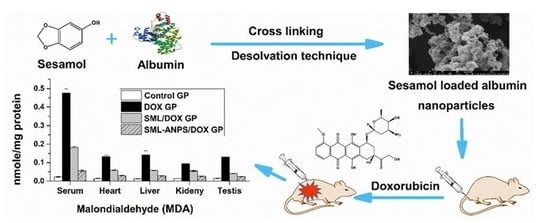
Sesamol Loaded Albumin Nanoparticles: A Boosted Protective Property in Animal Models of Oxidative Stress
Abstract
:1. Introduction
2. Results
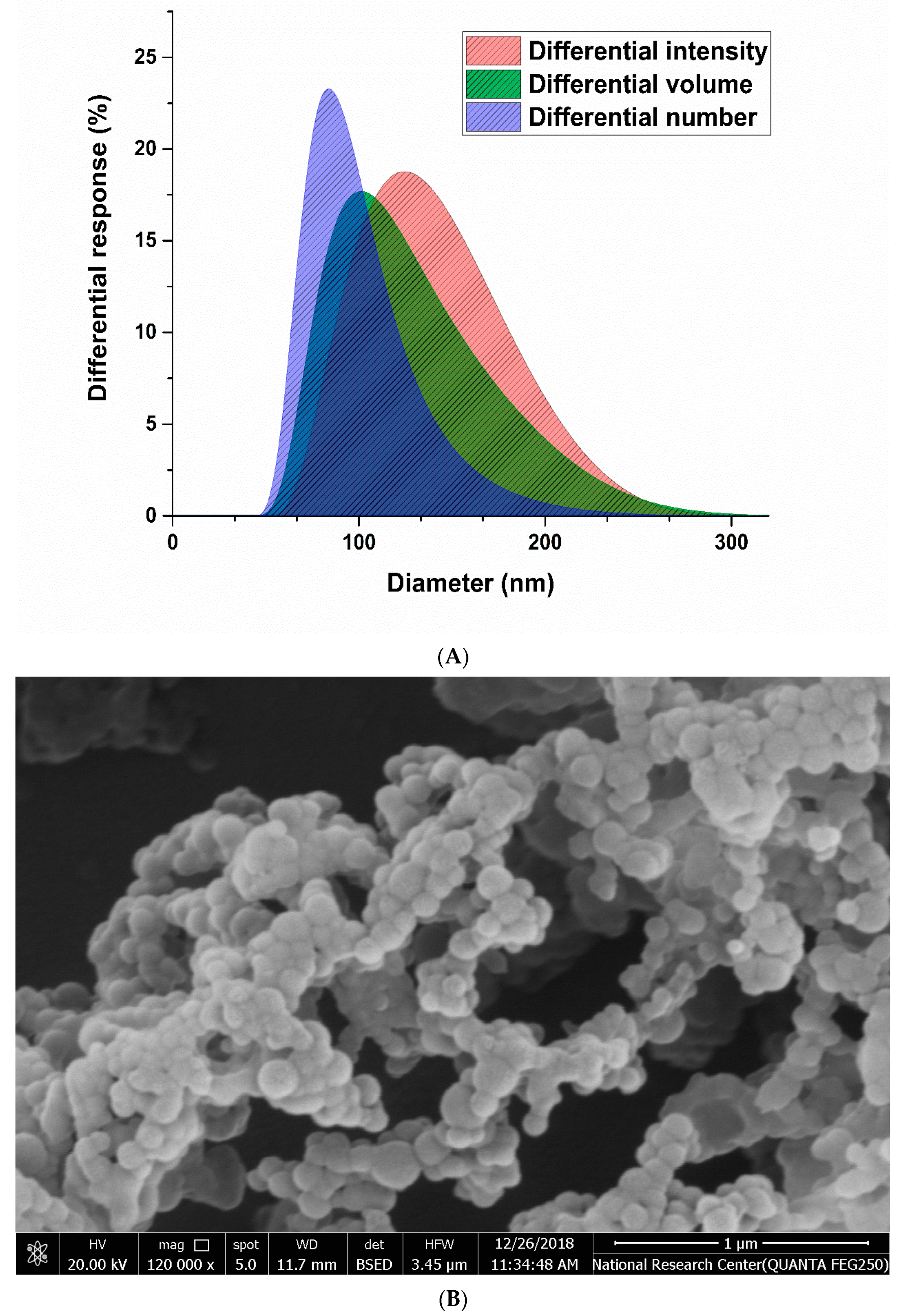
2.1. Characterization of SML-ANPs and Statistical Modeling
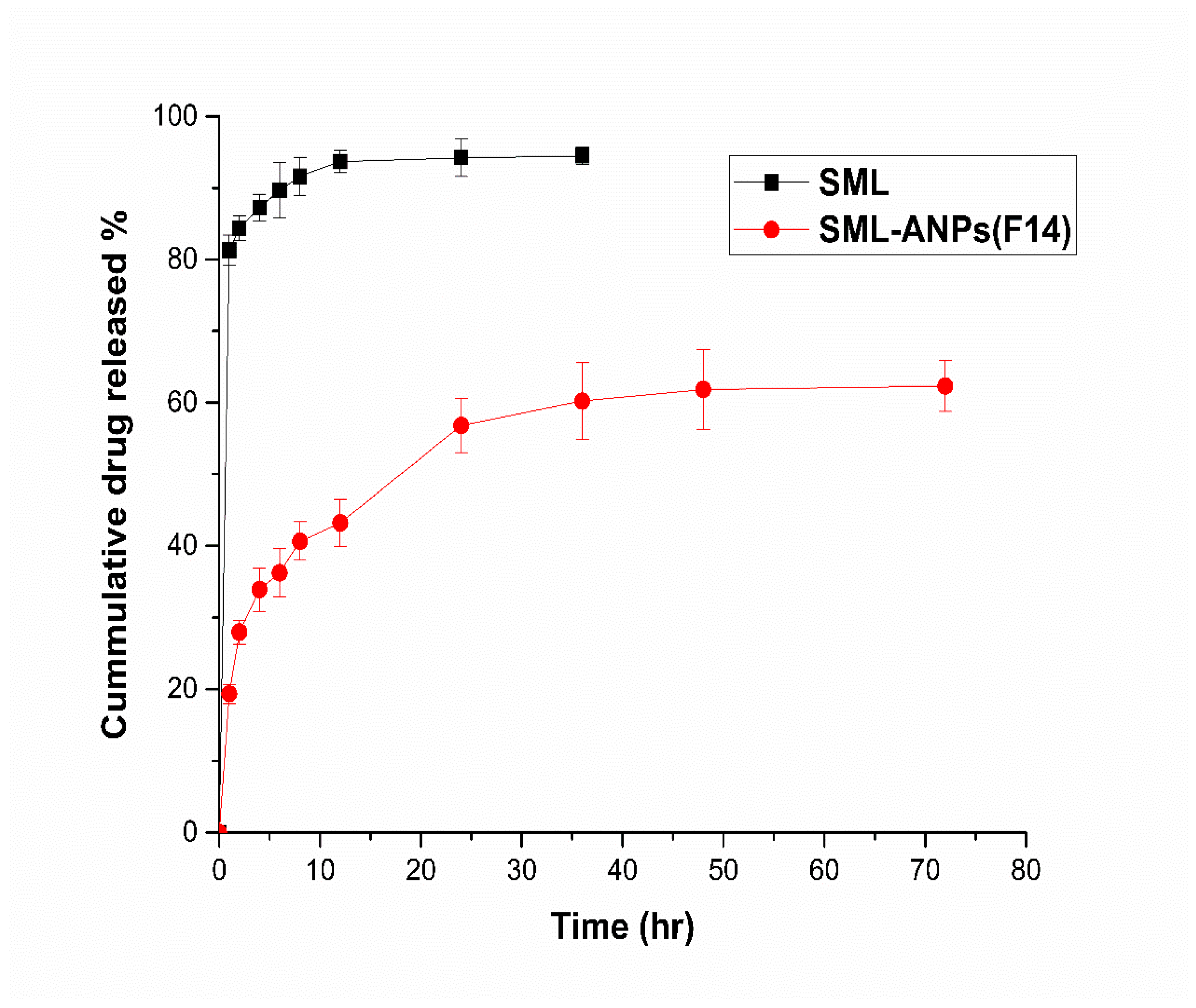
2.2. In Vitro Cumulative Release Study
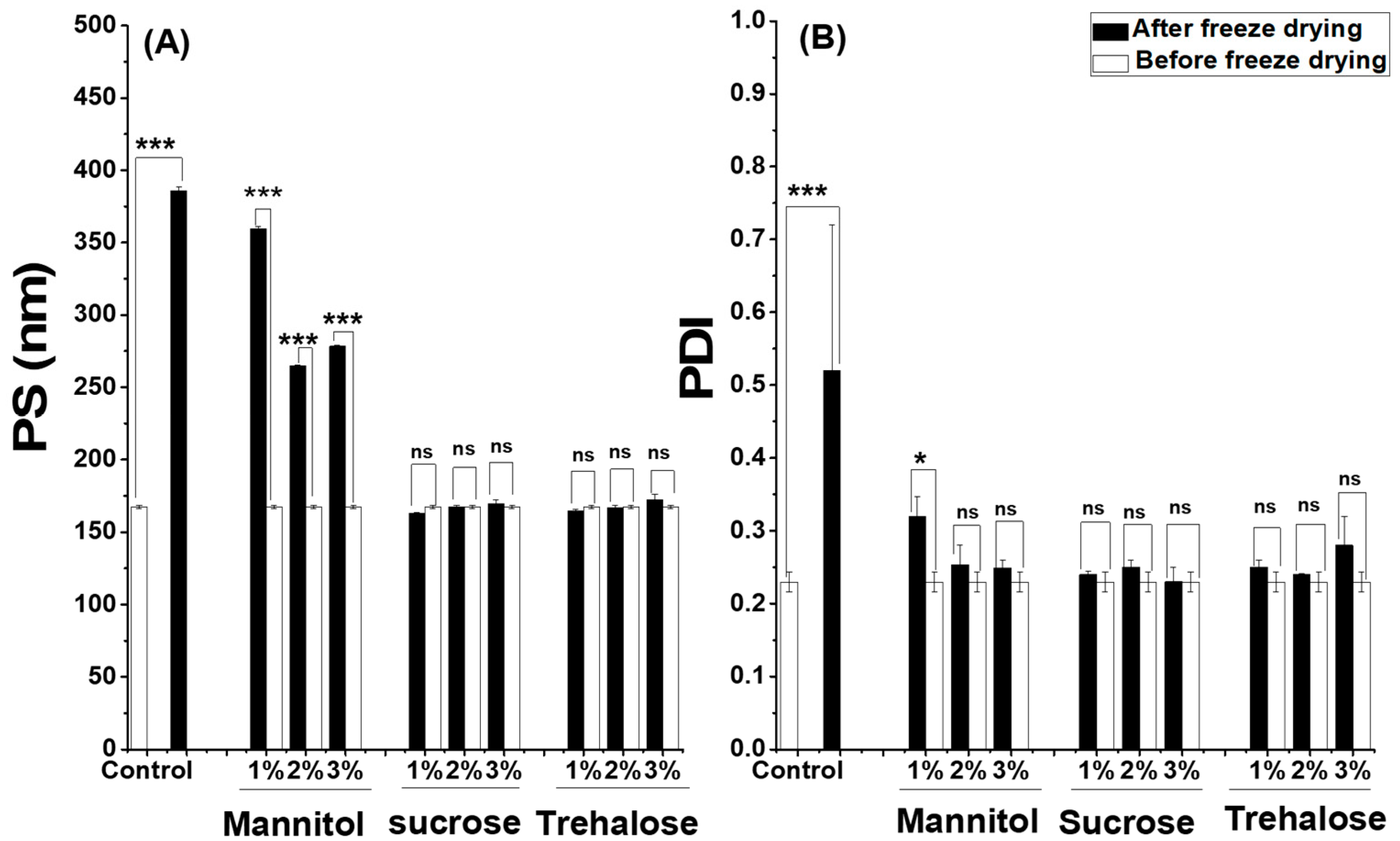
2.3. Freeze Drying and Stability Studies of SML-ANPs
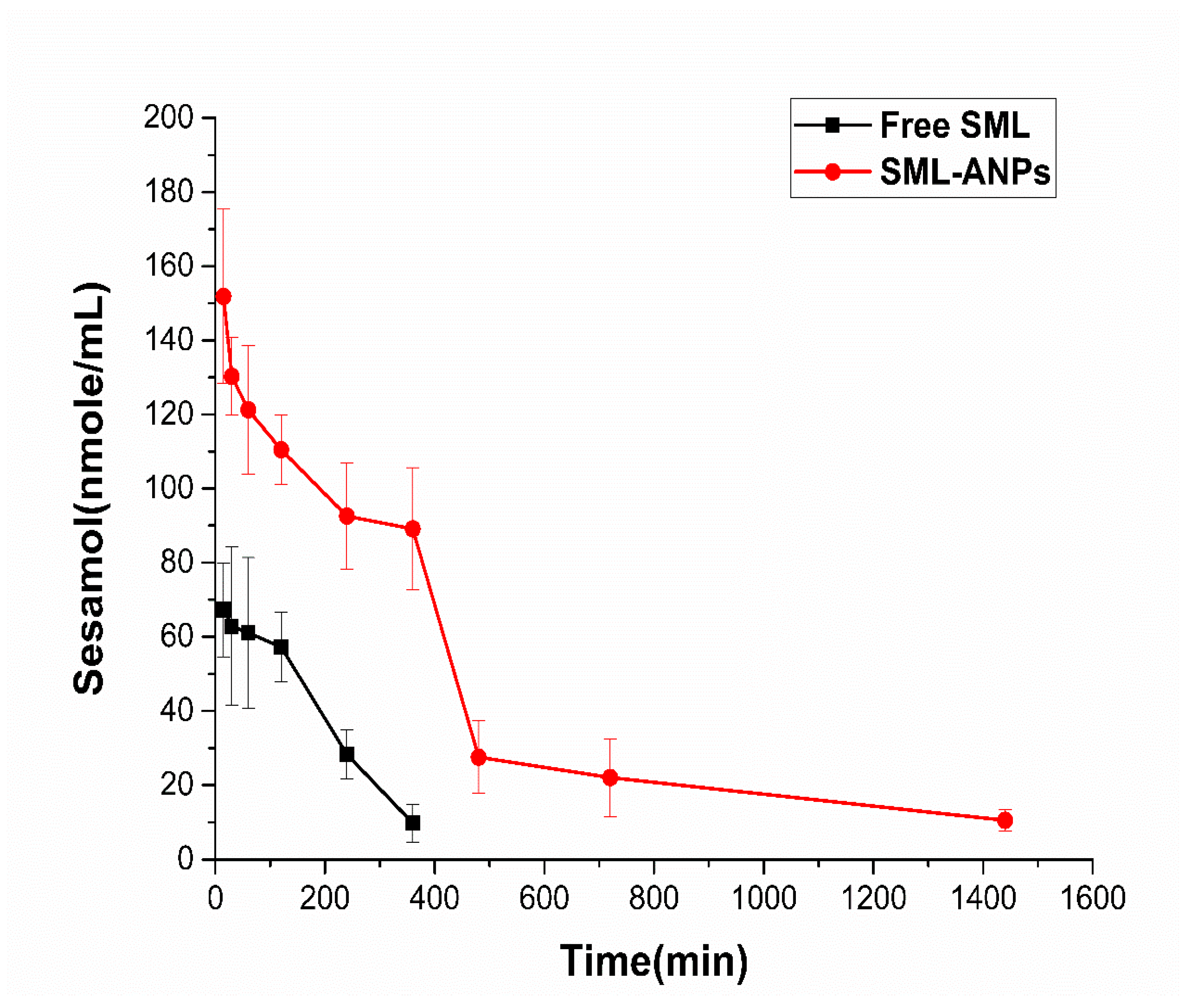
2.4. Pharmacokinetic Study Results
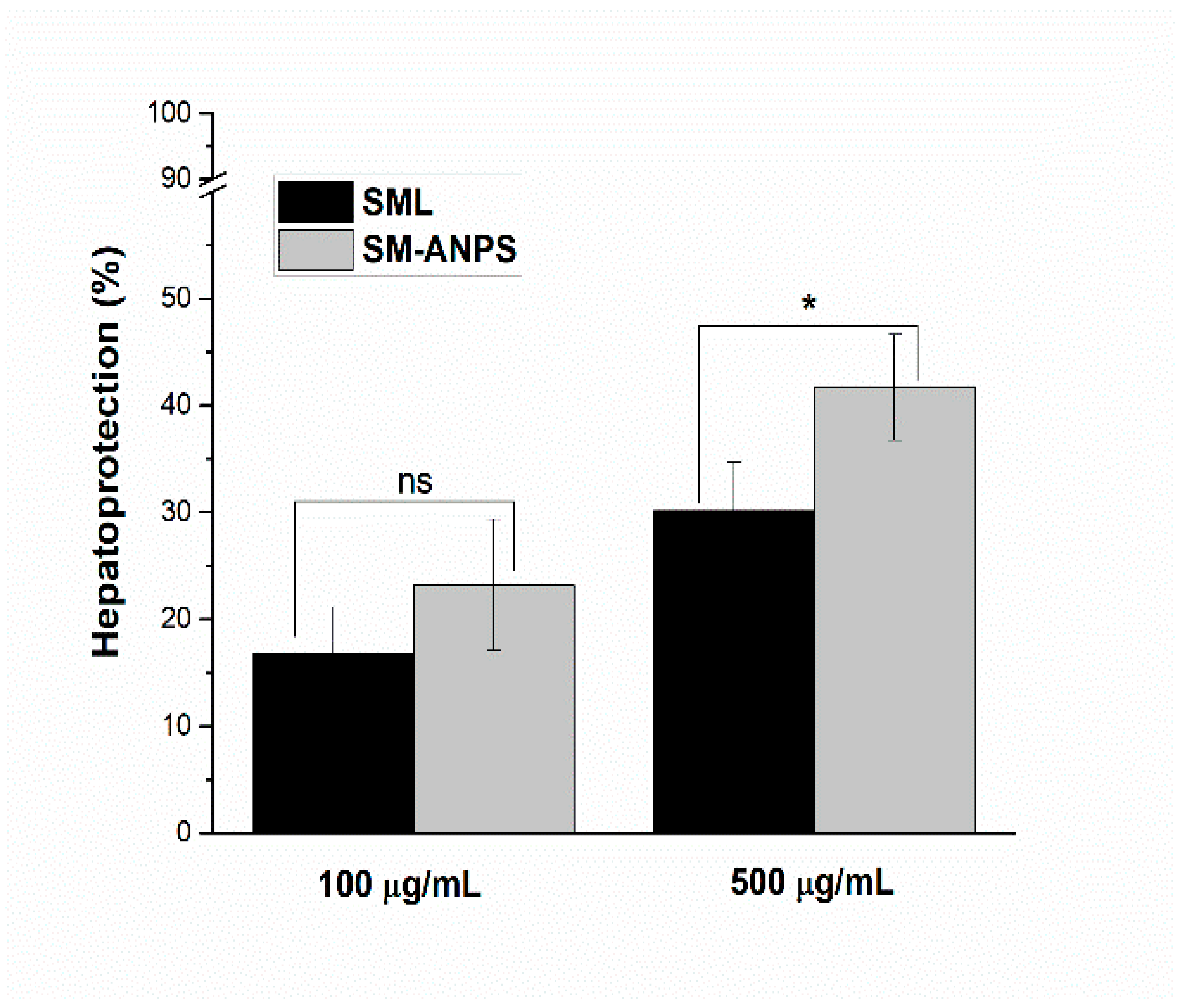
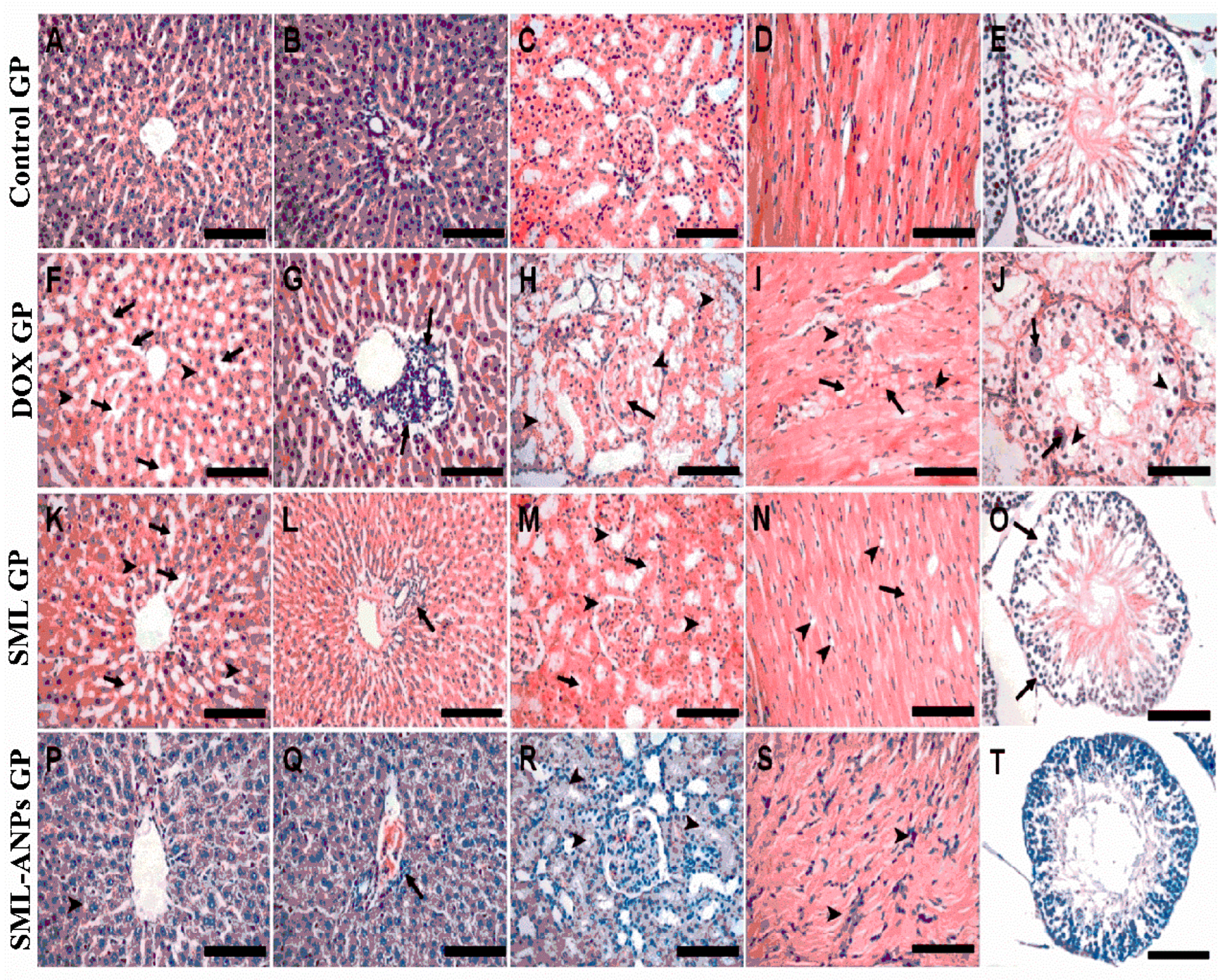
2.5. Evaluation of SML-ANPs Hepatoprotection
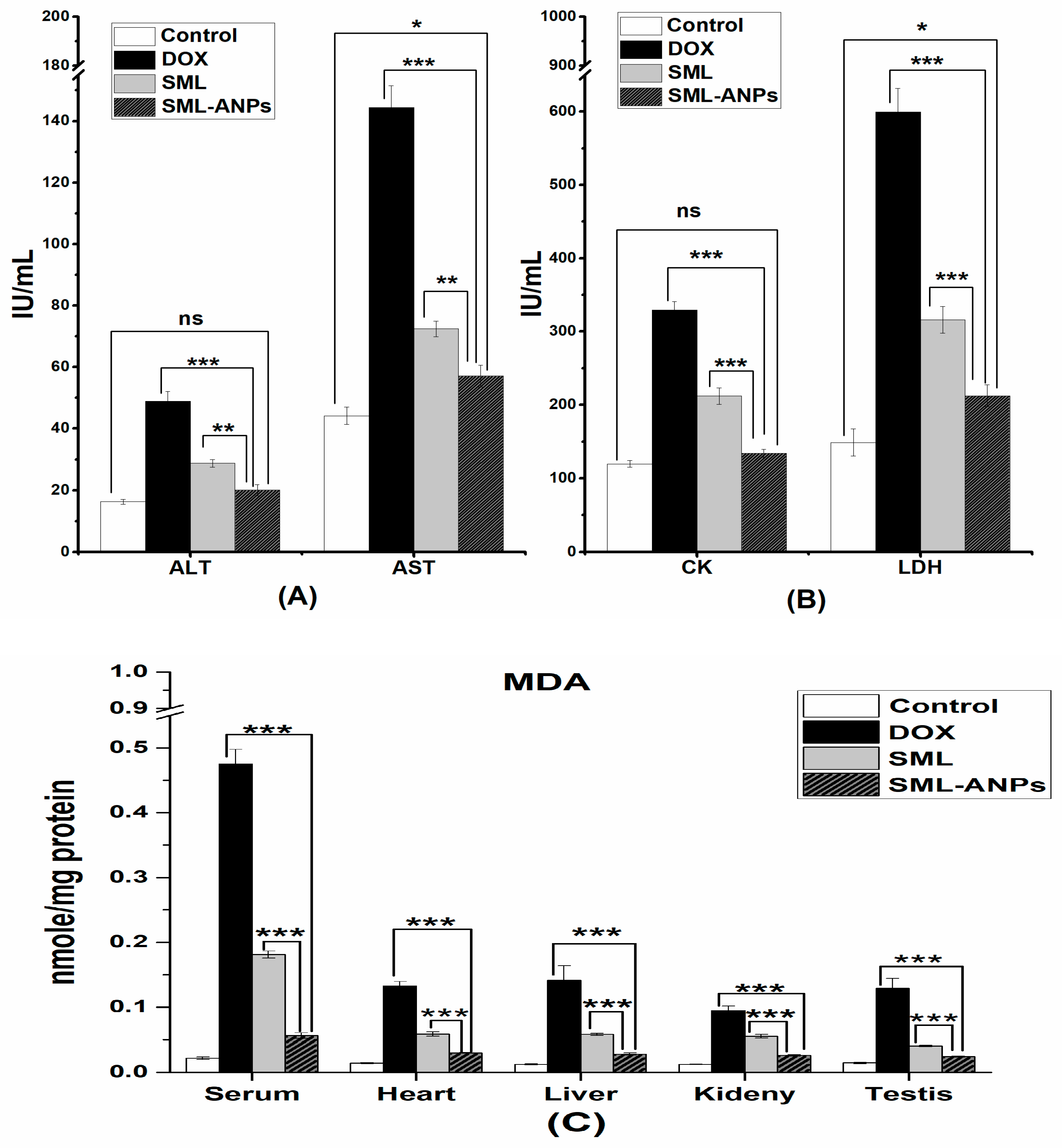
2.6. Biochemical Assessment
2.7. Changes in Animal Body Weight and Mortality Rates
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Fabrication of SML-ANPs
4.2.2. Characterizations of Albumin Nanoparticles
4.2.3. Design of Experiments (DOE) for Studying Critical Factors
4.2.4. In Vitro Drug Release of SML from Selected SML-ANPs
Release Kinetic Studies
4.2.5. Morphological Characterizations
4.2.6. Freeze Drying of the Selected SML-ANPs
4.2.7. Evaluating the Reconstituted SML-ANPs Stability
4.2.8. The Hepatoprotective Study of SML-ANPs
4.2.9. Pharmacokinetic Study
4.2.10. In Vivo Study of SML-ANPs
Biochemical Assessment of the Selected SML-ANPs Formulations
Histopathological Analysis
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Pinto-Ribeiro, L.; Silva, C.; Andrade, N.; Martel, F. α-tocopherol prevents oxidative stress-induced proliferative dysfunction in first-trimester human placental (HTR-8/SVneo) cells. Reprod. Biol. 2022, 22, 100602. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Salinas, F.L.; Perez-Gonzalez, A.; Acosta-Casique, A.; Ix-Ballote, A.; Diaz, A.; Treviño, S.; Rosas-Murrieta, N.H.; Millán-Perez-Peña, L.; Maycotte, P. Reactive oxygen species: Role in carcinogenesis, cancer cell signaling and tumor progression. Life Sci. 2021, 284, 119942. [Google Scholar] [CrossRef]
- Andreadi, A.; Bellia, A.; Di Daniele, N.; Meloni, M.; Lauro, R.; Della-Morte, D.; Lauro, D. The molecular link between oxidative stress, insulin resistance, and type 2 diabetes: A target for new therapies against cardiovascular diseases. Curr. Opin. Pharmacol. 2022, 62, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Songbo, M.; Lang, H.; Xinyong, C.; Bin, X.; Ping, Z.; Liang, S. Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicol. Lett. 2019, 307, 41–48. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative stress mitigation by antioxidants—An overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef] [PubMed]
