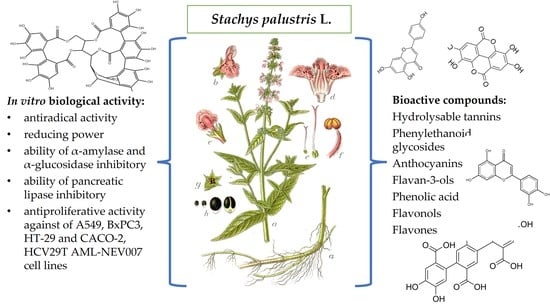
Flowers and Leaves Extracts of Stachys palustris L. Exhibit Stronger Anti-Proliferative, Antioxidant, Anti-Diabetic, and Anti-Obesity Potencies than Stems and Roots Due to More Phenolic Compounds as Revealed by UPLC-PDA-ESI-TQD-MS/MS
Abstract
:1. Introduction
2. Results and Discussion
2.1. Profile of Polyphenolic Compounds
2.1.1. Hydrolysable Tannins
2.1.2. Flavanones
2.1.3. Phenylethanoid Glycosides (PhGs)
2.1.4. Other Phenolic Acids, Anthocyanins, Flavonol, and Flavan-3-ol
2.2. Content of Polyphenolic Compounds and Polymeric Procyanidins
2.3. In Vitro Biological Activity
2.3.1. Antiradical and Reducing Potential
2.3.2. In Vitro Enzyme Inhibition
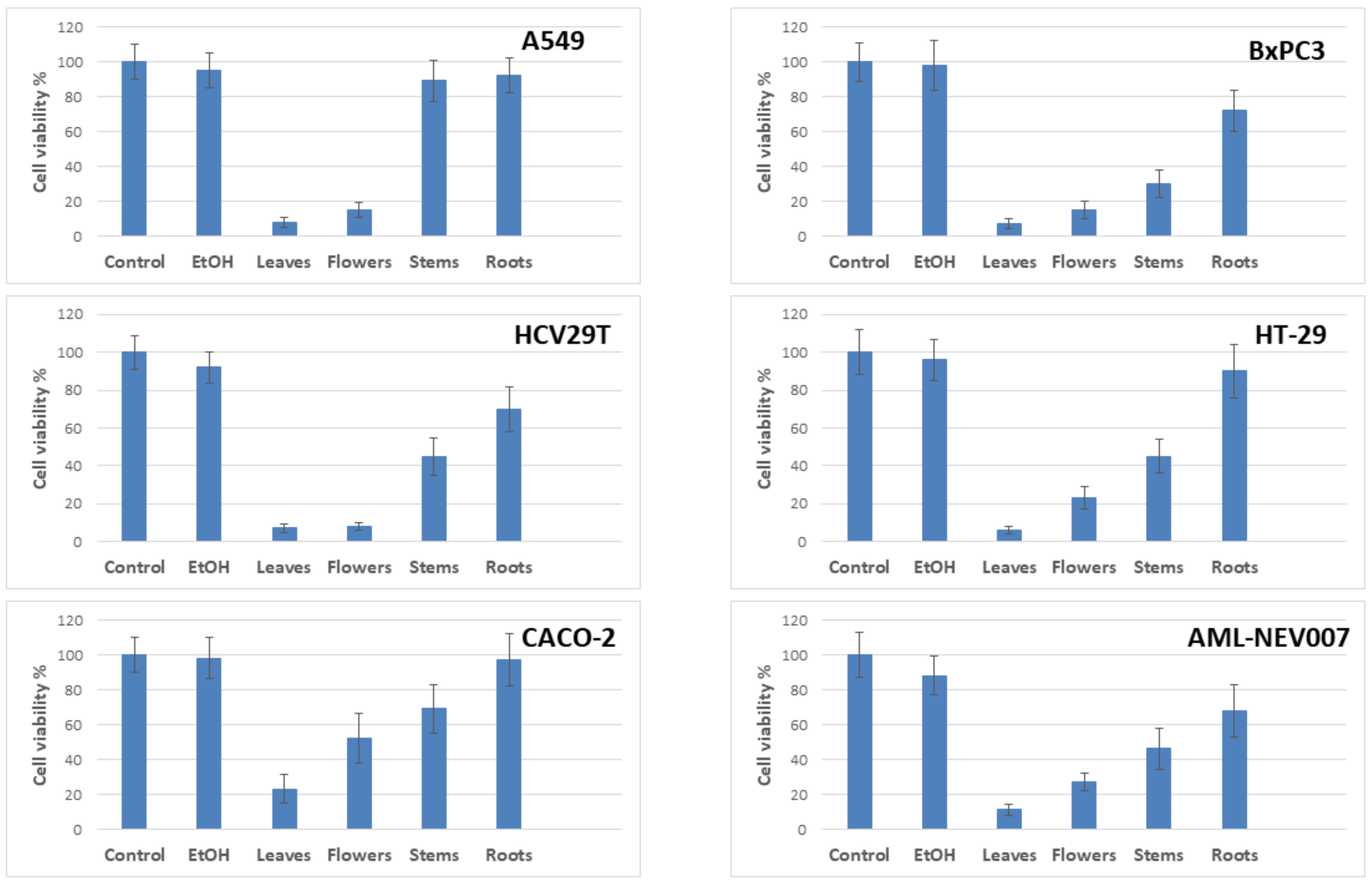
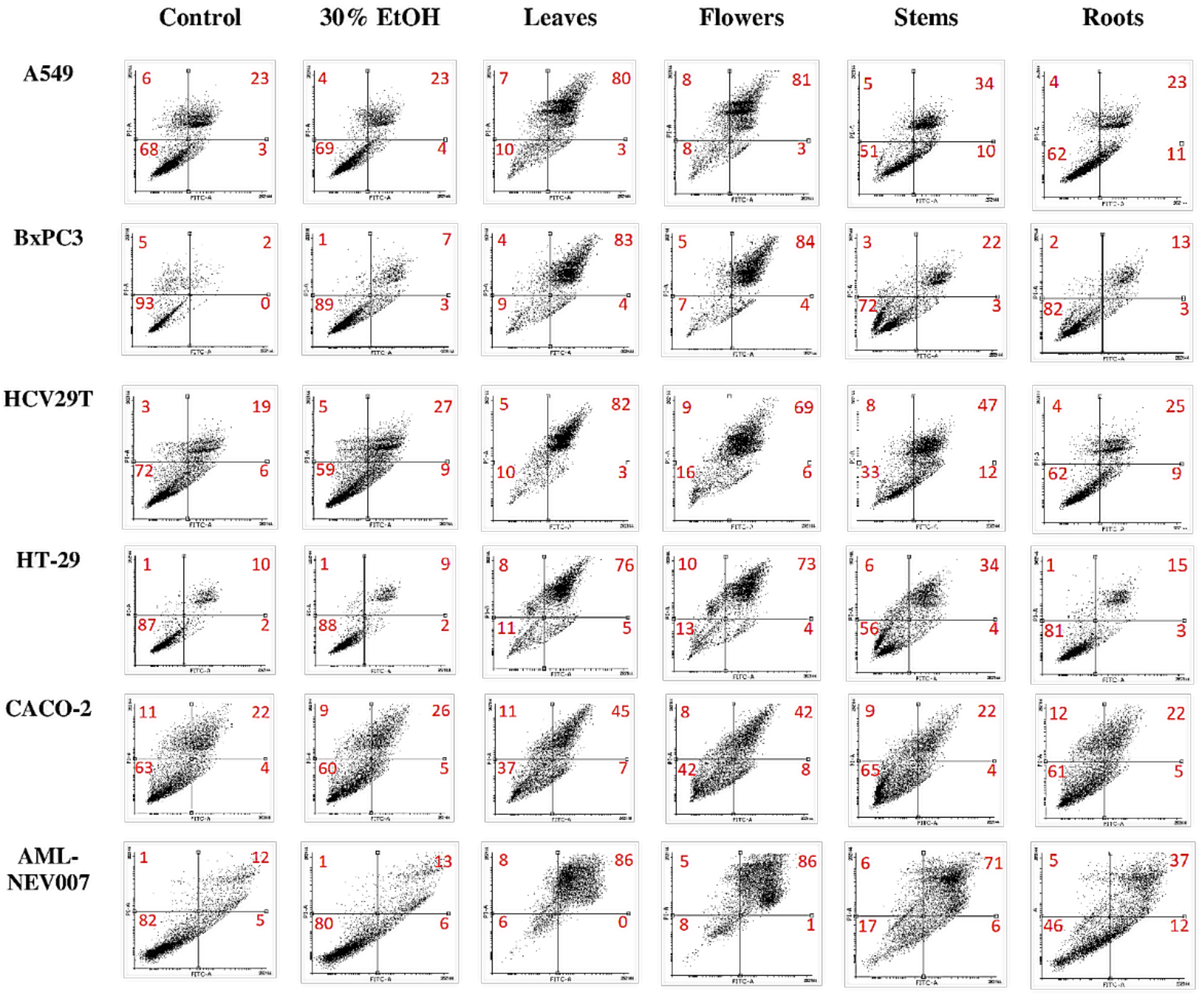
2.3.3. Anti-Proliferative Activity
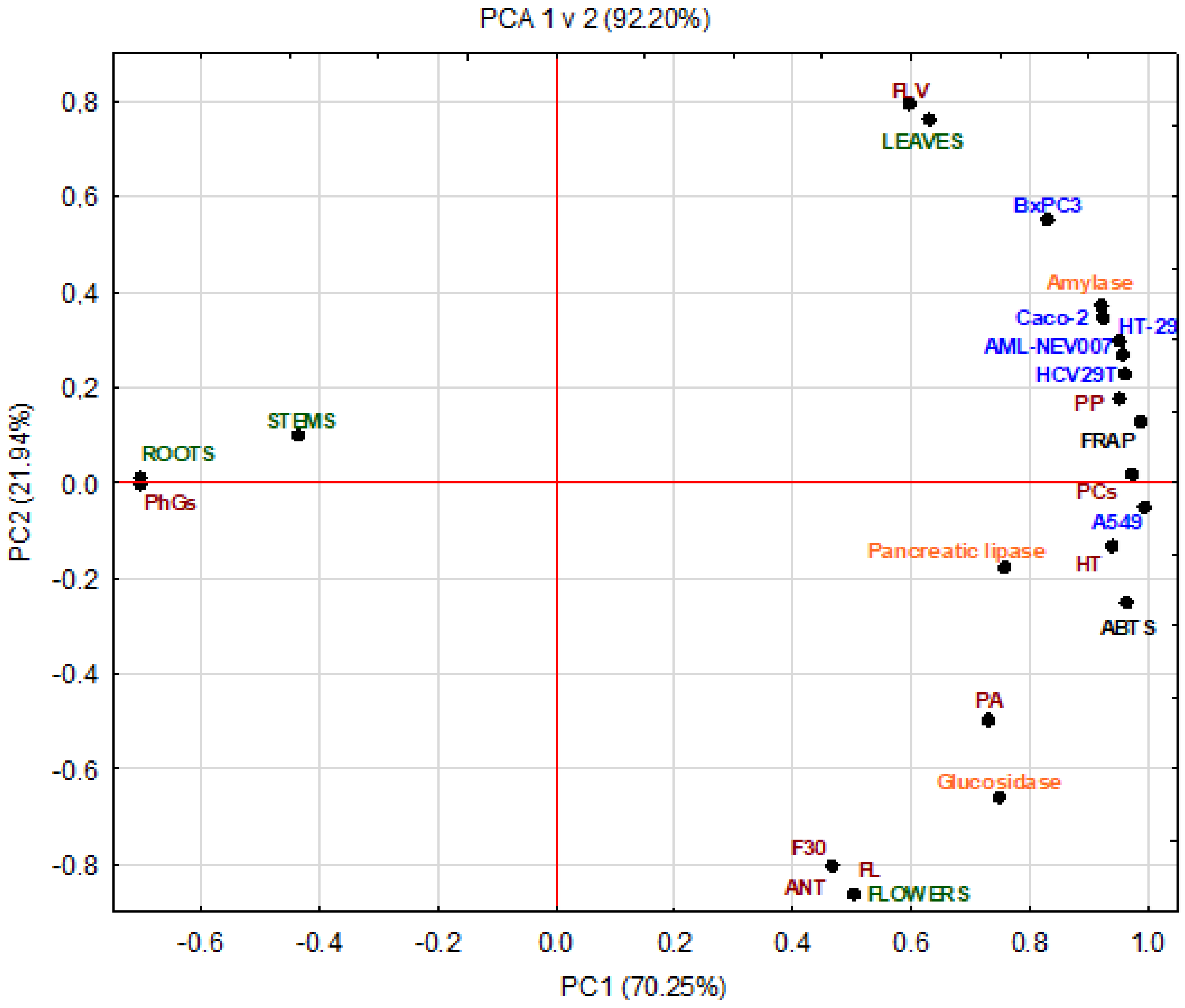
2.4. Multivariate Analysis
3. Materials and Methods
3.1. Chemicals, Material and Instruments
3.2. Color Parameter
3.3. Polyphenolic Compounds (PCs) by UPLC-ESI-TQD-MS/MS and Procyanidin Polymers (PP) by the Phloroglucinolysis Method
3.4. In Vitro Biological Activity
3.4.1. Extraction Procedure
3.4.2. Antioxidant Activity
Antiradical Activity
Reducing Potency
3.4.3. Ability of α-Amylase, α-Glucosidase, Pancreatic Lipase Inhibitors
3.4.4. Antiproliferative Potency
Cell Lines and Cell Culture
Determination of Cell Viability
Apoptosis Assay
3.5. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohammed, M.J.; Anand, U.; Altemimi, A.B.; Tripathi, V.; Guo, Y.; Pratap-Singh, A. Phenolic Composition, Antioxidant Capacity and Antibacterial Activity of White Wormwood (Artemisia herbaalba). Plants 2021, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Venditti, A.; Frezza, C.; Bianco, A.; Serafini, M.; Cianfaglione, K.; Nagy, D.U.; Iannarelli, R.; Caprioli, G.; Maggi, F. Polar constituents, essential oil and antioxidant activity of marsh woundwort (Stachys palustris L.). Chem. Biodivers. 2017, 14, e1600401. [Google Scholar] [CrossRef] [PubMed]
- Facciola, S. Cornocupia II. A Source Book of Edible Plants; Kampong: Vista, CA, USA, 1998. [Google Scholar]
- Łuczaj, Ł.J.; Svanberg, I.; Köhler, P. Marsh woundwort, Stachys palustris L.(Lamiaceae): An overlooked food plant. Genet. Resour. Crop Evol. 2011, 58, 783–793. [Google Scholar] [CrossRef] [Green Version]
- Usher, G. Dictionary of Plants Used by Man; Constable and Company Ltd.: London, UK, 1974. [Google Scholar]
- Feduraev, P.; Chupakhina, G.; Maslennikov, P.; Tacenko, N.; Skrypnik, L. Variation in phenolic compounds content and antioxidant activity of different plant organs from Rumex crispus L. and Rumex obtusifolius L. at different growth stages. Antioxidants 2019, 8, 237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Háznagy-Radnai, E.; Réthy, B.; Czigle, S.; Zupkó, I.; Wéber, E.; Martinek, T.; Falkay, G.; Máthé, I. Cytotoxic activities of Stachys species. Fitoterapia 2008, 79, 595–597. [Google Scholar] [CrossRef] [PubMed]
- Esposito, T.; Celano, R.; Pane, C.; Piccinelli, A.L.; Sansone, F.; Picerno, P.; Zaccardelli, M.; Aquino, R.P.; Mencherini, T. Chestnut (Castanea sativa Miller.) burs extracts and functional compounds: UHPLC-UV-HRMS profiling, antioxidant activity, and inhibitory effects on phytopathogenic fungi. Molecules 2019, 24, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Bajpai, V.; Kumar, S.; Sharma, K.R.; Kumar, B. Profiling of gallic and ellagic acid derivatives in different plant parts of Terminalia arjuna by HPLC-ESI-QTOF-MS/MS. Nat. Prod. Commun. 2016, 11, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Moilanen, J.; Sinkkonen, J.; Salminen, J.P. Characterization of bioactive plant ellagitannins by chromatographic, spectroscopic and mass spectrometric methods. Chemoecology 2013, 23, 165–179. [Google Scholar] [CrossRef]
- Lachowicz, S.; Oszmiański, J.; Rapak, A.; Ochmian, I. Profile and content of phenolic compounds in leaves, flowers, roots, and stems of Sanguisorba officinalis L. determined with the LC-DAD-ESI-QTOF-MS/MS analysis and their in vitro antioxidant, antidiabetic, antiproliferative potency. Pharmaceuticals 2020, 13, 191. [Google Scholar] [CrossRef]
- D’Urso, G.; Sarais, G.; Lai, C.; Pizza, C.; Montoro, P. LC-MS based metabolomics study of different parts of myrtle berry from Sardinia (Italy). J. Berry Res. 2017, 7, 217–229. [Google Scholar] [CrossRef]
- Del Bubba, M.; Checchini, L.; Chiuminatto, U.; Doumett, S.; Fibbi, D.; Giordani, E. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of polyphenolic composition of four cultivars of Fragaria vesca L. berries and their comparative evaluation. J. Mass Spectrom. 2012, 47, 1207–1220. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Chen, J. Screening and identification of acetylcholinesterase inhibitors from Terminalia chebula fruits based on ultrafiltration and ultrα-performance liquid chromatography-quadrupole time-of-flight mass spectrometry. Microchem. J. 2021, 168, 106438. [Google Scholar] [CrossRef]
