Applications in Which Aptamers Are Needed or Wanted in Diagnostics and Therapeutics
Abstract
:1. Introduction
2. Diagnostic Applications in Which Aptamers Are Needed or Desired
2.1. When No Commercial Antibodies Exist or Are Very Difficult to Obtain
2.2. When Antibodies Fail to Distinguish Closely Related Variant Targets
2.3. When Smaller Size Matters
3. Therapeutic Applications in Which Aptamers Are Needed or Desired
3.1. When the Target Is Too Toxic or Lethal for a Host Animal to Develop Antibodies
3.2. For Passive Immunity versus the More Expensive Humanized Monoclonal Antibodies
4. Discussion and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef]
- Jayasena, S.D. Aptamers: An emerging class of molecules that rival antibodies in diagnostics. Clin. Chem. 1999, 45, 1628–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Rossi, J. Aptamers as targeted therapeutics: Current potential and challenges. Nat. Rev. Drug Discov. 2017, 16, 181–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
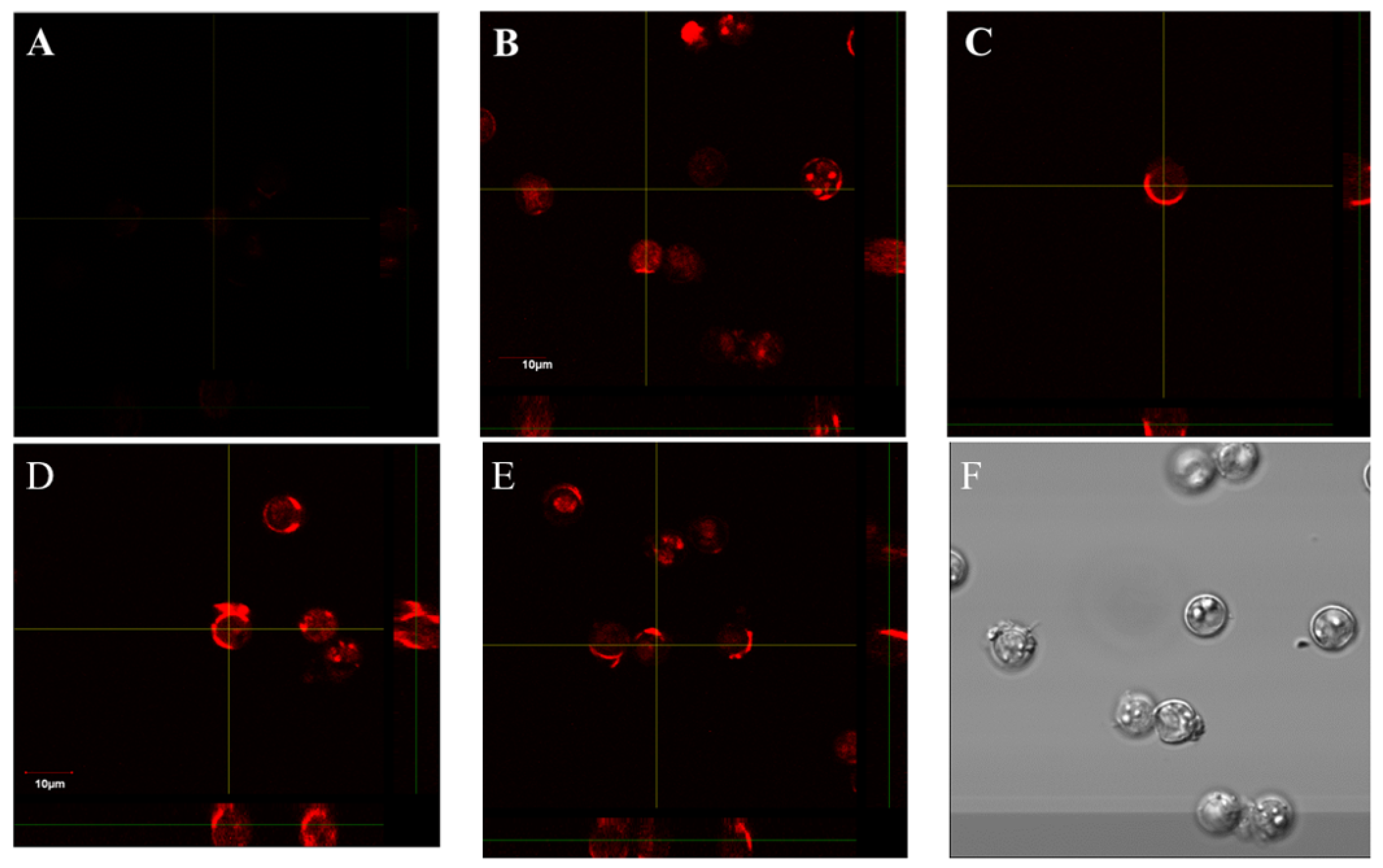
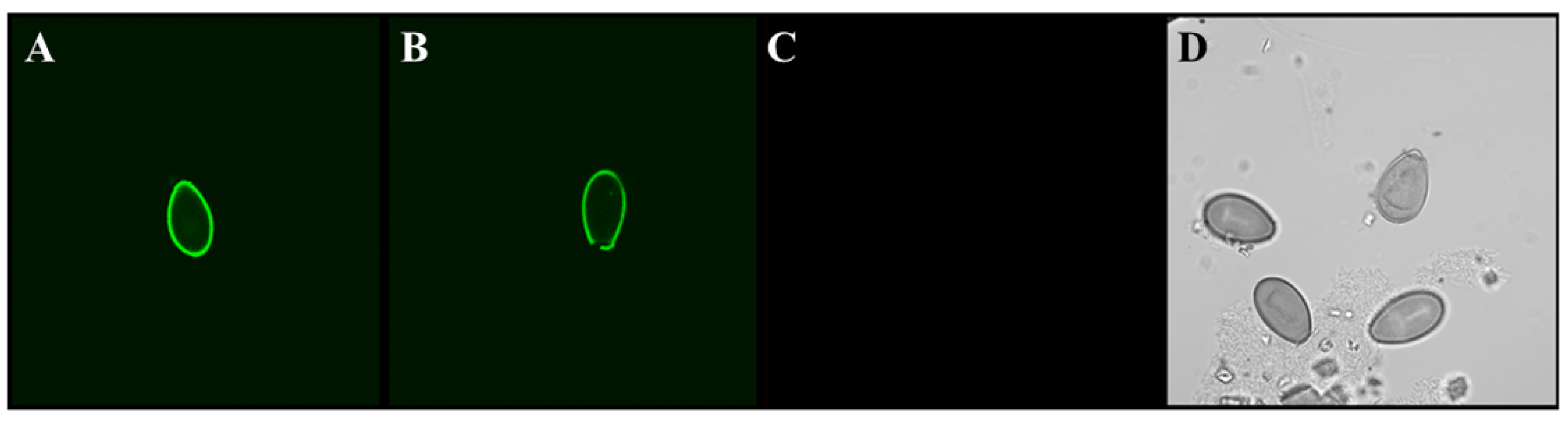
- Shields, J.M.; Olson, B.H. Cyclospora cayetanensis: A review of an emerging parasitic coccidian. Int. J. Parasitol. 2003, 33, 371–391. [Google Scholar] [CrossRef]
- Sanchez-Vega, J.T.; Cabrera-Fuentes, H.A.; Romero-Olmedo, A.J.; Ortiz-Frias, J.L.; Sokolina, F.; Barreto, G. Cyclospora cayetanensis: This emerging protozoan pathogen in Mexico. Am. J. Trop. Med. Hyg. 2014, 90, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Almeria, S.; Cinar, H.N.; Dubey, J.P. Cyclospora cayetanensis and Cyclosporiasis: An Update. Microorganisms 2019, 7, 317. [Google Scholar] [CrossRef] [Green Version]
- Hadjilouka, A.; Tsaltas, D. Cyclospora Cayetanensis-Major Outbreaks from Ready to Eat Fresh Fruits and Vegetables. Foods 2020, 9, 1703. [Google Scholar] [CrossRef]
- Li, J.; Cui, Z.; Qi, M.; Zhang, L. Advances in Cyclosporiasis Diagnosis and Therapeutic Intervention. Front. Cell. Infect. Microbiol. 2020, 10, 43. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Wang, L.; Zheng, H.; Xu, Z.; Roellig, D.M.; Li, N.; Frace, M.A.; Tang, K.; Arrowood, M.J.; Moss, D.M.; et al. Comparative genomics reveals Cyclospora cayetanensis possesses coccidia-like metabolism and invasion components but unique surface antigens. BMC Genom. 2016, 17, 316. [Google Scholar] [CrossRef] [Green Version]
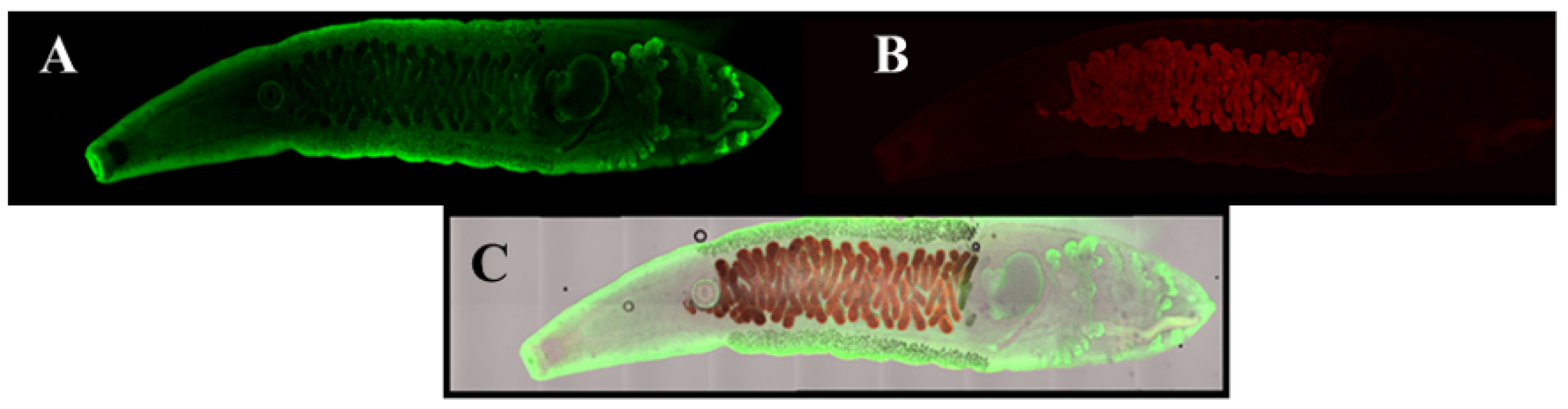
