Depression and Autoimmune Hypothyroidism—Their Relationship and the Effects of Treating Psychiatric and Thyroid Disorders on Changes in Clinical and Biochemical Parameters Including BDNF and Other Cytokines—A Systematic Review
Abstract
1. Introduction
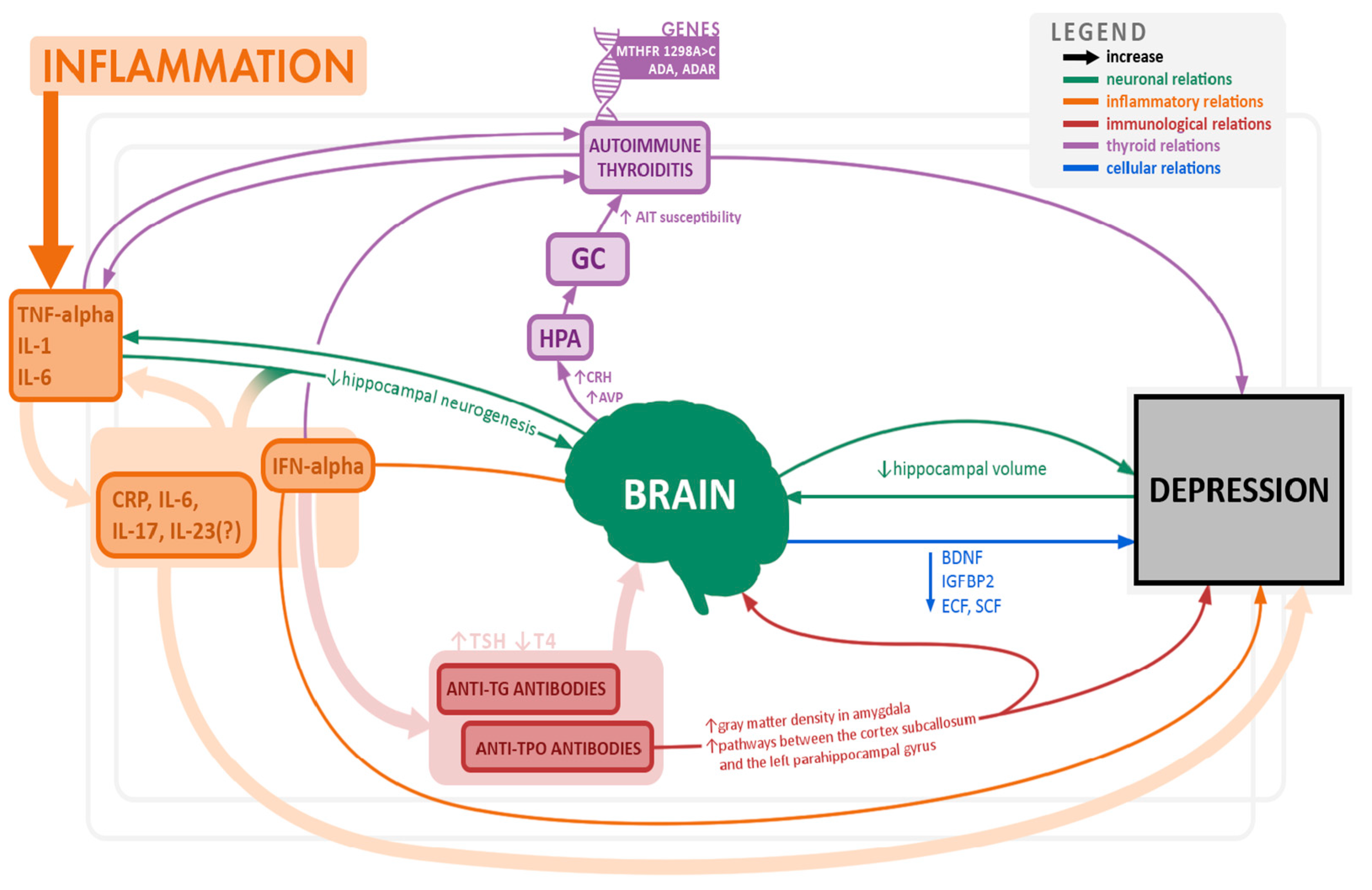
Autoimmune Hypothyroidism (AHT) and Depression
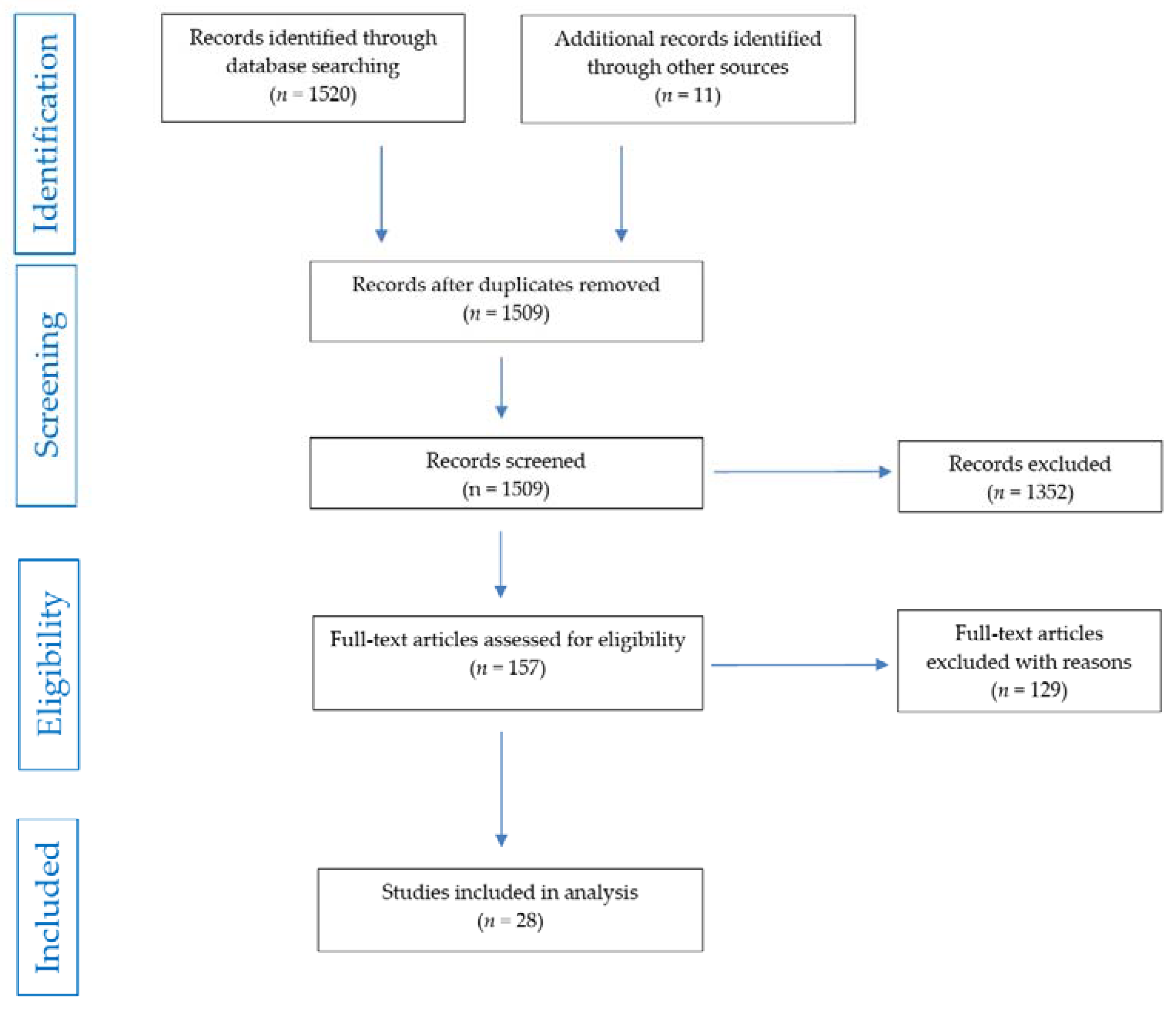
2. Methods
2.1. Review Question
2.2. Searches
2.3. Search Strategy
2.4. Condition or Domain Being Studied
2.5. Participants/Population
2.6. Intervention(s), Exposure(s)
2.7. Comparator(s)/Control
2.8. Types of Study to Be Included
2.9. Main Outcomes
2.10. Measures of Effect
2.11. Data Extraction
2.12. Risk of Bias (Quality) Assessment
2.13. Certainty Assessment
2.14. Strategy for Data Synthesis
2.15. Registration
3. Results and Discussion
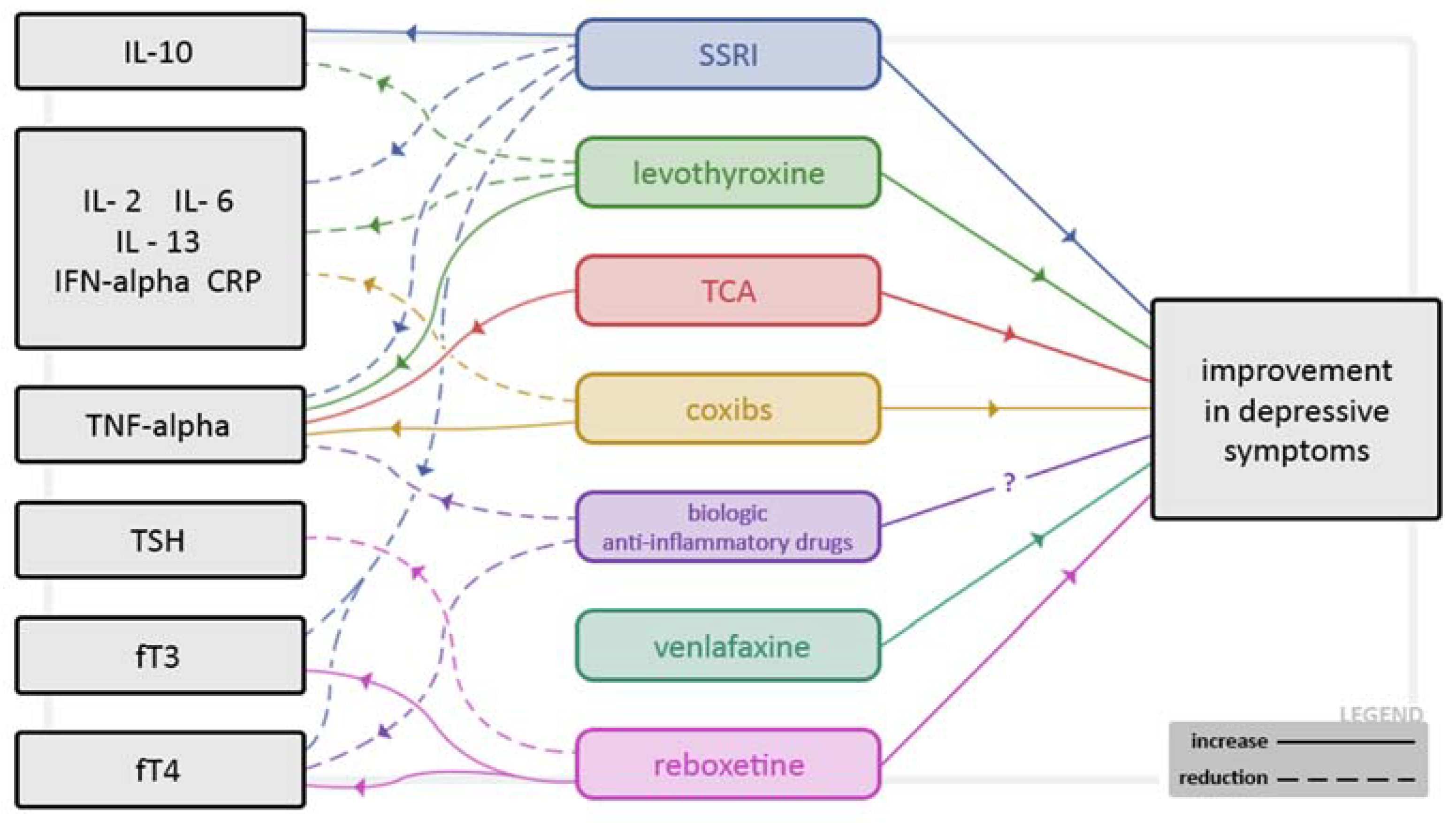
3.1. Inflammatory Processes, Cytokines and Growth Factors
3.2. Brain-Derived Neurotrophic Factor (BDNF) and Depression, AHT and Gender
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Boxtel, M.P.J.; Menheere, P.P.C.A.; Bekers, O.; Hogervorst, E.; Jolles, J. Thyroid Function, Depressed Mood, and Cognitive Performance in Older Individuals: The Maastricht Aging Study. Psychoneuroendocrinology 2004, 29, 891–898. [Google Scholar] [CrossRef]
- Hage, M.P.; Azar, S.T. The Link between Thyroid Function and Depression. J. Thyroid Res. 2012, 2012, 590648. [Google Scholar] [CrossRef]
- Kirkegaard, C.; Faber, J. The Role of Thyroid Hormones in Depression. Eur. J. Endocrinol. 1998, 138, 1–9. [Google Scholar] [CrossRef]
- Bhagwat, N.M.; Tayde, P.S.; Sharma, P.; Sharma, B.; Dalwadi, P.P.; Sonawane, A.; Subramanyam, A.; Chadha, M.; Varthakavi, P.K. Hypothyroidism and Depression: Are Cytokines the Link? Indian J. Endocrinol. Metab. 2017, 21, 886. [Google Scholar] [CrossRef] [PubMed]
- Boswell, E.B.; Anfinson, T.H.; Nemeroff, C.B. Depression Associated with Endocrine Disorder. In Depression and Physical Illness (Perspectives in Psychiatry Volume 6); Robertson, M.M., Katona, C.L.E., Eds.; John Wiley and Sons: Chichester, UK, 1996; pp. 256–292. [Google Scholar]
- Cleare, A.J.; McGregor, A.; O’Keane, V. Neuroendocrine Evidence for an Association between Hypothyroidism, Reduced Central 5-HT Activity and Depression. Clin. Endocrinol. (Oxf.) 1995, 43, 713–719. [Google Scholar] [CrossRef]
- Łojko, D.; Rybakowski, J. L-Thyroxine Augmentation of Serotonergic Antidepressants in Female Patients with Refractory Depression. J. Affect. Disord. 2007, 103, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Cooper-Kazaz, R.; Apter, J.T.; Cohen, R.; Karagichev, L.; Muhammed-Moussa, S.; Grupper, D.; Drori, T.; Newman, M.E.; Sackeim, H.A.; Glaser, B.; et al. Combined treatment with sertraline and liothyronine in major depression: A randomized, double-blind, placebo-controlled trial. Arch. Gen. Psychiatry 2007, 64, 679–688. [Google Scholar] [CrossRef]
- Kirim, S.; Keskek, S.Ö.; Köksal, F.; Haydardedeoglu, F.E.; Bozkirli, E.; Toledano, Y. Depression in Patients with Euthyroid Chronic Autoimmune Thyroiditis. Endocrine J. 2012, 59, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Dunn, D.; Turner, C. Hypothyroidism in Women. Nurs. Womens Health 2016, 20, 93–98. [Google Scholar] [CrossRef]
- Massolt, E.T.; Effraimidis, G.; Korevaar, T.I.; Wiersinga, W.M.; Visser, W.E.; Peeters, R.P.; Drexhage, H.A. Aberrant Levels of Hematopoietic/Neuronal Growth and Differentiation Factors in Euthyroid Women at Risk for Autoimmune Thyroid Disease. PLoS ONE 2016, 11, e0153892. [Google Scholar] [CrossRef]
- Carta, M.G.; Hardoy, M.C.; Carpiniello, B.; Murru, A.; Marci, A.R.; Carbone, F.; Deiana, L.; Cadeddu, M.; Mariotti, S. A Case Control Study on Psychiatric Disorders in Hashimoto Disease and Euthyroid Goitre: Not Only Depressive but Also Anxiety Disorders are Associated with thyroid autoimmunity. Clin. Pract. Epidemiol. Ment. Health 2005, 1, 23. [Google Scholar] [CrossRef] [PubMed]
- Benros, M.E.; Waltoft, B.L.; Nordentoft, M.; Østergaard, S.D.; Eaton, W.W.; Krogh, J.; Mortensen, P.B. Autoimmune Diseases and Severe Infections as Risk Factors for Mood Disorders. JAMA Psychiatry 2013, 70, 812. [Google Scholar] [CrossRef] [PubMed]
- Carta, M.G.; Loviselli, A.; Hardoy, M.C.; Massa, S.; Cadeddu, M.; Sardu, C.; Carpiniello, B.; Dell’Osso, L.; Mariotti, S. The Link between Thyroid Autoimmunity (Antithyroid Peroxidase Autoantibodies) with Anxiety and Mood Disorders in the Community: A Field of Interest for Public Health in the Future. BMC Psychiatry 2004, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Nemeroff, C.B.; Simon, J.S.; Haggerty, J.J.; Evans, D.L. Antithyroid Antibodies in Depressed Patients. Am. J. Psychiatry 1985, 142, 840–843. [Google Scholar] [CrossRef]
- Joffe, R.T. Antithyroid Antibodies in Major Depression. Acta Psychiatr. Scand. 1987, 76, 598–599. [Google Scholar] [CrossRef] [PubMed]
- Kupka, R.W.; Nolen, W.A.; Post, R.M.; McElroy, S.L.; Altshuler, L.L.; Denicoff, K.D.; Frye, M.A.; Keck, P.E.; Leverich, G.S.; Rush, A.J.; et al. High Rate of Autoimmune Thyroiditis in Bipolar Disorder: Lack of Association with Lithium Exposure. Biol. Psychiatry 2002, 51, 305–311. [Google Scholar] [CrossRef]
- Padmos, R.C.; Bekris, L.; Knijff, E.M.; Tiemeier, H.; Kupka, R.W.; Cohen, D.; Nolen, W.A.; Lernmark, Å.; Drexhage, H.A. A High Prevalence of Organ-Specific Autoimmunity in Patients with Bipolar Disorder. Biol. Psychiatry 2004, 56, 476–482. [Google Scholar] [CrossRef]
