Etodolac Fortified Sodium Deoxycholate Stabilized Zein Nanoplatforms for Augmented Repositioning Profile in Human Hepatocellular Carcinoma: Assessment of Bioaccessibility, Anti-Proliferation, Pro-Apoptosis and Oxidant Potentials in HepG2 Cells
Abstract
1. Introduction
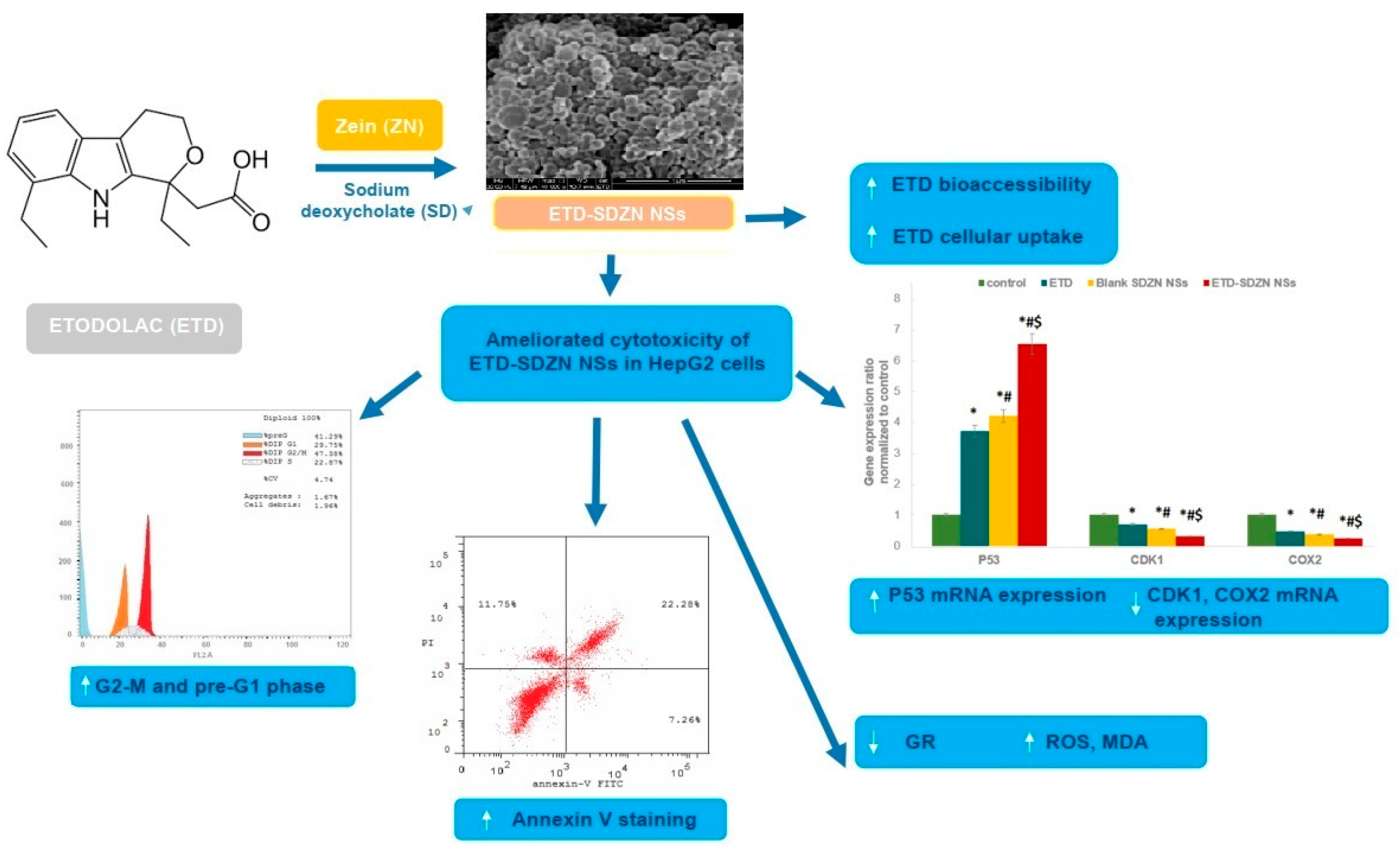
2. Results and Discussion
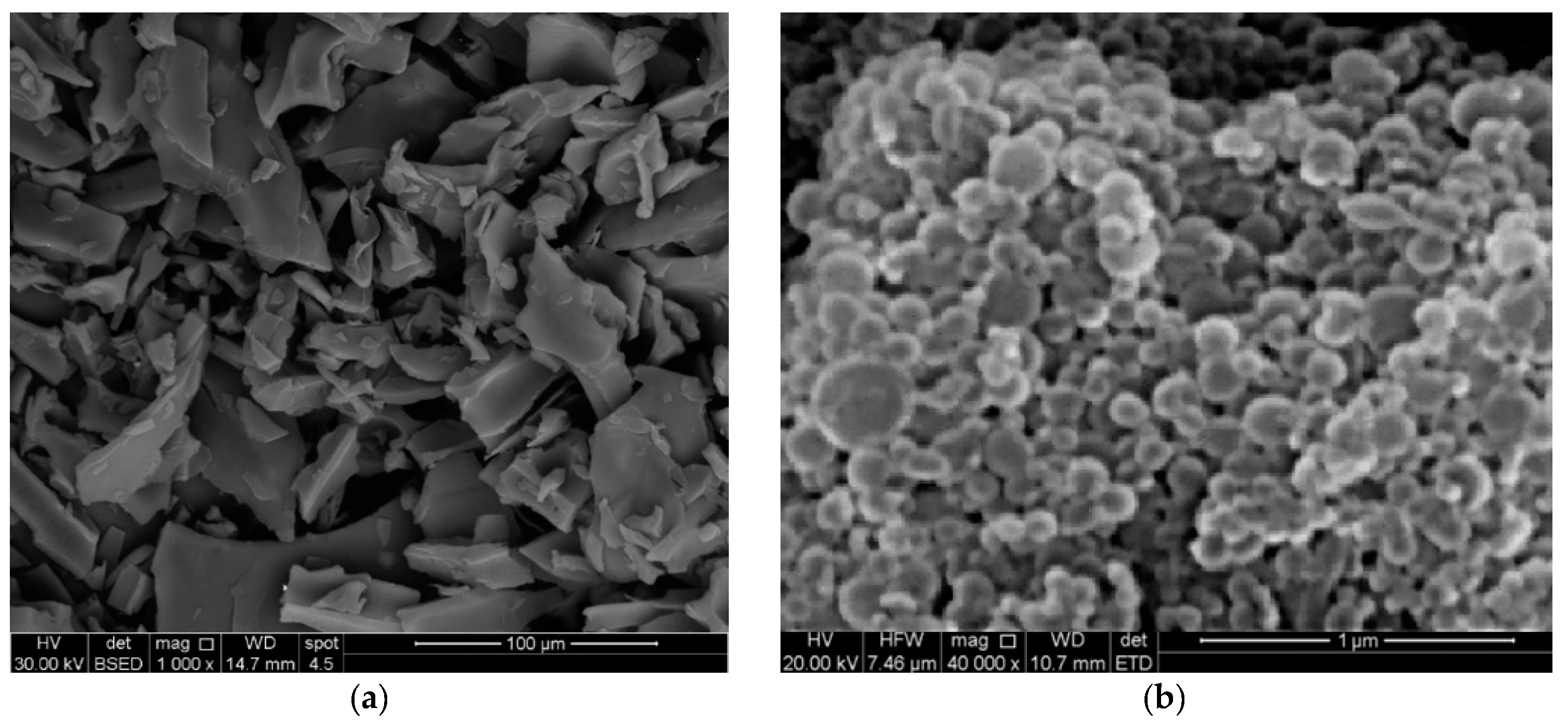
