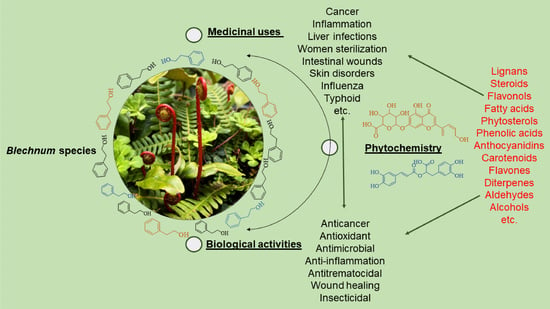
Traditional Uses, Phytochemistry, and Pharmacological Properties of the Genus Blechnum—A Narrative Review
Abstract
1. Introduction
1.1. Botanical and Taxonomic Description
1.2. Distribution and Conservation Status
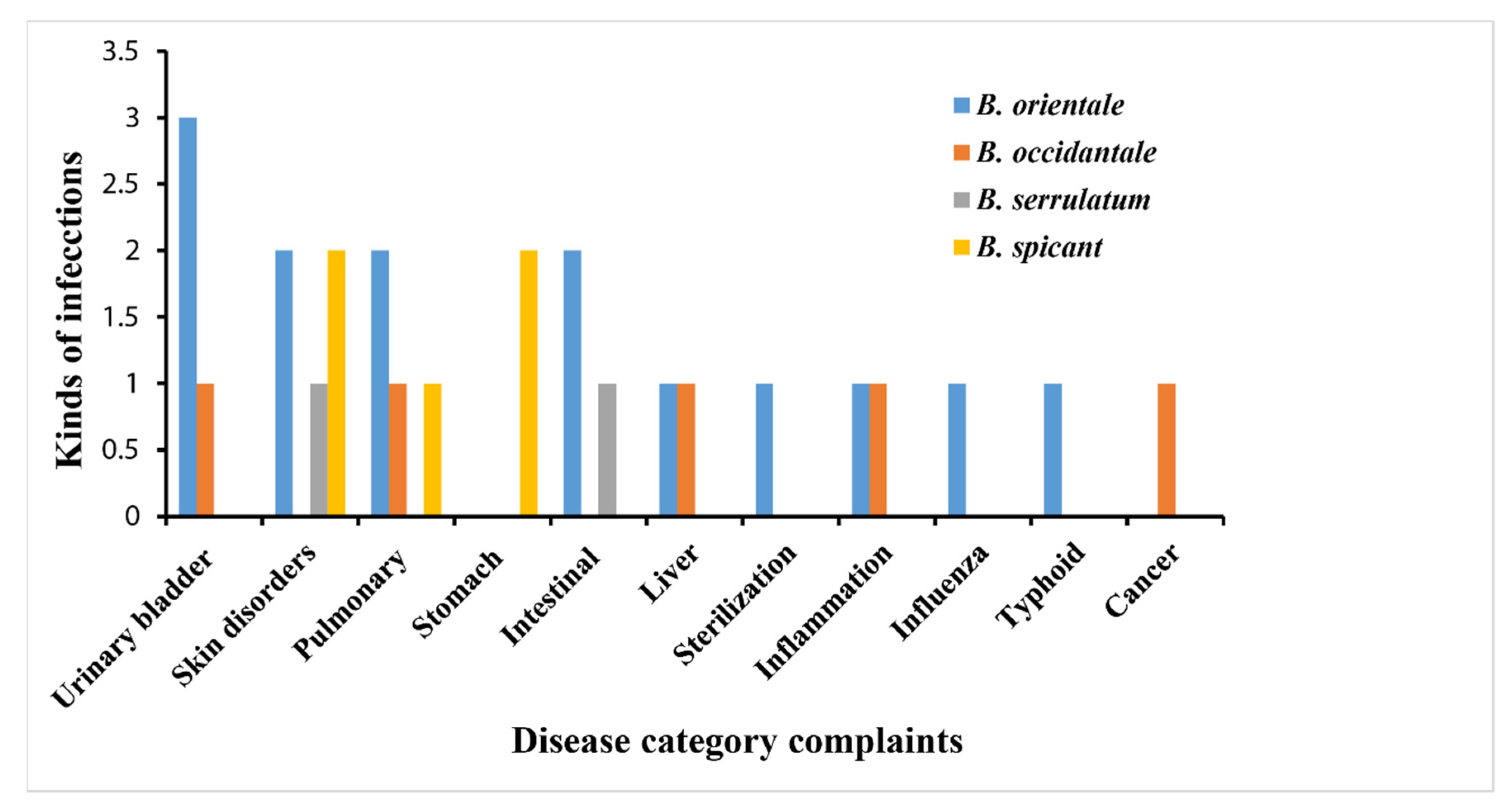
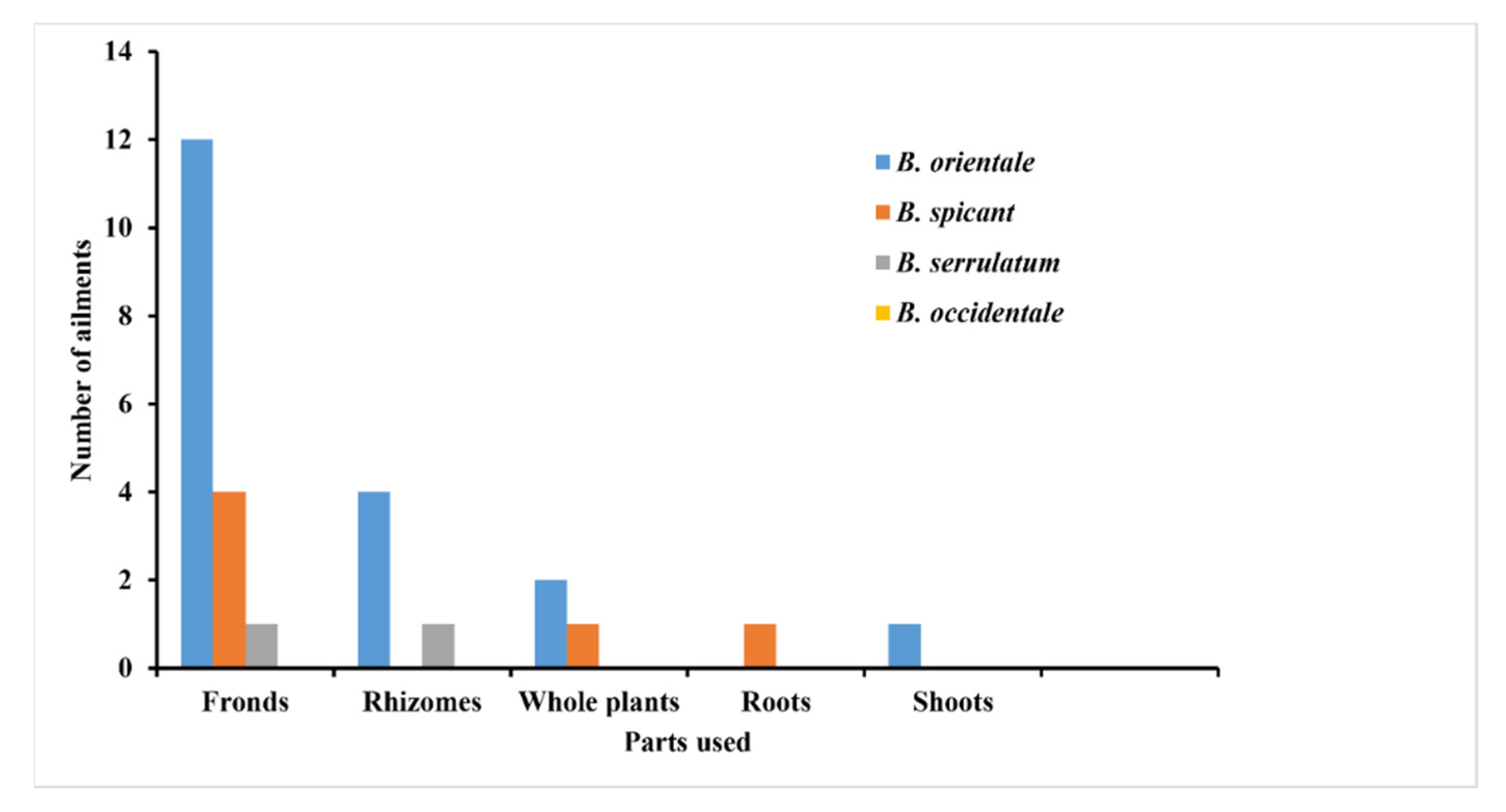
2. Traditional Uses of Blechnum Species
| Accepted Species | Region | Parts Used | Medicinal Use | References |
|---|---|---|---|---|
| B. spicant (L.) Roth. | Fronds | The leaflets are chewed to treat internal cancer, lungs, and stomach complaints. | [30] | |
| They are externally applied to treat skin sores. | [30] | |||
| Roots | Decoctions prepared from the roots are used as a remedy for diarrhea | [30] | ||
| United States of America | Whole plant | Treatment of skin ailments | [32] | |
| B. orientale (L.) C. Presl. | Malaysia. | Shoots | The shoots are pounded and used as a paste to cure boils | [31] |
| Fronds | The fronds are ground in cow’s milk to treat asthma | [1] | ||
| Applied in the form of a poultice to treat boils | [31] | |||
| Externally applied to cure blisters, boils, carbuncles, and sores | ||||
| The leaves are crushed and applied as medication for abscesses | [31,32,33] | |||
| India | Fronds | Urinary bladder complaints | [8,34] | |
| Hot decoction prepared from pinnae is used for its antiseptic action or applied externally over a boil to release pus. | [35] | |||
| The extracted juice is used to treat intestinal Wounds. | [36] | |||
| Rhizomes | It is orally administered to treat typhoid. | [9,32] | ||
| The prepared paste is applied to cure urinary bladder infections. | [36] | |||
| Philippines | Fronds | They are used for polynesia, diaphoretic, and operative actions | [8,28] | |
| China | Rhizomes | Are used as an anthelminthic to cure intestinal worms | [8,28] | |
| Papua New Guinea | Whole plant | Orally ingested for women’s sterilization | [32] | |
| United States of America | Treatment of influenza | [37] | ||
| B. occidentale L. | Brazil | - | The whole plant is frequently used as a therapy for pulmonary ailments, urinary disorders and liver infections. | [3,38,39] |
| - | - | Inflammation | [40] | |
| B. serrulatum Rich. | French Guiana | Rhizome | An infusion prepared from the rhizomes is used as a vermifuge. | [41] |
| Guyana | Fronds | Used to treat abscesses | [41] |
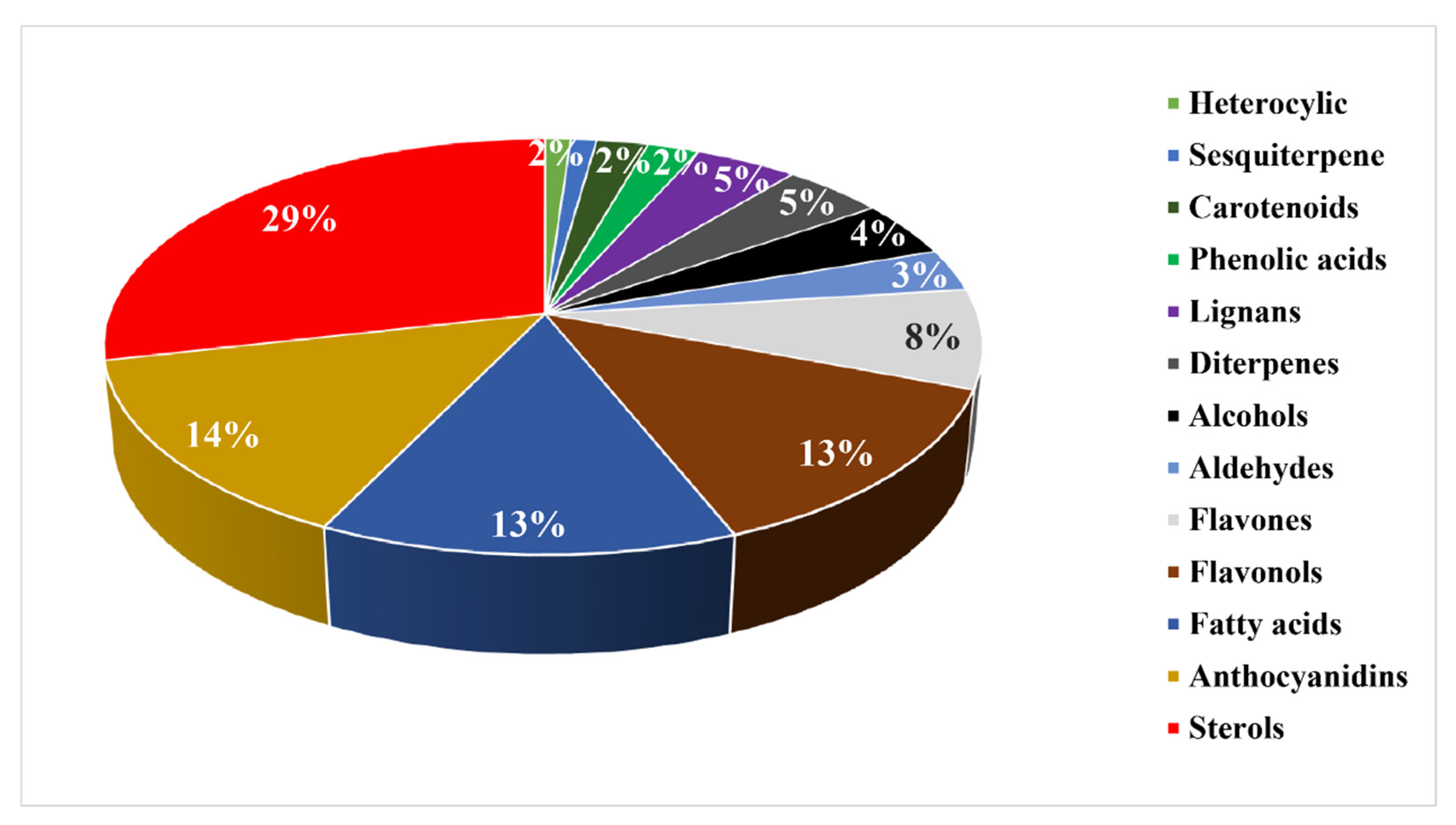
3. Secondary Metabolites
3.1. Phenolic Compounds
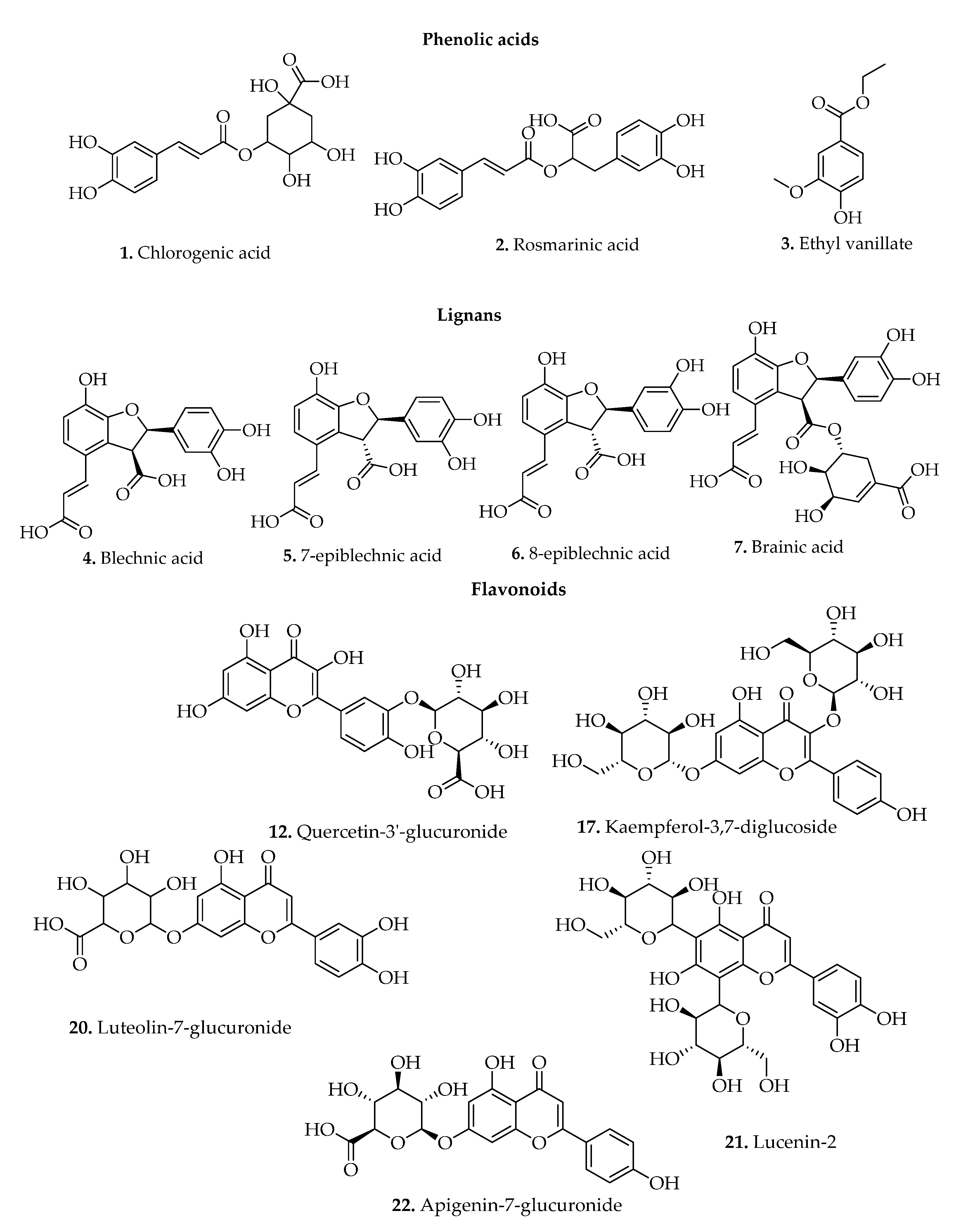
3.1.1. Simple Phenols/Phenol Derivatives
3.1.2. Lignans
3.1.3. Polyphenols
Flavonols
Flavones
Anthocyanins
3.2. Terpenoids
3.3. Sterols
3.4. Fatty Acids
| No. | Secondary Metabolite | Specimen | Part Used | Identification Method | References |
|---|---|---|---|---|---|
| Phenolic compounds | |||||
| (a). Phenolic acids | |||||
| 1. | Chlorogenic acid | B. binervatum (Poir.) C.V.Morton & Lellinger, B. brasiliense Desv, B. orientale L., B. discolor (Forst.) Keyserl, B. brasiliense Desv., B. spicant (L.) Roth, and B. occidentale L. | Fronds | TLC, HPLC-DAD-MS | [42,44] |
| 2. | Rosmarinic acid | B. binervatum (Poir.) C.V.Morton & Lellinger, B. brasiliense Desv., and B. occidentale L. | Fronds | TLC, HPLC-DAD-MS | [42,44,45] |
| 3. | Ethyl vanillate | B. spicant (L.) Roth | Aerial parts | GC-MS | [11] |
| (b). Lignans | |||||
| 4. | Blechnic acid | B. spicant (L.) Roth, and B. orientale L. | Fronds | TLC | [15,42] |
| 5. | 7-Epiblechnic acid | B. orientale L. | Fronds | [15] | |
| 6. | 8-Epiblechnic acid | B. orientale L. | Fronds | [15] | |
| 7. | Brainic acid | B. orientale L. | Fronds | [15] | |
| (c). Flavonols | |||||