- Vonk, R.; van der Schot, A.C.; Kahn, R.S.; Nolen, W.A.; Drexhage, H.A. Is Autoimmune Thyroiditis Part of the Genetic Vulnerability (or an Endophenotype) for Bipolar Disorder? Biol. Psychiatry 2007, 62, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Hillegers, M.H.J.; Reichart, C.G.; Wals, M.; Verhulst, F.C.; Ormel, J.; Nolen, W.A.; Drexhage, H.A. Signs of a Higher Prevalence of Autoimmune Thyroiditis in Female Offspring of Bipolar Parents. Eur. Neuropsychopharmacol. 2007, 17, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Fountoulakis, K.N.; Iacovides, A.; Grammaticos, P.; St Kaprinis, G.; Bech, P. Thyroid Function in Clinical Subtypes of Major Depression: An Exploratory Study. BMC Psychiatry 2004, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Tsigos, C.; Chrousos, G.P. Hypothalamic–Pituitary–Adrenal Axis, Neuroendocrine Factors and Stress. J. Psychosom. Res. 2002, 53, 865–871. [Google Scholar] [CrossRef]
- Maes, M.; Meltzer, H.; Cosyns, P.; Suy, E.; Schotte, C. An Evaluation of Basal Hypothalamic-Pituitary-Thyroid Axis Function in Depression: Results of a Large-Scaled and Controlled Study. Psychoneuroendocrinology 1993, 18, 607–620. [Google Scholar] [CrossRef]
- Kuijpens, J.L.; Vader, H.L.; Drexhage, H.A.; Wiersinga, W.M.; van Son, M.J.; Pop, V.J. Thyroid Peroxidase Antibodies during Gestation Are a Marker for Subsequent Depression Postpartum. Eur. J. Endocrinol. 2001, 145, 579–584. [Google Scholar] [CrossRef]
- Elenkov, I.J.; Wilder, R.L.; Bakalov, V.K.; Link, A.A.; Dimitrov, M.A.; Fisher, S.; Crane, M.; Kanik, K.S.; Chrousos, G.P. IL-12, TNF-α, and Hormonal Changes during Late Pregnancy and Early Postpartum: Implications for Autoimmune Disease Activity during These Times. J. Clin. Endocrinol. Metabol. 2001, 86, 4933–4938. [Google Scholar] [CrossRef]
- Siegmann, E.-M.; Müller, H.H.; Luecke, C.; Philipsen, A.; Kornhuber, J.; Grömer, T.W. Association of Depression and Anxiety Disorders with Autoimmune Thyroiditis. A Systematic Review and Meta-analysis. JAMA Psychiatry 2018, 75, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Bode, H.; Ivens, B.; Bschor, T.; Schwarzer, G.; Henssler, J.; Baethge, C. Association of Hypothyroidism and Clinical Depression: A Systematic Review and Meta-Analysis. JAMA Psychiatry. 2021, 78, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Shojaie, M.; Eshraghian, A. Primary Hypothyroidism Presenting with Torsades De Pointes Type Tachycardia: A Case Report. Cases J. 2008, 1, 298. [Google Scholar] [CrossRef] [PubMed]
- Pop, V.J.; Maartens, L.H.; Leusink, G.; van Son, M.J.; Knottnerus, A.A.; Ward, A.M.; Metcalfe, R.; Weetman, A.P. Are Autoimmune Thyroid Dysfunction and Depression Related? J. Clin. Endocrinol. Metab. 1998, 83, 3194–3197. [Google Scholar] [CrossRef] [PubMed]
- Zettinig, G.; Asenbaum, S.; Fueger, B.J.; Hofmann, A.; Diemling, M.; Mittlboeck, M.; Dudczak, R. Increased Prevalence of Sublinical Brain Perfusion Abnormalities in Patients with Autoimmune Thyroiditis: Evidence of Hashimoto’s Encephalitis? Clin. Endocrinol. (Oxf.) 2003, 59, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Engum, A.; Bjøro, T.; Mykletun, A.; Dahl, A.A. Thyroid Autoimmunity, Depression and Anxiety; Are There Any Connections? an Epidemiological Study of a Large Population. J. Psychosom. Res. 2005, 59, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Gulseren, S.; Gulseren, L.; Hekimsoy, Z.; Cetinay, P.; Ozen, C.; Tokatlioglu, B. Depression, Anxiety, Health-related Quality of Life, and Disability in Patients with Overt and Subclinical Thyroid Dysfunction. Arch. Med. Res. 2006, 37, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Bunevicius, R.; Peceliuniene, J.; Mickuviene, N.; Bunevicius, A.; Pop, V.J.; Girdler, S.S. Mood and Thyroid Immunity Assessed by Ultrasonographic Imaging in a Primary Health Care. J. Affect. Disord. 2007, 97, 85–90. [Google Scholar] [CrossRef][Green Version]
- van der Deure, W.M.; Appelhof, B.C.; Peeters, R.P.; Wiersinga, W.M.; Wekking, E.M.; Huyser, J.; Schene, A.H.; Tijssen, J.G.; Hoogendijk, W.J.; Visser, T.J.; et al. Polymorphisms in the Brain-specific Thyroid Hormone Transporter OATP1C1 are Associated with Fatigue and Depression in Hypothyroid Patients. Clin. Endocrinol. (Oxf.) 2008, 69, 804–811. [Google Scholar] [CrossRef]
- Schinhammer, S. Untersuchung Möglicher Zusammenhänge Zwischen Hashimoto-Thyreoiditis, Psychischen Störungen und Genetischen Varianten des Adenosinsystems; Friedrich-Alexander-Universität: Erlangen-Nürnberg, Germany, 2010. [Google Scholar]
- Watt, T.; Hegedüs, L.; Bjorner, J.B.; Groenvold, M.; Bonnema, S.J.; Rasmussen, A.K.; Feldt-Rasmussen, U. Is Thyroid Autoimmunity per se a Determinant of Quality of Life in Patients with Autoimmune Hypothyroidism? Eur. Thyroid J. 2012, 1, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Franke, J.C. Untersuchungen zur Entwicklung von Depression und Angststörungen bei Hashimoto-Thyreoiditis; Friedrich-Alexander-Universität: Erlangen-Nürnberg, Germany, 2013. [Google Scholar]
- Giynas Ayhan, M.; Uguz, F.; Askin, R.; Gonen, M.S. The Prevalence of Depression and Anxiety Disorders in Patients with Euthyroid Hashimoto’s Thyroiditis: A Comparative Study. Gen. Hosp. Psychiatry 2014, 36, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Medici, M.; Direk, N.; Visser, W.E.; Korevaar, T.I.; Hofman, A.; Visser, T.J.; Tiemeier, H.; Peeters, R.P. Thyroid Function within the Normal Range and the Risk of Depression: A Population-based Cohort Study. J. Clin. Endocrinol. Metab. 2014, 99, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Demartini, B.; Ranieri, R.; Masu, A.; Selle, V.; Scarone, S.; Gambini, O. Depressive Symptoms and Major Depressive Disorder in Patients Affected by Subclinical Hypothyroidism: A Cross-sectional Study. J. Nerv. Ment. Dis. 2014, 202, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Quinque, E.M. Brain, Mood and Cognition in Hypothyroidism; Max-Planck-Institut für Kognitions-und Neurowissenschaften: Leipzig, Germany, 2015. [Google Scholar]