- van der Zanden, S.Y.; Qiao, X.; Neefjes, J. New insights into the activities and toxicities of the old anticancer drug doxorubicin. FEBS J. 2021, 288, 6095–6111. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Sarkar, S.; Scott, L.; Danelisen, I.; Trush, M.A.; Jia, Z.; Li, Y.R. Doxorubicin redox biology: Redox cycling, topoisomerase inhibition, and oxidative stress. React. Oxyg. Species (Apex N.C.) 2016, 1, 189. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varela-López, A.; Battino, M.; Navarro-Hortal, M.D.; Giampieri, F.; Forbes-Hernández, T.Y.; Romero-Márquez, J.M.; Collado, R.; Quiles, J.L. An update on the mechanisms related to cell death and toxicity of doxorubicin and the protective role of nutrients. Food Chem. Toxicol. 2019, 134, 110834. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zou, H.; Huang, G.; Chen, G. Preparations and antioxidant activities of sesamol and it’s derivatives. Bioorg. Med. Chem. Lett. 2021, 31, 127716. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.S.; Hosny, E.N.; Khadrawy, Y.A.; Magdy, M.; Attia, Y.S.; Sayed, O.A.; AbdElaal, M. Protective effect of curcumin nanoparticles against cardiotoxicity induced by doxorubicin in rat. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165665. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Zanardelli, M.; Failli, P.; Ghelardini, C. Oxaliplatin-Induced Neuropathy: Oxidative Stress as Pathological Mechanism. Protective Effect of Silibinin. J. Pain 2012, 13, 276–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, T.G.; Singh, H.P.; Kaur, S.; Dhiman, S. Protective effects of sesamol against cisplatin-induced nephrotoxicity in rats: A mechanistic approach. Obes. Med. 2020, 19, 100269. [Google Scholar] [CrossRef]
- Tian, H.; Guo, R. Cardioprotective potential of sesamol against ischemia/reperfusion injury induced oxidative myocardial damage. Biomed. Res. 2017, 28, 2156–2163. [Google Scholar]
- Majdalawieh, A.F.; Mansour, Z.R. Sesamol, a major lignan in sesame seeds (Sesamum indicum): Anti-cancer properties and mechanisms of action. Eur. J. Pharmacol. 2019, 855, 75–89. [Google Scholar] [CrossRef]