2.1. Formulation and Characterization of ETD-SDZN NSs
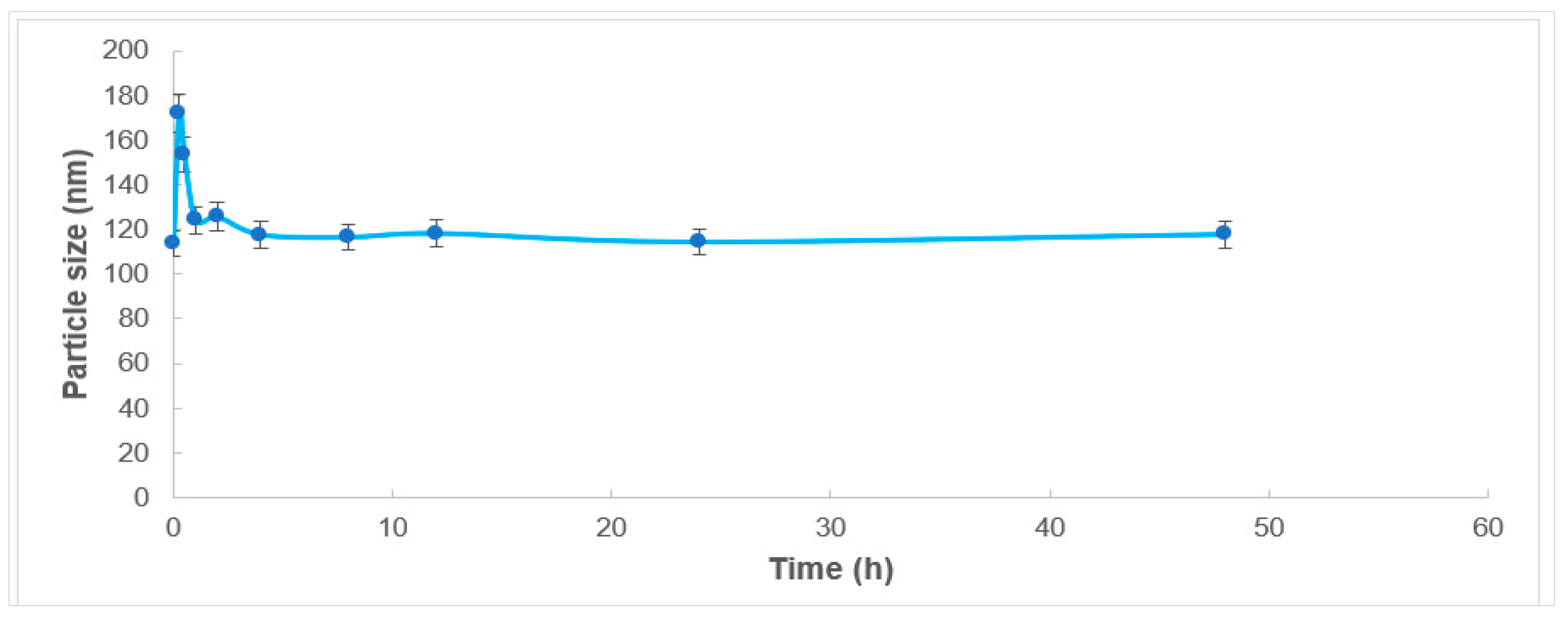
2.2. Serum Stability
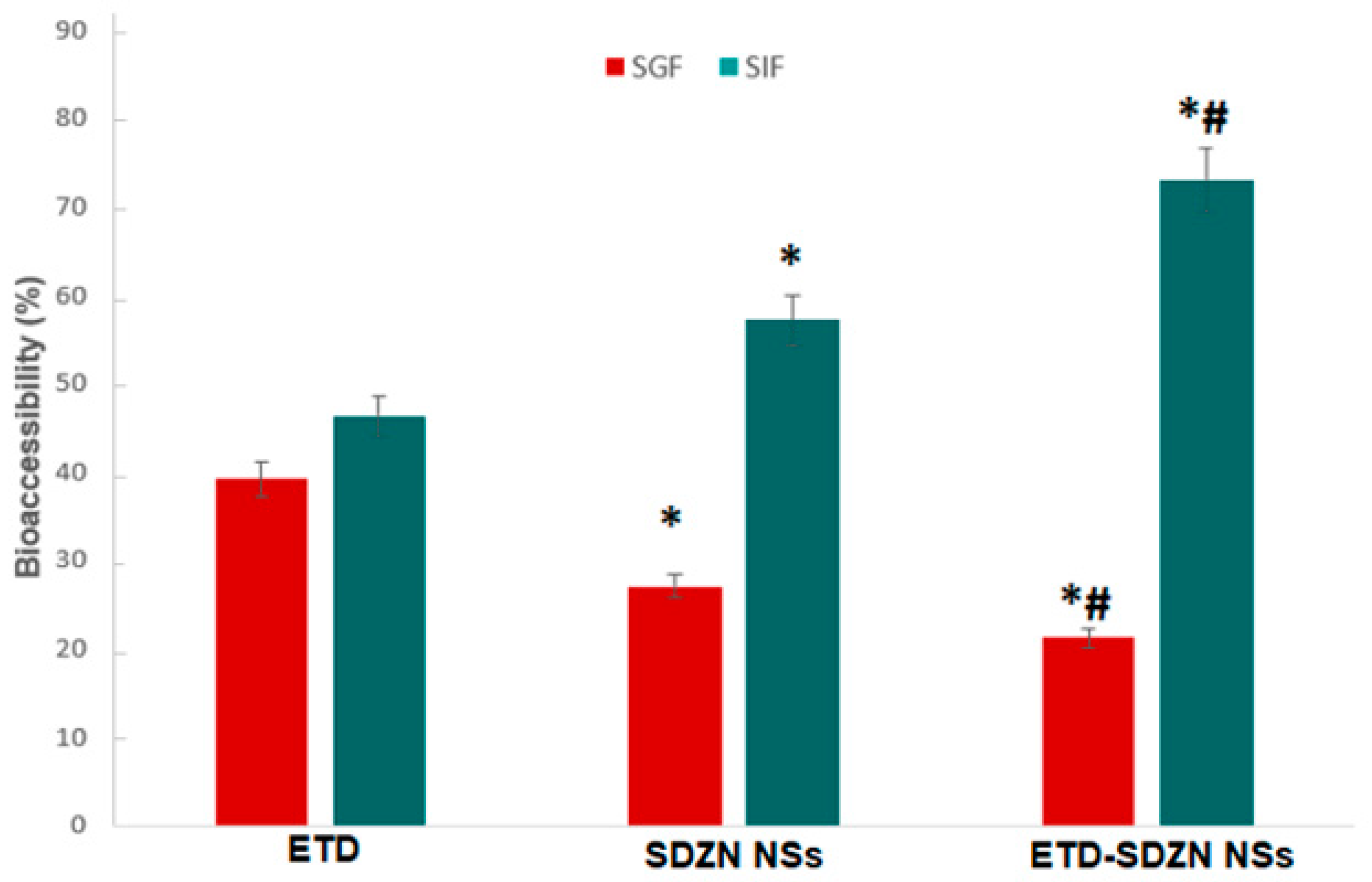
2.3. In Vitro Simulated Digestion Assay
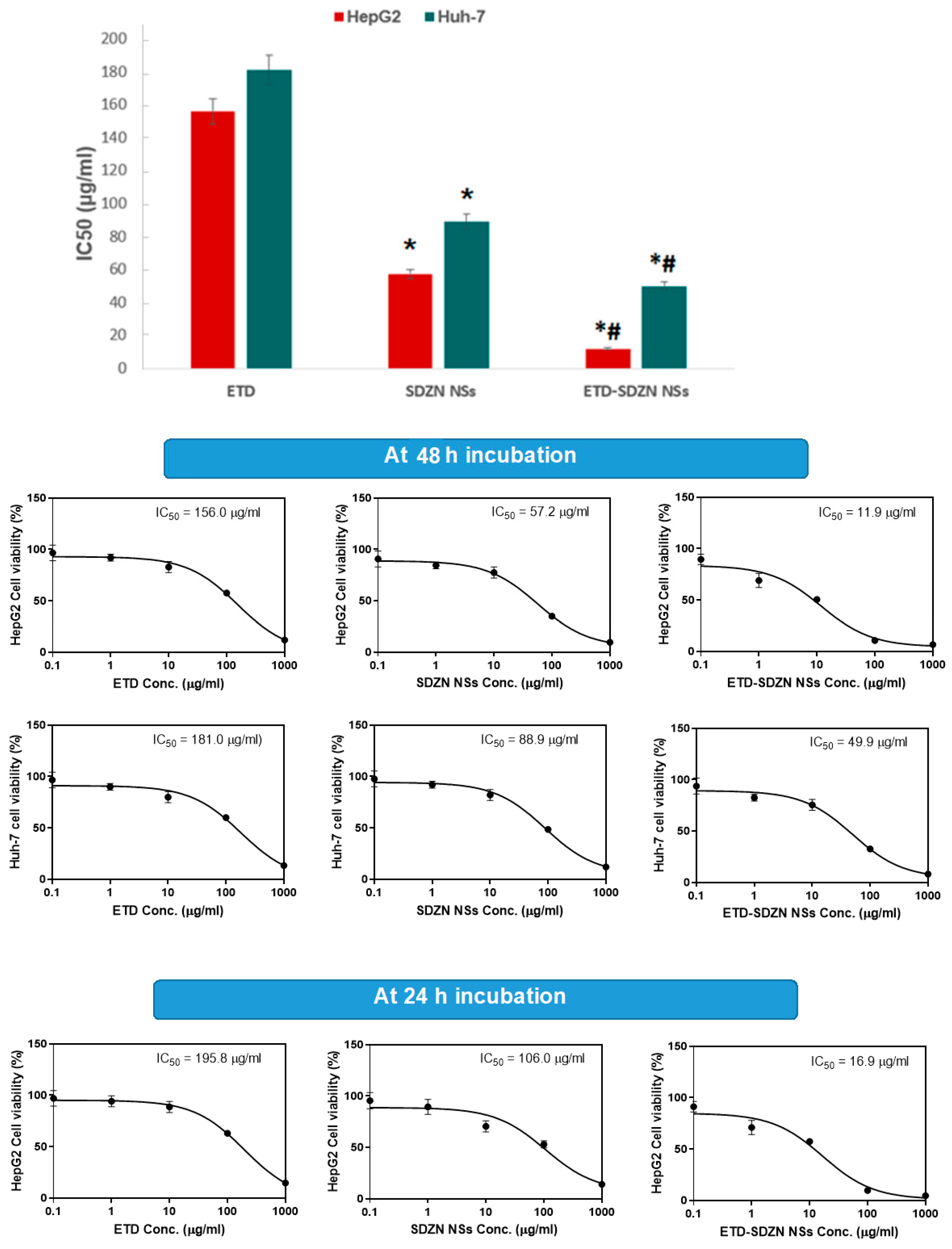
