Impaired Vitamin D Metabolism in Hospitalized COVID-19 Patients
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Population and Design
4.2. Laboratory Measurements
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, Y.-R.; Cao, Q.-D.; Hong, Z.-S.; Tan, Y.Y.; Chen, S.-D.; Jin, H.-D.; Tan, K.-S.; Wang, D.-Y.; Yan, Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—An update on the status. Mil. Med. Res. 2020, 7, 11. [Google Scholar] [CrossRef] [Green Version]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [Green Version]
- Hansdottir, S.; Monick, M.M. Vitamin D Effects on Lung Immunity and Respiratory Diseases. Vitam. Horm. 2011, 86, 217–237. [Google Scholar] [CrossRef] [Green Version]
- Bilezikian, J.P.; Bikle, D.; Hewison, M.; Lazaretti-castro, M.; Formenti, A.M. Vitamin D and COVID-19. Eur. J. Endocrinol. 2020, 183, R133–R147. [Google Scholar] [CrossRef]
- Chun, R.F.; Liu, P.T.; Modlin, R.L.; Adams, J.S.; Hewison, M. Impact of vitamin D on immune function: Lessons learned from genome-wide analysis. Front. Physiol. 2014, 5, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Sun, J.; Wang, X.; Zhang, T.; Zhao, M.; Li, H. Low vitamin D status is associated with coronavirus disease 2019 outcomes: A systematic review and meta-analysis. Int. J. Infect. Dis. 2021, 104, 58–64. [Google Scholar] [CrossRef]
- Teshome, A.; Adane, A.; Girma, B.; Mekonnen, Z.A. The Impact of Vitamin D Level on COVID-19 Infection: Systematic Review and Meta-Analysis. Front. Public Health 2021, 9, 624559. [Google Scholar] [CrossRef]
- Kaya, M.O.; Pamukçu, E.; Yakar, B. The role of vitamin D deficiency on the Covid-19: A systematic review and meta-analysis of observational studies. Epidemiol. Health 2021, 43, e2021074. [Google Scholar] [CrossRef]
- Szarpak, L.; Rafique, Z.; Gasecka, A.; Chirico, F.; Gawel, W.; Hernik, J.; Kaminska, H.; Filipiak, K.J.; Jaguszewski, M.J.; Szarpak, L. A systematic review and meta-analysis of effect of vitamin D levels on the incidence of COVID-19. Cardiol. J. 2021, 28, 647–654. [Google Scholar] [CrossRef]
- Akbar, M.R.; Wibowo, A.; Pranata, R.; Setiabudiawan, B. Low Serum 25-hydroxyvitamin D (Vitamin D) Level Is Associated With Susceptibility to COVID-19, Severity, and Mortality: A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 1–10. [Google Scholar] [CrossRef]
- Petrelli, F.; Luciani, A.; Perego, G.; Dognini, G.; Luigi, P.; Ghidini, A. Therapeutic and prognostic role of vitamin D for COVID-19 infection: A systematic review and meta-analysis of 43 observational studies. J. Steroid Biochem. Mol. Biol. 2021, 211, 105883. [Google Scholar] [CrossRef]
- Margarucci, L.M.; Montanari, E.; Gianfranceschi, G.; Caprara, C.; Valeriani, F.; Piccolella, A.; Lombardi, V.; Scaramucci, E.; Spica, V.R. The role of vitamin D in prevention of COVID-19 and its severity: An umbrella review. Acta Biomed. 2021, 92, e2021451. [Google Scholar] [CrossRef]
- Chiodini, I.; Gatti, D.; Soranna, D.; Merlotti, D.; Mingiano, C.; Fassio, A.; Falchetti, A.; Eller-Vainicher, C.; Rossini, M.; Persani, L.; et al. Vitamin D Status and SARS-CoV-2 Infection and COVID-19 Clinical Outcomes. Front. Public Health 2021, 9, 1–19. [Google Scholar] [CrossRef]
- Oscanoa, T.J.; Amado, J.; Vidal, X.; Laird, E.; Ghashut, R.A.; Romero-Ortuno, R. The relationship between the severity and mortality of SARS-COV-2 infection and 25-hydroxy vitamin D concentration–a metaanalysis. Adv. Respir. Med. 2021, 89, 175–187. [Google Scholar] [CrossRef]
- Wang, Z.; Joshi, A.; Leopold, K.; Jackson, S.; Christensen, S.; Nayfeh, T.; Mohammed, K.; Creo, A.; Tebben, P.; Kumar, S. Association of vitamin D deficiency with COVID-19 infection severity: Systematic review and meta-analysis. Clin. Endocrinol. 2021, 96, 1–7. [Google Scholar] [CrossRef]
- Bassatne, A.; Basbous, M.; Chakhtoura, M.; Zein, O.E.; Rahme, M.; Fuleihan, G.E.-H. The link between COVID-19 and VItamin D (VIVID): A systematic review and meta-analysis. Metab. Clin. Exp. 2021, 119, 154753. [Google Scholar] [CrossRef]
- Chen, J.; Mei, K.; Xie, L.; Yuan, P.; Ma, J.; Yu, P.; Zhu, W.; Zheng, C.; Liu, X. Low vitamin D levels do not aggravate COVID-19 risk or death, and vitamin D supplementation does not improve outcomes in hospitalized patients with COVID-19: A meta-analysis and GRADE assessment of cohort studies and RCTs. Nutr. J. 2021, 20, 1–10. [Google Scholar] [CrossRef]