- Chu, P.-Y.; Chien, S.-P.; Hsu, D.-Z.; Liu, M.-Y. Protective effect of sesamol on the pulmonary inflammatory response and lung injury in endotoxemic rats. Food Chem. Toxicol. 2010, 48, 1821–1826. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, H.N.; El-Bery, H.M.; Metwally, A.A.; Elshazly, M.; Hathout, R.M. Synthesis of CdS-modified chitosan quantum dots for the drug delivery of Sesamol. Carbohydr. Polym. 2019, 214, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Jan, K.C.; Ho, C.T.; Hwang, L.S. Elimination and metabolism of sesamol, a bioactive compound in sesame oil, in rats. Mol. Nutr. Food Res. 2009, 53, S36–S43. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-C.; Tsai, S.-Y.; Liu, I.-L.; Yu, C.-P.; Chao, P.-D.L. Metabolic transformation of sesamol and ex vivo effect on 2, 2′-azo-bis (2-amidinopropane) dihydrochloride-induced hemolysis. J. Agric. Food Chem. 2008, 56, 9636–9640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, T.; Jiang, C. Biomacromolecules as carriers in drug delivery and tissue engineering. Acta Pharm. Sin. B 2018, 8, 34–50. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, S.; Lee, S. Albumin nanoscience: Homing nanotechnology enabling targeted drug delivery and therapy. Arch. Pharmacal Res. 2020, 43, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, V.; García-Martinez, R.; Salvatella, X. Human serum albumin, systemic inflammation, and cirrhosis. J. Hepatol. 2014, 61, 396–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naguib, S.S.; Hathout, R.M.; Mansour, S. Optimizing novel penetration enhancing hybridized vesicles for augmenting the in-vivo effect of an anti-glaucoma drug. Drug Deliv. 2017, 24, 99–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varshochian, R.; Jeddi-Tehrani, M.; Mahmoudi, A.R.; Khoshayand, M.R.; Atyabi, F.; Sabzevari, A.; Esfahani, M.R.; Dinarvand, R. The protective effect of albumin on bevacizumab activity and stability in PLGA nanoparticles intended for retinal and choroidal neovascularization treatments. Eur. J. Pharm. Sci. 2013, 50, 341–352. [Google Scholar] [CrossRef]
- Abdel-Hafez, S.M.; Hathout, R.M.; Sammour, O.A. Towards better modeling of chitosan nanoparticles production: Screening different factors and comparing two experimental designs. Int. J. Biol. Macromol. 2014, 64, 334–340. [Google Scholar] [CrossRef]
