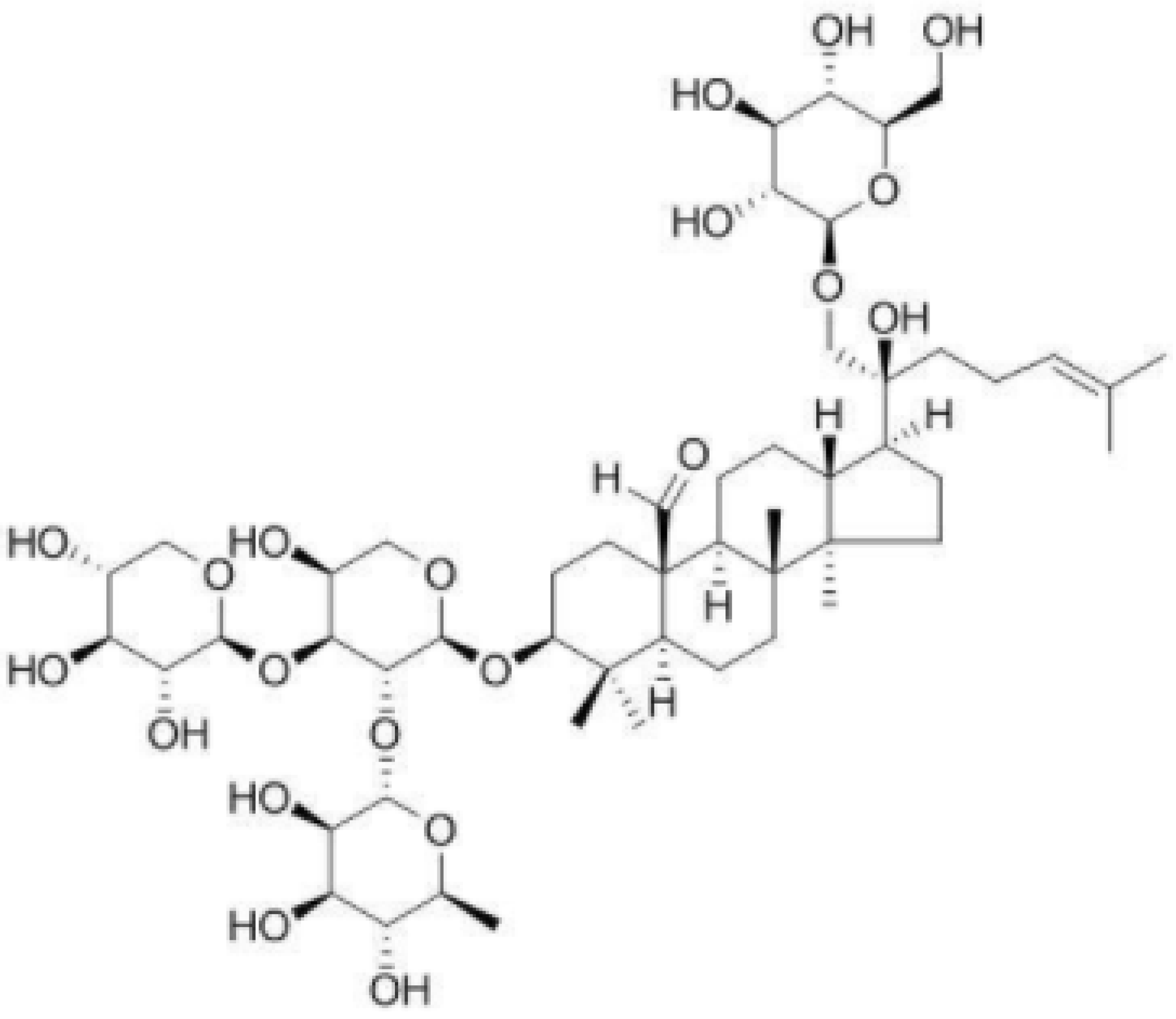
Gypenoside XLIX Ameliorate High-Fat Diet-Induced Atherosclerosis via Regulating Intestinal Microbiota, Alleviating Inflammatory Response and Restraining Oxidative Stress in ApoE−/− Mice
Abstract
:1. Introduction
2. Results
2.1. Body Weight, Food Intake, and Organ Weight
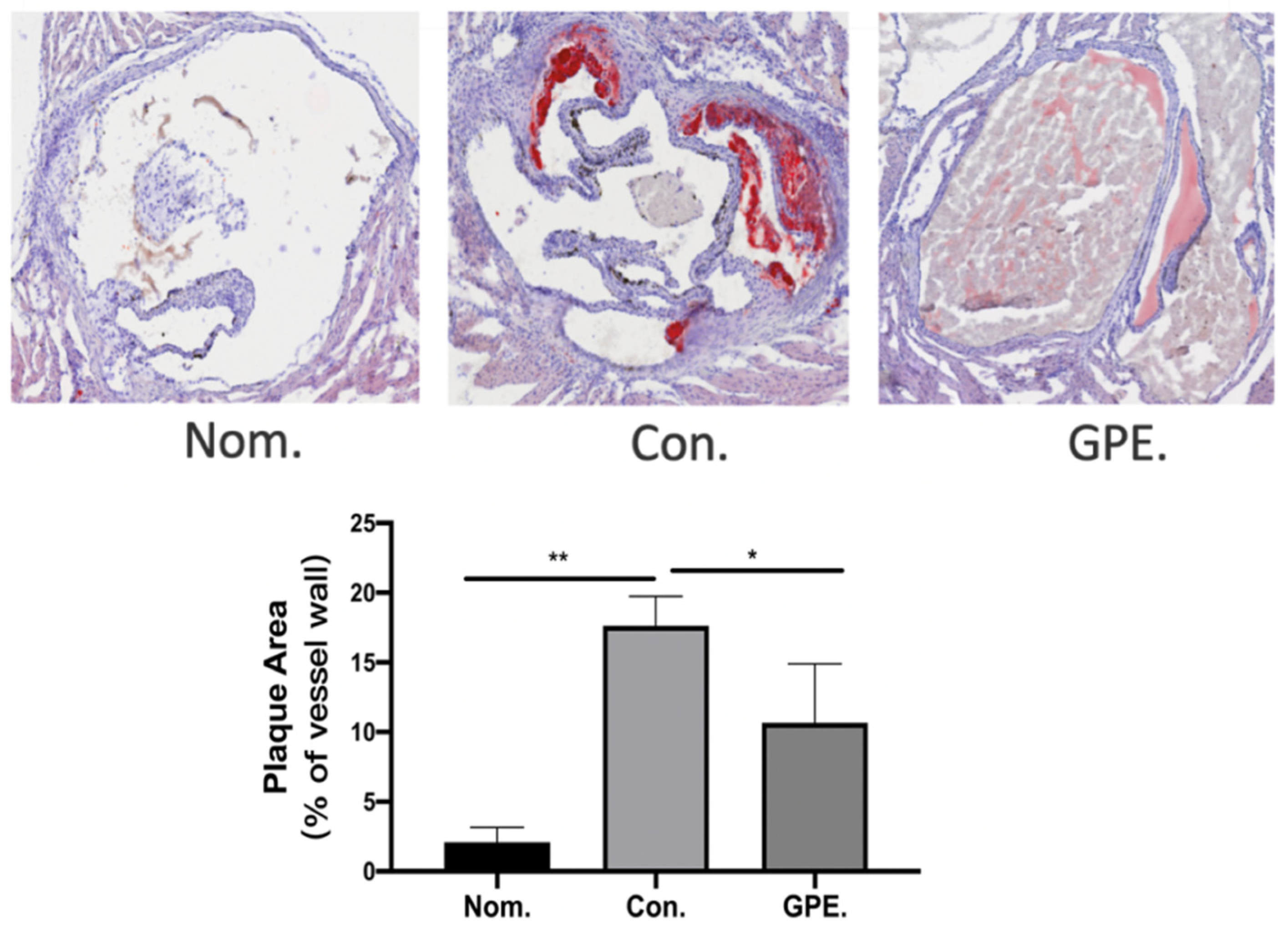
2.2. GPE Alleviated HFCD-Induced Atherosclerotic Lesions
2.3. GPE Had Lipid-Lowering Effects
2.4. GPE Inhibited TMA/TMAO Metabolism
2.5. Effect of GPE Treatment on Gut Microbiota in ApoE−/− Mice
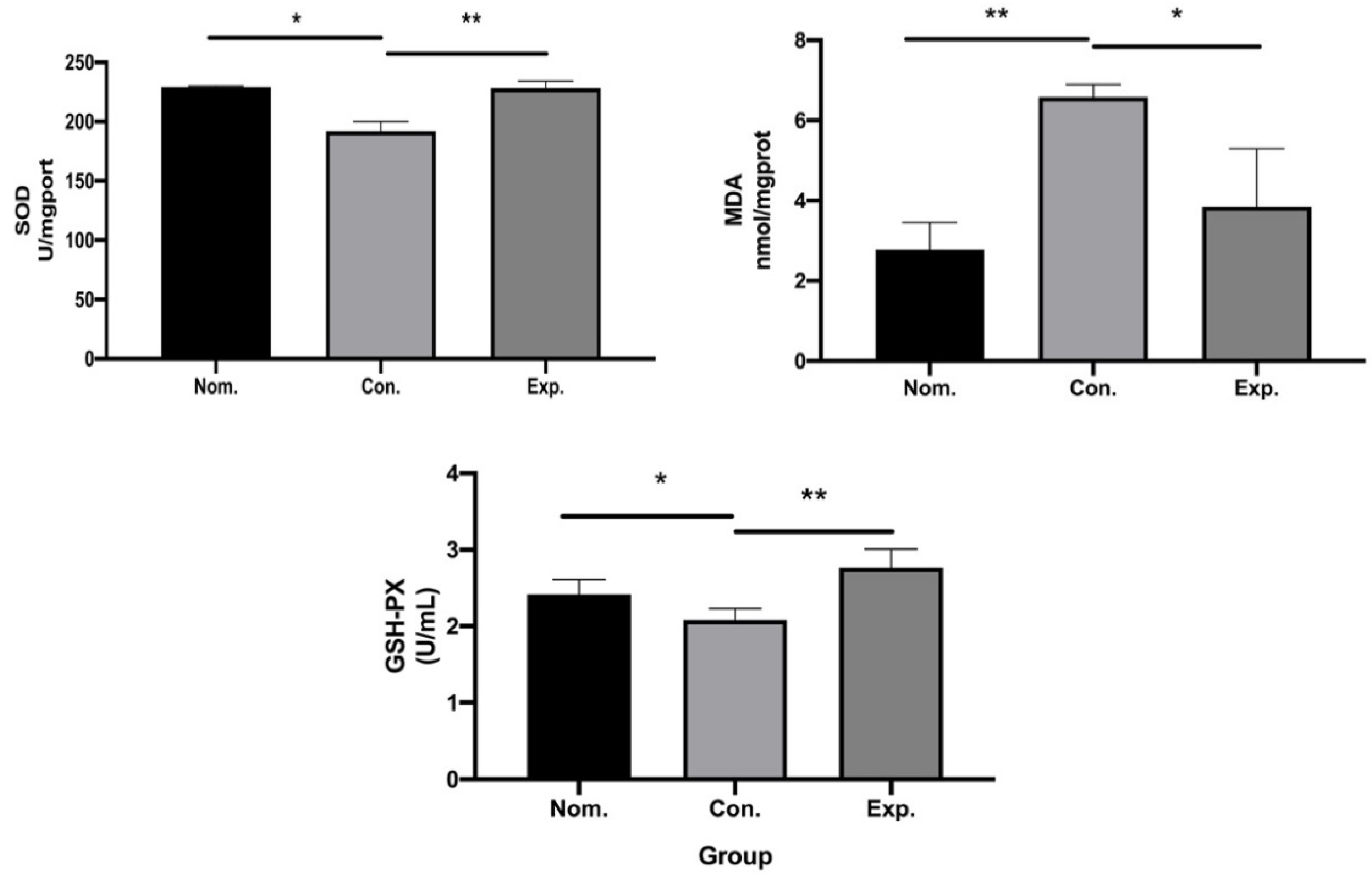
2.6. In Vivo Antioxidant Capacity
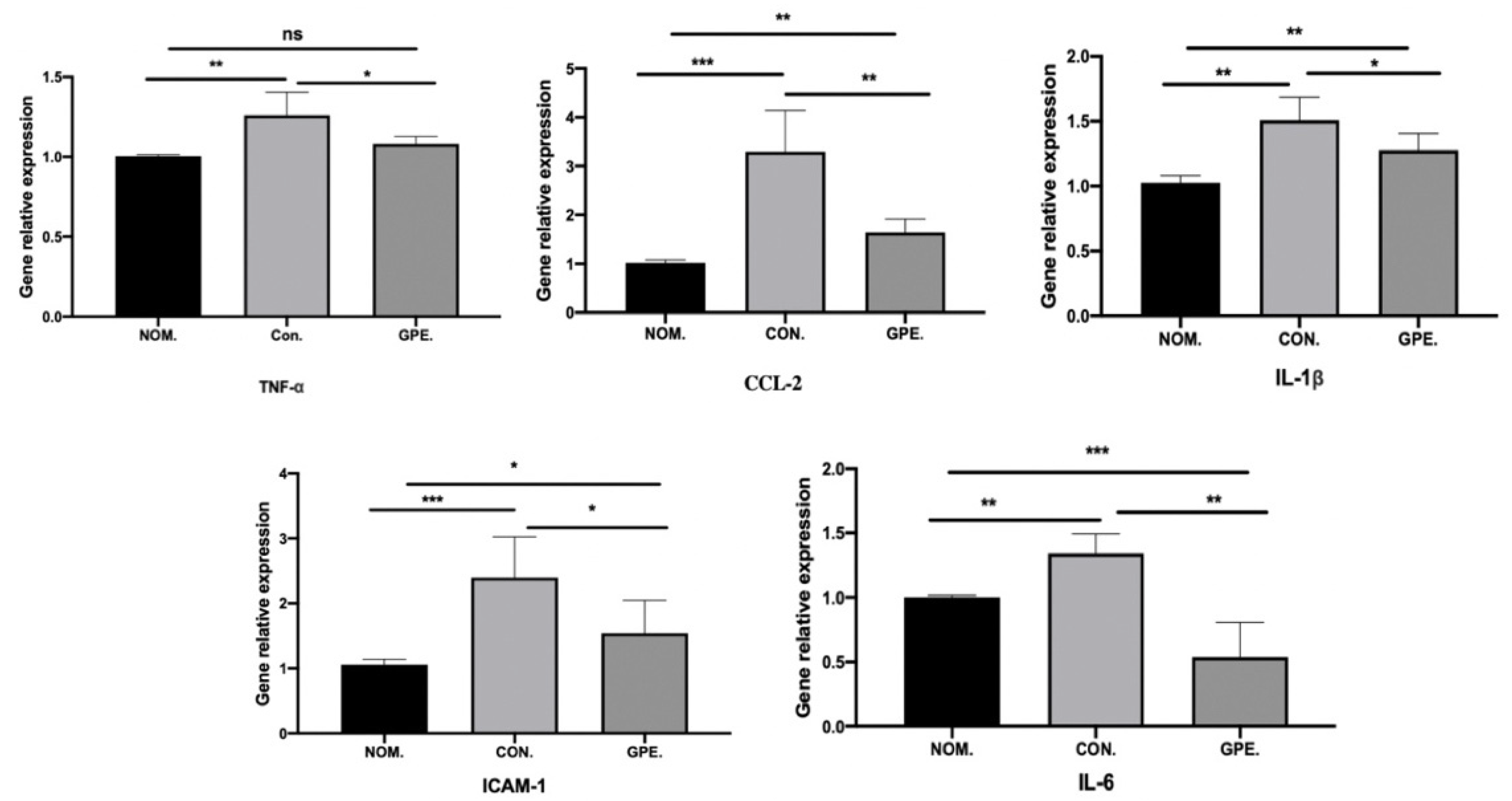
2.7. GPE Supplementation Reduces Inflammation in the Liver
2.8. Intestinal SCFA Metabolites
2.9. Significant Intestinal Metabolic Changes during GPE Intervention
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Ethics Statement
4.3. Animal and Choline Treatment
4.4. Biochemical Analysis
4.5. Analysis of Atherosclerotic Lesions
4.6. Detection of FMO3 and TMAO in Mice
4.7. The Effect of GPE on Gut Microbiota
4.8. In Vivo Antioxidant Capacity of GPE
4.9. RNA Isolation and Real-Time PCR
4.10. SCFA Analysis by HSGC/MS
4.11. GC-TOF-MS-Based Metabolomics Analyses
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sandoval-Gallegos, E.M.; Ramírez-Moreno, E.; Lucio, J.D.; Arias-Rico, J.; Cruz-Cansino, N.; Ortiz, M.I.; CariO-Cortés, R. In Vitro Bioaccessibility and Effect of Mangifera indica (Ataulfo) Leaf Extract on Induced Dyslipidemia. J. Med. Food 2018, 21, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The Impact of the Gut Microbiota on Human Health: An Integrative View. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Albany, C.J.; Trevelin, S.C.; Giganti, G.; Lombardi, G.; Scottà, C. Getting to the Heart of the Matter: The Role of Regulatory T-Cells (Tregs) in Cardiovascular Disease (CVD) and Atherosclerosis. Front. Immunol. 2019, 10, 2795. [Google Scholar] [CrossRef]
- Aziz, Q.; Doré, J.; Emmanuel, A.; Guarner, F.; Quigley, E.M.M. Gut microbiota and gastrointestinal health: Current concepts and future directions. Neurogastroenterol. Motil. 2013, 25, 4–15. [Google Scholar] [CrossRef]
- Heintz-Buschart, A.; Wilmes, P. Human Gut Microbiome: Function Matters. Trends Microbiol. 2018, 26, 563–574. [Google Scholar] [CrossRef]
- Kasahara, K.; Krautkramer, K.A.; Org, E.; Romano, K.A.; Kerby, R.L.; Vivas, E.I.; Mehrabian, M.; Denu, J.M.; Bäckhed, F.; Lusis, A.J. Interactions Between Roseburia Intestinalis and Diet Modulate Atherogenesis in a Murine Model. Nat. Microbiol. 2018, 3, 1461–1471. [Google Scholar] [CrossRef]
- Loscalzo, J. Gut Microbiota, the Genome, and Diet in Atherogenesis. N. Engl. J. Med. 2013, 368, 1647–1649. [Google Scholar] [CrossRef]
- Lee, H.-S.; Kim, M.-J. Selective Responses of Three Ginkgo biloba Leaf-Derived Constituents on Human Intestinal Bacteria. J. Agric. Food Chem. 2002, 50, 1840–1844. [Google Scholar] [CrossRef]
- Su, C.; Li, N.; Ren, R.; Wang, Y.; Su, X.; Lu, F.; Zong, R.; Yang, L.; Ma, X. Progress in the Medicinal Value, Bioactive Compounds, and Pharmacological Activities of Gynostemma Pentaphyllum. Molecules 2021, 26, 6249. [Google Scholar] [CrossRef]