| 8. | Quercetin-3,7-digalactoside | B. orientale L. | Fronds | TLC | [2,16] |
| 9. | Quercetin-7,4′-digalactoside | B. orientale L. | Fronds | TLC | [2,16] |
| 10. | Quercetin-3,4′-diglucoside | B. orientale L. | Fronds | TLC | [2,16] |
| 11. | Quercetin-3′,4′di methyl ether-3-glucoside | B. orientale L. | Fronds | TLC | [2,16] |
| 12. | Quercetin-3, glucuronide | B. orientale L. | Fronds | TLC | [2,16] |
| 13. | Quercetin 7′3′4′—Trimethoxy | B. orientale L. | Aerial parts | GC-MS | [23] |
| 14. | Quercetin 3-0-β-D-[6-0-caffeoylglucopyranoside] | B. novae-zelandiae T.C.Chambers & P.A.Farrant | Fronds | HPLC, NMR | [64] |
| 15. | Quercetin 3-0-β-D-[6-0-caffeoylgalactopyranoside] | B. novae-zelandiae T.C.Chambers & P.A.Farrant | Fronds | HPLC, NMR | [64] |
| 16. | Kaempferol-3,7-digalactoside | B. orientale L. | Fronds | TLC | [2,16] |
| 17. | Kaempferol-3,7-diglucoside | B. orientale L. | Fronds | TLC | [2,16] |
| 18. | Kaempferol-3,7-diglucuronide | B. orientale L. | Fronds | TLC | [2,16] |
| 19. | Kaempferol 3-0-β-D-glucuronopyranoside | B. novae-zelandiae T.C.Chambers & P.A.Farrant | Fronds | [64] | |
| (d). Flavones | |||||
| 20. | Luteolin-7-glucuronide | B. orientale L. | Fronds | TLC | [2,16] |
| 21. | Lucenin-2 (luteolin 6,8-di-C-glucoside) | B. orientale L. | Aerial parts | [23] | |
| 22. | Apigenin-7-glucuronide | B. orientale L. | Fronds | TLC | [2,16] |
| 23. | Isorhamnetin-3-glucoside | B. orientale L. | Fronds | TLC | [2,16] |
| 24. | Apigenin-7,4′-diglucoside | B. orientale L. | Fronds | TLC | [2,16] |
| 25. | Genkwanin-4′-glucuronide | B. orientale L. | Fronds | TLC | [2,16] |
| 26. | Acacetin-7-galactoside | B. orientale L. | Fronds | TLC | [2,16] |
| (e). Anthocyanidins | |||||
| 27. | Apigeninidin-5-glucoside | B. procerum (G.Forst.) Sw | Fronds | [17] | |
| 28. | Apigeninidin-7-glucoside | B. procerum (G.Forst.) Sw | Fronds | [17] | |
| 29. | Apigeninidin-5-diglycoside | B. procerum (G.Forst.) Sw | Fronds | [17] | |
| 30. | Apigeninidin-7-diglycoside | B. procerum (G.Forst.) Sw | Fronds | [17] | |
| 31. | Apigeninidin-5-rhamnoside glucoside | B. procerum (G.Forst.) Sw | Fronds | [17] | |
| 32. | Apigeninidin-5-7-glycoside | B. procerum (G.Forst.) Sw | Fronds | [17] | |
| 33. | Luteolinidin-5-glucoside | B. procerum (G.Forst.) Sw | Fronds | [17] | |
| 34. | Luteolinidin-7-glucoside | B. procerum (G.Forst.) Sw | Fronds | [17] | |
| 35. | Luteolinidin-5-diglycoside | B. procerum (G.Forst.) Sw | Fronds | [17] | |
| 36. | Luteolinidin-7-diglycoside | B. procerum (G.Forst.) Sw | Fronds | [17] | |
| 37. | Luteolinidin -5-rhamnoside glucoside | B. procerum (G.Forst.) Sw | Fronds | [17] | |
| 38. | Luteolinidin-5-7-glycoside | B. procerum (G.Forst.) Sw | Fronds | [17] | |
| 39. | Luteolinidin 5-0-β-D-[3-0- ß-D-glucopyranosyl-2-O-acetylglucopyranoside] | B. novae-zelandiae T.C.Chambers & P.A.Farrant | Fronds | HPLC, NMR | [64] |
| Terpenoids | |||||
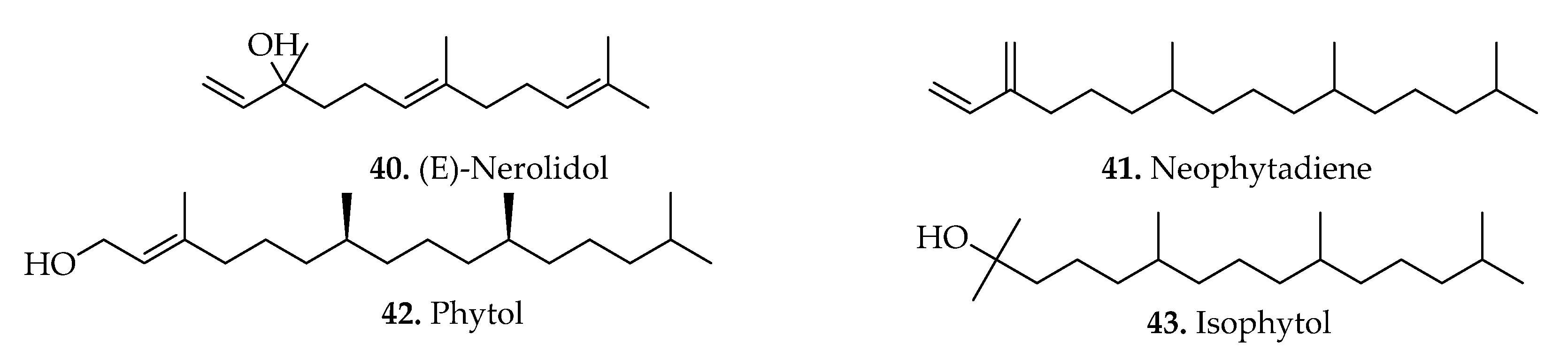
| (a). Sesquiterpene | |||||
| 40. | (E)-Nerolidol | B. spicant (L.) Roth | Aerial parts | GC-MS | [11] |
| Diterpenes | |||||
| 41. | Neophytadiene | B. penna-marina (Maxon & C.V.Morton) Kuhn, B. arcuatum Remy, B. mochaenum G.Kunkel, B. asperum (Klotzsch) J.W.Sturm, B. blechnoides (Lag.) C.Chr., B. hastatum Kaulf, B. microphyllum (Goldm.) C.V.Morton, B. chilense (Kaulf.) Mett., B. magellanicum, B. corralense Espinosa, and B. occidentale L. | Fronds | GC-MS | [12,18] |
| 42. | Phytol ((3,7,11,15-tetramethyl-2-hexadecen-1-ol) | B. penna-marina (Maxon & C.V.Morton) Kuhn, B. arcuatum Remy, B. mochaenum G.Kunkel, B. asperum (Klotzsch) J.W.Sturm, B. blechnoides (Lag.) C.Chr., B. hastatum Kaulf, B. microphyllum (Goldm.) C.V.Morton, B. chilense (Kaulf.) Mett., B. magellanicum (Desv.) Mett., and B. corralense Espinosa. | Fronds | GC-MS | [18] |
