Abstract
A new family of pyrazole-based compounds (1–15) was synthesized and characterized using different physicochemical analyses, such as FTIR, UV-Visible, 1H, 13C NMR, and ESI/LC-MS. The compounds were evaluated for their in vitro antifungal and antibacterial activities against several fungal and bacterial strains. The results indicate that some compounds showed excellent antibacterial activity against E. coli, S. aureus, C. freundii, and L. monocytogenes strains. In contrast, none of the compounds had antifungal activity. Molecular electrostatic potential (MEP) map analyses and inductive and mesomeric effect studies were performed to study the relationship between the chemical structure of our compounds and the biological activity. In addition, molecular docking and virtual screening studies were carried out to rationalize the antibacterial findings to characterize the modes of binding of the most active compounds to the active pockets of NDM1 proteins.
1. Introduction
Resistance to antibiotics pushes researchers to discover new antibacterial candidates as prospective treatments for different infectious diseases with another class of antibiotics with specific mechanisms of action.
Beta-lactam antibiotics [1,2], commonly known as penicillin-binding proteins (PBPs), act as mechanism-based inhibitors by targeting the cell wall-modifying DD-transpeptidases, susceptible to nucleophilic attack from long-lived acylated complexes. PBPs are responsible for the formation and integrity of the membrane surface’s rigid mesh-like peptidoglycan layer exterior. However, there is a mechanism of resistance to beta-lactam due to beta-lactamases. Where Ampicillin was selected as an example for this study, the selection was because it has an amine and carbonyl function similar to our first target compound (Figure 1) that binds to the penicillin-binding proteins. Commonly used broad-spectrum antibiotics are streptomycin [3,4,5,6,7,8,9] and cefotaxime [10,11], a third-generation cephalosporin [5,12,13,14] with less susceptibility to beta-lactamase [15,16,17] than ampicillin [5,18,19,20].
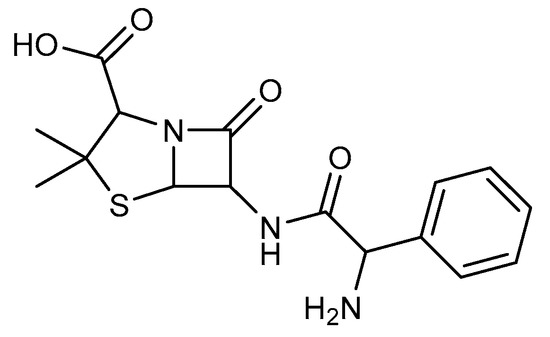
Figure 1.
Chemical structure of Ampicillin.
Infectious diseases are the main threat, especially in developing countries [21], and include listeriosis [4,22,23,24] caused by L. monocytogenes, septicemia, and meningitis caused by E. coli [8,25], bloodstream infections, and meninges caused by C. freundii [26,27,28,29]. These diseases commonly affect healthy, sensitive individuals, such as older people, pregnant women, and the immunosuppressed [11]. In addition, however, candidiasis and related fungal bloodstream infections are caused by Saccharomyces cerevisiae [30], Candida albicans, and Candida glabrata [31].
Pyrazole-based heterocyclic ligands have multiple biological applications. Many compounds prepared in our research group already have high efficiencies [32,33,34,35,36,37,38,39,40,41,42,43] as antibacterial or antifungal candidates [34,38,44,45,46] due to their nitrogen electron and proton acceptor abilities [32]. With limited facilities to investigate more experimental properties, molecular docking [43,47,48,49,50,51,52,53,54,55,56,57] becomes crucial for studying the binding modes and affinities between the prepared compounds and selected biological targets using the lock and key concept. In our study, various tripodal pyrazole ligands were prepared and characterized using FTIR, UV-visible, 1H, and 13C NMR, and then their toxicity predictions and the Lipinski rule of five agreement were determined. Finally, the molecular ligand-protein docking, through the New Delhi metallo β-lactamase hydrolysis of β-lactams antibiotics [17,54,58,59], was studied in two different active sites to investigate our studied compounds’ binding susceptibility to the hydrolase enzyme.
2. Results and Discussion
2.1. Antibacterial and Antifungal Activities
The antibacterial potential of the compounds against two Gram-negative bacterial strains (Escherichia coli and Citrobacter freundii) and two Gram-positive bacteria (Staphylococcus aureus and Listeria monocytogenes) was evaluated as described in the materials and methods section, and the results are displayed in Table 1. Only compounds 12 and 14 showed antibacterial activity when tested at 500 μM. Compound 12 was active against E. coli, S. aureus, and C. freundii but inactive against L. monocytogenes, whereas compound 14 was only active against L. monocytogenes.

Table 1.
The antibiotic activity of the active synthesized pyrazole ligands was determined using the broth macro dilution assay and the phenol red indicator.
The MICs of compounds 12 and 14 were then determined as described in the material and methods (Table 2). The MIC of compound 12 was 134.9 mg/L against E. coli, 168.7 mg/L against S. aureus, and 168.7 mg/L against C. freundii. For compound 14, the MIC against L. monocytogenes was 134.6 mg/L. Interestingly, the determination of the MBC of these compounds showed that they are bactericidal, as demonstrated by the ratio of MBC/MIC ≤ 2 (Table 2).

Table 2.
MIC and MBC values in mg/L of the studied compounds 12 and 14 against the used bacterial strains.
Regarding the antifungal activity, all the compounds were tested for toxicity against Saccharomyces cerevisiae and two species of Candida, Candida glabrata and Candida albicans, as described in Section 3.3.3. All compounds showed no antifungal activity against all three strains used. Together with the antibacterial activity analysis, these results suggest that compounds 1 to 15 lack antifungal activity, and only compounds 12 and 14 act specifically as antibacterial agents.
2.2. MEP Analysis of the Compounds 12, 14, Ampicillin, and Cefotaxime
Molecular electrostatic potential (MEP) maps of compounds 12, 14, Ampicillin, and cefotaxime have been generated (Figure 2) to determine and predict the reactive sites (nucleophilic or electrophilic) on the molecular system of the studied compounds.
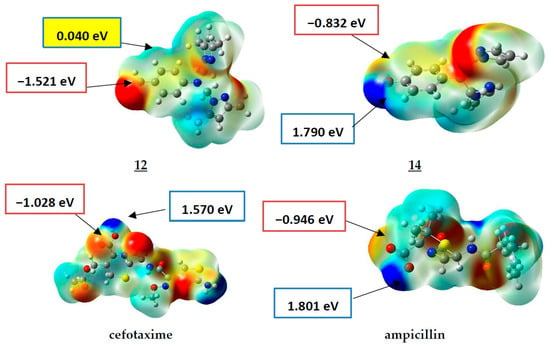
Figure 2.
MEP surfaces of compounds 12, 14, ampicillin, and cefotaxime (−4.300 × 10−3 (Red) to 4.300 × 10−3 (Blue)).
As presented in Figure 2, the positive electrostatic potential areas (in blue) are concentrated over the hydroxyl group of the drugs cefotaxime and Ampicillin with 1.570 and 1.801 eV (at an iso value of 0.0004 electrons/Å3). For compound 14, the highest value was about 1.790 eV (located on the hydroxyl group substituent on the phenyl ring), while a low value of 0.040 eV was estimated for compound 12.
The negative electrostatic potential areas are located over the carbonyl group of the acidic function of the drugs cefotaxime and Ampicillin, with values of −1.028 and −0.946 eV, respectively, with a higher value for the compound 12, with the value of −1.521 eV. In comparison, it was −0.832 eV for compound 14.
To sum up, compound 12 only has a higher negative charge value over the carbonyl. In contrast, compound 14, similar to the antibiotics cefotaxime and Ampicillin, has lower negative–positive (δ+-δ−) charge values over the hydroxyl and the acidic function. These results, which agree with the experimental results, give us information about the possible sites for binding modes to the biological targets that need molecular docking investigations.
In general, and in the light of these observations, we can postulate that the substitution by groups with different electronic effects (withdrawing-donating) is more beneficial in improving the antibacterial potency than the substitution by one group (Figure 3).
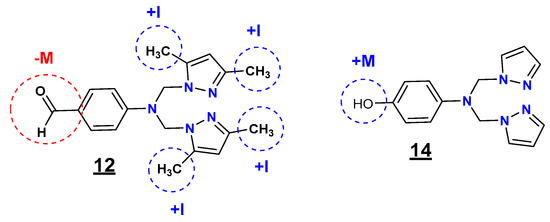
Figure 3.
Inductive and mesomeric effect study of the compounds 12 and 14.
Regarding the R1 (substituent on the phenyl ring) and R2 (substituent at positions 3 and 5 of the pyrazole moiety) substituents, the inductive and mesomeric effect study revealed that the presence of the formyl (CHO) group (electron-withdrawing effect (-M)) on the phenyl ring and the methyl (CH3) groups (electron-donating effect (+I)) on the pyrazole moieties at positions 3 and 5 (12) is highly favorable for the inhibitory potency against E. coli, S. aureus, and C. freundii strains. In contrast, the presence of the hydroxyl (OH) group (electron-donating effect (+M)) on the phenyl ring with non-substituted pyrazole moieties (14) resulted in selective antibacterial activity against L. monocytogenes.
2.3. ADME and Toxicity Predictions
ADME Predictions
For the ADME predictions, the physicochemical properties (MW: molecular weight expressed in Daltons; logP: octanol/water partition coefficient characterizing Lipophilicity; HDO: number of hydrogen bond donors; HAC: number of hydrogen bond acceptors; NRO: number of rotatable bonds; TPSA: total polar surface area) were calculated and are presented in Table 3 for the compounds 12 and 14 and the drugs streptomycin, Ampicillin, and cefotaxime as references.

Table 3.
The physicochemical properties of the compounds 12, 14, and the drugs streptomycin, Ampicillin, and cefotaxime.
In Table 4, compounds 12 and 14 have no violations of Lipinski’s rule of five [58,59], with MW = 337.42 and 269.30 ˂ 500, logP value of 3.0933 and 1.227 ˂ 5, H donor of 0 and 1 ˂ 5, H acceptor of 3 and 3 ˂ 10, number of rotatable bonds of 6 and 5 ˂ 10 and TPSA value of 55.95 and 59.11 Å2 ˂ 140 Å2. This comparison highlights that the two compounds 12 and 14 have better oral bioavailability than Ampicillin and are better than streptomycin.

Table 4.
The binding affinity values of the compounds 12, 14, and ampicillin within the two NDM1 chains A and B.
These results make compound 12 a better antibacterial candidate than cefotaxime with the same selective multitarget activity, but further toxicity predictions are required to validate these propositions.
2.4. Molecular Docking and Virtual Screening Studies
Ligand–protein docking simulations were carried out to determine the binding mode of the studied compounds with the catalytic sites of the selected receptors. Flexibility was allowed in all the rotatable bonds of the ligand; the protein was used as a rigid structure.
2.4.1. Docking against the NDM-1 β-lactamase (NDM1) Protein
NDM-1 β-lactamase hydrolysis docking study of the compounds 12 and 14 compared to Ampicillin.
