Antidepressant-like Activity of Patchouli Oil var. Tapak Tuan (Pogostemon cablin Benth) via Elevated Dopamine Level: A Study Using Rat Model
Abstract
:1. Introduction
2. Results
2.1. Components of iPO, POFs, and pPA
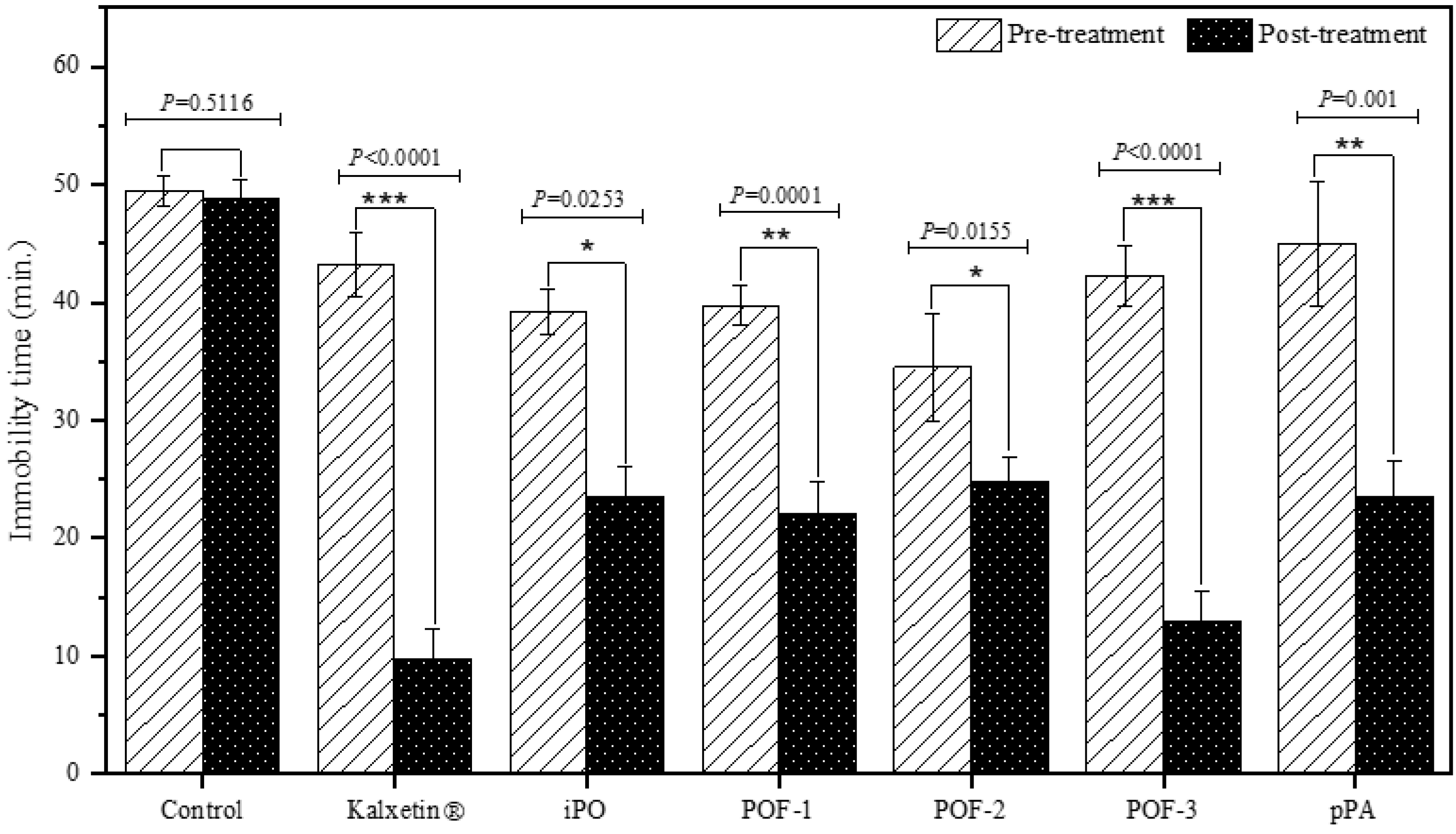
2.2. Effect of iPO, POFs, and pPA on Depression-Related Behavior
2.3. Effect of iPO, POFs, and pPA on Plasma Cortisol
2.4. Effect of iPO, POFs, and pPA on Neurotransmitters
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Study Design
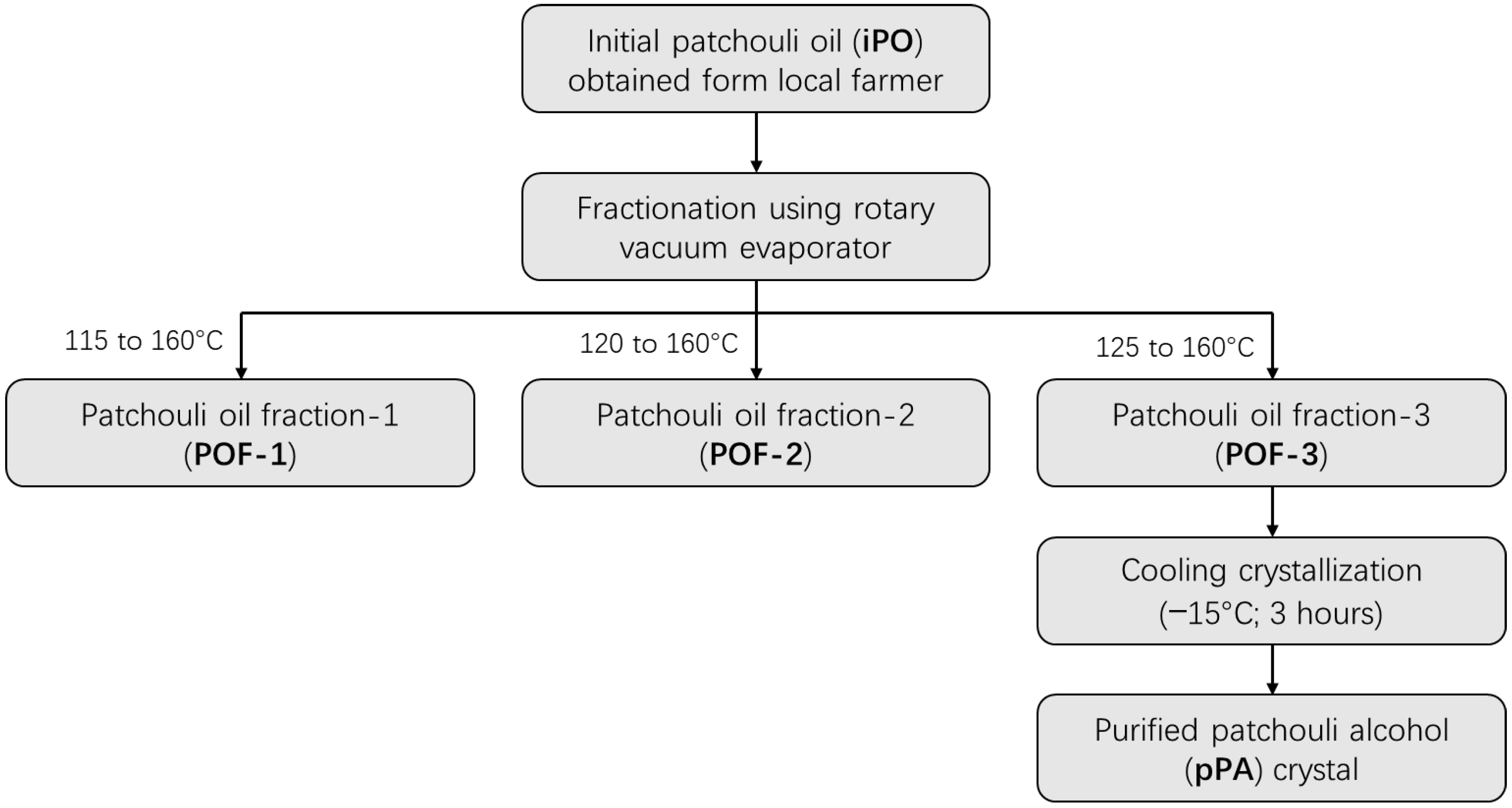
4.3. Preparation of POFs and pPA Crystal
4.4. TST and Exposure of PO-Based Aromatherapies
4.5. Determination of Plasma Cortisol and Brain Serotonin and Dopamine
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethical Clearance
References
- van Beek, T.A.; Joulain, D. The essential oil of patchouli, Pogostemon cablin: A review. Flavour Fragr. J. 2018, 33, 6–51. [Google Scholar] [CrossRef] [Green Version]
- Ernawati, E.; Masbar, R.; Majid, M.S.A.; Jamal, A. Production and marketing efficiency of patchouli oil industry in Indonesia. Reg. Sci. Inq. 2021, 13, 135–148. [Google Scholar]
- Nuryani, Y. Karakteristik empat aksesi nilam. Bul. Plasma Nutfah 2016, 12, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Ratnaningsih Dewi, A.; Nella, S.; Risdayanti; Siti, K. A stick formulation of a mixture of citronella oil, cananga oil and patchouli oil as aromatherapy. In Proceedings of the First International Conference on Health, Social Sciences and Technology (ICoHSST 2020), Palembang, Indonesia, 19 April 2021; pp. 257–261. [Google Scholar]
- Pandey, S.K.; Bhandari, S.; Sarma, N.; Begum, T.; Munda, S.; Baruah, J.; Gogoi, R.; Haldar, S.; Lal, M. Essential oil compositions, pharmacological importance and agro technological practices of Patchouli (Pogostemon cablin Benth.): A review. J. Essent. Oil Bear. Plants 2021, 24, 1212–1226. [Google Scholar] [CrossRef]
- Allard, M.E.; Katseres, J. Using essential oils to enhance nursing practice and for self-care. Am. J. Nurs. 2016, 116, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Vidana, D.I.; Ngai, S.P.; He, W.; Chow, J.K.; Lau, B.W.; Tsang, H.W. The Effectiveness of Aromatherapy for Depressive Symptoms: A Systematic Review. Evid. Based Complement. Altern. Med. 2017, 2017, 5869315. [Google Scholar] [CrossRef]
