Effect of Administration of Azithromycin and/or Probiotic Bacteria on Bones of Estrogen-Deficient Rats
Abstract
:1. Introduction
2. Results
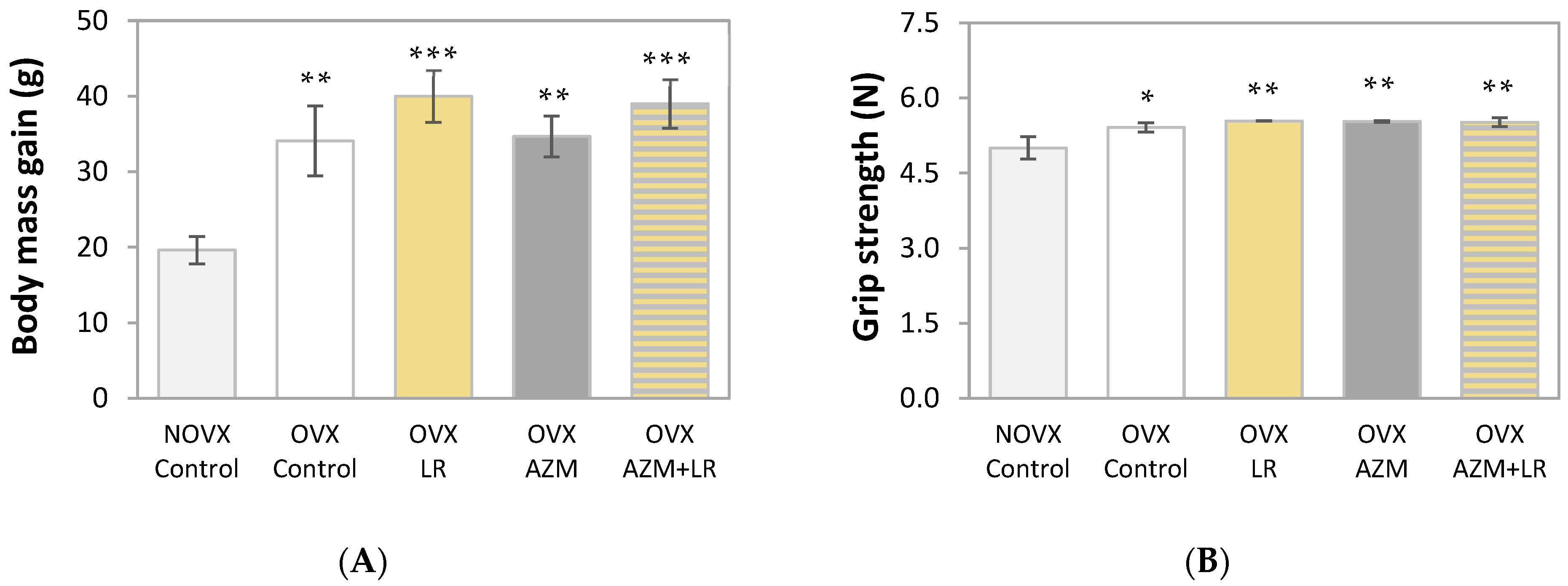
2.1. Effects of AZM and/or LR on Body Mass Gain and Grip Strength
2.2. Effect of AZM and/or LR on Serum Biochemical Parameters
2.3. Effects of AZM and/or LR on the Mass, Composition, Mineralization, and Density of the Femur
2.4. Effect of AZM and/or LR on the Histomorphometric Parameters of The Femur
2.5. Effects of AZM and/or LR on Bone Mechanical Properties
3. Discussion
4. Materials and Methods
4.1. Animals and Drugs Used
- NOVX control rats;
- OVX control rats;
- OVX rats treated with LR;
- OVX rats treated with AZM;
- OVX rats treated with AZM and LR.
4.2. Biochemical Studies
4.3. Bone Macrometric Parameters, Composition, and Mineralization Studies
4.4. Bone Histomorphometric Studies
4.5. Bone Mechanical Property Studies
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kallala, R.; Graham, S.M.; Nikkhah, D.; Kyrkos, M.; Heliotis, M.; Mantalaris, A.; Tsiridis, E. In Vitro and in Vivo Effects of Antibiotics on Bone Cell Metabolism and Fracture Healing. Expert Opin. Drug Saf. 2012, 11, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.J.; Guss, J.D.; Luna, M.; Goldring, S.R. Links between the Microbiome and Bone. J. Bone Miner. Res. 2016, 31, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Charles, J.F. Gut Microbiome and Bone: To Build, Destroy, or Both? Curr. Osteoporos. Rep. 2017, 15, 376–384. [Google Scholar] [CrossRef]
- Ohlsson, C.; Sjögren, K. Osteomicrobiology: A New Cross-Disciplinary Research Field. Calcif. Tissue Int. 2018, 102, 426–432. [Google Scholar] [CrossRef]
- Folwarczna, J.; Pytlik, M.; Janiec, W. Effects of Doxycycline on Development of Changes in Histomorphometric Parameters of Bones Induced by Bilateral Ovariectomy in Rats. Pol. J. Pharmacol. 2003, 55, 433–441. [Google Scholar] [PubMed]
- Pytlik, M.; Folwarczna, J.; Janiec, W. Effects of Doxycycline on Mechanical Properties of Bones in Rats with Ovariectomy-Induced Osteopenia. Calcif. Tissue Int. 2004, 75, 225–230. [Google Scholar] [CrossRef]
- Parnham, M.J.; Haber, V.E.; Giamarellos-Bourboulis, E.J.; Perletti, G.; Verleden, G.M.; Vos, R. Azithromycin: Mechanisms of Action and Their Relevance for Clinical Applications. Pharmacol. Ther. 2014, 143, 225–245. [Google Scholar] [CrossRef]
- McMullan, B.J.; Mostaghim, M. Prescribing Azithromycin. Aust. Prescr. 2015, 38, 87–89. [Google Scholar] [CrossRef] [Green Version]
- Landersdorfer, C.B.; Bulitta, J.B.; Kinzig, M.; Holzgrabe, U.; Sörgel, F. Penetration of Antibacterials into Bone. Clin. Pharmacokinet. 2009, 48, 89–124. [Google Scholar] [CrossRef]
- Cheung, P.S.; Si, E.C.; Hosseini, K. Anti-Inflammatory Activity of Azithromycin as Measured by Its NF-кB Inhibitory Activity. Ocul. Immunol. Inflamm. 2010, 18, 32–37. [Google Scholar] [CrossRef]
- Naderi, N.; Assayag, D.; Mostafavi-Pour-Manshadi, S.-M.-Y.; Kaddaha, Z.; Joubert, A.; Ouellet, I.; Drouin, I.; Li, P.Z.; Bourbeau, J. Long-Term Azithromycin Therapy to Reduce Acute Exacerbations in Patients with Severe Chronic Obstructive Pulmonary Disease. Respir. Med. 2018, 138, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.T.-J.; Ward, C.; Meachery, G.; Lordan, J.L.; Fisher, A.J.; Corris, P.A. Long-Term Effect of Azithromycin in Bronchiolitis Obliterans Syndrome. BMJ Open Respir. Res. 2019, 6, e000465. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.