Molecular Targets of Pinocembrin Underlying Its Regenerative Activities in Human Keratinocytes
Abstract
:1. Introduction
2. Results
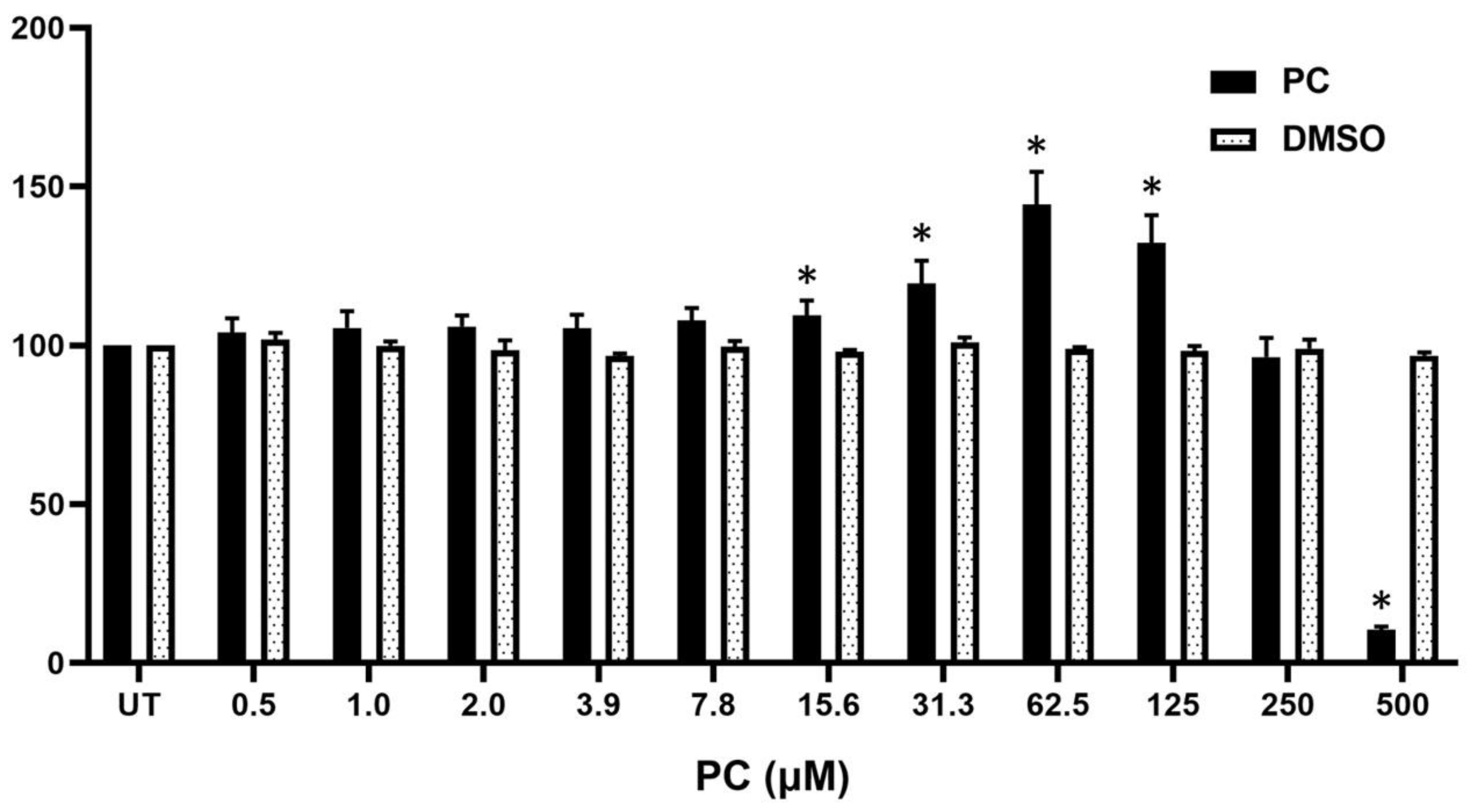
2.1. Pinocembrin Affects the Viability of Human Keratinocytes
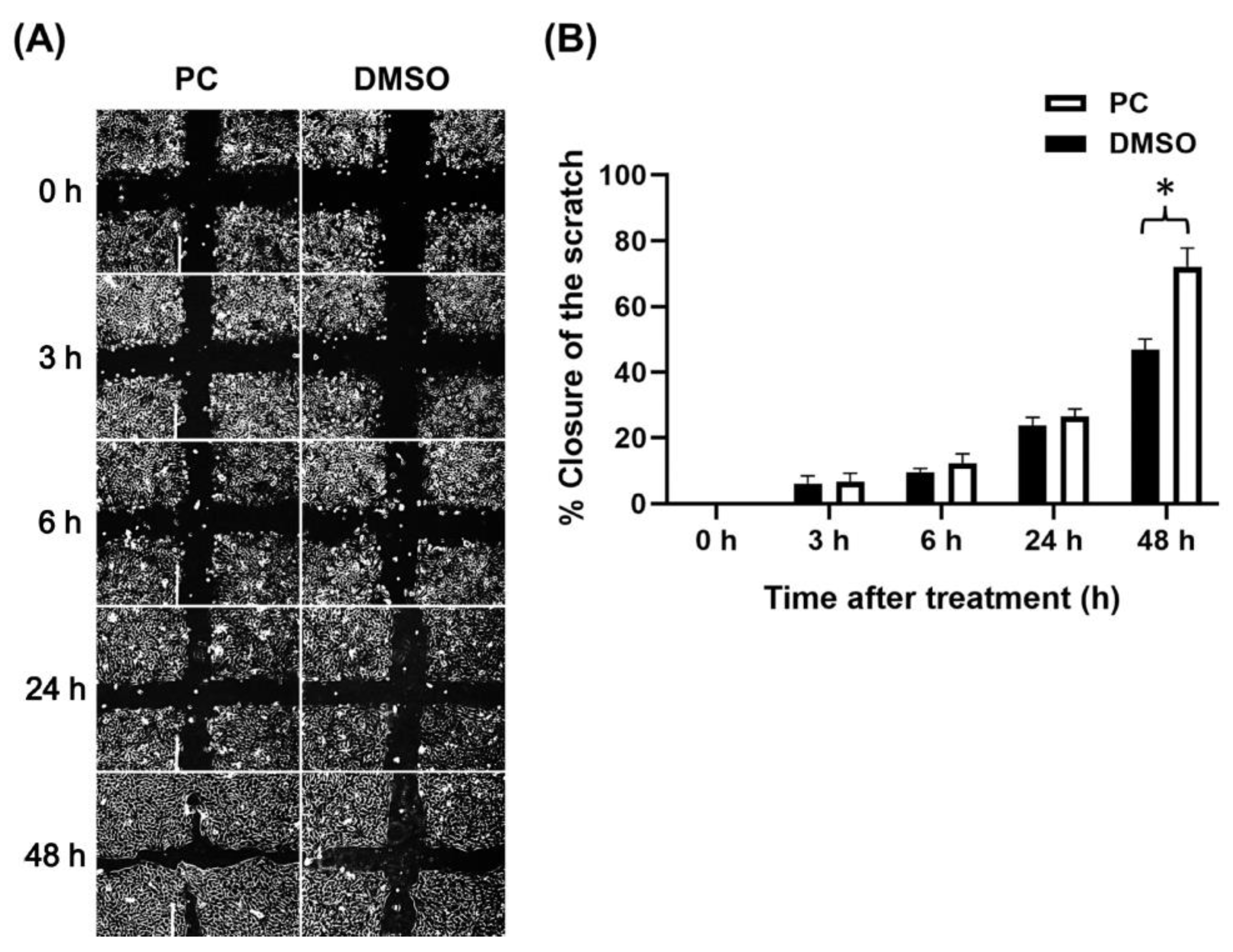
2.2. Effects of Pinocembrin on Accelerating Scratch Wound Closure of Human Keratinocyte Monolayer
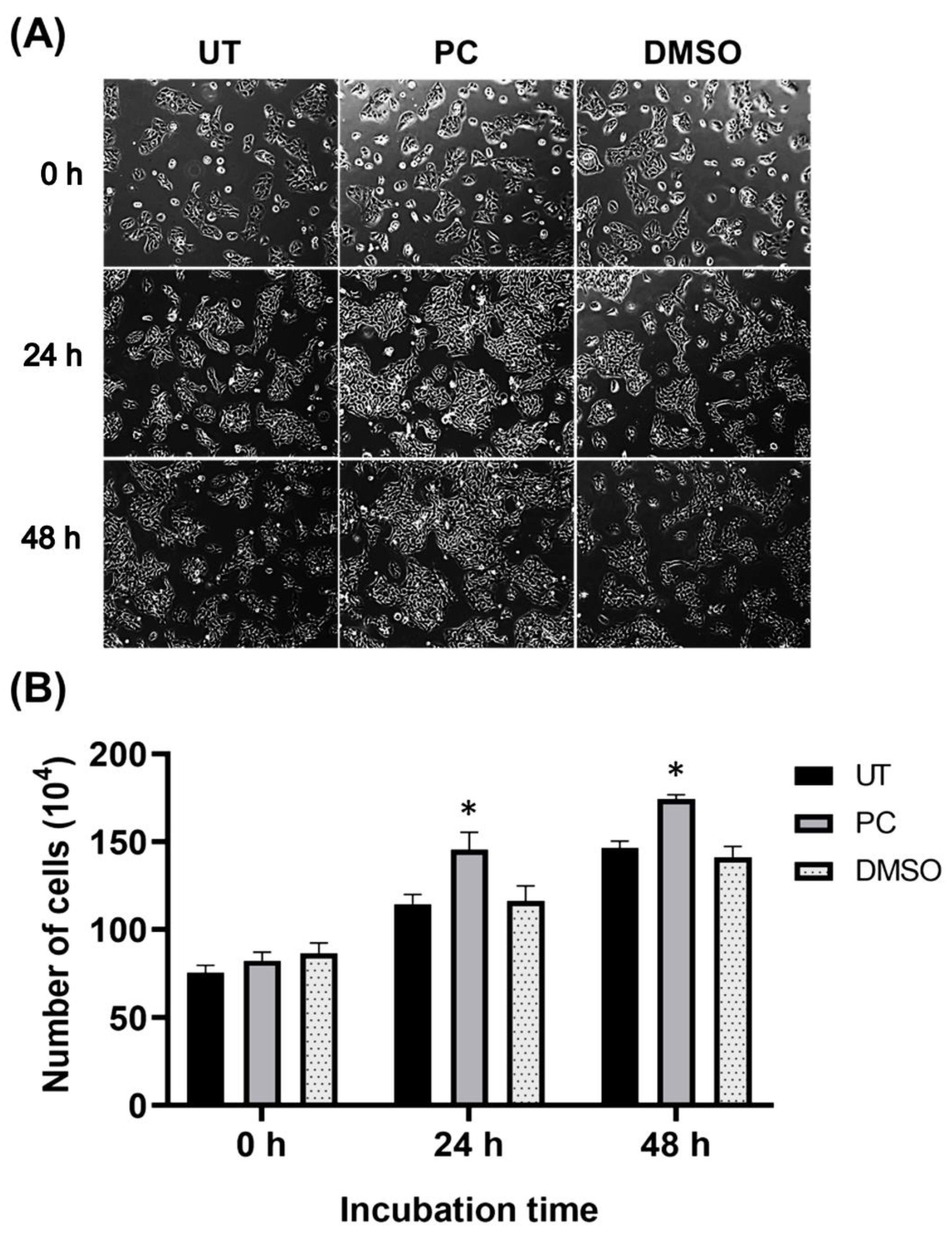
2.3. Pinocembrin Induces Proliferation and Increases the Size of HaCaT Colonies
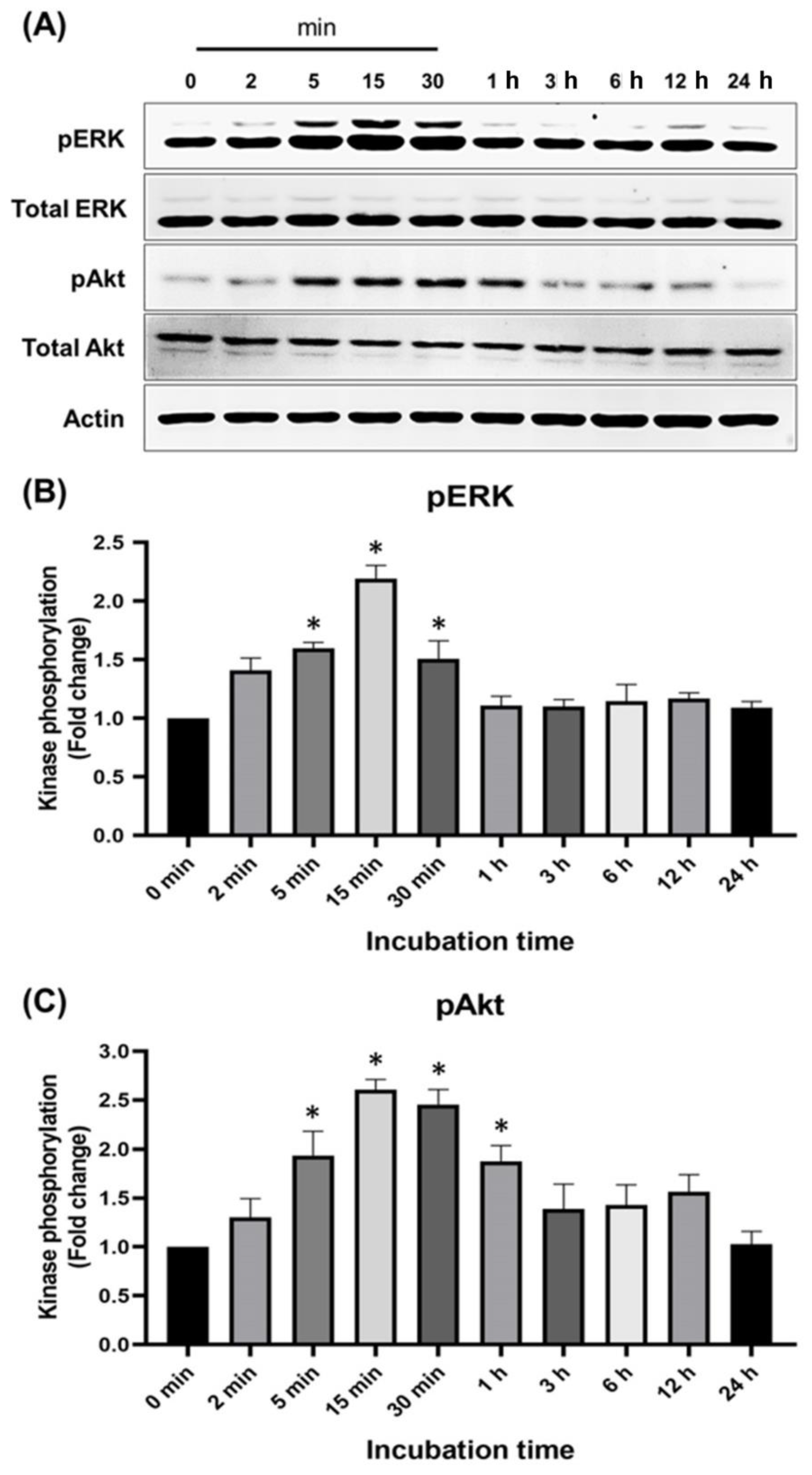
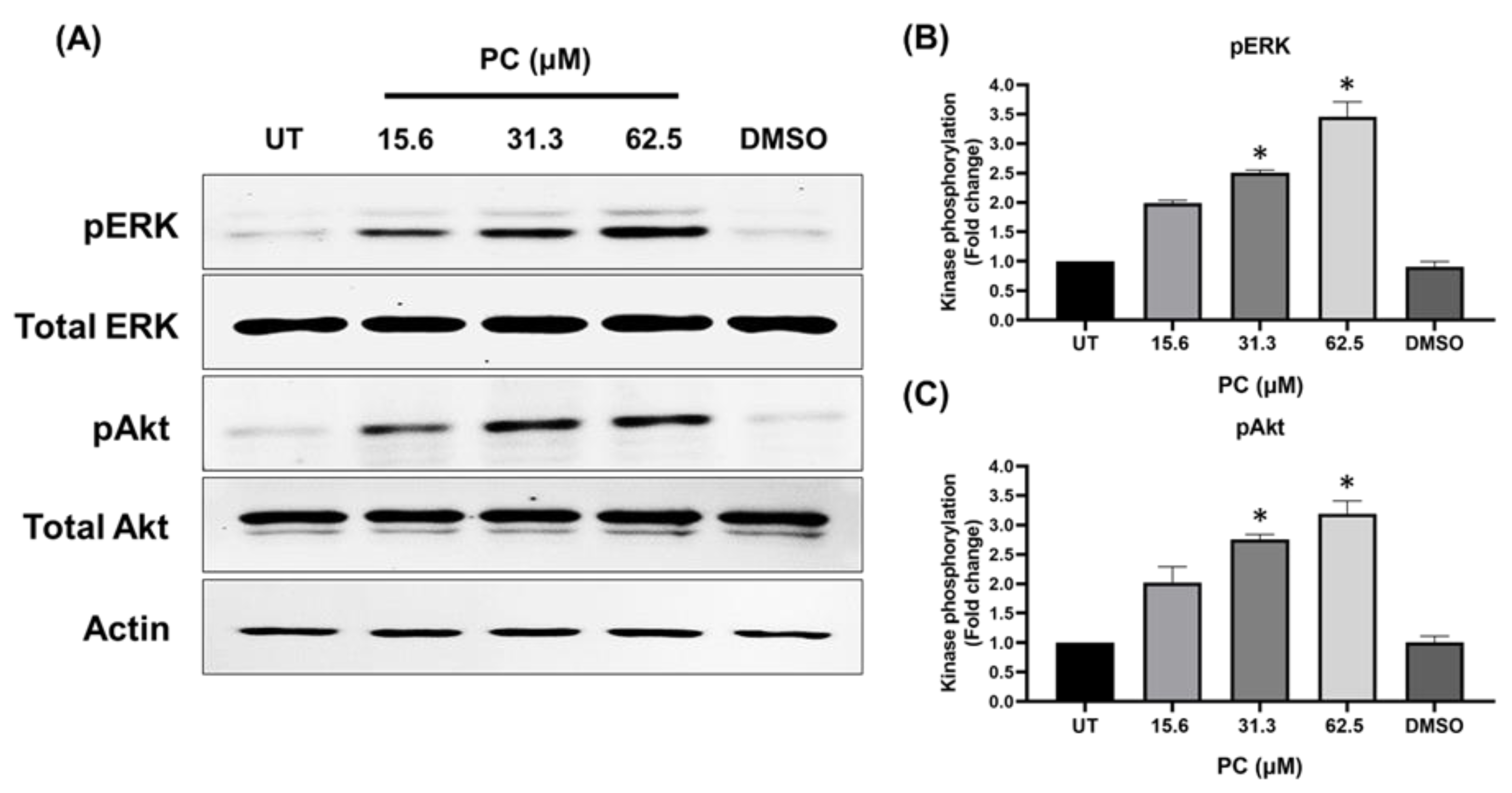
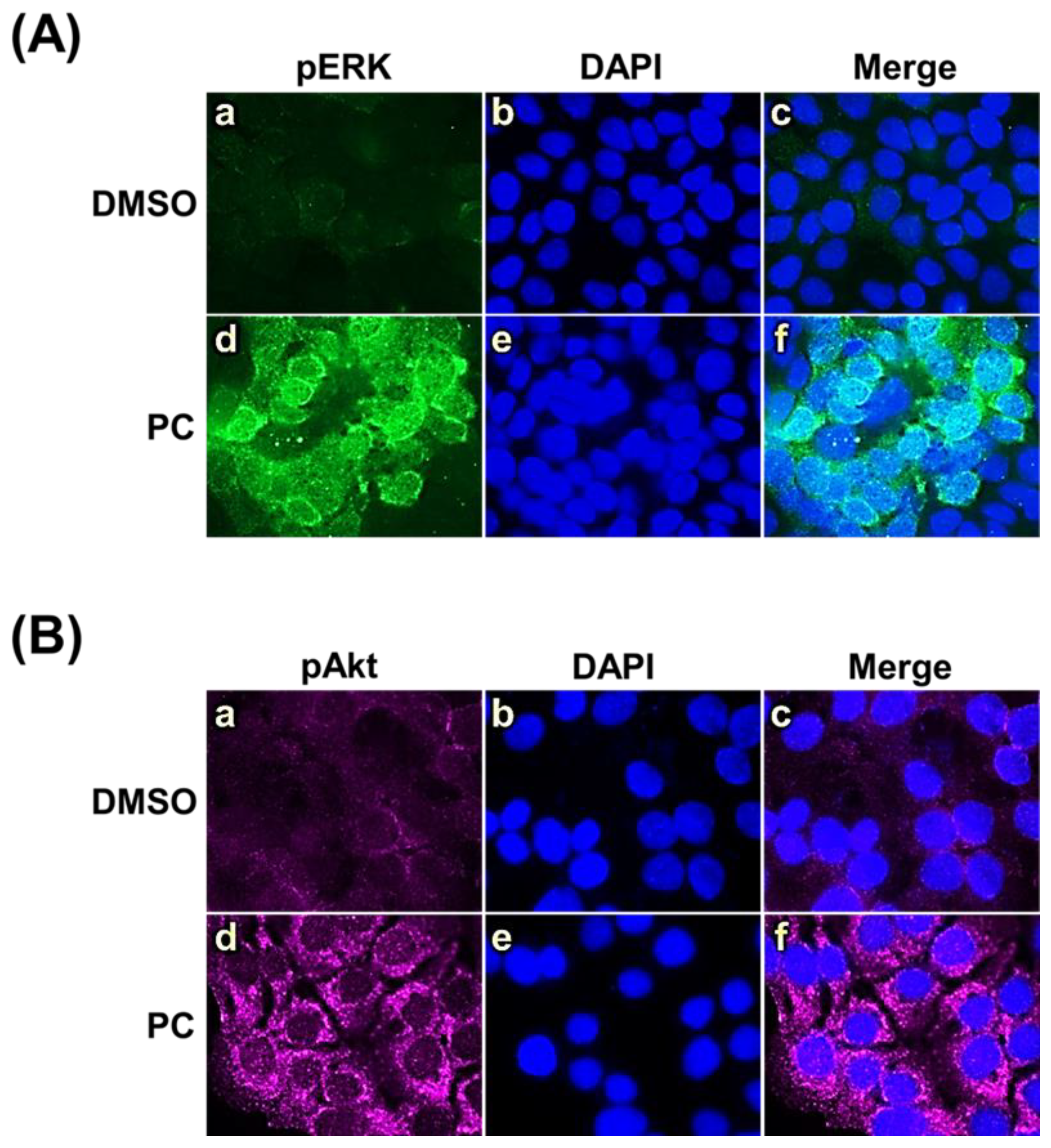
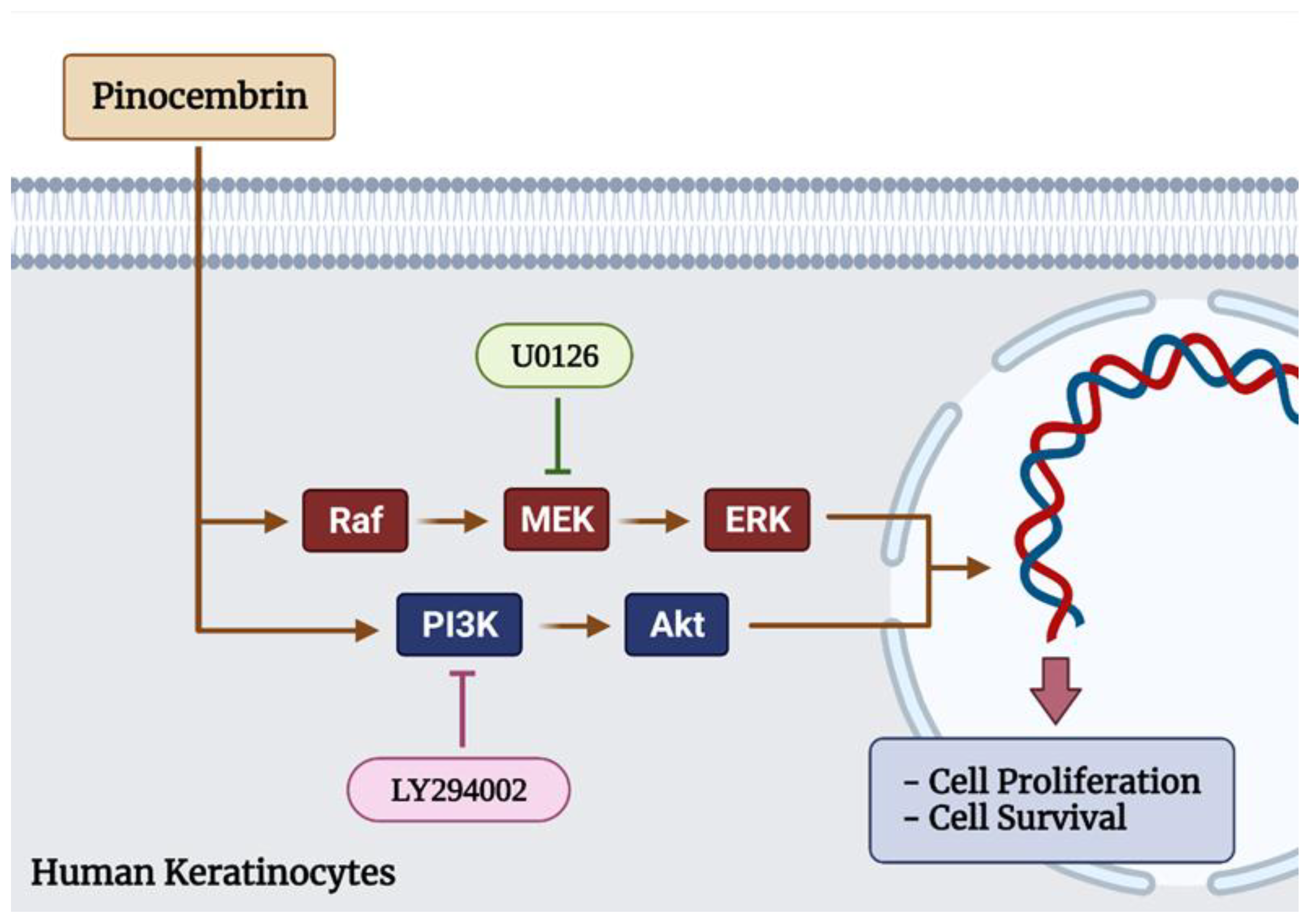
2.4. Effects of Pinocembrin on the Signaling Pathways Regulating Keratinocyte Proliferation
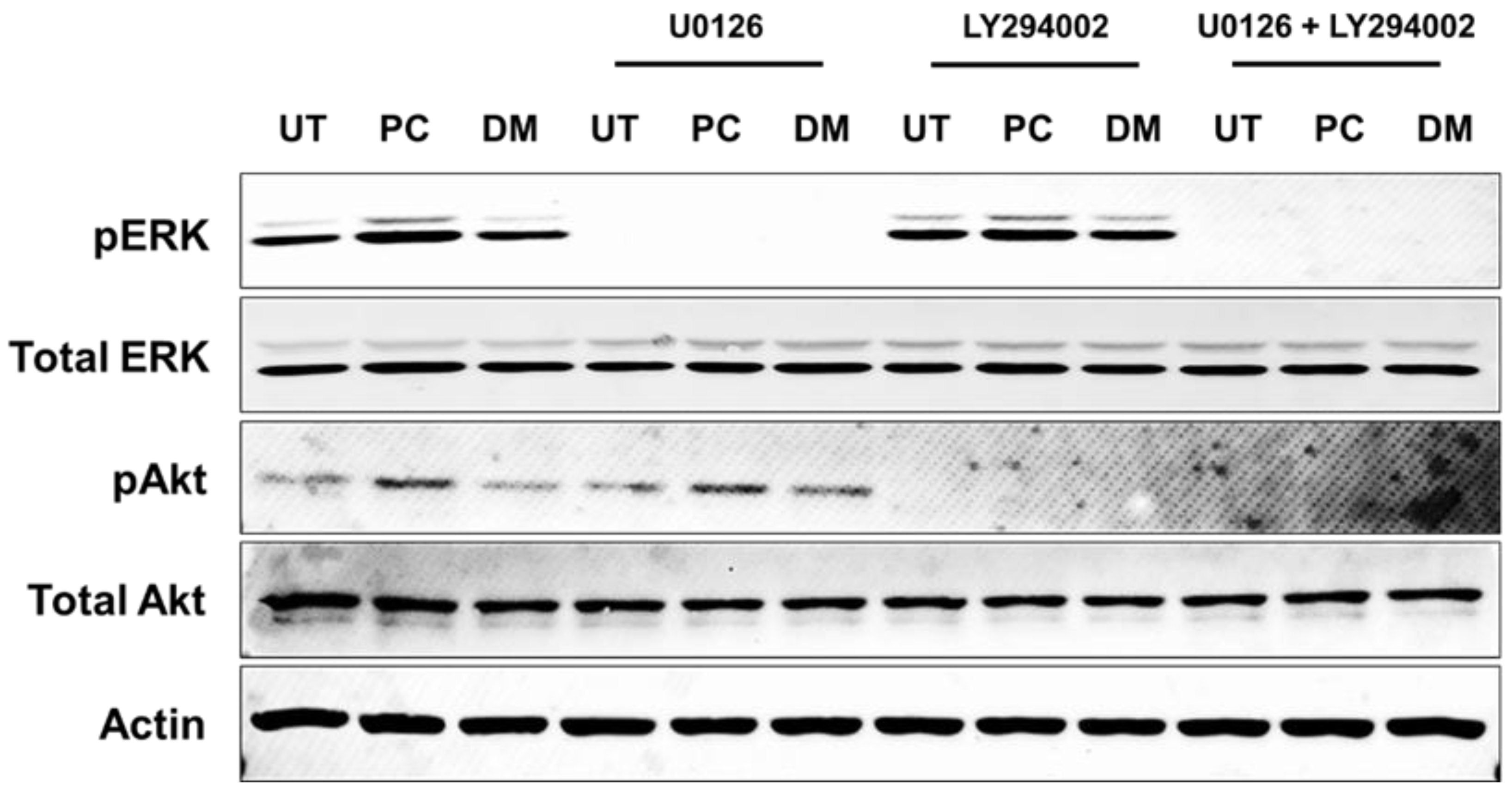
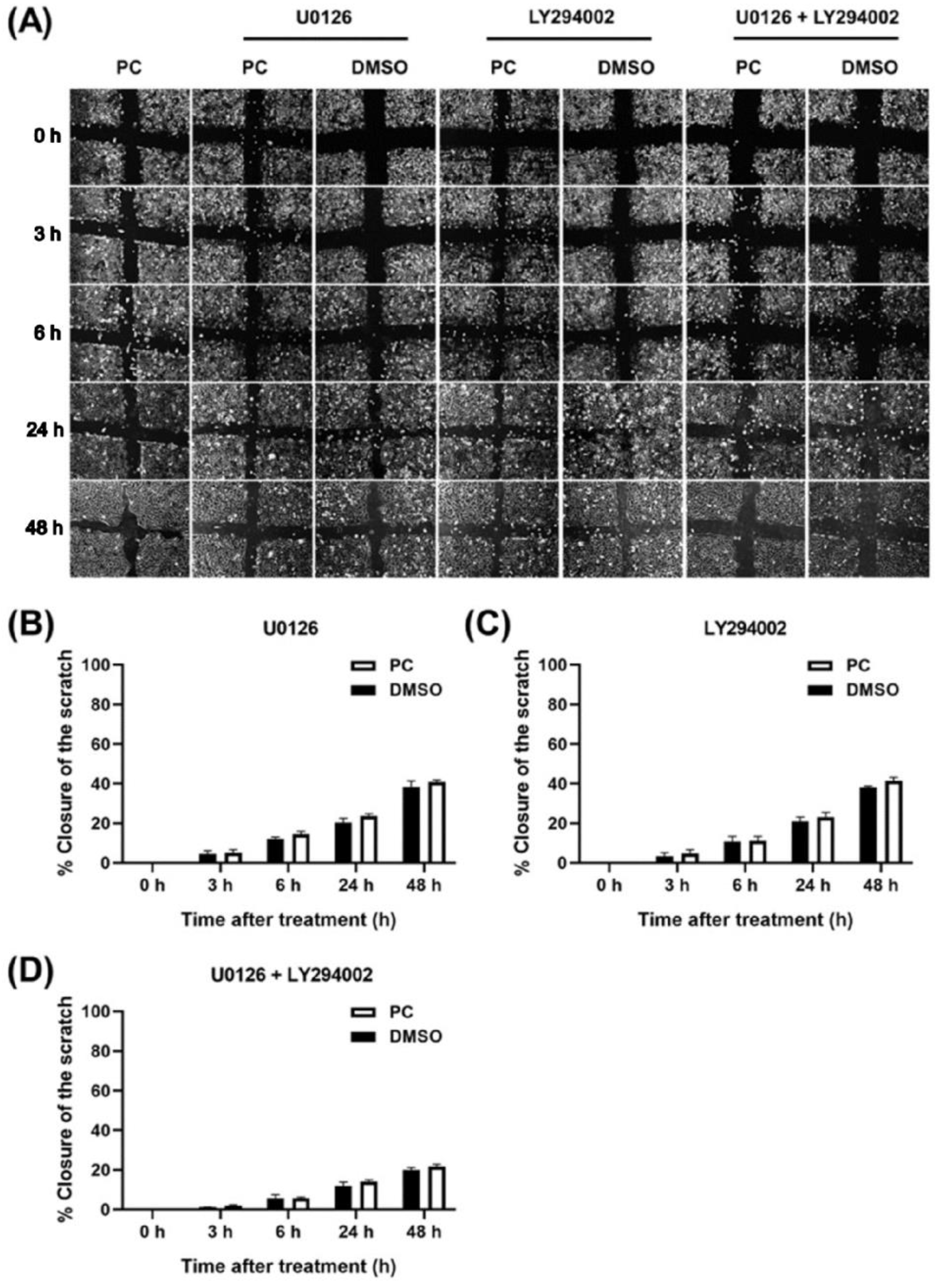
2.5. Inhibitors That Specifically Inhibit MEK and PI3K Completely Block ERK1/2 and Akt Activation and HaCaT Cell Proliferation Induced by Pinocembrin
3. Discussion
4. Materials and Methods
4.1. Pinocembrin Preparation
4.2. Cell Lines and Cell Cultures
4.3. Cell Viability Assay
4.4. Phase-Contrast Microscopy
4.5. Direct Measurement of Cell Number
4.6. Cellular Wound-Healing Activity Assay
4.7. Western Blot Analysis
4.8. Immunofluorescence Study
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kasuya, A.; Tokura, Y. Attempts to accelerate wound healing. J. Dermatol. Sci. 2014, 76, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirsner, R.S.; Eaglstein, W.H. The wound healing process. Dermatol. Clin. 1993, 11, 629–640. [Google Scholar] [CrossRef]