- Elbay, R.Y.; Kurtulmus, A.; Arpacioglu, S.; Karadere, E. Depression, anxiety, stress levels of physicians and associated factors in Covid-19 pandemics. Psychiatry Res. 2020, 290, 113130. [Google Scholar] [CrossRef]
- Bintari, D.C.; Sudibyo, D.A.; Karimah, A. Correlation between depression level and headache severity: A study among medical students during the COVID-19 pandemic. Narra J. 2021, 1, e64. [Google Scholar] [CrossRef]
- Malhi, G.S.; Morris, G.; Bell, E.; Hamilton, A. A new paradigm for achieving a rapid antidepressant response. Drugs 2020, 80, 755–764. [Google Scholar] [CrossRef]
- Wang, S.M.; Han, C.; Bahk, W.M.; Lee, S.J.; Patkar, A.A.; Masand, P.S.; Pae, C.U. Addressing the side effects of contemporary antidepressant drugs: A comprehensive review. Chonnam Med. J. 2018, 54, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Kohler-Forsberg, O.; Larsen, E.R.; Buttenschon, H.N.; Rietschel, M.; Hauser, J.; Souery, D.; Maier, W.; Farmer, A.; McGuffin, P.; Aitchison, K.J.; et al. Effect of antidepressant switching between nortriptyline and escitalopram after a failed first antidepressant treatment among patients with major depressive disorder. Br. J. Psychiatry 2019, 215, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Dome, P.; Tombor, L.; Lazary, J.; Gonda, X.; Rihmer, Z. Natural health products, dietary minerals and over-the-counter medications as add-on therapies to antidepressants in the treatment of major depressive disorder: A review. Brain Res. Bull. 2019, 146, 51–78. [Google Scholar] [CrossRef] [PubMed]
- Avram, S.; Stan, M.S.; Udrea, A.M.; Buiu, C.; Mernea, M. The study of natural compounds as antidepressants by bioinformatics methods. Biol. Life Sci. Forum 2021, 7, 17. [Google Scholar] [CrossRef]
- Mischoulon, D. Popular herbal and natural remedies used in psychiatry. Focus 2018, 16, 2–11. [Google Scholar] [CrossRef]