| 43. | Isophytol ((3,7,11,15-tetramethyl-1-hexadecen-3-ol) | B. penna-marina (Maxon & C.V.Morton) Kuhn, B. arcuatum Remy, B. mochaenum G.Kunkel, B. asperum (Klotzsch) J.W.Sturm, B. blechnoides (Lag.) C.Chr., B. hastatum Kaulf, B. microphyllum (Goldm.) C.V.Morton, B. chilense (Kaulf.) Mett., B. magellanicum (Desv.) Mett., B. corralense Espinosa, and B. occidentale L. | Fronds | GC-MS | [12,18] |
| 44. | 2-Hexadecene, 3,7,11,15-tetramethyl-, [R-[R*, R*-(E)]]- | B. orientale L. | Aerial parts | GC-MS | [23] |
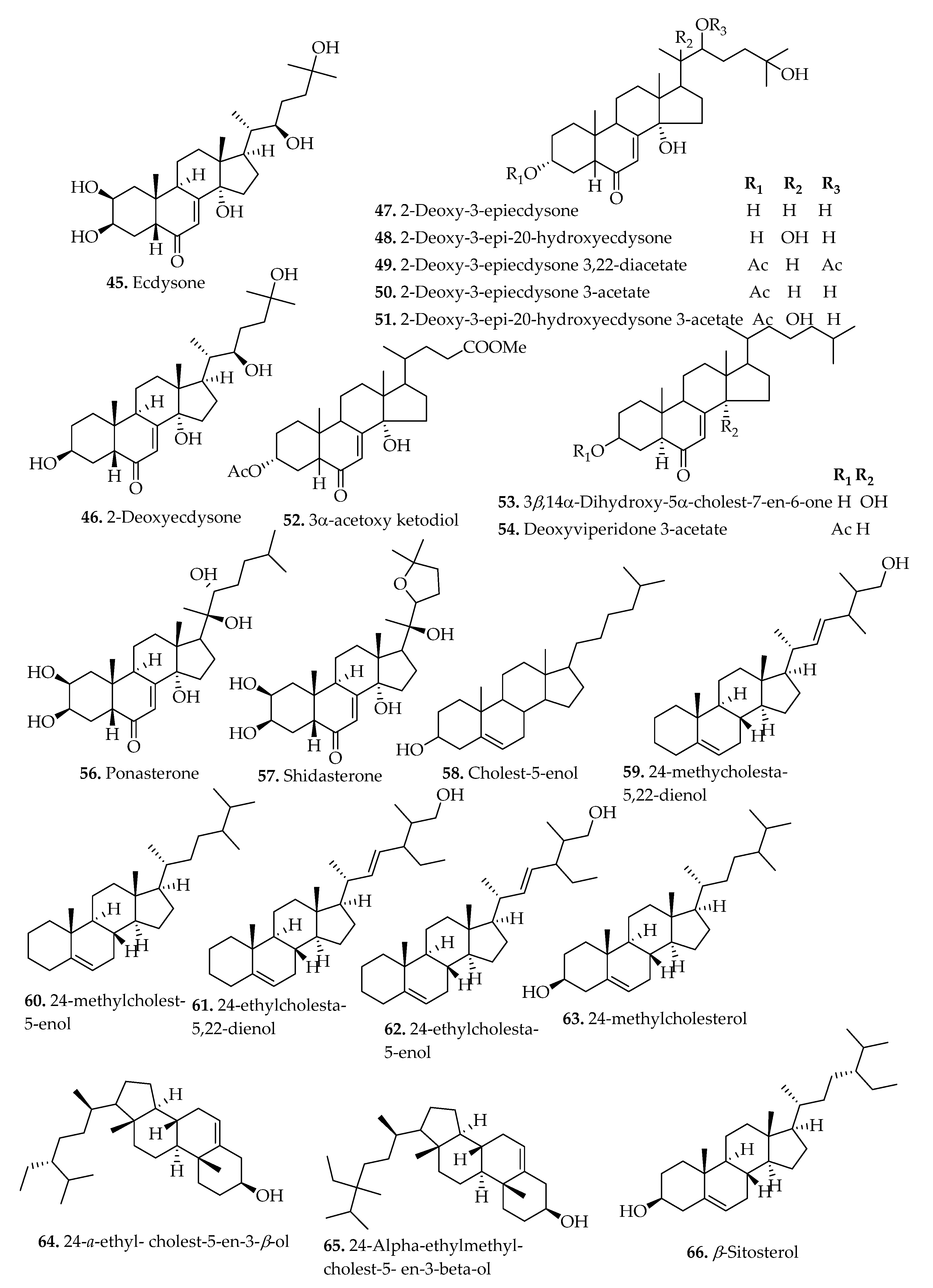
| Sterols | |||||
| 45. | Ecdysone | B. penna-marina (Maxon & C.V.Morton) Kuhn, B. arcuatum Remy, B. mochaenum G.Kunkel, B. asperum (Klotzsch) J.W.Sturm, B. blechnoides (Lag.) C.Chr., B. hastatum Kaulf, B. microphyllum (Goldm.) C.V.Morton, B. chilense (Kaulf.) Mett., B. magellanicum (Desv.) Mett., B. corralense Espinosa, B. vulcanicum (Blume) Kuhn, and B. minus (R.Br.) Ettingsh | Fronds | HPLC, GC-MS, NMR | [14,18,26] |
| 46. | 2-Deoxyecdysone (2-Deoxycrusteecdysone) | B. vulcanicum (Blume) Kuhn, B. minus (R.Br.) Ettingsh, B. arcuatum Remy, B. asperum (Klotzsch) J.W.Sturm, B. blechnoides (Lag.) C.Chr., B. chilense (Kaulf.) Mett., B. corralense Espinosa, B. hastatum Kaulf, B. magellanicum (Desv.) Mett., B. microphyllum (Goldm.) C.V.Morton, B. mochaenum G.Kunkel, and B. penna-marina (Maxon & C.V.Morton) Kuhn | Fronds | HPLC, GC-MS, NMR | [14,18,26] |
| 47. | 2-Deoxy-3-epiecdysone | B. vulcanicum (Blume) Kuhn | Fronds | HPLC | [14] |
| 48. | 2-Deoxy-3-epi-20-hydroxyecdysone | B. vulcanicum (Blume) Kuhn | Fronds | HPLC | [14] |
| 49. | 2-Deoxy-3-epiecdysone 3,22-diacetate | B. vulcanicum (Blume) Kuhn | Fronds | HPLC | [14] |
| 50. | 2-Deoxy-3-epiecdysone 3-acetate | B. vulcanicum (Blume) Kuhn | Fronds | HPLC | [14] |
| 51. | 2-Deoxy-3-epi-20-hydroxyecdysone 3-acetate | B. vulcanicum (Blume) Kuhn | Fronds | HPLC | [14] |
| 52. | 3α-Acetoxy ketodiol | B. vulcanicum (Blume) Kuhn | Fronds | [14] | |
| 53. | 3β,14α-Dihydroxy-5α-cholest-7-en-6-one | B. vulcanicum (Blume) Kuhn | Fronds | HPLC | [14] |
| 54. | Deoxyviperidone 3-acetate. | B. vulcanicum (Blume) Kuhn | Fronds | HPLC | [14] |
| 55. | 3β,14α-Dihydroxy-5β-cholest-7-en-6-one | B. vulcanicum (Blume) Kuhn | Fronds | HPLC | [14] |
| 56. | Ponasterone | B. penna-marina (Maxon & C.V.Morton) Kuhn, B. arcuatum Remy, B. mochaenum G.Kunkel, and B. asperum (Klotzsch) J.W.Sturm. B. blechnoides (Lag.) C.Chr., B. hastatum Kaulf, B. microphyllum (Goldm.) C.V.Morton, B. chilense (Kaulf.) Mett., B. magellanicum (Desv.) Mett., and B. corralense Espinosa. | Fronds | GC-MS, NMR | [18,26] |
| 57. | Shidasterone | B. penna-marina (Maxon & C.V.Morton) Kuhn, B. arcuatum Remy, B. mochaenum G.Kunkel, B. asperum (Klotzsch) J.W.Sturm, B. blechnoides (Lag.) C.Chr., B. hastatum Kaulf, B. microphyllum (Goldm.) C.V.Morton, B. chilense (Kaulf.) Mett., B. magellanicum (Desv.) Mett., and B. corralense Espinosa. | Fronds | GC-MS, NMR | [18,26] |
| 58. | Cholest-5-enol | B. orientale L. | Whole plant | GC | [13] |
| 59. | 24-methycholesta-5,22-dienol | B. orientale L. | Whole plant | GC | [13] |
| 60. | 24-methylcholest-5-enol | B. orientale L. | Whole plant | GC | [13] |
| 61. | 24,-ethylcholesta-5,22-dienol | B. orientale L. | Whole plant | GC | [13] |
| 62. | 24-ethylcholest-5-enol | B. orientale L. | Whole plant | GC | [13] |
| 63. | 24-methylcholesterol | B. orientale L. | Whole plant | GC | [13] |
| 64. | 24-α-ethyl-cholest-5-en-3β-ol | B. orientale L. | Whole plant | GC | [13] |