- Shi, C.; Wang, C.; Liu, H.; Li, Q.; Li, R.; Zhang, Y.; Liu, Y.; Shao, Y.; Wang, J. Selection of Appropriate Wound Dressing for Various Wounds. Front. Bioeng. Biotechnol. 2020, 8, 182. [Google Scholar] [CrossRef] [Green Version]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hackam, D.J.; Ford, H.R. Cellular, biochemical, and clinical aspects of wound healing. Surg. Infect. 2002, 3 (Suppl. S1), S23–S35. [Google Scholar] [CrossRef] [PubMed]
- Kawasumi, A.; Sagawa, N.; Hayashi, S.; Yokoyama, H.; Tamura, K. Wound healing in mammals and amphibians: Toward limb regeneration in mammals. Curr. Top. Microbiol. Immunol. 2013, 367, 33–49. [Google Scholar] [CrossRef]
- Goodson, W.H., 3rd; Hunt, T.K. Wound healing and aging. J. Investig. Dermatol. 1979, 73, 88–91. [Google Scholar] [CrossRef] [Green Version]
- Gerstein, A.D.; Phillips, T.J.; Rogers, G.S.; Gilchrest, B.A. Wound healing and aging. Dermatol. Clin. 1993, 11, 749–757. [Google Scholar] [CrossRef]
- Morikawa, T.; Funakoshi, K.; Ninomiya, K.; Yasuda, D.; Miyagawa, K.; Matsuda, H.; Yoshikawa, M. Medicinal foodstuffs. XXXIV. Structures of new prenylchalcones and prenylflavanones with TNF-alpha and aminopeptidase N inhibitory activities from Boesenbergia rotunda. Chem. Pharm. Bull. 2008, 56, 956–962. [Google Scholar] [CrossRef] [Green Version]
