Vascular Effects of Polyphenols from Agrimonia eupatoria L. and Role of Isoquercitrin in Its Vasorelaxant Potential in Human Arteries
Abstract
1. Introduction
2. Results
2.1. Phytochemical Profile of Infusion and EtOAc Fraction
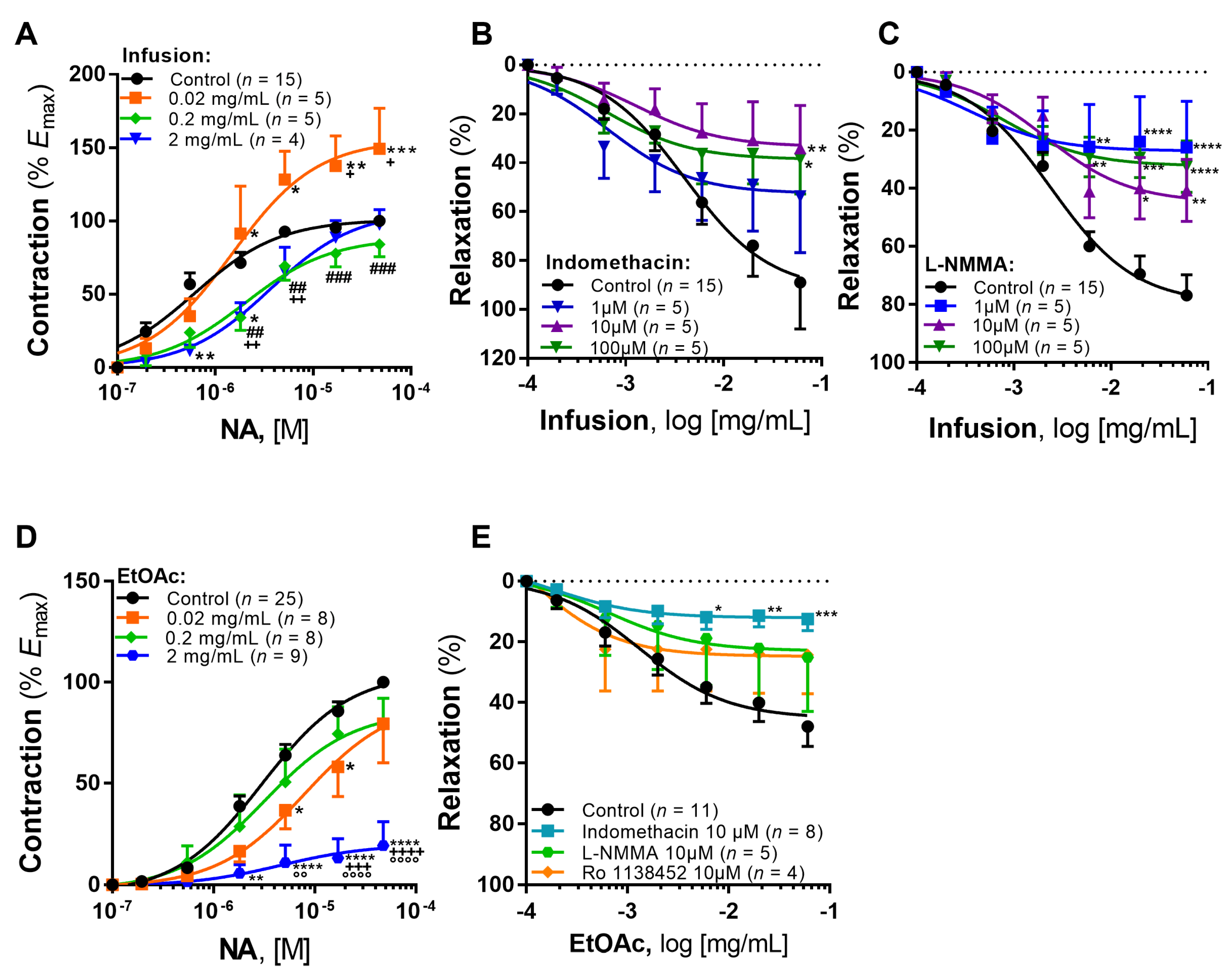
2.2. Vascular Effects
2.2.1. Infusion
2.2.2. EtOAc Fraction
2.2.3. Isoquercitrin, Tiliroside and p-Coumaric Acid
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. HPLC-PDA
4.3. Vascular Activity Studies
4.4. Analysis of Results
4.5. Reagents
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muruzović, M.; Mladenović, K.G.; Stefanović, O.D.; Vasić, S.M.; Čomić, L.R. Extracts of Agrimonia eupatoria L. as Sources of Biologically Active Compounds and Evaluation of Their Antioxidant, Antimicrobial, and Antibiofilm Activities. J. Food Drug Anal. 2016, 24, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Kiselova, Y.; Nashar, M.; Ivanova, D. Effects of Dietary Administration of Agrimonia eupatoria L. in Experimental Model of Metabolic Syndrome. Obes. Rev. 2011, 12, 155. [Google Scholar]
- Santos, T.N.; Costa, G.; Ferreira, J.P.; Liberal, J.; Francisco, V.; Paranhos, A.; Cruz, M.T.; Castelo-Branco, M.; Figueiredo, I.V.; Batista, M.T. Antioxidant, Anti-Inflammatory, and Analgesic Activities of Agrimonia eupatoria L. Infusion. Evid. Based Complement. Alternat. Med. 2017, 2017, 8309894. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Oliveira, P.; Fraga-Corral, M.; Pereira, A.G.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Prieto, M.A.; Simal-Gandara, J. Scientific Basis for the Industrialization of Traditionally Used Plants of the Rosaceae Family. Food Chem. 2020, 330, 127197. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.M.; Flatt, P.R. Actions of the Traditional Anti-Diabetic Plant, Agrimony eupatoria (Agrimony): Effects on Hyperglycaemia, Cellular Glucose Metabolism and Insulin Secretion. Br. J. Nutr. 1998, 80, 109–114. [Google Scholar] [CrossRef]