- Ittermann, T.; Völzke, H.; Baumeister, S.E.; Appel, K.; Grabe, H.J. Diagnosed Thyroid Disorders are Associated with Depression and Anxiety. Soc. Psychiatry Psychiatr. Epidemiol. 2015, 50, 1417–1425. [Google Scholar] [CrossRef]
- Fjaellegaard, K.; Kvetny, J.; Allerup, P.N.; Bech, P.; Ellervik, C. Well-being and Depression in Individuals with Subclinical Hypothyroidism and Thyroid Autoimmunity—A General Population Study. Nord. J. Psychiatry 2015, 69, 73–78. [Google Scholar] [CrossRef] [PubMed]
- van de Ven, A.C. Towards an Optimal TSH Level: Different Goals for Different Outcomes and for Different Populations? Radboud Universiteit Nijmegen: Nijmegen, The Netherlands, 2016. [Google Scholar]
- Krysiak, R.; Drosdzol-Cop, A.; Skrzypulec-Plinta, V.; Okopien, B. Sexual Function and Depressive Symptoms in Young Women with Thyroid Autoimmunity and Subclinical Hypothyroidism. Clin. Endocrinol. (Oxf.) 2016, 84, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Delitala, A.P.; Terracciano, A.; Fiorillo, E.; Orrù, V.; Schlessinger, D.; Cucca, F. Depressive Symptoms, Thyroid Hormone and Autoimmunity in a Population-based Cohort from Sardinia. J. Affect. Disord. 2016, 191, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, M.M.; Altinova, A.E.; Cavnar, B.; Bolayir, B.; Akturk, M.; Arslan, E.; Ozkan, C.; Cakir, N.; Balos Toruner, F. Is Thyroid Autoimmunity Itself Associated with Psychological Well-being in Euthyroid Hashimoto’s Thyroiditis? Endocr. J. 2017, 64, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Oh, S.S.; Park, E.C.; Jang, S.I. Sex Differences in the Association Between Thyroid-Stimulating Hormone Levels and Depressive Symptoms among the General Population with Normal Free T4 Levels. J. Affect. Disord. 2019, 249, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Dersch, R.; van Elst, L.T.; Hochstuhl, B.; Fiebich, B.L.; Stich, O.; Robinson, T.; Matysik, M.; Michel, M.; Runge, K.; Nickel, K.; et al. Anti-Thyroid Peroxidase and Anti-Thyroglobulin Autoantibodies in the Cerebrospinal Fluid of Patients with Unipolar Depression. J. Clin. Med. 2020, 9, 2391. [Google Scholar] [CrossRef] [PubMed]
- Minaldi, E.; D’Andrea, S.; Castellini, C.; Martorella, A.; Francavilla, F.; Francavilla, S.; Barbonetti, A. Thyroid Autoimmunity and Risk of Post-partum Depression: A Systematic Review and Meta-analysis of Longitudinal Studies. J. Endocrinol. Investig. 2020, 43, 271–277. [Google Scholar] [CrossRef]
- Hirtz, R.; Föcker, M.; Libuda, L.; Antel, J.; Öztürk, D.; Kiewert, C.; Munteanu, M.; Peters, T.; Führer, D.; Zwanziger, D.; et al. Increased Prevalence of Subclinical Hypothyroidism and Thyroid Autoimmunity in Depressed Adolescents: Results from a Clinical Cross-Sectional Study in Comparison to the General Pediatric Population. J. Clin. Psychiatry 2021, 82, 20m13511. [Google Scholar] [CrossRef] [PubMed]
- Kamyshna, I.I.; Pavlovych, L.B.; Kamyshnyi, A.M. Association Between NMDA Gene Polymorphism (rs4880213) and GRIN2B Blood Serum Levels in Thyroid Pathology Patients. J. Med. Life 2022, 15, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A Meta-Analysis of Cytokines in Major Depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef]
- Konca Degertekin, C.; Aktas Yilmaz, B.; Balos Toruner, F.; Kalkanci, A.; Turhan Iyidir, O.; Fidan, I.; Yesilyurt, E.; Cakır, N.; Kustimur, S.; Arslan, M. Circulating Th17 Cytokine Levels Are Altered in Hashimoto’s Thyroiditis. Cytokine 2016, 80, 13–17. [Google Scholar] [CrossRef]
- Fam, J.; Rush, A.J.; Burt, T.; Chan, E.S.; Siddiqui, F.J.; Assam, P.N.; Lai, O.F.; Chan, H.N.; Ng, B.Y.; Khoo, D.H. Thyroid Autoimmune Antibodies and Major Depressive Disorder in Women. Ann. Acad. Med. Singap. 2015, 44, 284–289. [Google Scholar]
- Ajjan, R.A.; Weetman, A.P. Cytokines in Thyroid Autoimmunity. Autoimmunity 2003, 36, 351–359. [Google Scholar] [CrossRef]
- Banks, W.A.; Kastin, A.J.; Broadwell, R.D. Passage of Cytokines Across the Blood-Brain Barrier. Neuroimmunomodulation 1995, 2, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Farr, S.A.; Morley, J.E. Entry of Blood-Borne Cytokines into the Central Nervous System: Effects on Cognitive Processes. Neuroimmunomodulation 2002, 10, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R. Cytokine-induced Sickness Behavior: Where Do We Stand? Brain Behav. Immun. 2001, 15, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Malberg, J.E.; Eisch, A.J.; Nestler, E.J.; Duman, R.S. Chronic Antidepressant Treatment Increases Neurogenesis in Adult Rat Hippocampus. J. Neurosci. 2000, 20, 9104–9110. [Google Scholar] [CrossRef]
- Saarelainen, T.; Hendolin, P.; Lucas, G.; Koponen, E.; Sairanen, M.; MacDonald, E.; Agerman, K.; Haapasalo, A.; Nawa, H.; Aloyz, R.; et al. Activation of the TrkB Neurotrophin Receptor Is Induced by Antidepressant Drugs and Is Required for Antidepressant-Induced Behavioral Effects. J. Neurosci. 2003, 23, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Ekdahl, C.T.; Claasen, J.-H.; Bonde, S.; Kokaia, Z.; Lindvall, O. Inflammation Is Detrimental for Neurogenesis in Adult Brain. Proc. Natl. Acad. Sci. USA 2003, 100, 13632–13637. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.; Marriott, M.; Nahmias, C.; MacQueen, G.M. Lower Hippocampal Volume in Patients Suffering From Depression: A Meta-Analysis. Am. J. Psychiatry 2004, 161, 598–607. [Google Scholar] [CrossRef]