- Li, K.; Ma, C.; Li, H.; Dev, S.; He, J.; Qu, X. Medicinal Value and Potential Therapeutic Mechanisms of Gynostemma pentaphyllum (Thunb.) Makino and Its Derivatives: An Overview. Curr. Top. Med. Chem. 2020, 19, 2855–2867. [Google Scholar] [CrossRef] [PubMed]
- Davidson, W.S.; Thompson, T. The Structure of Apolipoprotein A-I in High Density Lipoproteins. J. Biol. Chem. 2007, 282, 22249–22253. [Google Scholar] [CrossRef]
- Ohashi, R.; Mu, H.; Yao, Q.; Chen, C. Cellular and Molecular Mechanisms of Atherosclerosis with Mouse Models. Trends Cardiovasc. Med. 2004, 14, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I. Macrophage Death and Defective Inflammation Resolution in Atherosclerosis. Nat. Rev. Immunol. 2009, 10, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Mulvihill, E.E.; Assini, J.M.; Sutherland, B.G.; DiMattia, A.S.; Khami, M.; Koppes, J.B.; Sawyez, C.G.; Whitman, S.C.; Huff, M.W. Naringenin Decreases Progression of Atherosclerosis by Improving Dyslipidemia in High-Fat–Fed Low-Density Lipoprotein Receptor–Null Mice. Arter. Thromb. Vasc. Biol. 2010, 30, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Chanet, A.; Milenkovic, D.; Deval, C.; Potier, M.; Constans, J.; Mazur, A.; Bennetau-Pelissero, C.; Morand, C.; Bérard, A.M. Naringin, the Major Grapefruit Flavonoid, Specifically Affects Atherosclerosis Development in Diet-Induced Hypercholesterolemia in Mice. J. Nutr. Biochem. 2011, 23, 469–477. [Google Scholar] [CrossRef]
- Pettersson, K.; Björk, H. Inhibition of Platelet Accumulation By Β1-Adrenoceptor Blockade in the Thoracic Aorta of Rabbits Subjected to Experimental Sympathetic Activation. Cardiovasc. Drugs Ther. 1992, 6, 505–511. [Google Scholar] [CrossRef]
