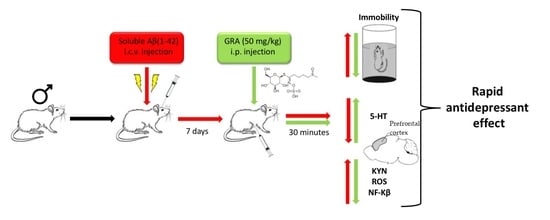
Glucoraphanin Triggers Rapid Antidepressant Responses in a Rat Model of Beta Amyloid-Induced Depressive-like Behaviour
Abstract
:1. Introduction
2. Results
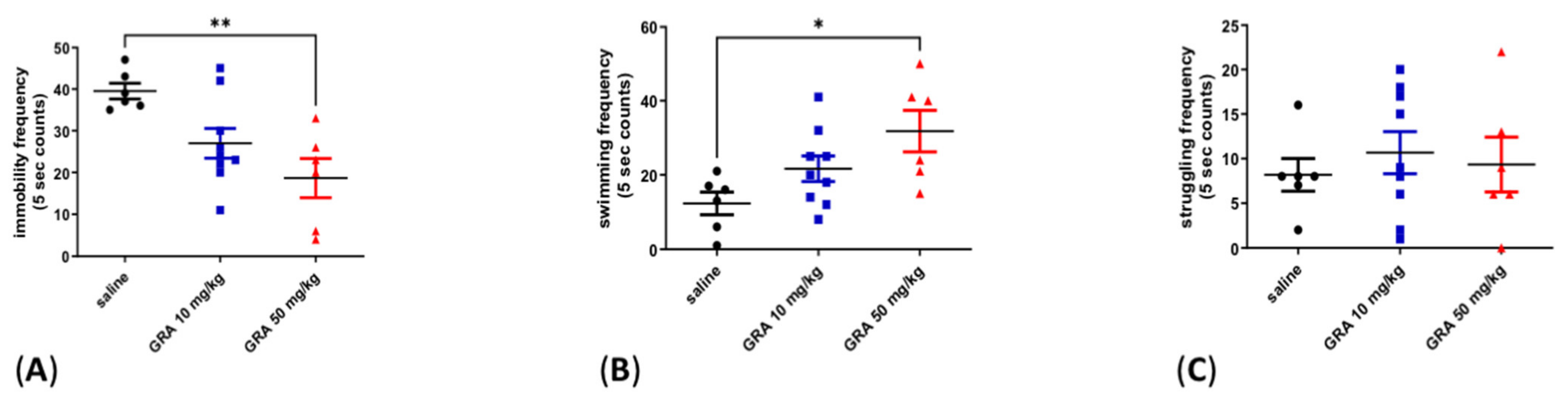
2.1. Effects of GRA on Depressive-like Behaviour in the FST
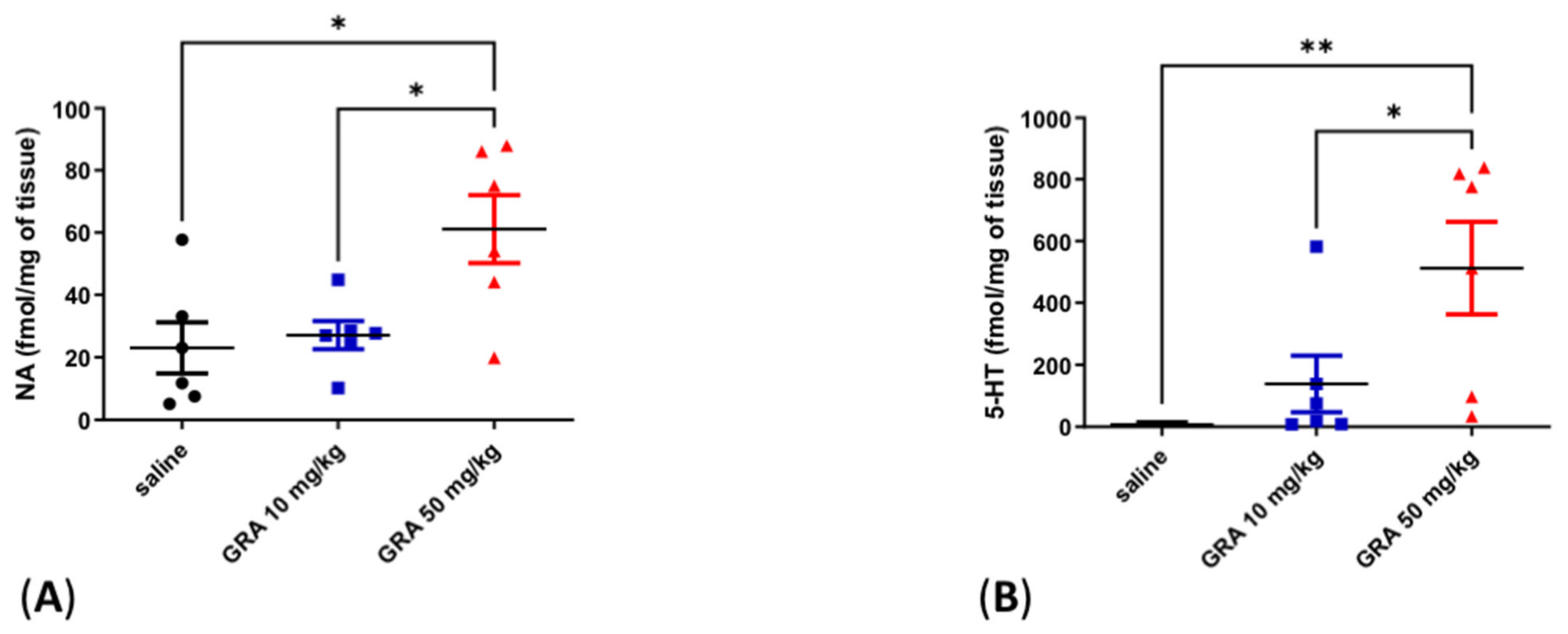
2.2. Effects of GRA on Cortical 5-HT and Noradrenaline (NA) Levels
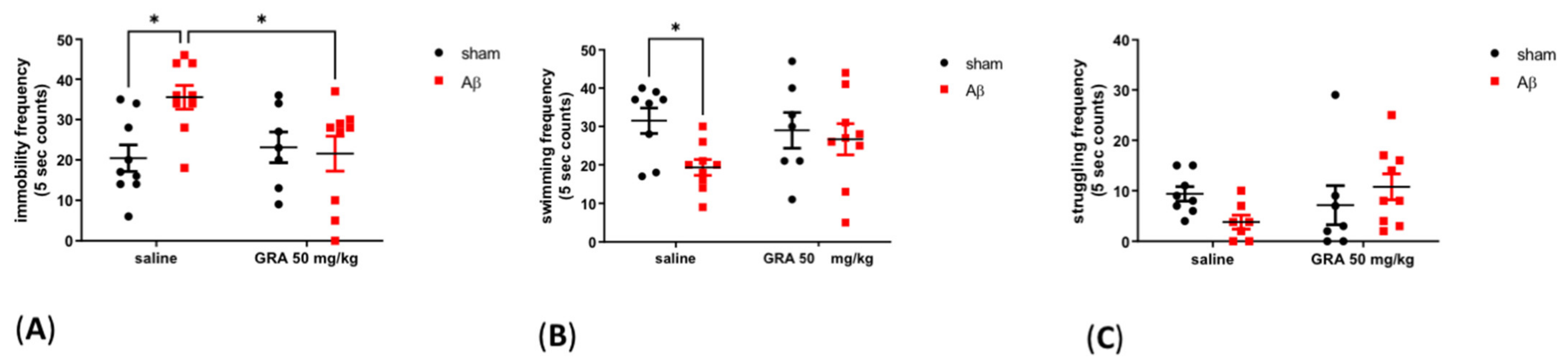
2.3. Effects of GRA on sAβ 1-42-Induced Depressive-like Behaviour in the FST
2.4. Effects of GRA on Cortical 5-HT and NA Levels in sAβ-1-42-Treated Animals
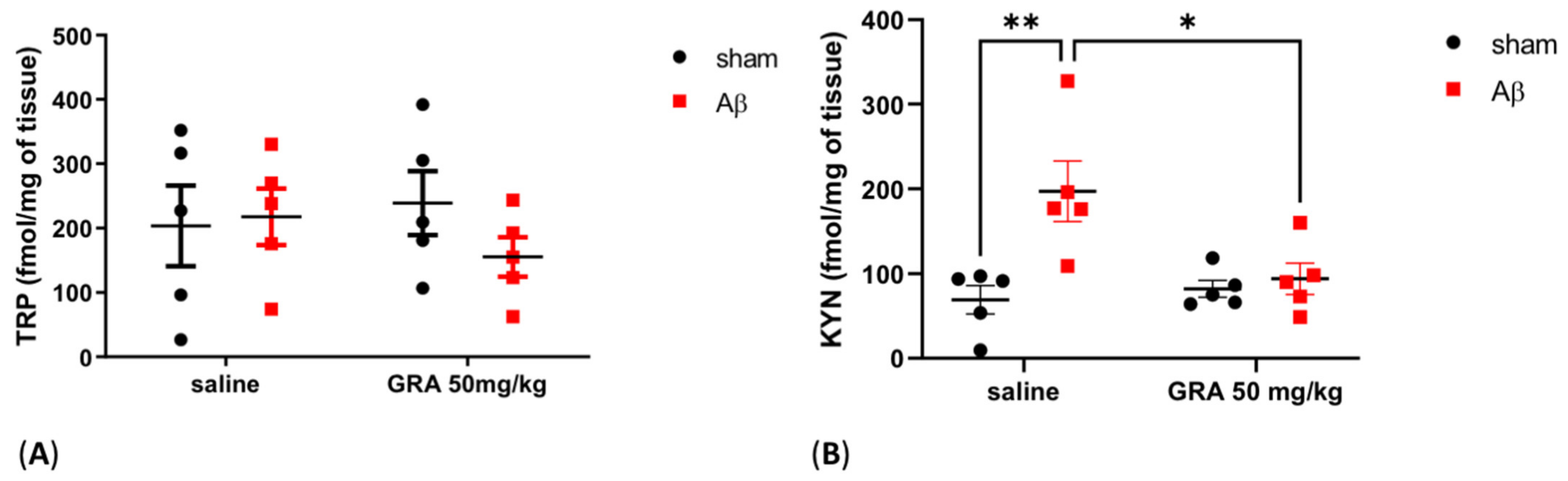
2.5. Effects of GRA on TRP and KYN Levels in sAβ-1-42-Treated Animals
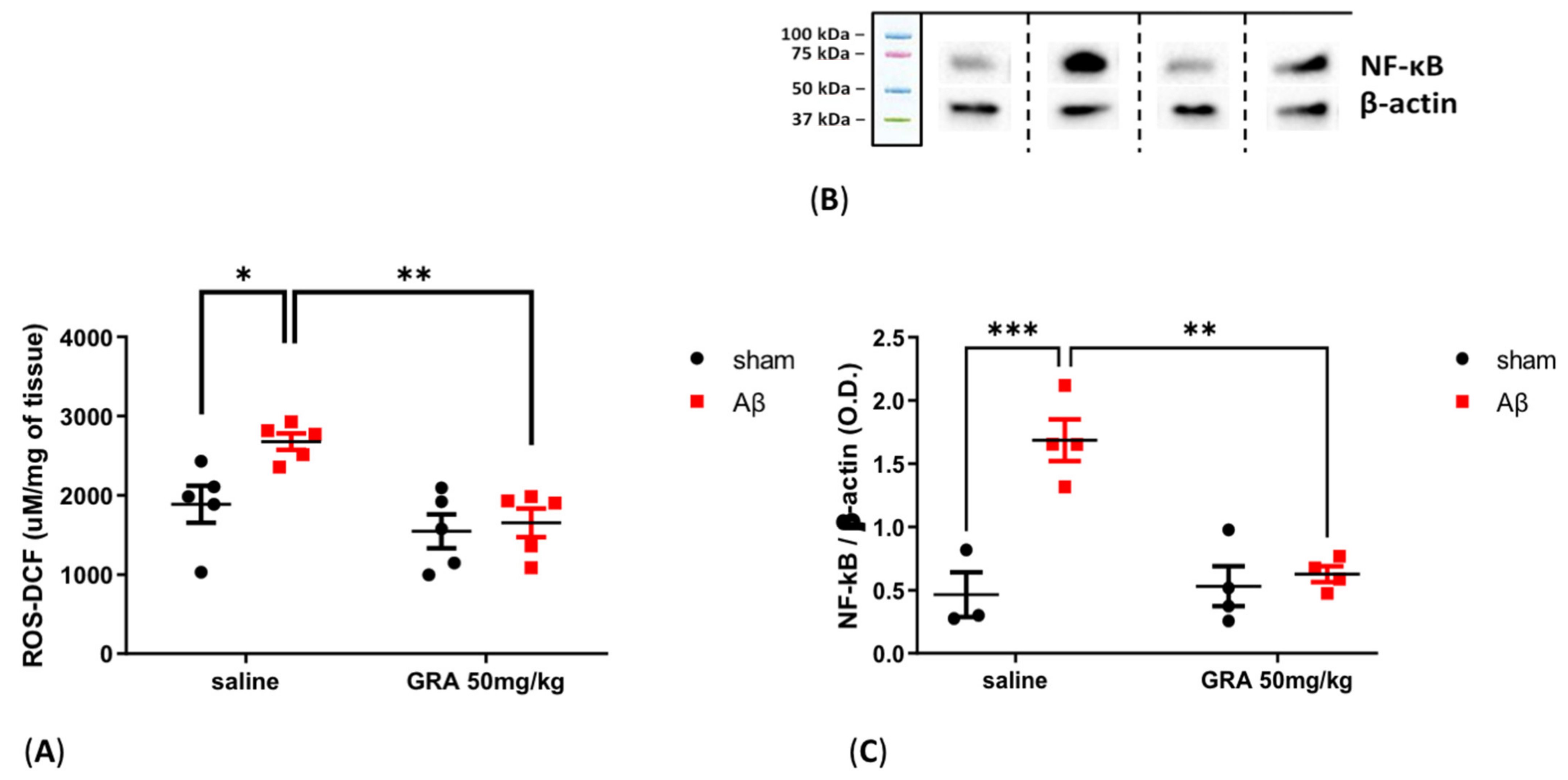
2.6. Effects of GRA on Cortical ROS Content and NF-kB Levels in sAβ-1-42-Treated Animals
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Surgery and sAβ 1-42 Infusion
4.3. Pharmacological Treatments and Experimental Design
4.4. Forced Swimming Test
4.5. Post Mortem Tissue Analyses
4.6. Neurochemical Analyses
4.7. ROS Measurement
4.8. Western Blotting
4.9. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shapiro, T.A.; Fahey, J.W.; Wade, K.L.; Stephenson, K.K.; Talalay, P. Chemoprotective Glucosinolates and Isothiocyanates of Broccoli Sprouts: Metabolism and Excretion in Humans. Cancer Epidemiol. Biomarkers Prev. 2001, 10, 501–508. [Google Scholar] [PubMed]
- Fahey, J.W.; Zhang, Y.; Talalay, P. Broccoli Sprouts: An Exceptionally Rich Source of Inducers of Enzymes That Protect against Chemical Carcinogens. Proc. Natl. Acad. Sci. USA 1997, 94, 10367–10372. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Connors, S.L.; Macklin, E.A.; Smith, K.D.; Fahey, J.W.; Talalay, P.; Zimmerman, A.W. Sulforaphane Treatment of Autism Spectrum Disorder (ASD). Proc. Natl. Acad. Sci. USA 2014, 111, 15550–15555. [Google Scholar] [CrossRef]
- Liu, H.; Zimmerman, A.W.; Singh, K.; Connors, S.L.; Diggins, E.; Stephenson, K.K.; Dinkova-Kostova, A.T.; Fahey, J.W. Biomarker Exploration in Human Peripheral Blood Mononuclear Cells for Monitoring Sulforaphane Treatment Responses in Autism Spectrum Disorder. Sci. Rep. 2020, 10, 5822. [Google Scholar] [CrossRef]
