Role of Calcimimetics in Treating Bone and Mineral Disorders Related to Chronic Kidney Disease
Abstract
1. Introduction
2. Pathogenesis of CKD–Mineral and Bone Disorder: PTH–Vitamin D Interaction Dysregulation as Glomerular Filtration Declines
2.1. Early-Stage CKD–Mineral and Bone Disorder: Low-Bone-Turnover Disease
2.2. Advanced CKD–Mineral and Bone Disorder: High-Bone-Turnover Disease Due to SHPT
2.3. Advanced CKD–Mineral and Bone Disorder: Low Bone Turnover Disease Due to Medical or Surgical Parathyroidectomy or Aluminum Intoxication
3. Role of CaSRs in SHPT
3.1. Action of CaMs
3.1.1. The Pharmacodynamics of CaMs
3.1.2. The Factors Influencing the Potency of CaMs
3.1.3. The Actions of CaMs beyond the Parathyroid Gland
Increasing CaSR Insertion within the Plasma Membrane
Increasing Dihydroxy Vitamin D3 (1,25(OH)2D3) within the Kidney and Parathyroid Glands
Inhibiting FGF23 of Osteocytes
Attenuating Intestinal Calcium Absorption through TRPV6
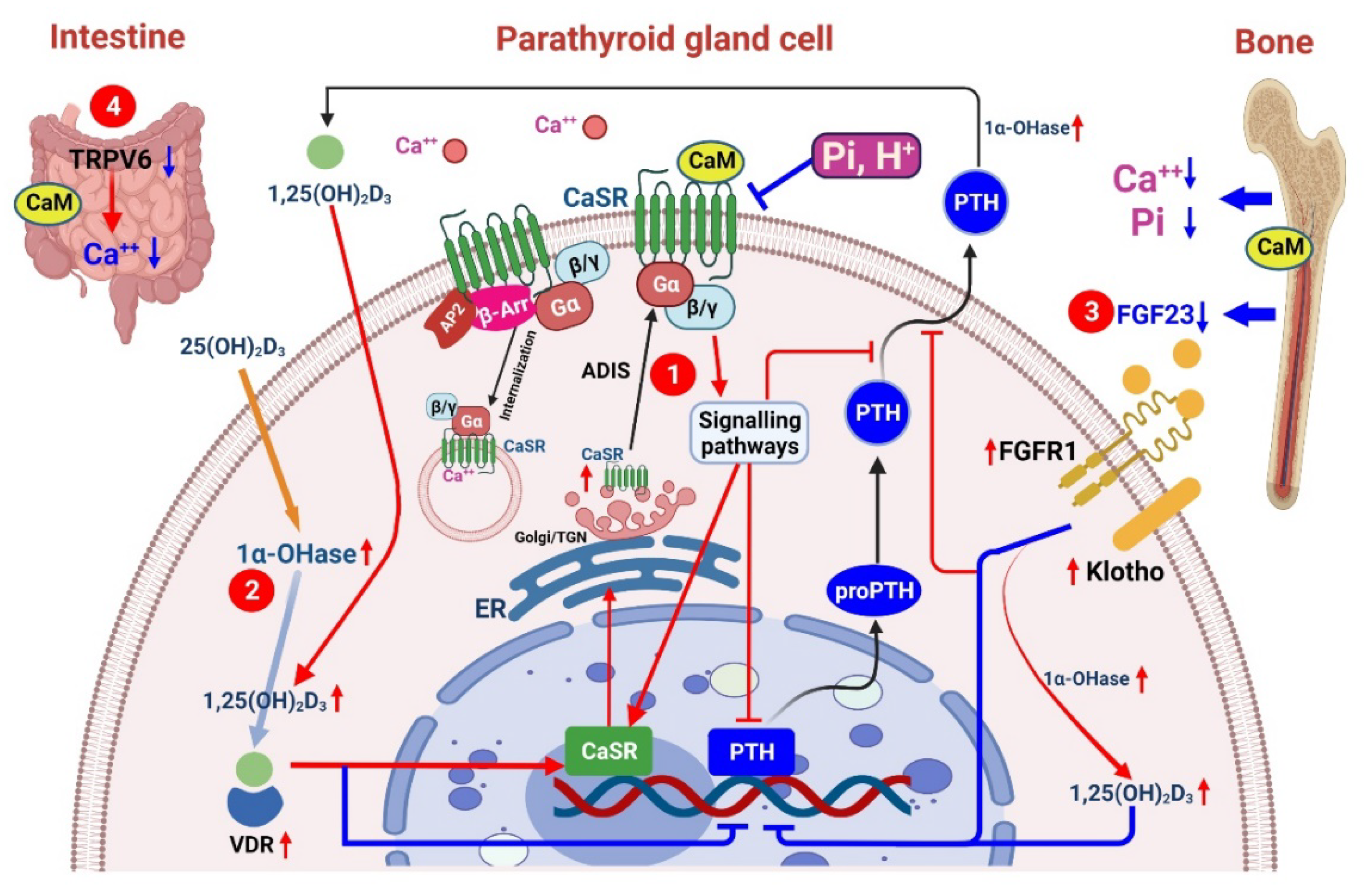
3.2. Effects of CaSRs on Renal Osteodystrophy
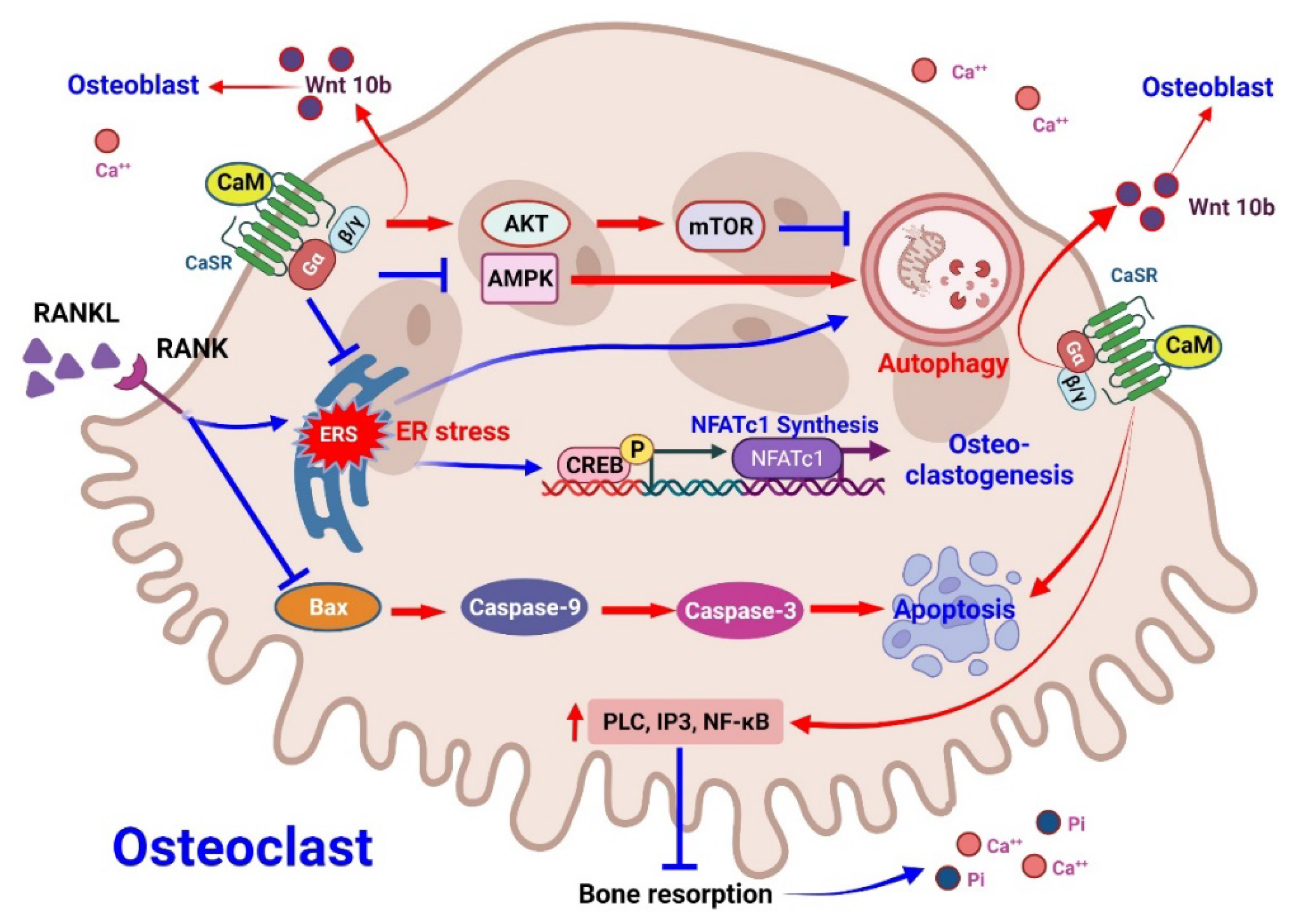
3.2.1. Effect of CaMs on Osteoclasts
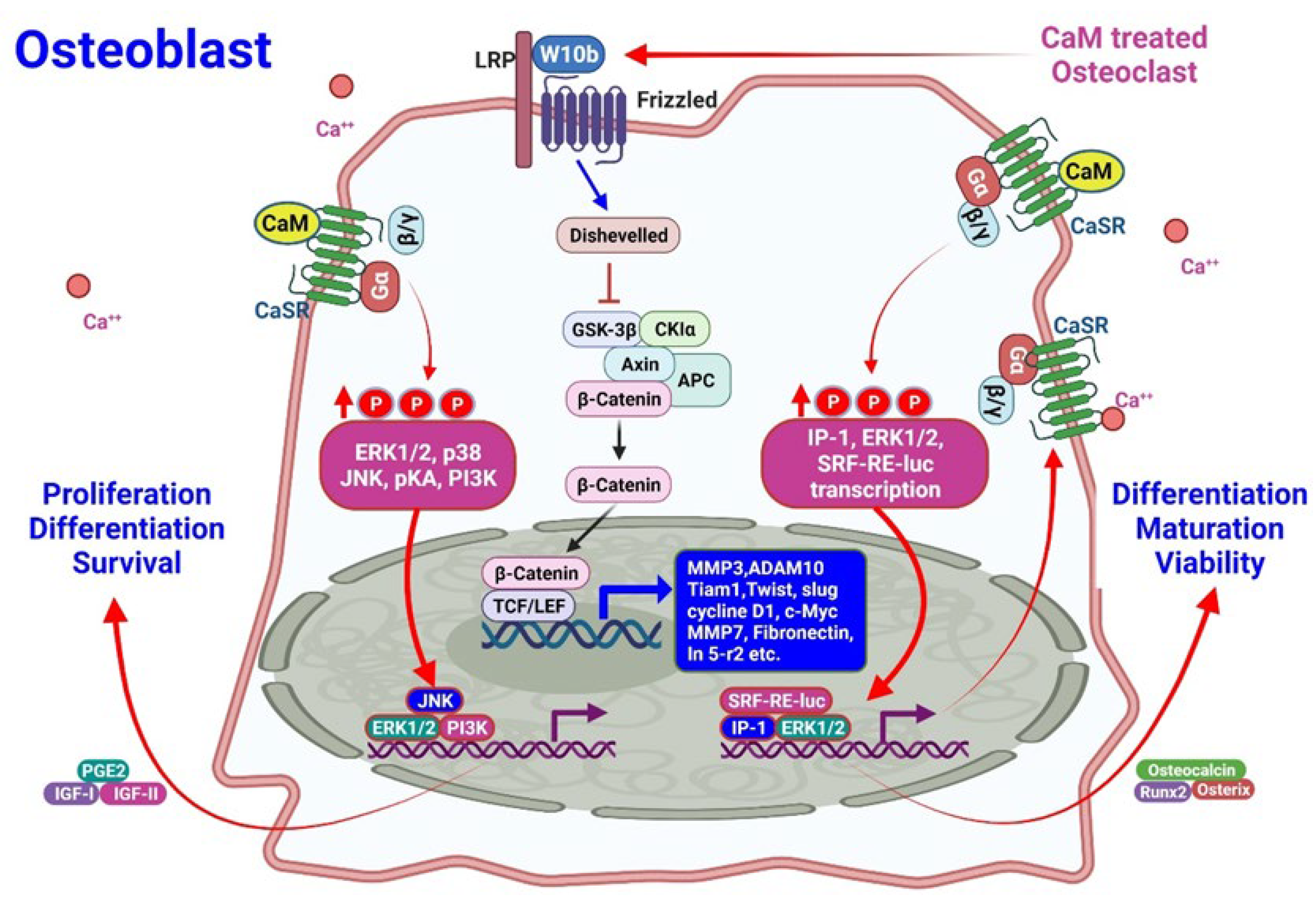
3.2.2. Effects of CaMs on Osteoblasts
3.2.3. Effects of CaMs on Bone Quality
3.2.4. Role in CaMs in Conjunction with Vitamin D for Bone Remodeling
3.3. Effects of CaSRs on Vascular Calcification
3.4. CaSRs and Left Ventricular Remodeling
3.5. Role of CaSRs in Renal Tubules and Vasculature
4. Clinical Efficacy of CaMs
4.1. CaMs in Renal Osteodystrophy/Fracture
4.2. CaMs in Cardiovascular Mortality
4.3. CaMs Compared with Parathyroidectomy
4.4. Current Guidelines on Treating CKD–MBD with Calcimimetics
5. Limitation of the CaMs in CKD–MBD
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADIS | agonist-driven insertional signaling |
| CaMs | Calcimimetics |
| CaSRs | Calcium-sensing receptors |
| CKD | Chronic kidney disease |
| CKD–MBD | Chronic kidney disease–mineral and bone disorder |
| ER stress | Endoplasmic reticulum stress |
| ERK | Extracellular signal-regulated protein kinases |
| ESRD | end stage renal disease |
| FGF23 | fibroblast growth factor 23 |
| GCM2 | glial cells missing-2 |
| GFR | glomerular filtration rate |
| JNK | c-Jun N-terminal kinase |
| MAPK | Mitogen-activated protein kinase |
| P1NP | procollagen type I N-terminal pro-peptide (PINP) |
| PTG | Parathyroid gland |
| PTH | parathyroid hormone |
| RAAS | renin–angiotensin–aldosterone system |
| Runx2 | Runt-related transcription factor 2 |
| SHPT | Secondary hyperparathyroidism |
| SRF-RE | serum response factor-response element |
| TGFα | Transforming growth factor-α |
| TRPV6 | Transient Receptor Potential Vanilloid subfamily member 6 |
| VFT | Venus flytrap |
| Wnt | Wingless-related integration site |
References
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2011 2017, 7, 1–59. [Google Scholar] [CrossRef]
- Patel, N.M.; Gutiérrez, O.M.; Andress, D.L.; Coyne, D.W.; Levin, A.; Wolf, M. Vitamin D deficiency and anemia in early chronic kidney disease. Kidney Int. 2010, 77, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Cannata-Andía, J.B.; Carrera, F. The Pathophysiology of Secondary Hyperparathyroidism and the Consequences of Uncontrolled Mineral Metabolism in Chronic Kidney Disease: The Role of COSMOS. NDT Plus 2008, 1, i2–i6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hou, Y.C.; Lu, C.L.; Lu, K.C. Mineral bone disorders in chronic kidney disease. Nephrology 2018, 23 (Suppl. S4), 88–94. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.F. Allosteric modulators of the extracellular calcium receptor. Drug Discov. Today. Technol. 2013, 10, e277–e284. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tang, W.; Zhou, J.; Stubbs, J.R.; Luo, Q.; Pi, M.; Quarles, L.D. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J. Am. Soc. Nephrol. JASN 2006, 17, 1305–1315. [Google Scholar] [CrossRef]
- Onsgard-Meyer, M.J.; Berndt, T.J.; Khraibi, A.A.; Knox, F.G. Phosphaturic effect of parathyroid hormone in the spontaneously hypertensive rat. Am. J. Physiol. 1994, 267, R78–R83. [Google Scholar] [CrossRef]
- Razzaque, M.S. The FGF23-Klotho axis: Endocrine regulation of phosphate homeostasis. Nat. Rev. Endocrinol. 2009, 5, 611–619. [Google Scholar] [CrossRef]