- Vangipuram, R.; Mask-Bull, L.; Kim, S.J. Cutaneous implications of essential oils. World J. Dermatol. 2017, 6, 27. [Google Scholar] [CrossRef]
- Wijaya, R.C. Lethal concentration 50% of pathchouli oil (Pogostemon cablin) towards zebrafish embryo (Danio rerio). Herb-Med. J. 2020, 3, 1–6. [Google Scholar] [CrossRef]
- Sah, S.P.; Mathela, C.S.; Chopra, K. Antidepressant effect of Valeriana wallichii patchouli alcohol chemotype in mice: Behavioural and biochemical evidence. J. Ethnopharmacol. 2011, 135, 197–200. [Google Scholar] [CrossRef]
- Sah, S.P.; Mathela, C.S.; Chopra, K. Involvement of nitric oxide (NO) signalling pathway in the antidepressant activity of essential oil of Valeriana wallichii Patchouli alcohol chemotype. Phytomedicine 2011, 18, 1269–1275. [Google Scholar] [CrossRef]
- Cahyono, E.; Rimawati, B.C.; Kusuma, E. Antidepressant activity of patchouli alcohol microcapsule. J. Phys. Conf. Ser. 2019, 1321, 022039. [Google Scholar] [CrossRef] [Green Version]
- Zhuo, J.; Chen, B.; Sun, C.; Jiang, T.; Chen, Z.; Liu, Y.; Nie, J.; Yang, H.; Zheng, J.; Lai, X.; et al. Patchouli alcohol protects against chronic unpredictable mild stress-induced depressant-like behavior through inhibiting excessive autophagy via activation of mTOR signaling pathway. Biomed. Pharm. 2020, 127, 110115. [Google Scholar] [CrossRef]
- Aguilar-Martinez, I.S.; Reyes-Mendez, M.E.; Herrera-Zamora, J.M.; Osuna-Lopez, F.; Virgen-Ortiz, A.; Mendoza-Munoz, N.; Gongora-Alfaro, J.L.; Moreno-Galindo, E.G.; Alamilla, J. Synergistic antidepressant-like effect of capsaicin and citalopram reduces the side effects of citalopram on anxiety and working memory in rats. Psychopharmacology 2020, 237, 2173–2185. [Google Scholar] [CrossRef] [PubMed]
- Aisyah, Y.; Anwar, S.H.; Annisa, Y. Increment of patchouli alcohol in patchouli oil by vacuum distillation fraction method. In Proceedings of the 3rd Annual International Conference Syiah Kuala University (AIC Unsyiah) 2013 in conjunction with the 2nd International Conference on Multidisciplinary Research (ICMR) 2013, Banda Aceh, Indonesia, 15 October 2013; pp. 25–29. [Google Scholar]