- Szarpak, L.; Filipiak, K.J.; Gasecka, A.; Gawel, W.; Koziel, D.; Jaguszewski, M.J.; Chmielewski, J.; Gozhenko, A.; Bielski, K.; Wroblewski, P.; et al. Vitamin D supplementation to treat SARS-CoV-2 positive patients. Evidence from meta-analysis. Cardiol. J. 2021, 29, 188–196. [Google Scholar] [CrossRef]
- Shah, K.; Saxena, D.; Mavalankar, D. Vitamin D supplementation, COVID-19 and disease severity: A meta-analysis. QJM 2021, 114, 175–181. [Google Scholar] [CrossRef]
- Rocha, A.P.d.; Atallah, A.N.; Aldrighi, J.M.; Pires, A.L.R.; Puga, M.E.d.S.; Pinto, A.C.P.N. Insufficient evidence for vitamin D use in COVID-19: A rapid systematic review. Int. J. Clin. Pract. 2021, 75, 1–11. [Google Scholar] [CrossRef]
- Rawat, D.; Roy, A.; Maitra, S.; Shankar, V.; Khanna, P.; Baidya, D.K. Vitamin D supplementation and COVID-19 treatment: A systematic review and meta-analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102189. [Google Scholar] [CrossRef]
- Stroehlein, J.K.; Wallqvist, J.; Iannizzi, C.; Mikolajewska, A.; Metzendorf, M.-I.; Benstoem, C.; Meybohm, P.; Becker, M.; Skoetz, N.; Stegemann, M.; et al. Vitamin D supplementation for the treatment of COVID-19: A living systematic review. Cochrane Database Syst. Rev. 2021, 5, CD015043. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Banerjee, M.; Bhadada, S.K.; Shetty, A.J.; Singh, B.; Vyas, A. Vitamin D supplementation and clinical outcomes in COVID-19: A systematic review and meta-analysis. J. Endocrinol. Investig. 2022, 45, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Dramé, M.; Cofais, C.; Hentzien, M.; Proye, E.; Coulibaly, P.S.; Demoustier-Tampère, D.; Destailleur, M.-H.; Lotin, M.; Cantagrit, E.; Cebille, A.; et al. Relation between vitamin D and COVID-19 in aged people: A systematic review. Nutrients 2021, 13, 1339. [Google Scholar] [CrossRef]
- Hosseini, B.; Abd, A.E.; Ducharme, F. Effects of Vitamin D Supplementation on COVID-19 Related Outcomes: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 2134. [Google Scholar] [CrossRef]
- Giannini, S.; Passeri, G.; Tripepi, G.; Sella, S.; Fusaro, M.; Arcidiacono, G.; Torres, M.O.; Michielin, A.; Prandini, T.; Baffa, V.; et al. Effectiveness of in-hospital cholecalciferol use on clinical outcomes in comorbid COVID-19 patients: A hypothesis-generating study. Nutrients 2021, 13, 219. [Google Scholar] [CrossRef]
- Azer, S.M.; Vaughan, L.E.; Tebben, P.J.; Sas, D.J. 24-Hydroxylase Deficiency Due to CYP24A1 Sequence Variants: Comparison with Other Vitamin D−mediated Hypercalcemia Disorders. J. Endocr. Soc. 2021, 5, 1–10. [Google Scholar] [CrossRef]
- Schlingmann, K.P.; Kaufmann, M.; Weber, S.; Lrwin, A.; Goos, C.; John, U.; Misselwitz, J.; Klaus, G.; Fehrenbach, H.; Wingin, A.M.; et al. Mutations in CYP24A1 and Idiopathic Infantile Hypercalcemia. N. Engl. J. Med. Orig. 2011, 365, 410–421. [Google Scholar] [CrossRef]
- Kaufmann, M.; Gallagher, J.C.; Peacock, M.; Schlingmann, K.-P.; Konrad, M.; DeLuca, H.F.; Sigueiro, R.; Lopez, B.; Mourino, A.; Maestro, M.; et al. Clinical utility of simultaneous quantitation of 25-hydroxyvitamin D and 24,25-dihydroxyvitamin D by LC-MS/MS involving derivatization with DMEQ-TAD. J. Clin. Endocrinol. Metab. 2014, 99, 2567–2574. [Google Scholar] [CrossRef]
- Kaufmann, M.; Morse, N.; Molloy, B.J.; Cooper, D.P.; Schlingmann, K.P.; Molin, A.; Kottler, M.L.; Gallagher, J.C.; Armas, L.; Jones, G. Improved Screening Test for Idiopathic Infantile Hypercalcemia Confirms Residual Levels of Serum 24,25-(OH)2D3 in Affected Patients. J. Bone Miner. Res. 2017, 32, 1589–1596. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, M.; Schlingmann, K.P.; Berezin, L.; Molin, A.; Sheftel, J.; Vig, M.; Gallagher, J.C.; Nagata, A.; Masoud, S.S.; Sakamoto, R.; et al. Differential diagnosis of vitamin D–related hypercalcemia using serum vitamin D metabolite profiling. J. Bone Miner. Res. 2021, 36, 1340–1350. [Google Scholar] [CrossRef] [PubMed]
- Cavalier, E.; Huyghebaert, L.; Rousselle, O.; Bekaert, A.-C.; Kovacs, S.; Vranken, L.; Peeters, S.; Goff, C.L.; Ladang, A. Simultaneous measurement of 25(OH)-vitamin D and 24,25(OH)2-vitamin D to define cut-offs for CYP24A1 mutation and vitamin D deficiency in a population of 1200 young subjects. Clin. Chem. Lab. Med. 2020, 58, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wang, D.; Yin, Y.; Cheng, Q.; Xie, S.; Yu, J.; Sun, D.; Cheng, X.; Qiu, L. Sources of variation evaluation of 24,25(OH)2D levels and the ratio of 25OHD to 24,25(OH)2D in apparently healthy Chinese adults: A multicenter cross-sectional study. J. Steroid Biochem. Mol. Biol. 2019, 192, 105407. [Google Scholar] [CrossRef] [PubMed]