- Goc, Z.; Szaroma, W.; Kapusta, E.; Dziubek, K. Protective Effects of Melatonin on the Activity of SOD, CAT, GSH-Px and GSH Content in Organs of Mice after Administration of SNP. Chin. J. Physiol. 2017, 60, 1–10. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Yang, C.; Hou, M.; Wang, F.; Wang, G.; Wang, S. Effect of Tectorigenin on MCP-1 and ICAM-1 mRNA Expression in Injured Vascular Endothelial Cells. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Med. 2010, 35, 2001–2003. [Google Scholar]
- Libby, P.; Okamoto, Y.; Rocha, V.Z.; Folco, E. Inflammation in Atherosclerosis: Transition from Theory to Practice. Circ. J. 2010, 74, 213–220. [Google Scholar] [CrossRef]
- Di Pietro, M.; Filardo, S.; De Santis, F.; Sessa, R. Chlamydia Pneumoniae Infection in Atherosclerotic Lesion Development Through Oxidative Stress: A Brief Overview. Int. J. Mol. Sci. 2013, 14, 15105–15120. [Google Scholar] [CrossRef] [PubMed]
- Ott, S.J.; El Mokhtari, N.E.; Musfeldt, M.; Hellmig, S.; Freitag, S.; Rehman, A.; Kühbacher, T.; Nikolaus, S.; Namsolleck, P.; Blaut, M.; et al. Detection of Diverse Bacterial Signatures in Atherosclerotic Lesions of Patients with Coronary Heart Disease. Circulation 2006, 113, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Jie, Z.; Xia, H.; Zhong, S.-L.; Feng, Q.; Li, S.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Zhao, H.; et al. The Gut Microbiome in Atherosclerotic Cardiovascular Disease. Nat. Commun. 2017, 8, 845. [Google Scholar] [CrossRef]