-P.; Xuan, N.; Wang, Y.; Zhang, G.; Cui, W. The Efficacy and Safety of Azithromycin in Asthma: A Systematic Review. J. Cell. Mol. Med. 2019, 23, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Cramer, C.L.; Patterson, A.; Alchakaki, A.; Soubani, A.O. Immunomodulatory Indications of Azithromycin in Respiratory Disease: A Concise Review for the Clinician. Postgrad. Med. 2017, 129, 493–499. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, V.J. Azithromycin as an Adjunct to Non-Surgical Periodontal Therapy: A Systematic Review. Aust. Dent. J. 2017, 62, 14–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagannathan, N.; Acharya, A.; Yi Farn, O.; Li, K.Y.; Nibali, L.; Pelekos, G. Disease Severity, Debridement Approach and Timing of Drug Modify Outcomes of Adjunctive Azithromycin in Non-Surgical Management of Chronic Periodontitis: A Multivariate Meta-Analysis. BMC Oral Health 2019, 19, 65. [Google Scholar] [CrossRef] [PubMed]
- Damle, B.; Vourvahis, M.; Wang, E.; Leaney, J.; Corrigan, B. Clinical Pharmacology Perspectives on the Antiviral Activity of Azithromycin and Use in COVID-19. Clin. Pharmacol. Ther. 2020, 108, 201–211. [Google Scholar] [CrossRef]
- Oliver, M.E.; Hinks, T.S.C. Azithromycin in Viral Infections. Rev. Med. Virol. 2021, 31, e2163. [Google Scholar] [CrossRef]
- Echeverría-Esnal, D.; Martin-Ontiyuelo, C.; Navarrete-Rouco, M.E.; De-Antonio Cuscó, M.; Ferrández, O.; Horcajada, J.P.; Grau, S. Azithromycin in the Treatment of COVID-19: A Review. Expert Rev. Anti. Infect. Ther. 2021, 19, 147–163. [Google Scholar] [CrossRef]
- Arshad, S.; Kilgore, P.; Chaudhry, Z.S.; Jacobsen, G.; Wang, D.D.; Huitsing, K.; Brar, I.; Alangaden, G.J.; Ramesh, M.S.; McKinnon, J.E.; et al. Treatment with Hydroxychloroquine, Azithromycin, and Combination in Patients Hospitalized with COVID-19. Int. J. Infect. Dis. 2020, 97, 396–403. [Google Scholar] [CrossRef]
- Fiolet, T.; Guihur, A.; Rebeaud, M.E.; Mulot, M.; Peiffer-Smadja, N.; Mahamat-Saleh, Y. Effect of Hydroxychloroquine with or without Azithromycin on the Mortality of Coronavirus Disease 2019 (COVID-19) Patients: A Systematic Review and Meta-Analysis. Clin. Microbiol. Infect. 2021, 27, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Rizk, J.G.; Kalantar-Zadeh, K.; Mehra, M.R.; Lavie, C.J.; Rizk, Y.; Forthal, D.N. Pharmaco-Immunomodulatory Therapy in COVID-19. Drugs 2020, 80, 1267–1292. [Google Scholar] [CrossRef] [PubMed]
- Bakadia, B.M.; He, F.; Souho, T.; Lamboni, L.; Ullah, M.W.; Boni, B.O.; Ahmed, A.A.Q.; Mukole, B.M.; Yang, G. Prevention and Treatment of COVID-19: Focus on Interferons, Chloroquine/Hydroxychloroquine, Azithromycin, and Vaccine. Biomed. Pharmacother. 2021, 133, 111008. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D. Human Gut Microbiome: Hopes, Threats and Promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef]
- Belkaid, Y.; Harrison, O.J. Homeostatic Immunity and the Microbiota. Immunity 2017, 46, 562–576. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.-X.; Wang, H.-Y.; Chen, T.-X. Interactions between Intestinal Microflora/Probiotics and the Immune System. Biomed. Res. Int. 2019, 2019, 6764919. [Google Scholar] [CrossRef] [Green Version]
- Pushpanathan, P.; Mathew, G.S.; Selvarajan, S.; Seshadri, K.G.; Srikanth, P. Gut Microbiota and Its Mysteries. Indian J. Med. Microbiol. 2019, 37, 268–277. [Google Scholar] [CrossRef]
- Weiss, G.A.; Hennet, T. Mechanisms and Consequences of Intestinal Dysbiosis. Cell. Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef] [Green Version]
- Cooney, O.D.; Nagareddy, P.R.; Murphy, A.J.; Lee, M.K.S. Healthy Gut, Healthy Bones: Targeting the Gut Microbiome to Promote Bone Health. Front. Endocrinol. 2021, 11, 620466. [Google Scholar] [CrossRef]