- Kuczmannová, A.; Balažová, A.; Račanská, E.; Kameníková, M.; Fialová, S.; Majerník, J.; Nagy, M.; Gál, P.; Mučaji, P. Agrimonia eupatoria L. and Cynara Cardunculus L. Water Infusions: Comparison of Anti-Diabetic Activities. Molecules 2016, 21, 1–12. [Google Scholar] [CrossRef]
- Mabry, T.; Markham, K.R.; Thomas, M.B. The Systematic Identification of Flavonoids; Part II; Springer: Berlin/Heidelberg, Germany, 1970. [Google Scholar]
- Couto, J.; Figueirinha, A.; Batista, M.T.; Paranhos, A.; Nunes, C.; Gonçalves, L.M.; Marto, J.; Fitas, M.; Pinto, P.; Ribeiro, H.M.; et al. Fragaria Vesca L. Extract: A Promising Cosmetic Ingredient with Antioxidant Properties. Antioxidants 2020, 9, 154. [Google Scholar] [CrossRef]
- Matos, P.; Figueirinha, A.; Paranhos, A.; Nunes, F.; Cruz, P.; Geraldes, C.F.G.C.; Cruz, M.T.; Batista, M.T. Bioactivity of Acanthus mollis—Contribution of Benzoxazinoids and Phenylpropanoids. J. Ethnopharmacol. 2018, 227, 198–205. [Google Scholar] [CrossRef]
- Novakovic, A.; Marinko, M.; Jankovic, G.; Stojanovic, I.; Milojevic, P.; Nenezic, D.; Kanjuh, V.; Yang, Q.; He, G.W. Endothelium-Dependent Vasorelaxant Effect of Procyanidin B2 on Human Internal Mammary Artery. Eur. J. Pharmacol. 2017, 807, 75–81. [Google Scholar] [CrossRef]
- Aldini, G.; Carini, M.; Piccoli, A.; Rossoni, G.; Facino, R.M. Procyanidins from Grape Seeds Protect Endothelial Cells from Peroxynitrite Damage and Enhance Endothelium-Dependent Relaxation in Human Artery: New Evidences for Cardio-Protection. Life Sci. 2003, 73, 2883–2898. [Google Scholar] [CrossRef]
- Correia, H.; González-Paramás, A.; Amaral, M.T.; Santos-Buelga, C.; Batista, M.T. Polyphenolic Profile Characterization of Agrimonia eupatoria L. by HPLC with Different Detection Devices. Biomed. Chromatogr. 2006, 20, 88–94. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mihaljević, Z.; Matić, A.; Stupin, A.; Frkanec, R.; Tavčar, B.; Kelava, V.; Bujak, I.T.; Kolobarić, N.; Kibel, A.; Drenjančević, I. Arachidonic Acid Metabolites of Cyp450 Enzymes and Hif-1α Modulate Endothelium-Dependent Vasorelaxation in Sprague-Dawley Rats under Acute and Intermittent Hyperbaric Oxygenation. Int. J. Mol. Sci. 2020, 21, 6353. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.L.; Gragasin, F.S.; Wu, X.; Wang, S.; McMurtry, S.; Kim, D.H.; Platonov, M.; Koshal, A.; Hashimoto, K.; Campbell, W.B.; et al. Endothelium-Derived Hyperpolarizing Factor in Human Internal Mammary Artery Is 11,12-Epoxyeicosatrienoic Acid and Causes Relaxation by Activating Smooth Muscle BKCa Channels. Circulation 2003, 107, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, P.; Li, J.; Lu, J.; Ge, J.; Zhao, Z.; Ma, X.; Wan, S.; Yao, X.; Shen, B. Epoxyeicosatrienoic Acids Act through TRPV4-TRPC1-KCa1.1 Complex to Induce Smooth Muscle Membrane Hyperpolarization and Relaxation in Human Internal Mammary Arteries. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Foudi, N.; Kotelevets, L.; Gomez, I.; Louedec, L.; Longrois, D.; Chastre, E.; Norel, X. Differential Reactivity of Human Mammary Artery and Saphenous Vein to Prostaglandin E2: Implication for Cardiovascular Grafts. Br. J. Pharmacol. 2011, 163, 826–834. [Google Scholar] [CrossRef]
- Foudi, N.; Ozen, G.; Amgoud, Y.; Louedec, L.; Choqueux, C.; Badi, A.; Kotelevets, L.; Chastre, E.; Longrois, D.; Norel, X. Decreased Vasorelaxation Induced by Iloprost during Acute Inflammation in Human Internal Mammary Artery. Eur. J. Pharmacol. 2017, 804, 31–37. [Google Scholar] [CrossRef]
- Kim, C.H.; Kim, J.E.; Song, Y.J. Antiviral Activities of Quercetin and Isoquercitrin against Human Herpesviruses. Molecules 2020, 25, 2379. [Google Scholar] [CrossRef]
- Valentová, K.; Vrba, J.; Bancířová, M.; Ulrichová, J.; Křen, V. Isoquercitrin: Pharmacology, Toxicology, and Metabolism. Food Chem. Toxicol. 2014, 68, 267–282. [Google Scholar] [CrossRef]
- Gasparotto, A.; Gasparotto, F.M.; Lourenço, E.L.B.; Crestani, S.; Stefanello, M.E.A.; Salvador, M.J.; da Silva-Santos, J.E.; Marques, M.C.A.; Kassuya, C.A.L. Antihypertensive Effects of Isoquercitrin and Extracts from Tropaeolum Majus L.: Evidence for the Inhibition of Angiotensin Converting Enzyme. J. Ethnopharmacol. 2011, 134, 363–372. [Google Scholar] [CrossRef]
