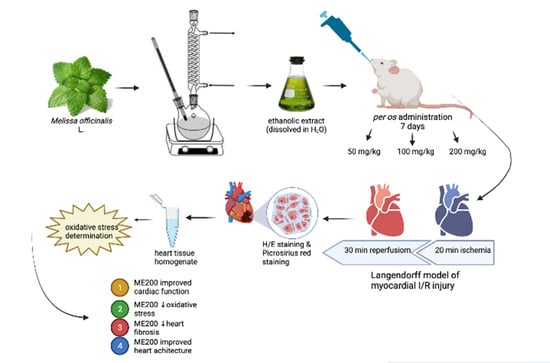
Short-Term Administration of Lemon Balm Extract Ameliorates Myocardial Ischemia/Reperfusion Injury: Focus on Oxidative Stress
Abstract
:1. Introduction
2. Results
2.1. Chemical Characterisation of the Examined Extract
2.2. Effects of Heart Preconditioning with ME on Cardiac Function
2.3. Effects of Heart Preconditioning with ME on Cardiac Redox State
2.4. Effects of Heart Preconditioning with ME on Heart Morphology
3. Discussion
4. Materials and Methods
4.1. Ethical Concerns
4.2. Preparation of the Melissa Officinalis Extract
4.3. Animals
4.4. Isolation of the Rat Heart and Langendorff Protocol
4.5. Cardiodynamic Measurements
4.6. Cardiac Redox State
4.7. Histological Analyses of the Heart (H/E Staining and Picrosirius Red Staining)
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiang, M.; Lu, Y.; Xin, L.; Gao, J.; Shang, C.; Jiang, Z.; Lin, H.; Fang, X.; Qu, Y.; Wang, Y.; et al. Role of Oxidative Stress in Reperfusion following Myocardial Ischemia and Its Treatments. Oxid. Med. Cell. Longev. 2021, 2021, 6614009. [Google Scholar] [CrossRef] [PubMed]
- Neri, M.; Fineschi, V.; Di Paolo, M.; Pomara, C.; Riezzo, I.; Turillazzi, E.; Cerretani, D. Cardiac oxidative stress and inflammatory cytokines response after myocardial infarction. Curr. Vasc. Pharmacol. 2015, 13, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Tibaut, M.; Mekis, D.; Petrovic, D. Pathophysiology of Myocardial Infarction and Acute Management Strategies. Cardiovasc. Hematol. Agents Med. Chem. 2017, 14, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Li, S.; Zhang, J.; Huang, Y.; Zhang, L.; Zhao, F.; Du, X.; Hou, J.; Zhang, T.; Shi, C.; et al. Current state and future perspective of cardiovascular medicines derived from natural products. Pharmacol. Ther. 2020, 216, 107698. [Google Scholar] [CrossRef]
- Sedighi, M.; Sewell, R.D.E.; Nazari, A.; Abbaszadeh, S.; Cheraghi, M.; Amini, A.; Heydari, Z.; Rafieian-Kopaei, M.A. Review on the Most Important Medicinal Plants Effective in Cardiac Ischemia-Reperfusion Injury. Curr. Pharm. Des. 2019, 25, 352–358. [Google Scholar] [CrossRef]
- Liu, H.; Guo, X.; Chu, Y.; Lu, S. Heart protective effects and mechanism of quercetin preconditioning on anti-myocardial ischemia reperfusion (IR) injuries in rats. Gene 2014, 545, 149–155. [Google Scholar] [CrossRef]