- Hannan, F.M.; Kallay, E.; Chang, W.; Brandi, M.L.; Thakker, R.V. The calcium-sensing receptor in physiology and in calcitropic and noncalcitropic diseases. Nat. Rev. Endocrinol. 2018, 15, 33–51. [Google Scholar] [CrossRef]
- Long, F. Building strong bones: Molecular regulation of the osteoblast lineage. Nat. Rev. Mol. Cell Biol. 2011, 13, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Compton, J.T.; Lee, F.Y. A review of osteocyte function and the emerging importance of sclerostin. J. Bone Jt. Surg. Am. Vol. 2014, 96, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Hadjidakis, D.J.; Androulakis, I.I. Bone remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef]
- Huang, J.C.; Sakata, T.; Pfleger, L.L.; Bencsik, M.; Halloran, B.P.; Bikle, D.D.; Nissenson, R.A. PTH differentially regulates expression of RANKL and OPG. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2004, 19, 235–244. [Google Scholar] [CrossRef]
- Silva, B.C.; Bilezikian, J.P. Parathyroid hormone: Anabolic and catabolic actions on the skeleton. Curr. Opin. Pharmacol. 2015, 22, 41–50. [Google Scholar] [CrossRef]
- Rauch, F. CHAPTER 15—Bone Histomorphometry. In Pediatric Bone; Glorieux, F.H., Pettifor, J.M., JÜPpner, H., Eds.; Academic Press: San Diego, CA, USA, 2003; pp. 359–374.I–VII. [Google Scholar] [CrossRef]
- Ott, S.M. Histomorphometric measurements of bone turnover, mineralization, and volume. Clin. J. Am. Soc. Nephrol. 2008, 3 (Suppl. S3), S151–S156. [Google Scholar] [CrossRef] [PubMed]
- Evenepoel, P.; D’Haese, P.; Brandenburg, V. Sclerostin and DKK1: New players in renal bone and vascular disease. Kidney Int. 2015, 88, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.M.; Zheng, J.Q.; Wu, C.C.; Lu, C.L.; Shyu, J.F.; Yung-Ho, H.; Wu, M.Y.; Chiu, I.J.; Wang, Y.H.; Lin, Y.F.; et al. Bone loss in chronic kidney disease: Quantity or quality? Bone 2016, 87, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Coen, G.; Mazzaferro, S.; Ballanti, P.; Sardella, D.; Chicca, S.; Manni, M.; Bonucci, E.; Taggi, F. Renal bone disease in 76 patients with varying degrees of predialysis chronic renal failure: A cross-sectional study. Nephrol. Dial. Transplant. 1996, 11, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Barreto, F.C.; Barreto, D.V.; Canziani, M.E.; Tomiyama, C.; Higa, A.; Mozar, A.; Glorieux, G.; Vanholder, R.; Massy, Z.; de Carvalho, A.B. Association between indoxyl sulfate and bone histomorphometry in pre-dialysis chronic kidney disease patients. J. Bras. Nefrol. 2014, 36, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Nii-Kono, T.; Iwasaki, Y.; Uchida, M.; Fujieda, A.; Hosokawa, A.; Motojima, M.; Yamato, H.; Kurokawa, K.; Fukagawa, M. Indoxyl sulfate induces skeletal resistance to parathyroid hormone in cultured osteoblastic cells. Kidney Int. 2007, 71, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Mozar, A.; Louvet, L.; Godin, C.; Mentaverri, R.; Brazier, M.; Kamel, S.; Massy, Z.A. Indoxyl sulphate inhibits osteoclast differentiation and function. Nephrol. Dial. Transplant. 2012, 27, 2176–2181. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-C.; Shyu, J.-F.; Lim, P.S.; Fang, T.-C.; Lu, C.-L.; Zheng, C.-M.; Hou, Y.-C.; Wu, C.-C.; Lin, Y.-F.; Lu, K.-C. Concentration and Duration of Indoxyl Sulfate Exposure Affects Osteoclastogenesis by Regulating NFATc1 via Aryl Hydrocarbon Receptor. Int. J. Mol. Sci. 2020, 21, 3486. [Google Scholar] [CrossRef] [PubMed]
- Niu, T.; Rosen, C.J. The insulin-like growth factor-I gene and osteoporosis: A critical appraisal. Gene 2005, 361, 38–56. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Sousa, K.M.; MacDougald, O.A.; Rosen, C.J. The many facets of PPARgamma: Novel insights for the skeleton. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E3–E9. [Google Scholar] [CrossRef] [PubMed]
- Drüeke, T.B.; Massy, Z.A. Changing bone patterns with progression of chronic kidney disease. Kidney Int. 2016, 89, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Volovelsky, O.; Cohen, G.; Kenig, A.; Wasserman, G.; Dreazen, A.; Meyuhas, O.; Silver, J.; Naveh-Many, T. Phosphorylation of Ribosomal Protein S6 Mediates Mammalian Target of Rapamycin Complex 1-Induced Parathyroid Cell Proliferation in Secondary Hyperparathyroidism. J. Am. Soc. Nephrol. JASN 2016, 27, 1091–1101. [Google Scholar] [CrossRef]