- Karlsson, F.; Fåk, F.; Nookaew, I.; Tremaroli, V.; Fagerberg, B.; Petranovic, D.; Bäckhed, F.; Nielsen, J. Symptomatic Atherosclerosis is Associated with an Altered Gut Metagenome. Nat. Commun. 2012, 3, 1245. [Google Scholar] [CrossRef]
- Hui, D.Y. Intestinal Phospholipid and Lysophospholipid Metabolism in Cardiometabolic Disease. Curr. Opin. Lipidol. 2016, 27, 507–512. [Google Scholar] [CrossRef]
- Jin, L.; Shi, X.; Yang, J.; Zhao, Y.; Xue, L.; Xu, L.; Cai, J. Gut Microbes in Cardiovascular Diseases and Their Potential Therapeutic Applications. Protein Cell 2021, 12, 346–359. [Google Scholar] [CrossRef]
- Anselmi, G.; Gagliardi, L.; Egidi, G.; Leone, S.; Gasbarrini, A.; Miggiano, G.A.D.; Galiuto, L. Gut Microbiota and Cardiovascular Diseases: A Critical Review. Cardiol. Rev. 2021, 29, 195–204. [Google Scholar] [CrossRef]
- Mullen, A.R.; Wheaton, W.W.; Jin, E.S.; Chen, P.-H.; Sullivan, L.B.; Cheng, T.; Yang, Y.; Linehan, W.M.; Chandel, N.S.; DeBerardinis, R.J. Reductive Carboxylation Supports Growth in Tumour Cells with Defective Mitochondria. Nature 2011, 481, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Heyse, M.; Schneider, C.; Monostori, P.; Schwarz, K.V.; Hauke, J.; Drüschler, K.; Berberich, A.; Zorn, M.; Ringleb, P.A.; Okun, J.G.; et al. Trimethylamine-N-Oxide Levels Are Similar in Asymptomatic vs. Symptomatic Cerebrovascular Atherosclerosis. Front. Neurol. 2021, 12, 617944. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.E.; Taesuwan, S.; Malysheva, O.V.; Bender, E.; Tulchinsky, N.F.; Yan, J.; Sutter, J.L.; Caudill, M.A. Trimethylamine-N-Oxide (TMAO) Response to Animal Source Foods Varies Among Healthy Young Men and Is Influenced by Their Gut Microbiota Composition: A Randomized