- Eng-Chong, T.; Yean-Kee, L.; Chin-Fei, C.; Choon-Han, H.; Sher-Ming, W.; Li-Ping, C.T.; Gen-Teck, F.; Khalid, N.; Abd Rahman, N.; Karsani, S.A.; et al. Boesenbergia rotunda: From Ethnomedicine to Drug Discovery. Evid. Based Complement. Alternat. Med. 2012, 2012, 473637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzotta, S.; Governa, P.; Borgonetti, V.; Marcolongo, P.; Nanni, C.; Gamberucci, A.; Manetti, F.; Pessina, F.; Carullo, G.; Brizzi, A.; et al. Pinocembrin and its linolenoyl ester derivative induce wound healing activity in HaCaT cell line potentially involving a GPR120/FFA4 mediated pathway. Bioorg. Chem. 2021, 108, 104657. [Google Scholar] [CrossRef] [PubMed]
- Rasul, A.; Millimouno, F.M.; Ali Eltayb, W.; Ali, M.; Li, J.; Li, X. Pinocembrin: A Novel Natural Compound with Versatile Pharmacological and Biological Activities. BioMed Res. Int. 2013, 2013, 379850. [Google Scholar] [CrossRef]
- Aguero, M.B.; Gonzalez, M.; Lima, B.; Svetaz, L.; Sanchez, M.; Zacchino, S.; Feresin, G.E.; Schmeda-Hirschmann, G.; Palermo, J.; Wunderlin, D.; et al. Argentinean propolis from Zuccagnia punctata Cav. (Caesalpinieae) exudates: Phytochemical characterization and antifungal activity. J. Agric. Food Chem. 2010, 58, 194–201. [Google Scholar] [CrossRef]
- Kanchanapiboon, J.; Kongsa, U.; Pattamadilok, D.; Kamponchaidet, S.; Wachisunthon, D.; Poonsatha, S.; Tuntoaw, S. Boesenbergia rotunda extract inhibits Candida albicans biofilm formation by pinostrobin and pinocembrin. J. Ethnopharmacol. 2020, 261, 113193. [Google Scholar] [CrossRef] [PubMed]