- Fathana, H.; Iqhrammullah, M.; Rahmi, R.; Adlim, M.; Lubis, S. Tofu wastewater-derived amino acids identification using LC-MS/MS and their uses in the modification of chitosan/TiO2 film composite. Chem. Data Collect. 2021, 35, 100754. [Google Scholar] [CrossRef]
- Manglani, N.; Deshmukh, V.; Kashyap, P. Evaluation of Anti-Depressant Activity of Pogostemon cablin (Labiatae). Int. J. PharmTech Res. 2011, 3, 58–61. [Google Scholar]
- Li, J.; Lu, C.; Gao, Z.; Feng, Y.; Luo, H.; Lu, T.; Sun, X.; Hu, J.; Luo, Y. SNRIs achieve faster antidepressant effects than SSRIs by elevating the concentrations of dopamine in the forebrain. Neuropharmacology 2020, 177, 108237. [Google Scholar] [CrossRef] [PubMed]
- Rincon-Cortes, M.; Grace, A.A. Antidepressant effects of ketamine on depression-related phenotypes and dopamine dysfunction in rodent models of stress. Behav. Brain Res. 2020, 379, 112367. [Google Scholar] [CrossRef] [PubMed]
- Field, T.; Hernandez-Reif, M.; Diego, M.; Schanberg, S.; Kuhn, C. Cortisol decreases and serotonin and dopamine increase following massage therapy. Int. J. Neurosci. 2005, 115, 1397–1413. [Google Scholar] [CrossRef]
- Padilla-de la Rosa, J.D.; Manzano-Alfaro, M.D.; Gomez-Huerta, J.R.; Arriola-Guevara, E.; Guatemala-Morales, G.; Cardador-Martinez, A.; Estarron-Espinosa, M. Innovation in a Continuous System of Distillation by Steam to Obtain Essential Oil from Persian Lime Juice (Citrus latifolia Tanaka). Molecules 2021, 26, 4172. [Google Scholar] [CrossRef]
- Qin, D.D.; Rizak, J.; Feng, X.L.; Yang, S.C.; Lu, L.B.; Pan, L.; Yin, Y.; Hu, X.T. Prolonged secretion of cortisol as a possible mechanism underlying stress and depressive behaviour. Sci. Rep. 2016, 6, 30187. [Google Scholar] [CrossRef]
- Pawluski, J.L.; van Donkelaar, E.; Abrams, Z.; Houbart, V.; Fillet, M.; Steinbusch, H.W.; Charlier, T.D. Fluoxetine dose and administration method differentially affect hippocampal plasticity in adult female rats. Neural Plast. 2014, 2014, 123026. [Google Scholar] [CrossRef]
- Kamińska, K.; Górska, A.; Noworyta-Sokołowska, K.; Wojtas, A.; Rogóż, Z.; Gołembiowska, K. The effect of chronic co-treatment with risperidone and novel antidepressant drugs on the dopamine and serotonin levels in the rats frontal cortex. Pharmacol. Rep. 2018, 70, 1023–1031. [Google Scholar] [CrossRef]