- Gasparotto, A., Jr.; dos Reis Piornedo, R.; Assreuy, J.; da Silva-Santos, J.E. Nitric Oxide and Kir6.1 Potassium Channel Mediate Isoquercitrin-Induced Endothelium-Dependent and Independent Vasodilation in the Mesenteric Arterial Bed of Rats. Eur. J. Pharmacol. 2016, 788, 328–334. [Google Scholar] [CrossRef]
- Duan, H.; Zhang, Q.; Liu, J.; Li, R.; Wang, D.; Peng, W.; Wu, C. Suppression of Apoptosis in Vascular Endothelial Cell, the Promising Way for Natural Medicines to Treat Atherosclerosis. Pharmacol. Res. 2021, 168, 105599. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Huang, S.; Xu, M.; Gong, Y.; Li, D.; Wan, C.; Wu, H.; Tang, Q. Isoquercitrin Protects HUVECs against High Glucose-Induced Apoptosis through Regulating P53 Proteasomal Degradation. Int J. Mol. Med. 2021, 48, 122. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, K.; Koyama, H.; Moriwaki, M.; Emura, K.; Okuyama, S.; Sato, E.; Inoue, M.; Shioi, A.; Nishizawa, Y. Atheroprotective and Plaque-Stabilizing Effects of Enzymatically Modified Isoquercitrin in Atherogenic ApoE-Deficient Mice. Nutrition 2009, 25, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, N.P.; Bondonno, C.P.; Rich, L.; Mas, E.; Shinde, S.; Ward, N.C.; Hodgson, J.M.; Croft, K.D. Acute Effects of Quercetin-3-O-Glucoside on Endothelial Function and Blood Pressure: A Randomized Dose-Response Study. Am. J. Clin. Nutr. 2016, 104, 97–103. [Google Scholar] [CrossRef]
- Bondonno, N.P.; Bondonno, C.P.; Ward, N.C.; Woodman, R.J.; Hodgson, J.M.; Croft, K.D. Enzymatically Modified Isoquercitrin Improves Endothelial Function in Volunteers at Risk of Cardiovascular Disease. Br. J. Nutr. 2020, 123, 182–189. [Google Scholar] [CrossRef]
- Roghani, M.; Baluchnejadmojarad, T.; Reza Vaez-Mahdavi, M.; Roghani-Dehkordi, F. Mechanisms Underlying Quercetin-Induced Vasorelaxation in Aorta of Subchronic Diabetic Rats: An in Vitro Study. Vascul. Pharmacol. 2004, 42, 31–35. [Google Scholar] [CrossRef]