- Barteková, M.; Carnická, S.; Pancza, D.; Ondrejcáková, M.; Breier, A.; Ravingerová, T. Acute treatment with polyphenol quercetin improves postischemic recovery of isolated perfused rat hearts after global ischemia. Can. J. Physiol. Pharmacol. 2010, 88, 465–471. [Google Scholar] [CrossRef]
- Liu, Y.; Song, Y.; Li, S.; Mo, L. Cardioprotective Effect of Quercetin against Ischemia/Reperfusion Injury Is Mediated Through NO System and Mitochondrial K-ATP Channels. Cell J. 2021, 23, 184–190. [Google Scholar]
- Zhao, L.; Zhou, Z.; Zhu, C.; Fu, Z.; Yu, D. Luteolin alleviates myocardial ischemia reperfusion injury in rats via Siti1/NLRP3/NF-κB pathway. Int. Immunopharmacol. 2020, 85, 106680. [Google Scholar] [CrossRef]
- Testai, L.; Martelli, A.; Cristofaro, M.; Breschi, M.C.; Calderone, V. Cardioprotective effects of different flavonoids against myocardial ischaemia/reperfusion injury in Langendorff-perfused rat hearts. J. Pharm. Pharmacol. 2013, 65, 750–756. [Google Scholar] [CrossRef]
- Wang, P.; Sun, J.; Lv, S.; Xie, T.; Wang, X. Apigenin Alleviates Myocardial Reperfusion Injury in Rats by Downregulating miR-15b. Med. Sci. Monit. 2019, 25, 2764–2776. [Google Scholar] [CrossRef] [PubMed]
- Ferenczyová, K.; Kindernay, L.; Vlkovičová, J.; Kaločayová, B.; Rajtík, T.; Barteková, M. Pharmacology of Catechins in Ischemia-Reperfusion Injury of the Heart. Antioxidants 2021, 10, 1390. [Google Scholar] [CrossRef] [PubMed]
- Quan, W.; Liu, H.X.; Zhang, W.; Lou, W.J.; Gong, Y.Z.; Yuan, C.; Shao, Q.; Wang, N.; Guo, C.; Liu, F. Cardioprotective effect of rosmarinic acid against myocardial ischaemia/reperfusion injury via suppression of the NF-κB inflammatory signalling pathway and ROS production in mice. Pharm. Biol. 2021, 59, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, D.; Ye, L.; Li, P.; Hao, W.; Chen, X.; Ma, J.; Wang, B.; Shang, J.; Li, D.; et al. Rosmarinic Acid Protects against Inflammation and Cardiomyocyte Apoptosis during Myocardial Ischemia/Reperfusion Injury by Activating Peroxisome Proliferator-Activated Receptor Gamma. Front. Pharmacol. 2017, 8, 456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyriakopoulos, G.; Valsami, G.; Tsalikidis, C.; Pitiakoudis, M.; Tsaroucha, A.K. Use of natural anti-oxidants in experimental animal models of hepatic ischemia-reperfusion injury. Ann. Med. Surg. 2020, 60, 592–599. [Google Scholar] [CrossRef]