| 65. | 24-Alphaethyl-methyl-cholest-5- en-3-beta-ol | B. orientale L. | Whole plant | GC | [13] |
| 66. | β-Sitosterol (Stigmast-5-en-3-ol) | B. penna-marina (Maxon & C.V.Morton) Kuhn, B. arcuatum Remy, B. mochaenum G.Kunkel, B. orientale L., B. asperum (Klotzsch) J.W.Sturm, B. blechnoides (Lag.) C.Chr., B. hastatum Kaulf, B. microphyllum (Goldm.) C.V.Morton, B. chilense (Kaulf.) Mett., B. magellanicum (Desv.) Mett., and B. corralense Espinosa. | Whole plant | GC, GC-MS | [13,18] |
| 67. | Stigmasterol (24- α-cholest-5-22-Dien-3-β-ol) | B. orientale L. | Whole plant | GC | [13] |
| 68. | Campesterol (ergost-5-en-3-ol) | B. occidentale L., B. binervatum (Poir.) C.V.Morton & Lellinger, and B. brasiliense Desv. | Fronds | GC-MS | [12] |
| 69. | 22-Dehydrocampesterol | B. orientale L. | Whole plant | GC | [13] |
| Fatty acids | |||||
| 70. | Palmitic acid (Hexadecanoic acid) | B. occidentale L., B. binervatum (Poir.) C.V.Morton & Lellinger, and B. brasiliense Desv. | Fronds | GC-MS | [12] |
| 71. | Methyl palmitate (Hexadecanoic acid, methyl ester) | B. occidentale L., B. binervatum (Poir.) C.V.Morton & Lellinger, and B. brasiliense Desv. | Fronds | GC-MS | [12] |
| 72. | Linolenic acid (9,12,15-octadecatrienoic acid) | B. occidentale L., B. binervatum (Poir.) C.V.Morton & Lellinger, and B. brasiliense Desv. | Fronds | GC-MS | [12] |
| 73. | Linoleic acid (9,12-octadecadienoic acid | B. occidentale L., B. binervatum (Poir.) C.V.Morton & Lellinger, and B. brasiliense Desv. | Fronds | GC-MS | [12] |
| 74. | Oleic acid (9-octadecenoic acid). | B. occidentale L., B. binervatum (Poir.) C.V.Morton & Lellinger, and B. brasiliense Desv. | Fronds | GC-MS | [12] |
| 75. | Methyl linoleate (9,12-octadecadienoic acid, methyl ester) | B. occidentale L., B. binervatum (Poir.) C.V.Morton & Lellinger, and B. brasiliense Desv. | Fronds | GC-MS | [12] |
| 76. | Methyl linolenate (9,12,15-octadecatrienoic acid, methyl ester) | B. occidentale L., B. binervatum (Poir.) C.V.Morton & Lellinger, and B. brasiliense Desv. | Fronds | GC-MS | [12] |
| 77. | Hexanedioic acid, bis (2-ethylhexyl) ester | B. occidentale L., B. binervatum (Poir.) C.V.Morton & Lellinger, and B. brasiliense Desv. | Fronds | GC-MS | [12] |
| 78. | Tetradecanoic acid 2,3-diacetoxy-propyl ester | B. occidentale L., B. binervatum (Poir.) C.V.Morton & Lellinger, and B. brasiliense Desv. | Fronds | GC-MS | [12] |
| 79. | 1,2,3-Propanetricarboxylic acid 2-hydroxy-, triethyl ester | B. orientale L. | Whole plant | GC-MS | [23] |
| 80. | Hexanedioic acid, mono (2-ethylhexyl) ester | B. orientale L. | Whole plant | GC-MS | [23] |
| 81. | Nonanoic acid | B. spicant (L.) Roth | Aerial parts | GC-MS | [11] |
| Alcohols | |||||
| 82. | 1-Octen-3-ol | B. spicant (L.) Roth | Aerial parts | GC-MS | [11] |
| 83. | 3-Octanol | B. spicant (L.) Roth | Aerial parts | GC-MS | [11] |
| 84. | (E)-2-Octenol | B. spicant (L.) Roth | Aerial parts | GC-MS | [11] |
| 85. | 3,7-Dimethyloctan-3-ol | B. spicant (L.) Roth | Aerial parts | GC-MS | [11] |
| Aldehydes | |||||
| 86. | (E)-2-Heptenal | B. spicant (L.) Roth | Aerial parts | GC-MS | [11] |
| 87. | 2-Phenylethanal | B. spicant (L.) Roth | Aerial parts | GC-MS | [11] |
| 88. | Benzaldehyde | B. spicant (L.) Roth | Aerial parts | GC-MS | [11] |
| Carotenoids | |||||
| 89. | Epoxy-α-ionone | B. spicant (L.) Roth | Aerial parts | GC-MS | [11] |
| 90. | 4-Hydroxyepoxy-β-ionol | B. spicant (L.) Roth | Aerial parts | GC-MS | [11] |
| Heterocyclic | |||||
| 91. | 3-benzoyl-4-methyl-6-ethyl-2(1H)-Pyridone | B. orientale L. | Whole plant | GC-MS | [23] |
3.5. Other Compounds
4. Pharmacological Activity
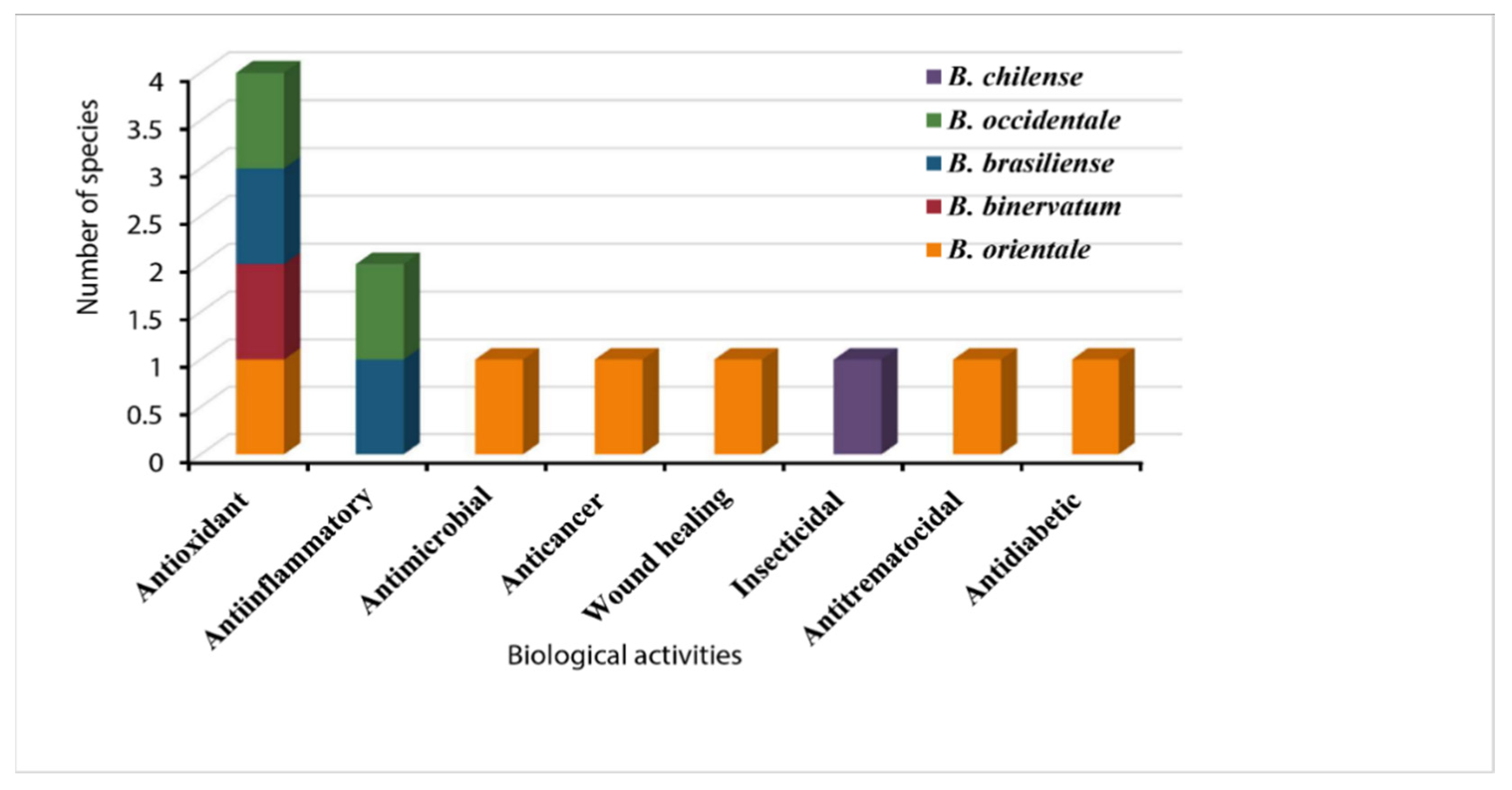