The three-dimensional structure of New Delhi metallo-β-lactamase (NDM1) [17,58,59] is represented in Figure 4, where two sequences, A and B, were co-crystalized with Ampicillin; so in this part, we studied the binding affinity of the compounds 12 and 14 compared to Ampicillin.
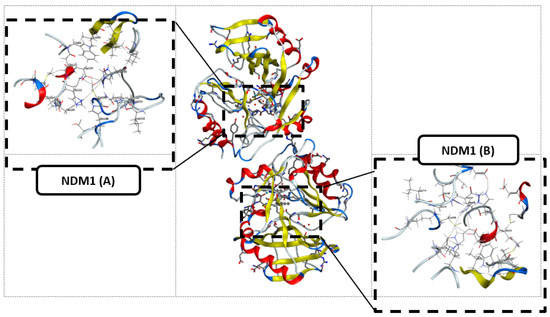
Figure 4.
The three-dimensional structure of New Delhi metallo-β-lactamase (NDM1) and the two selected active sites.
The protein preparation was performed by removing all the water, zinc, and OH molecules.
As previously mentioned in the docking study of the transpeptidase inhibition study, the NDM1 hydrolysis followed the same parameters, and the binding affinity results of both active sites are collected in Table 4.
From Table 5, compound 12 had a better affinity than compound 14 in both selected active sites, NDM1 A and B, with a binding affinity of −6.0075 and −6.6776 Kcal/mol, respectively. In addition, compared to Ampicillin, with −6.9737 and −6.7344 Kcal/mol, compound 12 was more readily hydrolyzed than compound 14.

Table 5.
Docking results of the compounds 12, 14, and ampicillin in the two NDM1 chains A and B.
First, compound 12 has two H-acceptors, bond 3.08 and 2.90 Å, respectively, between the carbonyl and the nitrogen ND1 and NE2 of His122 and His 189, as shown in Table 5 and Figure 5.
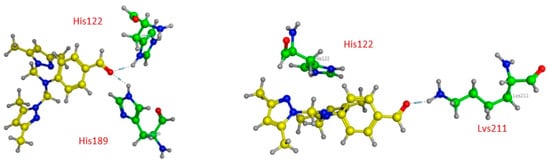
Figure 5.
Three-dimensional presentations of the binding modes between the compound 12 and NDM1.
Contrary to compound 12, compound 14, as represented in Figure 6, has only weak van der Waals bonds with Asn 220 and His 122, with a distance range of 3.60–3.90 Å, making this compound more stable against NDM1 hydrolysis.
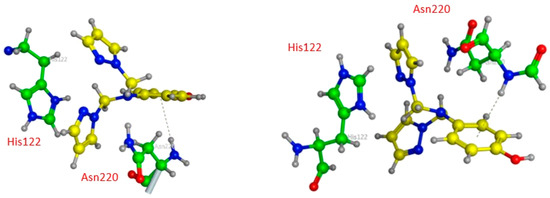
Figure 6.
Three-dimensional presentations of the binding modes between the compound 14 and NDM1.
As penicillin β-lactam antibiotics reported in the literature, Ampicillin seems to interact with more residues at the site (A) than at the site (B) of NDM1 protein, as represented in Table 5 and Figure 7. In the active pocket A, the compound forms seven bonds: one H-donor and one H-acceptor (of 2.90 and 3.55 Å, respectively) with Asp124 residue, two H-acceptor bonds with Lys211 amino acid with distances equal to 2.98 and 3.37 Å, one H-pi interaction (4.07 Å) between the carbon (C16 12) atom and 5-ring of His250, and two pi-H interactions between the 6-ring and the nitrogen and carbon atoms of Gln123 (4.08 and 3.78 Å). In contrast, Ampicillin was found to bind into the active pocket B of NDM1 only with three interactions: two by H-donor and H-acceptor bonds with Asp124 (2.99 and 3.55 Å) and one by an H-acceptor bond with Lys211 with a distance of 2.88 Å involving its oxygen atom (O1 1).
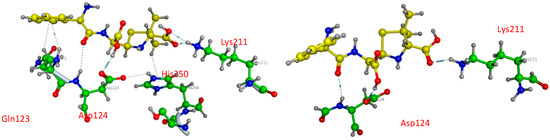
Figure 7.
Three-dimensional presentations of the binding modes between Ampicillin and NDM1.
From the docking study results, compound 12 is more sensitive to NDM1 hydrolysis, which inactivates its antibacterial activity against Listeria monocytogenes due to the common carbonyl function in all β-lactam antibiotics. Although otherwise, compound 14 has listericidal activity due to its weak binding with the selected NDM1, its specific mechanism needs more computational studies and biological assays for prediction.
2.4.2. Blind Docking/Virtual Screening against the NDM-1 β-lactamase (NDM1) Protein
As presented in Figure 8, the B-chain has considered the active site with all the docking poses. At the same time, there is good alignment between the ligand 12 and ampicillin docking poses with smooth variation, while ligand 14 is so far in a different site.
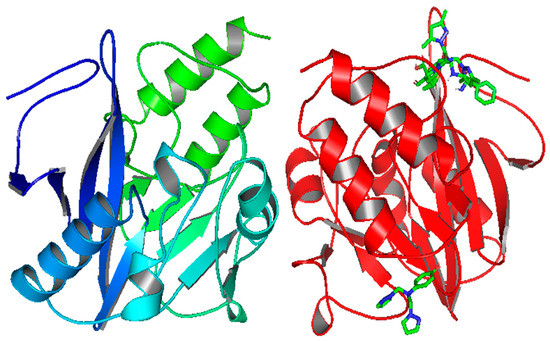
Figure 8.
Docking poses of the ligands 12, 14, and ampicillin in the NDM1 protein (PDB: 5ZGE).
Table 6 shows that the mode of binding interaction is the same as that of LYS216, which is bound with the nitrogen of pyrazole for the ligand 12 and the oxygen for Ampicillin with the same binding affinity of −7.1 Kcal/mol. On the other hand, ligand 14 has a lower −7.0 Kcal/mol value with amino acids such as ILE203 and LYS242.

Table 6.
Binding affinity and L–AA interaction of the ligands 12 and 14 and ampicillin with the chosen target, NDM1.
3. Materials and Methods
3.1. Analytical Procedures
A Bruker DPX 800 MHz Spectrometer recorded the 1HNMR (500 MHz, DMSO-d6) and 13CNMR (125 MHz, chloroform-d) spectra. Chemical shift (δ) values were stated in parts per million (ppm) using internal standard tetramethylsilane, according to the D2O exchange. Chemical shift (d) values were stated in parts per million (ppm) using internal standard tetramethylsilane. The D2O exchange confirmed the exchangeable protons (OH and NH). The FTIR analyses were performed using an FTIR 8400S spectrophotometer recorded in KBr pellets.
Many different pyrazole derivatives were synthesized and indexed.
3.2. Chemistry
The pyrazole derivatives (Figure 9) investigated in this work were prepared following the experimental procedure of the N-alkylation reaction described previously in the literature [43,46,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77]. First, all the compounds were prepared by condensation of primary amines with (3,5-dimethyl-1H-pyrazole-1-yl)methanol or (1H-pyrazole-1-yl)methanol in acetonitrile as a polar aprotic solvent that promotes SN2 reaction; after that, the compounds were purified either by diethyl ether or a DCM:water (3:1) mixture to obtain the final products, with yields varying from 15.22 to 99.41%.
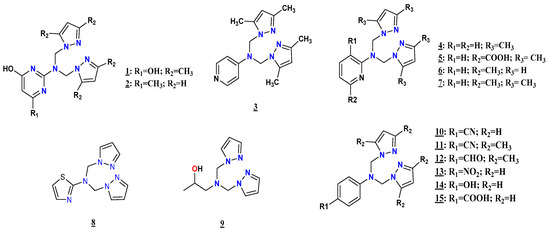
Figure 9.
Structures of the compounds 1–15.
2-(Bis((3,5-dimethyl-1H-pyrazol-1-yl)methyl)amino)pyrimidine-4,6-diol (1)
2-Aminopyrimidine-4,6-diol (1 g, 7.86 mmol) and (3,5-dimethyl-1H-pyrazol-1-yl)methanol (1.98 g, 15.72 mmol) were mixed together in acetonitrile (20 mL) under reflux for 4 h, and the solvent was evaporated, then recrystallized in diethyl ether, and then filtrated to obtain the final product (1.28 g, 47.4%), mp > 250 °C (diethyl ether); FTIR (KBr, cm−1): 3348 (-OH); 3149 (C-H); 1684 (C=C); 1560 (C-C); 1455 (C-N); 1266 (C=N); 1067 (N-N); 779 (=C-H); 1H NMR (DMSO-d6, 500 MHz) δ ppm: 6.47 (s, 4H, H-CH2); 5.76 (s, 4H, H-OH and Hpyrz-4)); 5.25 (s, 1H, Hpyrm); 2.14 (s, 12H, Hpyrz), 13C NMR (DMSO-d6, 125 MHz) δ ppm: 105.46 (Cpyrz); 70.38 (CH2); 10.75 (Cpyrz-5); 10.19 (Cpyrz-3). The elemental Analysis was calculated for C16H21N7O2 (M.wt 384.13); C-55.96; H-6.16; and N-28.55 were the % calculated. C-55.81; H-6.06; and N-28.55 were the % found.
2-(Bis((1H-pyrazol-1-yl)methyl)amino)-6-methylpyrimidin-4-ol (2)
2-Amino-6-methylpyrimidine-4-ol (1 g, 7.99 mmol) and (1H-pyrazol-1-yl)methanol (1.57 g, 15.98 mmol) were mixed together in acetonitrile (20 mL) under reflux for 4 h, and the solvent was evaporated, then recrystallized in diethyl ether, and then filtrated to obtain the final product (3.8 g, 91.22%): mp ˃ 250 °C (diethyl ether); FTIR (KBr, cm−1): 3328 et 3069 (O-H Free and linked); 2925 (C-H); 1656 (C=C); 1493 (C-C); 1383 (C-N); 1172 (C=N); 1049 (N-N); 763 (=C-H); 1H NMR (DMSO-d6, 400 MHz) δ ppm: 10.53 (s, 1H, H-OH); 7.61 (d, 2H, Hpyrz-5, JH-H = 4–6 Hz); 7.30 (d, 2H, Hpyrz-3, JH-H = 4–6 Hz); 6.10 (m, Hpyrm-4); 4.82 (s, H-CH2 and Hpyrm); 1.91 (s, CH3); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 137.65 (Cpyrz-3); 128.99 (Cpyrz-5); 104.67 (Cpyrz-4); 104.59 (Cpyrm); 73.69 (CH2); 21.59 (CH3).).The elemental Analysis was calculated for C9H9N3O4S (M.wt 255); C-54.73; H-5.30; and N-34.37 were the % calculated. C-54.65; H-5.21; N-34.29 were the % found.