- María Belén Agüero, L.S.; Baroni, V.; Lima, B.; Luna, L.; Zacchino, S.; Saavedra, P.; Wunderlin, D.; Feresin, G.E.; Tapia, A. Urban propolis from San Juan province (Argentina): Ethnopharmacological uses and antifungal activity against Candida and dermatophytes. Ind. Crops Prod. 2014, 57, 166–173. [Google Scholar] [CrossRef]
- Hooker, J.D. The Flora of British India; L. Reeve & Co.: London, UK, 1890; Volume 5. [Google Scholar] [CrossRef]
- Ruttanapattanakul, J.; Wikan, N.; Okonogi, S.; Na Takuathung, M.; Buacheen, P.; Pitchakarn, P.; Potikanond, S.; Nimlamool, W. Boesenbergia rotunda extract accelerates human keratinocyte proliferation through activating ERK1/2 and PI3K/Akt kinases. Biomed. Pharmacother. 2021, 133, 111002. [Google Scholar] [CrossRef] [PubMed]
- Punvittayagul, C.; Wongpoomchai, R.; Taya, S.; Pompimon, W. Effect of pinocembrin isolated from Boesenbergia pandurata on xenobiotic-metabolizing enzymes in rat liver. Drug Metab. Lett. 2011, 5, 1–5. [Google Scholar] [CrossRef]
- Gosain, A.; DiPietro, L.A. Aging and wound healing. World J. Surg. 2004, 28, 321–326. [Google Scholar] [CrossRef]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef]
- Fisher, G.J.; Wang, Z.Q.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of premature skin aging induced by ultraviolet light. N. Engl. J. Med. 1997, 337, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Swift, M.E.; Burns, A.L.; Gray, K.L.; DiPietro, L.A. Age-related alterations in the inflammatory response to dermal injury. J. Investig. Dermatol. 