Controlled Trial. Mol. Nutr. Food Res. 2017, 61, 1600324. [Google Scholar] [CrossRef]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A Database of Systems Pharmacology for Drug Discovery from Herbal Medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic Biomarker Discovery and Explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Hao, R.; Du, X.; Wang, Q.; Deng, Y.; Sun, R.; Zheng, Z. GC–TOF/MS-Based Metabolomics Studies on the Effect of Protein Sources in Formulated Diet for Pearl Oyster Pinctada Fucata Martensii. Aquaculture 2018, 486, 139–147. [Google Scholar] [CrossRef]
- Wen, B.; Jin, S.R.; Chen, Z.Z.; Gao, J.Z. Physiological Responses to Cold Stress in the Gills of Discus Fish (Symphysodon Aequifasciatus) Revealed by Conventional Biochemical Assays and Gc-Tof-Ms Metabolomics. Sci. Total Environ. 2018, 640, 1372–1381. [Google Scholar] [CrossRef] [PubMed]






| CON. | GPE. | p Value | |
|---|---|---|---|
| Shannon | 3.21 ± 0.02 | 4.39 ± 0.16 | 0.01 |
| Simpson | 0.12 ± 0.03 | 0.03 ± 0.01 | 0.04 |
| Chao | 204.30 ± 7.72 | 451.03 ± 10.13 | 0.01 |
| Coverage | 0.99 ± 0.00 | 0.99 ± 0.00 |
| Metabolite | Fold Change |
|---|---|
| D-Cellobiose | 22.449 |
| Tyramine | 13.37 |
| Melibiose | 13.235 |
| Dihydro-cholesterol | 12.633 |
| 5-Methoxytryptamine | 10.771 |
| 4-O-Hexopyranosylhex-2-ulofuranose | 9.1412 |
| D-Galacturonic cid | 8.8722 |
| Hydroxyproline | 7.9615 |
| 3-Methyl-2-oxobutanoic acid | 5.8605 |
| Hexose | 5.4677 |
| 3-Methyl-2-oxovalerate | 5.4058 |
| Oxalacetic acid | 5.0682 |
| L-Phenylalanine | 3.7558 |
| 3-Methyl-2-oxopentanoate | 3.7309 |
| 4-Methyl-2-oxopentanoate | 3.6265 |