4.1. Antioxidant Activity
4.2. Antimicrobial Activity
4.2.1. Antibacterial Activity
4.2.2. Antifungal Activity
4.3. Anti-Inflammatory Activity
4.4. Anticancer Activity
4.5. Wound Healing Activity
4.6. Insecticidal Activity
4.7. Antitrematocidal Activity
4.8. Other Activities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baskaran, X.; Vigila, A.G.E.O.; Zhang, S. A review of the use of pteridophytes for treating human ailments. J. Zhejiang Univ. Sci. B 2018, 19, 85–119. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.Y.; Lim, Y.Y.; Kim, K.H. Blechnum Orientale Linn—A fern with potential as antioxidant, anticancer and antibacterial agent. BMC Complement. Altern. Med. 2010, 10, 15. [Google Scholar] [CrossRef]
- Regina, N.F.; Barros, T.A.A.; Lucchese, A.M.; Oliveira, C.E.C.; Santos, R.R.; Soares, M.B.P.; Villarreal, C.F. Antiinflammatory and antinociceptive activities of Blechnum occidentale L. extract. J. Ethnopharmacol. 2009, 125, 102–107. [Google Scholar] [CrossRef]
- Bresciani, L.F.V.; Priebe, J.P.; Yunes, R.A.; Magro, J.D.; Monache, F.D.; de Campos, F.; Cechinel-Filho, V. Pharmacological and phytochemical evaluation of Adiantum cuneatum growing in Brazil. Zeitschrift Naturforsch. 2003, 58, 191–194. [Google Scholar] [CrossRef]
- Rajesh, N.V.; Vasantha, S.; Jeyathilakan, N. Screening of in vitro antitrematodal activities of compounds and secondary metabolites isolated from selected Pteridophytes. Vet. Ital. 2020, 56, 271–287. [Google Scholar]
- Brownsey, P.J.; Perrie, L.R. Ophioglossaceae. In Flora of New Zealand—Ferns and Lycophytes. Fascicle 14; Breitwieser, I., Heenan, P.B., Wilton, A.D., Eds.; Manaaki Whenua Press: Lincoln, New Zealand, 2015. [Google Scholar]
- Mendoza-Ruiz, A. Morphogenesis of the gametophytes of eight Mexican species of Blechnum (Blechnaceae). Acta Bot. Mex. 2009, 88, 59–72. [Google Scholar] [CrossRef][Green Version]
- Benjamin, A.; Manickam, V.S. Medicinal pteridophytes from the Western Ghats. Indian J. Tradit. Knowl. 2007, 6, 611–618. [Google Scholar]
- Upreti, K.; Jalal, J.S.; Tewari, L.M.; Joshi, G.C.; Pangtey, Y.P.S.; Tewari, G. Ethnomedicinal uses of Pteridophytes of Kumaun Himalaya, Uttarakhand, India. J. Am. Sci. 2009, 5, 167–170. [Google Scholar]
- Salehi, B.; Quispe, C.; Sharifi-Rad, J.; Cruz-Martins, N.; Nigam, M.; Mishra, A.P.; Konovalov, D.A.; Orobinskaya, V.; Abu-Reidah, I.M.; Zam, W. Phytosterols: From preclinical evidence to potential clinical applications. Front. Pharmacol. 2021, 11, 1819. [Google Scholar] [CrossRef]
- Fons, F.; Froissard, D.; Bessière, J.; Buatois, B.; Fons, F.; Froissard, D.; Bessière, J.; Buatois, B.; Rapior, S. Biodiversity of volatile organic compounds from five French ferns. Nat. Prod. Commun. 2010, 5, 1655–1658. [Google Scholar] [CrossRef]
- Maria, J.; Andrade, D.M.; Maurmann, N.; Pranke, P.; Casanova, I.C. Identification of compounds from non-polar fractions of Blechnum spp and a multitarget approach involving enzymatic modulation and oxidative stress. J. Pharm. Pharmacol. 2017, 69, 89–98. [Google Scholar] [CrossRef]
- Chiu, P.; Patterson, W.; Salt, T.A. Sterol composition of pteridophytes. Phytochemistry 1988, 27, 819–822. [Google Scholar] [CrossRef]
- Russell, G.B.; Greenwood, D.R.; Lane, G.A.; Blunt, J.W.; Munro, M.H.G. 2-Deoxy-3-epiecdysone from the fern Blechnum vulcanicum. Phytochemistry 1981, 20, 2407–2410. [Google Scholar] [CrossRef]
- Wada, H.; Kido, T.; Tanaka, N.; Murakami, T.; Saiki, Y.; Chen, C.-M. Chemical and chemotaxonomical studies of ferns. LXXXI. Characteristic lignans of Blechnaceous ferns. Chem. Pharm. Bull. 1992, 40, 2099–2101. [Google Scholar] [CrossRef]