- Seeler, M.J.; Christiansen, K.; Wegmann, R.; Bohnet, H.G. Persönlichkeitsmerkmale, körperliche Beschwerden und mikrosomaler Schilddrüsen-Antikörper-Titer bei frisch entbundenen Frauen [Personality markers, physical complaints and microsomal thyroid antibody titer in postpartum women]. Z. Geburtshilfe Neonatol. 1996, 200, 138–143. (In German) [Google Scholar]
- Baldissarelli, J.; Mânica, A.; Pillat, M.M.; Bagatini, M.D.; Leal, D.B.; Abdalla, F.H.; Morsch, V.M.; Ulrich, H.; Bornemann, C.P.; Chitolina Schetinger, M.R. Increased Cytokines Production and Oxidative Stress Are Related with Purinergic Signaling and Cell Survival in Post-Thyroidectomy Hypothyroidism. Mol. Cell. Endocrinol. 2020, 499, 110594. [Google Scholar] [CrossRef]
- Marchiori, R.C.; Pereira, L.A.; Naujorks, A.A.; Rovaris, D.L.; Meinerz, D.F.; Duarte, M.M.; Rocha, J.B. Improvement of Blood Inflammatory Marker Levels in Patients with Hypothyroidism under Levothyroxine Treatment. BMC Endocr. Disord. 2015, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Mikoś, H.; Mikoś, M.; Obara-Moszyńska, M.; Niedziela, M. The Role of the Immune System and Cytokines Involved in the Pathogenesis of Autoimmune Thyroid Disease (AITD). Endokrynol. Pol. 2014, 65, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Chiou, S.-H.; Chen, S.-J.; Peng, C.-H.; Chang, Y.-L.; Ku, H.-H.; Hsu, W.-M.; Ho, L.L.-T.; Lee, C.-H. Fluoxetine Up-Regulates Expression of Cellular FLICE-Inhibitory Protein and Inhibits LPS-Induced Apoptosis in Hippocampus-Derived Neural Stem Cell. Biochem. Biophys. Res. Commun. 2006, 343, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.L.; Ignatowski, T.A.; Sud, R.; Spengler, R.N. An Antidepressant Mechanism of Desipramine Is to Decrease Tumor Necrosis Factor-α Production Culminating in Increases in Noradrenergic Neurotransmission. Neuroscience 2005, 133, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.L.; Ignatowski, T.A.; Sud, R.; Spengler, R.N. Brain-Derived Tumor Necrosis Factor-α and Its Involvement in Noradrenergic Neuron Functioning Involved in the Mechanism of Action of an Antidepressant. J. Pharmacol. Exp. Ther. 2004, 310, 1216–1225. [Google Scholar] [CrossRef]
- Karakatsoulis, G.N.; Tsapakis, E.M.; Mitkani, C.; Fountoulakis, K.N. Subclinical Thyroid Dysfunction and Major Depressive Disorder. Hormones (Athens) 2021, 20, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Eker, S.S.; Akkaya, C.; Sarandol, A.; Cangur, S.; Sarandol, E.; Kirli, S. Effects of Various Antidepressants on Serum Thyroid Hormone Levels in Patients with Major Depressive Disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Caye, A.; Pilz, L.K.; Maia, A.L.; Hidalgo, M.P.; Furukawa, T.A.; Kieling, C. The Impact of Selective Serotonin Reuptake Inhibitors on the Thyroid Function Among Patients with Major Depressive Disorder: A Systematic Review and Meta-Analysis. Eur. Neuropsychopharmacol. 2020, 33, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Maia, A.L.; Goemann, I.M.; Meyer, E.L.; Wajner, S.M. Deiodinases: The Balance of Thyroid Hormone: Type 1 Iodothyronine Deiodinase in Human Physiology and Disease. J. Endocrinol. 2011, 209, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Yuan, R.; Ren, D.; Bi, Y.; Niu, W.; Guo, Z.; Wu, X.; Xu, F.; Sun, Q.; Ma, G.; et al. A Novel NR3C2 Polymorphism and The Increased Thyroid-Stimulating Hormone Concentration are Associated with Venlafaxine Treatment Outcome in Chinese Han MDD Patients. Psychiatry Res. 2020, 284, 112690. [Google Scholar] [CrossRef] [PubMed]
- Riepe, F.G.; Finkeldei, J.; de Sanctis, L.; Einaudi, S.; Testa, A.; Karges, B.; Peter, M.; Viemann, M.; Grötzinger, J.; Sippell, W.G.; et al. Elucidating the Underlying Molecular Pathogenesis of NR3C2 Mutants Causing Autosomal Dominant Pseudohypoaldosteronism Type 1. J. Clin. Endocrinol. Metab. 2006, 91, 4552–4561. [Google Scholar] [CrossRef] [PubMed]
- Papakostas, G.I.; Cooper-Kazaz, R.; Appelhof, B.C.; Posternak, M.A.; Johnson, D.P.; Klibanski, A.; Lerer, B.; Fava, M. Simultaneous Initiation (Coinitiation) of Pharmacotherapy with Triiodothyronine and A Selective Serotonin Reuptake Inhibitor for Major Depressive Disorder: A Quantitative Synthesis of Double-Blind Studies. Int. Clin. Psychopharmacol. 2009, 24, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.B.; Aftab, A.; Radhakrishnan, R.; Widge, A.; Rodriguez, C.I.; Carpenter, L.L.; Nemeroff, C.B.; McDonald, W.M.; Kalin, N.H. APA Council of Research Task Force on Novel Biomarkers and Treatments. Hormonal Treatments for Major Depressive Disorder: State of the Art. Am. J. Psychiatry 2020, 177, 686–705. [Google Scholar] [CrossRef]
- Müller, N.; Riedel, M.; Schwarz, M.J. Psychotropic Effects of COX-2 Inhibitors—A Possible New Approach for the Treatment of Psychiatric Disorders. Pharmacopsychiatry 2004, 37, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Müller, N.; Schwarz, M.J.; Dehning, S.; Douhe, A.; Cerovecki, A.; Goldstein-Müller, B.; Spellmann, I.; Hetzel, G.; Maino, K.; Kleindienst, N.; et al. The Cyclooxygenase-2 Inhibitor Celecoxib Has Therapeutic Effects in Major Depression: Results of a Double-Blind, Randomized, Placebo Controlled, Add-on Pilot Study to Reboxetine. Mol. Psychiatry 2006, 11, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Bavaresco, D.V.; Uggioni, M.; Ferraz, S.D.; Marques, R.; Simon, C.S.; Dagostin, V.S.; Grande, A.J.; da Rosa, M.I. Efficacy of Infliximab in Treatment-Resistant Depression: A Systematic Review and Meta-Analysis. Pharmacol. Biochem. Behav. 2020, 188, 172838. [Google Scholar] [CrossRef] [PubMed]