| Malbit | 3.5755 |
| Deoxyadenosine | 3.5743 |
| Galactosyl-glycerol | 3.5642 |
| L-Gulcono-1,4-lactone | 3.2961 |
| N-Methyl-DL-alanine | 3.0154 |
| D-Lyxose | 2.9811 |
| L-Iditol | 2.9403 |
| 2-O-Methyl-D-mannopyranosa | 2.5005 |
| Pentose | 2.4453 |
| Deoxyinosine | 2.323 |
| Pantothenic acid | 2.1301 |
| Pseudo-uridine | 2.0331 |
| Thymine | 1.9163 |
| D-Tagatose | 1.8483 |
| D-Glucose | 1.7846 |
| 2,5,7,8-Tetramethyl-2-(5,9,13-trimethyltetradecyl)-3,4-dihydro-2h-chromen-6-ol | 1.7703 |
| Linoleate | 1.7462 |
| Aldohexose | 1.5223 |
| Aminomalonate | 1.2711 |
| Di-isopropanolamine | 1.268 |
| Glycerol | 0.6751 |
| Hexa-decanoic acid | 0.6641 |
| Propane-1,3-diol | 0.66263 |
| Lactic acid | 0.66201 |
| gamma-Aminobutyric acid | 0.59821 |
| L-Alanyl-L-alanine | 0.53075 |
| Glycolic acid | 0.52183 |
| Octadecanoic acid | 0.51757 |
| Malic acid | 0.46053 |
| Chimyl alcohol | 0.41685 |
| 4-Hydroxylphenyllactic acid | 0.38248 |
| Cholestanol | 0.3759 |
| Batyl alcohol | 0.37026 |
| 2,3-Bisphospho-glyceric acid | 0.29243 |
| Creatine | 0.28688 |
| 2,3-Diaminopropionic acid | 0.26765 |
| 3-Epicholic acid | 0.19065 |
| Icosanoic acid | 0.18807 |
| 11beta-Hydroxyandrostenedione | 0.15355 |
| 4-Hydroxyphenylacetic acid | 0.14495 |
| 1-Eicosanol | 0.13899 |
| Butenedioic acid | 0.11864 |
| 2-Deoxy-D-galactose | 0.11572 |
| L-Aspartate | 0.097594 |
| Octadecanol | 0.070298 |
| 3,4-Dihydroxyphenylacetic acid | 0.028664 |
| Cholan-24-oic acid, 3,7,12-trihydroxy-, (3alpha,5beta,7alpha,12alpha)- | 0.0058382 |
| Cholic acid | 0.0040922 |
| Gene | Primer Sequence (5′-3′) |
|---|---|