- Zheng, S.; Zhu, Y.; Zhao, Z.; Wu, Z.; Okanurak, K.; Lv, Z. Liver fluke infection and cholangiocarcinoma: A review. Parasitol. Res. 2017, 116, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Sripa, B.; Suwannatrai, A.T.; Sayasone, S.; Do, D.T.; Khieu, V.; Yang, Y. Current status of human liver fluke infections in the Greater Mekong Subregion. Acta Trop. 2021, 224, 106133. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.G.; Richarte, A. Aptamer-quantum dot lateral flow test strip development for rapid and sensitive detection of pathogenic E. coli via intimin, O157-specific LPS and Shiga toxin 1 aptamers. Curr. Bionanotechnol. 2015, 1, 80–86. [Google Scholar] [CrossRef]
- Bruno, J.G. Application of DNA Aptamers and Quantum Dots to Lateral Flow Test Strips for Detection of Foodborne Pathogens with Improved Sensitivity versus Colloidal Gold. Pathogens 2014, 3, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Jenison, R.D.; Gill, S.C.; Pardi, A.; Polisky, B. High-resolution molecular discrimination by RNA. Science 1994, 263, 1425–1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruno, J.G.; Carrillo, M.P.; Phillips, T.; Edge, A. Discrimination of recombinant from natural human growth hormone using DNA aptamers. J. Biomol. Tech. 2011, 22, 27–36. [Google Scholar]
- Hepner, F.; Cszasar, E.; Roitinger, E.; Lubec, G. Mass spectrometrical analysis of recombinant human growth hormone (Genotropin®) reveals amino acid substitutions in 2% of the expressed protein. Proteome Sci. 2005, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Kalra, P.; Dhiman, A.; Cho, W.C.; Bruno, J.G.; Sharma, T.K. Simple Methods and Rational Design for Enhancing Aptamer Sensitivity and Specificity. Front. Mol. Biosci. 2018, 5, 41. [Google Scholar] [CrossRef]
- Bruno, J.G. Integration of multiple computer modeling software programs for characterization of a brain natriuretic peptide sandwich DNA aptamer complex. J. Mol. Recognit. 2019, 32, e2809. [Google Scholar] [CrossRef]
- Lee, J.F.; Stovall, G.M.; Ellington, A.D. Aptamer therapeutics advance. Curr. Opin. Chem. Biol. 2006, 10, 282–289. [Google Scholar] [CrossRef]
- de Castro, M.A.G.; Höbartner, C.; Opazo, F. Aptamers provide superior stainings of cellular receptors studied under super-resolution microscopy. PLoS ONE 2017, 12, e0173050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barahona, F.; Bardliving, C.L.; Phifer, A.; Bruno, J.G.; Batt, C.A. An Aptasensor Based on Polymer-Gold Nanoparticle Composite Microspheres for the Detection of Malathion Using Surface-Enhanced Raman Spectroscopy. Ind. Biotechnol. 2013, 9, 42–50. [Google Scholar] [CrossRef]