N,N-bis((3,5-dimethyl-1H-pyrazol-1-yl)methyl)pyridin-4-amine (3) [61]
4-Aminopyridine (0.5 g, 5.31 mmol) and (3,5-dimethyl-1H-pyrazol-1-yl)methanol (1.34 g, 10.62 mmol) were mixed together in acetonitrile (20 mL) under reflux for 4 h, and the solvent was evaporated, then recrystallized in diethyl ether, and then filtrated to obtain the final product (0.48 g, 29.04%): mp 100–102 °C (diethyl ether); FTIR (KBr, cm−1): 2359 (C-H); 1648 (C=C); 1559 (C-C); 1454 (C-N); 1310 (C=N); 1071 (N-N); 807 (=C-H); 1H NMR (CDCl3, 500 MHz) δ ppm: 7.66 (d, 1H, Hpyrn-3, JH-H = 5–6 Hz); 7.24 (d, 1H, Hpyrn-2, JH-H = 5–6 Hz); 6.24 (s, 1H, Hpyrz-4); 5.66 (s, 2H, H-CH2); 2.34 (s, 3H, CH3−5); 2.09 (s, 3H, CH3-3); 13C NMR (CDCl3, 125 MHz) δ ppm: 167.73 (Cpyrn-1); 156.11 (Cpyrn-2); 140.41 (Cpyrz-3); 138.13 (Cpyrz-5); 113.78 (Cpyrn-3); 105.50 (Cpyrz-4); 57.61 (2H, CH2); 14.02 (Cpyrz-3); 10.96 (Cpyrz-5).
N,N-bis((3,5-dimethyl-1H-pyrazol-1-yl)methyl)pyridin-2-amine (4) [78,79]
2-Aminopyridine (0.5 g, 5.31 mmol) and (3,5-dimethyl-1H-pyrazol-1-yl)methanol (1.34 g, 10.62 mmol) were mixed together in acetonitrile (20 mL) at room temperature for 4 days, and the solvent was evaporated, then recrystallized in diethyl ether, and then filtered to obtain the final product (1.51 g, 92.13%): mp 88–90 °C (diethyl ether); FTIR (KBr, cm−1): 1609 (C=C); 1530 (C-C); 1423 (C-N); 1291 (C=N); 1067 (N-N); 772 (=C-H); 1H NMR (CDCl3, 500 MHz) δ ppm: 8.01 (d, 1H, Hpyrn-3, JH-H = 5–6 Hz); 7.31 (d, 1H, Hpyrn-4, JH-H = 4–6 Hz); 6.54 (dd, 1H, Hpyrn-5, JH-H = 4–6 Hz and JH-H = 5–6 Hz); 6.45 (d, 1H, Hpyrn-2, JH-H = 4–6 Hz); 6.38 (d, Hpyrn-6, JH-H = 5–6 Hz); 5.67 (s, 1H, Hpyrz-4); 5.50 (s, 2H, CH2); 2.35 (s, 3H, CH3-5); 2.27 (s, 3H, CH3-3); 13C NMR (CDCl3, 125 MHz) δ ppm: 156.65 (Cpyrn-1); 148.42 (Cpyrn-2); 147.41 (Cpyrz-3); 139.81 (Cpyrz-5); 137.49 (Cpyrn-4); 114.17 (Cpyrn-3); 109.03 (Cpyrn-2); 106.16 (Cpyrz-4); 54.33 (2H, CH2); 13.43 (Cpyrz-3); 11.12 (Cpyrz-5).
2-(Bis((3,5-dimethyl-1H-pyrazol-1-yl)methyl)amino) nicotinic acid (5)
3-Amino-4-methylnicotinic acid (1 g, 7.24 mmol) and (3,5-dimethyl-1H-pyrazol-1-yl)methanol (1.82 g, 14.48 mmol) were mixed together in acetonitrile (20 mL) under reflux for 4 h, and the solvent was evaporated, then recrystallized in diethyl ether, and then filtrated to obtain the final product (0.37 g, 52.1%): mp 78–80 °C (diethyl ether); 1H NMR (DMSO-d6, 400 MHz) δ ppm: 7.87 (s, 2H, Hpyrn-4 and 6); 6.69 (s, 5H, Hpyrn-5, 2 CH2); 5.37 (s, 2H, Hpyrz-4); 2.12 (s, 12H, Hpyrz-3, 5); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 164.32 (COOH); 163.09 (Cpyrn-1); 154.75 (Cpyrn-4, Cpyrz-3); 110.89 (Cpyrn-5, COOH, Cpyrz-4); 54.24 (2 CH2); 20.67 (4 CH3). The elemental Analysis was calculated for C18H22N6O2 (M.wt 354.43); C- C-61.00, H-6.26, and N-23.71 were the % calculated. C-61.00; H-6.26; and N-23.71 were the % found.
N,N-bis((1H-pyrazol-1-yl)methyl)-6-methylpyridin-2-amine (6) [61]
2-Amino-6-methylpyrimidine (0.5 g, 4.75 mmol) and (1H-pyrazol-1-yl)methanol (0.93 g, 9.51 mmol) were mixed together in acetonitrile (20 mL) at room temperature for 4 days, and the solvent was evaporated, then recrystallized in diethyl ether, and then filtrated to obtain the final product (0.19 g, 15.22%): mp 78–80 °C (diethyl ether); FTIR (KBr, cm−1): 3300 (N-H); 1614 (C=C); 1532 (C-C); 1473 (C-N); 1281 (C=N); 1082 (N-N); 747 (=C-H); 1H NMR (CDCl3, 500 MHz) δ ppm: 7.66 (d, 2H, Hpyrz-5, JH-H = 4–6 Hz); 7.42 (d, 2H, Hpyrz-3, JH-H = 5–6 Hz); 7.19 (dd, 1H, Hpyrn-2, JH-H = 6–8 Hz and JH-H = 8–10 Hz); 6.45 (d, 1H, Hpyrn-3, JH-H = 4–6 Hz); 6.24 (dd, 2H, Hpyrz-4, JH-H = 4–6 Hz and JH-H = 5–6 Hz); 2.33 (s, 3H, Hpyrn-CH3); 6.13 (d, 1H, Hpyrn-6, JH-H = 4–6 Hz); 5.63 (s, 2H, H-CH2); 13C NMR (CDCl3, 125 MHz) δ ppm: 156.49 (Cpyrz-5); 156.09 (Cpyrn-3); 132.46 (Cpyrn-6); 73.79 (Cpyrz-4); 59 (CH2); 57.8 (Cpyrn-2); 23.76 (Cpyrn-CH3).
N,N-bis((3,5-dimethyl-1H-pyrazol-1-yl)methyl)-6-methylpyridin-2-amine (7) [61]
2-Amino-6-methylpyridine (0.5 g, 4.62 mmol) and (3,5-dimethyl-1H-pyrazol-1-yl)methanol (1.17 g, 9.24 mmol) were mixed together in acetonitrile (20 mL) at room temperature for 4 days, and the solvent was evaporated, then recrystallized in diethyl ether, and then filtrated to obtain the final product (1.03 g, 68.7%): mp 96–98 °C (diethyl ether); FTIR (KBr, cm−1): 3288 (N-H); 1612 (C=C); 1537 (C-C); 1433 (C-N); 1336 (C=N); 1072 (N-N); 774 (=C-H); 1H NMR (CD2Cl2, 500 MHz) δ ppm: 7.33 (dd, 1H, Hpyrn-3, JH-H = 4–6 Hz and JH-H = 5–7 Hz); 6.53 (d, 1H, Hpyrn-2, JH-H = 4–6 Hz); 6.38 (d, Hpyrn-6, JH-H = 9–10 Hz); 5.88 (s, 1H, Hpyrz-4); 5.41 (s, 2H, H-CH2); 2.48 (s, 3H, Hpyrn-CH3); 2.39 and 2.35 (s, 3H, Hpyrz-5); 2.20 and 2.18 (s, 3H, Hpyrz-3); 13C NMR (CD2Cl2, 125 MHz) δ ppm: 156.15 (C-N); 148.33 (Cpyrz-3); 139.56 (Cpyrz-5); 137.56 (Cpyrn-3); 113.25 (Cpyrn-6); 106.96 (Cpyrz-4); 104.69 (1H, Cpyrn-2); 70.67 (2H, CH2); 23.90 (3H, Cpyrn-CH3); 13.90 (Cpyrz-3); 10.85 (Cpyrz-5).
N,N-bis((1H-pyrazol-1-yl)methyl)thiazol-2-amine (8) [80]
2-Aminothiazole (1 g, 9.98 mmol) and (1H-pyrazol-1-yl)methanol (1.96 g, 19.97 mmol) were mixed together in acetonitrile (20 mL) under reflux for 4 h, and the solvent was evaporated, then recrystallized in diethyl ether, and then filtrated to obtain the final product: (2.58 g, 99.41%): mp 84–86 °C (diethyl ether); FTIR (KBr, cm−1): 3025 (C-H); 1560 (C=C); 1540 (C-C); 1386 (C-N); 1159 (C=N); 1050 (N-N); 754 (=C-H); 692 (C-S); 1H NMR (CD2Cl2, 500 MHz) δ ppm: 7.79 (d, 2H, Hpyrz-5, JH-H = 4–6 Hz); 7.55 (s, 1H, Hthi-2); 7.17 (d, 2H, Hpyrz-3, JH-H = 6–7 Hz); 6.62 (1H, Hthi-5); 6.28 (dd, 2H, Hpyrz-4,); 5.56 (s, 4H, H-CH2); 13C NMR (CD2Cl2, 125 MHz) δ ppm: 167.75 (C-N); 139.80 (2H, Cpyrz-3); 138.79 (1H, Cthi-2); 129.53 (Cpyrz-5); 109.76 (1H, Cthi-5); 105.42 (Cpyrz-4); 59.81 (CH2).
1-(Bis((1H-pyrazol-1-yl)methyl)amino)propan-2-ol (9) [81,82,83]
3-Amino propan-2-ol (1 g, 13.31 mmol) and (3,5-dimethyl-1H-pyrazol-1-yl)methanol (3.36 g, 26.62 mmol) were mixed together in acetonitrile (20 mL) under reflux for 4 h, and the solvent was evaporated, then recrystallized in diethyl ether, and then filtrated to obtain the final product: yellow oil; 1H NMR (DMSO-d6, 400 MHz) δ ppm: 7.58 (d, 3H, Hprop-3 and OH, JH-H = 6–7 Hz); 7.42 (p, 2H, Hpyrz-5, JH-H = 6–8 Hz); 6.24 (d, 2H, Hpyrz-4, JH-H = 5–6 Hz); 4.89 (m, 4H, Hprop-2, JH-H = 5–6 Hz); 4.38 (s, 1H, Hprop-1); 2.46 (d, 2H, CH2, JH-H = 4–6 Hz); 0.97 (s, 3H, H-CH3); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 138.83 (Cpyrz-5); 129.64 (Cpyrz-3); 105.45 (pyrz-4); 66.08 (CH2); 65.99 (Cprop-2); 54.05 (CH2); 21.55 (CH3).