- Francic, V.; Ursem, S.R.; Dirks, N.F.; Keppel, M.H.; Theiler-Schwetz, V.; Trummer, C.; Pandis, M.; Borzan, V.; Grübler, M.R.; Verheyen, N.D.; et al. The effect of vitamin D supplementation on its metabolism and the vitamin D metabolite ratio. Nutrients 2019, 11, 2539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginsberg, C.; Katz, R.; de Boer, I.H.; Kestenbaum, B.R.; Chonchol, M.; Shlipak, M.G.; Sarnak, M.J.; Hoofnagle, A.N.; Rifkin, D.E.; Garimella, P.S.; et al. The 24,25 to 25-hydroxyvitamin D ratio and fracture risk in older adults: The cardiovascular health study. Bone 2018, 107, 124–130. [Google Scholar] [CrossRef]
- Ginsberg, C.; Hoofnagle, A.N.; Katz, R.; Hughes-Austin, J.; Miller, L.M.; Becker, J.O.; Kritchevsky, S.B.; Shlipak, M.G.; Sarnak, M.J.; Ix, J.H. The Vitamin D Metabolite Ratio Is Associated With Changes in Bone Density and Fracture Risk in Older Adults. J. Bone Miner. Res. 2021, 36, 1–8. [Google Scholar] [CrossRef]
- Tang, J.C.Y.; Jackson, S.; Walsh, N.P.; Greeves, J.; Franser, W.D.; Facilityteam, B. The dynamic relationships between the active and catabolic vitamin D metabolites, their ratios, and associations with PTH. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Zelzer, S.; Prüller, F.; Curcic, P.; Sloup, Z.; Holter, M.; Herrmann, M.; Mangge, H. Vitamin D metabolites and clinical outcome in hospitalized COVID-19 patients. Nutrients 2021, 13, 2129. [Google Scholar] [CrossRef]
- Gallelli, L.; Mannino, G.C.; Luciani, F.; Sire, A.d.; Mancuso, E.; Gangemi, P.; Cosco, L.; Monea, G.; Averta, C.; Minchella, P.; et al. Vitamin d serum levels in subjects tested for SARS-CoV-2: What are the differences among acute, healed, and negative COVID-19 patients? a multicenter real-practice study. Nutrients 2021, 13, 3932. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, M.C.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
- Pigarova, E.A.; Rozhinskaya, L.Y.; Belaya, J.E.; Dzeranova, L.K.; Karonova, T.; Llyin, A.V.; Melnichenko, G.A.; Dedov, I.I. Russian Association of Endocrinologists recommendations for diagnosis, treatment and prevention of vitamin D deficiency in adults. Probl. Endocrinol. 2016, 62, 60–84. [Google Scholar] [CrossRef]
- Dirks, N.F.; Martens, F.; Vanderschueren, D.; Billen, J.; Pauwels, S.; Ackermans, M.T.; Endert, E.; Heijer, M.d.; Blankenstein, M.A.; Heijboer, A.C. Determination of human reference values for serum total 1,25-dihydroxyvitamin D using an extensively validated 2D ID-UPLC–MS/MS method. J. Steroid Biochem. Mol. Biol. 2016, 164, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.C.Y.; Nicholls, H.; Piec, I.; Washbourne, C.J.; Dutton, J.J.; Jackson, S.; Greeves, J.; Franser, W.D. Reference intervals for serum 24,25-dihydroxyvitamin D and the ratio with 25-hydroxyvitamin D established using a newly developed LC–MS/MS method. J. Nutr. Biochem. 2017, 46, 21–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suplotova, L.; Avdeeva, V.; Pigarova, E.; Rozhinskaya, L.; Karonova, T.; Troshina, E. The first Russian multicenter non-interventional registry study to study the incidence of vitamin D deficiency and insufficiency in Russian Federation. Ter. Arkh. 2021, 93, 1209–1216. [Google Scholar] [CrossRef]
- Mercola, J.; Grant, W.B.; Wagner, C.L. Evidence regarding vitamin D and risk of COVID-19 and its severity. Nutrients 2020, 12, 3361. [Google Scholar] [CrossRef]
- Hernández, J.L.; Nan, D.; Fernandez-Ayala, M.; García-Unzueta, M.; Hernández-Hernández, M.A.; López-Hoyos, M.; Muñoz-Cacho, P.; Olmos, J.M.; Gutiérrez-Cuadra, M.; Ruiz-Cubillán, J.J.; et al. Vitamin D Status in Hospitalized Patients with SARS-CoV-2 Infection. J. Clin. Endocrinol. Metab. 2020, 106, 1343–1353. [Google Scholar] [CrossRef]
- Portale, A.A.; Halloran, B.P.; Morris, R.C.; Lonergan, E.T. Effect of aging on the metabolism of phosphorus and 1,25-dihydroxyvitamin D in healthy men. Am. J. Physiol.–Endocrinol. Metab. 1996, 270, E483–E490. [Google Scholar] [CrossRef]