- Boushell, V.; Pang, S.; He, L. Aptamer-Based SERS Detection of Lysozyme on a Food-Handling Surface. J. Food Sci. 2017, 82, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.M.; Campbell, J.L.; Burkitt, S.; Qiu, Z.; Kang, S.; Mehraein, M.; Miyasato, D.; Salinas, H.; Liu, J.T.C.; Zavaleta, C. A Raman Imaging Approach Using CD47 Antibody-Labeled SERS Nanoparticles for Identifying Breast Cancer and Its Potential to Guide Surgical Resection. Nanomaterials 2018, 8, 953. [Google Scholar] [CrossRef] [Green Version]
- Kukushkin, V.I.; Ivanov, N.M.; Novoseltseva, A.A.; Gambaryan, A.S.; Yaminsky, I.V.; Kopylov, A.M.; Zavyalova, E.G. Highly sensitive detection of influenza virus with SERS aptasensor. PLoS ONE 2019, 14, e0216247. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Park, S.-G.; Choi, N.; Moon, J.-I.; Dang, H.; Das, A.; Lee, S.; Kim, D.-G.; Chen, L.; Choo, J. SERS imaging-based aptasensor for ultrasensitive and reproducible detection of influenza virus A. Biosens. Bioelectron. 2020, 167, 112496. [Google Scholar] [CrossRef]
- Muhammad, M.; Huang, Q. A review of aptamer-based SERS biosensors: Design strategies and applications. Talanta 2021, 227, 122188. [Google Scholar] [CrossRef]
- Chen, H.; Park, S.K.; Joung, Y.; Kang, T.; Lee, M.K.; Choo, J. SERS-based dual-mode DNA aptasensors for rapid classification of SARS-CoV-2 and influenza A/H1N1 infection. Sens. Actuators B Chem. 2022, 355, 131324. [Google Scholar] [CrossRef]
- Nardou, E.; Vouagner, D.; Jurdyc, A.-M.; Berthelot, A.; Pillonnet, A.; Sablonière, V.; Champagnon, B. Distance dependence of the Surface Enhanced Raman Scattering effect observed in amorphous TiO2 on nanostructured gold. Opt. Mater. 2011, 33, 1907–1910. [Google Scholar] [CrossRef]
- Liu, F.-M.; Köllensperger, P.; Green, M.; Cass, T.; Cohen, L. A note on distance dependence in surface enhanced Raman spectroscopy. Chem. Phys. Lett. 2006, 430, 173–176. [Google Scholar] [CrossRef]
- Kovacs, G.J.; Loutfy, R.O.; Vincett, P.S.; Jennings, C.; Aroca, R. Distance dependence of SERS enhancement factor from Langmuir-Blodgett monolayers on metal island films: Evidence for the electromagnetic mechanism. Langmuir ACS J. Surf. Colloids 1986, 2, 689–694. [Google Scholar] [CrossRef]
- Xu, X.; Ma, X.; Wang, H.; Wang, Z. Aptamer based SERS detection of Salmonella typhimurium using DNA-assembled gold nanodimers. Mikrochim. Acta 2018, 185, 325. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, Q.; Chen, J.; Ni, X.; Dai, J. A DNA Aptamer Based Method for Detection of SARS-CoV-2 Nucleocapsid Protein. Virol. Sin. 2020, 35, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.G.; Phillips, T.; Montez, T. Preliminary Development of DNA Aptamers to Inhibit Phospholipase A2 Activity of Bee and Cobra Venoms. J. Bionanosci. 2015, 9, 270–275. [Google Scholar] [CrossRef]