- Luo, W.; Tao, Y.; Chen, S.; Luo, H.; Li, X.; Qu, S.; Chen, K.; Zeng, C. Rosmarinic Acid Ameliorates Pulmonary Ischemia/Reperfusion Injury by Activating the PI3K/Akt Signaling Pathway. Front. Pharmacol. 2022, 13, 860944. [Google Scholar] [CrossRef]
- Khodai, M.; Ahmadvand, H.; Tamjidipour, A.; Hasanvand, A. Rosmarinic acid ameliorates renal ischemia reperfusion damage in rats. J. Nephropharm. 2019, 9, e15. [Google Scholar]
- Shakeri, A.; Sahebkar, A.; Javadi, B. Melissa officinalis L.—A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2016, 188, 204–228. [Google Scholar] [CrossRef]
- Draginic, N.; Jakovljevic, V.; Andjic, M.; Jeremic, J.; Srejovic, I.; Rankovic, M.; Tomovic, M.; Nikolic Turnic, T.; Svistunov, A.; Bolevich, S.; et al. Melissa officinalis L. as a Nutritional Strategy for Cardioprotection. Front. Physiol. 2021, 12, 661778. [Google Scholar] [CrossRef]
- Joukar, S.; Asadipour, H.; Sheibani, M.; Najafipour, H.; Dabiri, S. The effects of Melissa officinalis (lemon balm) pretreatment on the resistance of the heart to myocardial injury. Pharm. Biol. 2016, 54, 1005–1013. [Google Scholar] [CrossRef] [Green Version]
- Hamza, A.A.; Ahmed, M.M.; Elwey, H.M.; Amin, A. Melissa officinalis protects against doxorubicin-induced cardiotoxicity in rats and potentiates its anticancer activity on MCF-7 cells. PLoS ONE 2016, 11, e0167049. [Google Scholar] [CrossRef] [PubMed]
- Miraj, S.; Rafieian-Kopaei; Kiani, S. Melissa officinalis L: A Review Study with an Antioxidant Prospective. J. Evid. Based Complementary Altern. Med. 2017, 22, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Draginic, N.; Andjic, M.; Jeremic, J.; Zivkovic, V.; Kocovic, A.; Tomovic, M.; Bozin, B.; Kladar, N.; Bolevich, S.; Jakovljevic, V.; et al. Anti-inflammatory and Antioxidant Effects of Melissa officinalis Extracts: A Comparative Study. Iran. J. Pharm. Res. 2022, 21, e126561. [Google Scholar] [CrossRef]
- Chen, C.; Yu, L.T.; Cheng, B.R.; Xu, J.L.; Cai, Y.; Jin, J.L.; Feng, R.L.; Xie, L.; Qu, X.Y.; Li, D.; et al. Promising Therapeutic Candidate for Myocardial Ischemia/Reperfusion Injury: What Are the Possible Mechanisms and Roles of Phytochemicals? Front. Cardiovasc. Med. 2022, 8, 792592. [Google Scholar] [CrossRef]
- Joukar, S.; Asadipour, H. Evaluation of Melissa officinalis (Lemon Balm) Effects on Heart Electrical System. Res. Cardiovasc. Med. 2015, 4, e27013. [Google Scholar] [CrossRef]