- Ito, H. Metabolites of the ellagitannin geraniin and their antioxidant activities. Planta Med. 2011, 77, 1110–1115. [Google Scholar] [CrossRef] [Green Version]
- Muccilli, V.; Cardullo, N.; Spatafora, C.; Cunsolo, V.; Tringali, C. α-Glucosidase inhibition and antioxidant activity of an oenological commercial tannin. Extraction, fractionation and analysis by HPLC/ESI-MS/MS and 1H NMR. Food Chem. 2017, 215, 50–60. [Google Scholar] [CrossRef]
- Santos, L.S.; Alves Filho, E.G.; Ribeiro, P.R.; Zocolo, G.J.; Silva, S.M.; de Lucena, E.M.; Alves, R.E.; de Brito, E.S. Chemotaxonomic evaluation of different species from the Myrtaceae family by UPLC-qToF/MS-MS coupled to supervised classification based on genus. Biochem. Syst. Ecol. 2020, 90, 104028. [Google Scholar] [CrossRef]
- Troalen, L.G.; Phillips, A.S.; Peggie, D.A.; Barran, P.E.; Hulme, A.N. Historical textile dyeing with Genista tinctoria L.: A comprehensive study by UPLC-MS/MS analysis. Anal. Methods 2014, 6, 8915–8923. [Google Scholar] [CrossRef] [Green Version]
- Hooi Poay, T.; Sui Kiong, L.; Cheng Hock, C. Characterisation of galloylated cyanogenic glucosides and hydrolysable tannins from leaves of Phyllagathis rotundifolia by LC-ESI-MS/MS. Phytochem. Anal. 2011, 22, 516–525. [Google Scholar] [CrossRef]
- Kool, M.M.; Comeskey, D.J.; Cooney, J.M.; McGhie, T.K. Structural identification of the main ellagitannins of a boysenberry (Rubus loganbaccus× baileyanus Britt.) extract by LC–ESI-MS/MS, MALDI-TOF-MS and NMR spectroscopy. Food Chem. 2010, 119, 1535–1543. [Google Scholar] [CrossRef]
- Mullen, W.; Yokota, T.; Lean, M.E.; Crozier, A. Analysis of ellagitannins and conjugates of ellagic acid and quercetin in raspberry fruits by LC–MSn. Phytochemistry 2003, 64, 617–624. [Google Scholar] [CrossRef]
- Li, L.; Tsao, R.; Yang, R.; Liu, C.; Young, J.C.; Zhu, H. Isolation and purification of phenylethanoid glycosides from Cistanche deserticola by high-speed counter-current chromatography. Food Chem. 2008, 108, 702–710. [Google Scholar] [CrossRef]
- Piwowarczyk, R.; Ochmian, I.; Lachowicz, S.; Kapusta, I.; Sotek, Z.; Błaszak, M. Phytochemical parasite-host relations and interactions: A Cistanche armena case study. Sci. Total Environ. 2020, 716, 137071. [Google Scholar] [CrossRef]
- Kırmızıbekmez, H.; Montoro, P.; Piacente, S.; Pizza, C.; Dönmez, A.; Çalış, İ. Identification by HPLC-PAD-MS and quantification by HPLC-PAD of phenylethanoid glycosides of five Phlomis species. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2005, 16, 1–6. [Google Scholar] [CrossRef]
- Karioti, A.; Bolognesi, L.; Vincieri, F.F.; Bilia, A.R. Analysis of the constituents of aqueous preparations of Stachys recta by HPLC–DAD and HPLC–ESI-MS. J. Pharm. Biomed. Anal. 2010, 53, 15–23. [Google Scholar] [CrossRef]
- Shakeri, A.; D’Urso, G.; Taghizadeh, S.F.; Piacente, S.; Norouzi, S.; Soheili, V.; Asili, J.; Salarbashi, D. LC-ESI/LTQOrbitrap/MS/MS and GC–MS profiling of Stachys parviflora L. and evaluation of its biological activities. J. Pharm. Biomed. Anal. 2019, 168, 209–216. [Google Scholar] [CrossRef]
- Miyase, T.; Yamamoto, R.; Ueno, A. Phenylethanoid glycosides from Stachys officinalis. Phytochemistry 1996, 43, 475–479. [Google Scholar] [CrossRef]
- Yang, L.; He, J. Lagopsis supina extract and its fractions exert prophylactic effects against blood stasis in rats via anti-coagulation, anti-platelet activation and anti-fibrinolysis and chemical characterization by UHPLC-qTOF-MS/MS. Biomed. Pharmacother. 2020, 132, 110899. [Google Scholar] [CrossRef]
- Nicoue, E.E.; Savard, S.; Belkacemi, K. Anthocyanins in wild blueberries of Quebec: Extraction and identification. J. Agric. Food Chem. 2007, 55, 5626–5635. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Zeng, X.; Yang, L.; Deng, Y. Chemical profiling of bioactive constituents in Sarcandra glabra and its preparations using ultra-high-pressure liquid chromatography coupled with LTQ Orbitrap mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 2439–2447. [Google Scholar] [CrossRef]
- Delazar, A.; Celik, S.; Göktürk, R.S.; Unal, N.; Nahar, L.; Sarker, S.D. Two acylated flavonoid glycosides from Stachys bombycina, and their free radical scavenging activity. Die Pharm.-Int. J. Pharm. Sci. 2005, 60, 878–880. [Google Scholar] [CrossRef]
- Marin, P.D.; Grayer, R.J.; Grujic-Jovanovic, S.; Kite, G.C.; Veitch, N.C. Glycosides of tricetin methyl ethers as chemosystematic markers in Stachys subgenus Betonica. Phytochemistry 2004, 65, 1247–1253. [Google Scholar] [CrossRef]