- Khoo, N.K.H.; White, C.R.; Pozzo-Miller, L.; Zhou, F.; Constance, C.; Inoue, T.; Patel, R.P.; Parks, D.A. Dietary Flavonoid Quercetin Stimulates Vasorelaxation in Aortic Vessels. Free Rad. Biol. Med. 2010, 49, 339–347. [Google Scholar] [CrossRef]
- Yuan, T.Y.; Niu, Z.R.; Chen, D.; Chen, Y.C.; Zhang, H.F.; Fang, L.H.; Du, G.H. Vasorelaxant Effect of Quercetin on Cerebral Basilar Artery in Vitro and the Underlying Mechanisms Study. J. Asian Nat. Prod. Res. 2018, 20, 477–487. [Google Scholar] [CrossRef]
- Iozzi, D.; Schubert, R.; Kalenchuk, V.U.; Neri, A.; Sgaragli, G.; Fusi, F.; Saponara, S. Quercetin Relaxes Rat Tail Main Artery Partly via a PKG-Mediated Stimulation of KCa1.1 Channels. Acta Physiol. 2013, 208, 329–339. [Google Scholar] [CrossRef]
- Hou, X.; Liu, Y.; Niu, L.; Cui, L.; Zhang, M. Enhancement of Voltage-Gated K+ Channels and Depression of Voltage-Gated Ca2+ Channels Are Involved in Quercetin-Induced Vasorelaxation in Rat Coronary Artery. Planta Med. 2014, 80, 465–472. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.H.; El-Mahdy, M.A.; Abd-Ellah, M.F.; Helal, G.K.; Khalifa, F.; Hamada, F.M.A. Influence of p-Coumaric Acid on Doxorubicin-Induced Oxidative Stress in Rat’s Heart. Pharmacol. Res. 2003, 48, 461–465. [Google Scholar] [CrossRef]
- Luceri, C.; Giannini, L.; Lodovici, M.; Antonucci, E.; Abbate, R.; Masini, E.; Dolara, P. p-Coumaric Acid, a Common Dietary Phenol, Inhibits Platelet Activity In Vitro and In Vivo. Br. J. Nutr. 2007, 97, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.C.; Pereira, A.C.; Rezende, B.A.; da Silva, J.P.F.; Cruz, J.S.; de Souza, M.D.F.V.; Gomes, R.A.; Teles, Y.C.F.; Cortes, S.F.; Lemos, V.S. Mechanism of the Antihypertensive and Vasorelaxant Effects of the Flavonoid Tiliroside in Resistance Arteries. Planta Med. 2013, 79, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Lagunas-Herrera, H.; Tortoriello, J.; Herrera-Ruiz, M.; Belen Martínez-Henández, G.; Zamilpa, A.; Santamaría, L.A.; Lorenzana, M.G.; Lombardo-Earl, G.; Jiménez-Ferrer, E. Acute and Chronic Antihypertensive Effect of Fractions, Tiliroside and Scopoletin from Malva parviflora. Biol. Pharm. Bull. 2019, 18, 18–25. [Google Scholar] [CrossRef]