- Sedighi, M.; Faghihi, M.; Rafieian-Kopaei, M.; Rasoulian, B.; Nazari, A. Cardioprotective Effect of Ethanolic Leaf Extract of Melissa Officinalis L Against Regional Ischemia-Induced Arrhythmia and Heart Injury after Five Days of Reperfusion in Rats. Iran. J. Pharm. Res. 2019, 18, 1530–1542. [Google Scholar]
- Gazola, R.; Machado, D.; Ruggiero, C.; Singi, G.; Macedo Alexandre, M. Lippia alba, Melissa officinalis and Cymbopogon citratus: Effects of the aqueous extracts on the isolated hearts of rats. Pharmacol. Res. 2004, 50, 477–580. [Google Scholar] [CrossRef]
- Draginic, N.D.; Jakovljevic, V.L.; Jeremic, J.N.; Srejovic, I.M.; Andjic, M.M.; Rankovic, M.R.; Sretenovic, J.Z.; Zivkovic, V.I.; Ljujic, B.T.; Mitrovic, S.L.; et al. Melissa officinalis L. Supplementation Provides Cardioprotection in a Rat Model of Experimental Autoimmune Myocarditis. Oxid. Med. Cell Longev. 2022, 2022, 1344946. [Google Scholar] [CrossRef]
- Safaeian, L.; Sajjadi, S.E.; Javanmard, S.H.; Montazeri, H.; Samani, F. Protective effect of Melissa officinalis extract against H2O2-induced oxidative stress in human vascular endothelial cells. Res. Pharm. Sci. 2016, 11, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Ersoy, S.; Orhan, I.; Turan, N.N.; Sahan, G.; Ark, M.; Tosun, F. Endothelium-dependent induction of vasorelaxation by Melissa officinalis L. ssp. officinalis in rat isolated thoracic aorta. Phytomedicine 2008, 15, 1087–1092. [Google Scholar] [CrossRef]
- Li, X.L.; Liu, J.X.; Li, P.; Zheng, Y.Q. Protective effect of rosmarinic acid on hypoxia/reoxygenation injury in cardiomyocytes. Zhongguo Zhong Yao Za Zhi 2014, 39, 1897–1901. [Google Scholar] [PubMed]
- Zhang, X.; Ma, Z.G.; Yuan, Y.P.; Xu, S.C.; Wei, W.Y.; Song, P.; Kong, C.Y.; Deng, W.; Tang, Q.Z. Rosmarinic acid attenuates cardiac fibrosis following long-term pressure overload via AMPKα/Smad3 signaling. Cell Death. Dis. 2018, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, A.; Al Masri, D.S.; Farhan, H.; Nasser, M.; Rammal, H.; Annan, H. Effect of different ethanol concentrations, using different extraction techniques, on the antioxidant capacity of Lebanese Eryngium creticum. J. Pharm. Chem. Biol. Sci. 2015, 3, 262–271. [Google Scholar]