- Safwat, S.; Hathout, R.M.; Ishak, R.A.; Mortada, N.D. Augmented simvastatin cytotoxicity using optimized lipid nanocapsules: A potential for breast cancer treatment. J. Liposome Res. 2017, 27, 1–10. [Google Scholar] [CrossRef]
- Karami, K.; Jamshidian, N.; Hajiaghasi, A.; Amirghofran, Z. BSA nanoparticles as controlled release carriers for isophethalaldoxime palladacycle complex; synthesis, characterization, in vitro evaluation, cytotoxicity and release kinetics analysis. New J. Chem. 2020, 44, 4394–4405. [Google Scholar] [CrossRef]
- Kayani, Z.; Firuzi, O.; Bordbar, A.-K. Doughnut-shaped bovine serum albumin nanoparticles loaded with doxorubicin for overcoming multidrug-resistant in cancer cells. Int. J. Biol. Macromol. 2018, 107, 1835–1843. [Google Scholar] [CrossRef]
- Elmasry, S.R.; Hathout, R.M.; Abdel-Halim, M.; Mansour, S. In vitro transdermal delivery of sesamol using oleic acid chemically-modified gelatin nanoparticles as a potential breast cancer medication. J. Drug Deliv. Sci. Technol. 2018, 48, 30–39. [Google Scholar] [CrossRef]
- Allison, A.C. Structure and Function of Plasma Proteins; Springer: New York, NY, USA, 1974; Volume 1. [Google Scholar]
- Amighi, F.; Emam-Djomeh, Z.; Labbafi-Mazraeh-Shahi, M. Effect of different cross-linking agents on the preparation of bovine serum albumin nanoparticles. J. Iran. Chem. Soc. 2020, 17, 1223–1235. [Google Scholar] [CrossRef]
- Sadeghi, R.; Moosavi-Movahedi, A.; Emam-Jomeh, Z.; Kalbasi, A.; Razavi, S.; Karimi, M.; Kokini, J. The effect of different desolvating agents on BSA nanoparticle properties and encapsulation of curcumin. J. Nanopart. Res. 2014, 16, 2565. [Google Scholar] [CrossRef]
- Anhorn, M.G.; Mahler, H.-C.; Langer, K. Freeze drying of human serum albumin (HSA) nanoparticles with different excipients. Int. J. Pharm. 2008, 363, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Shepherd, D.; Sun, J.; Ouellette, D.; Grant, K.L.; Tang, X.; Pikal, M.J. Mechanism of protein stabilization by sugars during freeze-drying and storage: Native structure preservation, specific interaction, and/or immobilization in a glassy matrix? J. Pharm. Sci. 2005, 94, 1427–1444. [Google Scholar] [CrossRef] [PubMed]