- Menter, A.; Augustin, M.; Signorovitch, J.; Yu, A.P.; Wu, E.Q.; Gupta, S.R.; Bao, Y.; Mulani, P. The Effect of Adalimumab on Reducing Depression Symptoms in Patients with Moderate to Severe Psoriasis: A Randomized Clinical Trial. J. Am. Acad. Dermatol. 2010, 62, 812–818. [Google Scholar] [CrossRef]
- Gordon, K.B.; Armstrong, A.W.; Han, C.; Foley, P.; Song, M.; Wasfi, Y.; You, Y.; Shen, Y.K.; Reich, K. Anxiety and Depression in Patients with Moderate-to-Severe Psoriasis and Comparison of Change from Baseline After Treatment with Guselkumab vs. Adalimumab: Results from The Phase 3 VOYAGE 2 Study. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1940–1949. [Google Scholar] [CrossRef] [PubMed]
- Tiosano, S.; Yavne, Y.; Watad, A.; Langevitz, P.; Lidar, M.; Feld, J.; Tishler, M.; Aamar, S.; Elkayam, O.; Balbir-Gurman, A.; et al. The Impact of Tocilizumab on Anxiety and Depression in Patients with Rheumatoid Arthritis. Eur. J. Clin. Investig. 2020, 50, e13268. [Google Scholar] [CrossRef] [PubMed]
- Behrens, F.; Burmester, G.R.; Feuchtenberger, M.; Kellner, H.; Kuehne, C.; Liebhaber, A.; Wassenberg, S.; Gerlach, J.; Zortel, M.; Hofmann, M.W.; et al. Characterisation of Depressive Symptoms in Rheumatoid Arthritis Patients Treated with Tocilizumab During Routine Daily Care. Clin. Exp. Rheumatol. 2021; Online ahead of print. [Google Scholar]
- Knight, J.M.; Costanzo, E.S.; Singh, S.; Yin, Z.; Szabo, A.; Pawar, D.S.; Hillard, C.J.; Rizzo, J.D.; D’Souza, A.; Pasquini, M.; et al. The IL-6 Antagonist Tocilizumab is Associated with Worse Depression and Related Symptoms in The Medically Ill. Transl. Psychiatry 2021, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Steeland, S.; Libert, C.; Vandenbroucke, R.E. A New Venue of TNF Targeting. Int. J. Mol. Sci. 2018, 19, 1442. [Google Scholar] [CrossRef] [PubMed]
- Paschou, S.A.; Palioura, E.; Kothonas, F.; Myroforidis, A.; Loi, V.; Poulou, A.; Goumas, K.; Effraimidis, G.; Vryonidou, A. The Effect of Anti-TNF Therapy on Thyroid Function in Patients with Inflammatory Bowel Disease. Endocr. J. 2018, 65, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- Akinci, B.; Comlekci, A.; Yener, S.; Bayraktar, F.; Demir, T.; Ozcan, M.A.; Yuksel, F.; Yesil, S. Hashimoto’s Thyroiditis, but not Treatment of Hypothyroidism, is Associated with Altered TGF-beta1 Levels. Arch. Med. Res. 2008, 39, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Mon, M.A.; Gomez-Lahoz, A.M.; Orozco, A.; Lahera, G.; Sosa-Reina, M.D.; Diaz, D.; Albillos, A.; Quintero, J.; Molero, P.; Monserrat, J.; et al. Blunted Expansion of Regulatory T Lymphocytes Is Associated with Increased Bacterial Translocation in Patients with Major Depressive Disorder. Front. Psychiatry 2021, 11, 591962. [Google Scholar] [CrossRef] [PubMed]
- Małczyńska, P.; Piotrowicz, Z.; Drabarek, D.; Langfort, J.; Chalimoniuk, M. The Role of the Brain-Derived Neurotrophic Factor (BDNF) in Neurodegenerative Processes and in the Neuroregeneration Mechanisms Induced by Increased Physical Activity. Postepy Biochem. 2019, 65, 2–8. [Google Scholar] [CrossRef]
- Shirayama, Y.; Chen, A.C.-H.; Nakagawa, S.; Russell, D.S.; Duman, R.S. Brain-Derived Neurotrophic Factor Produces Antidepressant Effects in Behavioral Models of Depression. J. Neurosci. 2002, 22, 3251–3261. [Google Scholar] [CrossRef] [PubMed]
- Matrisciano, F.; Bonaccorso, S.; Ricciardi, A.; Scaccianoce, S.; Panaccione, I.; Wang, L.; Ruberto, A.; Tatarelli, R.; Nicoletti, F.; Girardi, P.; et al. Changes in BDNF Serum Levels in Patients with Major Depression Disorder (MDD) after 6 Months Treatment with Sertraline, Escitalopram, or Venlafaxine. J. Psychiatr. Res. 2009, 43, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, Y.; Rizavi, H.S.; Conley, R.R.; Roberts, R.C.; Tamminga, C.A.; Pandey, G.N. Altered Gene Expression of Brain-Derived Neurotrophic Factor and Receptor Tyrosine Kinase B in Postmortem Brain of Suicide Subjects. Arch. Gen. Psychiatry 2003, 60, 804–815. [Google Scholar] [CrossRef]
- Huang, T.-L.; Lee, C.-T.; Liu, Y.-L. Serum Brain-Derived Neurotrophic Factor Levels in Patients with Major Depression: Effects of Antidepressants. J. Psychiatr. Res. 2008, 42, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Kavalali, E.T.; Monteggia, L.M. Synaptic Mechanisms Underlying Rapid Antidepressant Action of Ketamine. Am. J. Psychiatry 2012, 169, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- Lasley, S.M.; Gilbert, M.E. Developmental Thyroid Hormone Insufficiency Reduces Expression of Brain-Derived Neurotrophic Factor (BDNF) in Adults but Not in Neonates. Neurotoxicol. Teratol. 2011, 33, 464–472. [Google Scholar] [CrossRef]
- Hung, P.-L.; Huang, C.-C.; Huang, H.-M.; Tu, D.-G.; Chang, Y.-C. Thyroxin Treatment Protects Against White Matter Injury in The Immature Brain via Brain-Derived Neurotrophic Factor. Stroke 2013, 44, 2275–2283. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Kang, E.-S.; Fava, M.; Mischoulon, D.; Nierenberg, A.A.; Lee, D.; Heo, J.-Y.; Jeon, H.J. Thyroid Stimulating Hormone and Serum, Plasma, and Platelet Brain-Derived Neurotrophic Factor during a 3-Month Follow-up in Patients with Major Depressive Disorder. J. Affect. Disord. 2014, 169, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Gitlin, M.; Altshuler, L.L.; Frye, M.A.; Suri, R.; Huynh, E.L.; Fairbanks, L.; Bauer, M.; Korenman, S. Peripheral Thyroid Hormones and Response to Selective Serotonin Reuptake Inhibitors. J. Psychiatry Neurosci. 2004, 29, 383–386. [Google Scholar] [PubMed]