- Singh, V.P.; Jain, N.K.; Kulkarni, S.K. On the antinociceptive effect of fluoxetine, a selective serotonin reuptake inhibitor. Brain Res. 2001, 915, 218–226. [Google Scholar] [CrossRef]
- Ramsey, J.T.; Shropshire, B.C.; Nagy, T.R.; Chambers, K.D.; Li, Y.; Korach, K.S. Essential oils and health. Yale J. Biol. Med. 2020, 93, 291–305. [Google Scholar] [PubMed]
- Ali, B.; Al-Wabel, N.A.; Shams, S.; Ahamad, A.; Khan, S.A.; Anwar, F. Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop. Biomed. 2015, 5, 601–611. [Google Scholar] [CrossRef] [Green Version]
- O’Leary, O.F.; Cryan, J.F. The Tail-Suspension Test: A Model for Characterizing Antidepressant Activity in Mice. In Mood and Anxiety Related Phenotypes in Mice; Neuromethods: Washington, DC, USA, 2009; pp. 119–137. [Google Scholar]
- Pinheiro, B.G.; Silva, A.S.; Souza, G.E.; Figueiredo, J.G.; Cunha, F.Q.; Lahlou, S.; da Silva, J.K.; Maia, J.G.; Sousa, P.J. Chemical composition, antinociceptive and anti-inflammatory effects in rodents of the essential oil of Peperomia serpens (Sw.) Loud. J. Ethnopharmacol. 2011, 138, 479–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Rocca, V.; da Fonseca, D.V.; Silva-Alves, K.S.; Ferreira-da-Silva, F.W.; de Sousa, D.P.; Santos, P.L.; Quintans-Junior, L.J.; Leal-Cardoso, J.H.; de Almeida, R.N. Geraniol Induces Antinociceptive Effect in Mice Evaluated in Behavioural and Electrophysiological Models. Basic Clin. Pharmacol. Toxicol. 2017, 120, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Oshima, T.; Ito, M. Sedative effects of l-menthol, d-camphor, phenylethyl alcohol, and geraniol. J. Nat. Med. 2021, 75, 319–325. [Google Scholar] [CrossRef]
- Deng, X.Y.; Xue, J.S.; Li, H.Y.; Ma, Z.Q.; Fu, Q.; Qu, R.; Ma, S.P. Geraniol produces antidepressant-like effects in a chronic unpredictable mild stress mice model. Physiol. Behav. 2015, 152, 264–271. [Google Scholar] [CrossRef]