- Savchik, E.Y.; Kalinina, T.B.; Drozd, N.N.; Makarov, V.A.; Zav’yalova, E.G.; Lapsheva, E.N.; Mudrik, N.N.; Babij, A.V.; Pavlova, G.V.; Golovin, A.V.; et al. Aptamer RA36 inhibits of human, rabbit, and rat plasma coagulation activated with thrombin or snake venom coagulases. Bull. Exp. Biol. Med. 2013, 156, 44–48. [Google Scholar] [CrossRef]
- Ye, F.; Zheng, Y.; Wang, X.; Tan, X.; Zhang, T.; Xin, W.; Wang, J.; Huang, Y.; Fan, Q.; Wang, J. Recognition of Bungarus multicinctus venom by a DNA aptamer against beta-bungarotoxin. PLoS ONE 2014, 9, e105404. [Google Scholar] [CrossRef]
- Bruno, J.G. Potential Inherent Stimulation of the Innate Immune System by Nucleic Acid Aptamers and Possible Corrective Approaches. Pharmaceuticals 2018, 11, 62. [Google Scholar] [CrossRef] [Green Version]
- Binning, J.M.; Wang, T.; Luthra, P.; Shabman, R.S.; Borek, D.M.; Liu, G.; Xu, W.; Leung, D.W.; Basler, C.F.; Amarasinghe, G.K. Development of RNA aptamers targeting Ebola virus VP35. Biochemistry 2013, 52, 8406–8419. [Google Scholar] [CrossRef] [Green Version]
- Negri, P.; Chen, G.; Kage, A.; Nitsche, A.; Naumann, D.; Xu, B.; Dluhy, R.A. Direct optical detection of viral nucleoprotein binding to an anti-influenza aptamer. Anal. Chem. 2012, 84, 5501–5508. [Google Scholar] [CrossRef] [Green Version]
- Musafia, B.; Oren-Banaroya, R.; Noiman, S. Designing anti-influenza aptamers: Novel quantitative structure activity relationship approach gives insights into aptamer-virus interaction. PLoS ONE 2014, 9, e97696. [Google Scholar] [CrossRef]
- Jeon, S.H.; Kayhan, B.; Ben-Yedidia, T.; Arnon, R. A DNA aptamer prevents influenza infection by blocking the receptor binding region of the viral hemagglutinin. J. Biol. Chem. 2004, 279, 48410–48419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopinath, S.C.; Kawasaki, K.; Kumar, P.K. Selection of RNA-aptamer against human influenza B virus. Nucleic Acids Symp. Ser. 2005, 49, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, S.C.; Misono, T.S.; Kawasaki, K.; Mizuno, T.; Imai, M.; Odagiri, T.; Kumar, P.K. An RNA aptamer that distinguishes between closely related human influenza viruses and inhibits haemagglutinin-mediated membrane fusion. J. Gen. Virol. 2006, 87, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, S.C.; Sakamaki, Y.; Kawasaki, K.; Kumar, P.K. An efficient RNA aptamer against human influenza B virus hemagglutinin. J. Biochem. 2006, 139, 837–846. [Google Scholar] [CrossRef]