- Shiina, A.; Kanahara, N.; Sasaki, T.; Oda, Y.; Hashimoto, T.; Hasegawa, T.; Yoshida, T.; Iyo, M.; Hashimoto, K. An Open Study of Sulforaphane-Rich Broccoli Sprout Extract in Patients with Schizophrenia. Clin. Psychopharmacol. Neurosci. 2015, 13, 62–67. [Google Scholar] [CrossRef]
- Morroni, F.; Tarozzi, A.; Sita, G.; Bolondi, C.; Zolezzi Moraga, J.M.; Cantelli-Forti, G.; Hrelia, P. Neuroprotective Effect of Sulforaphane in 6-Hydroxydopamine-Lesioned Mouse Model of Parkinson’s Disease. Neurotoxicology 2013, 36, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hettinger, C.L.; Zhang, D.; Rezvani, K.; Wang, X.; Wang, H. Sulforaphane Enhances Proteasomal and Autophagic Activities in Mice and Is a Potential Therapeutic Reagent for Huntington’s Disease. J. Neurochem. 2014, 129, 539–547. [Google Scholar] [CrossRef]
- Kim, H.V.; Kim, H.Y.; Ehrlich, H.Y.; Choi, S.Y.; Kim, D.J.; Kim, Y. Amelioration of Alzheimer’s Disease by Neuroprotective Effect of Sulforaphane in Animal Model. Amyloid 2013, 20, 7–12. [Google Scholar] [CrossRef]
- Mahgoub, N.; Alexopoulos, G.S. The Amyloid Hypothesis: Is There a Role for Anti-Amyloid Treatment in Late-Life Depression? Am. J. Geriatr. Psychiatry 2016, 24, 239–247. [Google Scholar] [CrossRef]
- Colaianna, M.; Tucci, P.; Zotti, M.; Morgese, M.; Schiavone, S.; Govoni, S.; Cuomo, V.; Trabace, L. Soluble Βamyloid1-42: A Critical Player in Producing Behavioural and Biochemical Changes Evoking Depressive-Related State?: Soluble Βamyloid and Emotional Profile. Br. J. Pharmacol. 2010, 159, 1704–1715. [Google Scholar] [CrossRef] [Green Version]
- Morgese, M.G.; Schiavone, S.; Trabace, L. Emerging Role of Amyloid Beta in Stress Response: Implication for Depression and Diabetes. Eur. J. Pharmacol. 2017, 817, 22–29. [Google Scholar] [CrossRef]