- Knudsen, J.K.; Leutscher, P.; Sørensen, S. Gut Microbiota in Bone Health and Diabetes. Curr. Osteoporos. Rep. 2021, 19, 462–479. [Google Scholar] [CrossRef]
- Li, S.; Mao, Y.; Zhou, F.; Yang, H.; Shi, Q.; Meng, B. Gut Microbiome and Osteoporosis: A Review. Bone Jt. Res. 2020, 9, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Chassaing, B.; Tyagi, A.M.; Vaccaro, C.; Luo, T.; Adams, J.; Darby, T.M.; Weitzmann, M.N.; Mulle, J.G.; Gewirtz, A.T.; et al. Sex Steroid Deficiency-Associated Bone Loss Is Microbiota Dependent and Prevented by Probiotics. J. Clin. Investig. 2016, 126, 2049–2063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, A.T.; Castelo, P.M.; Ribeiro, D.A.; Ferreira, C.M. Influence of Oral and Gut Microbiota in the Health of Menopausal Women. Front. Microbiol. 2017, 8, 1884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Jia, X.; Mo, L.; Liu, C.; Zheng, L.; Yuan, Q.; Zhou, X. Intestinal Microbiota: A Potential Target for the Treatment of Postmenopausal Osteoporosis. Bone Res. 2017, 5, 17046. [Google Scholar] [CrossRef]
- Britton, R.A.; Irwin, R.; Quach, D.; Schaefer, L.; Zhang, J.; Lee, T.; Parameswaran, N.; McCabe, L.R. Probiotic L. reuteri Treatment Prevents Bone Loss in a Menopausal Ovariectomized Mouse Model. J. Cell. Physiol. 2014, 229, 1822–1830. [Google Scholar] [CrossRef] [Green Version]
- Dar, H.Y.; Shukla, P.; Mishra, P.K.; Anupam, R.; Mondal, R.K.; Tomar, G.B.; Sharma, V.; Srivastava, R.K. Lactobacillus acidophilus Inhibits Bone Loss and Increases Bone Heterogeneity in Osteoporotic Mice via Modulating Treg-Th17 Cell Balance. Bone Rep. 2018, 8, 46–56. [Google Scholar] [CrossRef]
- Sapra, L.; Dar, H.Y.; Bhardwaj, A.; Pandey, A.; Kumari, S.; Azam, Z.; Upmanyu, V.; Anwar, A.; Shukla, P.; Mishra, P.K.; et al. Lactobacillus Rhamnosus Attenuates Bone Loss and Maintains Bone Health by Skewing Treg-Th17 Cell Balance in Ovx Mice. Sci. Rep. 2021, 11, 1807. [Google Scholar] [CrossRef]
- Sözen, T.; Özışık, L.; Başaran, N.Ç. An Overview and Management of Osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef]
- D’Amelio, P.; Grimaldi, A.; Di Bella, S.; Brianza, S.Z.M.; Cristofaro, M.A.; Tamone, C.; Giribaldi, G.; Ulliers, D.; Pescarmona, G.P.; Isaia, G. Estrogen Deficiency Increases Osteoclastogenesis Up-Regulating T Cells Activity: A Key Mechanism in Osteoporosis. Bone 2008, 43, 92–100. [Google Scholar] [CrossRef]
- Li, L.; Wang, Z. Ovarian Aging and Osteoporosis. Adv. Exp. Med. Biol. 2018, 1086, 199–215. [Google Scholar] [CrossRef]
- Föger-Samwald, U.; Dovjak, P.; Azizi-Semrad, U.; Kerschan-Schindl, K.; Pietschmann, P. Osteoporosis: Pathophysiology and Therapeutic Options. EXCLI J. 2020, 19, 1017–1037. [Google Scholar] [CrossRef] [PubMed]
- Hsu, E.; Pacifici, R. From Osteoimmunology to Osteomicrobiology: How the Microbiota and the Immune System Regulate Bone. Calcif. Tissue Int. 2018, 102, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, L.; Rouleau, M.; Wakkach, A.; Blin-Wakkach, C. Gut Microbiome and Bone. Jt. Bone Spine 2019, 86, 43–47. [Google Scholar] [CrossRef]
- Tousen, Y.; Matsumoto, Y.; Nagahata, Y.; Kobayashi, I.; Inoue, M.; Ishimi, Y. Resistant Starch Attenuates Bone Loss in Ovariectomised Mice by Regulating the Intestinal Microbiota and Bone-Marrow Inflammation. Nutrients 2019, 11, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, K.; Buerger, M.; Stallmach, A.; Bruns, T. Effects of Antibiotics on Gut Microbiota. Dig. Dis. 2016, 34, 260–268. [Google Scholar] [CrossRef]
- Yoon, M.Y.; Yoon, S.S. Disruption of the Gut Ecosystem by Antibiotics. Yonsei Med. J. 2018, 59, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, A.; Dachang, W.; Xin, Y. Determining the Role of a Probiotic in the Restoration of Intestinal Microbial Balance by Molecular and Cultural Techniques. Genet. Mol. Res. 2015, 14, 1526–1537. [Google Scholar] [CrossRef]