- Jean, G.; Souberbielle, J.C.; Chazot, C. Vitamin D in chronic kidney disease and dialysis patients. Nutrients 2017, 9, 328. [Google Scholar] [CrossRef]
- Parikh, S.J.; Edelman, M.; Uwaifo, G.I.; Freedman, R.J.; Semega-Janneh, M.; Reynolds, J.; Yanovski, J.A. The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. J. Clin. Endocrinol. Metab. 2004, 89, 1196–1199. [Google Scholar] [CrossRef] [Green Version]
- Mulligan, J.K.; Pasquini, W.N.; Carroll, W.W.; Williamson, T.; Reaves, N.; Patel, K.J.; Mappus, E.; Schlosser, R.J.; Atkinson, C. Dietary vitamin D3 deficiency exacerbates sinonasal inflammation and alters local 25(OH)D3 metabolism. PLoS ONE 2017, 12, e0186374. [Google Scholar] [CrossRef]
- Vijayendra Chary, A.; Hemalatha, R.; Seshacharyulu, M.; Vasudeva Murali, M.; Jayaprakash, D.; Dinesh Kumar, B. Vitamin D deficiency in pregnant women impairs regulatory T cell function. J. Steroid Biochem. Mol. Biol. 2015, 147, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M. Vitamin D and the intracrinology of innate immunity. Mol. Cell Endocrinol. 2010, 321, 103–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.H.; Tang, X.; Wasnik, S.; Wang, X.; Zhang, J.; Xu, Y.; Lau, K.-H.W.; Nguyen, H.B.; Baylink, D.J. Mechanistic study of the cause of decreased blood 1,25-Dihydroxyvitamin D in sepsis. BMC Infect. Dis. 2019, 19, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, H.B.; Eshete, B.; Lau, K.H.W.; Sai, A.; Villarin, M.; Baylink, D. Serum 1,25-Dihydroxyvitamin D: An Outcome Prognosticator in Human Sepsis. PLoS ONE 2013, 8, e64348. [Google Scholar] [CrossRef]
- Subramanian, S.; Rhodes, J.M.; Taylor, J.M.; Milan, A.M.; Lane, S.; Hewison, M.; Chun, R.F.; Jorgensen, A.; Richardson, P.; Nitchingham, D.; et al. Vitamin D, vitamin D–binding protein, free vitamin D and COVID-19 mortality in hospitalized patients. Am. J. Clin. Nutr. 2022, 115, 1367–1377. [Google Scholar] [CrossRef]
- Dahl, B.; Schiødt, F.V.; Ott, P.; Fank, W.; William, L.; Jody, B.; Grant, O. Plasma concentration of Gc-globulin is associated with organ dysfunction and sepsis after injury. Crit. Care Med. 2003, 31, 152–156. [Google Scholar] [CrossRef]
- Dahl, B.; Schiødt, F.V.; Rudolph, S.; Ott, P.; Kiær, T.; Heslet, L. Trauma stimulates the synthesis of Gc-globulin. Intensive Care Med. 2001, 27, 394–399. [Google Scholar] [CrossRef]
- Kelly, A.; Levine, M.A. Hypocalcemia in the critically ill patient. J. Intensive Care Med. 2013, 28, 166–177. [Google Scholar] [CrossRef]
- Filippo, L.d.; Formenti, A.M.; Rovere-Querini, P.; Carlucci, M.; Conte, C.; Ciceri, F.; Zangrillo, A.; Giustina, A. Hypocalcemia is highly prevalent and predicts hospitalization in patients with COVID-19. Endocrine 2020, 68, 475–478. [Google Scholar] [CrossRef]
- Filippo, L.D.; Allora, A.; Locatelli, M.; Querini, P.R.; Frara, S.; Banfi, G.; Giustina, A. Hypocalcemia in COVID-19 is associated with low vitamin D levels and impaired compensatory PTH response. Endocrine 2021, 74, 219–225. [Google Scholar] [CrossRef]
- Hashemipour, S.; Kiani, S.; Shahsavari, P.; Afshar, S.; Ghobadi, A.; Khairkhahan, S.M.R.H.; Badri, M.; Farzam, S.S.; Sohrabi, H.; Seddighi, M.; et al. Hypocalcemia in hospitalized patients with COVID-19: Roles of hypovitaminosis D and functional hypoparathyroidism. J. Bone Miner. Metab. 2022, 40, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Roizen, J.D.; Li, D.; O’Lear, L.; Javaid, M.K.; Shaw, N.J.; Ebeling, P.R.; Nguyen, H.H.; Rodda, C.P.; Thummel, K.E.; Thacher, T.D.; et al. CYP3A4 mutation causes Vitamin D-dependent rickets type 3. J. Clin. Investig. 2018, 128, 1913–1918. [Google Scholar] [CrossRef]
- Rahmaniyan, M.; Patrick, K.; Bell, N.H. Characterization of recombinant CYP2C11: A vitamin D 25-hydroxylase and 24-hydroxylase. Am. J. Physiol.-Endocrinol. Metab. 2005, 288, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Tuckey, R.C.; Cheng, C.Y.S.; Slominski, A.T. The serum vitamin D metabolome: What we know and what is still to discover. J. Steroid Biochem. Mol. Biol. 2019, 186, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Temporary guidelines – prevention, diagnosis and treatment of new coronavirus infection (COVID-19). Ministry of Health of Russia. Version VI. 28.04.2020. Available online: https://static-1.rosminzdrav.ru/system/attachments/attaches/000/050/116/original/28042020_MR_COVID-19_v6.pdf (accessed on 20 July 2022).