- Šliumpaitė, I.; Venskutonis, P.R.; Murkovic, M.; Ragažinskienė, O. Antioxidant properties and phenolic composition of wood betony (Betonica officinalis L., syn. Stachys officinalis L.). Ind. Crops Prod. 2013, 50, 715–722. [Google Scholar] [CrossRef]
- Tahir, N.I.; Shaari, K.; Abas, F.; Parveez, G.K.A.; Ishak, Z.; Ramli, U.S. Characterization of apigenin and luteolin derivatives from oil palm (Elaeis guineensis Jacq.) leaf using LC–ESI-MS/MS. J. Agric. Food Chem. 2012, 60, 11201–11210. [Google Scholar] [CrossRef] [PubMed]
- Orsini, F.; Vovk, I.; Glavnik, V.; Jug, U.; Corradini, D. HPTLC, HPTLC-MS/MS and HPTLC-DPPH methods for analyses of flavonoids and their antioxidant activity in Cyclanthera pedata leaves, fruits and dietary supplement. J. Liq. Chromatogr. Relat. Technol. 2019, 42, 290–301. [Google Scholar] [CrossRef]
- Demirtas, I.; Gecibesler, I.H.; Yaglioglu, A.S. Antiproliferative activities of isolated flavone glycosides and fatty acids from Stachys byzantina. Phytochem. Lett. 2013, 6, 209–214. [Google Scholar] [CrossRef]
- Lin, L.Z.; Harnly, J.; Zhang, R.W.; Fan, X.E.; Chen, H.J. Quantitation of the hydroxycinnamic acid derivatives and the glycosides of flavonols and flavones by UV absorbance after identification by LC-MS. J. Agric. Food Chem. 2012, 60, 544–553. [Google Scholar] [CrossRef]
- Sun, D.; Dong, L.; Guo, P.; Yan, W.; Wang, C.; Zhang, Z. Simultaneous determination of four flavonoids and one phenolic acid in rat plasma by LC–MS/MS and its application to a pharmacokinetic study after oral administration of the Herba Desmodii Styracifolii extract. J. Chromatogr. B 2013, 932, 66–73. [Google Scholar] [CrossRef]
- Arthur, H.; Joubert, E.; De Beer, D.; Malherbe, C.J.; Witthuhn, R.C. Phenylethanoid glycosides as major antioxidants in Lippia multiflora herbal infusion and their stability during steam pasteurisation of plant material. Food Chem. 2011, 127, 581–588. [Google Scholar] [CrossRef]
- Amessis-Ouchemoukh, N.; Abu-Reidah, I.M.; Quirantes-Piné, R.; Rodríguez-Pérez, C.; Madani, K.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Tentative characterisation of iridoids, phenylethanoid glycosides and flavonoid derivatives from Globularia alypum L. (Globulariaceae) leaves by LC-ESI-QTOF-MS. Phytochem. Anal. 2014, 25, 389–398. [Google Scholar] [CrossRef]
- Bahadori, M.B.; Kirkan, B.; Sarikurkcu, C.; Ceylan, O. Metabolite profiling and health benefits of Stachys cretica subsp. mersinaea as a medicinal food. Ind. Crops Prod. 2019, 131, 85–89. [Google Scholar] [CrossRef]
- Carev, I.; Sarikurkcu, C. LC-MS/MS Profiles and in vitro biological activities of extracts of an endemic species from Turkey: Stachys cretica ssp. anatolica. Plants 2021, 10, 1054. [Google Scholar] [CrossRef]
- Saltveit, M.E. Synthesis and metabolism of phenolic compounds. Fruit Veg. Phytochem. Chem. Hum. Health 2017, 2, 115. [Google Scholar]
- Chepel, V.; Lisun, V.; Skrypnik, L. Changes in the content of some groups of phenolic compounds and biological activity of extracts of various parts of heather (Calluna vulgaris (L.) Hull) at different growth stages. Plants 2020, 9, 926. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Zengin, G. Polyphenol profile and biological activity comparisons of different parts of Astragalus macrocephalus subsp. finitimus from Turkey. Biology 2020, 9, 231. [Google Scholar] [CrossRef]
- Khanavi, M.; Hajimahmoodi, M.; Cheraghi-Niroomand, M.; Kargar, Z.; Ajani, Y.; Hadjiakhoondi, A.; Oveisi, M.R. Comparison of the antioxidant activity and total phenolic contents in some Stachys species. Afr. J. Biotechnol. 2009, 8, 1143–1147. [Google Scholar]
- Oracz, J.; Zyzelewicz, D.; Nebesny, E. The content of polyphenolic compounds in cocoa beans (Theobroma cacao L.), depending on variety, growing region, and processing operations: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1176–1192. [Google Scholar] [CrossRef]
- Dušek, K.; Dušková, E.; Smékalová, K. Variability of morphological characteristic and content of active substances in Betonica officinalis L. in the czech republic. Agriculture/Pol’nohospodárstvo 2009, 55, 102–110. [Google Scholar]
- Franz, C.H.; Novak, J.; Hajdari, A.; Mustafa, B. Total flavonoids, total phenolics and antioxidant activity of Betonica officinalis L. from Kosovo. In Proceedings of the IV International Symposium on Breeding Research on Medicinal and Aromatic Plants-ISBMAP2009, Ljubljana, Slovenia, 17–21 June 2009; Volume 860, pp. 75–80. [Google Scholar]
- Elfalleh, W.; Kirkan, B.; Sarikurkcu, C. Antioxidant potential and phenolic composition of extracts from Stachys tmolea: An endemic plant from Turkey. Ind. Crops Prod. 2019, 127, 212–216. [Google Scholar] [CrossRef]