- Hasballah, K.; Sarong, M.; Rusly, R.; Fitria, H.; Maida, D.R.; Iqhrammullah, M. Antiproliferative activity of triterpenoid and steroid compounds from ethyl acetate extract of Calotropis gigantea root bark against P388 murine leukemia cell lines. Sci. Pharm. 2021, 89, 21. [Google Scholar] [CrossRef]
- Ghosh, S.; Kumar, A.; Sachan, N.; Chandra, P. Anxiolytic and antidepressant-like effects of essential oil from the fruits of Piper nigrum Linn. (Black pepper) in mice: Involvement of serotonergic but not GABAergic transmission system. Heliyon 2021, 7, e06884. [Google Scholar] [CrossRef]
- Parente, M.S.R.; Custodio, F.R.; Cardoso, N.A.; Lima, M.J.A.; Melo, T.S.; Linhares, M.I.; Siqueira, R.M.P.; Nascimento, A.A.D.; Catunda Junior, F.E.A.; Melo, C.T.V. Antidepressant-Like Effect of Lippia sidoides CHAM (Verbenaceae) Essential Oil and Its Major Compound Thymol in Mice. Sci. Pharm. 2018, 86, 27. [Google Scholar] [CrossRef] [Green Version]
- Birmann, P.T.; Casaril, A.M.; Zugno, G.P.; Acosta, G.G.; Severo Sabedra Sousa, F.; Collares, T.; Seixas, F.K.; Jacob, R.G.; Bruning, C.A.; Savegnago, L.; et al. Flower essential oil of Tagetes minuta mitigates oxidative stress and restores BDNF-Akt/ERK2 signaling attenuating inflammation- and stress-induced depressive-like behavior in mice. Brain Res. 2022, 1784, 147845. [Google Scholar] [CrossRef] [PubMed]
- El-Farhan, N.; Rees, D.A.; Evans, C. Measuring cortisol in serum, urine and saliva—Are our assays good enough? Ann. Clin. Biochem 2017, 54, 308–322. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, S.; Kirschbaum, C.; Bohme, C.; Petrowski, K. Cortisol stress response in post-traumatic stress disorder, panic disorder, and major depressive disorder patients. Psychoneuroendocrinology 2017, 83, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Belujon, P.; Grace, A.A. Dopamine System Dysregulation in Major Depressive Disorders. Int. J. Neuropsychopharmacol. 2017, 20, 1036–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duval, F.; Mokrani, M.C.; Erb, A.; Danila, V.; Lopera, F.G.; Foucher, J.R.; Jeanjean, L.C. Thyroid axis activity and dopamine function in depression. Psychoneuroendocrinology 2021, 128, 105219. [Google Scholar] [CrossRef] [PubMed]