- Cavus, I.; Duman, R.S. Influence of Estradiol, Stress, and 5-HT2A Agonist Treatment on Brain-Derived Neurotrophic Factor Expression in Female Rats. Biol. Psychiatry 2003, 54, 59–69. [Google Scholar] [CrossRef]
- Maes, M.; Bosmans, E.; Meltzer, H.Y.; Scharpé, S.; Suy, E. Interleukin-1 Beta: A Putative Mediator of HPA Axis Hyperactivity in Major Depression? Am. J. Psychiatry 1993, 150, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
| Symptoms | Assessment Findings | Diagnostic Findings |
|---|---|---|
| Depression | Dry, coarse skin | Hyponatremia |
| Fatigue | Reduced body and scalp hair | Macrocytic anemia |
| Weight gain | Dull facial expression | Decreased memory |
| Constipation | Bradycardia | Hyperprolactinemia |
| Muscle cramps, arthralgias | Goiter | Elevated creatine kinase level |
| Menorrhagia | Macroglossia | Pituitary gland enlargement |
| Infertility | Ascites | Delayed bone age |
| Sexual dysfunction | Galactorrhea | Hypercholesterolemia |
| Cold intolerance | Slow relaxation of tendon reflexes | |
| Carpal tunnel syndrome | Nonpitting edema of lower extremities | |
| Sleep disorders | Hoarseness |
| Author | Year | Sample Size | Characteristic of Participants | Results and Conclusions | Reference |
|---|---|---|---|---|---|
| Pop et al. | 1998 | 583 women | A total of 58 women had elevated levels of anti-TPO antibodies. Age group: 47–54 years old. | This study showed that women with elevated levels of anti-TPO antibodies are more prone to developing depression, while postmenopausal age does not increase this risk. | [29] |
| Zetting et al. | 2003 | 76 | A total of 41 patients with autoimmune thyroiditis and 35 patients in the control group. | Brain impaired perfusion has been confirmed in patients with AHT. The presence of cerebral hypoperfusion suggests cerebral vasculitis as the most likely pathogenic cause. | [30] |
| Carta et al. | 2004 | 222 | In all, 16.6% of the studied patients had elevated levels of anti-TPO antibodies. | A relationship has been demonstrated between the presence of a lifetime diagnosis of depressive disorders and the level of anti-TPO antibodies. | [13] |
| Carta et al. | 2005 | 190 | A total of 19 patients were diagnosed with Hashimoto’s disease (HD) in euthyroidism, 19 patients with euthyroid neutral goiter, 152 people in the control group. | Patients diagnosed with HD in euthyroidism showed a higher incidence of depressive episodes throughout life. | [11] |
| Engum et al. | 2005 | 30,175 | In all 995 of the study group had elevated levels of anti-TPO antibodies. Age group: 40–84. | In the group with elevated levels of anti-TPO antibodies, the incidence of depression was not higher than in the general population. | [31] |
| Gulseren et al. | 2006 | 160 | A total of 33 patients with overt hypothyroidism, 43 patients with subclinical hypothyroidism, 51 patients with overt hyperthyroidism, 13 patients with subclinical hyperthyroidism and a healthy control group of 20 patients. | Achieving euthyroidism reduces depressive symptoms. In this study, the causes of hypothyroidism were not mentioned—the levels of TSH, fT3 and fT4 were examined. | [32] |
| Bunevicius et al. | 2007 | 474 | Of the 474 randomly selected primary care patients, 348 were female, 95 of them were postmenopausal, 67 had a history of endocrine disease; 68 patients had a history of mental disorders. 84 patients used psychotropic drugs, including 69 benzodiazepines; none of the patients used lithium. Six women were diagnosed with hypothyroidism and were treated with L-thyroxine. | The results of this study indicate that ultrasound-assessed thyroid autoimmunity is associated with the symptoms of mood disorders in primary care patients, especially in premenopausal women. | [33] |
| van der Deure et al. | 2008 | 141 | Patients with AHT treated with levothyroxine or combination of levothyroxine and liothyronine. | OATP1C1 polymorphisms are associated with fatigue and depression in patients with primary autoimmune hypothyroidism. | [34] |
| Schinhammer et al. | 2010 | 96 | 67 patients were diagnosed with HD and 30 patients in the control group, age group 18–80. | Patients with HD have an increased risk of developing depression. This study showed that the MTHRF 1298C/C gene polymorphism in patients with HD may be associated with the etiology of depression. There seems to be an association between the MTHFR 677C/C and COMT A/A polymorphisms and Hashimoto’s disease. Gene polymorphism in the folate/homocysteine system appears to play a role in HD and associated depressive disorders. | [35] |
| Kirim et al. | 2012 | 201 | Patients diagnosed with HD in the euthyroid stage, age group 18–65 years. | Increased frequency and severity of symptoms of depression in patients diagnosed with Hashimoto’s disease in the euthyroid stage. | [8] |
| Watt et al. | 2012 | 199 | Patients with AHT. | Health-related quality of life (Qol) in patients with autoimmune hypothyroidism was related to anti-TPO antibodies level but not to thyroid function. Level of anti-TPO antibodies was significantly associated with several QoL outcomes such as depressivity, anxiety, emotional susceptibility and impaired social life. In the multivariate model, the anti-TPO antibodies levels were related to e.g., depressivity and anxiety. | [36] |