2.4. Cytotoxicity Assay
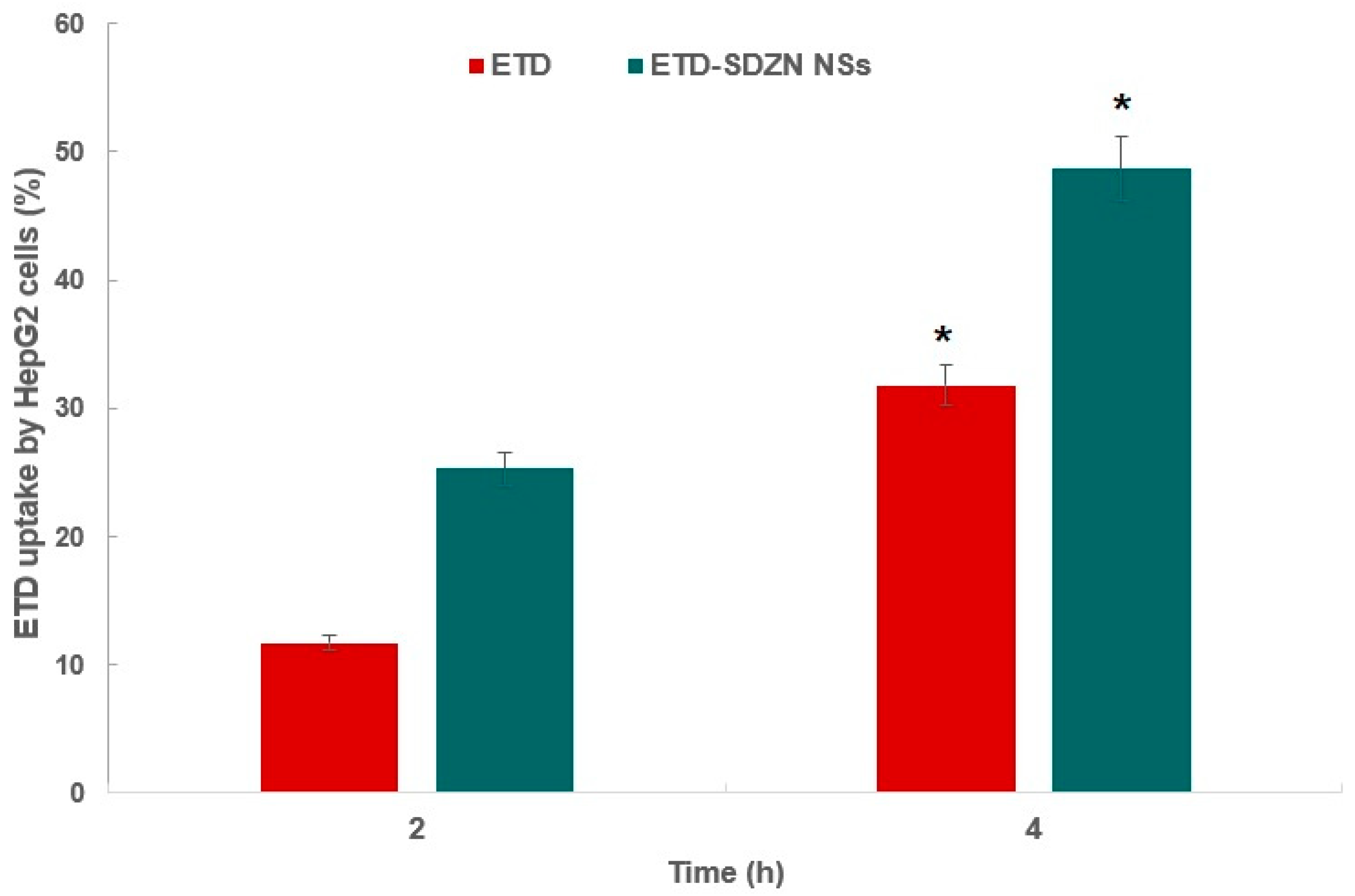
2.5. Cellular Uptake Analysis
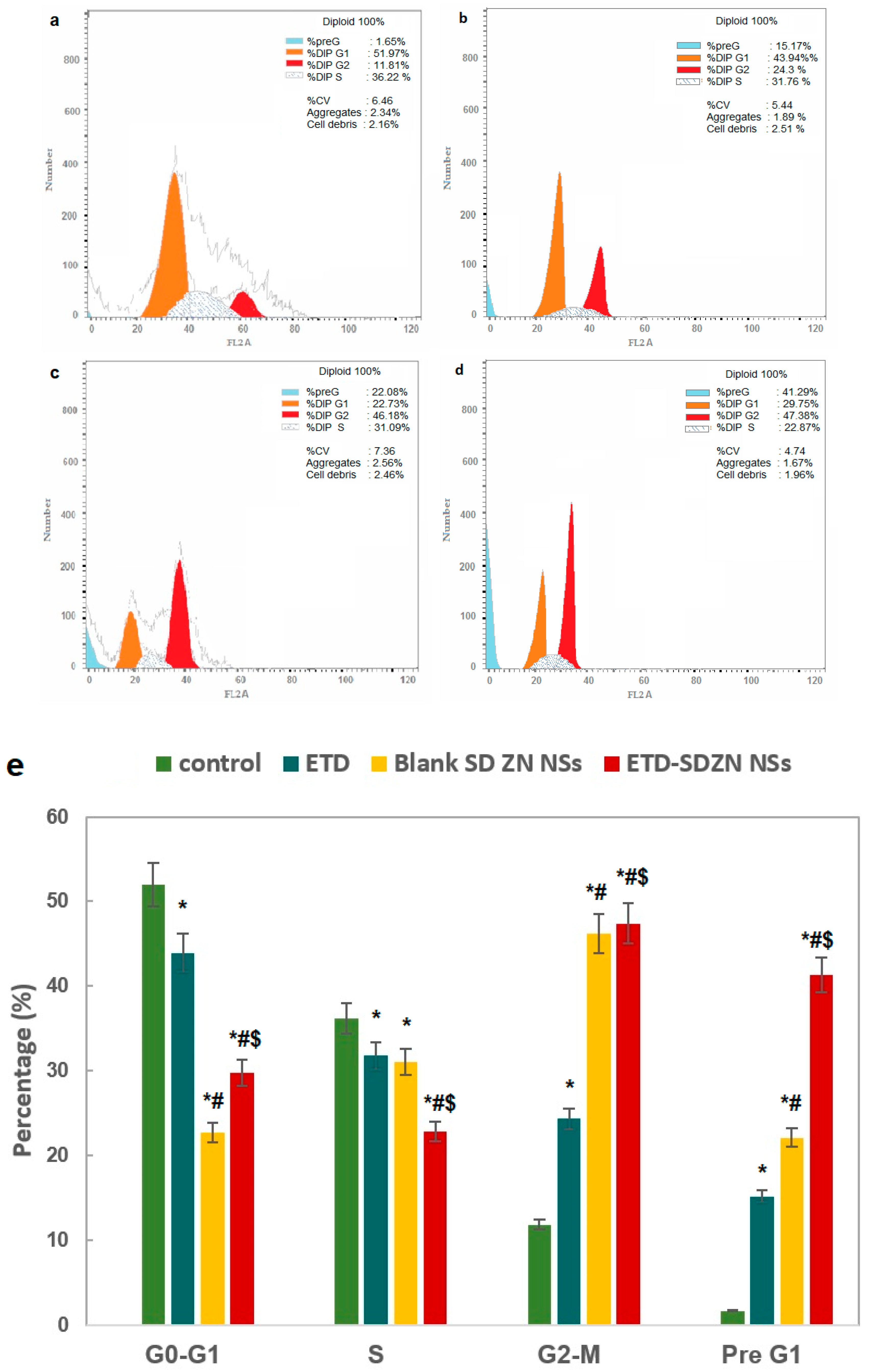
2.6. Cell Cycle Progression Analysis