- Yusuf, U.K. Flavonoid glycosides in the fern Blechnum orientale Linn. Am. Fern J. 1994, 84, 69–70. [Google Scholar] [CrossRef]
- Crowden, R.K.; Jarman, S.J. 3-deoxyanthocyanins from the fern Blechnum procerum. Phytochemistry 1974, 13, 1947–1948. [Google Scholar] [CrossRef]
- Flores-González, M.; Torres-Benítez, A.; Simirgiotis, M. Terpenic Compounds in Chilean Species of the Genus Blechnum (Pteridophyta: Blechnaceae) with Neuroprotective. Multidiscip. Digit. Publ. Inst. Proc. 2021, 71, 8535. [Google Scholar] [CrossRef]
- Deepa, J.; Parashurama, T.R.; Krishnappa, M.; Nataraja, S. Antimicrobial efficacy of Blechnum orientale L. Int. J. Pharma Bio Sci. 2013, 4, P475–P479. [Google Scholar]
- Aini, N.; Shukor, A.; Stanslas, J. Cytotoxic Potential on Breast Cancer Cells Using Selected Forest Species Found in Malaysia. Int. J. Cancer Res. 2008, 4, 103–109. [Google Scholar] [CrossRef]
- Zapata, N.; Ceballos, R.; Cespedes, C.; Alarcon, J.; Leyton, A. Insecticidal activity and growth regulatory of Blechnum chilense (Blechnaceae) and Condalia microphylla Cav. (Rhamnaceae) extracts, on larvae of Galleria mellonella (Linnaeus) (Lepidoptera: Pyralidae). Boletín Latinoam. Caribe Plantas Med. Aromáticas 2016, 15, 77–87. [Google Scholar]
- Lai, H.Y.; Lim, Y.Y.; Kim, K.H. Potential dermal wound healing agent in Blechnum orientale Linn. Complement. Altern. Med. 2011, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Devi, R.K.; Vasantha, S.; Panneerselvam, A.; Rajesh, N.V.; Jeyathilakan, N. Phytochemical constituents and in vitro trematocidal activity of Blechnum orientale Linn. against Gastrothylax crumenifer. Ann. Phytomed. 2016, 5, 127–134. [Google Scholar]
- Fraser-Jenkins, C.R. Rare and Threatened Pteridophytes of Asia 2. Endangered Species of India—The Higher IUCN Categories. Bull. Natl. Mus. Nat. Sci. Ser. B 2012, 38, 153–181. [Google Scholar]
- Galán, J.M.G.Y.; Prada, C.; Rolleri, C.; Ainouche, A.; Vicent, M. cpDNA supports the identification of the major lineages of American Blechnum (Blechnaceae, Polypodiopsida) established by morphology. Turk. J. Bot. 2013, 37, 769–777. [Google Scholar] [CrossRef]
- Monsalve, Z.I.; Parada, K.; Lamilla, C. Insect Growth Regulatory Activity of Blechnum chilense. NPC Nat. Prod. Commun. 2011, 8, 1085–1088. [Google Scholar] [CrossRef]
- Ho, R.; Teai, T.; Bianchini, J.-P.; Lafont, R.; Raharivelomanana, P. Ferns: From traditional uses to pharmaceutical development, chemical identification of active principles. In Working with Ferns; Springer: Berlin/Heidelberg, Germany, 2011; pp. 321–346. [Google Scholar]
- Maridass, M.; Ghantikumar, S. Antibacterial activity of leaves of Blechnum orientale L. Pharmacol. Newslett. 2008, 3, 58–60. [Google Scholar]
- Gupta, A. Applications and uses of active ingredients from medicinal plants. Indian J. Nov. Drug Deliv. 2016, 6, 106–111. [Google Scholar]
- May, L.W. The economic uses and associated folklore of ferns and fern allies. Bot. Rev. 1978, 44, 491–528. [Google Scholar] [CrossRef]
- Ahmad, F.B.; Holdsworth, D.K.; Ahmad, F.B.; Holdsworth, D.K. Medicinal Plants of Sabah, East Malaysia–Part. Pharm. Biol. 2003, 41, 340–346. [Google Scholar] [CrossRef]
- Kumar, D.G.; Syafiq, A.M.; Ruhaiyem, Y.; Shahnaz, M. Blechnum orientale Linn. An Important Edible Medicinal Fern. Int. J. Pharm. Phytochem. Res. 2015, 7, 723–726. [Google Scholar]
- Ong, H.C.; Lina, E.; Milow, P. Traditional knowledge and usage of medicinal plants among the Semai Orang Asli at Kampung Batu 16, Tapah, Perak, Malaysia. Stud. Ethno-Med. 2012, 6, 207–211. [Google Scholar] [CrossRef]
- Dixit, R.D.; Vohra, J.N. A Dictionary of the Pteridophytes of India; Botanical Survey of India, Department of Environment: Kolkata, India, 1984.