- Benabderrahim, M.A.; Sarikurkcu, C.; Elfalleh, W.; Ozer, M.S.; Ceylan, O. Phenolic composition and biological activities of Turkish endemic plant: Stachys cretica subsp. kutahyensis. S. Afr. J. Bot. 2021, 138, 124–128. [Google Scholar] [CrossRef]
- Kumar, D.; Bhat, Z.A. Apigenin 7-glucoside from Stachys tibetica Vatke and its anxiolytic effect in rats. Phytomedicine 2014, 21, 1010–1014. [Google Scholar] [CrossRef]
- Madunić, J.; Madunić, I.V.; Gajski, G.; Popić, J.; Garaj-Vrhovac, V. Apigenin: A dietary flavonoid with diverse anticancer properties. Cancer Lett. 2018, 413, 11–22. [Google Scholar] [CrossRef]
- Bouasla, I.; Hamel, T.; Barour, C.; Bouasla, A.; Hachouf, M.; Bouguerra, O.M.; Messarah, M. Evaluation of solvent influence on phytochemical content and antioxidant activities of two Algerian endemic taxa: Stachys marrubiifolia Viv. and Lamium flexuosum Ten. (Lamiaceae). Eur. J. Integr. Med. 2021, 42, 101267. [Google Scholar] [CrossRef]
- Taylor, K.; Rowland, P. Biological flora of the British Isles: Stachys palustris L. J. Ecol. 2011, 99, 1081–1090. [Google Scholar] [CrossRef]
- Kukić, J.; Petrović, S.; Niketić, M. Antioxidant activity of four endemic Stachys taxa. Biol. Pharm. Bull. 2006, 29, 725–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saravanakumar, K.; Park, S.; Mariadoss, A.V.A.; Sathiyaseelan, A.; Veeraraghavan, V.P.; Kim, S.; Wang, M.H. Chemical composition, antioxidant, and anti-diabetic activities of ethyl acetate fraction of Stachys riederi var. japonica (Miq.) in streptozotocin-induced type 2 diabetic mice. Food Chem. Toxicol. 2021, 155, 112374. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Yadav, M.; Kumar, S.; Sharma, D.; Yadav, J.P. In vitro antioxidant activities and GC-MS analysis of different solvent extracts of Acacia nilotica leaves. Indian J. Pharm. Sci. 2018, 80, 892–902. [Google Scholar] [CrossRef]
- Khan, S.; Nazir, M.; Saleem, H.; Raiz, N.; Saleem, M.; Anjum, S.M.M.; Zengin, G.; Mukhtar, M.; Tiusif, M.I.; Mahomoodally, F.M.; et al. Valorization of the antioxidant, enzyme inhibition and phytochemical propensities of Berberis calliobotrys Bien. ex Koehne: A multifunctional approach to probe for bioactive natural products. Ind. Crops Prod. 2019, 141, 111693. [Google Scholar] [CrossRef]
- Bahadori, M.B.; Kirkan, B.; Sarikurkcu, C. Phenolic ingredients and therapeutic potential of Stachys cretica subsp. smyrnaea for the management of oxidative stress, Alzheimer’s disease, hyperglycemia, and melasma. Ind. Crops Prod. 2019, 127, 82–87. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Kocak, M.S.; Uren, M.C.; Calapoglu, M.; Tepe, A.S. Potential sources for the management global health problems and oxidative stress: Stachys byzantina and S. iberica subsp. iberica var. densipilosa. Eur. J. Integr. Med. 2016, 8, 631–637. [Google Scholar] [CrossRef]
- Kokhdan, E.P.; Sadeghi, H.; Ghafoori, H.; Sadeghi, H.; Danaei, N.; Javadian, H.; Aghamaali, M.R. Cytotoxic effect of methanolic extract, alkaloid and terpenoid fractions of Stachys pilifera against HT-29 cell line. Res. Pharm. Sci. 2018, 13, 404. [Google Scholar]
- Khanavi, M.; Manayi, A.; Lotfi, M.; Abbasi, R.; Majdzadeh, M.; Ostad, S.N. Investigation of cytotoxic activity in four Stachys species from Iran. Iran. J. Pharm. Res. IJPR 2012, 11, 589. [Google Scholar]
- Gullett, N.P.; Amin, A.R.; Bayraktar, S.; Pezzuto, J.M.; Shin, D.M.; Khuri, F.R.; Aggarwal, B.B.; Surh, Y.-U.; Kucuk, O. Cancer prevention with natural compounds. Semin. Oncol. 2010, 37, 258–281. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [Green Version]
- Aiello, P.; Sharghi, M.; Mansourkhani, S.M.; Ardekan, A.P.; Jouybari, L.; Daraei, N.; Peiro, K.; Mohamadian, S.; Rezaei, M.; Heidari, M.; et al. Medicinal plants in the prevention and treatment of colon cancer. Oxid. Med. Cell. Longev. 2019, 2019, 2075614. [Google Scholar] [CrossRef] [Green Version]
- Bystrická, J.; Vollmannová, A.; Margitanová, E. Dynamics of polyphenolics formation in different plant parts and different growth phases of selected buckwheat cultivars. Acta Agric. Slov. 2010, 95, 225. [Google Scholar] [CrossRef]
- Ochmian, I.; Błaszak, M.; Lachowicz, S.; Piwowarczyk, R. The impact of cultivation systems on the nutritional and phytochemical content, and microbiological contamination of highbush blueberry. Sci. Rep. 2020, 10, 16696. [Google Scholar] [CrossRef]