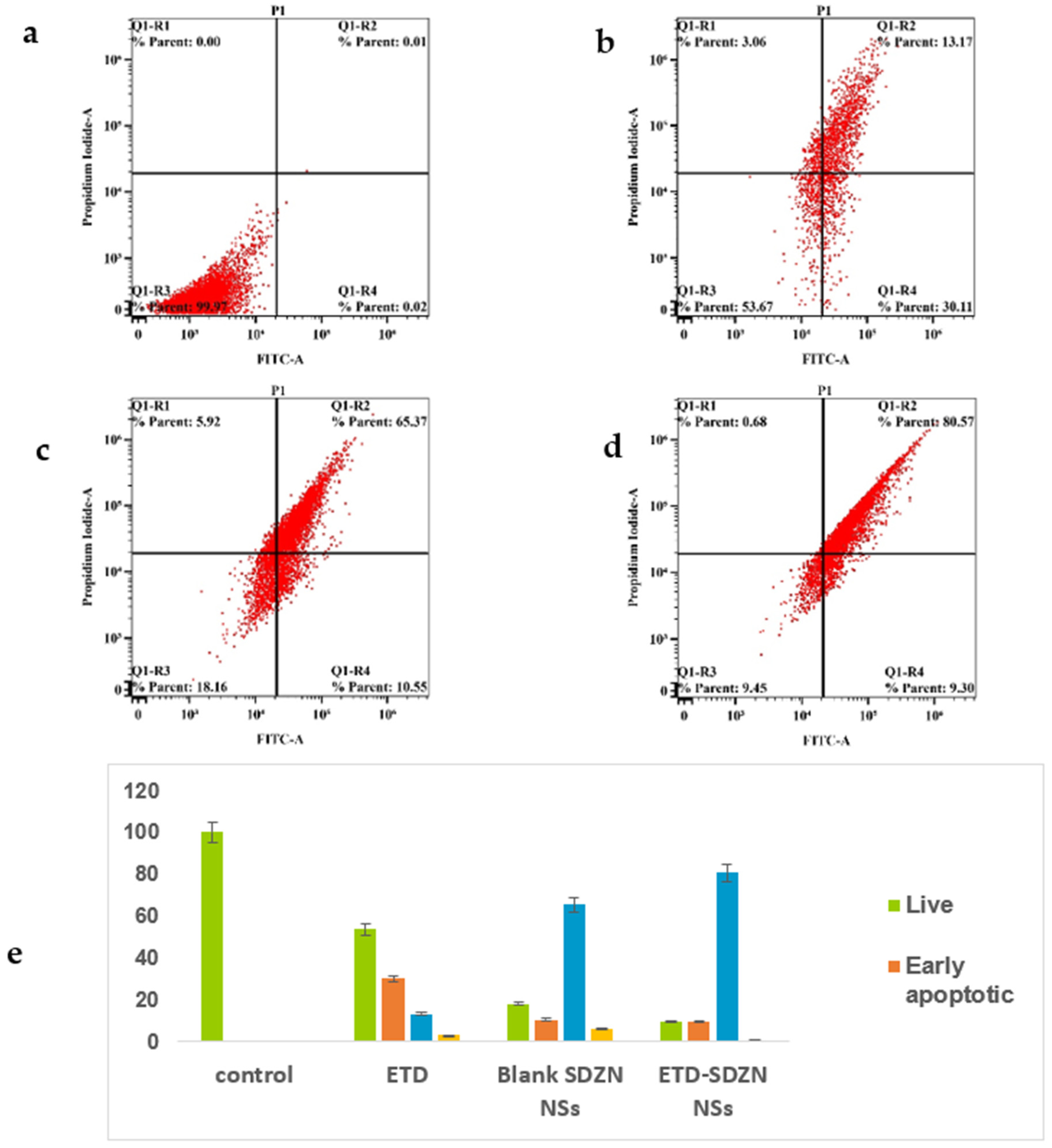
2.7. Annexin-V Assay
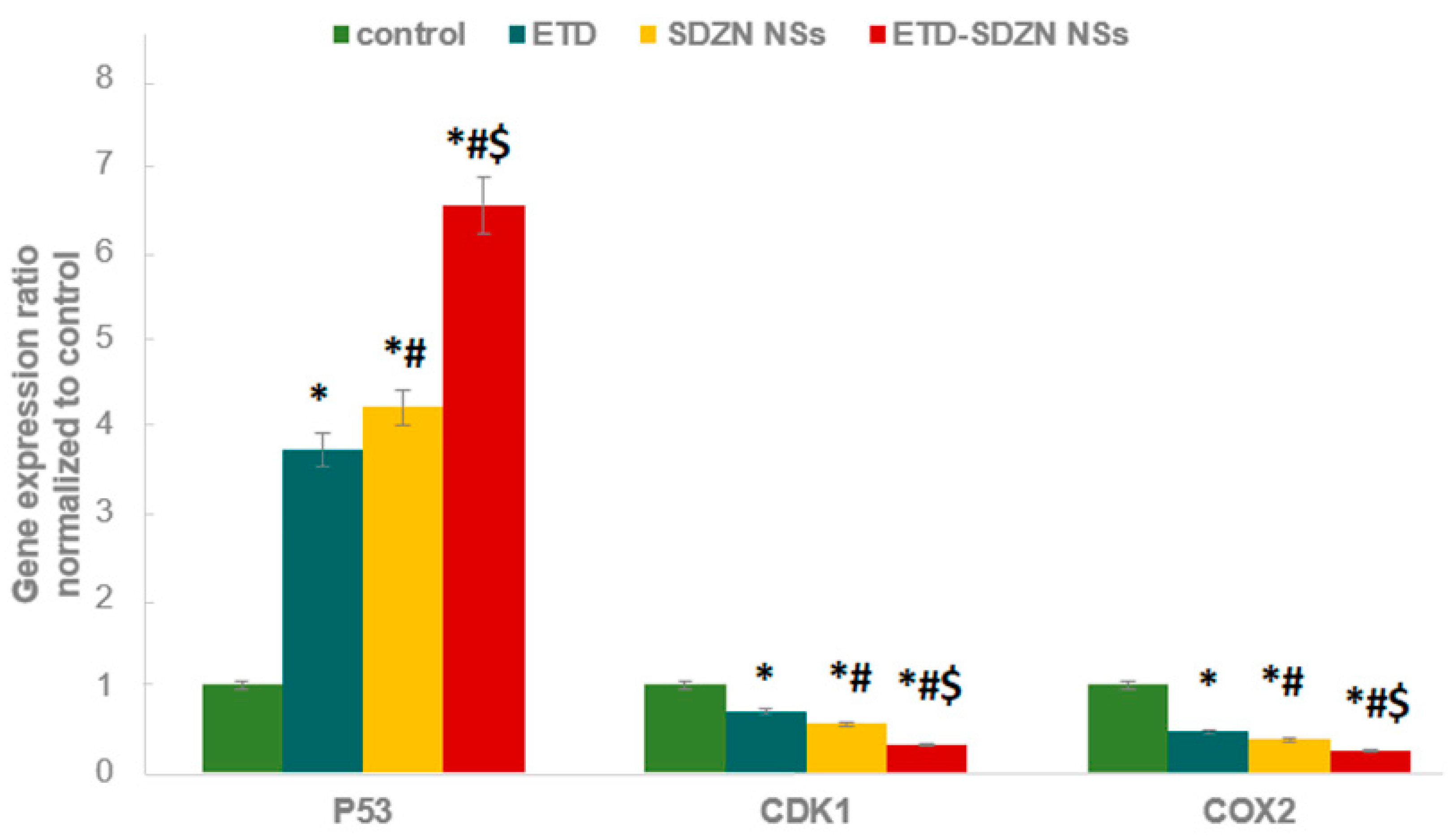
2.8. mRNA Expression of Apoptosis-Related Genes by Quantitative Real-Time Polymerase Chain Reaction (RT-PCR)