- Morgese, M.G.; Tucci, P.; Colaianna, M.; Zotti, M.; Cuomo, V.; Schiavone, S.; Trabace, L. Modulatory Activity of Soluble Beta Amyloid on HPA Axis Function in Rats. Curr. Pharm. Des. 2014, 20, 2539–2546. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, S.; Tucci, P.; Mhillaj, E.; Bove, M.; Trabace, L.; Morgese, M.G. Antidepressant Drugs for Beta Amyloid-Induced Depression: A New Standpoint? Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 78, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Gao, Q.; Zhao, P.; Gao, Y.; Xi, Y.; Wang, X.; Liang, Y.; Shi, H.; Ma, Y. Sulforaphane Produces Antidepressant- and Anxiolytic-like Effects in Adult Mice. Behav. Brain Res. 2016, 301, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Zhang, J.; Ishima, T.; Dong, C.; Yang, C.; Ren, Q.; Ma, M.; Han, M.; Wu, J.; Suganuma, H.; et al. Role of Keap1-Nrf2 Signaling in Depression and Dietary Intake of Glucoraphanin Confers Stress Resilience in Mice. Sci. Rep. 2016, 6, 30659. [Google Scholar] [CrossRef]
- Ressler, K.J.; Nemeroff, C.B. Role of Serotonergic and Noradrenergic Systems in the Pathophysiology of Depression and Anxiety Disorders. Depress. Anxiety 2000, 12 (Suppl. 1), 2–19. [Google Scholar] [CrossRef]
- Dantzer, R. Role of the Kynurenine Metabolism Pathway in Inflammation-Induced Depression: Preclinical Approaches. Curr. Top. Behav. Neurosci. 2017, 31, 117–138. [Google Scholar] [CrossRef]
- Vécsei, L.; Szalárdy, L.; Fülöp, F.; Toldi, J. Kynurenines in the CNS: Recent Advances and New Questions. Nat. Rev. Drug Discov. 2013, 12, 64–82. [Google Scholar] [CrossRef]
- Duan, Z.; Zhang, S.; Liang, H.; Xing, Z.; Guo, L.; Shi, L.; Du, L.; Kuang, C.; Takikawa, O.; Yang, Q. Amyloid β Neurotoxicity Is IDO1–Kyn–AhR Dependent and Blocked by IDO1 Inhibitor. Sig. Transduct. Target. Ther. 2020, 5, 96. [Google Scholar] [CrossRef]
- Morgese, M.G.; Bove, M.; Francavilla, M.; Schiavone, S.; Dimonte, S.; Colia, A.L.; Bevilacqua, M.; Trabace, L.; Tucci, P. Sublingual AKBA Exerts Antidepressant Effects in the Aβ-Treated Mouse Model. Biomolecules 2021, 11, 686. [Google Scholar] [CrossRef]