- Cui, Z.Q.; Ren, Q.; Wei, H.P.; Chen, Z.; Deng, J.Y.; Zhang, Z.P.; Zhang, X.E. Quantum dot-aptamer nanoprobes for recognizing and labeling influenza A virus particles. Nanoscale 2011, 3, 2454–2457. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, S.; Yoon, H.; Kim, K.B.; Kalme, S.S.; Oh, S.; Song, C.S.; Kim, D.E. Selection of an antiviral RNA aptamer against hemagglutinin of the subtype H5 avian influenza virus. Nucleic Acid Ther. 2011, 21, 395–402. [Google Scholar] [CrossRef]
- Li, X.; Lu, D.; Sheng, Z.; Chen, K.; Guo, X.; Jin, M.; Han, H. A fast and sensitive immunoassay of avian influenza virus based on label-free quantum dot probe and lateral flow test strip. Talanta 2012, 100, 1–6. [Google Scholar] [CrossRef]
- Woo, H.M.; Kim, K.S.; Lee, J.M.; Shim, H.S.; Cho, S.J.; Lee, W.K.; Ko, H.W.; Keum, Y.S.; Kim, S.Y.; Pathinayake, P.; et al. Single-stranded DNA aptamer that specifically binds to the influenza virus NS1 protein suppresses interferon antagonism. Antivir. Res. 2013, 100, 337–345. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, J.; He, Y.; Chen, S.; Jiang, Y.; Zhao, Y.; Zhao, S. Protein-binding aptamer assisted signal amplification for the detection of influenza A (H1N1) DNA sequences based on quantum dot fluorescence polarization analysis. Analyst 2013, 138, 4722–4727. [Google Scholar] [CrossRef]
- Kwon, H.M.; Lee, K.H.; Han, B.W.; Han, M.R.; Kim, D.E. An RNA aptamer that specifically binds to the glycosylated hemagglutinin of avian influenza virus and suppresses viral infection in cells. PLoS ONE 2014, 9, e97574. [Google Scholar] [CrossRef] [Green Version]
- Suenaga, E.; Kumar, P.K. An aptamer that binds efficiently to the hemagglutinins of highly pathogenic avian influenza viruses (H5N1 and H7N7) and inhibits hemagglutinin-glycan interactions. Acta Biomater. 2014, 10, 1314–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diba, F.S.; Kim, S.; Lee, H.J. Amperometric bioaffinity sensing platform for avian influenza virus proteins with aptamer modified gold nanoparticles on carbon chips. Biosens. Bioelectron. 2015, 72, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Feng, X.; Yan, X.; Liu, K.; Deng, L. A DNA Aptamer Against Influenza A Virus: An Effective Inhibitor to the Hemagglutinin-Glycan Interactions. Nucleic Acid Ther. 2016, 26, 166–172. [Google Scholar] [CrossRef]
- Tseng, Y.T.; Wang, C.H.; Chang, C.P.; Lee, G.B. Integrated microfluidic system for rapid detection of influenza H1N1 virus using a sandwich-based aptamer assay. Biosens. Bioelectron. 2016, 82, 105–111. [Google Scholar] [CrossRef]