2001, 117, 1027–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swift, M.E.; Kleinman, H.K.; DiPietro, L.A. Impaired wound repair and delayed angiogenesis in aged mice. Lab. Investig. 1999, 79, 1479–1487. [Google Scholar] [PubMed]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Fife, C.E.; Cartel, M.J.; Walker, D.; Thomson, B. Wound Care Outcomes and Associated Cost Among Patients Treated in US Outpatient Wound Centers: Data from the US Wound Registry. Wounds 2012, 24, 10–17. [Google Scholar]
- Zhao, P.; Sui, B.D.; Liu, N.; Lv, Y.J.; Zheng, C.X.; Lu, Y.B.; Huang, W.T.; Zhou, C.H.; Chen, J.; Pang, D.L.; et al. Anti-aging pharmacology in cutaneous wound healing: Effects of metformin, resveratrol, and rapamycin by local application. Aging Cell 2017, 16, 1083–1093. [Google Scholar] [CrossRef]
- Nimlamool, W.; Potikanond, S.; Ruttanapattanakul, J.; Wikan, N.; Okonogi, S.; Jantrapirom, S.; Pitchakarn, P.; Karinchai, J. Curcuma amarissima Extract Activates Growth and Survival Signal Transduction Networks to Stimulate Proliferation of Human Keratinocyte. Biology 2021, 10, 289. [Google Scholar] [CrossRef]
- Nimlamool, W.; Chansakaow, S.; Potikanond, S.; Wikan, N.; Hankittichai, P.; Ruttanapattanakul, J.; Thaklaewphan, P. The Leaf Extract of Mitrephora chulabhorniana Suppresses Migration and Invasion and Induces Human Cervical Cancer Cell Apoptosis through Caspase-Dependent Pathway. BioMed Res. Int. 2022, 2022, 2028082. [Google Scholar] [CrossRef]
