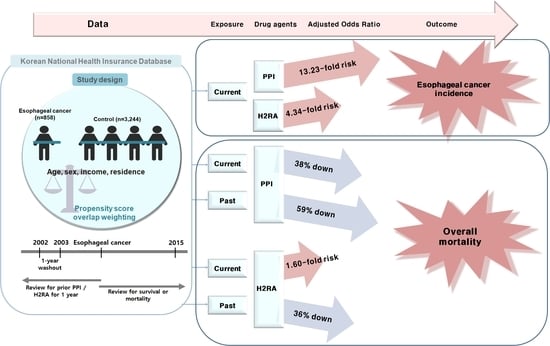
Possible Association between the Use of Proton Pump Inhibitors and H2 Receptor Antagonists, and Esophageal Cancer: A Nested Case–Control Study Using a Korean National Health Screening Cohort
Abstract
:1. Introduction
2. Results
2.1. Baseline Characteristics
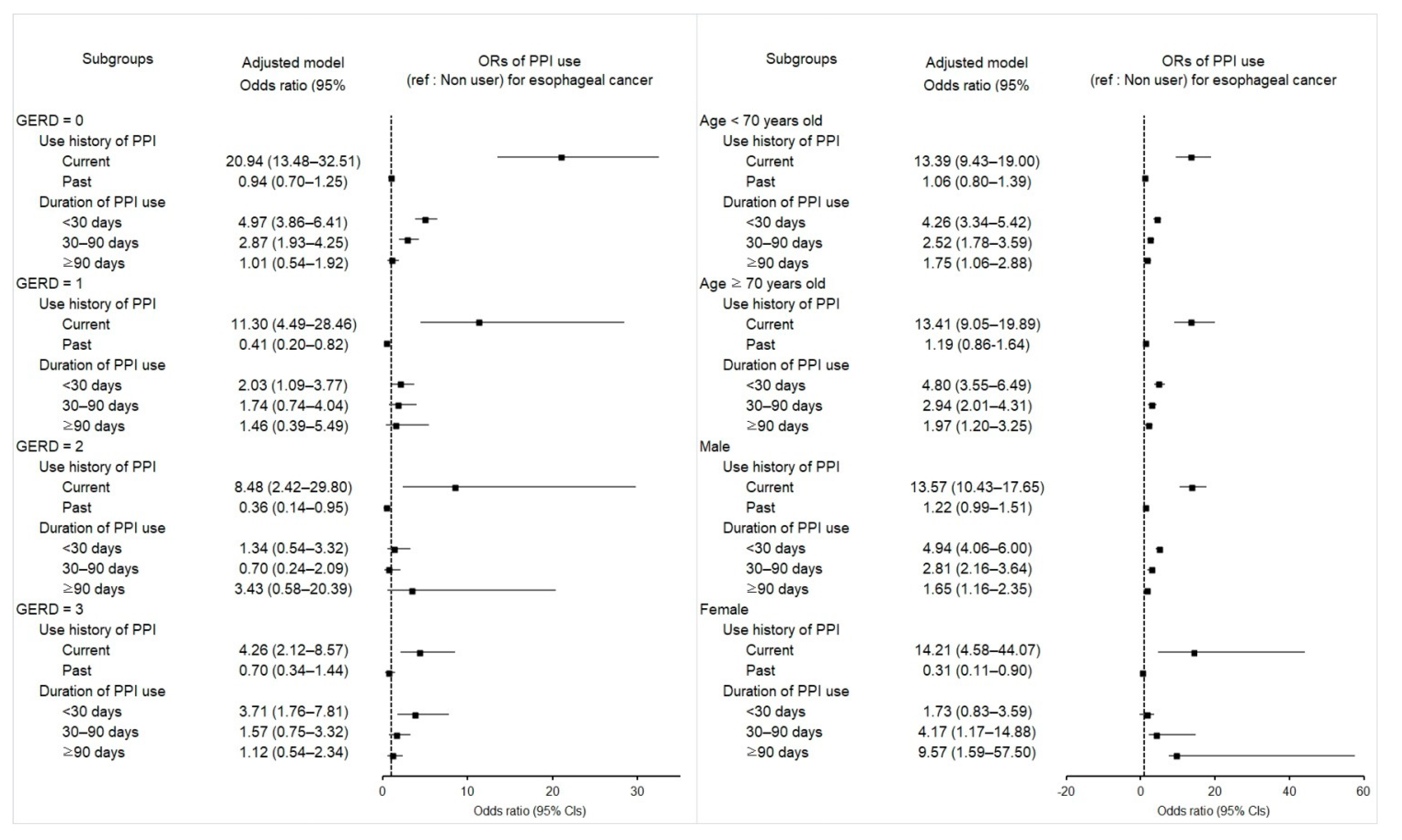
2.2. Associations of Prior Use of PPI and Its Duration with Esophageal Cancer
| Characteristics | N of Esophageal Cancer | N of Control | Odds Ratios for Esophageal Cancer (95% CI) | |||
|---|---|---|---|---|---|---|
| Exposure/Total (%) | Exposure/Total(%) | Crude | p | Overlap Weighted Model † | p | |
| Total participants (n = 4055) | ||||||
| Exposure | ||||||
| Current | 279/811 (34.4) | 107/3244 (3.3) | 16.1 (12.61–20.56) | <0.001 * | 13.23 (10.25–17.06) | <0.001 * |
| Past | 85/811 (10.5) | 376/3244 (11.6) | 1.40 (1.08–1.80) | 0.011 * | 1.15 (0.93–1.41) | 0.194 |
| Duration of PPI use (days) | ||||||
| <30 | 231/811 (28.5) | 253/3244 (7.8) | 5.64 (4.60–6.92) | <0.001 * | 4.59 (3.81–5.53) | <0.001 * |
| 30–90 | 83/811 (10.2) | 141/3244 (4.3) | 3.64 (2.72–4.85) | <0.001 * | 2.79 (2.17–3.60) | <0.001 * |
| ≥90 | 50/811 (6.2) | 89/3244 (2.7) | 3.47 (2.42–4.98) | <0.001 * | 1.80 (1.28–2.52) | 0.001 * |
| GERD = 0 (n = 3304) | ||||||
| Exposure | ||||||
| Current | 102/507 (20.1) | 38/2797 (1.4) | 18.6 (12.62–27.41) | <0.001 * | 20.94 (13.48–32.51) | <0.001 * |
| Past | 30/507 (5.9) | 161/2797 (5.8) | 1.29 (0.86–1.93) | 0.216 | 0.94 (0.70–1.25) | 0.673 |
| Duration of PPI use (days) | ||||||
| <30 | 99/507 (19.5) | 121/2797 (4.3) | 5.67 (4.26–7.55) | <0.001 * | 4.97 (3.86–6.41) | <0.001 * |