- Egstrand, S.; Nordholm, A.; Morevati, M.; Mace, M.L.; Hassan, A.; Naveh-Many, T.; Rukov, J.L.; Gravesen, E.; Olgaard, K.; Lewin, E. A molecular circadian clock operates in the parathyroid gland and is disturbed in chronic kidney disease associated bone and mineral disorder. Kidney Int. 2020, 98, 1461–1475. [Google Scholar] [CrossRef]
- Arcidiacono, M.V.; Sato, T.; Alvarez-Hernandez, D.; Yang, J.; Tokumoto, M.; Gonzalez-Suarez, I.; Lu, Y.; Tominaga, Y.; Cannata-Andia, J.; Slatopolsky, E.; et al. EGFR activation increases parathyroid hyperplasia and calcitriol resistance in kidney disease. J. Am. Soc. Nephrol. JASN 2008, 19, 310–320. [Google Scholar] [CrossRef]
- Dusso, A.S.; Pavlopoulos, T.; Naumovich, L.; Lu, Y.; Finch, J.; Brown, A.J.; Morrissey, J.; Slatopolsky, E. p21(WAF1) and transforming growth factor-alpha mediate dietary phosphate regulation of parathyroid cell growth. Kidney Int. 2001, 59, 855–865. [Google Scholar] [CrossRef]
- Iwasaki-Ishizuka, Y.; Yamato, H.; Nii-Kono, T.; Kurokawa, K.; Fukagawa, M. Downregulation of parathyroid hormone receptor gene expression and osteoblastic dysfunction associated with skeletal resistance to parathyroid hormone in a rat model of renal failure with low turnover bone. Nephrol. Dial. Transplant. 2005, 20, 1904–1911. [Google Scholar] [CrossRef] [PubMed]
- Malluche, H.H.; Monier-Faugere, M.C.; Koszewski, N.J. Use and indication of vitamin D and vitamin D analogues in patients with renal bone disease. Nephrol. Dial. Transplant. 2002, 17 (Suppl. S10), 6–9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Andress, D.L. Adynamic bone in patients with chronic kidney disease. Kidney Int. 2008, 73, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Haarhaus, M.; Evenepoel, P. Differentiating the causes of adynamic bone in advanced chronic kidney disease informs osteoporosis treatment. Kidney Int. 2021, 100, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Malluche, H.H.; Porter, D.S.; Pienkowski, D. Evaluating bone quality in patients with chronic kidney disease. Nat. Rev. Nephrol. 2013, 9, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.T.; Riccardi, D. New concepts in calcium-sensing receptor pharmacology and signalling. Br. J. Pharmacol. 2012, 165, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M.; MacLeod, R.J. Extracellular calcium sensing and extracellular calcium signaling. Physiol. Rev. 2001, 81, 239–297. [Google Scholar] [CrossRef] [PubMed]
- Canaff, L.; Hendy, G.N. Calcium-sensing receptor gene transcription is up-regulated by the proinflammatory cytokine, interleukin-1beta. Role of the NF-kappaB PATHWAY and kappaB elements. J. Biol. Chem. 2005, 280, 14177–14188. [Google Scholar] [CrossRef]
- Canaff, L.; Zhou, X.; Mosesova, I.; Cole, D.E.; Hendy, G.N. Glial cells missing-2 (GCM2) transactivates the calcium-sensing receptor gene: Effect of a dominant-negative GCM2 mutant associated with autosomal dominant hypoparathyroidism. Hum. Mutat. 2009, 30, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Hendy, G.N.; Canaff, L. Calcium-Sensing Receptor Gene: Regulation of Expression. Front. Physiol. 2016, 7, 394. [Google Scholar] [CrossRef]
- Brown, A.J.; Zhong, M.; Finch, J.; Ritter, C.; McCracken, R.; Morrissey, J.; Slatopolsky, E. Rat calcium-sensing receptor is regulated by vitamin D but not by calcium. Am. J. Physiol. 1996, 270, F454–F460. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Porta, A.; Mady, L.J.; Seth, T. Vitamin D and intestinal calcium absorption. Mol. Cell. Endocrinol. 2011, 347, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Silver, J. Pathogenesis of parathyroid dysfunction in end-stage renal disease. Adv. Ren. Replace. Ther. 2002, 9, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Naveh-Many, T.; Rahamimov, R.; Livni, N.; Silver, J. Parathyroid cell proliferation in normal and chronic renal failure rats. The effects of calcium, phosphate, and vitamin D. J. Clin. Investig. 1995, 96, 1786–1793. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Seo, U.H.; Kim, W.Y.; Woo, S.U.; Lee, J.B. Calcium-sensing receptor and apoptosis in parathyroid hyperplasia of patients with secondary hyperparathyroidism. J. Int. Med. Res. 2013, 41, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.F.; Heaton, W.H.; Miller, M.; Fox, J.; Balandrin, M.F.; Van Wagenen, B.C.; Colloton, M.; Karbon, W.; Scherrer, J.; Shatzen, E.; et al. Pharmacodynamics of the type II calcimimetic compound cinacalcet HCl. J. Pharmacol. Exp. Ther. 2004, 308, 627–635. [Google Scholar] [CrossRef]
