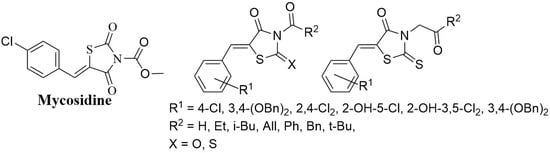
Antifungal Thiazolidines: Synthesis and Biological Evaluation of Mycosidine Congeners
Abstract
Share and Cite
Levshin, I.B.; Simonov, A.Y.; Lavrenov, S.N.; Panov, A.A.; Grammatikova, N.E.; Alexandrov, A.A.; Ghazy, E.S.M.O.; Savin, N.A.; Gorelkin, P.V.; Erofeev, A.S.; et al. Antifungal Thiazolidines: Synthesis and Biological Evaluation of Mycosidine Congeners. Pharmaceuticals 2022, 15, 563. https://doi.org/10.3390/ph15050563
Levshin IB, Simonov AY, Lavrenov SN, Panov AA, Grammatikova NE, Alexandrov AA, Ghazy ESMO, Savin NA, Gorelkin PV, Erofeev AS, et al. Antifungal Thiazolidines: Synthesis and Biological Evaluation of Mycosidine Congeners. Pharmaceuticals. 2022; 15(5):563. https://doi.org/10.3390/ph15050563
Chicago/Turabian StyleLevshin, Igor B., Alexander Y. Simonov, Sergey N. Lavrenov, Alexey A. Panov, Natalia E. Grammatikova, Alexander A. Alexandrov, Eslam S. M. O. Ghazy, Nikita A. Savin, Peter V. Gorelkin, Alexander S. Erofeev, and et al. 2022. "Antifungal Thiazolidines: Synthesis and Biological Evaluation of Mycosidine Congeners" Pharmaceuticals 15, no. 5: 563. https://doi.org/10.3390/ph15050563
APA StyleLevshin, I. B., Simonov, A. Y., Lavrenov, S. N., Panov, A. A., Grammatikova, N. E., Alexandrov, A. A., Ghazy, E. S. M. O., Savin, N. A., Gorelkin, P. V., Erofeev, A. S., & Polshakov, V. I. (2022). Antifungal Thiazolidines: Synthesis and Biological Evaluation of Mycosidine Congeners. Pharmaceuticals, 15(5), 563. https://doi.org/10.3390/ph15050563