| 30–90 | 26/507 (5.1) | 49/2797 (1.8) | 3.68 (2.26–5.99) | <0.001 * | 2.87 (1.93–4.25) | <0.001 * |
| ≥90 | 7/507 (1.4) | 29/2797 (1.0) | 1.67 (0.73–3.84) | 0.226 | 1.01 (0.54–1.92) | 0.968 |
| GERD = 1 (n = 281) | ||||||
| Exposure | ||||||
| Current | 56/107 (52.3) | 14/174 (8.0) | 10.42 (5.13–21.20) | <0.001 * | 11.3 (4.49–28.46) | <0.001 * |
| Past | 18/107 (16.8) | 74/174 (42.5) | 0.63 (0.33–1.22) | 0.171 | 0.41 (0.20–0.82) | 0.013 * |
| Duration of PPI use (days) | ||||||
| <30 | 53/107 (49.5) | 59/174 (33.9) | 2.34 (1.36–4.04) | 0.002 * | 2.03 (1.09–3.77) | 0.026 * |
| 30–90 | 15/107 (14.0) | 23/174 (13.2) | 1.70 (0.79–3.65) | 0.174 | 1.74 (0.74–4.04) | 0.202 |
| ≥90 | 6 / 107 (5.6) | 6/174 (3.4) | 2.61 (0.78–8.66) | 0.118 | 1.46 (0.39–5.49) | 0.579 |
| GERD = 2 (n = 174) | ||||||
| Exposure | ||||||
| Current | 44/76 (57.9) | 13/98 (13.3) | 5.42 (2.35–12.46) | <0.001 * | 8.48 (2.42–29.80) | 0.001 * |
| Past | 12/76 (15.8) | 53/98 (54.1) | 0.36 (0.16–0.84) | 0.018 * | 0.36 (0.14–0.95) | 0.038 * |
| Duration of PPI use (days) | ||||||
| <30 | 38/76 (50.0) | 38/98 (38.8) | 1.60 (0.78–3.28) | 0.199 | 1.34 (0.54–3.32) | 0.534 |
| 30–90 | 12/76 (15.8) | 23/98 (23.5) | 0.83 (0.34–2.04) | 0.692 | 0.70 (0.24–2.09) | 0.527 |
| ≥90 | 6/76 (7.9) | 5/98 (5.1) | 1.92 (0.52–7.13) | 0.330 | 3.43 (0.58–20.39) | 0.176 |
| GERD ≥ 3 (n = 296) | ||||||
| Exposure | ||||||
| Current | 77/121 (63.6) | 42/175 (24.0) | 4.34 (2.26–8.36) | <0.00* | 4.26 (2.12–8.57) | <0.001 * |
| Past | 25/121 (20.7) | 88/175 (50.3) | 0.67 (0.34–1.35) | 0.265 | 0.70 (0.34–1.44) | 0.328 |
| Duration of PPI use (days) | ||||||
| <30 | 41/121 (33.9) | 35/175 (20.0) | 2.77 (1.38–5.59) | 0.00 * | 3.71 (1.76–7.81) | 0.001 * |