- Gannon, S.C.; Cantley, M.D.; Haynes, D.R.; Hirsch, R.; Bartold, P.M. Azithromycin Suppresses Human Osteoclast Formation and Activity in Vitro. J. Cell. Physiol. 2013, 228, 1098–1107. [Google Scholar] [CrossRef] [Green Version]
- Park, H.S.; Lee, Y.S.; Choi, E.Y.; Choi, J. Il; Choi, I.S.; Kim, S.J. Subantibiotic Dose of Azithromycin Attenuates Alveolar Bone Destruction and Improves Trabecular Microarchitectures in a Rat Model of Experimental Periodontitis: A Study Using Micro-Computed Tomography. Int. Immunopharmacol. 2017, 47, 212–217. [Google Scholar] [CrossRef]
- Meng, T.; Zhou, Y.; Li, J.; Hu, M.; Li, X.; Wang, P.; Jia, Z.; Li, L.; Liu, D. Azithromycin Promotes the Osteogenic Differentiation of Human Periodontal Ligament Stem Cells after Stimulation with TNF-α. Stem Cells Int. 2018, 2018, 7961962. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, R. Periodontal Healing and Bone Regeneration in Response to Azithromycin. Aust. Dent. J. 2010, 55, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, M.; Strong, J.M.; Alabi, D.A.; Hernandez, C.J. The Gut Microbiome and Bone Strength. Curr. Osteoporos. Rep. 2020, 18, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Yang, R.; Xu, X.; Zhou, X. The Microbiota-Gut-Bone Axis and Bone Health. J. Leukoc. Biol. 2021, 110, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Behera, J.; Ison, J.; Tyagi, S.C.; Tyagi, N. The Role of Gut Microbiota in Bone Homeostasis. Bone 2020, 135, 115317. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.; Vaquette, C.; Hashimi, S.; Rathnayake, I.; Huygens, F.; Hutmacher, D.W.; Ivanovski, S. Antimicrobial and Immunomodulatory Surface-Functionalized Electrospun Membranes for Bone Regeneration. Adv. Healthc. Mater. 2017, 6, 1601345. [Google Scholar] [CrossRef]
- Kato, K.; Ozaki, M.; Nakai, K.; Nagasaki, M.; Nakajima, J.; Koshi, R.; Tanaka, H.; Kawato, T.; Tonogi, M. Effect of Azithromycin on Mineralized Nodule Formation in MC3T3-E1 Cells. Curr. Issues Mol. Biol. 2021, 43, 1451–1459. [Google Scholar] [CrossRef]
- Doan, T.; Hinterwirth, A.; Arzika, A.M.; Cotter, S.Y.; Ray, K.J.; O’Brien, K.S.; Zhong, L.; Chow, E.D.; Zhou, Z.; Cummings, S.L.; et al. Mass Azithromycin Distribution and Community Microbiome: A Cluster-Randomized Trial. Open Forum Infect. Dis. 2018, 5, ofy182. [Google Scholar] [CrossRef] [Green Version]
- Doan, T.; Arzika, A.M.; Ray, K.J.; Cotter, S.Y.; Kim, J.; Maliki, R.; Zhong, L.; Zhou, Z.; Porco, T.C.; Vanderschelden, B.; et al. Gut Microbial Diversity in Antibiotic-Naive Children after Systemic Antibiotic Exposure: A Randomized Controlled Trial. Clin. Infect. Dis. 2017, 64, 1147–1153. [Google Scholar] [CrossRef] [Green Version]
- McDonnell, L.; Gilkes, A.; Ashworth, M.; Rowland, V.; Harries, T.H.; Armstrong, D.; White, P. Association between Antibiotics and Gut Microbiome Dysbiosis in Children: Systematic Review and Meta-Analysis. Gut Microbes 2021, 13, e1870402. [Google Scholar] [CrossRef]
- Ammor, M.S.; Flórez, A.B.; Van Hoek, A.H.A.M.; De Los Reyes-Gavilán, C.G.; Aarts, H.J.M.; Margolles, A.; Mayo, B. Molecular Characterization of Intrinsic and Acquired Antibiotic Resistance in Lactic Acid Bacteria and Bifidobacteria. J. Mol. Microbiol. Biotechnol. 2008, 14, 6–15. [Google Scholar] [CrossRef] [Green Version]
- Gueimonde, M.; Sánchez, B.; De los Reyes-Gavilán, C.G.; Margolles, A. Antibiotic Resistance in Probiotic Bacteria. Front. Microbiol. 2013, 4, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.; Tomar, S.K.; Sangwan, V.; Goswami, P.; Singh, R. Antibiotic Resistance of Lactobacillus Sp. Isolated from Commercial Probiotic Preparations. J. Food Saf. 2016, 36, 38–51. [Google Scholar] [CrossRef]