- Bradic, J.; Zivkovic, V.; Srejovic, I.; Jakovljevic, V.; Petkovic, A.; Turnic, T.N.; Jeremic, J.; Jeremic, N.; Mitrovic, S.; Sobot, T.; et al. Protective Effects of Galium verum L. Extract against Cardiac Ischemia/Reperfusion Injury in Spontaneously Hypertensive Rats. Oxid. Med. Cell. Longev. 2019, 2019, 4235405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sretenovic, J.; Zivkovic, V.; Srejovic, I.; Milosavljevic, Z. The effects of high doses of nandrolone decanoate on cardiac muscle tissue. Ser. J. Clin. Exp. Res. 2016, 17, 303–308. [Google Scholar] [CrossRef]
- Mishra, P.; Singh, U.; Pandey, C.M.; Mishra, P.; Pandey, G. Application of student’s t-test, analysis of variance, and covariance. Ann. Card. Anaesth. 2019, 22, 407–411. [Google Scholar] [CrossRef]



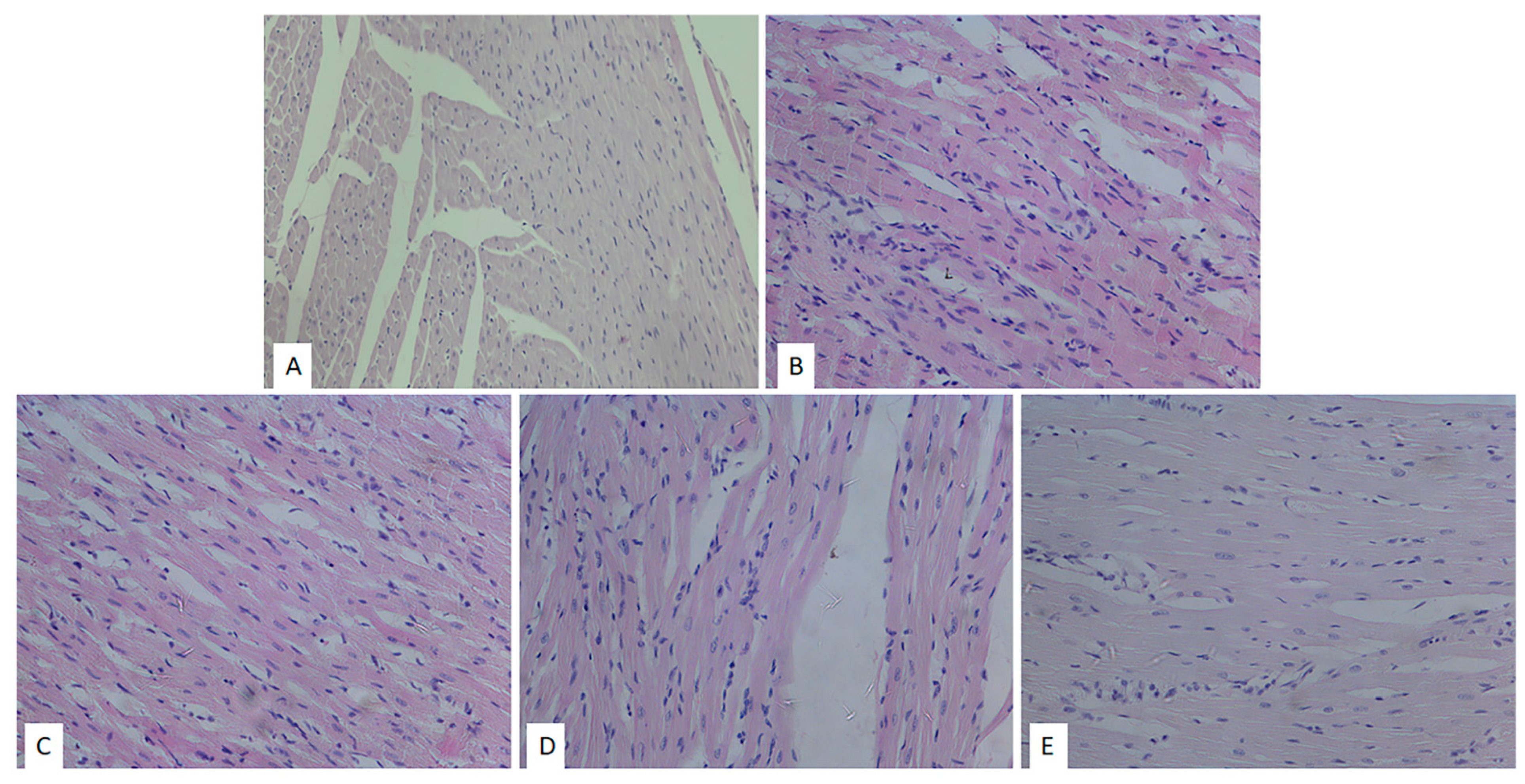


| Group | Degenerative Changes | Expanded Interstitium | Stromae Hypercellularity | Muscle Fibers Hypertrophy |
|---|---|---|---|---|
| SHAM | — | — | — | — |
| CTRL | +++ | + | +++ | +++ |
| ME50 | ++ | + | ++ | ++ |
| ME100 | ++ | - | ++ | ++ |
| ME200 | + | - | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Draginic, N.; Milosavljevic, I.; Andjic, M.; Jeremic, J.; Nikolic, M.; Sretenovic, J.; Kocovic, A.; Srejovic, I.; Zivkovic, V.; Bolevich, S.; et al. Short-Term Administration of Lemon Balm Extract Ameliorates Myocardial Ischemia/Reperfusion Injury: Focus on Oxidative Stress. Pharmaceuticals 2022, 15, 840. https://doi.org/10.3390/ph15070840
Draginic N, Milosavljevic I, Andjic M, Jeremic J, Nikolic M, Sretenovic J, Kocovic A, Srejovic I, Zivkovic V, Bolevich S, et al. Short-Term Administration of Lemon Balm Extract Ameliorates Myocardial Ischemia/Reperfusion Injury: Focus on Oxidative Stress. Pharmaceuticals. 2022; 15(7):840. https://doi.org/10.3390/ph15070840
Chicago/Turabian StyleDraginic, Nevena, Isidora Milosavljevic, Marijana Andjic, Jovana Jeremic, Marina Nikolic, Jasmina Sretenovic, Aleksandar Kocovic, Ivan Srejovic, Vladimir Zivkovic, Sergey Bolevich, and et al. 2022. "Short-Term Administration of Lemon Balm Extract Ameliorates Myocardial Ischemia/Reperfusion Injury: Focus on Oxidative Stress" Pharmaceuticals 15, no. 7: 840. https://doi.org/10.3390/ph15070840
APA StyleDraginic, N., Milosavljevic, I., Andjic, M., Jeremic, J., Nikolic, M., Sretenovic, J., Kocovic, A., Srejovic, I., Zivkovic, V., Bolevich, S., Bolevich, S., Curcic, S., & Jakovljevic, V. (2022). Short-Term Administration of Lemon Balm Extract Ameliorates Myocardial Ischemia/Reperfusion Injury: Focus on Oxidative Stress. Pharmaceuticals, 15(7), 840. https://doi.org/10.3390/ph15070840