- Povaliaeva, A.; Pigarova, E.; Zhukov, A.; Bogdanov, V.; Dzeranova, L.; Mel’nikova, O.; Pekareva, E.; Malysheva, N.; Ioutsi, V.; Nikankina, L.; et al. Evaluation of vitamin D metabolism in patients with type 1 diabetes mellitus in the setting of cholecalciferol treatment. Nutrients 2020, 12, 3873. [Google Scholar] [CrossRef] [PubMed]
- Thode, J.; Juul-Jørgensen, B.; Bhatia, H.M.; Kjaerulf-Nielsen, M.; Bartels, P.D.; Fogh-Andersen, N.; Siggaard-Andersen, O. Comparison of serum total calcium, albumin-corrected total calcium, and ionized calcium in 1213 patients with suspected calcium disorders. Scand. J. Clin. Lab. Investig. 1989, 49, 217–223. [Google Scholar] [CrossRef]
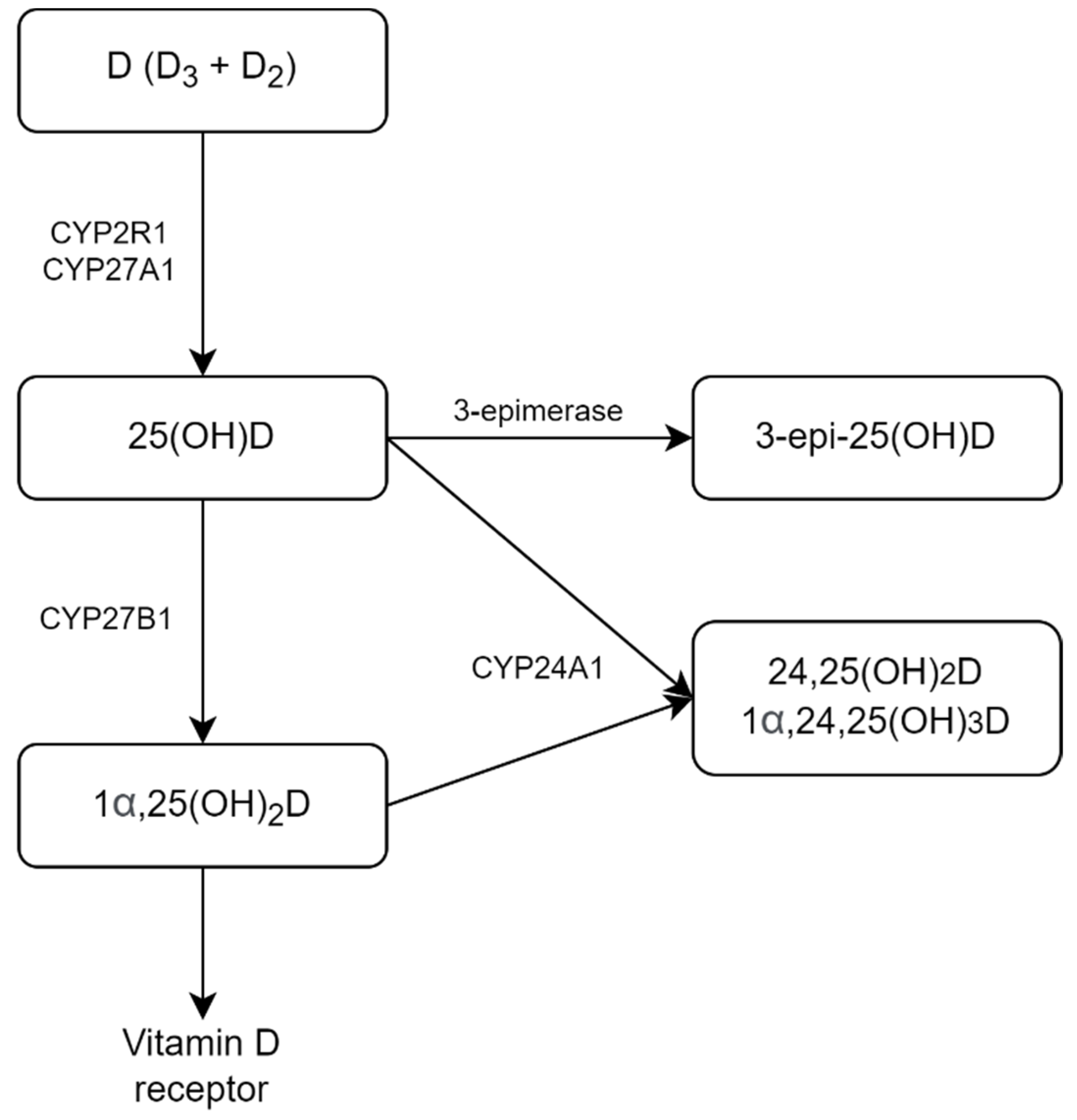
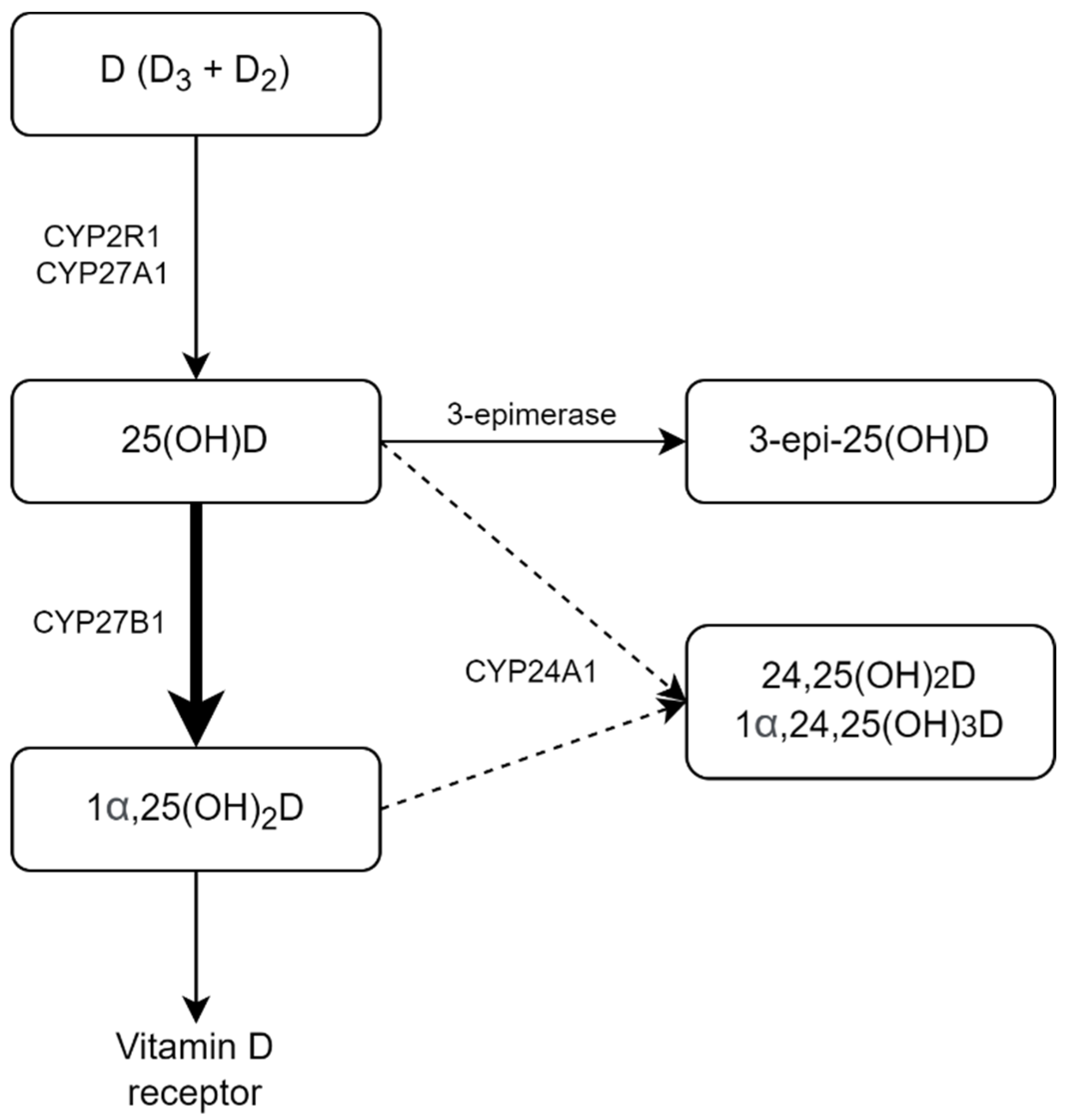

| Parameter | COVID-19 Group (n = 119) | Reference Group (n = 44) | p-Value |
|---|---|---|---|
| Age, years | 61 [47; 73] | 26 [24; 31] | <0.001 |
| Sex (male/female), n | 61/58 | 12/32 | 0.008 |
| Body mass index, kg/m2 | 28.9 [24.9; 32.8] | 21.5 [19.6; 25.7] | <0.001 |
| Parameter | Value |
|---|---|
| Evaluation at the time of admission | |
| Time from symptom onset to hospitalization, days | 9 [6; 11] |
| No. (%) of PCR positive | 53 (45%) |
| No. (%) CT positive | 115 (97%) |
| NEWS, points | 4 [2; 6] |
| Body temperature, °C | 37.4 [36.6; 38.0] |
| Respiratory rate, per minute | 22 [20; 26] |
| Systolic blood pressure, mmHg | 130 [119; 140] |
| Diastolic blood pressure, mmHg | 80 [72; 90] |
| Lung involvement, % | 28 [14; 42] |
| No. (%) of requiring respiratory support | 54 (45%) |