- Ruttanapattanakul, J.; Wikan, N.; Chinda, K.; Jearanaikulvanich, T.; Krisanuruks, N.; Muangcha, M.; Okonogi, S.; Potikanond, S.; Nimlamool, W. Essential Oil from Zingiber ottensii Induces Human Cervical Cancer Cell Apoptosis and Inhibits MAPK and PI3K/AKT Signaling Cascades. Plants 2021, 10, 1419. [Google Scholar] [CrossRef] [PubMed]
- Thaklaewphan, P.; Ruttanapattanakul, J.; Monkaew, S.; Buatoom, M.; Sookkhee, S.; Nimlamool, W.; Potikanond, S. Kaempferia parviflora extract inhibits TNF-alpha-induced release of MCP-1 in ovarian cancer cells through the suppression of NF-kappaB signaling. Biomed. Pharmacother. 2021, 141, 111911. [Google Scholar] [CrossRef] [PubMed]
- Governa, P.; Carullo, G.; Biagi, M.; Rago, V.; Aiello, F. Evaluation of the In Vitro Wound-Healing Activity of Calabrian Honeys. Antioxidants 2019, 8, 36. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.M.; Ao, M.Z.; Li, W.; Yu, L.J. Effect of glabridin from Glycyrrhiza glabra on learning and memory in mice. Planta Med. 2008, 74, 377–380. [Google Scholar] [CrossRef] [Green Version]
- Drewes, S.E.; van Vuuren, S.F. Antimicrobial acylphloroglucinols and dibenzyloxy flavonoids from flowers of Helichrysum gymnocomum. Phytochemistry 2008, 69, 1745–1749. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.K.; Koo, M.H.; Abreu, J.A.; Ikegaki, M.; Cury, J.A.; Rosalen, P.L. Antimicrobial activity of propolis on oral microorganisms. Curr. Microbiol. 