- Block, G.A.; Bushinsky, D.A.; Cunningham, J.; Drueke, T.B.; Ketteler, M.; Kewalramani, R.; Martin, K.J.; Mix, T.C.; Moe, S.M.; Patel, U.D.; et al. Effect of Etelcalcetide vs Placebo on Serum Parathyroid Hormone in Patients Receiving Hemodialysis With Secondary Hyperparathyroidism: Two Randomized Clinical Trials. JAMA 2017, 317, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.C.; London, G.M.; Parfrey, P.S.; Block, G.A.; Correa-Rotter, R.; Dehmel, B.; Drüeke, T.B.; Floege, J.; Kubo, Y.; Mahaffey, K.W.; et al. Effects of cinacalcet on atherosclerotic and nonatherosclerotic cardiovascular events in patients receiving hemodialysis: The EValuation Of Cinacalcet HCl Therapy to Lower CardioVascular Events (EVOLVE) trial. J. Am. Heart Assoc. 2014, 3, e001363. [Google Scholar] [CrossRef]
- Bellasi, A.; Cozzolino, M.; Malberti, F.; Cancarini, G.; Esposito, C.; Guastoni, C.M.; Ondei, P.; Pontoriero, G.; Teatini, U.; Vezzoli, G.; et al. New scenarios in secondary hyperparathyroidism: Etelcalcetide. Position paper of working group on CKD-MBD of the Italian Society of Nephrology. J. Nephrol. 2020, 33, 211–221. [Google Scholar] [CrossRef]
- Ellaithy, A.; Gonzalez-Maeso, J.; Logothetis, D.A.; Levitz, J. Structural and Biophysical Mechanisms of Class C G Protein-Coupled Receptor Function. Trends Biochem. Sci. 2020, 45, 1049–1064. [Google Scholar] [CrossRef]
- Negrea, L. Active Vitamin D in Chronic Kidney Disease: Getting Right Back Where We Started from? Kidney Dis. 2019, 5, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Floege, J.; Ketteler, M. Vascular calcification in patients with end-stage renal disease. Nephrol. Dial. Transplant. 2004, 19, v59–v66. [Google Scholar] [CrossRef] [PubMed]
- Friedl, C.; Zitt, E. Role of etelcalcetide in the management of secondary hyperparathyroidism in hemodialysis patients: A review on current data and place in therapy. Drug Des. Dev. Ther. 2018, 12, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Steddon, S.J.; Cunningham, J. Calcimimetics and calcilytics--fooling the calcium receptor. Lancet 2005, 365, 2237–2239. [Google Scholar] [CrossRef]
- Centeno, P.P.; Herberger, A.; Mun, H.-C.; Tu, C.; Nemeth, E.F.; Chang, W.; Conigrave, A.D.; Ward, D.T. Phosphate acts directly on the calcium-sensing receptor to stimulate parathyroid hormone secretion. Nat. Commun. 2019, 10, 4693. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.C.; Shieh, S.D.; Li, B.L.; Chu, P.; Jan, S.Y.; Lin, Y.F. Rapid correction of metabolic acidosis in chronic renal failure: Effect on parathyroid hormone activity. Nephron 1994, 67, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Campion, K.L.; McCormick, W.D.; Warwicker, J.; Khayat, M.E.B.; Atkinson-Dell, R.; Steward, M.C.; Delbridge, L.W.; Mun, H.-C.; Conigrave, A.D.; Ward, D.T. Pathophysiologic Changes in Extracellular pH Modulate Parathyroid Calcium-Sensing Receptor Activity and Secretion <em>via</em> a Histidine-Independent Mechanism. J. Am. Soc. Nephrol. 2015, 26, 2163. [Google Scholar] [CrossRef]
- Breitwieser, G.E. Minireview: The intimate link between calcium sensing receptor trafficking and signaling: Implications for disorders of calcium homeostasis. Mol. Endocrinol. 2012, 26, 1482–1495. [Google Scholar] [CrossRef]
- Lee, J.J.; Liu, X.; O’Neill, D.; Beggs, M.R.; Weissgerber, P.; Flockerzi, V.; Chen, X.Z.; Dimke, H.; Alexander, R.T. Activation of the calcium sensing receptor attenuates TRPV6-dependent intestinal calcium absorption. JCI Insight 2019, 5, e128013. [Google Scholar] [CrossRef]
- Wada, M.; Nagano, N.; Furuya, Y.; Chin, J.; Nemeth, E.F.; Fox, J. Calcimimetic NPS R-568 prevents parathyroid hyperplasia in rats with severe secondary hyperparathyroidism. Kidney Int. 2000, 57, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, T.; Ohkido, I.; Nakashima, A.; Saito, Y.; Okabe, M.; Yokoo, T. Severe chronic kidney disease environment reduced calcium-sensing receptor expression in parathyroid glands of adenine-induced rats even without high phosphorus diet. BMC Nephrol. 2020, 21, 219. [Google Scholar] [CrossRef]