| 30–90 | 30/121 (24.8) | 46/175 (26.3) | 1.54 (0.76–3.13) | 0.228 | 1.57 (0.75–3.32) | 0.234 |
| ≥90 | 31/121 (25.6) | 49/175 (28.0) | 1.50 (0.74–3.02) | 0.258 | 1.12 (0.54–2.34) | 0.761 |
| Characteristics | N of Esophageal Cancer | N of Control | Odds Ratios for Esophageal Cancer (95% CI) | |||
|---|---|---|---|---|---|---|
| Exposure/Total (%) | Exposure/Total (%) | Crude | p | Overlap Weighted Model † | p | |
| Total participants (n = 4055) | ||||||
| Exposure history | ||||||
| Current | 387/811 (47.7) | 493/3244 (15.2) | 5.51 (4.52–6.72) | <0.001 * | 4.34 (3.67–5.14) | <0.001 * |
| Past | 220/811 (27.1) | 1318/3244 (40.6) | 1.17 (0.96–1.44) | 0.128 | 0.99 (0.84–1.16) | 0.871 |
| Duration of H2RA use (days) | ||||||
| <30 | 344/811 (42.4) | 1133/3244 (34.9) | 2.13 (1.76–2.58) | <0.001 * | 1.91 (1.64–2.21) | <0.001 * |
| 30–90 | 144/811 (17.8) | 394/3244 (12.1) | 2.57 (2.02–3.27) | <0.001 * | 1.93 (1.58–2.35) | <0.001 * |
| ≥90 | 119/811 (14.7) | 284/3244 (8.8) | 2.94 (2.27–3.82) | <0.001 * | 2.09 (1.69–2.59) | <0.001 * |
| GERD = 0 (n = 3304) | ||||||
| Exposure history | ||||||
| Current | 230/507 (45.4) | 379/2797 (13.6) | 5.58 (4.41–7.07) | <0.001 * | 4.74 (3.94–5.72) | <0.001 * |
| Past | 129/507 (25.4) | 1056/2797 (37.8) | 1.12 (0.88–1.44) | 0.358 | 1.01 (0.85–1.20) | 0.922 |
| Duration of H2RA use (days) | ||||||
| <30 | 223/507 (44.0) | 946/2797 (33.8) | 2.17 (1.73–2.71) | <0.001 * | 2.00 (1.70–2.35) | <0.001 * |
| 30–90 | 72 /507 (14.2) | 293/2797 (10.5) | 2.26 (1.66–3.08) | <0.001 * | 1.77 (1.41–2.23) | <0.001 * |
| ≥90 | 64 /507 (12.6) | 196/2797 (7.0) | 3.00 (2.16–4.18) | <0.001 * | 2.51 (1.96–3.22) | <0.001 * |
| GERD = 1 (n = 281) | ||||||