- Morgese, M.G.; Schiavone, S.; Mhillaj, E.; Bove, M.; Tucci, P.; Trabace, L. N-3 PUFA Diet Enrichment Prevents Amyloid Beta-Induced Depressive-like Phenotype. Pharmacol. Res. 2018, 129, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Fertan, E.; Stover, K.R.J.; Brant, M.G.; Stafford, P.M.; Kelly, B.; Diez-Cecilia, E.; Wong, A.A.; Weaver, D.F.; Brown, R.E. Effects of the Novel IDO Inhibitor DWG-1036 on the Behavior of Male and Female 3xTg-AD Mice. Front. Pharmacol. 2019, 10, 1044. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Valentino, R.J.; Lucki, I. Assessing Substrates Underlying the Behavioral Effects of Antidepressants Using the Modified Rat Forced Swimming Test. Neurosci. Biobehav. Rev. 2005, 29, 547–569. [Google Scholar] [CrossRef]
- Mhillaj, E.; Morgese, M.G.; Tucci, P.; Furiano, A.; Luongo, L.; Bove, M.; Maione, S.; Cuomo, V.; Schiavone, S.; Trabace, L. Celecoxib Prevents Cognitive Impairment and Neuroinflammation in Soluble Amyloid β-Treated Rats. Neuroscience 2018, 372, 58–73. [Google Scholar] [CrossRef]
- Morgese, M.G.; Bove, M.; Di Cesare Mannelli, L.; Schiavone, S.; Colia, A.L.; Dimonte, S.; Mhillaj, E.; Sikora, V.; Tucci, P.; Ghelardini, C.; et al. Precision Medicine in Alzheimer’s Disease: Investigating Comorbid Common Biological Substrates in the Rat Model of Amyloid Beta-Induced Toxicity. Front. Pharmacol. 2022, 12, 799561. [Google Scholar] [CrossRef] [PubMed]
- Farzi, A.; Reichmann, F.; Meinitzer, A.; Mayerhofer, R.; Jain, P.; Hassan, A.M.; Fröhlich, E.E.; Wagner, K.; Painsipp, E.; Rinner, B.; et al. Synergistic Effects of NOD1 or NOD2 and TLR4 Activation on Mouse Sickness Behavior in Relation to Immune and Brain Activity Markers. Brain Behav. Immun. 2015, 44, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Holtzclaw, W.D.; Wehage, S.L.; Wade, K.L.; Stephenson, K.K.; Talalay, P. Sulforaphane Bioavailability from Glucoraphanin-Rich Broccoli: Control by Active Endogenous Myrosinase. PLoS ONE 2015, 10, e0140963. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; O’Connor, J.C.; Lawson, M.A.; Kelley, K.W. Inflammation-Associated Depression: From Serotonin to Kynurenine. Psychoneuroendocrinology 2011, 36, 426–436. [Google Scholar] [CrossRef]
- Sun, Y.; Drevets, W.; Turecki, G.; Li, Q.S. The Relationship between Plasma Serotonin and Kynurenine Pathway Metabolite Levels and the Treatment Response to Escitalopram and Desvenlafaxine. Brain Behav. Immun. 2020, 87, 404–412. [Google Scholar] [CrossRef]