- Mary, A.; Objois, T.; Brazier, M.; Bennis, Y.; Boudot, C.; Lenglet, G.; Paccou, J.; Bugnicourt, J.-M.; Choukroun, G.; Drueke, T.B.; et al. Decreased monocyte calcium sensing receptor expression in patients with chronic kidney disease is associated with impaired monocyte ability to reduce vascular calcification. Kidney Int. 2021, 99, 1382–1391. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.; Merkel, S.; Leitolf, H.; Haller, H. The effect of cinacalcet on bone remodeling and renal function in transplant patients with persistent hyperparathyroidism. Transplantation 2011, 91, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Suzuki, K.; Sumi, M.; Tokumoto, A.; Shigeno, K.; Himeno, Y.; Sugimoto, T. Bone metabolism after cinacalcet administration in patients with secondary hyperparathyroidism. J. Bone Miner. Metab. 2010, 28, 49–54. [Google Scholar] [CrossRef]
- Hung, K.C.; Chang, J.F. Therapeutic Effect of Calcimimetics on Osteoclast-Osteoblast Crosslink in Chronic Kidney Disease and Mineral Bone Disease. Int. J. Mol. Sci. 2020, 21, 8712. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Tocados, J.M.; Rodríguez-Ortiz, M.E.; Almadén, Y.; Pineda, C.; Martínez-Moreno, J.M.; Herencia, C.; Vergara, N.; Pendón-Ruiz de Mier, M.V.; Santamaría, R.; Rodelo-Haad, C.; et al. Calcimimetics maintain bone turnover in uremic rats despite the concomitant decrease in parathyroid hormone concentration. Kidney Int. 2019, 95, 1064–1078. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H.; Shima, N.; Nakagawa, N.; Yamaguchi, K.; Kinosaki, M.; Mochizuki, S.; Tomoyasu, A.; Yano, K.; Goto, M.; Murakami, A.; et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. USA 1998, 95, 3597–3602. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhu, W.; Li, S.; Ma, J.; Zhang, H.; Li, Z.; Zhang, L.; Zhang, B.; Li, Z.; Liang, X.; et al. Bovine parathyroid hormone enhances osteoclast bone resorption by modulating V-ATPase through PTH1R. Int. J. Mol. Med. 2016, 37, 284–292. [Google Scholar] [CrossRef]
- Del Fattore, A.; Teti, A.; Rucci, N. Osteoclast receptors and signaling. Arch. Biochem. Biophys. 2008, 473, 147–160. [Google Scholar] [CrossRef]
- Zhao, W.; Byrne, M.H.; Boyce, B.F.; Krane, S.M. Bone resorption induced by parathyroid hormone is strikingly diminished in collagenase-resistant mutant mice. J. Clin. Investig. 1999, 103, 517–524. [Google Scholar] [CrossRef]
- Rybchyn, M.S.; Brennan-Speranza, T.C.; Mor, D.; Cheng, Z.; Chang, W.; Conigrave, A.D.; Mason, R.S. The mTORC2 Regulator Homer1 Modulates Protein Levels and Sub-Cellular Localization of the CaSR in Osteoblast-Lineage Cells. Int. J. Mol. Sci. 2021, 22, 6509. [Google Scholar] [CrossRef]
- Zheng, C.-M.; Hsu, Y.-H.; Wu, C.-C.; Lu, C.-L.; Liu, W.-C.; Zheng, J.-Q.; Lin, Y.-F.; Chiu, H.-W.; Chang, T.-J.; Shyu, J.-F.; et al. Osteoclast-Released Wnt-10b Underlies Cinacalcet Related Bone Improvement in Chronic Kidney Disease. Int. J. Mol. Sci. 2019, 20, 2800. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, M.; Yamaguchi, T.; Kaji, H.; Sugimoto, T.; Chihara, K. Involvement of calcium-sensing receptor in osteoblastic differentiation of mouse MC3T3-E1 cells. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E608–E616. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.-W.; Hou, Y.-C.; Lu, C.-L.; Lu, K.-C.; Liu, W.-C.; Shyu, J.-F.; Chang, J.-F.; Zheng, C.-M. Cinacalcet improves bone parameters through regulation of osteoclast endoplasmic reticulum stress, autophagy, and apoptotic pathways in chronic kidney disease-mineral and bone disorder. J. Bone Miner. Res. 2021, 37, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Komiya, Y.; Habas, R. Wnt signal transduction pathways. Organogenesis 2008, 4, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.G.; Carneiro, B.A.; Mota, J.M.; Costa, R.; Leite, C.A.; Barroso-Sousa, R.; Kaplan, J.B.; Chae, Y.K.; Giles, F.J. Wnt/beta-catenin pathway: Modulating anticancer immune response. J. Hematol. Oncol. 2017, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Pan, W. GSK3: A multifaceted kinase in Wnt signaling. Trends Biochem. Sci. 2010, 35, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Banse, X.; Sims, T.J.; Bailey, A.J. Mechanical properties of adult vertebral cancellous bone: Correlation with collagen intermolecular cross-links. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2002, 17, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Mallipattu, S.K.; Uribarri, J. Advanced glycation end product accumulation: A new enemy to target in chronic kidney disease? Curr. Opin. Nephrol. Hypertens. 2014, 23, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Mitome, J.; Yamamoto, H.; Saito, M.; Yokoyama, K.; Marumo, K.; Hosoya, T. Nonenzymatic Cross-Linking Pentosidine Increase in Bone Collagen and Are Associated with Disorders of Bone Mineralization in Dialysis Patients. Calcif. Tissue Int. 2011, 88, 521. [Google Scholar] [CrossRef] [PubMed]