| Franke et al. | 2013 | 57 | 36 patients diagnosed with HD and 21 patients diagnosed with neutral goiter. | The study shows increased expression of the ADA (adenosine deaminase gene) and ADAR (adenosine deaminase gene, RNA specific) genes in patients diagnosed with HD compared to patients with neutral goiter, which may explain the increased incidence of depression in patients with HD. | [37] |
| Giynas Ayhan et al. | 2014 | 164 | 51 patients diagnosed with HD in euthyroidism, 45 patients with euthyroid neutral goiter, 68 patients in the control group. | Depressive disorders are more common in euthyroid patients diagnosed with HD, suggesting that they may be associated not only with abnormal levels of thyroid hormones, but also with the process of autoimmunity. | [38] |
| Medici et al. | 2014 | 7983 | 1503 people had the level of anti-TPO tested, the study included Caucasian people aged 55+ (people with dementia were excluded). | Older people with low normal TSH levels have more comorbid depressive symptoms and a significantly increased risk of developing a depressive syndrome later in life. Low TSH levels are an important risk factor for the development of depression in the elderly. Autoimmunity of the thyroid gland (as assessed by elevated levels of anti-TPO antibodies) was not associated with an increased risk of depression. | [39] |
| Demartini et al. | 2014 | 246 | A total of 123 patients with subclinical hypothyroidism, including 106 patients diagnosed with HD, 12 with non-autoimmune hypothyroidism and 5 with nodular goiter, and 123 controls without diagnosed thyroid disease. Patients diagnosed with intellectual disability and dementia were excluded. | More than twice more patients diagnosed with subclinical hypothyroidism had at least mild depression. | [40] |
| Quinque et al. | 2015 | 36 | 18 patients treated for AHT and 18 patients in the control group. Structural and functional MRI and neuropsychological tests were performed to assess mood and cognitive function. | Properly treated patients report more depressive symptoms compared with healthy controls. Mood changes were not associated with brain structure and function in brain regions specific to depression. Higher levels of anti-TPO antibodies are associated with higher gray matter density in the right amygdala and increased connections between the cortex subcallosum and the left post-hippocampal gyrus. Duration of treatment was associated with the development of structural and functional changes in brain areas associated with depression and untreated hypothyroidism. Autoimmunity and the duration of treatment are possible factors explaining the occurrence of psychiatric symptoms in patients receiving long-term treatment for hypothyroidism. | [41] |
| Itterman et al. | 2015 | 2142 | The analysis included 498 patients with previously diagnosed thyroid dysfunction—247 people were taking medication, 223 had thyroid nodules, 74 were diagnosed with hyperthyroidism, and 70 with hypothyroidism—without specifying the causes of the disorders. | Untreated, diagnosed hypothyroidism is associated with a higher risk of depressive symptoms. TSH and anti-TPO levels were not significantly associated with the risk of depression. | [42] |
| Fjaellegaard et al. | 2015 | 8214 | The patients were divided into four groups: 1. Euthyroid patients with normal levels of anti-TPO antibodies (7015 patients), 2. Euthyroid patients with elevated levels of anti-TPO antibodies (619 patients), 3. Patients with subclinical hypothyroidism and normal levels of anti-TPO antibodies (378 patients), 4. Patients with subclinical hypothyroidism and elevated levels of anti-TPO antibodies (202 patients). | This study showed no significant differences in the incidence of depression in euthyroid patients and patients diagnosed with subclinical hypothyroidism. Euthyroid women with elevated levels of anti-TPO antibodies had statistically significantly better well-being than patients with normal levels of anti-TPO antibodies. | [43] |
| Van de Ven et al. | 2016 | 906 | Age group 50–70, relationship between the presence of anti-TPO antibodies, TSH and fT4 levels and the risk of depression was examined. | Presence of anti-TPO antibodies may be a marker of susceptibility to depression. Lack of correlation between thyroid function and incidence of depression. | [44] |
| Krysiak et al. | 2016 | 86 | Age group: women aged 20–40, 68 patients were divided into four groups: 1. Patients in euthyroidism diagnosed with HD, 2. Patients with non-autoimmune hypothyroidism, 3. Patients with autoimmune hypothyroidism, 4. Control group—18 patients. | The Beck Depression Inventory (BDI) total score was highest in group 3, and higher in groups 1 and 2 than in group 4. Anti-TPO antibody levels were directly proportional to serum TSH levels and the BDI total score and the number of patients with depressive symptoms. | [45] |
| Delitala et al. | 2016 | 3138 | The group included patients who were not taking thyroid medications or antidepressants. The levels of TSH, fT4 and anti-TPO antibodies were assessed. | No relationship was found between the level of anti-TPO antibodies and the occurrence of depressive symptoms. On the other hand, a U-shaped relationship was found between the level of fT4 and the occurrence of depressive symptoms in comparison with the average values of fT4—both high and low values of fT4 were associated with a greater number of depressive symptoms. | [46] |