- Eastell, R.; O’Neill, T.W.; Hofbauer, L.C.; Langdahl, B.; Reid, I.R.; Gold, D.T.; Cummings, S.R. Postmenopausal Osteoporosis. Nat. Rev. Dis. Prim. 2016, 2, 16069. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C. From Estrogen-Centric to Aging and Oxidative Stress: A Revised Perspective of the Pathogenesis of Osteoporosis. Endocr. Rev. 2010, 31, 266–300. [Google Scholar] [CrossRef] [Green Version]
- Breuil, V.; Ticchioni, M.; Testa, J.; Roux, C.H.; Ferrari, P.; Breittmayer, J.P.; Albert-Sabonnadière, C.; Durant, J.; De Perreti, F.; Bernard, A.; et al. Immune Changes in Post-Menopausal Osteoporosis: The Immunos Study. Osteoporos. Int. 2010, 21, 805–814. [Google Scholar] [CrossRef]
- Uehara, I.A.; Soldi, L.R.; Silva, M.J.B. Current Perspectives of Osteoclastogenesis through Estrogen Modulated Immune Cell Cytokines. Life Sci. 2020, 256, 117921. [Google Scholar] [CrossRef]
- Folwarczna, J.; Pytlik, M.; Zych, M.; Cegieła, U.; Kaczmarczyk-Sedlak, I.; Nowińska, B.; Śliwiński, L. Favorable Effect of Moderate Dose Caffeine on the Skeletal System in Ovariectomized Rats. Mol. Nutr. Food Res. 2013, 57, 1772–1784. [Google Scholar] [CrossRef]
- Janas, A.; Kruczek, E.; Londzin, P.; Borymski, S.; Czuba, Z.P.; Folwarczna, J. Negligible Effect of Estrogen Deficiency on Development of Skeletal Changes Induced by Type 1 Diabetes in Experimental Rat Models. Mediat. Inflamm. 2020, 2020, 2793804. [Google Scholar] [CrossRef]
- Sengupta, P. The Laboratory Rat: Relating Its Age with Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar]
- Quinn, R. Comparing Rat’s to Human’s Age: How Old Is My Rat in People Years? Nutrition 2005, 21, 775–777. [Google Scholar] [CrossRef]
- Folwarczna, J.; Konarek, N.; Freier, K.; Karbowniczek, D.; Londzin, P.; Janas, A. Effects of Loratadine, a Histamine H1 Receptor Antagonist, on the Skeletal System of Young Male Rats. Drug Des. Devel. Ther. 2019, 13, 3357–3367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Londzin, P.; Siudak, S.; Cegieła, U.; Pytlik, M.; Janas, A.; Waligóra, A.; Folwarczna, J. Phloridzin, an Apple Polyphenol, Exerted Unfavorable Effects on Bone and Muscle in an Experimental Model of Type 2 Diabetes in Rats. Nutrients 2018, 10, 1701. [Google Scholar] [CrossRef] [Green Version]
- Londzin, P.; Kisiel-Nawrot, E.; Kocik, S.; Janas, A.; Trawczyński, M.; Cegieła, U.; Folwarczna, J. Effects of Diosgenin on the Skeletal System in Rats with Experimental Type 1 Diabetes. Biomed. Pharmacother. 2020, 129, 110342. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Kim, J.-Y.; Kim, B.K.; Lee, I.O.; Park, N.H.; Kim, S.H. Lactobacillus-Fermented Milk Products Attenuate Bone Loss in an Experimental Rat Model of Ovariectomy-Induced Post-Menopausal Primary Osteoporosis. J. Appl. Microbiol. 2021, 130, 2041–2062. [Google Scholar] [CrossRef]
- Gatej, S.M.; Marino, V.; Bright, R.; Fitzsimmons, T.R.; Gully, N.; Zilm, P.; Gibson, R.J.; Edwards, S.; Bartold, P.M. Probiotic Lactobacillus Rhamnosus GG Prevents Alveolar Bone Loss in a Mouse Model of Experimental Periodontitis. J. Clin. Periodontol. 2018, 45, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Cosme-Silva, L.; Dal-Fabbro, R.; Cintra, L.T.A.; Ervolino, E.; Plazza, F.; Mogami Bomfim, S.; Duarte, P.C.T.; Junior, V.E.D.S.; Gomes-Filho, J.E. Reduced Bone Resorption and Inflammation in Apical Periodontitis Evoked by Dietary Supplementation with Probiotics in Rats. Int. Endod. J. 2020, 53, 1084–1092. [Google Scholar] [CrossRef]