- Finch, J.L.; Tokumoto, M.; Nakamura, H.; Yao, W.; Shahnazari, M.; Lane, N.; Slatopolsky, E. Effect of paricalcitol and cinacalcet on serum phosphate, FGF-23, and bone in rats with chronic kidney disease. Am. J. Physiol. Ren. Physiol. 2010, 298, F1315–F1322. [Google Scholar] [CrossRef] [PubMed]
- Henley, C.; Davis, J.; Miller, G.; Shatzen, E.; Cattley, R.; Li, X.; Martin, D.; Yao, W.; Lane, N.; Shalhoub, V. The calcimimetic AMG 641 abrogates parathyroid hyperplasia, bone and vascular calcification abnormalities in uremic rats. Eur. J. Pharmacol. 2009, 616, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Allard, L.; Demoncheaux, N.; Machuca-Gayet, I.; Georgess, D.; Coury-Lucas, F.; Jurdic, P.; Bacchetta, J. Biphasic Effects of Vitamin D and FGF23 on Human Osteoclast Biology. Calcif. Tissue Int. 2015, 97, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Bernardor, J.; Flammier, S.; Ranchin, B.; Gaillard, S.; Platel, D.; Peyruchaud, O.; Machuca-Gayet, I.; Bacchetta, J. Inhibition of Osteoclast Differentiation by 1.25-D and the Calcimimetic KP2326 Reveals 1.25-D Resistance in Advanced CKD. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2020, 35, 2265–2274. [Google Scholar] [CrossRef] [PubMed]
- Smajilovic, S.; Hansen, J.L.; Christoffersen, T.E.; Lewin, E.; Sheikh, S.P.; Terwilliger, E.F.; Brown, E.M.; Haunso, S.; Tfelt-Hansen, J. Extracellular calcium sensing in rat aortic vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2006, 348, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Molostvov, G.; James, S.; Fletcher, S.; Bennett, J.; Lehnert, H.; Bland, R.; Zehnder, D. Extracellular calcium-sensing receptor is functionally expressed in human artery. Am. J. Physiol.-Ren. Physiol. 2007, 293, F946–F955. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.U.; Kirton, J.P.; Wilkinson, F.L.; Towers, E.; Sinha, S.; Rouhi, M.; Vizard, T.N.; Sage, A.P.; Martin, D.; Ward, D.T.; et al. Calcification is associated with loss of functional calcium-sensing receptor in vascular smooth muscle cells. Cardiovasc. Res. 2009, 81, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.Y.; Hui, J.; Wang, L.M.; Tang, N.; Zhong, H.; Liu, Y.M.; Li, Z.; Feng, Q.; He, F. Reduced Expression of the Extracellular Calcium-Sensing Receptor (CaSR) Is Associated with Activation of the Renin-Angiotensin System (RAS) to Promote Vascular Remodeling in the Pathogenesis of Essential Hypertension. PLoS ONE 2016, 11, e0157456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Chow, S.K.H.; Leung, K.S.; Lee, H.H.; Cheung, W.H. An animal model of co-existing sarcopenia and osteoporotic fracture in senescence accelerated mouse prone 8 (SAMP8). Exp. Gerontol. 2017, 97, 1–8. [Google Scholar] [CrossRef]
- Lu, M.; Leng, B.; He, X.; Zhang, Z.; Wang, H.; Tang, F. Calcium Sensing Receptor-Related Pathway Contributes to Cardiac Injury and the Mechanism of Astragaloside IV on Cardioprotection. Front. Pharmacol. 2018, 9, 1163. [Google Scholar] [CrossRef]
- Ortiz-Capisano, M.C.; Reddy, M.; Mendez, M.; Garvin, J.L.; Beierwaltes, W.H. Juxtaglomerular cell CaSR stimulation decreases renin release via activation of the PLC/IP3 pathway and the ryanodine receptor. Am. J. Physiol. Ren. Physiol. 2012, 304, F248–F256. [Google Scholar] [CrossRef]
- Mendoza, F.J.; Martinez-Moreno, J.; Almaden, Y.; Rodriguez-Ortiz, M.E.; Lopez, I.; Estepa, J.C.; Henley, C.; Rodriguez, M.; Aguilera-Tejero, E. Effect of calcium and the calcimimetic AMG 641 on matrix-Gla protein in vascular smooth muscle cells. Calcif. Tissue Int. 2011, 88, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-C.; Lu, C.-L.; Zheng, C.-M.; Chen, R.-M.; Lin, Y.-F.; Liu, W.-C.; Yen, T.-H.; Chen, R.; Lu, K.-C. Emerging Role of Vitamins D and K in Modulating Uremic Vascular Calcification: The Aspect of Passive Calcification. Nutrients 2019, 11, 152. [Google Scholar] [CrossRef] [PubMed]