| Yalcin et al. | 2017 | 124 | 93 patients diagnosed with euthyroid HD for at least 3 months and 31 patients in the control group. | The level of TSH was statistically higher in patients diagnosed with HD, no differences in the level of fT4 were observed in the group of patients with HD and in the control group. In 17.3% of patients diagnosed with HD and in 4.3% of patients in the control group, depression was diagnosed. Autoimmunity itself may have an impact on the risk of depression in patients diagnosed with HD in the euthyroid stage. | [47] |
| Bhagwat et al. | 2017 | 66 | 33 patients with autoimmune hypothyroidism and 33 patients from the control group. | In 57% of patients diagnosed with AHT, mild to moderate depression was diagnosed (MADRS > 11 points). After 6 months of treatment with tyroxin, 42% of these patients had remission of symptoms. The decrease in inflammatory markers correlated with the remission of depression. | [4] |
| Lee et al. | 2019 | 1651 | The study group was divided into three groups depending on the level of TSH. Anti-TPO antibodies and fT4 levels were also tested in all patients. | Depressive symptoms were observed less frequently in patients with positive anti-TPO antibodies and the highest TSH concentrations than in the group of patients with the lowest TSH concentrations. Men with the highest TSH level were less than twice as likely to develop depressive symptoms than in the group with the lowest TSH levels. In women with the highest TSH level 35% less often depressive symptoms than in the group with the lowest TSH level. Gender may play an important role in the relationship between TSH levels and depressive symptoms. | [48] |
| Dersch et al. | 2020 | 100 | 100 patients with unipolar endogenous major depression or treatment-resistant depression, including: 25 patients with first depressive episode (6 of them patients with psychotic symptoms) and 75 patients with recurrent depression (18 of them were patients with psychotic symptoms). | This study provides evidence of intrathecal synthesis of anti-thyroid antibodies in a subset of patients with unipolar depression. This may indicate central immunization in a subset of patients diagnosed with HD. | [49] |
| Minaldi et al. | 2020 | 2932 | A meta-analysis of five studies (449 women with anti-TPO antibodies and 2483 women without anti-TPO antibodies). | Thyroid autoimmunity (anti-TPO antibodies -positive) during pregnancy and in the weeks after childbirth is associated with an increased risk of developing post-partum depression. | [50] |
| Hirtz et al. | 2021 | 360 | Adolescents (11–19 years) with at least mild depression (BDI-II score > 13) compared to a representative reference cohort without evidence of mental health impairment. | Study found a higher prevalence of thyroid peroxidase antibody positivity in depressed adolescents compared to mentally healthy participants. The prevalence of subclinical hypothyroidism was higher in depressed adolescents vs. mentally healthy participants, but no other types of thyroid dysfunction had a higher prevalence. The prevalence of subclinical hypothyroidism and of thyroid autoimmunity in depressed adolescents is increased. | [51] |
| Kamyshna | 2022 | 153 | Patients with various forms of thyroid pathology including AHT. | The CT genotype of the glutamate ionotropic receptor NMDA type subunit 1, GRIN1 (rs4880213) was predominant in the patients with autoimmune diseases of the thyroid gland. GRIN2B (glutamate ionotropic receptor NMDA type subunit 2B) serum levels in ELISA were probable increased in AHT by 1.58 times compared with controls, while significantly (by 3.45 times) decreased in patients with postoperative hypothyroidism. The C allele of rs4880213 was more frequent than the T allele among patients with thyroid disease. GRIN2B levels were significantly different in patients of different groups depending on thyroid pathology. Study found direct close correlation (r = 0.635) between GRIN2B and anti-TPO levels (p < 0.001) and direct close correlation (r = 0.527) between GRIN2B and anti-TG levels in the blood (p < 0.001). | [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotkowska, Z.; Strzelecki, D. Depression and Autoimmune Hypothyroidism—Their Relationship and the Effects of Treating Psychiatric and Thyroid Disorders on Changes in Clinical and Biochemical Parameters Including BDNF and Other Cytokines—A Systematic Review. Pharmaceuticals 2022, 15, 391. https://doi.org/10.3390/ph15040391
Kotkowska Z, Strzelecki D. Depression and Autoimmune Hypothyroidism—Their Relationship and the Effects of Treating Psychiatric and Thyroid Disorders on Changes in Clinical and Biochemical Parameters Including BDNF and Other Cytokines—A Systematic Review. Pharmaceuticals. 2022; 15(4):391. https://doi.org/10.3390/ph15040391
Chicago/Turabian StyleKotkowska, Zofia, and Dominik Strzelecki. 2022. "Depression and Autoimmune Hypothyroidism—Their Relationship and the Effects of Treating Psychiatric and Thyroid Disorders on Changes in Clinical and Biochemical Parameters Including BDNF and Other Cytokines—A Systematic Review" Pharmaceuticals 15, no. 4: 391. https://doi.org/10.3390/ph15040391
APA StyleKotkowska, Z., & Strzelecki, D. (2022). Depression and Autoimmune Hypothyroidism—Their Relationship and the Effects of Treating Psychiatric and Thyroid Disorders on Changes in Clinical and Biochemical Parameters Including BDNF and Other Cytokines—A Systematic Review. Pharmaceuticals, 15(4), 391. https://doi.org/10.3390/ph15040391