- Tyagi, A.M.; Yu, M.; Darby, T.M.; Vaccaro, C.; Li, J.-Y.; Owens, J.A.; Hsu, E.; Adams, J.; Weitzmann, M.N.; Jones, R.M.; et al. The Microbial Metabolite Butyrate Stimulates Bone Formation via T Regulatory Cell-Mediated Regulation of WNT10B Expression. Immunity 2018, 49, 1116–1131. [Google Scholar] [CrossRef] [Green Version]
- Doyle, C.J.; Fitzsimmons, T.R.; Marchant, C.; Dharmapatni, A.A.S.S.K.; Hirsch, R.; Bartold, P.M. Azithromycin Suppresses P. gingivalis LPS-Induced pro-Inflammatory Cytokine and Chemokine Production by Human Gingival Fibroblasts in Vitro. Clin. Oral Investig. 2015, 19, 221–227. [Google Scholar] [CrossRef]
- Soysa, N.S.; Alles, N.; Shimokawa, H.; Jimi, E.; Aoki, K.; Ohya, K. Inhibition of the Classical NF-кB Pathway Prevents Osteoclast Bone-Resorbing Activity. J. Bone Miner. Metab. 2009, 27, 131–139. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A Simple Practice Guide for Dose Conversion between Animals and Human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Milić, A.; Mihaljević, V.B.; Ralić, J.; Bokulić, A.; Nožinić, D.; Tavčar, B.; Mildner, B.; Munić, V.; Malnar, I.; Padovan, J. A Comparison of in Vitro ADME Properties and Pharmacokinetics of Azithromycin and Selected 15-Membered Ring Macrolides in Rodents. Eur. J. Drug Metab. Pharmacokinet. 2014, 39, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Parvaneh, K.; Ebrahimi, M.; Sabran, M.R.; Karimi, G.; Hwei, A.N.M.; Abdul-Majeed, S.; Ahmad, Z.; Ibrahim, Z.; Jamaluddin, R. Probiotics (Bifidobacterium Longum) Increase Bone Mass Density and Upregulate Sparc and Bmp-2 Genes in Rats with Bone Loss Resulting from Ovariectomy. Biomed. Res. Int. 2015, 2015, 897639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.-G.; Lee, T.-H.; Kim, J.-H.; Seok, J.-W.; Lee, S.-H.; Kim, Y.-H.; Kim, J.-E.; Chung, M.-J.; Yeo, M.-H. The Effects of a Mineral Supplement (Aquamin F®) and Its Combination with Multi-Species Lactic Acid Bacteria (LAB) on Bone Accretion in an Ovariectomized Rat Model. J. Exp. Biomed. Sci. 2010, 16, 213–220. [Google Scholar]
- Lara-Castillo, N.; Johnson, M.L. Bone-Muscle Mutual Interactions. Curr. Osteoporos. Rep. 2020, 18, 408–421. [Google Scholar] [CrossRef] [PubMed]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, M.A. Standardized Nomenclature, Symbols, and Units for Bone Histomorphometry: A 2012 Update of the Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2013, 28, 2–17. [Google Scholar] [CrossRef] [Green Version]
- Turner, C.H.; Burr, D.B. Basic Biomechanical Measurements of Bone: A Tutorial. Bone 1993, 14, 595–608. [Google Scholar] [CrossRef]
- Stürmer, E.K.; Seidlová-Wuttke, D.; Sehmisch, S.; Rack, T.; Wille, J.; Frosch, K.H.; Wuttke, W.; Stürmer, K.M. Standardized Bending and Breaking Test for the Normal and Osteoporotic Metaphyseal Tibias of the Rat: Effect of Estradiol, Testosterone, and Raloxifene. J. Bone Miner. Res. 2006, 21, 89–96. [Google Scholar] [CrossRef]
| Parameter | NOVX Rats | OVX Rats | |||
|---|---|---|---|---|---|
| Control | Control | LR | AZM | AZM + LR | |
| CTX-I (ng/mL) | 21.73 ± 1.86 | 26.34 ± 2.03 | 27.42 ± 2.04 | 30.52 ± 2.47 | 26.83 ± 2.91 |
| Osteocalcin (ng/mL) | 339.46 ± 15.97 | 458.87 ± 21.82 ** | 491.08 ± 35.93 *** | 553.56 ± 31.28 *** ● | 494.98 ± 27.13 *** |
| ALP (U/L) | 71.35 ± 5.59 | 112.24 ± 8.80 *** | 91.38 ± 4.58 * ● | 108.84 ± 7.07 *** | 101.04 ± 5.03 ** |
| Calcium (mg/dL) | 11.05 ± 0.13 | 11.13 ± 0.14 | 11.19 ± 0.09 | 11.10 ± 0.24 | 10.90 ± 0.16 |
| Inorganic phosphorus (mg/dL) | 6.45 ± 0.26 | 6.23 ± 0.28 | 6.79 ± 0.26 | 7.17 ± 0.29 ● | 7.49 ± 0.26 ** ●● |