- Wu, F.; Yuan, H.; Zhou, C.; Mao, M.; Liu, Q.; Shen, H.; Cen, Y.; Qin, Z.; Ma, L.; Li, L.S. Multiplexed detection of influenza A virus subtype H5 and H9 via quantum dot-based immunoassay. Biosens. Bioelectron. 2016, 77, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Mao, M.; Liu, Q.; Shi, L.; Cen, Y.; Qin, Z.; Ma, L. Ultra Sensitive Detection of Influenza A Virus Based on Cdse/Zns Quantum Dots Immunoassay. SOJ Biochem. 2016, 2, 6. [Google Scholar] [CrossRef]
- Hmila, I.; Wongphatcharachai, M.; Laamiri, N.; Aouini, R.; Marnissi, B.; Arbi, M.; Sreevatsan, S.; Ghram, A. A novel method for detection of H9N2 influenza viruses by an aptamer-real time-PCR. J. Virol. Methods 2017, 243, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Kim, J.; Ryu, I.; Woo, H.M.; Lee, T.G.; Jung, W.; Yim, S.; Jeong, Y.J. An Aptamer-Based Electrochemical Sensor That Can Distinguish Influenza Virus Subtype H1 from H5. J. Microbiol. Biotechnol. 2017, 27, 2037–2043. [Google Scholar] [CrossRef]
- Bhardwaj, J.; Chaudhary, N.; Kim, H.; Jang, J. Subtyping of influenza A H1N1 virus using a label-free electrochemical biosensor based on the DNA aptamer targeting the stem region of HA protein. Anal. Chim. Acta 2019, 1064, 94–103. [Google Scholar] [CrossRef]
- Bai, Z.; Wei, H.; Yang, X.; Zhu, Y.; Peng, Y.; Yang, J.; Wang, C.; Rong, Z.; Wang, S. Rapid Enrichment and Ultrasensitive Detection of Influenza A Virus in Human Specimen using Magnetic Quantum Dot Nanobeads Based Test Strips. Sens. Actuators B Chem. 2020, 325, 128780. [Google Scholar] [CrossRef]
- Kwon, J.; Lee, Y.; Lee, T.; Ahn, J.H. Aptamer-Based Field-Effect Transistor for Detection of Avian Influenza Virus in Chicken Serum. Anal. Chem. 2020, 92, 5524–5531. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.V.T.; Dao, T.D.; Trinh, T.T.T.; Choi, D.Y.; Yu, S.T.; Park, H.; Yeo, S.J. Sensitive detection of influenza a virus based on a CdSe/CdS/ZnS quantum dot-linked rapid fluorescent immunochromatographic test. Biosens. Bioelectron. 2020, 155, 112090. [Google Scholar] [CrossRef] [PubMed]
- Bizyaeva, A.A.; Bunin, D.A.; Moiseenko, V.L.; Gambaryan, A.S.; Balk, S.; Tashlitsky, V.N.; Arutyunyan, A.M.; Kopylov, A.M.; Zavyalova, E.G. The Functional Role of Loops and Flanking Sequences of G-Quadruplex Aptamer to the Hemagglutinin of Influenza a Virus. Int. J. Mol. Sci. 2021, 22, 2409. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Fauzi, H.; Fukuda, K.; Sekiya, S.; Kakiuchi, N.; Shimotohno, K.; Taira, K.; Kusakabe, I.; Nishikawa, S. The RNA aptamer-binding site of hepatitis C virus NS3 protease. Biochem. Biophys. Res. Commun. 2000, 279, 557–562. [Google Scholar] [CrossRef]