4-(Bis((1H-pyrazol-1-yl)methyl)amino)benzonitrile (10) [76,84]
4-Aminobenzonitrile (1 g, 8.47 mmol) and (1H-pyrazol-1-yl)methanol (1.66 g, 16.94 mmol) were mixed together in acetonitrile (20 mL) under reflux for 4 h, and the solvent was evaporated, then recrystallized in DCM:water (3:1 washed three times in 15:5 mL), and then filtrated to obtain the final product (0.89 g, 96.64%): mp 130–132 °C (diethyl ether); FTIR: 1365.65 cm−1 (CN(benzene)); 2218.42 cm−1 (C≡N); 2854.74 cm−1 (C-H(benzene)); 1455.86 cm−1 (C=C(benzene)); 1277.43 cm−1 (N-N); 1609.56 cm−1 (C=N); 2922.37 cm−1 (C-H (CH2) asym); 2854.03 cm−1 (C-H (CH2) sym); 822.486 cm−1 (H (benzene)); UV-Visible (λ nm): 201.77 (C=N: Transition n→σ*); 206.78 (C=C: Transition π→π*); 245.24 (C=C: Transition π→π*); 250.74 (C=C: Transition π→π*); 264.74 (C=N: Transition n→π *); 1H NMR (300 MHz, DMSO-d6) δ ppm: 5.994 (s, 2H, H-CH2); 6.234 (t, 4H, Hpyrz-4, JH-H = 2–3 Hz); 6.9015 (d, 2H, Hbz-2, JH-H = 6.5–7.5 Hz); 7.36 (d, 2H, Hpyrz-3, JH-H = 9–10 Hz); 7.457 (d, 2H, Hbz-3, JH-H = 9.5–10.5 Hz); 7.8 (d, 2H, Hpyrz-5, JH-H = 8–9 Hz); 13C NMR (75 MHz, DMSO-d6) δ ppm: 65.58 (C-CH2); 98.62 (Cbz-1); 105.99 (Cpyrz-4); 113.50 (Cbz-2); 119.90 (Cbz-4); 129.73 (Cpyrz-5); 133.75 (Cbz-3); 139.31 (Cpyrz-3); 150.91 (Cpyrz-5); MS [M+] (m/z): 278.86 [M+].
4-(Bis((3,5-dimethyl-1H-pyrazol-1-yl)methyl)amino)benzonitrile (11) [61,76,84]
4-Aminobenzonitrile (1 g, 8.46 mmol) and 2 equiv. of (3,5-dimethyl-1H-pyrazol-1-yl)methanol (2.13 g, 16.92 mmol) were mixed together in acetonitrile (20 mL) under reflux for 4 h, and the solvent was evaporated, then recrystallized in DCM:water (3:1 washed three times in 15:5 mL), and then filtrated to obtain the final product (1.22 g, 86.93%): mp 143–145 °C; FTIR (KBr, ν (cm−1)): 1365,65 cm−1 (CN (benzene)); 2218.21 cm−1 (C≡N); 2854.74 cm−1 (C-H(benzene)); 1458.23 cm−1 (C=C(benzene)); 1271 cm−1 (N-N); 1610.61 cm−1 (C=N); 2924.15 cm−1 (C-H (CH2) asym); 2854.74 cm−1 (C-H (CH2) sym); 2934.24 cm−1 (C-H (CH3)); 825.56 cm−1 (H (benzene)); UV-Visible (λ nm): 207.77 (C=N: Transition n → σ*); 247.27 (C=C: Transition π →π*); 266.73 (C=N: Transition n →π *); 1H NMR (300 MHz, DMSO-d6) δ ppm: 6.9425 (d, 2H, Hbz-1, JH-H = 8–9 Hz); 7.485 (d, 2H, Hbz-2, JH-H = 9–10 Hz); 2.26 (s, 2H, Hpyrz-3); 2.477 (s, 2H, Hpyrz-5); 5.779 (s, 2H, Hpyrz-4); 5.33 (d, 4H, H-CH2, JH-H = 6–7 Hz); 13C NMR (75 MHz, DMSO-d6) δ ppm: 11.17 (CH3-5); 13.78 (CH3-3); 62.87 (C-CH2); 100.95 (Cbz-1); 106.15 (Cpyrz-4); 113.38 (Cbz-2); 120.02 (Cbz-4); 133.63 (Cbz-3); 139.18 (Cpyrz-5); 146.20 (Cpyrz-3); 151.20 (Cbz-1); MS [M+] (m/z): 334.8 [M+].
4-(Bis((3,5-dimethyl-1H-pyrazol-1-yl)methyl)amino)benzaldehyde (12) [61]
4-Aminobenzaldehyde (1 g, 8.25 mmol) and (3,5-dimethyl-1H-pyrazol-1-yl)methanol (2.08 g, 16.5 mmol) were mixed together in acetonitrile (20 mL) under reflux for 4 h, and the solvent was evaporated, then recrystallized in diethyl ether, and then filtrated to obtain the final product (1.8 g, 85%): mp 96–98 °C (diethyl ether); 1H NMR (DMSO-d6, 500 MHz) δ ppm: 9.69 (s, 1H, OH); 7.71 (d, 2H, Hbz-2, JH-H = 5–6 Hz); 6.81 (d, 2H, Hbz-3, JH-H = 5–6 Hz); 5.83 (s, 2H, Hpyrz-4); 5.24 (s, 4H, H-CH2); 2.25 (s, 6H, CH3-5); 2.10 (s, 6H, CH3-3); 13C NMR (DMSO-d6, 125 MHz) δ ppm: 189.85 (C=O); 154.21 (Cbz-1); 145.91 (Cbz-3); 138.68 (Cpyrz-3); 131.52 (Cpyrz-5); 124.51 (Cbz-4); 111.05 (Cbz-2); 13.21 (CH3-3); 10.22 (CH3-5).
N,N-bis((1H-pyrazol-1-yl)methyl)-4-nitroaniline (13) [61]
4-Nitroaniline (1 g, 7.24mmol) and (1H-pyrazol-1-yl)methanol (1.42 g, 14.48 mmol) were mixed together in acetonitrile (20 mL) under reflux for 4 h, and the solvent was evaporated, then recrystallized in diethyl ether, and then filtrated to obtain the final product (0.88 g, 82.05%): mp 86–88 °C (diethyl ether); 1H NMR (DMSO-d6, 400 MHz) δ ppm: 8.04 (d, 2H, Hbz-2); 7.88 (d, 2H, Hpyrz-5); 7.80 (Hpyrz-3); 6.96 (d, 2H, Hbz-3); 6.28 (t, 2H, Hpyrz-4); 5.61 (s, 4H, H-CH2); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 152.82 (Cbz-1); 139.02 (Cpyrz-3); 138.92 (Cbz-4); 129.44 (Cbz-2); 125.85 (Cpyrz-5); 112.13 (Cbz-3); 105.60 (Cpyrz-4); 57.86 (CH2).
4-(Bis((1H-pyrazol-1-yl)methyl)amino)phenol (14) [66,69,85,86,87]
4-Aminophenol (0.9g, 8.24mmol) and (1H-pyrazol-1-yl)methanol (1.62 g, 16.49 mmol) were mixed together in acetonitrile (20 mL) under reflux for 4h, and the solvent was evaporated, then recrystallized in diethyl ether, and then filtrated to obtain the final product (1.8 g, 81.1%): mp 102–104 °C (diethyl ether), FTIR (KBr, cm−1): 3113 (O-H); 2300 (C-H); 1659 (C=C); 1509 (C-C); 1393 (C-N); 1183 (C=N); 1039 (N-N); 750 (=C-H), 1H NMR (DMSO, 500 MHz) δ ppm: 8.75 (s, 1H, OH); 7.66 (d, 2H, Hbz-5, JH-H = 4–6 Hz); 7.30 (d, 2H, Hbz-3, JH-H = 5–6 Hz); 7.15 (d, 2H, Hpyrz-5, JH-H = 4–6 Hz); 6.96 (d, 2H, Hpyrz-3, JH-H = 5–7 Hz); 6.31 (dd, 2H, Hpyrz-4, JH-H = 4–6 Hz and JH-H = 6–7 Hz); 5.99 (s, 4H, H-CH2), 13C NMR (DMSO, 125 MHz) δ ppm: 167.79 (Cbz-1); 139.68 (Cbz-3); 138.99 (Cbz-1); 130.48 (Cbz-5); 129.32 (Cpyrz-5); 110.42 (Cpyrz-3); 105.91 (Cpyrz-4); 59.09 (CH2).
4-(Bis((1H-pyrazol-1-yl)methyl)amino)benzoic acid (15)
4-Aminobenzoic acid (1 g, 7.29 mmol) and (1H-pyrazol-1-yl)methanol (1.43 g, 14.58 mmol) were mixed together in acetonitrile (20 mL) under reflux for 4 h, and the solvent was evaporated. then recrystallized in diethyl ether, and then filtrated to obtain the final product (1.24 g, 82.05%): mp 164–166 °C (diethyl ether), FTIR (KBr, cm−1): 3268 (O-H); 1699 (C=O); 1609 (C=C); 1522 (C-C); 1376 (C-N); 1182 (C=N); 951 (N-N); 764 (=C-H), 1H NMR (CD2Cl2, 500 MHz) δ ppm: 8.02 (d, 2H, Hbz-3, JH-H = 9–10 Hz); 7.60 (d, 2H, Hpyrz-5, JH-H = 8–10 Hz); 7.55 (d, 2H, Hpyrz-3, JH-H = 5–6 Hz); 7.28 (d, 2H, Hbz-2, JH-H = 5–7 Hz); 6.33 (dd, 2H, Hpyrz-4, JH-H = 6–8 Hz and JH-H = 14–15 Hz); 5.88 (s, 4H, H-CH2), 13C NMR (CD2Cl2, 125 MHz) δ ppm: 140.03 (Cbz-1); 139.96 (Cpyrz-3); 132 (Cbz-2); 128.88 (Cpyrz-5); 113,74 (Cbz-4); 112.62 (Cbz-3); 106.36 (Cpyrz-4); 66.69 (C-CH2). The elemental Analysis was calculated for C15H15N5O2 (M.wt 297.12); C-60.60, H-5.09, and N-23.56 were the % calculated. C-60.58; H-5.12; and N-23.45 were the % found.
3.3. Biological Evaluation
3.3.1. Antibacterial Assay
The microdilution method with phenol red [88] evaluated the antibacterial effect against four bacterial strains: Listeria monocytogenes, Escherichia coli, Staphylococcus aureus, and Citrobacter freundii. First, the bacterial isolate was cultivated in liquid Luria–Bertani medium (LB) overnight at 37 °C under aeration. Then, a suspension containing 106 CFU/mL of bacteria cells was prepared. Next, an aliquot from this bacterial suspension was added to test tubes containing phenol red medium and the compound to be tested. After an incubation of 24 h at 37 °C, the color of the culture remains red in the absence of growth, indicating that the tested compound has antibacterial activity against the tested strain. However, if there is bacterial growth, the culture becomes yellow due to the acidification of the medium and indicating that the tested compound lacks antibacterial activity against the tested strain. All the experiments were repeated twice for each drug, including the antibiotic streptomycin as a positive control, and means were calculated.
3.3.2. Determination of the Minimum Inhibitory Concentration (MIC) and the Minimum Bactericidal Concentration (MBC)
The MIC (the lowest drug concentration that inhibits bacterial growth after incubation at 37 °C for 24 h) and the MBC (the lowest drug concentration that kills 99% of bacteria after 24 h of incubation) were obtained as described in the literature [89,90].