- Simões, D.M.; Malheiros, J.; Antunes, P.E.; Figueirinha, A.; Cotrim, M.D.; Fonseca, D.A. Vascular Activity of Infusion and Fractions of Cymbopogon citratus (DC) Stapf. in Human Arteries. J. Ethnopharmacol. 2020, 258, 112947. [Google Scholar] [CrossRef]
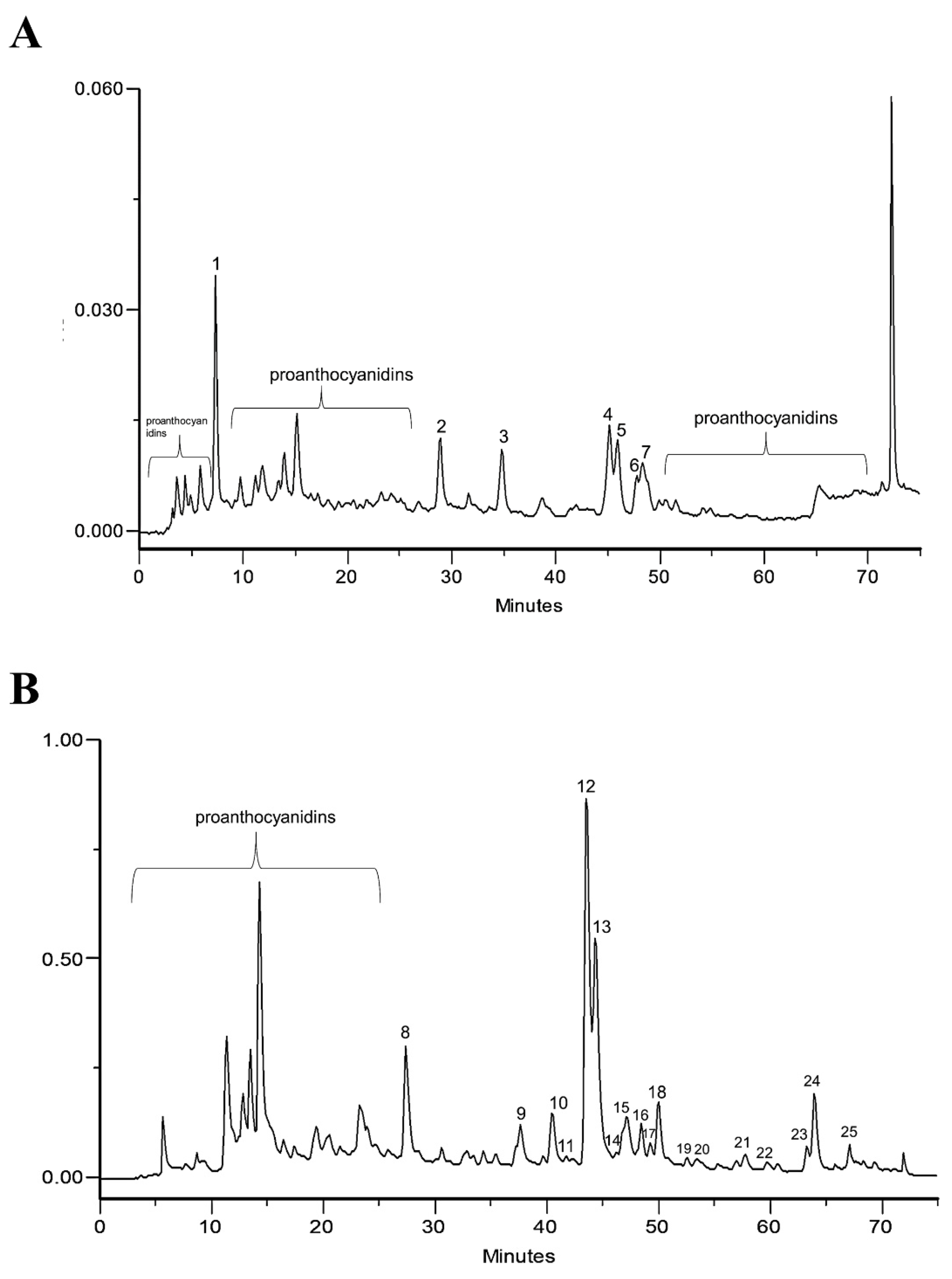

| Compound (Tentative Identification) | μg of Compound per 100 g of Extract | μg of Compound per 2 mg of Extract/mL 1 | |
|---|---|---|---|
| Infusion | |||
| Peak 2 | p-coumaric acid | 73.00 | 1.470 |
| Peak 3 | Quercetin derivatives 2 | 150.7 | 3.140 |
| Peak 4 | Isoquercitrin | 1500 | 30.16 |
| Peak 5 | Quercetin derivatives 2 | 270.8 | 5.550 |
| Peak 6 | Tiliroside | 147.0 | 2.940 |
| EtOAc fraction | |||
| Peak 8 | p-coumaric acid | 70.00 | 1.400 |
| Peak 10 | Quercetin derivatives 2 | 157.9 | 3.150 |
| Peak 12 | Isoquercitrin | 1400 | 28.00 |
| Peak 13 | Quercetin derivatives 2 | 333.5 | 6.670 |
| Peak 16 | Tiliroside | 138.0 | 2.760 |
| Studies (Incubation with Infusion or Compound) | Concentration | Maximal Effect 1 | Potency 2 | n |
|---|---|---|---|---|
| Influence on adrenergic contraction (infusion) | Control | 100.00 ± 0.00 | 6.21 ± 0.07 | 15 |
| 0.02 mg/mL | 149.18 ± 27.76 *** | 5.83 ± 0.18 | 5 | |
| 0.2 mg/mL | 84.08 ± 8.55 ### | 5.69 ± 0.14 * | 5 | |
| 2 mg/mL | 97.65 ± 10.15 + | 5.44 ± 0.14 *** | 4 | |
| Role of COX (indomethacin) | Control | 89.99 ± 18.89 | 2.40 ± 0.17 | 15 |
| 1 μM | 53.77 ± 22.91 | 3.18 ± 0.36 | 5 | |
| 10 μM | 34.03 ± 17.36 ** | 2.89 ± 0.44 | 5 | |
| 100 μM | 38.44 ± 14.18 * | 3.19 ± 0.29 | 5 | |
| Role of NO (L-NMMA) | Control | 76.88 ± 6.98 | 2.62 ± 0.09 | 15 |
| 1 μM | 26.00 ± 15.91 **** | 3.45 ± 0.56 | 5 | |
| 10 μM | 40.73 ± 10.62 ** | 2.67 ± 0.24 | 5 | |
| 100 μM | 32.51 ± 8.7 **** | 3.12 ± 0.22 | 5 |
| Studies (Incubation with Fraction or Compound) | Concentration | Maximal Effect 1 | Potency 2 | n |
|---|---|---|---|---|
| Influence on adrenergic contraction (EtOAc fraction) | Control | 100.00 ± 0.00 | 5.51 ± 0.06 | 25 |
| 0.02 mg/mL | 79.43 ± 19.32 ++++ | 5.09 ± 0.26 | 8 | |