- Guillemin, G.J.; Smythe, G.A.; Veas, L.A.; Takikawa, O.; Brew, B.J. A Beta 1-42 Induces Production of Quinolinic Acid by Human Macrophages and Microglia. Neuroreport 2003, 14, 2311–2315. [Google Scholar] [CrossRef]
- Morgese, M.G.; Schiavone, S.; Bove, M.; Colia, A.L.; Dimonte, S.; Tucci, P.; Trabace, L. N-3 PUFA Prevent Oxidative Stress in a Rat Model of Beta-Amyloid-Induced Toxicity. Pharmaceuticals 2021, 14, 339. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Rojo, A.I.; Wells, G.; Hayes, J.D.; Cousin, S.P.; Rumsey, W.L.; Attucks, O.C.; Franklin, S.; Levonen, A.-L.; Kensler, T.W.; et al. Therapeutic Targeting of the NRF2 and KEAP1 Partnership in Chronic Diseases. Nat. Rev. Drug Discov. 2019, 18, 295–317. [Google Scholar] [CrossRef] [PubMed]
- Seminotti, B.; Saso, L. Nuclear Factor Erythroid-Related Factor 2 Signaling in the Neuropathophysiology of Inherited Metabolic Disorders. Front. Cell. Neurosci. 2021, 15, 14. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Lucarini, E.; Micheli, L.; Mosca, I.; Ambrosino, P.; Soldovieri, M.V.; Martelli, A.; Testai, L.; Taglialatela, M.; Calderone, V.; et al. Effects of Natural and Synthetic Isothiocyanate-Based H2S-Releasers against Chemotherapy-Induced Neuropathic Pain: Role of Kv7 Potassium Channels. Neuropharmacology 2017, 121, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Citi, V.; Martelli, A.; Testai, L.; Marino, A.; Breschi, M.C.; Calderone, V. Hydrogen Sulfide Releasing Capacity of Natural Isothiocyanates: Is It a Reliable Explanation for the Multiple Biological Effects of Brassicaceae? Planta Med. 2014, 80, 610–613. [Google Scholar] [CrossRef]
- Lucarini, E.; Micheli, L.; Trallori, E.; Citi, V.; Martelli, A.; Testai, L.; De Nicola, G.R.; Iori, R.; Calderone, V.; Ghelardini, C.; et al. Effect of Glucoraphanin and Sulforaphane against Chemotherapy-Induced Neuropathic Pain: Kv7 Potassium Channels Modulation by H2S Release in vivo: Glucosinolates against Chemotherapy-Induced Neuropathy. Phytother. Res. 2018, 32, 2226–2234. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C.; Papapetropoulos, A. International Union of Basic and Clinical Pharmacology. CII: Pharmacological Modulation of H2S Levels: H2S Donors and H2S Biosynthesis Inhibitors. Pharmacol. Rev. 2017, 69, 497–564. [Google Scholar] [CrossRef]