- Kapusta, I.; Cebulak, T.; Oszmiański, J. Characterization of polish wines produced from the interspecific hybrid grapes grown in south-east Poland. Eur. Food Res. Technol. 2018, 244, 441–455. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Nakai, M.; Fukui, Y.; Asami, S.; Toyoda-Ono, Y.; Iwashita, T.; Shibata, H.; Mitsunaga, T.; Hashimoto, F.; Kiso, Y. Inhibitory effects of oolong tea polyphenols on pancreatic lipase in vitro. J. Agric. Food Chem. 2005, 53, 4593–4598. [Google Scholar] [CrossRef]
- Podsedek, A.; Majewska, I.; Redzynia, M.; Sosnowska, D.; Koziołkiewicz, M. In vitro inhibitory effect on digestive enzymes and antioxidant potential of commonly consumed fruits. J. Agric. Food Chem. 2014, 62, 4610–4617. [Google Scholar] [CrossRef]
- Nickavar, B.; Yousefian, N. Evaluation of α-amylase inhibitory activities of selected antidiabetic medicinal plants. J. Verbrauch. Lebensm. 2011, 6, 191–195. [Google Scholar] [CrossRef]
| Identified Compounds | Rr [min] | Δd [nm] | [M-H]−/MS-MS | Ref. |
|---|---|---|---|---|
| Hydrolysable tannins | ||||
| Grandinin | 2.49 | 203 | 1065/975/931/301 | [8,9,10,11,12,13,14,15,16,17,18,19] |
| Grandinin isomer | 2.71 | 227 | 1065/975/931/301 | [8,9,10,11,12,13,14,15,16,17,18,19] |
| Grandinin isomer | 2.77 | 203 | 1065/975/931/301 | [8,9,10,11,12,13,14,15,16,17,18,19] |
| Castalagin/vescalagin isomer | 2.83 | 224 | 933/915/631/301 | [8,9,10,11,12,13,14,15,16,17,18,19] |
| Vescalagin | 2.93 | 224 | 933/915/631/613/569/467/301 | [8,9,10,11,12,13,14,15,16,17,18,19] |
| Castalagin/vescalagin isomer | 3.01 | 224 | 933/915/613/569/467/301 | [8,9,10,11,12,13,14,15,16,17,18,19] |
| Castalagin/vescalagin (HHDP–NHTP–glucose) isomer | 3.25 | 224 | 933/915/889/871/631/613/467/301 | [8,9,10,11,12,13,14,15,16,17,18,19] |
| Pedunculagin isomer (diHHDP-glucose) | 3.36 | 232 | 783/481/301 | [8,9,10,11,12,13,14,15,16,17,18,19] |
| Castalagin/vescalagin isomer | 3.43 | 204 | 933/915/889/631/301 | [8,9,10,11,12,13,14,15,16,17,18,19] |
| Cocciferin d2 isomer (HHDP-NHTP-glucose-galloyldiHHDP-glucose) | 3.59 | 224 | 933/915/631/301 | [8] |
| Castalagin/vescalagin isomer | 3.69 | 204 | 933/631/461/301 | [8,9,10,11,12,13,14,15,16,17,18,19] |
| Pedunculagin isomer (diHHDP-glucose) | 3.95 | 204 | 783/481/301 | [8,9,10,11,12,13,14,15,16,17,18,19] |
| Castalagin/vescalagin isomer | 4.02 | 208 | 933/915/631/301 | [8,9,10,11,12,13,14,15,16,17,18,19] |
| Pedunculagin isomer (diHHDP-glucose) | 4.24 | 230, 275 | 783/481/301 | [8,9,10,11,12,13,14,15,16,17,18,19] |
| Castalagin/Vescalagin isomer | 4.29 | 245 | 933/915/871/613/569/301 | [8,9,10,11,12,13,14,15,16,17,18,19] |
| Casuarictin (galloyl-DiHHDP-glucose) | 4.45 | 205 | 935/783/633/301 | [8,9,10,11,12,13,14,15,16,17,18,19] |
| Cocciferin d2 isomer (HHDP-NHTP-glucose-galloyldiHHDP-glucose) | 4.51 | 223 | 933/915/631/390/301 | [8] |
| Chebulanin | 4.54 | 205 | 651/481/463/337/319/275/169 | [8,9,10,11,12,13,14,15,16,17,18,19] |
| Castalagin/vescalagin isomer | 4.58 | 212/270 | 933/631/569/301 | [8,9,10,11,12,13,14,15,16,17,18,19] |
| Chebulanin | 4.60 | 206 | 651/481/463/337/319/275/169 | [9,11,12,13,14,15,16,17,18,19] |
| Sanguiin H-10 isome (digalloyltriHHDPdiglucose) | 4.87 | 315 | 1567/1265/1103/933/631/481/301 | [20,21] |
| Castalagin/vescalagin isomer | 4.93 | 216 | 933/633/481/301 | [8] |
| Castalagin/Vescalagin isomer | 5.05 | 276/353 | 933/915/871/631/613/467/301 | [8] |
| Casuarinin (diHHDP-galloyl-glucose) | 5.18 | 217 | 935/783/633/481/301 | [8,9,10,11,12,13,14,15,16,17,18,19] |
| Castalagin/vescalagin isomer | 5.33 | 220 | 933/783/633/434/301 | [8] |
| Pedunculagin (diHHDP-glucose) | 5.43 | 313 | 783/707/633/481/301 | [8,9,10,11,12,13,14,15,16,17,18,19] |
| Sanguiin H-10 isomer (digalloyltriHHDPdiglucose) | 5.51 | 240 | 1567/783/631/481/301 | [20,21] |
| Roburin E | 5.63 | 230 | 1064/301 | [8,9,10,11,12,13,14,15,16,17,18,19] |
| Vescalagin isomer | 5.91 | 218 | 933/631/467/301 | [8,9,10,11,12,13,14,15,16,17,18,19] |
| Castalagin isomer | 6.16 | 222 | 933/631/467/301 | [8,9,10,11,12,13,14,15,16,17,18,19] |
| Geraniin isomer | 6.25 | 209 | 951/933/633/301/257 | [8,9,10,11,12,13,14,15,16,17,18,19] |
| Casuarinin/potentilin (galloyl-diHHDP-glucose) | 6.51 | 279 | 935/783/633/301 | [8,9,10,11,12,13,14,15,16,17,18,19] |
| Tellimagrandin I (digalloyl-HHDP-glucose) | 6.53 | 218/277 | 785/615/483/301 | [8,9,10,11,12,13,14,15,16,17,18,19] |
| Pentagalloyl-glucose | 6.95 | 280 | 939/787/769/617/599/447 | [8,9,10,11,12,13,14,15,16,17,18,19] |
| Tellimagrandin I isomer (digalloyl-HHDP-glucose) | 6.99 | 218/277 | 785/615/483/301 | [8,9,10,11,12,13,14,15,16,17,18,19] |
| Trigalloyl-HHDP-glucose | 7.07 | 280 | 937/767/635/465/301 | [8,14] |
| Trigalloyl-HHDP-glucose | 7.74 | 280 | 937/767/635/465/301 | [8,14] |
| Trigalloyl-HHDP-glucose | 7.80 | 281 | 937/767/635/465/301 | [8,14] |
| Trigalloyl-HHDP-glucose | 7.93 | 281 | 937/767/635/465/301 | [8,14] |