- Llabot, J.M.; Luis de Redin, I.; Agüeros, M.; Dávila Caballero, M.J.; Boiero, C.; Irache, J.M.; Allemandi, D. In vitro characterization of new stabilizing albumin nanoparticles as a potential topical drug delivery system in the treatment of corneal neovascularization (CNV). J. Drug Deliv. Sci. Technol. 2019, 52, 379–385. [Google Scholar] [CrossRef]
- Duarte, A.R.; Chenet, A.L.; de Almeida, F.J.S.; Andrade, C.M.B.; de Oliveira, M.R. The inhibition of heme oxigenase-1 (HO-1) abolishes the mitochondrial protection induced by sesamol in LPS-treated RAW 264.7 cells. Chem.-Biol. Interact. 2018, 296, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Nayak, P.G.; Paul, P.; Bansal, P.; Kutty, N.G.; Pai, K.S.R. Sesamol prevents doxorubicin-induced oxidative damage and toxicity on H 9c2 cardiomyoblasts. J. Pharm. Pharmacol. 2013, 65, 1083–1093. [Google Scholar] [CrossRef]
- Patravale, V.; Dandekar, P.; Jain, R. Nanotoxicology: Evaluating Toxicity Potential of Drug-Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2012; pp. 123–155. [Google Scholar]
- Tremblay, A.R.; Delbes, G. In vitro study of doxorubicin-induced oxidative stress in spermatogonia and immature Sertoli cells. Toxicol. Appl. Pharmacol. 2018, 348, 32–42. [Google Scholar] [CrossRef]
- Kumar, B.; Kuhad, A.; Chopra, K. Neuropsychopharmacological effect of sesamol in unpredictable chronic mild stress model of depression: Behavioral and biochemical evidences. Psychopharmacology 2011, 214, 819–828. [Google Scholar] [CrossRef]
- Ren, B.; Yuan, T.; Diao, Z.; Zhang, C.; Liu, Z.; Liu, X. Protective effects of sesamol on systemic oxidative stress-induced cognitive impairments via regulation of Nrf2/Keap1 pathway. Food Funct. 2018, 9, 5912–5924. [Google Scholar] [CrossRef]
- Mo, Y.; Barnett, M.E.; Takemoto, D.; Davidson, H.; Kompella, U.B. Human serum albumin nanoparticles for efficient delivery of Cu, Zn superoxide dismutase gene. Mol. Vis. 2007, 13, 746. [Google Scholar]
- Fadel, M.; Fadeel, D.A.; Ibrahim, M.; Hathout, R.M.; El-Kholy, A.I. One-Step Synthesis of Polypyrrole-Coated Gold Nanoparticles for Use as a Photothermally Active Nano-System. Int. J. Nanomed. 2020, 15, 2605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negrette-Guzmán, M. Combinations of the antioxidants sulforaphane or curcumin and the conventional antineoplastics cisplatin or doxorubicin as prospects for anticancer chemotherapy. Eur. J. Pharmacol. 2019, 859, 172513. [Google Scholar] [CrossRef] [PubMed]