3.3.3. Antifungal Assay
The prepared ligands were evaluated for their antifungal activity using liquid cell culture against Saccharomyces cerevisiae and Candida species: Candida glabrata and Candida albicans. The growth rate of fungal cells in liquid culture was monitored by absorbance measurements at 600 nm (OD600) using a V-1200 spectrophotometer (Shanghai Mapada Instruments Co., Ltd., Shanghai, China). The antifungal activity was described (Bouchal et al., 2019 [88]). Briefly, cells were cultured in the presence and absence of 500 µM of each compound for 24 h, and OD600 measurements were then used to monitor the growth rate. Growth in the presence of a compound was expressed as a percentage relative to the untreated control. All experiments were repeated at least twice, and means were calculated.
3.4. Computational Studies
3.4.1. In Silico ADME-Tox Predictions
In silico screening was performed to predict the studied compounds’ properties (absorption, distribution, metabolism, excretion) of the ADME [9,88,91,92,93,94,95,96,97,98,99,100,101,102,103,104]
Additionally, using the SwissADME web tool (www.swissadme.ch/, accessed on 20 April 2022) [105,106,107], lipophilicity (logP) was calculated using the Marvin sketch program, while the toxicity predictions were made using the PROTOX online tool (http://tox.charite.de/protox_II/, accessed on 20 April 2022) [108,109,110,111,112,113] based on the functional group similarity of the existing molecules tested in vitro and in vivo in the database. The three most similar compounds were taken for toxicity prediction.
3.4.2. DFT, Molecular Ligand–Protein Docking, and Virtual Screening Studies
The chemical structures of the studied molecules were sketched using Gaussview 6.0, then optimized using the DFT/B3LYP method with 6-31G(d,p) basis sets in the Gaussian 09W software [114]. The docking study performed with New Delhi metallo-β-lactamase was conducted in two different active sites of the crystal structure of NDM-1 at pH 5.5 (Bis-Tris) in a complex with hydrolyzed Ampicillin (PDB: 5ZGE) with a resolution of 1.00 Å.
Blind docking/virtual screening was considered to target the previous protein (5ZGE), which was prepared in Autodock 4 [115] default parameters, and the whole protein was used for the grid box (Table S1), with Perl as a launcher for all the ligands in Autodock Vina [116,117,118,119,120].
4. Conclusions
Fifteen compounds based on pyrazole derivatives were prepared with good yield and characterized using different physicochemical analyses, such as FTIR, UV-Visible, 1H and 13C NMR, and MS. These compounds were evaluated for their antifungal and antibacterial activities against several fungal and bacterial strains. None of the compounds had antifungal activity. Interestingly, compounds 12 and 14 displayed intense antibacterial activity. In addition, compound 12 presented excellent antibacterial activity against E. coli, S. aureus, and C. freundii, with inactivity against L. monocytogenes and cephalosporins antibiotics. In contrast, compound 14 showed tremendous antibacterial potential against L. monocytogenes, with no effect against the other bacterial strains.
Compound 14′s listericidal activity could be due to the presence of the hydroxyl as a substituent on the phenyl ring, an electron donor group (+M) that causes oxidative stress to the bacterial strain the production of hydroxyl radicals. In contrast, compound 12 has carbonyl as an electron-withdrawing group (-M), methyl as an electron donor group (+I), and a bulky substituent. Furthermore, from the MEP surface analysis, compound 12 only has a higher negative charge value over the carbonyl. In contrast, compound 14, similar to the antibiotics cefotaxime and Ampicillin, has a close negative-positive (δ+-δ−) higher charge value over the hydroxyl and the acidic function.
From ADME and toxicity predictions, compounds 12 and 14 have no violations of Lipinski’s rule of five, better than streptomycin, with three violations, and cefotaxime, with one offense. In contrast, compounds 12 and 14 have lower predicted LD50 than Ampicillin and cefotaxime, with less toxicity (class 4) than streptomycin (class 3), even though they are both active as carcinogens with mutagenic activity for compound 14 and have no binding probability with all the toxicity targets better than Ampicillin and cefotaxime, which have probable binding with toxicity targets.
From the docking results, compound 12 has a better affinity with both active protein sites, which have an H-acceptor bond, than compound 14, with ligand exposure. However, Ampicillin and cefotaxime still have the best values, with more H-donors and H-acceptors; otherwise, compound 12 is hydrolyzed by NDM1 hydrolysis and inactivates its antibacterial activity against Listeria monocytogenes due to the presence of the carbonyl function, which is common in all β-lactam antibiotics. On the other hand, compound 14 has listericidal activity with a specific mechanism that needs more computational studies and biological assays for prediction; from blind docking/virtual screening studies, the mode of binding interaction is the same as that of LYS216, which is bound with the nitrogen of pyrazole for the ligand 12 and the oxygen for Ampicillin with the same binding affinity of −7.1 Kcal/mol. On the other hand, ligand 14 has a lower −7.0 Kcal/mol value with amino acids such as ILE203 and LYS242.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ph15070803/s1, Table S1: Blind docking/virtual screening as Perl configuration and GRID box parameters; File S1: 1HNMR and 13CNMR spectra of compounds.
Author Contributions
Y.K. methodology, formal analysis, software, resources, original draft preparation, writing—review and editing. M.B. validation, investigation, and visualization. F.A. software, data curation, and resources. M.E.K. software, data curation, supervision, A.E., validation, formal analysis, and funding acquisition, and R.T. supervision, validation, and conceptualization. M.H.A. visualization, writing—review and editing, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by MESRSFC (Ministère de l’Enseignement Supérieur, de la Recherche Scientifique et de la Formation des Cadres), the CNRST (Center National pour la Recherche Scientifique et Technique) and Taif University, Taif, Saudi Arabia (grant number TURSP2020/91).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lu, Z.; Wanang, H.; Zhang, A.; Liu, X.; Zhou, W.; Yang, C.; Guddat, L.; Yang, H.; Schofield, C.J.; Rao, Z. Structures of Mycobacterium tuberculosis Penicillin-Binding Protein 3 in Complex with Five beta-Lactam Antibiotics Reveal Mechanism of Inactivation. Mol. Pharmacol. 2020, 97, 287–294. [Google Scholar] [CrossRef]
- Antipin, R.L.; Beshnova, D.A.; Petrov, R.A.; Shiryaeva, A.S.; Andreeva, I.P.; Grigorenko, V.G.; Rubtsova, M.Y.; Majouga, A.G.; Lamzin, V.S.; Egorov, A.M. Synthesis, SAR and molecular docking study of novel non-beta-lactam inhibitors of TEM type beta-lactamase. Bioorganic Med. Chem. Lett. 2017, 27, 1588–1592. [Google Scholar] [CrossRef]
- Kogut, M.; Harris, M. Effects of Streptomycin in Bacterial Cultures Growing at Different Rates; Interaction with Bacterial Ribosomes in vivo. Eur. J. Biochem. 1969, 9, 42–49. [Google Scholar] [CrossRef]
- Mazumdar, K.; Dastidar, S.G.; Park, J.H.; Dutta, N.K. The anti-inflammatory non-antibiotic helper compound diclofenac: An antibacterial drug target. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 881–891. [Google Scholar] [CrossRef]
- Chudobova, D.; Dastidar, S.G.; Park, J.H.; Dutta, N.K. Effect of Ampicillin, streptomycin, penicillin and tetracycline on metal resistant and non-resistant Staphylococcus aureus. Int. J. Environ. Res. Public Health 2014, 11, 3233–3255. [Google Scholar] [CrossRef]
- Hoerr, V.; Duggan, G.E.; Zbytnuik, L.; Poon, K.K.H.; Grobe, C.; Neugebauer, U.; Methling, K.; Loffler, B.; Vogel, H.J. Characterization and prediction of the mechanism of action of antibiotics through NMR metabolomics. BMC Microbiol. 2016, 16, 82. [Google Scholar] [CrossRef]
- Stern, A.L.; Van der Verren, S.E.; Näsvall, J.; Gutiérrez-de-Terán, H.; Selmer, M. Structural mechanism of AadA, a dual-specificity aminoglycoside adenylyltransferase from Salmonella enterica. J. Biol. Chem. 2018, 293, 11481–11490. [Google Scholar] [CrossRef]
- Cho, S.; Hiott, L.M.; Barrett, J.B.; McMillan, E.A.; House, S.L.; Humayoun, S.B.; Adams, E.S.; Jackson, C.R.; Frye, J.G. Prevalence and characterization of Escherichia coli isolated from the Upper Oconee Watershed in Northeast Georgia. PLoS ONE 2018, 13, e0197005. [Google Scholar] [CrossRef]
- Bendaif, H.; Melhaoui, A.; Ramdani, M.; Elmsellem, H.; Douez, C.; El Ouadi, Y. Antibacterial activity and virtual screening by molecular docking of lycorine from Pancratium foetidum Pom (Moroccan endemic Amaryllidaceae). Microb. Pathog. 2018, 115, 138–145. [Google Scholar] [CrossRef]
- Alexandre, H.L.; Kuzin, P.; Kelly, J.A.; Knox, J.R. Binding of Cephalothin and Cefotaxime to D-Ala-D-Ala-Peptidase Reveals a Functional Basis of a Natural Mutation in a Low-Affinity Penicillin-Binding Protein and in Extended-Spectrum P-Lactamases. Biochemistry 1995, 34, 9532–9540. [Google Scholar]
- Pacifici, G.M.; Marchini, G. Clinical Pharmacology of Cefotaxime in Neonates and Infants: Effects and Pharmacokinetics. Int. J. Pediatr. 2017, 5, 6111–6138. [Google Scholar]
- Wangoye, K.; Mwesigye, J.; Tungotyo, M.; Twinomujuni Samba, S. Chronic wound isolates and their minimum inhibitory concentrations againgst third generation cephalosporins at a tertiary hospital in Uganda. Sci. Rep. 2022, 12, 1195. [Google Scholar] [CrossRef]
- Shahbaz, K. Cephalosporins: Pharmacology and chemistry. Pharm. Biol. Eval. 2017, 4, 234–238. [Google Scholar] [CrossRef]
- Mohamed, S.B.; Adlan, T.A.; Khalafalla, N.A.; Abdall, N.I.; Ali, Z.S.A.; Ka, A.M.; Hassan, M.M.; Elnour, M.A.B. Proteomics and Docking Study Targeting Penicillin-Binding Protein and Penicillin-Binding Protein2a of Methicillin-Resistant Staphylococcus aureus Strain SO-1977 Isolated from Sudan. Evol. Bioinform. 2019, 15, 1–13. [Google Scholar] [CrossRef]