- Shil, S.; Choudhury, M.D. Ethnomedicinal Importance of Pteridophytes Used by Reang tribe of Tripura, North East India. Ethnobot. Leafl. 2009, 2009, 10. [Google Scholar]
- Karthik, V.; Raju, K.; Ayyanar, M.; Gowrishankar, K.; Sekar, T. Ethnomedicinal uses of pteridophytes in kolli hills, Eastern Ghats of Tamil Nadu, India. J. Nat. Prod. Plant Resour 2011, 1, 50–55. [Google Scholar]
- Tsai, H.-H.; Hwang, S.-M. Compositions of Matter Useful in the Treatment of Viral Infections Derived from Plant. U.S. Patent 5989556, 23 November 1999. [Google Scholar]
- Barros, I.C.L.; Andrade, L.H.C. Pteridófitas Medicinais (Samambaias, Avencas e Plantas Afins). Federal University of Pernambuco: Recife, Brazil, 1997. [Google Scholar]
- Carlos, A.; David, S. Antioxidant activity of Blechnum chilense (Kaulf.) Mett. Curcuma domestica Valeton and Tagetes verticillata Lag. & Rodriguez. Boletín Latinoam. Caribe Plantas Med. Aromáticas 2011, 10, 315–324. [Google Scholar]
- Goswami, H.K.; Sen, K.; Mukhopadhyay, R. Pteridophytes: Evolutionary boon as medicinal plants. Plant Genet. Resour. 2016, 14, 328–355. [Google Scholar] [CrossRef]
- DeFilipps, R.A.; Maina, S.L.; Crepin, J. Medicinal Plants of the Guianas (Guyana, Surinam, French Guiana); Smithsonian Institution: Washington, DC, USA, 2004. [Google Scholar]
- Bohm, B.A. Phenolic compounds in ferns—III: An examination of some ferns for caffeic acid derivatives. Phytochemistry 1968, 7, 1825–1830. [Google Scholar] [CrossRef]
- Minatel, I.O.; Borges, C.V.; Ferreira, M.I.; Gomez, H.A.G.; Chen, C.-Y.O.; Lima, G.P.P. Phenolic compounds: Functional properties, impact of processing and bioavailability. Phenolic Compd. Biol. Act 2017, 8, 1–24. [Google Scholar]
- Andrade, J.M.M.; Biegelmeyer, R.; Dresch, R.R.; Maurmann, N.; Pranke, P.; Henriques, A.T. In vitro Antioxidant and Enzymatic Approaches to Evaluate Neuroprotector Potential of Blechnum Extracts Without Cytotoxicity to Human Stem Cells. Pharmacogn. Mag. 2016, 12, 171. [Google Scholar] [CrossRef]
- Andrade, J.M.M.; Passos, S.C.; Rubio, M.A.K.; Mendonça, J.N.; Lopes, N.P.; Henriques, A.T. Combining in vitro and in silico approaches to evaluate the multifunctional profile of rosmarinic acid from Blechnum brasiliense on targets related to neurodegeneration. Chem.-Biol. Interact. 2016, 254, 135–145. [Google Scholar] [CrossRef]
- Petersen, M.; Simmonds, M.S.J. Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef]
- Caro-León, J.; Espinosa-Cano, E.; Rosa, M.; Huerta-Madroñal, M.; Blanca, V. Chitosan—Rosmarinic acid conjugates with antioxidant, anti-inflammatory and photoprotective properties. Carbohydr. Polym. 2021, 273, 118619. [Google Scholar] [CrossRef]
- Augusto, A.; Lataliza, B.; De Assis, P.M.; Laurindo, R.; Cristina, E.; Gonçalves, D.; Dutra, R.C. Antidepressant-like effect of rosmarinic acid during LPS- induced neuroinflammatory model: The potential role of cannabinoid receptors/PPAR- γ signaling pathway. Phytotherapy Res. 2021, 35, 6974–6989. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Meng, X.; Chen, S.; Huang, D.; Xia, Y.; Zhu, S. Antioxidant activities of chlorogenic acid derivatives with different acyl donor chain lengths and their stabilities during in vitro simulated gastrointestinal digestion. Food Chem. 2021, 357, 129904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, N.; Xin, N.; Li, Q.; Zhang, T.; Ye, H.; Zhao, C. Complexation of chlorogenic acid enhances the antiproliferative effect of lactoferrin to colon cancer cells. Food Biosci. 2022, 46, 101601. [Google Scholar] [CrossRef]
- Masoomzadeh, F.; Khan, B.A.; Alshahrani, S.M.; Alqahtani, A.; Ebrahimzadeh, M.A.; Khalili, M. Protective effects of rutin and chlorogenic acid against antihypoxic conditions in mice. Pak. J. Pharm. Sci. 2021, 34, 1679–1683. [Google Scholar]
- Vassão, D.G.; Kim, K.-W.; Davin, L.B.; Lewis, N.G. Lignans (neolignans) and allyl/propenyl phenols: Biogenesis, structural biology, and biological/human health considerations. In Natural Products Structural Diversity—I. Secondary Metabolites: Organization and Biosynthesis; Elsevier: Amsterdam, The Netherlands, 2010; Volume 1, pp. 815–928. [Google Scholar]
- Waswa, E.N.; Li, J.; Mkala, E.M.; Wanga, V.O.; Mutinda, E.S.; Nanjala, C.; Odago, W.O.; Katumo, D.M.; Gichua, M.K.; Gituru, R.W. Ethnobotany, phytochemistry, pharmacology, and toxicology of the genus Sambucus L. (Viburnaceae). J. Ethnopharmacol. 2022, 292, 115102. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Cappellini, F.; Reiner, Ž.; Zorzan, D.; Imran, M.; Sener, B.; Kilic, M.; El-Shazly, M.; Fahmy, N.M. The therapeutic potential of anthocyanins: Current approaches based on their molecular mechanism of action. Front. Pharmacol. 2020, 11, 1300. [Google Scholar] [CrossRef]
- Swinny, E.E.; Bloor, S.J.; Wong, H. H and 13C NMR assignments for the 3-deoxyanthocyanins, luteolinidin-5-glucoside and apigeninidin-5-glucoside. Magn. Reson. Chem. 2000, 38, 1031–1033. [Google Scholar] [CrossRef]
- Thurman, E.M. Analysis of Terpenes in Hemp (Cannabis sativa) by Gas Chromatography/Mass Spectrometry: Isomer Identification Analysis. Compr. Anal. Chem. 2020, 90, 197–233. [Google Scholar] [CrossRef]
- Krist, S.; Banovac, D.; Tabanca, N.; Wedge, D.E.; Gochev, V.K.; Wanner, J.; Schmidt, E.; Jirovetz, L. Antimicrobial activity of nerolidol and its derivatives against airborne microbes and further biological activities. Nat. Prod. Commun. 2015, 10, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.-K.; Tan, L.T.-H.; Chan, K.-G.; Lee, L.-H.; Goh, B.-H. Nerolidol: A sesquiterpene alcohol with multi-faceted pharmacological and biological activities. Molecules 2016, 21, 529. [Google Scholar] [CrossRef]
- Ferrer, A.; Altabella, T.; Arró, M.; Boronat, A. Emerging roles for conjugated sterols in plants. Prog. Lipid Res. 2017, 67, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Watanabe, B.; Nakagawa, Y.; Miyagawa, H. Synthesis of ponasterone A derivative with various steroid skeleton moieties and evaluation of their binding to the ecdysone receptor of Kc cells. Steroids 2008, 73, 1452–1464. [Google Scholar] [CrossRef] [PubMed]
- Burdge, G.C.; Calder, P.C. Introduction to Fatty Acids and Lipids. World Rev. Nutr. Diet. 2015, 112, 1–16. [Google Scholar] [CrossRef]
- Karimi, E.; Jaafar, H.Z.E.; Ghasemzadeh, A.; Ebrahimi, M. Fatty acid composition, antioxidant and antibacterial properties of the microwave aqueous extract of three varieties of Labisia pumila Benth. Biol. Res. 2015, 48, 1–6. [Google Scholar] [CrossRef]
- Voronkov, A.S.; Ivanova, T. V Fatty Acids Composition of the Epiphytic Ferns, Platycerium bifurcatum and Asplenium nidus, and the Terrestrial Fern, Asplenium trichomanes. Am. Fern J. 2021, 111, 117–128. [Google Scholar] [CrossRef]
- Swinny, E.E. A Novel Acetylated 3-Deoxyanthocyanidin Laminaribioside from the Fern. Z. Naturforsch. 2001, 56, 177–180. [Google Scholar] [CrossRef]
- Oskoueian, E.; Abdullah, N.; Hendra, R.; Karimi, E. Bioactive Compounds, Antioxidant, Xanthine Oxidase Inhibitory, Tyrosinase Inhibitory and Anti-Inflammatory Activities of Selected Agro-Industrial By-products. Int. J. Mol. Sci. 2011, 12, 8610–8625. [Google Scholar] [CrossRef]
- Rahmawati, D.; Rifky, N.A.; Marpaung, A.M. Extraction and stability analysis of antioxidant activity from Stenochlaena palustris. In Proceedings of the International Postgraduate Symposium on Food, Agriculture and Biotechnology Extraction, Maha Sarakham, Thailand, 30–31 August 2017; pp. 45–52. [Google Scholar]