- Pugazhendhi, A.; Edison, T.N.J.I.; Velmurugan, B.K.; Jacob, J.A.; Karuppusamy, I. Toxicity of Doxorubicin (Dox) to different experimental organ systems. Life Sci. 2018, 200, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bruschi, M.L. 5-Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Cambridge, UK, 2015; pp. 63–86. [Google Scholar] [CrossRef]
- Abdelkader, A.; El-Mokhtar, M.A.; Abdelkader, O.; Hamad, M.A.; Elsabahy, M.; El-Gazayerly, O.N. Ultrahigh antibacterial efficacy of meropenem-loaded chitosan nanoparticles in a septic animal model. Carbohydr. Polym. 2017, 174, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Dadparvar, M.; Wagner, S.; Wien, S.; Worek, F.; von Briesen, H.; Kreuter, J. Freeze-drying of HI-6-loaded recombinant human serum albumin nanoparticles for improved storage stability. Eur. J. Pharm. Biopharm. 2014, 88, 510–517. [Google Scholar] [CrossRef]
- Reese, J.A.; Byard, J.L. Isolation and culture of adult hepatocytes from liver biopsies. In Vitro 1981, 17, 935. [Google Scholar] [CrossRef]
- Shen, L.; Hillebrand, A.; Wang, D.Q.-H.; Liu, M. Isolation and primary culture of rat hepatic cells. JoVE (J. Vis. Exp.) 2012, 64, 3917. [Google Scholar] [CrossRef]
- Safwat, S.; Ishak, R.A.H.; Hathout, R.M.; Mortada, N.D. Nanostructured lipid carriers loaded with simvastatin: Effect of PEG/glycerides on characterization, stability, cellular uptake efficiency and in vitro cytotoxicity. Drug Dev. Ind. Pharm. 2017, 43, 1112–1125. [Google Scholar] [CrossRef]
- Chao, H.-H.; Liu, J.-C.; Hong, H.-J.; Lin, J.-W.; Chen, C.-H.; Cheng, T.-H. L-carnitine reduces doxorubicin-induced apoptosis through a prostacyclin-mediated pathway in neonatal rat cardiomyocytes. Int. J. Cardiol. 2011, 146, 145–152. [Google Scholar] [CrossRef]
- Shabalala, S.C.; Dludla, P.V.; Muller, C.J.; Nxele, X.; Kappo, A.P.; Louw, J.; Johnson, R. Aspalathin ameliorates doxorubicin-induced oxidative stress in H9c2 cardiomyoblasts. Toxicol. Vitr. 2019, 55, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Geetha, T.; Singh, N.; Deol, P.K.; Kaur, I.P. Biopharmaceutical profiling of sesamol: Physiochemical characterization, gastrointestinal permeability and pharmacokinetic evaluation. RSC Adv. 2015, 5, 4083–4091. [Google Scholar] [CrossRef]