| SpO2, % | 94 [92; 96] |
| C-reactive protein, mg/L | 75.5 [31.8; 139.6] |
| D-dimer, ng/mL | 282 [164; 463] |
| Prothrombin time, s | 12.2 [11.5; 13.2] |
| Inpatient setting | |
| Bed-days, n | 12 [10; 15] |
| No. (%) of receiving antibiotic treatment | 96 (81%) |
| No. (%) of receiving anticoagulant treatment | 88 (74%) |
| No. (%) of treated with hydroxychloroquine | 36 (30%) |
| No. (%) of treated with immunobiological drugs | 21 (18%) |
| No. (%) of transferred to intensive care unit | 11 (9%) |
| No. (%) of fatal outcomes | 10 (8%) |
| Laboratory Parameter | COVID-19 Group (n = 119) | Reference Group (n = 44) | Normal Range | p-Value |
|---|---|---|---|---|
| Creatinine, μmol/L | 121 [88; 148] | 70 [65; 78] | 63–110 (male) 50–98 (female) | <0.001 |
| Total calcium, mmol/L | 2.19 [2.12; 2.30] | 2.40 [2.34; 2.47] | 2.15–2.55 | <0.001 |
| Albumin, g/L | 39.5 [36; 42] | 47 [46; 49] | 35–50 | <0.001 |
| Albumin-adjusted calcium, mmol/L | 2.23 [2.17; 2.28] | 2.25 [2.20; 2.31] | 2.15–2.55 | 0.06 |
| Phosphorus, mmol/L | 1.07 [0.93; 1.21] | 1.14 [1.02; 1.26] | 0.74–1.52 | 0.04 |
| PTH, pg/mL | 45.7 [29.8; 67.6] | 40.8 [32.2; 52.2] | 15–65 | 0.65 |
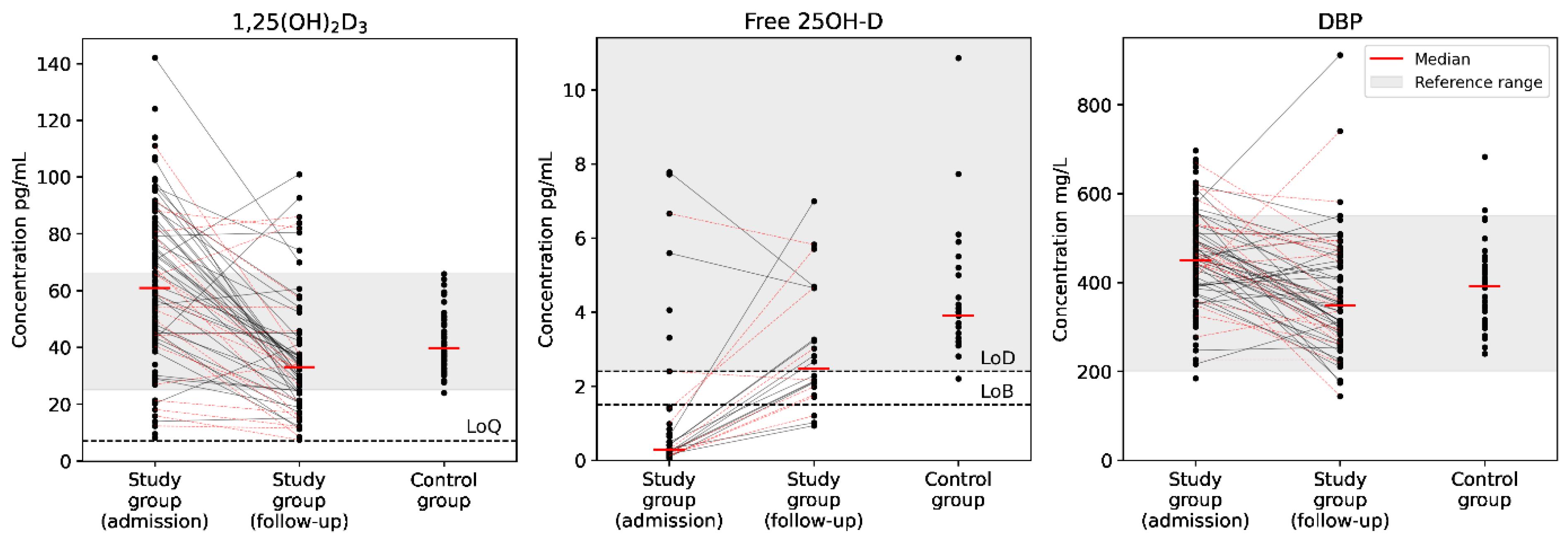
| Laboratory Parameter | COVID-19 Group, Admission (n = 119) | COVID-19 Group, Follow-Up (n = 62) | Reference Group (n = 44) | Normal Range | p-Value (Mann-Whitney) 1 | p-Value (Wilcoxon) |
|---|---|---|---|---|---|---|
| Free 25OH-D, pg/mL | <LoB | 2.5 [2.0; 4.7] | 3.9 [3.2; 4.4] | 2.4–35 2 | <0.001; 0.004 | 0.002 |
| DBP, mg/L | 450 [386; 515] | 348 [283; 449] | 392 [311; 433] | 200–550 2 | <0.001; 0.13 | <0.001 |