| Chebulagic acid (galloyl-chebuloyl-HHDP-glucose) | 8.00 | 220/272 | 953/785/633/463/337/301/169 | [14] |
| Phenylethanoid glycosides | ||||
| Echinacoside | 6.27 | 329 | 785/623/461/161 | [22,23] |
| Betonyoside E | 6.32 | 326 | 785/639//621/609/193/161 | [22,23] |
| Stachysoside A | 6.38 | 272 | 755/623/461/593/179/161 | [24] |
| B-OH-Forsythoside B methylether | 7.29 | 330 | 785/755/623/347/161 | [22,23] |
| Stachysoside A | 7.41 | 330 | 755/623/593/461/179/161 | [24] |
| Isoacteoside (isoverbascoside) | 7.60 | 330 | 623/461/161 | [2,25,26,27] |
| B-OH-Forsythoside B methylether | 7.80 | 323 | 785/755/623/347/161 | [22,23] |
| Forsythoside B isomer | 7.91 | 330 | 755/623/607/593/461/161 | [22] |
| Forsythoside B isomer | 7.97 | 325 | 755/623/593/461/161 | [22] |
| Forsythoside B isomer | 8.07 | 326 | 755/623/461/447/161 | [24] |
| Cistanoside A | 8.22 | 328 | 799/637/623/475/315 | [22,23] |
| Alyssonoside | 8.34 | 329 | 769/593/575/447/315/161 | [24] |
| Alyssonoside isomer | 8.59 | 329 | 769/593/575/447/315/161 | [24] |
| Martynoside | 9.34 | 283 | 651/475/457/328/161 | [24] |
| Samioside | 10.02 | 329 | 755/593/461/315/161 | [24] |
| Leucoseptoside A | 10.29 | 313 | 637/461/315/193/175/161 | [24,28] |
| Stachysoside E | 10.43 | 319 | 669/625/583/380/264 | [24] |
| Anthocyanins | ||||
| Delphinidin 3-O-glucoside | 3.65 | 520 | 465/303 | [29] |
| Malvidin 3-O-diglucoside | 4.51 | 525 | 665/493/331 | [29] |
| Cyanidin 3-O-glucoside | 5.06 | 517 | 449/287 | [29] |
| Malvidin 3-O-acetylglucoside | 6.00 | 525 | 535/331 | [29] |
| Flavan-3-ols | ||||
| (-)Epicatchin | 4.83 | 280 | 289 | [8] |
| Phenolic acid | ||||
| Ellagic acid glucoside | 5.74 | 370 | 463/301 | [25] |
| Ellagic acid pentoside | 6.05 | 254/362 | 433/301 | [25] |
| Ellagic acid | 7.52 | 255/365 | 301 | [25] |
| 3,4-dicaffeoyl quinic acid | 8.91 | 324 | 515/353/191/179 | [25] |
| 3,5-dicaffeoyl quinic acid | 9.04 | 324 | 515/353/191/179 | [25] |
| 4,5-dicaffeoyl quinic acid | 11.07 | 324 | 515/353/191/179 | [25] |
| Flavonols | ||||
| Kaempferol hexose glucuronide | 10.85 | 343 | 623/285 | [30] |
| Flavones | ||||
| Chrysoeriol acetyl-allopyranosyl-glucopyranoside | 5.82 | 269/332 | 677/299 | [31,32] |
| Luteolin-6-C-galactoside | 6.59 | 269/349 | 447/357/327/297/285 | [33,34] |
| Luteolin-6-C-glucoside | 6.74 | 267/347 | 447/357/327/299/285 | [33,34] |
| Apigenin-6-C-galactoside | 7.29 | 268/336 | 431/341/311/283/269 | [35] |
| Apigenin-6-C-glucoside | 7.43 | 269/336 | 431/341/311/283/269 | [35] |
| Apigenin 7-O-β-D-(6-p-coumaroyl)-glucopyranoside | 8.26 | 325 | 577/432/407/269 | [31,36] |
| Apigenin acetyl-allosyl-glucoside | 9.01 | 332 | 635/269 | [26] |
| Chrysoeriol 7-O-acetylallosylglucoside | 10.84 | 311 | 667/299 | [25] |
| 4′-O-methylisoscutellarein-diacetyl-allosyl-glucopyranoside | 8.62 | 346 | 707/299 | [25,26] |
| 4′-O-methylisoscutellarein-acetyl-allosyl-glucopyranoside | 8.87 | 329 | 665/485/299 | [25,26] |
| Isoscutellarein-acetylallosyl-(glucopyranoside)apiose | 9.19 | 276/330 | 651/429/285 | [25,26] |
| Isoscutellarein-acetylallosyl-(glucopyranoside)apiose isommer | 9.32 | 326 | 651/637/429/285 | [25,26] |
| Isoscutellarein-acetylallosyl-(glucopyranoside)apiose isommer | 9.44 | 329 | 651/607/429/285 | [25,26] |
| Isoscutellarein-acetylallosyl-(glucopyranoside)apiose isommer | 9.49 | 329 | 651/607/429/285 | [25,26] |
| Isoscutellarein-acetylallosyl-(glucopyranoside)apiose isommer | 9.63 | 329 | 651/285 | [25,26] |
| Isoscutellarein-acetylallosyl-(glucopyranoside)apiose isommer | 9.72 | 329 | 651/285 | [25,26] |
| Isoscutellarein-acetylallosyl-(glucopyranoside)apiose isommer | 9.79 | 314 | 651/285 | [25,26] |
| Isoscutellarein-acetylallosyl-glucopyranoside | 9.85 | 328 | 651/285 | [25,26] |
| Isoscutellarein- acetylallosyl-glucopyranoside isommer | 10.10 | 329 | 651/285 | [25,26] |
| 4′-O-methylisoscutellarein-acetyl-allosyl-glucopyranoside isomer | 11.23 | 277/305 | 665/299 | [25,26] |
| Polyphenolic Compounds | Flowers | Leaves | Stems | Roots |
|---|---|---|---|---|
| Hydrolysable Tannins (HT) | ||||
| Grandinin | 172.25 ± 2.07a a | 76.69 ± 0.92b | 62.67 ± 0.75c | 32.34 ± 0.39d |
| Grandinin isommer | 399.62 ± 4.80b | 533.26 ± 6.40a | 230.27 ± 2.76c | nd |
| Grandinin isommer | 265.51 ± 3.19a | nd b | 71.98 ± 0.86c | 120.43 ± 1.45b |
| Castalagin/vescalagin isomer | 214.78 ± 2.58b | 494.02 ± 5.93a | 212.20 ± 2.55b | nd |
| Vescalagin | 2370.75 ± 18.97a | 1528.04 ± 12.22b | 1236.86 ± 9.89c | 403.8 ± 3.23d |
| Castalagin/vescalagin isomer | 36.41 ± 0.44c | 224.02 ± 2.69a | 95.17 ± 1.14b | nd |
| Castalagin/vescalagin (HHDP–NHTP–glucose) isomer | 500.80 ± 6.01a | 129.82 ± 1.56c | 220.30 ± 2.64b | nd |
| Pedunculagin isomer (diHHDP-glucose) | nd | nd | nd | 78.36 ± 0.94a |
