pharmaceuticals. 2022; 15(9):1055. https://doi.org/10.3390/ph15091055
Chicago/Turabian StyleWeber, Bennet Y., Gábor B. Brenner, Bernadett Kiss, Tamás G. Gergely, Nabil V. Sayour, Huimin Tian, András Makkos, Anikó Görbe, Péter Ferdinandy, and Zoltán Giricz. 2022. "Rosiglitazone Does Not Show Major Hidden Cardiotoxicity in Models of Ischemia/Reperfusion but Abolishes Ischemic Preconditioning-Induced Antiarrhythmic Effects in Rats In Vivo" Pharmaceuticals 15, no. 9: 1055. https://doi.org/10.3390/ph15091055
APA StyleWeber, B. Y., Brenner, G. B., Kiss, B., Gergely, T. G., Sayour, N. V., Tian, H., Makkos, A., Görbe, A., Ferdinandy, P., & Giricz, Z. (2022). Rosiglitazone Does Not Show Major Hidden Cardiotoxicity in Models of Ischemia/Reperfusion but Abolishes Ischemic Preconditioning-Induced Antiarrhythmic Effects in Rats In Vivo. Pharmaceuticals, 15(9), 1055. https://doi.org/10.3390/ph15091055