- Reimold, M.; Batra, A.; Knobel, A.; Smolka, M.N.; Zimmer, A.; Mann, K.; Solbach, C.; Reischl, G.; Schwärzler, F.; Gründer, G.; et al. Anxiety is associated with reduced central serotonin transporter availability in unmedicated patients with unipolar major depression: A [11C] DASB PET study. Mol. Psychiatry 2008, 13, 606–613. [Google Scholar] [CrossRef]
- Zmudzka, E.; Salaciak, K.; Sapa, J.; Pytka, K. Serotonin receptors in depression and anxiety: Insights from animal studies. Life Sci. 2018, 210, 106–124. [Google Scholar] [CrossRef]
- Can, A.; Dao, D.T.; Terrillion, C.E.; Piantadosi, S.C.; Bhat, S.; Gould, T.D. The tail suspension test. J. Vis. Exp. 2012, 59, e3769. [Google Scholar] [CrossRef] [Green Version]
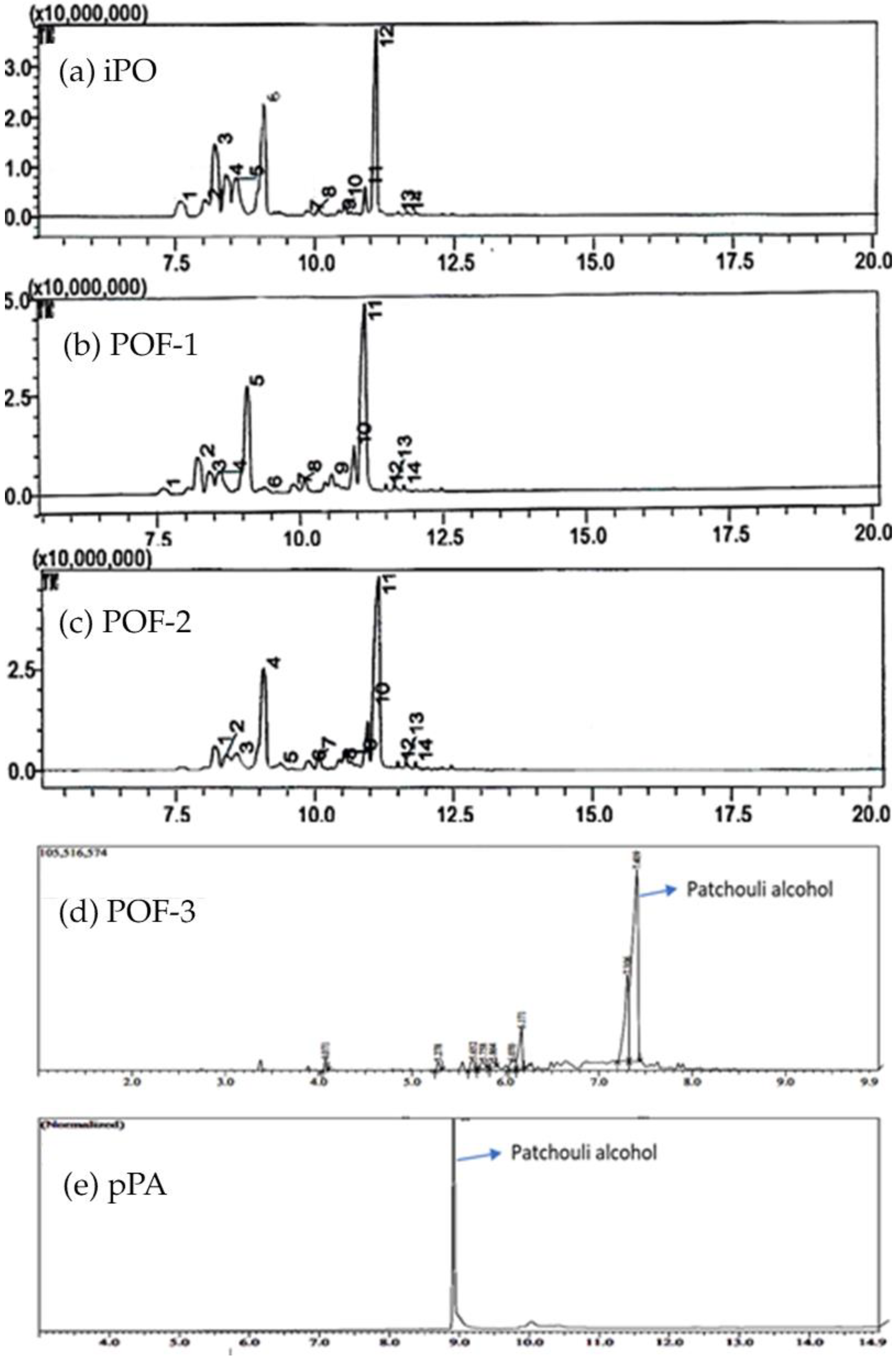
| Chemical Compounds | Percentage of Area (%) | ||||
|---|---|---|---|---|---|
| iPO | POF-1 | POF-2 | POF-3 | pPA | |
| Patchouli alcohol | 28.68 | 42.81 | 49.29 | 60.66 | 100 |
| δ-Guaiene | 24.87 | 25.56 | 27.51 | - | - |
| Ledol | - | - | - | 21.54 | |
| α-Guaiene | 16.89 | 8.73 | 4.58 | - | - |
| Seychellene | 8.32 | 3.99 | 0.91 | 1.71 | - |
| γ-Elemene | - | - | - | 7.83 | |
| α-Patchoulene | 7.29 | - | - | 1.43 | - |
| β-Patchoulene | 5.05 | - | - | - | - |
| (-)-Globulol | - | 5.77 | 6.42 | - | |
| Champacol | - | 3.05 | 1.53 | - | - |
| Globulol | 2.94 | - | 1.85 | - | - |
| α-Gurjunene | - | - | - | 2.54 | - |
| α-Selinene | - | - | - | 2.00 | - |
| Caryophyllene | 1.99 | - | 0.78 | - | - |
| 8-Isopropenyl-1,5-dimethyl-cyclodeca-1,5-diene | - | 1.78 | - | - | - |
| But-3-enal, 2-methyl-4-(2,6,6-trimethyl-1-cyclohexenyl)- | - | 1.75 | 1.96 | - | - |
| Aromadendrene, dehydro- | - | 1.48 | 1.23 | - | - |
| (-)-β-Elemene | - | - | - | 1.25 | - |
| 2,3,3-Trimethyl-2-(3-methyl-buta-1,3- dienyl)-cyclohexanone | 1.10 | - | - | - | - |
| Longipinocarveol, trans- | 1.08 | - | 2.34 | - | - |
| trans-Geraniol | - | - | - | 1.05 | |
| (-)-Globulol | 0.82 | 1.81 | - | - | - |
| (-)-Spathulenol | 0.41 | 1.95 | - | - | - |
| 2-Methyl-3-isopropenylcyclohexanol | 0.34 | 0.50 | 0.60 | - | - |
| 2(1H)Naphthalenone, 3,5,6,7,8,8a- hexahydro-4,8a-dimethyl-6-(1- methylethenyl)- | 0.22 | 0.43 | 0.45 | - | - |