| Castalagin/vescalagin isomer | 945.11 ± 1.89b | 1041.14 ± 2.08a | 617.74 ± 1.24c | 51.56 ± 0.62d |
| Cocciferin d2 isomer (HHDP-NHTP-glucose-galloyldiHHDP-glucose) | 1552.66 ± 18.63a | 1388.25 ± 16.66b | 867.36 ± 10.41c | 230.08 ± 2.76d |
| Castalagin/vescalagin isomer | 636.42 ± 7.64b | 990.62 ± 11.89a | 572.37 ± 6.87c | 37.70 ± 0.45d |
| Pedunculagin isomer (diHHDP-glucose) | 63.94 ± 0.77b | 80.22 ± 0.96a | 27.38 ± 0.33c | 2.59 ± 0.03d |
| Castalagin/vescalagin isomer | 162.72 ± 1.95c | 298.50 ± 3.58a | 137.30 ± 1.65b | 16.72 ± 0.20c |
| Pedunculagin isomer (diHHDP-glucose) | 110.36 ± 1.32b | 143.92 ± 1.73a | 47.51 ± 0.57c | 6.05 ± 0.07d |
| Castalagin/Vescalagin isomer | 100.25 ± 1.20b | 117.42 ± 1.41a | 57.71 ± 0.69c | 13.61 ± 0.16d |
| Casuarictin (galloyl-diHHDP-glucose) | 33.64 ± 0.40b | 85.27 ± 1.02a | 22.76 ± 0.27c | 34.51 ± 0.41b |
| Cocciferin d2 isomer (HHDP-NHTP-glucose-galloyldiHHDP-glucose) | 21.33 ± 0.26b | 29.19 ± 0.35a | 8.57 ± 0.10c | nd |
| Chebulanin | nd | nd | nd | 2.62 ± 0.03a |
| Castalagin/vescalagin isomer | 40.36 ± 0.48a | nd | nd | nd |
| Chebulanin | nd | nd | nd | 12.15 ± 0.15a |
| Sanguiin H-10 isomer (digalloyltriHHDPdiglucose) | 4.73 ± 0.06c | 9.11 ± 0.11b | 3.84 ± 0.05cd | 18.68 ± 0.22a |
| Castalagin/vescalagin isomer | 27.11 ± 0.33a | 28.71 ± 0.34a | 17.43 ± 0.21b | 4.79 ± 0.06c |
| Castalagin/Vescalagin isomer | 18.71 ± 0.22a | 1.32 ± 0.02b | 0.95 ± 0.01b | nd |
| Casuarinin (diHHDP-galloyl-glucose) | 38.79 ± 0.47a | 18.53 ± 0.22b | 7.82 ± 0.09c | 3.42 ± 0.04d |
| Castalagin/vescalagin isomer | 18.55 ± 0.22a | 15.14 ± 0.18b | 8.00 ± 0.10c | 5.39 ± 0.06d |
| Pedunculagin (diHHDP-glucose) | 5.76 ± 0.07b | 11.64 ± 0.14a | 5.43 ± 0.07b | 1.47 ± 0.02c |
| Sanguiin H-10 isomer (digalloyltriHHDPdiglucose) | 8.20 ± 0.10b | 45.22 ± 0.54a | 3.68 ± 0.04c | nd |
| Roburin E | 27.43 ± 0.33a | 20.53 ± 0.25b | 4.11 ± 0.05c | nd |
| Vescalagin isomer | 5.48 ± 0.07c | 8.96 ± 0.11a | 6.31 ± 0.08b | nd |
| Castalagin isomer | 26.95 ± 0.32a | 14.71 ± 0.18b | 4.85 ± 0.06c | 2.54 ± 0.03d |
| Geraniin isomer | 1.63 ± 0.02b | 5.28 ± 0.06a | 4.57 ± 0.05a | nd |
| Casuarinin/potentilin (galloyl-diHHDP-glucose) | nd | 1.39 ± 0.02b | 3.85 ± 0.05a | 1.59 ± 0.02b |
| Tellimagrandin I (digalloyl-HHDP-glucose) | 5.38 ± 0.06a | nd | nd | 2.32 ± 0.03b |
| Pentagalloyl-glucose | 1.83 ± 0.02a | nd | nd | nd |
| Tellimagrandin I isomer (digalloyl-HHDP-glucose) | 5.28 ± 0.06a | nd | nd | nd |
| Trigalloyl-HHDP-glucose | 8.55 ± 0.10a | 4.79 ± 0.06b | 1.94 ± 0.02c | nd |
| Trigalloyl-HHDP-glucose | 6.40 ± 0.08a | 1.30 ± 0.02b | 0.75 ± 0.01c | nd |
| Trigalloyl-HHDP-glucose | 6.23 ± 0.07a | 3.23 ± 0.04b | 0.61 ± 0.01c | nd |
| Trigalloyl-HHDP-glucose | 25.87 ± 0.31a | nd | nd | nd |
| Chebulagic acid (galloyl-chebuloyl-HHDP-glucose) | 75.04 ± 0.90a | 3.33 ± 0.04b | 1.64 ± 0.02c | nd |
| Phenylethanoid glycosides (PhG) | ||||
| Echinacoside | nd | nd | nd | 1.11 ± 0.01a |
| Betonyoside E | 1.53 ± 0.02a | 1.44 ± 0.02a | 0.57 ± 0.01bc | 1.10 ± 0.01b |
| Stachysoside A | 6.62 ± 0.08a | nd | nd | nd |
| B-OH-Forsythoside B methylether | nd | nd | nd | 15.01 ± 0.18a |
| Stachysoside A | nd | nd | nd | 30.72 ± 0.37a |
| Isoacteoside (isoverbascoside) | nd | nd | nd | 114.26 ± 1.37a |
| B-OH-Forsythoside B methylether | nd | nd | nd | 1.04 ± 0.01a |
| Forsythoside B | nd | nd | 5.76 ± 0.07b | 10.84 ± 0.13a |
| Forsythoside B | nd | nd | nd | 1.75 ± 0.02a |
| Forsythoside B isomer | nd | nd | nd | 2.69 ± 0.03a |
| Cistanoside A | nd | nd | nd | 1.33 ± 0.02a |
| Alyssonoside | nd | nd | nd | 13.05 ± 0.16a |
| Alyssonoside isomer | nd | nd | nd | 12.45 ± 0.15a |
| Martynoside | nd | nd | nd | 4.17 ± 0.05a |
| Samioside | nd | nd | nd | 0.92 ± 0.01a |
| Leucoseptoside A | nd | 1.76 ± 0.02a | nd | nd |
| Stachysoside E | nd | 1.70 ± 0.02a | nd | nd |
| Anthocyanins (ANT) | ||||
| Delphinidin 3-O-glucoside | 3.36 ± 0.04a | nd | nd | nd |
| Malvidin 3-O-diglucoside | 9.99 ± 0.12a | nd | nd | nd |
| Cyanidin 3-O-glucoside | 3.07 ± 0.04a | nd | nd | nd |
| Malvidin 3-O-acetylglucoside | 3.57 ± 0.04a | nd | nd | nd |
| Flavan-3-ols (F3O) | ||||
| (-)-Epicatchin | 31.95 ± 0.38a | 4.81 ± 0.06c | 13.69 ± 0.16b | nd |
| Phenolic acid (PA) | ||||
| Ellagic acid glucoside | nd | 4.24 ± 0.05a | 0.71 ± 0.01b | nd |
| Ellagic acid pentoside | 5.35 ± 0.06a | 3.56 ± 0.04b | 0.83 ± 0.01c | 0.84 ± 0.01c |
| Ellagic acid | 48.45 ± 0.58a | 26.35 ± 0.32b | 7.18 ± 0.09c | 3.16 ± 0.04d |
| 3,4-dicaffeoyl quinic acid | 0.64 ± 0.01c | 1.41 ± 0.02b | 0.38 ± 0.00c | 21.85 ± 0.26a |
| 3,5-dicaffeoyl quinic acid | 1.98 ± 0.02b | 2.21 ± 0.03a | 1.16 ± 0.01c | 2.03 ± 0.02b |
| 4,5-dicaffeoyl quinic acid | 0.89 ± 0.01a | 0.25 ± 0.00a | 0.30 ± 0.00a | 0.18 ± 0.00a |