2.9. Biochemical Estimation of Oxidative Stress
3. Materials and Methods
3.1. Materials and Cell Lines
3.2. Formulation of ETD-SDZN NSs
3.3. Characterization of ETD-SDZN NSs
3.3.1. Particle Size Analysis
3.3.2. Encapsulation Efficiency (E.E.)
3.3.3. Surface Morphology
3.4. Colloidal Stability
3.5. In Vitro Simulated Digestion Assay
3.6. Cytotoxicity Assay
3.7. Cellular Uptake Analysis
3.8. Cell Cycle Progression Analysis
3.9. Annexin-V Assay
3.10. mRNA Expression of Apoptosis-Related Genes by Quantitative Real-Time Polymerase Chain Reaction (RT-PCR)
3.11. Glutathione Reductase Enzyme Assay
3.12. Reactive Oxygen Species Assay (ROS) and Malondialdehyde (MDA) Assays
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bertolini, F.; Sukhatme, V.P.; Bouche, G. Drug repurposing in oncology—Patient and health systems opportunities. Nat. Rev. Clin. Oncol. 2015, 12, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Ashburn, T.T.; Thor, K.B. Drug repositioning: Identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004, 3, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. Partnering between pharma peers on the rise. Nat. Rev. Drug Discov. 2011, 10, 561–563. [Google Scholar] [CrossRef] [PubMed]
- Nosengo, N. Can you teach old drugs new tricks? Nat. News 2016, 534, 314. [Google Scholar] [CrossRef]
- Balogh, J.; Victor, D., III; Asham, E.; Burroughs, S.; Boktour, M.; Saharia, A.; Li, X.; Ghobrial, R.; Monsour, H. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 7. [Google Scholar]
- Bruix, J.; Gores, G.J.; Mazzaferro, V. Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut 2014, 63, 844–855. [Google Scholar] [CrossRef]
- Liver Cancer Study Group of Japan. Primary liver cancer in Japan. Clinicopathologic features and results of surgical treatment. Ann. Surg. 1990, 211, 277–287. [Google Scholar]
- Fabregat, I. Dysregulation of apoptosis in hepatocellular carcinoma cells. World J. Gastroenterol. 2009, 15, 513. [Google Scholar] [CrossRef]
- Ikeguchi, M.; Hirooka, Y.; Kaibara, N. Quantitative analysis of apoptosis-related gene expression in hepatocellular carcinoma. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2002, 95, 1938–1945. [Google Scholar] [CrossRef]
- Brown, J.M.; Attardi, L.D. The role of apoptosis in cancer development and treatment response. Nat. Rev. Cancer 2005, 5, 231–237. [Google Scholar] [CrossRef]
- Kim, R.; Emi, M.; Tanabe, K. The role of apoptosis in cancer cell survival and therapeutic outcome. Cancer Biol. Ther. 2006, 5, 1429–1442. [Google Scholar] [CrossRef] [PubMed]
- Gerl, R.; Vaux, D.L. Apoptosis in the development and treatment of cancer. Carcinogenesis 2005, 26, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Balfour, J.A.; Buckley, M.M. Etodolac. A reappraisal of its pharmacology and therapeutic use in rheumatic diseases and pain states. Drugs 1991, 42, 274–299. [Google Scholar] [CrossRef] [PubMed]
- He, T.-C.; Chan, T.A.; Vogelstein, B.; Kinzler, K.W. PPARδ is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell 1999, 99, 335–345. [Google Scholar] [CrossRef]
- Sevinç, S.K.; Orun, O.; Tiber, P.M.; Çıkla-Süzgün, P.; Küçükgüzel, Ş.G. Anti-Cancer Acitivity of Etodolac and Its Derivatives on Prostate and Colorectal Cancer Cell Lines. Proceedings 2018, 25, 1573. [Google Scholar]
- Schattenkirchner, M. An updated safety profile of etodolac in several thousand patients. Eur. J. Rheumatol. Inflamm. 1990, 10, 56–65. [Google Scholar]
- Elzoghby, A.; Freag, M.; Mamdouh, H.; Elkhodairy, K. Zein-based nanocarriers as potential natural alternatives for drug and gene delivery: Focus on cancer therapy. Curr. Pharm. Des. 2017, 23, 5261–5271. [Google Scholar] [CrossRef]
- Algandaby, M.M.; Al-Sawahli, M.M. Augmentation of anti-proliferative, pro-apoptotic and oxidant profiles induced by piceatannol in human breast carcinoma MCF-7 cells using zein nanostructures. Biomed. Pharmacother. 2021, 138, 111409. [Google Scholar] [CrossRef]
- Algandaby, M.M.; Al-Sawahli, M.M.; Ahmed, O.A.; Fahmy, U.A.; Abdallah, H.M.; Hattori, M.; Abdel-Naim, A.B. Curcumin-zein nanospheres improve liver targeting and antifibrotic activity of curcumin in carbon tetrachloride-induced mice liver fibrosis. J. Biomed. Nanotechnol. 2016, 12, 1746–1757. [Google Scholar] [CrossRef]
- Hashem, F.M.; Al-Sawahli, M.M.; Nasr, M.; Ahmed, O.A. Optimized zein nanospheres for improved oral bioavailability of atorvastatin. Int. J. Nanomed. 2015, 10, 4059. [Google Scholar]
- Lucio, D.; Martínez-Ohárriz, M.C.; Jaras, G.; Aranaz, P.; González-Navarro, C.J.; Radulescu, A.; Irache, J.M. Optimization and evaluation of zein nanoparticles to improve the oral delivery of glibenclamide. In vivo study using C. elegans. Eur. J. Pharm. Biopharm. 2017, 121, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.J.; Jones, O.G. Stabilizing zein nanoparticle dispersions with ι-carrageenan. Food Hydrocoll. 2017, 69, 28–35. [Google Scholar] [CrossRef]