| 25OH-D3, ng/mL | 10.8 [6.2; 15.6] | 9.4 [5.2; 13.0] | 10.9 [8.4; 13.1] | >30 3 | 0.88; 0.18 | <0.001 |
| 3-epi-25OH-D3, ng/mL | 0.6 [0.4; 1.0] | 0.5 [0.2; 0.8] | 0.6 [0.4; 0.8] | not available | 0.91; 0.48 | <0.001 |
| 24,25(OH)2D3, ng/mL | 0.4 [0.1; 0.8] | 0.3 [0.1; 0.8] | 0.5 [0.3; 0.9] | 0.5–5.6 4 | 0.07; 0.02 | <0.001 |
| 1,25(OH)2D3, pg/mL | 61 [44; 81] | 33 [21; 45] | 40 [35; 50] | 25–66 4 | <0.001; 0.004 | <0.001 |
| 25OH-D3/ 24,25(OH)2D3 | 25.9 [19.0; 46.2] | 28.6 [19.0; 52.2] | 18.6 [14.6; 34.7] | 7–23 4 | 0.001; <0.001 | 0.07 |
| 25OH-D3/ 1,25(OH)2D3 | 175 [112; 260] | 279 [165; 449] | 272 [200; 433] | not available | <0.001; <0.001 | <0.001 |
| Laboratory Parameter | Moderately Severe (n = 85) 1 | Severe (n = 34) 1 | Normal Range | p-Value (Mann–Whitney) 1 |
|---|---|---|---|---|
| Creatinine, μmol/L | 124 [83; 151] | 114 [101; 143] | 63–110 (male) 50–98 (female) | 0.68 |
| Total calcium, mmol/L | 2.20 [2.14; 2.31] | 2.15 [2.04; 2.23] | 2.15–2.55 | 0.04 |
| Albumin, g/L | 40 [36; 42] | 39 [36; 42] | 35–50 | 0.28 |
| Albumin-adjusted calcium, mmol/L | 2.24 [2.17; 2.29] | 2.21 [2.15; 2.26] | 2.15–2.55 | 0.16 |
| Phosphorus, mmol/L | 1.07 [0.94; 1.19] | 1.09 [0.90; 1.25] | 0.74–1.52 | 0.63 |
| Free 25OH-D, pg/mL | <LoB 2.7 [2.1; 3.3] | <LoB 2.2 [1.8; 4.7] | 2.4–35 2 | 0.80 0.90 |
| DBP, mg/L | 450 [391; 509] 342 [290; 434] | 442 [355; 531] 353 [267; 473] | 200–550 2 | 0.91 0.75 |
| 25OH-D3, ng/mL | 10.9 [6.4; 16] 9.8 [5.7; 14.1] | 8.8 [5.7; 15.5] 9.1 [3.3; 12.4] | >30 3 | 0.29 0.48 |