1998, 36, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Youn, K.; Jun, M. Biological Evaluation and Docking Analysis of Potent BACE1 Inhibitors from Boesenbergia rotunda. Nutrients 2019, 11, 662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jost, M.; Huggett, T.M.; Kari, C.; Rodeck, U. Matrix-independent survival of human keratinocytes through an EGF receptor/MAPK-kinase-dependent pathway. Mol. Biol. Cell 2001, 12, 1519–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, M.; Chen, W.L.; Takatori, A.; Peng, Z.; Zhang, L.; Mongan, M.; Parthasarathy, R.; Sartor, M.; Miller, M.; Yang, J.; et al. A role for the mitogen-activated protein kinase kinase kinase 1 in epithelial wound healing. Mol. Biol. Cell 2006, 17, 3446–3455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czech, M.P. PIP2 and PIP3: Complex roles at the cell surface. Cell 2000, 100, 603–606. [Google Scholar] [CrossRef] [Green Version]
- Insall, R.H.; Weiner, O.D. PIP3, PIP2, and cell movement—Similar messages, different meanings? Dev. Cell 2001, 1, 743–747. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Wang, H.; Zhao, X.; Shi, Y.; Jin, M.; Wan, B.; Xu, H.; Cheng, Y.; Ge, H.; Zhang, Y. Identification of G-protein-coupled receptor 120 as a tumor-promoting receptor that induces angiogenesis and migration in human colorectal carcinoma. Oncogene 2013, 32, 5541–5550. [Google Scholar] [CrossRef]
- Gao, B.; Huang, Q.; Jie, Q.; Lu, W.G.; Wang, L.; Li, X.J.; Sun, Z.; Hu, Y.Q.; Chen, L.; Liu, B.H.; et al. GPR120: A bi-potential mediator to modulate the osteogenic and adipogenic differentiation of BMMSCs. Sci. Rep. 2015, 5, 