- Ahmed, O.A.; Hosny, K.M.; Al-Sawahli, M.M.; Fahmy, U.A. Optimization of caseinate-coated simvastatin-zein nanoparticles: Improved bioavailability and modified release characteristics. Drug Des. Dev. Ther. 2015, 9, 655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, J.; Liu, D.; Zhu, W.; Guan, S.; Fan, L.; Cai, D. Targeted delivery of honokiol by zein/hyaluronic acid core-shell nanoparticles to suppress breast cancer growth and metastasis. Carbohydr. Polym. 2020, 240, 116325. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.; Paolino, D.; Iannone, M.; Palma, E.; Fresta, M.; Cosco, D. Sodium deoxycholate-decorated zein nanoparticles for a stable colloidal drug delivery system. Int. J. Nanomed. 2018, 13, 601. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Li, H.; Zhu, J.; Liu, C.; Sun, X.; Wang, D.; Xu, Y. Fabrication and characterization of zein nanoparticles by dextran sulfate coating as vehicles for delivery of curcumin. Int. J. Biol. Macromol. 2020, 151, 1074–1083. [Google Scholar] [CrossRef]
- Hassan, M.; Watari, H.; AbuAlmaaty, A.; Ohba, Y.; Sakuragi, N. Apoptosis and molecular targeting therapy in cancer. BioMed Res. Int. 2014, 2014, 150845. [Google Scholar] [CrossRef]
- Hashem, F.M.; Al-Sawahli, M.M.; Nasr, M.; Ahmed, O.A. Custom fractional factorial designs to develop atorvastatin self-nanoemulsifying and nanosuspension delivery systems–enhancement of oral bioavailability. Drug Des. Dev. Ther. 2015, 9, 3141. [Google Scholar]
- Gagliardi, A.; Bonacci, S.; Paolino, D.; Celia, C.; Procopio, A.; Fresta, M.; Cosco, D. Paclitaxel-loaded sodium deoxycholate-stabilized zein nanoparticles: Characterization and in vitro cytotoxicity. Heliyon 2019, 5, e02422. [Google Scholar] [CrossRef]
- Kutbi, H.I.; Kammoun, A.K.; El-Telbany, D.F. Amelioration of Pterostilbene Antiproliferative, Proapoptotic, and Oxidant Potentials in Human Breast Cancer MCF7 Cells Using Zein Nanocomposites. Int. J. Nanomed. 2021, 16, 3059. [Google Scholar] [CrossRef]
- Lim, S.; Park, J.; Shim, M.K.; Um, W.; Yoon, H.Y.; Ryu, J.H.; Kim, K. Recent advances and challenges of repurposing nanoparticle-based drug delivery systems to enhance cancer immunotherapy. Theranostics 2019, 9, 7906. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Guo, H. Preparation of new 5-fluorouracil-loaded zein nanoparticles for liver targeting. Int. J. Pharm. 2011, 404, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Davidov-Pardo, G.; Joye, I.J.; Espinal-Ruiz, M.; McClements, D.J. Effect of maillard conjugates on the physical stability of zein nanoparticles prepared by liquid antisolvent coprecipitation. J. Agric. Food Chem. 2015, 63, 8510–8518. [Google Scholar] [CrossRef]
- Davidov-Pardo, G.; Peérez-Ciordia, S.; Marín-Arroyo, M.R.; McClements, D.J. Improving resveratrol bioaccessibility using biopolymer nanoparticles and complexes: Impact of protein–carbohydrate Maillard conjugation. J. Agric. Food Chem. 2015, 63, 3915–3923. [Google Scholar] [CrossRef]
- Dhanapal, J.; Balaraman Ravindrran, M. Chitosan/poly (lactic acid)-coated piceatannol nanoparticles exert an in vitro apoptosis activity on liver, lung and breast cancer cell lines. Artif. Cells Nanomed. Biotechnol. 2018, 46, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G.; Maynard, A.; Donaldson, K.; Castranova, V.; Fitzpatrick, J.; Ausman, K.; Yang, H. Principles for characterizing the potential human health effects from exposure to nanomaterials: Elements of a screening strategy. Part. Fibre Toxicol. 2005, 2, 8. [Google Scholar] [CrossRef]
- Fernández-Carneado, J.; Kogan, M.J.; Castel, S.; Giralt, E. Potential peptide carriers: Amphipathic proline-rich peptides derived from the N-terminal domain of γ-zein. Angew. Chem. Int. Ed. 2004, 43, 1811–1814. [Google Scholar] [CrossRef]
- Conner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44. [Google Scholar] [CrossRef]
- Kanwar, J.R.; Samarasinghe, R.M.; Sehgal, R.; Kanwar, R.K. Nano-lactoferrin in diagnostic, imaging and targeted delivery for cancer and infectious diseases. J. Cancer Sci. Ther. 2012, 4, 31–42. [Google Scholar] [CrossRef]
- Roy, D.; Arason, G.; Chowdhury, B.; Mitra, A.; Calaf, G. Profiling of cell cycle genes of breast cells exposed to etodolac. Oncol. Rep. 2010, 23, 1383–1391. [Google Scholar] [CrossRef][Green Version]
- Mabrouk, A.A.; Tadros, M.I.; El-Refaie, W.M. Improving the Efficacy of Cyclooxegenase-2 Inhibitors in the Management of Oral Cancer: Insights into the Implementation of Nanotechnology and Mucoadhesion. J. Drug Deliv. Sci. Technol. 2020, 61, 102240. [Google Scholar] [CrossRef]
- Adhim, Z.; Matsuoka, T.; Bito, T.; Shigemura, K.; Lee, K.-M.; Kawabata, M.; Shirakawa, T. In vitro and in vivo inhibitory effect of three Cox-2 inhibitors and epithelial-to-mesenchymal transition in human bladder cancer cell lines. Br. J. Cancer 2011, 105, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.J. p53, the cellular gatekeeper for growth and division. Cell 1997, 88, 323–331. [Google Scholar] [CrossRef]
- Wang, X.W.; Hussain, S.P.; Huo, T.-I.; Wu, C.-G.; Forgues, M.; Hofseth, L.J.; Harris, C.C. Molecular pathogenesis of human hepatocellular carcinoma. Toxicology 2002, 181, 43–47. [Google Scholar] [CrossRef]
- Mukawa, K.; Fujii, S.; Tominaga, K.; Yoshitake, N.; Abe, A.; Kono, T.; Fujimori, T. Inhibitory effects of the cyclooxygenase-2 inhibitor, etodolac, on colitis-associated tumorigenesis in p53-deficient mice treated with dextran sulfate sodium. Oncol. Rep. 2008, 19, 393–399. [Google Scholar] [CrossRef][Green Version]
- Tsaur, I.; Makarević, J.; Hudak, L.; Juengel, E.; Kurosch, M.; Wiesner, C.; Blaheta, R.A. The cdk1-cyclin B complex is involved in everolimus triggered resistance in the PC3 prostate cancer cell line. Cancer Lett. 2011, 313, 84–90. [Google Scholar] [CrossRef]