- Ziegelstein, R.C.; Xiong, Y.; He, C.; Hu, Q. Expression of a functional extracellular calcium-sensing receptor in human aortic endothelial cells. Biochem. Biophys. Res. Commun. 2006, 342, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Hénaut, L.; Boudot, C.; Massy, Z.A.; Lopez-Fernandez, I.; Dupont, S.; Mary, A.; Drüeke, T.B.; Kamel, S.; Brazier, M.; Mentaverri, R. Calcimimetics increase CaSR expression and reduce mineralization in vascular smooth muscle cells: Mechanisms of action. Cardiovasc. Res. 2014, 101, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Zhang, X.; Zhang, X.; Liu, W.; Fu, Y.; Liu, Y.; Shi, Z.; Chi, J.; Zhao, M.; Yin, X. Role of the calcium sensing receptor in cardiomyocyte apoptosis via mitochondrial dynamics in compensatory hypertrophied myocardium of spontaneously hypertensive rat. Biochem. Biophys. Res. Commun. 2017, 487, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Tang, N.; Xi, D.; Zhao, Y.; Liu, Y.; Wang, L.; Tang, Y.; Zhang, X.; Zhong, H.; He, F. Calcimimetic R568 improved cardiac remodeling by classic and novel renin-angiotensin system in spontaneously hypertensive rats. Exp. Biol. Med. 2019, 244, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, C.; Lin, Y.; Xi, Y.; Li, H.; Shi, S.; Li, H.; Zhang, W.; Zhao, Y.; Tian, Y.; et al. Suppression of calcium-sensing receptor ameliorates cardiac hypertrophy through inhibition of autophagy. Mol. Med. Rep. 2016, 14, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Capasso, G.; Geibel, P.J.; Damiano, S.; Jaeger, P.; Richards, W.G.; Geibel, J.P. The calcium sensing receptor modulates fluid reabsorption and acid secretion in the proximal tubule. Kidney Int. 2013, 84, 277–284. [Google Scholar] [CrossRef]
- Loupy, A.; Ramakrishnan, S.K.; Wootla, B.; Chambrey, R.; de la Faille, R.; Bourgeois, S.; Bruneval, P.; Mandet, C.; Christensen, E.I.; Faure, H.; et al. PTH-independent regulation of blood calcium concentration by the calcium-sensing receptor. J. Clin. Investig. 2012, 122, 3355–3367. [Google Scholar] [CrossRef] [PubMed]
- Rothe, H.; Shapiro, W.; Sun, W.Y.; Matalon, A. CaSR polymorphism Arg990Gly and response to calcimimetic agents in end-stage kidney disease patients with secondary hyperparathyroidism and in cell culture. Pers. Med. 2008, 5, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Okechukwu, C.; Awumey, E.M. Calcium-sensing receptor (CaSR) expression and Renin/Angiotensin System (RAS) in salt-sensitive rats. FASEB J. 2019, 33, 577.6. [Google Scholar] [CrossRef]
- Watanabe, S.; Fukumoto, S.; Chang, H.; Takeuchi, Y.; Hasegawa, Y.; Okazaki, R.; Chikatsu, N.; Fujita, T. Association between activating mutations of calcium-sensing receptor and Bartter’s syndrome. Lancet 2002, 360, 692–694. [Google Scholar] [CrossRef]
- Fukagawa, M.; Yumita, S.; Akizawa, T.; Uchida, E.; Tsukamoto, Y.; Iwasaki, M.; Koshikawa, S. Cinacalcet (KRN1493) effectively decreases the serum intact PTH level with favorable control of the serum phosphorus and calcium levels in Japanese dialysis patients. Nephrol. Dial. Transplant. 2008, 23, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Peacock, M.; Bilezikian, J.P.; Klassen, P.S.; Guo, M.D.; Turner, S.A.; Shoback, D. Cinacalcet Hydrochloride Maintains Long-Term Normocalcemia in Patients with Primary Hyperparathyroidism. J. Clin. Endocrinol. Metab. 2005, 90, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Messa, P.; Macário, F.; Yaqoob, M.; Bouman, K.; Braun, J.; von Albertini, B.; Brink, H.; Maduell, F.; Graf, H.; Frazão, J.M.; et al. The OPTIMA study: Assessing a new cinacalcet (Sensipar/Mimpara) treatment algorithm for secondary hyperparathyroidism. Clin. J. Am. Soc. Nephrol. 2008, 3, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Fishbane, S.; Shapiro, W.B.; Corry, D.B.; Vicks, S.L.; Roppolo, M.; Rappaport, K.; Ling, X.; Goodman, W.G.; Turner, S.; Charytan, C. Cinacalcet HCl and concurrent low-dose vitamin D improves treatment of secondary hyperparathyroidism in dialysis patients compared with vitamin D alone: The ACHIEVE study results. Clin. J. Am. Soc. Nephrol. 2008, 3, 1718–1725. [Google Scholar] [CrossRef]
- Itano, Y.; Kato, S.; Tsuboi, M.; Kasuga, H.; Tsuruta, Y.; Sato, F.; Hishida, M.; Ishimoto, T.; Kosugi, T.; Ando, M.; et al. A Prospective, Randomized Clinical Trial of Etelcalcetide in Patients Receiving Hemodialysis With Secondary Hyperparathyroidism (the DUET Trial). Kidney Int. Rep. 2020, 5, 2168–2177. [Google Scholar] [CrossRef] [PubMed]