- Takuli, P.; Khulbe, K.; Kumar, P.; Parki, A.; Syed, A.; Elgorban, A.M. Phytochemical profiling, antioxidant and antibacterial efficacy of a native Himalayan Fern: Woodwardia unigemmata (Makino) Nakai. Saudi J. Biol. Sci. 2020, 27, 1961–1967. [Google Scholar] [CrossRef]
- Halder, K.; Chakraborty, S. An account of antioxidant potential in pteridophytes: A biochemical perspective. Int. J. Bioinform. Biol. Sci. 2018, 6, 15–24. [Google Scholar] [CrossRef]
- Lai, H.Y.; Lim, Y.Y.; Tan, S.P. Antioxidative, tyrosinase inhibiting and antibacterial activities of leaf extracts from medicinal ferns. Biosci. Biotechnol. Biochem. 2009, 73, 1362–1366. [Google Scholar] [CrossRef]
- Mothana, R.A.; Lindequist, U.; Gruenert, R.; Bednarski, P.J. Studies of the in vitro anticancer, antimicrobial and antioxidant potentials of selected Yemeni medicinal plants from the island Soqotra. BMC Complement. Altern. Med. 2009, 9, 1–11. [Google Scholar] [CrossRef]
- Grierson, D.S.; Afolayan, A.J. Antibacterial activity of some indigenous plants used for the treatment of wounds in the Eastern Cape, South Africa. J. Ethnopharmacol. 1999, 66, 103–106. [Google Scholar] [CrossRef]
- Kaur, G.J.; Arora, D.S. Antibacterial and phytochemical screening of Anethum graveolens, Foeniculum vulgare and Trachyspermum ammi. BMC Complement. Altern. Med. 2009, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.A.A.; Jülich, W.-D.; Kusnick, C.; Lindequist, U. Screening of Yemeni medicinal plants for antibacterial and cytotoxic activities. J. Ethnopharmacol. 2001, 74, 173–179. [Google Scholar] [CrossRef]
- Kelmanson, J.E.; Jäger, A.K.; van Staden, J. Zulu medicinal plants with antibacterial activity. J. Ethnopharmacol. 2000, 69, 241–246. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Netea, M.G.; Balkwill, F.; Chonchol, M.; Cominelli, F.; Donath, M.Y.; Giamarellos-Bourboulis, E.J.; Golenbock, D.; Gresnigt, M.S.; Heneka, M.T.; Hoffman, H.M.; et al. A guiding map for inflammation. Nat. Immunol. 2017, 18, 826–831. [Google Scholar] [CrossRef]
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020, 20, 95–112. [Google Scholar] [CrossRef]
- Miranda, G.M.; Bessa, J.R.; Teles, Y.C.F.; Cocou, S.; Alexandre, M.; Goncalves, M.S.; Ribeiro-filho, J. Inclusion Complexes of Non-Steroidal Anti-Inflammatory Drugs with Cyclodextrins: A Systematic Review. Biomolecules 2021, 11, 361. [Google Scholar] [CrossRef] [PubMed]
- Dogné, J.-M.; Supuran, C.T.; Pratico, D. Adverse cardiovascular effects of the coxibs. J. Med. Chem. 2005, 48, 2251–2257. [Google Scholar] [CrossRef] [PubMed]
- Martín Arias, L.H.; Martín González, A.; Sanz Fadrique, R.; Vazquez, E.S. Cardiovascular risk of nonsteroidal anti-inflammatory drugs and classical and selective cyclooxygenase-2 inhibitors: A meta-analysis of observational studies. J. Clin. Pharmacol. 2019, 59, 55–73. [Google Scholar] [CrossRef]
- Schäcke, H.; Döcke, W.-D.; Asadullah, K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol. Ther. 2002, 96, 23–43. [Google Scholar] [CrossRef]
- Fasolo, J.M.M.A.; Fernanda, A.; Vizuete, K.; Rico, E.P.; Rambo, R.B.S.; Toson, N.S.B.; Santos, E.; De Oliveira, D.L.; Gonçalves, C.A.S.; Schapoval, E.E.S.; et al. Comparative Biochemistry and Physiology, Part C Anti-inflammatory effect of rosmarinic acid isolated from Blechnum brasiliense in adult zebrafish brain. Comp. Biochem. Physiol. Part C 2021, 239, 108874. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Tyavambiza, C.; Dube, P.; Goboza, M.; Meyer, S.; Madiehe, A.M.; Meyer, M. Wound Healing Activities and Potential of Selected African Medicinal Plants and Their Synthesized Biogenic Nanoparticles. Plants 2021, 10, 2635. [Google Scholar] [CrossRef]
- Akkol, E.K.; Koca, U.; Peşin, I.; Yılmazer, D.; Toker, G.; Yeşilada, E. Exploring the wound healing activity of Arnebia densiflora (Nordm.) Ledeb. by in vivo models. J. Ethnopharmacol. 2009, 124, 137–141. [Google Scholar] [CrossRef]
- Demilew, W.; Adinew, G.M.; Asrade, S. Evaluation of the wound healing activity of the crude extract of leaves of Acanthus polystachyus Delile (Acanthaceae). Evid.-Based Complement. Altern. Med. 2018, 2018, 2047896. [Google Scholar] [CrossRef]
- Arnason, J.T.; Philogene, B.J.R.; Morand, P. Insecticides of Plant Origin; American Chemistry Society: Washington, DC, USA, 1989. [Google Scholar]
- Mordue, A.J. Azadirachtin—A review of its mode of action in insects. In Practice Oriented Results on Use and Production of Neem-Ingredients and Pheromones; Duck&Graphic: Giessen, Germany, 1998; pp. 1–4. [Google Scholar]
- Pardede, A.; Adfa, M.; Kusnanda, A.J.; Ninomiya, M.; Koketsu, M. Isolation of secondary metabolites from Stenochlaena palustris stems and structure-activity relationships of 20-hydroxyecdysone derivatives on antitermite activity. Holzforschung 2018, 72, 899–904. [Google Scholar] [CrossRef]
- Arnault, C.; Sláma, K. Dietary effects of phytoecdysones in the leek-moth, Acrolepiopsis assectella Zell. (Lepidoptera: Acrolepiidae). J. Chem. Ecol. 1986, 12, 1979–1986. [Google Scholar] [CrossRef] [PubMed]
- De-Fu, C.; Ming-Xue, S.; Wen-Fu, X. Pesticidal character of phytoecdysteroids from Ajuga multiflora Bunge (Labiatae) on larvae of Cryptorrhynchus lapathi L. (Coleoptera: Curculionidae). J. For. Res. 2002, 13, 177–182. [Google Scholar] [CrossRef]
- Descoins, C., Jr. Perception de substances antiappétentes par des chenilles de lépidoptères phytophages. L’Année Biol. 2001, 40, 55–73. [Google Scholar] [CrossRef]
- Kalpana Devi, R.; Vasantha, S.; Panneerselvam, A.; Rajesh, N.V.; Jeyathilakan, N.; Venkataramanan, R. Gastrothylax crumenifer: Ultrastructure and histopathology study of in vitro trematodicidal effect of Microlepia speluncae (L.) Moore. J. Appl. Anim. Res. 2017, 46, 427–434. [Google Scholar] [CrossRef]
- Singh, T.U.; Kumar, D.; Tandan, S.K. Paralytic effect of alcoholic extract of Allium sativum and Piper longum on liver amphistome, Gigantocotyle explanatum. Indian J. Pharmacol. 2008, 40, 64. [Google Scholar]
- Chai, T.; Yeoh, L.; Ismaliza, N.; Ismail, M.; Manan, F.A.; Wong, F. Evaluation of Glucosidase Inhibitory and Cytotoxic Potential of Five Selected Edible and Medicinal Ferns. Trop. J. Pharm. Res. 2015, 14, 449–454. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waswa, E.N.; Muema, F.W.; Odago, W.O.; Mutinda, E.S.; Nanjala, C.; Mkala, E.M.; Amenu, S.G.; Ding, S.-X.; Li, J.; Hu, G.-W. Traditional Uses, Phytochemistry, and Pharmacological Properties of the Genus Blechnum—A Narrative Review. Pharmaceuticals 2022, 15, 905. https://doi.org/10.3390/ph15070905
Waswa EN, Muema FW, Odago WO, Mutinda ES, Nanjala C, Mkala EM, Amenu SG, Ding S-X, Li J, Hu G-W. Traditional Uses, Phytochemistry, and Pharmacological Properties of the Genus Blechnum—A Narrative Review. Pharmaceuticals. 2022; 15(7):905. https://doi.org/10.3390/ph15070905
Chicago/Turabian StyleWaswa, Emmanuel Nyongesa, Felix Wambua Muema, Wyclif Ochieng Odago, Elizabeth Syowai Mutinda, Consolata Nanjala, Elijah Mbandi Mkala, Sarah Getachew Amenu, Shi-Xiong Ding, Jing Li, and Guang-Wan Hu. 2022. "Traditional Uses, Phytochemistry, and Pharmacological Properties of the Genus Blechnum—A Narrative Review" Pharmaceuticals 15, no. 7: 905. https://doi.org/10.3390/ph15070905
APA StyleWaswa, E. N., Muema, F. W., Odago, W. O., Mutinda, E. S., Nanjala, C., Mkala, E. M., Amenu, S. G., Ding, S.-X., Li, J., & Hu, G.-W. (2022). Traditional Uses, Phytochemistry, and Pharmacological Properties of the Genus Blechnum—A Narrative Review. Pharmaceuticals, 15(7), 905. https://doi.org/10.3390/ph15070905