- Nagpure, B.V.; Bian, J.-S. Brain, Learning, and Memory: Role of H2S in Neurodegenerative Diseases. In Chemistry, Biochemistry and Pharmacology of Hydrogen Sulfide; Moore, P.K., Whiteman, M., Eds.; Handbook of Experimental Pharmacology; Springer International Publishing: Cham, Switzerland, 2015; Volume 230, pp. 193–215. ISBN 978-3-319-18143-1. [Google Scholar]
- Wang, Y.; Wang, S.; Xin, Y.; Zhang, J.; Wang, S.; Yang, Z.; Liu, C. Hydrogen Sulfide Alleviates the Anxiety-like and Depressive-like Behaviors of Type 1 Diabetic Mice via Inhibiting Inflammation and Ferroptosis. Life Sci. 2021, 278, 119551. [Google Scholar] [CrossRef]
- Rose, P.; Moore, P.K.; Zhu, Y.Z. H2S Biosynthesis and Catabolism: New Insights from Molecular Studies. Cell. Mol. Life Sci. 2017, 74, 1391–1412. [Google Scholar] [CrossRef]
- Austgen, J.R.; Hermann, G.E.; Dantzler, H.A.; Rogers, R.C.; Kline, D.D. Hydrogen Sulfide Augments Synaptic Neurotransmission in the Nucleus of the Solitary Tract. J. Neurophysiol. 2011, 106, 1822–1832. [Google Scholar] [CrossRef] [Green Version]
- Morgese, M.G.; Colaianna, M.; Mhillaj, E.; Zotti, M.; Schiavone, S.; D’Antonio, P.; Harkin, A.; Gigliucci, V.; Campolongo, P.; Trezza, V.; et al. Soluble Beta Amyloid Evokes Alteration in Brain Norepinephrine Levels: Role of Nitric Oxide and Interleukin-1. Front. Neurosci. 2015, 9, 428. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Nadrigny, F.; Regen, T.; Martinez-Hernandez, A.; Dumitrescu-Ozimek, L.; Terwel, D.; Jardanhazi-Kurutz, D.; Walter, J.; Kirchhoff, F.; Hanisch, U.-K.; et al. Locus Ceruleus Controls Alzheimer’s Disease Pathology by Modulating Microglial Functions through Norepinephrine. Proc. Natl. Acad. Sci. USA 2010, 107, 6058–6063. [Google Scholar] [CrossRef] [PubMed]