- Dhara, L.; Tripathi, A.; Pal, A. Molecular characterization and in silico analysis of naturally occurring TEM beta-lactamase variants among pathogenic Enterobacteriaceae infecting Indian patients. BioMed Res. Int. 2013, 2013, 783540. [Google Scholar] [CrossRef]
- Danishuddin, M.; Khan, A.U. Molecular modeling and docking analysis of beta-lactamases with inhibitors: A comparative study. Silico Biol. 2011, 11, 273–280. [Google Scholar]
- Thakur, P.K.; Kumar, J.; Ray, D.; Anjum, F.; Hassan, M.I. Search of potential inhibitor against New Delhi metallo-beta-lactamase 1 from a series of antibacterial natural compounds. J. Nat. Sci. Biol. Med. 2013, 4, 51–56. [Google Scholar]
- Temple, M.E.; Nahata, M.C. Treatment of listeriosis. Ann. Pharmacother. 2000, 34, 656–661. [Google Scholar] [CrossRef]
- Ahrén, I.L.; Karlsson, E.; Forsgren, A.; Riesbeck, K. Comparison of the antibacterial activities of Ampicillin, ciprofloxacin, clarithromycin, telithromycin and quinupristin/dalfopristin against intracellular non-typeable Haemophilus influenzae. J. Antimicrob. Chemother. 2002, 50, 903–906. [Google Scholar] [CrossRef][Green Version]
- Sutherland, R.; Rolinson, G.N. Activity of Ampicillin in vitro compared with other antibiotics. J. Clin. Pathol. 1964, 17, 461–465. [Google Scholar] [CrossRef]
- Rao, L.; Tian, R.; Chen, X. Cell-Membrane-Mimicking Nanodecoys against Infectious Diseases. ACS Nano 2020, 14, 2569–2574. [Google Scholar] [CrossRef] [PubMed]
- Lungu, B.; O’Bryan, C.A.; Muthaiyan, A.; Milillo, S.R.; Johnson, M.G.; Crandall, P.G.; Ricke, S.C. Listeria monocytogenes: Antibiotic resistance in food production. Foodborne Pathog. Dis. 2011, 8, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Koster, S.; van Pee, K.; Hudel, M.; Leustik, M.; Rhinow, D.; Kuhlbrandt, W.; Chakraborty, T.; Yildiz, O. Crystal structure of listeriolysin O reveals molecular details of oligomerization and pore formation. Nat. Commun. 2014, 5, 3690. [Google Scholar] [CrossRef]
- Radoshevich, L.; Cossart, P. Listeria monocytogenes: Towards a complete picture of its physiology and pathogenesis. Nat. Rev. Microbiol. 2018, 16, 32–46. [Google Scholar] [CrossRef]
- Lv, H.; Ning, B. Pathogenesis of bloodstream infection in children with blood cancer. Exp. Ther. Med. 2013, 5, 201–204. [Google Scholar] [CrossRef][Green Version]
- Anufrieva, N.V.; Faleev, N.G.; Morozova, E.A.; Bazhulina, N.P.; Revtovich, S.V.; Timofeev, V.P.; Tkachev, Y.V.; Nikulin, A.D.; Demidkina, T.V. The role of active site tyrosine 58 in Citrobacter freundii methionine γ-lyase. Biochim. Biophys. Acta 2015, 1854, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Morozova, E.A.; Bazhulina, N.P.; Anufrieva, N.V.; Mamaeva, D.V.; Tkachev, Y.V.; Streltsov, S.A.; Timofeev, V.P.; Faleev, N.G.; Demidkina, T.V. Kinetic and spectral parameters of interaction of Citrobacter freundii methionine γ-lyase with amino acids. Biochemistry 2010, 75, 1272–1280. [Google Scholar] [CrossRef]
- Revtovich, S.V.; Morozova, E.A.; Kulikova, V.V.; Anufrieva, N.V.; Osipova, T.I.; Koval, V.S.; Nikylin, A.D.; Demidkima, T.V. Crystal structure of mutant form Cys115His of Citrobacter freundii methionine γ-lyase complexed with L-norleucine. Biochim. Biophys. Acta Proteins Proteom. 2017, 1865, 1123–1128. [Google Scholar] [CrossRef]
- Liu, L.H.; Wang, N.Y.; Wu, A.Y.; Lin, C.C.; Lee, C.M.; Liu, C.P. Citrobacter freundii bacteremia: Risk factors of mortality and prevalence of resistance genes. J. Microbiol. Immunol. Infect. 2018, 51, 565–572. [Google Scholar] [CrossRef]
- Munoz, P.; Bouza, E.; Cuenca-Estrella, M.; Eiros, J.M.; Perez, M.J.; Sanchez-Somolinos, M.; Rincon, C.; Hortal, J.; Pelaez, T. Saccharomyces cerevisiae fungemia: An emerging infectious disease. Clin. Infect. Dis. 2005, 40, 1625–1634. [Google Scholar] [CrossRef]
- Fidel, P.L.; Vazquez, J.A.; Sobel, J.D. Candida glabrata: Review of Epidemiology, Pathogenesis, and Clinical Disease with Comparison toC. Albicans. Clin. Microbiol. Rev. 1999, 12, 80–96. [Google Scholar] [CrossRef]
- Wu, D.; Jin, F.; Lu, W.; Zhu, J.; Li, C.; Wang, W.; Tang, Y.; Jiang, H.; Huang, J.; Liu, G.; et al. Synthesis, structure-activity relationship, and pharmacophore modeling studies of pyrazole-3-carbohydrazone derivatives as dipeptidyl peptidase IV inhibitors. Chem. Biol. Drug Des. 2012, 79, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Tighadouini, S.; Benabbes, R.; Tillard, M.; Eddike, D.; Haboubi, K.; Karrouchi, K.; Radi, S. Synthesis, crystal structure, DFT studies and biological activity of (Z)-3-(3-bromophenyl)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxyprop-2-en-1-one. Chem. Cent. J. 2018, 12, 122. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.; Al-Majid, A.M.; Al-Qahtany, B.M.; Ali, M.; Teleb, M.; Al-agamy, M.H.; Naz, S.; Ul-Haq, Z. Synthesis, antimicrobial activity, pharmacophore modeling and molecular docking studies of new pyrazole-dimedone hybrid architectures. Chem. Cent. J. 2018, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Karrouchi, K.; Radi, S.; Ramli, Y.; Taoufik, J.; Mabkhot, Y.N.; Al-Aizari, F.A.; Ansar, M. Synthesis and Pharmacological Activities of Pyrazole Derivatives: A Review. Molecules 2018, 23, 134. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.B.P.; Gulati, K.; Joshi, N.; Deb, D.K.; Rambabu, D.; Kaminsky, W.; Poluri, K.M.; Kollipara, M.R. Synthesis and biological studies of ruthenium, rhodium and iridium metal complexes with pyrazole-based ligands displaying unpredicted bonding modes. Inorg. Chim. Acta 2017, 462, 223–235. [Google Scholar]
- Bekhit, A.A.; Hymete, A.; Asfaw, H.; Ael, D.B. Synthesis and biological evaluation of some pyrazole derivatives as anti-malarial agents. Arch. Pharm. 2012, 345, 147–154. [Google Scholar] [CrossRef]
- El Shehry, M.F.; Ghorab, M.M.; Abbas, S.Y.; Fayed, E.A.; Shedid, S.A.; Ammar, Y.A. Quinoline derivatives bearing pyrazole moiety: Synthesis and biological evaluation as possible antibacterial and antifungal agents. Eur. J. Med. Chem. 2018, 143, 1463–1473. [Google Scholar] [CrossRef]
- Brahmbhatt, G.C.; Sutariya, T.R.; Atara, H.D.; Parmar, N.J.; Gupta, V.K.; Lagunes, I.; Padrón, J.M.; Murumkar, P.R.; Yadav, M.R. New pyrazolyl-dibenzo[b,e][1,4]diazepinones: Room temperature one-pot Synthesis and biological evaluation. Mol. Divers. 2020, 24, 355–377. [Google Scholar] [CrossRef]
- Dai, H.; Chen, J.; Li, G.; Ge, S.; Shi, Y.; Fang, Y.; Ling, Y. Design, Synthesis, and bioactivities of novel oxadiazole-substituted pyrazole oximes. Bioorganic Med. Chem. Lett. 2017, 27, 950–953. [Google Scholar] [CrossRef]
- Aggarwal, R.; Kumar, V.; Kumar, R.; Singh, S.P. Approaches towards the Synthesis of 5-aminopyrazoles. Beilstein J. Org. Chem. 2011, 7, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Kumar, S. 5-Aminopyrazole as precursor in design and Synthesis of fused pyrazoloazines. Beilstein J. Org. Chem. 2018, 14, 203–242. [Google Scholar] [CrossRef]
- Kaddouri, Y.; Abrigach, F.; Yousfi, E.B.; El Kodadi, M.; Touzani, R. New thiazole, pyridine and pyrazole derivatives as antioxidant candidates: Synthesis, DFT calculations and molecular docking study. Heliyon 2020, 6, e03185. [Google Scholar] [CrossRef] [PubMed]
- Elshaier, Y.A.; Barakat, A.; Al-Qahtany, B.M.; Al-Majid, A.M.; Al-Agamy, M.H. Synthesis of Pyrazole-Thiobarbituric Acid Derivatives: Antimicrobial Activity and Docking Studies. Molecules 2016, 21, 1337. [Google Scholar] [CrossRef] [PubMed]
- Brahmbhatt, H.; Molnar, M.; Pavić, V. Pyrazole nucleus fused tri-substituted imidazole derivatives as antioxidant and antibacterial agents. Karbala Inter. J. Moder. Sci. 2018, 4, 200–206. [Google Scholar] [CrossRef]
- Abrigach, F.; Rokni, Y.; Takfaoui, A.; Khoutoul, M.; Doucet, H.; Asehraou, A.; Touzani, R. In vitro screening, homology modeling and molecular docking studies of some pyrazole and imidazole derivatives. Biomed. Pharmacother. 2018, 103, 653–661. [Google Scholar] [CrossRef]
- Govender, H.; Mocktar, C.; Kumalo, H.M.; Koorbanally, N.A. Synthesis, antibacterial activity and docking studies of substituted quinolone thiosemicarbazones. Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 1074–1081. [Google Scholar] [CrossRef]
- Chandrasekar, K.; Kumar, B.; Saravanan, A.; Victor, A.; Sivaraj, S.; Haridoss, M.; Priyadurairaj, P.; Hemalatha, C.N.; Muthukumar, V.A. Evalution and Molecular Docking of Benzimidazole and its Derivatives as a Potent Antibacterial Agent. Biomed. Pharmacol. J. 2019, 12, 1835–1847. [Google Scholar] [CrossRef]
- Grein, F.; Schneider, T.; Sahl, H.G. Docking on Lipid II—A Widespread Mechanism for Potent Bactericidal Activities of Antibiotic Peptides. J. Mol. Biol. 2019, 431, 3520–3530. [Google Scholar] [CrossRef]
- Hassan, A.S.; Askar, A.A.; Nossier, E.S.; Naglah, A.M.; Moustafa, G.O.; Al-Omar, M.A. Antibacterial Evaluation, In Silico Characters and Molecular Docking of Schiff Bases Derived from 5-aminopyrazoles. Molecules 2019, 24, 3130. [Google Scholar] [CrossRef]
- Al-Khafaji, K.; Taskin Tok, T. Understanding the mechanism of amygdalin’s multifunctional anti-cancer action using computational approach. J. Biomol. Struct. Dyn. 2021, 39, 1600–1610. [Google Scholar] [CrossRef] [PubMed]
- Ghorab, M.M.; Soliman, A.M.; Alsaid, M.S.; Askar, A.A. Synthesis, antimicrobial activity and docking study of some novel 4-(4,4-dimethyl-2,6-dioxocyclohexylidene)methylamino derivatives carrying biologically active sulfonamide moiety. Arab. J. Chem. 2020, 13, 545–556. [Google Scholar] [CrossRef]
- Rezki, N.; Al-blewi, F.F.; Al-Sodies, S.A.; Alnuzha, A.K.; Messali, M.; Ali, I.; Aouad, M.R. Synthesis, Characterization, DNA Binding, Anticancer, and Molecular Docking Studies of Novel Imidazolium-Based Ionic Liquids with Fluorinated Phenylacetamide Tethers. ACS Omega 2020, 5, 4807–4815. [Google Scholar] [CrossRef]