- Sekiya, S.; Nishikawa, F.; Fukuda, K.; Nishikawa, S. Structure/function analysis of an RNA aptamer for hepatitis C virus NS3 protease. J. Biochem. 2003, 133, 351–359. [Google Scholar] [CrossRef]
- Ahn, D.G.; Jeon, I.J.; Kim, J.D.; Song, M.S.; Han, S.R.; Lee, S.W.; Jung, H.; Oh, J.W. RNA aptamer-based sensitive detection of SARS coronavirus nucleocapsid protein. Analyst 2009, 134, 1896–1901. [Google Scholar] [CrossRef]
- Park, J.H.; Jee, M.H.; Kwon, O.S.; Keum, S.J.; Jang, S.K. Infectivity of hepatitis C virus correlates with the amount of envelope protein E2: Development of a new aptamer-based assay system suitable for measuring the infectious titer of HCV. Virology 2013, 439, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Song, J.; Wei, X.; Huang, M.; Sun, M.; Zhu, L.; Lin, B.; Shen, H.; Zhu, Z.; Yang, C. Discovery of Aptamers Targeting the Receptor-Binding Domain of the SARS-CoV-2 Spike Glycoprotein. Anal. Chem. 2020, 92, 9895–9900. [Google Scholar] [CrossRef]
- Schmitz, A.; Weber, A.; Bayin, M.; Breuers, S.; Fieberg, V.; Famulok, M.; Mayer, G. A SARS-CoV-2 Spike Binding DNA Aptamer that Inhibits Pseudovirus Infection by an RBD-Independent Mechanism. Angew. Chem. Int. Ed. 2021, 60, 10279–10285. [Google Scholar] [CrossRef]
- Bruno, J.G.; Carrillo, M.P.; Richarte, A.M.; Phillips, T.; Andrews, C.; Lee, J.S. Development, screening, and analysis of DNA aptamer libraries potentially useful for diagnosis and passive immunity of arboviruses. BMC Res. Notes 2012, 5, 633. [Google Scholar] [CrossRef] [Green Version]
- Eulberg, D.; Buchner, K.; Maasch, C.; Klussmann, S. Development of an automated in vitro selection protocol to obtain RNA-based aptamers: Identification of a biostable substance P antagonist. Nucleic Acids Res. 2005, 33, e45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wochner, A.; Cech, B.; Menger, M.; Erdmann, V.A.; Glokler, J. Semi-automated selection of DNA aptamers using magnetic particle handling. BioTechniques 2007, 43, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Hünniger, T.; Wessels, H.; Fischer, C.; Paschke-Kratzin, A.; Fischer, M. Just in time-selection: A rapid semiautomated SELEX of DNA aptamers using magnetic separation and BEAMing. Anal. Chem. 2014, 86, 10940–10947. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.T.; Lee, W.B.; Tsai, Y.C.; Chuang, Y.J.; Hsu, K.F.; Lee, G.B. An automated microfluidic system for selection of aptamer probes against ovarian cancer tissues. Biomicrofluidics 2019, 13, 014114. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.S.; Tsai, Y.C.; Hsu, K.F.; Lee, G.B. Optimization of aptamer selection on an automated microfluidic system with cancer tissues. Lab Chip 2021, 21, 725–734. [Google Scholar] [CrossRef]