| 3-epi-25OH-D3, ng/mL | 0.6 [0.4; 1.1] 0.6 [0.4; 1.0] | 0.7 [0.3; 0.8] 0.6 [0.2; 0.8] | not available | 0.44 0.59 |
| 24,25(OH)2D3, ng/mL | 0.5 [0.1; 0.9] 0.4 [0.1; 0.9] | 0.3 [0.2; 0.7] 0.3 [0.1; 0.7] | 0.5–5.6 4 | 0.26 0.33 |
| 1,25(OH)2D3, pg/mL | 64 [46; 83] 34 [24; 43] | 54 [41; 79] 32 [15; 50] | 25–66 4 | 0.18 0.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Povaliaeva, A.; Bogdanov, V.; Pigarova, E.; Dzeranova, L.; Katamadze, N.; Malysheva, N.; Ioutsi, V.; Nikankina, L.; Rozhinskaya, L.; Mokrysheva, N. Impaired Vitamin D Metabolism in Hospitalized COVID-19 Patients. Pharmaceuticals 2022, 15, 906. https://doi.org/10.3390/ph15080906
Povaliaeva A, Bogdanov V, Pigarova E, Dzeranova L, Katamadze N, Malysheva N, Ioutsi V, Nikankina L, Rozhinskaya L, Mokrysheva N. Impaired Vitamin D Metabolism in Hospitalized COVID-19 Patients. Pharmaceuticals. 2022; 15(8):906. https://doi.org/10.3390/ph15080906
Chicago/Turabian StylePovaliaeva, Alexandra, Viktor Bogdanov, Ekaterina Pigarova, Larisa Dzeranova, Nino Katamadze, Natalya Malysheva, Vitaliy Ioutsi, Larisa Nikankina, Liudmila Rozhinskaya, and Natalia Mokrysheva. 2022. "Impaired Vitamin D Metabolism in Hospitalized COVID-19 Patients" Pharmaceuticals 15, no. 8: 906. https://doi.org/10.3390/ph15080906
APA StylePovaliaeva, A., Bogdanov, V., Pigarova, E., Dzeranova, L., Katamadze, N., Malysheva, N., Ioutsi, V., Nikankina, L., Rozhinskaya, L., & Mokrysheva, N. (2022). Impaired Vitamin D Metabolism in Hospitalized COVID-19 Patients. Pharmaceuticals, 15(8), 906. https://doi.org/10.3390/ph15080906