- Zhang, F.; Zhai, T.; Haider, S.; Liu, Y.; Huang, Z.J. Synergistic Effect of Chlorogenic Acid and Caffeic Acid with Fosfomycin on Growth Inhibition of a Resistant Listeria monocytogenes Strain. ACS Omega 2020, 5, 7537–7544. [Google Scholar] [CrossRef] [PubMed]
- Gurung, A.B.; Ali, M.A.; Lee, J.; Al-Hemaid, F.; Abul Farah, M.; Al-Anazi, K.M. Molecular docking elucidates the plausible mechanisms underlying the anticancer properties of acetyldigitoxigenin from Adenium obesum. Saudi J. Biol. Sci. 2020, 27, 1907–1911. [Google Scholar] [CrossRef] [PubMed]
- Gowda, K.; Swarup, H.A.; Nagarakere, S.C.; Rangappa, S.; Kanchugarkoppal, R.S.; Kempegowda, M. Structural studies of 2,5-disubstituted 1,3,4-thiadiazole derivatives from dithioesters under the mild condition: Studies on antioxidant, antimicrobial activities, and molecular docking. Synth. Commun. 2020, 50, 1528–1544. [Google Scholar] [CrossRef]
- Abusetta, A.; Alumairi, J.; Alkaabi, M.Y.; Al Ajeil, R.; Abu Shkaidim, A.; Akram, D.; Pajak, J.; Ghattas, M.A.; Atatreh, N.; AlNeyadi, S.S. Design, Synthesis, in Vitro Antibacterial Activity, and Docking Studies of New Rhodanine Derivatives. Open J. Med. Chem. 2020, 10, 15–34. [Google Scholar]
- Zhang, H.; Ma, G.; Zhu, Y.; Zeng, L.; Ahmad, A.; Wang, C.; Pang, B.; Fang, H.; Zhao, L.; Hao, Q. Active-Site Conformational Fluctuations Promote the Enzymatic Activity of NDM-1. Antimicrob. Agents Chemother. 2018, 62, e01579-18. [Google Scholar] [CrossRef]
- Sun, Z.; Hu, L.; Sankaran, B.; Prasad, B.V.V.; Palzkill, T. Differential active site requirements for NDM-1 beta-lactamase hydrolysis of carbapenem versus penicillin and cephalosporin antibiotics. Nat. Commun. 2018, 9, 4524. [Google Scholar] [CrossRef]
- El Kodadi, M.; Malek, F.; Touzani, R.; Ramdani, A. Synthesis of new tripodal ligand 5-(bis(3,5-dimethyl-1H-pyrazol-1-ylmethyl)amino)pentan-1-ol, catecholase activities studies of three functional tripodal pyrazolyl N-donor ligands, with different copper (II) salts. Catal. Commun. 2008, 9, 966–969. [Google Scholar] [CrossRef]
- Kaddouri, Y.; Abrigach, F.; Ouahhoud, S.; Benabbes, R.; El Kodadi, M.; Alsalme, A.; Al-Zaqri, N.; Warad, I.; Touzani, R. Synthesis, characterization, reaction mechanism prediction and biological study of mono, bis and tetrakis pyrazole derivatives against Fusarium oxysporum f. sp. Albedinis with conceptual DFT and ligand-protein docking studies. Bioorganic Chem. 2021, 110, 104696. [Google Scholar] [CrossRef] [PubMed]
- Touzani, R.; Ramdani, A.; Ben-Hadda, T.; El Kadiri, S.; Maury, O.; Le Bozec, H.; Dixneuf, P.H. Efficient synthesis of new nitrogen donor containing tripods under microwave irradiation and without solvent. Synth. Commun. 2001, 31, 1315–1321. [Google Scholar] [CrossRef]
- Lamsayah, M.; Khoutoul, M.; Abrigach, F.; Oussaid, A.; Touzani, R. Selective liquid-liquid extraction of Fe(II) and Cd(II) using N,N’-Pyrazole bidentate ligands with theoretical study investigations. Separ. Sci. Technol. 2015, 50, 2170–2176. [Google Scholar]
- Khoutoul, M.; Abrigach, F.; Zarrouk, A.; Benchat, N.-E.; Lamsayah, M.; Touzani, R. New nitrogen-donnor pyrazole ligands for excellent liquid-liquid extraction of Fe2+ ions from aqueous solution, with theoretical study. Res. Chem. Interm. 2015, 41, 3319–3334. [Google Scholar] [CrossRef]
- Garbacia, S.; Hillairet, C.; Touzani, R.; Lavastre, O. New nitrogen-rich tripodal molecules based on bis(pyrazol-1-ylmethyl)amines with substituents modulating steric hindrances and electron density of donor sites. Collect. Czechoslov. Chem. Commun. 2005, 70, 34–40. [Google Scholar] [CrossRef]
- Bouabdallah, I.; Touzani, R.; Zidane, I.; Ramdani, A. Synthesis of new tripodal ligand: N,N-bis[(1,5-dimethylpyrazol-3-yl)methyl]benzylamine. Catecholase activity of two series of tripodal ligands with some copper (II) salts. Catal. Commun. 2007, 8, 707–712. [Google Scholar] [CrossRef]
- El Kodadi, M.; Benamar, M.; Ibrahim, B.; Zyad, A.; Malek, F.; Touzani, R.; Ramdani, A.; Melhaoui, A. New Synthesis of two tridentate bipyrazolic compounds and their cytotoxic activity tumor cell lines. Nat. Prod. Res. 2007, 21, 947–952. [Google Scholar] [CrossRef]
- Touzani, R.; Garbacia, S.; Lavastre, O.; Yadav, V.K.; Carboni, B. Efficient solution phase combinatorial access to a library of pyrazole- and triazole-containing compounds. J. Comb. Chem. 2003, 5, 375–378. [Google Scholar] [CrossRef]
- Touzani, R.; Vasapollo, G.; Scorrano, S.; Del Sole, R.; Manera, M.G.; Rella, R.; El Kadiri, S. New complexes based on tridentate bispyrazole ligand for optical gas sensing. Mater. Chem. Phys. 2011, 126, 375–380. [Google Scholar] [CrossRef]
- Boussalah, N.; Touzani, R.; Bouabdallah, I.; El Kadiri, S.; Ghalem, S. Oxidation catalytic properties of new amino acid based on pyrazole tripodal ligands. Inter. J. Acad. Res. 2009, 1, 137–143. [Google Scholar]
- Boussalah, N.; Touzani, R.; Bouabdallah, I.; El Kadiri, S.; Ghalem, S. Synthesis, structure and catalytic properties of tripodal amino-acid derivatized pyrazole-based ligands. J. Mol. Catal. A Chem. 2009, 306, 113–117. [Google Scholar] [CrossRef]
- Hammouti, B.; Dafali, A.; Touzani, R.; Bouachrine, M. Inhibition of copper corrosion by bipyrazole compound in aerated 3% NaCl. J. Saudi Chem. Soc. 2012, 16, 413–418. [Google Scholar] [CrossRef]
- Boussalah, N.; Touzani, R.; Souna, F.; Himri, I.; Bouakka, M.; Hakkou, A.; Ghalem, S.; El Kadiri, S. Antifungal activities of amino acid ester functional pyrazolyl compounds against Fusarium oxysporum f.sp. albedinis and Saccharomyces cerevisiae yeast. J. Saudi Chem. Soc. 2013, 17, 17–21. [Google Scholar] [CrossRef]
- Abrigach, F.; Bouchal, B.; Riant, O.; Mace, Y.; Takfaoui, A.; Radi, S.; Oussaid, A.; Bellaoui, M.; Touzani, R. New N,N,N’,N’-tetradentate Pyrazoly Agents: Synthesis and Evaluation of their Antifungal and Antibacterial Activities. Med. Chem. 2016, 12, 83–89. [Google Scholar] [CrossRef]
- Abrigach, F.; Karzazi, Y.; Benabbes, R.; El Youbi, M.; Khoutoul, M.; Taibi, N.; Karzazi, N.; Benchat, N.; Bouakka, M.; Saalaoui, E.; et al. Synthesis, biological screening, POM, and 3D-QSAR analyses of some novel pyrazolic compounds. Med. Chem. Res. 2017, 26, 1784–1795. [Google Scholar] [CrossRef]
- Kaddouri, Y.; Abrigach, F.; Mechbal, N.; Karzazi, Y.; El Kodadi, M.; Aouniti, A.; Touzani, R. Pyrazole Compounds: Synthesis, molecular structure, chemical reactivity, experimental and theoretical DFT FTIR spectra. Mater. Today Proc. 2019, 13, 956–963. [Google Scholar] [CrossRef]
- El Ati, R.; Takfaoui, A.; El Kodadi, M.; Touzani, R.; Yousfi, E.B.; Almalki, A.A.; Ben Hadda, T. Catechol oxidase and Copper(I/II) Complexes Derived from Bipyrazol Ligand: Synthesis, Molecular Structure Investigation of New Biomimetic Functional Model and Mechanistic Study. Mater. Today Proc. 2019, 13, 129–1237. [Google Scholar] [CrossRef]
- Toubi, Y.; Touzani, R.; Radi, S.; El Kadiri, S. Synthesis, characterization and catecholase activity of copper (II) complexes with bispyrazole tri-podal ligands. J. Mater. Environ. Sci. 2012, 3, 328–341. [Google Scholar]
- Radi, S.; Toubi, Y.; Draoui, N.; Feron, O.; Riant, O. One-pot synthesis and in vitro antitumor activity of some bipyrazolic tripodal derivatives. Lett. Drug Des. Disc. 2012, 9, 305–309. [Google Scholar]
- Kalanithi, M.; Rajarajan, M.; Tharmaraj, P.; Johnson Raja, S. Synthesis, spectroscopic characterization, analgesic, and antimicrobial activities of Co(II), Ni(II), and Cu(II) complexes of 2-[N,N-bis-(3,5-dimethyl-pyrazolyl-1-methyl)]aminothiazole. Med. Chem. Res. 2015, 24, 1578–1585. [Google Scholar] [CrossRef]
- Ghosh, D.; Kundu, N.; Maity, G.; Choi, K.-Y.; Caneschi, A.; Endo, A.; Chaudhury, M. Synthesis, Structure, Magnetism, and Spectroscopic Properties of Heterobinuclear Copper(II)-Zinc(II) Complexes and Their Copper(II)-Copper(II) Analogues in Asymmetric Ligand Environments. Inorg. Chem. 2004, 43, 6015–6023. [Google Scholar] [CrossRef] [PubMed]
- Malek, F.; Draoui, N.; Feron, O.; Radi, S. Tridentate bipyrazole compounds with a side-arm as a new class of antitumor agents. Res. Chem. Intermed. 2014, 40, 681–687. [Google Scholar] [CrossRef]
- Harit, T.; Cherfi, M.; Isaad, J.; Riahi, A.; Malek, F. New generation of functionalized bipyrazolic tripods: Synthesis and study of their coordination properties towards metal cations. Tetrahedron 2012, 68, 4037–4041. [Google Scholar] [CrossRef]
- Kaddouri, Y.; Takfaoui, A.; El Ati, R.; Abrigach, F.; Lamsayah, M.; Touzani, R. Synthesis of four new tridentate pyrazolic ligands. J. Mar. Chim. Heter. 2017, 16, 100–104. [Google Scholar]
- Bouabdallah, I.; Touzani, R.; Zidane, I.; Ramdani, A.; Jalbout, A.F.; Trzaskowski, B. New comparative theoretical calculations of some N,N-bis[(3,5-dimethylpyrazol-1-yl)methyl]phenylamines tripodal ligands. J. Mater. Environ. Sci. 2013, 4, 671–674. [Google Scholar]
- Touzani, R.; El Kadiri, S.; Zerrouki, A.; Scorrano, S.; Vasapollo, G.; Manera, M.G.; Casino, F.; Rella, R. Optical and morphological characterization of bispyrazole thin films for gas sensing applications. Arab. J. Chem. 2014, 7, 695–700. [Google Scholar] [CrossRef]
- Mouadili, A.; Attayibat, A.; El Kadiri, S.; Radi, S.; Touzani, R. Catecholase activity investigations using in situ copper complexes with pyrazole and pyridine based ligands. Appl. Catal. A 2013, 454, 93–99. [Google Scholar] [CrossRef]
- Bouchal, B.; Abrigach, F.; Takfaoui, A.; Errahhali, M.E.; Errahhali, M.E.; Dixneuf, P.H.; Doucet, H.; Touzani, R.; Bellaoui, M. Identification of novel antifungal agents: Antimicrobial evaluation, SAR, ADME-Tox and molecular docking studies of a series of imidazole derivatives. BMC Chem. 2019, 13, 100. [Google Scholar] [CrossRef]
- Biyiti, L.; Meko’o, D.; Tamzc, V.; Amvam Zollo, P. Recherche de l’activité antibactérienne de quatre plantes médicinales camerounaises. Pharm. Med. Trad. Afr. 2004, 13, 11–20. [Google Scholar]
- Berche, P.; Gaillard, J.; Simonet, M. Les bactéries des infections humaines. Editeur: Flammarion. Médecine Sci. 1991, 660. Available online: https://lib.ugent.be/en/catalog/rug01:000199668 (accessed on 17 February 2022).