| Selina-6-en-4-ol | - | 0.39 | 0.55 | - | - |
| Group | Cortisol | Serotonin | Dopamine | |||
|---|---|---|---|---|---|---|
| Level, Mean ± SD (ng/mL) | p-Value | Level, Mean ± SD (ng/mL) | p-Value | Level, Mean ± SD (ng/mL) | p-Value | |
| Normal | 23.32 ± 2.27 | NA | 22.84 ± 4.36 | NA | 220.98 ± 2.36 | NA |
| Control | 121.61 ± 30.54 | ## 0.0073 | 20.72 ± 1.74 | 0.4177 | 182.24 ± 9.51 | ## 0.0025 |
| Kalxetin® | 169.49 ± 24.59 | 0.0522 | 17.14 ± 1.02 | * 0.0172 | 191.54 ± 15.79 | 0.3600 |
| iPO | 99.85 ± 27.35 | 0.3296 | 23.80 ± 3.66 | 0.1976 | 211.71 ± 17.93 | * 0.0374 |
| POF-1 | 153.98 ± 125.98 | 0.6485 | 20.88 ± 2.04 | 0.9103 | 192.86 ± 15.92 | 0.3049 |
| POF-2 | 196.69 ± 22.52 | 0.5544 | 17.68 ± 0.90 | * 0.0309 | 133.55 ± 5.88 | * 0.0492 |
| POF-3 | 81.27 ± 45.02 | 0.1951 | 23.71 ± 2.01 | 0.0662 | 223.36 ± 19.18 | * 0.0155 |
| pPA | 123.58 ± 41.20 | 0.9415 | 24.43 ± 4.12 | 0.1718 | 215.62 ± 28.45 | 0.0962 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astuti, P.; Khairan, K.; Marthoenis, M.; Hasballah, K. Antidepressant-like Activity of Patchouli Oil var. Tapak Tuan (Pogostemon cablin Benth) via Elevated Dopamine Level: A Study Using Rat Model. Pharmaceuticals 2022, 15, 608. https://doi.org/10.3390/ph15050608
Astuti P, Khairan K, Marthoenis M, Hasballah K. Antidepressant-like Activity of Patchouli Oil var. Tapak Tuan (Pogostemon cablin Benth) via Elevated Dopamine Level: A Study Using Rat Model. Pharmaceuticals. 2022; 15(5):608. https://doi.org/10.3390/ph15050608
Chicago/Turabian StyleAstuti, Puji, Khairan Khairan, Marthoenis Marthoenis, and Kartini Hasballah. 2022. "Antidepressant-like Activity of Patchouli Oil var. Tapak Tuan (Pogostemon cablin Benth) via Elevated Dopamine Level: A Study Using Rat Model" Pharmaceuticals 15, no. 5: 608. https://doi.org/10.3390/ph15050608
APA StyleAstuti, P., Khairan, K., Marthoenis, M., & Hasballah, K. (2022). Antidepressant-like Activity of Patchouli Oil var. Tapak Tuan (Pogostemon cablin Benth) via Elevated Dopamine Level: A Study Using Rat Model. Pharmaceuticals, 15(5), 608. https://doi.org/10.3390/ph15050608