| Flavonols (FL) | ||||
| Kaempferol hexose glucuronide | 7.36 ± 0.09a | nd | nd | nd |
| Flavones (FLN) | ||||
| Chrysoeriol acetyl-allopyranosyl-glucopyranoside | nd | 3.86 ± 0.05a | 3.29 ± 0.04a | 1.02 ± 0.01b |
| Luteolin-6-C-galactoside | 22.57 ± 0.27c | 462.91 ± 5.55a | 72.75 ± 0.87b | 0.46 ± 0.01d |
| Luteolin-6-C-glucoside | 45.72 ± 0.55c | 267.68 ± 3.21a | 40.74 ± 0.49b | nd |
| Apigenin-6-C-galactoside | 17.59 ± 0.21c | 237.74 ± 2.85a | 39.72 ± 0.48b | nd |
| Apigenin-6-C-glucoside | 14.16 ± 0.17c | 394.48 ± 4.73a | 65.50 ± 0.79b | nd |
| Apigenin 7-O-β-D-(6-p-coumaroyl)-glucopyranoside | 1.39 ± 0.02c | 2.70 ± 0.03a | 1.52 ± 0.02b | nd |
| Apigenin acetyl-allosyl-glucoside | nd | nd | nd | 3.72 ± 0.04a |
| Chrysoeriol 7-O-acetylallosylglucoside | nd | 2.01 ± 0.02a | nd | nd |
| 4′-O-methylisoscutellarein-diacetyl-allosyl-glucopyranoside | 36.59 ± 0.44a | 31.61 ± 0.38b | 17.67 ± 0.21c | nd |
| 4′-O-methylisoscutellarein-acetyl-allosyl-glucopyranoside | nd | nd | nd | 5.97 ± 0.07a |
| Isoscutellarein-acetylallosyl-(glucopyranoside)apiose | 0.37 ± 0.00c | 2.96 ± 0.04b | 0.80 ± 0.01c | 93.21 ± 1.12a |
| Isoscutellarein-acetylallosyl-(glucopyranoside)apiose isommer | nd | nd | nd | 2.91 ± 0.03a |
| Isoscutellarein-acetylallosyl-(glucopyranoside)apiose isommer | nd | nd | nd | 18.45 ± 0.22a |
| Isoscutellarein-acetylallosyl-(glucopyranoside)apiose isommer | nd | nd | nd | 11.22 ± 0.13a |
| Isoscutellarein-acetylallosyl-(glucopyranoside)apiose isommer | nd | nd | nd | 3.58 ± 0.04a |
| Isoscutellarein-acetylallosyl-(glucopyranoside)apiose isommer | nd | nd | nd | 4.42 ± 0.05a |
| Isoscutellarein-acetylallosyl-(glucopyranoside)apiose isommer | nd | nd | nd | 1.13 ± 0.01a |
| Isoscutellarein-acetylallosyl-glucopyranoside | nd | nd | nd | 5.18 ± 0.06a |
| Isoscutellarein-acetylallosyl-glucopyranoside isommer | nd | nd | nd | 2.69 ± 0.03a |
| 4′-O-methylisoscutellarein-acetyl-allosyl-glucopyranoside isomer | nd | nd | nd | 14.21 ± 0.17a |
| Procyjanidyny polimery (PP) | 336.61 ± 4.04b | 444.87 ± 5.34a | 102.29 ± 1.23d | 133.55 ± 1.6c |
| Degree of polymerization (DP) | 2.96c | 2.07d | 3.72b | 4.74a |
| The total sum of phenolic compounds | 8544.63b | 9252.11a | 4938.76c | 1622.94d |
| Parts of Plant | α-Amylase [IC50 (mg/mL)] | α-Glucosidase [IC50 (mg/mL)] | Pancreatic Lipase [IC50 (mg/mL)] | ABTS [mmol TE/g d.m.] | FRAP [mmol TE/g d.m.] |
|---|---|---|---|---|---|
| Leaves | 6.85 ± 0.11a a | 12.71 ± 0.20b | 27.46 ± 0.44a | 15.55 ± 0.25b | 7.25 ± 0.12a |
| Flowers | 8.14 ± 0.13b | 11.20 ± 0.18a | 46.23 ± 0.74c | 18.49 ± 0.30a | 5.57 ± 0.09b |
| Stems | 16.43 ± 0.26c | 19.09 ± 0.31c | 38.90 ± 0.62b | 7.81 ± 0.12c | 1.37 ± 0.02c |
| Roots | 26.34 ± 0.42d | 34.81 ± 0.56d | 47.94 ± 0.77c | 4.10 ± 0.07d | 0.39 ± 0.01d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lachowicz-Wiśniewska, S.; Pratap-Singh, A.; Kapusta, I.; Kruszyńska, A.; Rapak, A.; Ochmian, I.; Cebulak, T.; Żukiewicz-Sobczak, W.; Rubiński, P. Flowers and Leaves Extracts of Stachys palustris L. Exhibit Stronger Anti-Proliferative, Antioxidant, Anti-Diabetic, and Anti-Obesity Potencies than Stems and Roots Due to More Phenolic Compounds as Revealed by UPLC-PDA-ESI-TQD-MS/MS. Pharmaceuticals 2022, 15, 785. https://doi.org/10.3390/ph15070785
Lachowicz-Wiśniewska S, Pratap-Singh A, Kapusta I, Kruszyńska A, Rapak A, Ochmian I, Cebulak T, Żukiewicz-Sobczak W, Rubiński P. Flowers and Leaves Extracts of Stachys palustris L. Exhibit Stronger Anti-Proliferative, Antioxidant, Anti-Diabetic, and Anti-Obesity Potencies than Stems and Roots Due to More Phenolic Compounds as Revealed by UPLC-PDA-ESI-TQD-MS/MS. Pharmaceuticals. 2022; 15(7):785. https://doi.org/10.3390/ph15070785
Chicago/Turabian StyleLachowicz-Wiśniewska, Sabina, Anubhav Pratap-Singh, Ireneusz Kapusta, Angelika Kruszyńska, Andrzej Rapak, Ireneusz Ochmian, Tomasz Cebulak, Wioletta Żukiewicz-Sobczak, and Paweł Rubiński. 2022. "Flowers and Leaves Extracts of Stachys palustris L. Exhibit Stronger Anti-Proliferative, Antioxidant, Anti-Diabetic, and Anti-Obesity Potencies than Stems and Roots Due to More Phenolic Compounds as Revealed by UPLC-PDA-ESI-TQD-MS/MS" Pharmaceuticals 15, no. 7: 785. https://doi.org/10.3390/ph15070785
APA StyleLachowicz-Wiśniewska, S., Pratap-Singh, A., Kapusta, I., Kruszyńska, A., Rapak, A., Ochmian, I., Cebulak, T., Żukiewicz-Sobczak, W., & Rubiński, P. (2022). Flowers and Leaves Extracts of Stachys palustris L. Exhibit Stronger Anti-Proliferative, Antioxidant, Anti-Diabetic, and Anti-Obesity Potencies than Stems and Roots Due to More Phenolic Compounds as Revealed by UPLC-PDA-ESI-TQD-MS/MS. Pharmaceuticals, 15(7), 785. https://doi.org/10.3390/ph15070785