| Total protein (g/dL) | 5.20 ± 0.11 | 5.14 ± 0.09 | 5.07 ± 0.11 | 4.93 ± 0.08 | 4.65 ± 0.10 *** ●●● ## |
| ALT (U/L) | 50.03 ± 7.66 | 53.12 ± 5.22 | 49.69 ± 4.05 | 48.73 ± 3.56 | 56.87 ± 5.99 |
| AST (U/L) | 168.15 ± 18.84 | 163.11 ± 17.58 | 198.77 ± 15.20 | 191.77 ± 14.88 | 206.33 ± 19.50 |
| Parameter | NOVX Rats | OVX Rats | |||
|---|---|---|---|---|---|
| Control | Control | LR | AZM | AZM + LR | |
| Bone mass (g) | 0.616 ± 0.016 | 0.622 ± 0.012 | 0.650 ± 0.013 | 0.626 ± 0.017 | 0.623 ± 0.011 |
| Bone mass/body mass ratio (g/100 g) & | 0.302 ± 0.004 | 0.274 ± 0.012 ** | 0.274 ± 0.007 * | 0.268 ± 0.006 ** | 0.268 ± 0.006 ** |
| Bone mineral mass (g) | 0.284 ± 0.006 | 0.276 ± 0.004 | 0.289 ± 0.007 | 0.285 ± 0.007 | 0.282 ± 0.007 |
| Bone organic substance mass (g) | 0.147 ± 0.004 | 0.147 ± 0.002 | 0.155 ± 0.003 | 0.151 ± 0.004 | 0.149 ± 0.002 |
| Bone water mass (g) | 0.185 ± 0.007 | 0.199 ± 0.013 | 0.205 ± 0.006 | 0.191 ± 0.006 | 0.192 ± 0.003 |
| Bone mineral mass/ body mass ratio (g/100 g) & | 0.139 ± 0.001 | 0.121 ± 0.003 *** | 0.122 ± 0.002 *** | 0.122 ± 0.003 *** | 0.121 ± 0.003 *** |
| Mineral mass/bone mass ratio (g/g) | 0.461 ± 0.004 | 0.445 ± 0.009 | 0.445 ± 0.006 | 0.455 ± 0.004 | 0.452 ± 0.004 |
| Organic substance mass/ bone mass ratio (g/g) | 0.239 ± 0.001 | 0.238 ± 0.005 | 0.239 ± 0.003 | 0.241 ± 0.002 | 0.239 ± 0.002 |
| Water mass/bone mass ratio (g/g) | 0.300 ± 0.005 | 0.318 ± 0.013 | 0.316 ± 0.005 | 0.304 ± 0.004 | 0.308 ± 0.004 |
| Calcium content (g/g of bone mineral) | 0.371 ± 0.006 | 0.368 ± 0.006 | 0.364 ± 0.003 | 0.363 ± 0.005 | 0.367 ± 0.005 |
| Phosphorus content (g/g of bone mineral) | 0.143 ± 0.003 | 0.143 ± 0.002 | 0.143 ± 0.002 | 0.142 ± 0.003 | 0.142 ± 0.001 |
| Bone density (g/cm3) | 1.606 ± 0.007 | 1.586 ± 0.007 | 1.573 ± 0.012 | 1.585 ± 0.007 | 1.584 ± 0.009 |
| Bone mineral density (g/cm3) | 0.716 ± 0.009 | 0.690 ± 0.009 | 0.675 ± 0.015 | 0.690 ± 0.008 | 0.688 ± 0.013 |
| Bone | Parameter | NOVX Rats | OVX Rats | |||
|---|---|---|---|---|---|---|
| Control | Control | LR | AZM | AZM + LR | ||
| Femoral diaphysis | Ct.Ar (mm2) | 4.77 ± 0.05 | 4.94 ± 0.10 | 5.08 ± 0.16 | 5.05 ± 0.09 | 4.89 ± 0.08 |
| Ma.Ar (mm2) | 2.78 ± 0.12 | 2.61 ± 0.08 | 2.58 ± 0.07 | 2.70 ± 0.11 | 2.64 ± 0.08 | |
| Tt.Ar (mm2) | 7.55 ± 0.13 | 7.54 ± 0.13 | 7.65 ± 0.19 | 7.75 ± 0.17 | 7.53 ± 0.11 | |
| Ma.Ar/Tt.Ar | 0.367 ± 0.010 | 0.346 ± 0.008 | 0.337 ± 0.009 | 0.347 ± 0.009 | 0.351 ± 0.008 | |
| Femoral epiphysis | BV/TV (%) | 30.04 ± 1.50 | 25.18 ± 1.63 * | 23.77 ± 1.22 ** | 31.30 ± 2.15 ●● | 24.93 ± 1.30 * ^^ |
| Tb.Th (μm) | 63.44 ± 3.66 | 58.61 ± 3.21 | 55.31 ± 2.24 | 70.75 ± 3.69 ●● | 61.66 ± 2.51 | |
| Tb.Sp (μm) | 148.88 ± 7.28 | 176.40 ± 7.68 * | 179.65 ± 8.08 * | 159.22 ± 11.02 | 189.61 ± 11.41 ** ^ | |
| Tb.N (1/mm) | 4.79 ± 0.19 | 4.29 ± 0.12 * | 4.30 ± 0.16 * | 4.41 ± 0.17 | 4.04 ± 0.15 ** | |
| Femoral metaphysis | BV/TV (%) | 32.09 ± 1.52 | 25.65 ± 1.34 ** | 26.02 ± 1.83 * | 28.27 ± 1.35 | 25.01 ± 2.02 ** |
| Tb.Th (μm) | 55.42 ± 2.11 | 53.71 ± 2.84 | 51.46 ± 2.33 | 54.84 ± 2.59 | 53.45 ± 2.76 | |
| Tb.Sp (μm) | 119.75 ± 7.26 | 158.71 ± 10.34 ** | 152.83 ± 14.67 * | 141.11 ± 7.66 | 167.49 ± 13.04 ** | |
| Tb.N (1/mm) | 5.83 ± 0.28 | 4.84 ± 0.24 ** | 5.05 ± 0.28 | 5.22 ± 0.28 | 4.67 ± 0.29 ** | |
| Parameter | NOVX Rats | OVX Rats | |||
|---|---|---|---|---|---|
| Control | Control | LR | AZM | AZM + LR | |
| Young’s modulus (MPa) | 3284 ± 336 | 2354 ± 149 * | 1974 ± 216 ** | 2561 ± 374 | 2447 ± 378 |
| Yield point load (N) | 57.8 ± 9.3 | 33.3 ± 2.5 *** | 48.2 ± 3.5 ● | 41.6 ± 3.1 * | 30.7 ± 3.4 *** # |
| Displacement for yield point load (mm) | 0.39 ± 0.07 | 0.25 ± 0.02 | 0.51 ± 0.08 ●●● | 0.31 ± 0.02 | 0.26 ± 0.03 ## |