- Shigematsu, T.; Akizawa, T.; Uchida, E.; Tsukamoto, Y.; Iwasaki, M.; Koshikawa, S. Long-Term Cinacalcet HCl Treatment Improved Bone Metabolism in Japanese Hemodialysis Patients with Secondary Hyperparathyroidism. Am. J. Nephrol. 2009, 29, 230–236. [Google Scholar] [CrossRef]
- Tsuruta, Y.; Okano, K.; Kikuchi, K.; Tsuruta, Y.; Akiba, T.; Nitta, K. Effects of cinacalcet on bone mineral density and bone markers in hemodialysis patients with secondary hyperparathyroidism. Clin. Exp. Nephrol. 2013, 17, 120–126. [Google Scholar] [CrossRef]
- Chertow, G.M.; Block, G.A.; Correa-Rotter, R.; Drüeke, T.B.; Floege, J.; Goodman, W.G.; Herzog, C.A.; Kubo, Y.; London, G.M.; Mahaffey, K.W.; et al. Effect of Cinacalcet on Cardiovascular Disease in Patients Undergoing Dialysis. N. Engl. J. Med. 2012, 367, 2482–2494. [Google Scholar] [CrossRef] [PubMed]
- Moe, S.M.; Wetherill, L.; Decker, B.S.; Lai, D.; Abdalla, S.; Long, J.; Vatta, M.; Foroud, T.M.; Chertow, G.M. Calcium-Sensing Receptor Genotype and Response to Cinacalcet in Patients Undergoing Hemodialysis. Clin. J. Am. Soc. Nephrol. CJASN 2017, 12, 1128–1138. [Google Scholar] [CrossRef]
- Grzegorzewska, A.E.; Frycz, B.A.; Świderska, M.; Niepolski, L.; Mostowska, A.; Jagodziński, P.P. Calcium-sensing receptor gene (CASR) polymorphisms and CASR transcript level concerning dyslipidemia in hemodialysis patients: A cross-sectional study. BMC Nephrol. 2019, 20, 436. [Google Scholar] [CrossRef] [PubMed]
- Ruderman, I.; Smith, E.R.; Toussaint, N.D.; Hewitson, T.D.; Holt, S.G. Longitudinal changes in bone and mineral metabolism after cessation of cinacalcet in dialysis patients with secondary hyperparathyroidism. BMC Nephrol. 2018, 19, 113. [Google Scholar] [CrossRef]
- Lau, W.L.; Obi, Y.; Kalantar-Zadeh, K. Parathyroidectomy in the Management of Secondary Hyperparathyroidism. Clin. J. Am. Soc. Nephrol. 2018, 13, 952–961. [Google Scholar] [CrossRef]
- Keutgen, X.M.; Buitrago, D.; Filicori, F.; Kundel, A.; Elemento, O.; Fahey, T.J., 3rd; Zarnegar, R. Calcimimetics versus parathyroidectomy for treatment of primary hyperparathyroidism: Retrospective chart analysis of a prospective database. Ann. Surg. 2012, 255, 981–985. [Google Scholar] [CrossRef]
- Cruzado, J.M.; Moreno, P.; Torregrosa, J.V.; Taco, O.; Mast, R.; Gómez-Vaquero, C.; Polo, C.; Revuelta, I.; Francos, J.; Torras, J.; et al. A Randomized Study Comparing Parathyroidectomy with Cinacalcet for Treating Hypercalcemia in Kidney Allograft Recipients with Hyperparathyroidism. J. Am. Soc. Nephrol. 2016, 27, 2487–2494. [Google Scholar] [CrossRef]
- Cañadillas, S.; Canalejo, A.; Santamaría, R.; Rodríguez, M.E.; Estepa, J.C.; Martín-Malo, A.; Bravo, J.; Ramos, B.; Aguilera-Tejero, E.; Rodríguez, M.; et al. Calcium-Sensing Receptor Expression and Parathyroid Hormone Secretion in Hyperplastic Parathyroid Glands from Humans. J. Am. Soc. Nephrol. 2005, 16, 2190. [Google Scholar] [CrossRef]
- Fukagawa, M.; Yokoyama, K.; Koiwa, F.; Taniguchi, M.; Shoji, T.; Kazama, J.J.; Komaba, H.; Ando, R.; Kakuta, T.; Fujii, H.; et al. Clinical practice guideline for the management of chronic kidney disease-mineral and bone disorder. Ther. Apher. Dial. 2013, 17, 247–288. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Y.-C.; Zheng, C.-M.; Chiu, H.-W.; Liu, W.-C.; Lu, K.-C.; Lu, C.-L. Role of Calcimimetics in Treating Bone and Mineral Disorders Related to Chronic Kidney Disease. Pharmaceuticals 2022, 15, 952. https://doi.org/10.3390/ph15080952
Hou Y-C, Zheng C-M, Chiu H-W, Liu W-C, Lu K-C, Lu C-L. Role of Calcimimetics in Treating Bone and Mineral Disorders Related to Chronic Kidney Disease. Pharmaceuticals. 2022; 15(8):952. https://doi.org/10.3390/ph15080952
Chicago/Turabian StyleHou, Yi-Chou, Cai-Mei Zheng, Hui-Wen Chiu, Wen-Chih Liu, Kuo-Cheng Lu, and Chien-Lin Lu. 2022. "Role of Calcimimetics in Treating Bone and Mineral Disorders Related to Chronic Kidney Disease" Pharmaceuticals 15, no. 8: 952. https://doi.org/10.3390/ph15080952
APA StyleHou, Y.-C., Zheng, C.-M., Chiu, H.-W., Liu, W.-C., Lu, K.-C., & Lu, C.-L. (2022). Role of Calcimimetics in Treating Bone and Mineral Disorders Related to Chronic Kidney Disease. Pharmaceuticals, 15(8), 952. https://doi.org/10.3390/ph15080952