- Gourishetti, K.; Keni, R.; Nayak, P.G.; Jitta, S.R.; Bhaskaran, N.A.; Kumar, L.; Kumar, N.; Krishnadas, N.; Shenoy, R.R. Sesamol-loaded PLGA nanosuspension for accelerating wound healing in diabetic foot ulcer in rats. Int. J. Nanomed. 2020, 15, 9265. [Google Scholar] [CrossRef] [PubMed]
- Jan, K.-C.; Ho, C.-T.; Hwang, L.S. Bioavailability and tissue distribution of sesamol in rat. J. Agric. Food Chem. 2008, 56, 7032–7037. [Google Scholar] [CrossRef]
- Ismail, D.I. Histological study on doxorubicin-induced testicular toxicity and the protective role of sesamol in rats. Egypt. J. Histol. 2016, 39, 38–49. [Google Scholar] [CrossRef]
- Razavi-Azarkhiavi, K.; Iranshahy, M.; Sahebkar, A.; Shirani, K.; Karimi, G. The protective role of phenolic compounds against doxorubicin-induced cardiotoxicity: A comprehensive review. Nutr. Cancer 2016, 68, 892–917. [Google Scholar] [CrossRef]
- Singh, N.; Khullar, N.; Kakkar, V.; Kaur, I.P. Sesamol loaded solid lipid nanoparticles: A promising intervention for control of carbon tetrachloride induced hepatotoxicity. BMC Complementary Altern. Med. 2015, 15, 142. [Google Scholar] [CrossRef] [Green Version]
- El-Moselhy, M.A.; El-Sheikh, A.A. Protective mechanisms of atorvastatin against doxorubicin-induced hepato-renal toxicity. Biomed. Pharmacother. 2014, 68, 101–110. [Google Scholar] [CrossRef]
- Safwat, S.; Ishak, R.A.; Hathout, R.M.; Mortada, N.D. Statins anti-cancer targeted delivery systems: Re-purposing an old molecule. J. Pharm. Pharmacol. 2017, 69, 613–624. [Google Scholar] [CrossRef] [Green Version]
- Hassanzadeh, P.; Atyabi, F.; Dinarvand, R.; Dehpour, A.R.; Azhdarzadeh, M.; Dinarvand, M. Application of nanostructured lipid carriers: The prolonged protective effects for sesamol in in vitro and in vivo models of ischemic stroke via activation of PI3K signalling pathway. DARU J. Pharm. Sci. 2017, 25, 25. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Chen, T.; Deng, F.; Wan, J.; Tang, Y.; Yuan, P.; Zhang, L. Synthesis, characterization, and in vitro evaluation of curcumin-loaded albumin nanoparticles surface-functionalized with glycyrrhetinic acid. Int. J. Nanomed. 2015, 10, 5475. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Meng-Xia, X.; Dong, Z.; Yuan, L.; Xiao-Yu, L.; Xing, C. Spectroscopic studies on the interaction of cinnamic acid and its hydroxyl derivatives with human serum albumin. J. Mol. Struct. 2004, 692, 71–80. [Google Scholar] [CrossRef]