- Jhang, K.A.; Lee, E.O.; Kim, H.-S.; Chong, Y.H. Norepinephrine Provides Short-Term Neuroprotection against Aβ1-42 by Reducing Oxidative Stress Independent of Nrf2 Activation. Neurobiol. Aging 2014, 35, 2465–2473. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Su, M.; Wang, Y.; Li, X.; Zhang, Y.; Du, X.; Zhang, H. Selective Modulation of K+ Channel Kv7.4 Significantly Affects the Excitability of DRN 5-HT Neurons. Front. Cell. Neurosci. 2017, 11, 405. [Google Scholar] [CrossRef] [PubMed]
- Heiss, E.; Herhaus, C.; Klimo, K.; Bartsch, H.; Gerhäuser, C. Nuclear Factor Kappa B Is a Molecular Target for Sulforaphane-Mediated Anti-Inflammatory Mechanisms. J. Biol. Chem. 2001, 276, 32008–32015. [Google Scholar] [CrossRef]
- Zambrano, S.; De Toma, I.; Piffer, A.; Bianchi, M.E.; Agresti, A. NF-ΚB Oscillations Translate into Functionally Related Patterns of Gene Expression. eLife 2016, 5, e09100. [Google Scholar] [CrossRef]
- Shih, V.F.-S.; Kearns, J.D.; Basak, S.; Savinova, O.V.; Ghosh, G.; Hoffmann, A. Kinetic Control of Negative Feedback Regulators of NF-ΚB/RelA Determines Their Pathogen- and Cytokine-Receptor Signaling Specificity. Proc. Natl. Acad. Sci. USA 2009, 106, 9619–9624. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Li, X.-J. Intracellular Degradation of Misfolded Proteins in Polyglutamine Neurodegenerative Diseases. Brain Res. Rev. 2008, 59, 245–252. [Google Scholar] [CrossRef]
- Rubinsztein, D.C. The Roles of Intracellular Protein-Degradation Pathways in Neurodegeneration. Nature 2006, 443, 780–786. [Google Scholar] [CrossRef]
- Kwak, M.-K.; Cho, J.-M.; Huang, B.; Shin, S.; Kensler, T.W. Role of Increased Expression of the Proteasome in the Protective Effects of Sulforaphane against Hydrogen Peroxide-Mediated Cytotoxicity in Murine Neuroblastoma Cells. Free Radic. Biol. Med. 2007, 43, 809–817. [Google Scholar] [CrossRef]
- Gan, N.; Wu, Y.-C.; Brunet, M.; Garrido, C.; Chung, F.-L.; Dai, C.; Mi, L. Sulforaphane Activates Heat Shock Response and Enhances Proteasome Activity through Up-Regulation of Hsp27. J. Biol. Chem. 2010, 285, 35528–35536. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Elsevier: Amsterdam, The Netherlands; Academic Press: London, UK, 2005; ISBN 978-0-08-047412-0. [Google Scholar]