14080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsuma, S.; Hatae, N.; Yano, T.; Ruike, Y.; Kimura, M.; Hirasawa, A.; Tsujimoto, G. Free fatty acids inhibit serum deprivation-induced apoptosis through GPR120 in a murine enteroendocrine cell line STC-1. J. Biol. Chem. 2005, 280, 19507–19515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Nimlamool, W.; Miller, C.J.; Lou, H.J.; Turk, B.E. Rational Redesign of a Functional Protein Kinase-Substrate Interaction. ACS Chem. Biol. 2017, 12, 1194–1198. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruttanapattanakul, J.; Wikan, N.; Potikanond, S.; Nimlamool, W. Molecular Targets of Pinocembrin Underlying Its Regenerative Activities in Human Keratinocytes. Pharmaceuticals 2022, 15, 954. https://doi.org/10.3390/ph15080954
Ruttanapattanakul J, Wikan N, Potikanond S, Nimlamool W. Molecular Targets of Pinocembrin Underlying Its Regenerative Activities in Human Keratinocytes. Pharmaceuticals. 2022; 15(8):954. https://doi.org/10.3390/ph15080954
Chicago/Turabian StyleRuttanapattanakul, Jirapak, Nitwara Wikan, Saranyapin Potikanond, and Wutigri Nimlamool. 2022. "Molecular Targets of Pinocembrin Underlying Its Regenerative Activities in Human Keratinocytes" Pharmaceuticals 15, no. 8: 954. https://doi.org/10.3390/ph15080954
APA StyleRuttanapattanakul, J., Wikan, N., Potikanond, S., & Nimlamool, W. (2022). Molecular Targets of Pinocembrin Underlying Its Regenerative Activities in Human Keratinocytes. Pharmaceuticals, 15(8), 954. https://doi.org/10.3390/ph15080954