- Bruno, J.G.; Carrillo, M.P.; Phillips, T. In vitro antibacterial effects of antilipopolysaccharide DNA aptamer-C1qrs complexes. Folia Microbiol. 2008, 53, 295–302. [Google Scholar] [CrossRef]
- Stecker, J.R.; Savage, A.A.; Bruno, J.G.; Garcia, D.M.; Koke, J.R. Dynamics and visualization of MCF7 adenocarcinoma cell death by aptamer-C1q-mediated membrane attack. Nucleic Acid Ther. 2012, 22, 275–282. [Google Scholar] [CrossRef]
- Bruno, J.G. Aptamer-biotin-streptavidin-C1q complexes can trigger the classical complement pathway to kill cancer cells. Vitr. Cell. Dev. Biol. Anim. 2010, 46, 107–113. [Google Scholar] [CrossRef]
- Bruno, J.G.; Carrillo, M.P.; Crowell, R. Preliminary development of DNA aptamer-Fc conjugate opsonins. J. Biomed. Mater. Res. Part A 2009, 90, 1152–1161. [Google Scholar] [CrossRef]
- Kristian, S.A.; Hwang, J.H.; Hall, B.; Leire, E.; Iacomini, J.; Old, R.; Galili, U.; Roberts, C.; Mullis, K.B.; Westby, M.; et al. Retargeting pre-existing human antibodies to a bacterial pathogen with an alpha-Gal conjugated aptamer. J. Mol. Med. 2015, 93, 619–631. [Google Scholar] [CrossRef] [Green Version]
- Dobler, R.K.; Maki, W.C. Mars health care delivery systems: Aptamers provide critical technology. In Proceedings of the 12th NASA Symposium of VLSA Design, Coeur d’Alene, ID, USA, 4–5 October 2005. [Google Scholar]
- Olsen, T.R.; Tapia-Alveal, C.; Yang, K.-A.; Zhang, X.; Pereira, L.J.; Farmakidis, N.; Pei, R.; Stojanovic, M.N.; Lin, Q. Integrated Microfluidic Selex Using Free Solution Electrokinetics. J. Electrochem. Soc. 2017, 164, B3122–B3129. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, J.G. Applications in Which Aptamers Are Needed or Wanted in Diagnostics and Therapeutics. Pharmaceuticals 2022, 15, 693. https://doi.org/10.3390/ph15060693
Bruno JG. Applications in Which Aptamers Are Needed or Wanted in Diagnostics and Therapeutics. Pharmaceuticals. 2022; 15(6):693. https://doi.org/10.3390/ph15060693
Chicago/Turabian StyleBruno, John G. 2022. "Applications in Which Aptamers Are Needed or Wanted in Diagnostics and Therapeutics" Pharmaceuticals 15, no. 6: 693. https://doi.org/10.3390/ph15060693
APA StyleBruno, J. G. (2022). Applications in Which Aptamers Are Needed or Wanted in Diagnostics and Therapeutics. Pharmaceuticals, 15(6), 693. https://doi.org/10.3390/ph15060693