- De Nicola, G.R.; Rollin, P.; Mazzon, E.; Iori, R. Novel Gram-Scale Production of Enantiopure R-Sulforaphane from Tuscan Black Kale Seeds. Molecules 2014, 19, 6975–6986. [Google Scholar] [CrossRef] [PubMed]
- Yankelevitch-Yahav, R.; Franko, M.; Huly, A.; Doron, R. The Forced Swim Test as a Model of Depressive-like Behavior. JoVE 2015, 97, e52587. [Google Scholar] [CrossRef] [PubMed]
- Morgese, M.G.; Schiavone, S.; Maffione, A.B.; Tucci, P.; Trabace, L. Depressive-like Phenotype Evoked by Lifelong Nutritional Omega-3 Deficiency in Female Rats: Crosstalk among Kynurenine, Toll-like Receptors and Amyloid Beta Oligomers. Brain Behav. Immun. 2020, 87, 444–454. [Google Scholar] [CrossRef]
- Kim, J. Pre-Clinical Neuroprotective Evidences and Plausible Mechanisms of Sulforaphane in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 2929. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tucci, P.; Bove, M.; Sikora, V.; Dimonte, S.; Morgese, M.G.; Schiavone, S.; Di Cesare Mannelli, L.; Ghelardini, C.; Trabace, L. Glucoraphanin Triggers Rapid Antidepressant Responses in a Rat Model of Beta Amyloid-Induced Depressive-like Behaviour. Pharmaceuticals 2022, 15, 1054. https://doi.org/10.3390/ph15091054
Tucci P, Bove M, Sikora V, Dimonte S, Morgese MG, Schiavone S, Di Cesare Mannelli L, Ghelardini C, Trabace L. Glucoraphanin Triggers Rapid Antidepressant Responses in a Rat Model of Beta Amyloid-Induced Depressive-like Behaviour. Pharmaceuticals. 2022; 15(9):1054. https://doi.org/10.3390/ph15091054
Chicago/Turabian StyleTucci, Paolo, Maria Bove, Vladyslav Sikora, Stefania Dimonte, Maria Grazia Morgese, Stefania Schiavone, Lorenzo Di Cesare Mannelli, Carla Ghelardini, and Luigia Trabace. 2022. "Glucoraphanin Triggers Rapid Antidepressant Responses in a Rat Model of Beta Amyloid-Induced Depressive-like Behaviour" Pharmaceuticals 15, no. 9: 1054. https://doi.org/10.3390/ph15091054
APA StyleTucci, P., Bove, M., Sikora, V., Dimonte, S., Morgese, M. G., Schiavone, S., Di Cesare Mannelli, L., Ghelardini, C., & Trabace, L. (2022). Glucoraphanin Triggers Rapid Antidepressant Responses in a Rat Model of Beta Amyloid-Induced Depressive-like Behaviour. Pharmaceuticals, 15(9), 1054. https://doi.org/10.3390/ph15091054