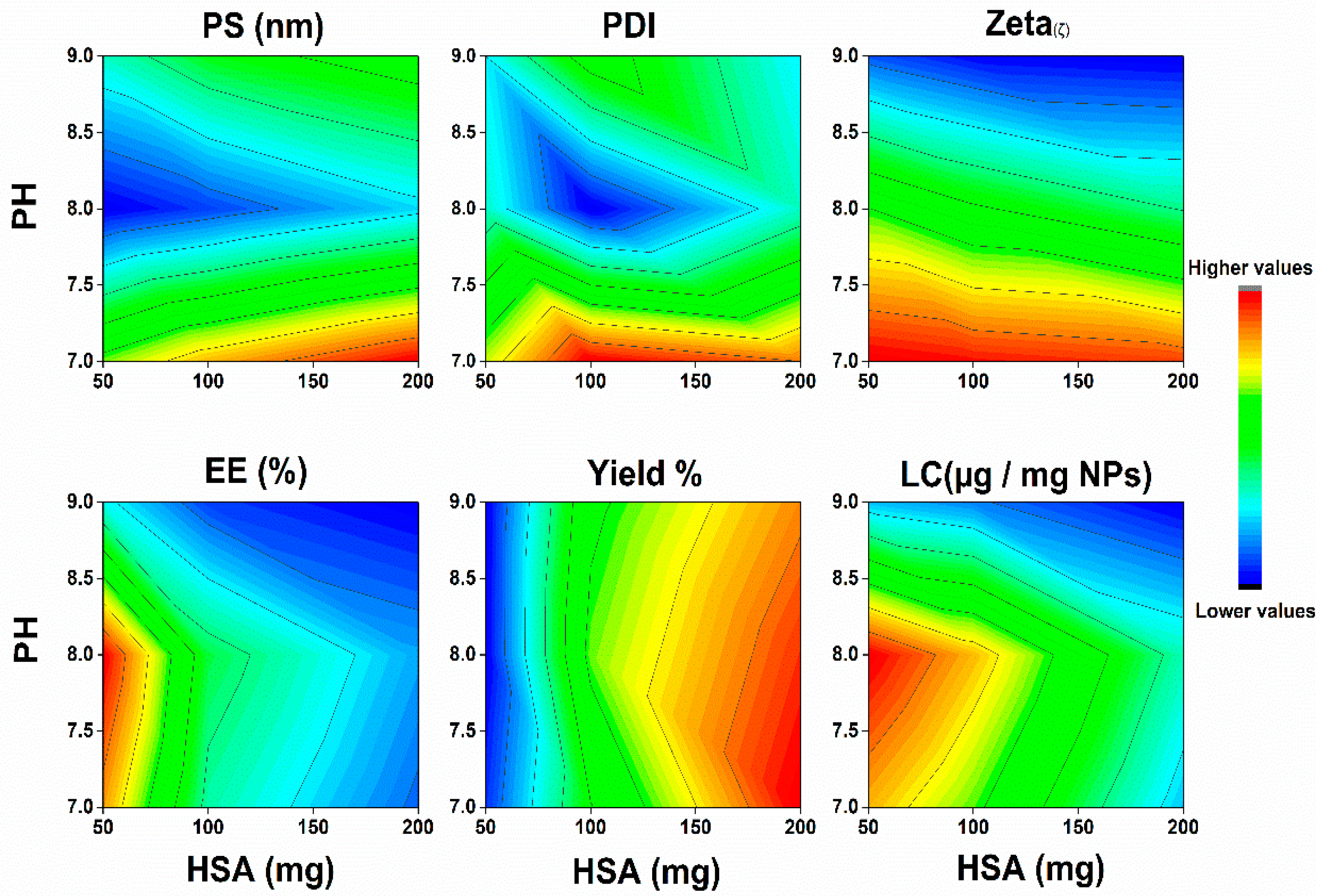
| Responses | PS | PDI | Zeta | %EE | %Yield | LC 1 |
|---|---|---|---|---|---|---|
| Order | Quadratic | Quadratic | Linear | Quadratic | Quadratic | Quadratic |
| Significance | extremely significant (p < 0.001) | ns | extremely significant (p < 0.001) | extremely significant (p < 0.001) | extremely significant (p < 0.001) | extremely significant (p < 0.001) |
| R2 | 0.994 | 0.649 | 0.969 | 0.972 | 0.983 | 0.942 |
| Adjusted R2 | 0.989 | 0.338 | 0.963 | 0.947 | 0.968 | 0.890 |
| Predicted R2 | 0.969 | −0.468 | 0.953 | 0.871 | 0.923 | 0.736 |
| Adequate precision | 45.801 | 3.998 | 34.93 | 18.01 | 23.926 | 12.855 2 |
| Parameters | t1/2(h) * | AUC (µg/mL h) * | MRT(h) * | CL (mL/h) * |
|---|---|---|---|---|
| Free SML | 1.3 ± 0.13 | 19.5 ± 4.2 | 2.6 ± 0.03 | 528.2 ± 115.5 |
| SML-ANPs | 6.1 ± 0.44 | 88.7 ± 12.2 | 8.5 ± 0.63 | 114.2 ± 15.9 |
| Wt.(g)/Survival Rate | Control GP | DOX GP * | SML GP ns | SML-ANPs GP ns,1,2 |
|---|---|---|---|---|
| Body weight | 151.7 ± 11.02 | 101.26 ± 5.61 | 146.5 ± 14.12 | 162.44 ± 15.49 |
| Survival rate | 10/10 | 8/10 | 9/10 | 10/10 |
| Independent Variable | Level (−1) | Level (0) | Level (+1) |
|---|---|---|---|
| PH | 7 | 8 | 9 |
| HSA (mg/mL) | 25 | 50 | 100 |
| D.A | Ethanol | - | Ethanol/acetone |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaher, S.; Soliman, M.E.; Elsabahy, M.; Hathout, R.M. Sesamol Loaded Albumin Nanoparticles: A Boosted Protective Property in Animal Models of Oxidative Stress. Pharmaceuticals 2022, 15, 733. https://doi.org/10.3390/ph15060733
Zaher S, Soliman ME, Elsabahy M, Hathout RM. Sesamol Loaded Albumin Nanoparticles: A Boosted Protective Property in Animal Models of Oxidative Stress. Pharmaceuticals. 2022; 15(6):733. https://doi.org/10.3390/ph15060733
Chicago/Turabian StyleZaher, Sara, Mahmoud E. Soliman, Mahmoud Elsabahy, and Rania M. Hathout. 2022. "Sesamol Loaded Albumin Nanoparticles: A Boosted Protective Property in Animal Models of Oxidative Stress" Pharmaceuticals 15, no. 6: 733. https://doi.org/10.3390/ph15060733
APA StyleZaher, S., Soliman, M. E., Elsabahy, M., & Hathout, R. M. (2022). Sesamol Loaded Albumin Nanoparticles: A Boosted Protective Property in Animal Models of Oxidative Stress. Pharmaceuticals, 15(6), 733. https://doi.org/10.3390/ph15060733