- Martínez-García, D.; Manero-Rupérez, N.; Quesada, R.; Korrodi-Gregório, L.; Soto-Cerrato, V. Therapeutic strategies involving survivin inhibition in cancer. Med. Res. Rev. 2019, 39, 887–909. [Google Scholar] [CrossRef]
- Castellone, M.D.; Teramoto, H.; Williams, B.O.; Druey, K.M.; Gutkind, J.S. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-ß-catenin signaling axis. Science 2005, 310, 1504–1510. [Google Scholar] [CrossRef]
- Jänne, P.A.; Mayer, R.J. Chemoprevention of colorectal cancer. N. Engl. J. Med. 2000, 342, 1960–1968. [Google Scholar] [CrossRef]
- Schwab, R.B.; Kato, S.; Crain, B.; Pu, M.; Messer, K.; Weidner, N.; Parker, B.A. A window-of-opportunity biomarker study of etodolac in resectable breast cancer. Cancer Med. 2015, 4, 1583–1588. [Google Scholar] [CrossRef]
- Orun, O.; Tiber, P.M.; Sevinç, S.K. Apoptotic Effects of Etodolac in Breast Cancer Cell Cultures. In Nonsteroidal Anti-Inflammatory Drugs; IntechOpen: London, UK, 2017. [Google Scholar]
- Costa, D.; Gomes, A.; Reis, S.; Lima, J.L.; Fernandes, E. Hydrogen peroxide scavenging activity by non-steroidal anti-inflammatory drugs. Life Sci. 2005, 76, 2841–2848. [Google Scholar] [CrossRef] [PubMed]
- Marnett, L.J. Oxyradicals and DNA damage. Carcinogenesis 2000, 21, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Sarıözkan, S.; Türk, G.; Çıkla-Süzgün, P.; Güvenç, M.; Yüce, A.; Yay, A.; Küçükgüzel, Ş.G. Effect of etodolac hydrazone, a new compound synthesised from etodolac, on spermatozoon quality, testicular lipid peroxidation, apoptosis and spermatozoon DNA integrity. Andrologia 2016, 48, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 1–32. [Google Scholar] [CrossRef]
- Balan, P.; Nimila, I.C.; Prasanna, M.L.; Rani, M.V.; Rajasekar, S. RP-HPLC method development and validation of etodolac and paracetamol in combined dosage form. Asian J. Res. Chem. 2011, 4, 1073–1076. [Google Scholar]
| Formula | ETD:ZN Ratio | SD Concentration (w/v%) | Mean Particle Size (nm) | Zeta Potential (mV) | Poly-Dispersity Index | Encapsulation Efficiency (%) |
|---|---|---|---|---|---|---|
| E1 | 3:1 | 1 | 265.1 ± 22.1 | 22.6 ± 1.8 | 0.67 ± 0.02 | 81.4 ± 4.9 |
| E2 | 3:1 | 2.5 | 291.3 ±15.7 | 19.2 ± 1.3 | 0.51 ± 0.01 | 87.6 ± 2.8 |
| E3 | 3:1 | 5 | 387 ± 17.8 | 31.4 ± 1.2 | 0.38 ± 0.02 | 88.3 ± 2.3 |
| E4 | 1:1 | 1 | 248.2 ± 15.9 | 21.1 ± 0.9 | 0.42 ± 0.02 | 90.7 ± 4.1 |
| E5 | 1:1 | 2.5 | 277.3 ± 20.8 | 24.0 ± 1.8 | 0.36 ± 0.01 | 92.1 ± 3.6 |
| E6 | 1:1 | 5 | 286.9 ± 23.5 | 25.9 ± 1.6 | 0.39 ± 0.02 | 90.7 ± 5.1 |
| E7 | 1:3 | 1 | 113.6 ± 7.4 | 32.7 ± 2.3 | 0.19 ± 0.01 | 93.3 ± 5.2 |
| E8 | 1:3 | 2.5 | 141.0 ± 5.5 | 35.6 ± 1.2 | 0.21 ± 0.01 | 92.6 ± 5.6 |
| E9 | 1:3 | 5 | 193.4 ± 13.1 | 31.4 ± 2.7 | 0.27 ± 0.02 | 93.0 ± 6.9 |
| GR (µU/106 Cells) | ROS (Pg/106 Cells) | MDA (nmol/106 Cells) | |
|---|---|---|---|
| Control | 1.83 ± 0.16 | 178.21 ± 11.7 | 1.39 ± 0.82 |
| ETD | 1.62 * ± 0.08 | 188.49 * ± 9.3 | 2.16 * ± 0.17 |
| SDZN NSs | 1.35 *,# ± 0.38 | 195.63 * ± 7.8 | 2.34 *,# ± 0.41 |
| ETD-SDZN NSs | 1.29 *,#,$ ± 0.09 | 209.71 *,#,$ ± 12.4 | 2.79 *,#,$ ± 0.22 |
| Primer | Sequence | |
|---|---|---|
| CASP3 | Forward primer | TTCATTATTCAGGCCTGCCGAGG |
| Reverse primer | TTCTGACAGGCCATGTCATCCTCA | |
| p53 | Forward primer | CCCCTCCTGGCCCCTGTCATCTTC |
| Reverse primer | GCAGCGCCTCACAACCTCCGTCAT | |
| CDK1 | Forward primer | TGGATCTGAAGAAATACTTGGATTCTA |
| Reverse primer | CAATCCCCTGTAGGATTTGG | |
| COX-2 | Forward primer | CTCAGACAGCAAAGCCTACC |
| Reverse primer | TGACTCCTTTCTCCGCAACA | |
| Β-actin | Forward primer | TCCGTCGCCGGTCCACACCC |
| Reverse primer | TCACCAACTGGGACGATATG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kammoun, A.K.; Hegazy, M.A.; Khedr, A.; Awan, Z.A.; Khayat, M.T.; Al-Sawahli, M.M. Etodolac Fortified Sodium Deoxycholate Stabilized Zein Nanoplatforms for Augmented Repositioning Profile in Human Hepatocellular Carcinoma: Assessment of Bioaccessibility, Anti-Proliferation, Pro-Apoptosis and Oxidant Potentials in HepG2 Cells. Pharmaceuticals 2022, 15, 916. https://doi.org/10.3390/ph15080916
Kammoun AK, Hegazy MA, Khedr A, Awan ZA, Khayat MT, Al-Sawahli MM. Etodolac Fortified Sodium Deoxycholate Stabilized Zein Nanoplatforms for Augmented Repositioning Profile in Human Hepatocellular Carcinoma: Assessment of Bioaccessibility, Anti-Proliferation, Pro-Apoptosis and Oxidant Potentials in HepG2 Cells. Pharmaceuticals. 2022; 15(8):916. https://doi.org/10.3390/ph15080916
Chicago/Turabian StyleKammoun, Ahmed K., Maha A. Hegazy, Alaa Khedr, Zuhier Ahmed Awan, Maan T. Khayat, and Majid Mohammad Al-Sawahli. 2022. "Etodolac Fortified Sodium Deoxycholate Stabilized Zein Nanoplatforms for Augmented Repositioning Profile in Human Hepatocellular Carcinoma: Assessment of Bioaccessibility, Anti-Proliferation, Pro-Apoptosis and Oxidant Potentials in HepG2 Cells" Pharmaceuticals 15, no. 8: 916. https://doi.org/10.3390/ph15080916
APA StyleKammoun, A. K., Hegazy, M. A., Khedr, A., Awan, Z. A., Khayat, M. T., & Al-Sawahli, M. M. (2022). Etodolac Fortified Sodium Deoxycholate Stabilized Zein Nanoplatforms for Augmented Repositioning Profile in Human Hepatocellular Carcinoma: Assessment of Bioaccessibility, Anti-Proliferation, Pro-Apoptosis and Oxidant Potentials in HepG2 Cells. Pharmaceuticals, 15(8), 916. https://doi.org/10.3390/ph15080916