| Energy for yield point load (mJ) | 12.8 ± 4.5 | 4.4 ± 0.5 ** | 11.8 ± 2.3 ● | 6.0 ± 0.7 * | 4.2 ± 0.8 * # |
| Stress for yield point load (MPa) | 47.5 ± 8.0 | 26.4 ± 2.1 *** | 33.4 ± 8.4 * | 30.1 ± 2.9 ** | 23.4 ± 3.3 *** |
| Maximum load (N) | 101.6 ± 6.6 | 61.3 ± 2.7 *** | 65.6 ± 3.0 *** | 65.9 ± 2.5 *** | 63.5 ± 5.1 *** |
| Displacement for maximum load (mm) | 0.78 ± 0.07 | 0.83 ± 0.04 | 0.94 ± 0.06 | 0.79 ± 0.05 | 0.87 ± 0.05 |
| Energy for maximum load (mJ) | 42.6 ± 4.8 | 33.8 ± 2.5 | 36.9 ± 3.8 | 32.1 ± 1.6 | 35.1 ± 3.1 |
| Stress for maximum load (MPa) | 83.8 ± 7.5 | 48.8 ± 2.7 *** | 45.1 ± 1.6 *** | 48.2 ± 2.8 *** | 47.6 ± 4.6 *** |
| Fracture load (N) | 70.9 ± 5.6 | 47.2 ± 2.1 *** | 52.8 ± 3.0 ** | 48.0 ± 2.7 *** | 54.1 ± 4.6 ** |
| Displacement for fracture load (mm) | 1.09 ± 0.09 | 1.25 ± 0.07 | 1.34 ± 0.08 | 1.19 ± 0.05 | 1.19 ± 0.06 |
| Energy for fracture load (mJ) | 68.8 ± 6.8 | 56.7 ± 3.7 | 59.2 ± 3.9 | 55.6 ± 2.9 | 52.7 ± 3.3 |
| Stress for fracture load (MPa) | 58.8 ± 6.2 | 37.9 ± 2.7 *** | 36.5 ± 2.2 *** | 35.4 ± 2.8 *** | 41.1 ± 4.6 ** |
| Parameter | NOVX Rats | OVX Rats | |||
|---|---|---|---|---|---|
| Control | Control | LR | AZM | AZM + LR | |
| Young’s modulus (MPa) | 12,587 ± 953 | 11,288 ± 685 | 11,039 ± 707 | 10,396 ± 502 | 11,328 ± 396 |
| Yield point load (N) | 54.7 ± 5.2 | 54.1 ± 3.8 | 63.5 ± 1.0 ● | 65.8 ± 2.0 * ● | 60.6 ± 1.6 |
| Displacement for yield point load (mm) | 0.26 ± 0.02 | 0.25 ± 0.02 | 0.29 ± 0.01 | 0.30 ± 0.01 | 0.27 ± 0.00 |
| Energy for yield point load (mJ) | 7.7 ± 0.9 | 7.2 ± 0.8 | 9.2 ± 0.3 | 9.3 ± 0.4 | 8.2 ± 0.3 |
| Stress for yield point load (MPa) | 175.1 ± 19.0 | 151.4 ± 11.1 | 172.4 ± 8.6 | 163.4 ± 5.1 | 166.2 ± 5.3 |
| Maximum load (N) | 70.5 ± 3.0 | 64.3 ± 3.7 | 73.7 ± 2.3 ● | 76.1 ± 2.7 ●● | 73.4 ± 1.6 ● |
| Displacement for maximum load (mm) | 0.41 ± 0.01 | 0.40 ± 0.03 | 0.42 ± 0.03 | 0.41 ± 0.02 | 0.44 ± 0.02 |
| Energy for maximum load (mJ) | 16.6 ± 3.8 | 16.8 ± 2.0 | 18.7 ± 2.2 | 17.3 ± 1.0 | 20.0 ± 1.2 |
| Stress for maximum load (MPa) | 217.8 ± 9.0 | 179.7 ± 11.2 ** | 198.5 ± 7.7 | 188.4 ± 4.6 * | 201.3 ± 6.2 |
| Fracture load (N) | 57.0 ± 5.3 | 48.9 ± 3.6 | 56.3 ± 4.2 | 55.5 ± 3.9 | 52.4 ± 2.8 |
| Displacement for fracture load (mm) | 0.74 ± 0.07 | 0.72 ± 0.05 | 0.82 ± 0.05 | 0.78 ± 0.06 | 0.92 ± 0.08 |
| Energy for fracture load (mJ) | 35.7 ± 3.6 | 34.1 ± 3.3 | 42.1 ± 2.1 | 39.9 ± 3.3 | 49.2 ± 5.2 * ●● |
| Stress for fracture load (MPa) | 171.7 ± 8.9 | 136.4 ± 10.7 ** | 149.3 ± 7.5 | 135.8 ± 5.2 ** | 143.1 ± 6.6 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cegieła, U.; Londzin, P.; Janas, A.; Pytlik, M.; Folwarczna, J. Effect of Administration of Azithromycin and/or Probiotic Bacteria on Bones of Estrogen-Deficient Rats. Pharmaceuticals 2022, 15, 915. https://doi.org/10.3390/ph15080915
Cegieła U, Londzin P, Janas A, Pytlik M, Folwarczna J. Effect of Administration of Azithromycin and/or Probiotic Bacteria on Bones of Estrogen-Deficient Rats. Pharmaceuticals. 2022; 15(8):915. https://doi.org/10.3390/ph15080915
Chicago/Turabian StyleCegieła, Urszula, Piotr Londzin, Aleksandra Janas, Maria Pytlik, and Joanna Folwarczna. 2022. "Effect of Administration of Azithromycin and/or Probiotic Bacteria on Bones of Estrogen-Deficient Rats" Pharmaceuticals 15, no. 8: 915. https://doi.org/10.3390/ph15080915
APA StyleCegieła, U., Londzin, P., Janas, A., Pytlik, M., & Folwarczna, J. (2022). Effect of Administration of Azithromycin and/or Probiotic Bacteria on Bones of Estrogen-Deficient Rats. Pharmaceuticals, 15(8), 915. https://doi.org/10.3390/ph15080915