- Ennadir, J.; Hassikou, R.; Bouazza, F.; Arahou, M.; Al Askari, G.; Khedid, K. Évaluation in vitro de l’activité antibactérienne des extraits aqueux et organiques des graines de Nigella sativa L. et de Foeniculum vulgare Mill. Phytothér 2014, 12, 302–308. [Google Scholar] [CrossRef]
- Bender, A.; Glen, R.C. Molecular similarity: A key technique in molecular informatics. Org. Biomol. Chem. 2004, 2, 3204–3218. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.P.; Hersey, A.; Montanari, D.; Overington, J. Probing the links between in vitro potency, ADMET and physicochemical parameters. Nat. Rev. Drug Discov. 2011, 10, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Fei, J.; Zhou, L.; Liu, T.; Tang, X.Y. Pharmacophore modeling, virtual screening, and molecular docking studies for discovery of novel Akt2 inhibitors. Int. J. Med. Sci. 2013, 10, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Skariyachan, S.; Pachiappan, A.; Joy, J.; Bhaduri, R.; Aier, I.; Vasist, K.S. Investigating the therapeutic potential of herbal leads against drug resistant Listeria monocytogenes by computational virtual screening and in vitro assays. J. Biomol. Struct. Dyn. 2015, 33, 2682–2694. [Google Scholar] [CrossRef]
- Leelananda, S.P.; Lindert, S. Computational methods in drug discovery. Beilstein J. Org. Chem. 2016, 12, 2694–2718. [Google Scholar] [CrossRef]
- Fares, M.; Said, M.A.; Alsherbiny, M.A.; Eladwy, R.A.; Almahli, H.; Abdel-Aziz, M.M.; Ghabbour, H.A.; Eldehna, W.M.; Abdel-Aziz, H.A. Synthesis, Biological Evaluation and Molecular Docking of Certain Sulfones as Potential Nonazole Antifungal Agents. Molecules 2016, 21, 114. [Google Scholar] [CrossRef]
- Nastasă, C.; Vodnar, D.C.; Ionuţ, I.; Stana, A.; Benedec, D.; Tamaian, R.; Oniga, O.; Tiperciuc, B. Antibacterial Evaluation and Virtual Screening of New Thiazolyl-Triazole Schiff Bases as Potential DNA-Gyrase Inhibitors. Int. J. Mol. Sci. 2018, 19, 222. [Google Scholar] [CrossRef]
- Aggarwal, S.; Paliwal, D.; Kaushik, D.; Gupta, G.K.; Kumar, A. Pyrazole Schiff Base Hybrids as Anti-Malarial Agents: Synthesis, In Vitro Screening and Computational Study. Comb. Chem. High Throughput Screen. 2018, 21, 194–203. [Google Scholar] [CrossRef]
- Hessler, G.; Baringhaus, K.H. Artificial Intelligence in Drug Design. Molecules 2018, 23, 2520. [Google Scholar] [CrossRef]
- Muchtaridi, M.; Dermawan, D.; Yusuf, M. Molecular Docking, 3D Structure-Based Pharmacophore Modeling, and ADME Prediction of Alpha Mangostin and Its Derivatives against Estrogen Receptor Alpha. J. Young-Pharm. 2018, 10, 252–259. [Google Scholar] [CrossRef]
- Holm, L. Benchmarking fold detection by DaliLite v.5. Bioinformatics 2019, 35, 5326–5327. [Google Scholar] [CrossRef] [PubMed]
- Sangpheak, K.; Tabtimmai, L.; Seetaha, S.; Rungnim, C.; Chavasiri, W.; Wolschann, P.; Choowongkomon, K.; Riungrotmongkol, T. Biological Evaluation and Molecular Dynamics Simulation of Chalcone Derivatives as Epidermal Growth Factor-Tyrosine Kinase Inhibitors. Molecules 2019, 24, 1092. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Kumar, S.; Narasimhan, B.; Ramasamy, K.; Lim, S.M.; Shah, S.A.A.; Mani, V. 4-(4-Bromophenyl)-thiazol-2-amine derivatives: Synthesis, biological activity and molecular docking study with ADME profile. BMC Chem. 2019, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Curreli, F.; Ahmed, S.; Victor, S.M.B.; Ilusupov, I.R.; Belov, D.S.; Markov, P.O.; Kurkin, A.V.; Altieri, A.; Bebnath, A.K. Preclinical Optimization of gp120 Entry Antagonists as anti-HIV-1 Agents with Improved Cytotoxicity and ADME Properties through Rational Design, Synthesis, and Antiviral Evaluation. J. Med. Chem. 2020, 63, 1724–1749. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Abdellattif, M.H.; Abdel-Rahman, A.A.H.; Arief, M.M.H.; Mouneir, S.M.; Ali, A.; Hussien, M.A.; Okasha, R.M.; Afifi, T.H.; Hagar, M. Novel 2-Hydroselenonicotinonitriles and Selenopheno[2, 3-b]pyridines: Efficient Synthesis, Molecular Docking-DFT Modeling, and Antimicrobial Assessment. Front. Chem. 2021, 9, 672503. [Google Scholar] [CrossRef]
- Drwal, M.N.; Banerjee, P.; Dunkel, M.; Wettig, M.R.; Preissner, R. ProTox: A web server for the in silico prediction of rodent oral toxicity. Nucleic Acids Res. 2014, 42, W53–W58. [Google Scholar] [CrossRef]
- Shehab, W.S.; Abdellattif, M.H.; Mouneir, S.M. Heterocyclization of polarized system: Synthesis, antioxidant and anti-inflammatory 4-(pyridin-3-yl)-6-(thiophen-2-yl) pyrimidine-2-thiol derivatives. Chem. Cent. J. 2018, 12, 68. [Google Scholar] [CrossRef]
- Yeni, S.; Merdekawati, F. In Silico Study of Pyrazolylaminoquinazoline Toxicity by Lazar, Protox, and Admet Predictor. J. Appl. Pharm. Sci. 2018, 8, 119–129. [Google Scholar]
- Abdellattif, M.H.; Shahbaaz, M.; Arief, M.M.H.; Hussien, M.A. Oxazinethione Derivatives as a Precursor to Pyrazolone and Pyrimidine Derivatives: Synthesis, Biological Activities, Molecular Modeling, ADME, and Molecular Dynamics Studies. Molecules 2021, 26, 5482. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.D.S.; Costa, J.D.S.; Silva, R.C.; da Costa, G.V.; Rodrigues, A.B.L.; Rabelo, E.D.M.; Souto, R.N.P.; Taft, C.A.; Silva, C.H.T.D.P.D.; Rosa, J.M.C.; et al. Identification of Potential Inhibitors from Pyriproxyfen with Insecticidal Activity by Virtual Screening. Pharmaceuticals 2019, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Rizvi, S.M.D.; Shakil, S.; Haneef, M. A simple click by click protocol to perform docking: Autodock 4.2 made easy for non-bioinformaticians. EXCLI J. 2013, 12, 831–857. [Google Scholar] [PubMed]
- Vieira, T.F.; Sousa, S.F. Comparing AutoDock and Vina in Ligand/Decoy Discrimination for Virtual Screening. Appl. Sci. 2019, 9, 4538. [Google Scholar] [CrossRef]
- Kaddouri, Y.; Abrigach, F.; Ouahhoud, S.; Benabbes, R.; El Kodadi, M.; Alsalme, A.; Al-Zaqri, N.; Warad, I.; Touzani, R. Mono-Alkylated Ligands Based on Pyrazole and Triazole Derivatives Tested Against Fusarium oxysporum f. sp. albedinis: Synthesis, Characterization, DFT, and Phytase Binding Site Identification Using Blind Docking/Virtual Screening for Potent Fophy Inhibitors. Front. Chem. 2020, 8, 559262. [Google Scholar]
- Hall, C.; Nelson, D.M.; Ye, X.; Baker, K.; DeCaprio, J.A.; Seeholzer, S.; Lipinski, M.; Adams, P.D. Hira, the human homologue of yeast hir1p and hir2p, is a novel cycin-cdk2 substrate whose expresssion blocks S-phase progresssion. Mol. Cel. Biol. 2001, 21, 1854–1865. [Google Scholar] [CrossRef]
- DeGoey, D.A.; Chen, H.J.; Cox, P.B.; Wendt, M.D. Beyond the Rule of 5: Lessons Learned from AbbVie’s Drugs and Compound Collection. J. Med. Chem. 2018, 61, 2636–2651. [Google Scholar] [CrossRef]
- Caron, G.; Digiesi, V.; Solaro, S.; Ermondi, G. Flexibility in early drug discovery: Focus on the beyond-Rule-of-5 chemical space. Drug Discov. Today 2020, 25, 621–627. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).