| 0.2 mg/mL | 78.40 ± 13.63 °°°° | 5.49 ± 0.27 | 8 | |
| 2 mg/mL | 19.35 ± 11.82 **** | 5.30 ± 0.73 | 9 | |
| Role of mediators in vasorelaxation | Control | 47.95 ± 6.55 | 2.88 ± 0.22 | 11 |
| Indomethacin | 10 μM | 12.45 ± 3.78 *** | 3.75 ± 0.76 | 8 |
| L-NMMA | 10 μM | 25.14 ± 17.80 | 3.19 ± 1.25 | 5 |
| Ro 1138452 | 10 μM | 24.33 ± 12.75 | 4.00 ± 1.51 | 4 |
| Standard Compound | Range Concentrations (μg/mL) | n 1 | Slope | Intercept | R2 | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|---|---|---|
| p-coumaric acid | 2.5–15 | 6 | 8.95 × 106 | 3.90 × 106 | 0.9960 | 0.49 ± 0.32 | 2.66 ± 0.21 |
| Quercetin | 2.5–125 | 5 | 3.10 × 106 | 2.62 × 106 | 0.9932 | 8.18 ± 3.66 | 29.26 ± 3.26 |
| Tiliroside | 5–25 | 6 | 3.65 × 106 | 1.11 × 106 | 0.9979 | 1.30 ± 0.36 | 3.65 ± 0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malheiros, J.; Simões, D.M.; Antunes, P.E.; Figueirinha, A.; Cotrim, M.D.; Fonseca, D.A. Vascular Effects of Polyphenols from Agrimonia eupatoria L. and Role of Isoquercitrin in Its Vasorelaxant Potential in Human Arteries. Pharmaceuticals 2022, 15, 638. https://doi.org/10.3390/ph15050638
Malheiros J, Simões DM, Antunes PE, Figueirinha A, Cotrim MD, Fonseca DA. Vascular Effects of Polyphenols from Agrimonia eupatoria L. and Role of Isoquercitrin in Its Vasorelaxant Potential in Human Arteries. Pharmaceuticals. 2022; 15(5):638. https://doi.org/10.3390/ph15050638
Chicago/Turabian StyleMalheiros, Jéssica, Daniela M. Simões, Pedro E. Antunes, Artur Figueirinha, Maria Dulce Cotrim, and Diogo A. Fonseca. 2022. "Vascular Effects of Polyphenols from Agrimonia eupatoria L. and Role of Isoquercitrin in Its Vasorelaxant Potential in Human Arteries" Pharmaceuticals 15, no. 5: 638. https://doi.org/10.3390/ph15050638
APA StyleMalheiros, J., Simões, D. M., Antunes, P. E., Figueirinha, A., Cotrim, M. D., & Fonseca, D. A. (2022). Vascular Effects of Polyphenols from Agrimonia eupatoria L. and Role of Isoquercitrin in Its Vasorelaxant Potential in Human Arteries. Pharmaceuticals, 15(5), 638. https://doi.org/10.3390/ph15050638