| IL-1β | FOR: GGTCAAAGGTTTGGAAGCAG REV: TGTGAAATGCCACCTTTTGA |
| IL-6 | FOR: AGGGTCTGGGCCATAGAACT REV: CCACCACGCTCTTCTGTCTAC |
| TNF-α | FOR: AGGGTCTGGGCCATAGAACT REV: CCACCACGCTCTTCTGTCTAC |
| ICAM-1 | FOR: AACAGTTCACCTGCACGGAC REV: GTCACCGTTGTGATCCCTG |
| Ccl2 | FOR: ATTGGGATCATCTTGCTGGT REV: CCTGCTGTTCACAGTTGCC |
| β-actin | FOR: GCTGTGCTATGTTGCTCTAG REV: CGCTCGTTGCCAATAGTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, M.; Heng, X.; Jin, J.; Chu, W. Gypenoside XLIX Ameliorate High-Fat Diet-Induced Atherosclerosis via Regulating Intestinal Microbiota, Alleviating Inflammatory Response and Restraining Oxidative Stress in ApoE−/− Mice. Pharmaceuticals 2022, 15, 1056. https://doi.org/10.3390/ph15091056
Gao M, Heng X, Jin J, Chu W. Gypenoside XLIX Ameliorate High-Fat Diet-Induced Atherosclerosis via Regulating Intestinal Microbiota, Alleviating Inflammatory Response and Restraining Oxidative Stress in ApoE−/− Mice. Pharmaceuticals. 2022; 15(9):1056. https://doi.org/10.3390/ph15091056
Chicago/Turabian StyleGao, Ming, Xing Heng, Jing Jin, and Weihua Chu. 2022. "Gypenoside XLIX Ameliorate High-Fat Diet-Induced Atherosclerosis via Regulating Intestinal Microbiota, Alleviating Inflammatory Response and Restraining Oxidative Stress in ApoE−/− Mice" Pharmaceuticals 15, no. 9: 1056. https://doi.org/10.3390/ph15091056
APA StyleGao, M., Heng, X., Jin, J., & Chu, W. (2022). Gypenoside XLIX Ameliorate High-Fat Diet-Induced Atherosclerosis via Regulating Intestinal Microbiota, Alleviating Inflammatory Response and Restraining Oxidative Stress in ApoE−/− Mice. Pharmaceuticals, 15(9), 1056. https://doi.org/10.3390/ph15091056