| Exposure history | ||||||
| Current | 55/107 (51.4) | 45/174 (25.9) | 1.32 (0.67–2.61) | 0.419 | 1.49 (0.69–3.24) | 0.312 |
| Past | 28/107 (26.2) | 103/174 (59.2) | 0.29 (0.15–0.59) | 0.001 * | 0.28 (0.13–0.62) | 0.002 * |
| Duration of H2RA use (days) | ||||||
| <30 | 50/107 (46.7) | 86/174 (49.4) | 0.63 (0.33–1.21) | 0.167 | 0.71 (0.34–1.46) | 0.350 |
| 30–90 | 20/107 (18.7) | 32/174 (18.4) | 0.68 (0.31–1.49) | 0.332 | 0.80 (0.33–1.98) | 0.635 |
| ≥90 | 13/107 (12.1) | 30/174 (17.2) | 0.47 (0.20–1.10) | 0.083 | 0.53 (0.21–1.38) | 0.196 |
| GERD = 2 (n = 174) | ||||||
| Exposure history | ||||||
| Current | 41/76 (53.9) | 23/98 (23.5) | 2.67 (1.15–6.24) | 0.023 * | 3.22 (0.97–10.66) | 0.056 |
| Past | 21/76 (27.6) | 54/98 (55.1) | 0.58 (0.25–1.36) | 0.210 | 0.56 (0.19–1.65) | 0.292 |
| Duration of H2RA use (days) | ||||||
| <30 | 29/76 (38.2) | 39/98 (39.8) | 1.12 (0.49–2.56) | 0.796 | 0.79 (0.27–2.32) | 0.667 |
| 30–90 | 22/76 (28.9) | 30/98 (30.6) | 1.10 (0.46–2.63) | 0.830 | 1.61 (0.49–5.25) | 0.432 |
| ≥90 | 11/76 (14.5) | 8/98 (8.2) | 2.06 (0.66–6.41) | 0.211 | 2.15 (0.49–9.38) | 0.309 |
| GERD ≥ 3 (n = 296) | ||||||
| Exposure history | ||||||
| Current | 61/121 (50.4%) | 46/175 (26.3) | 1.77 (0.86–3.64) | 0.121 | 1.62 (0.77–3.38) | 0.202 |
| Past | 42/121 (34.7%) | 105/175 (60.0) | 0.53 (0.26–1.08) | 0.082 | 0.57 (0.28–1.18) | 0.131 |
| Duration of H2RA use (days) | ||||||
| <30 | 42/121 (34.7%) | 62/175 (35.4) | 0.90 (0.44–1.87) | 0.783 | 1.32 (0.62–2.79) | 0.467 |
| 30–90 | 30/121 (24.8%) | 39/175 (22.3) | 1.03 (0.47–2.23) | 0.949 | 0.86 (0.38–1.96) | 0.726 |
| ≥90 | 31/121 (25.6%) | 50/175 (28.6) | 0.83 (0.39–1.76) | 0.623 | 0.60 (0.27–1.34) | 0.212 |
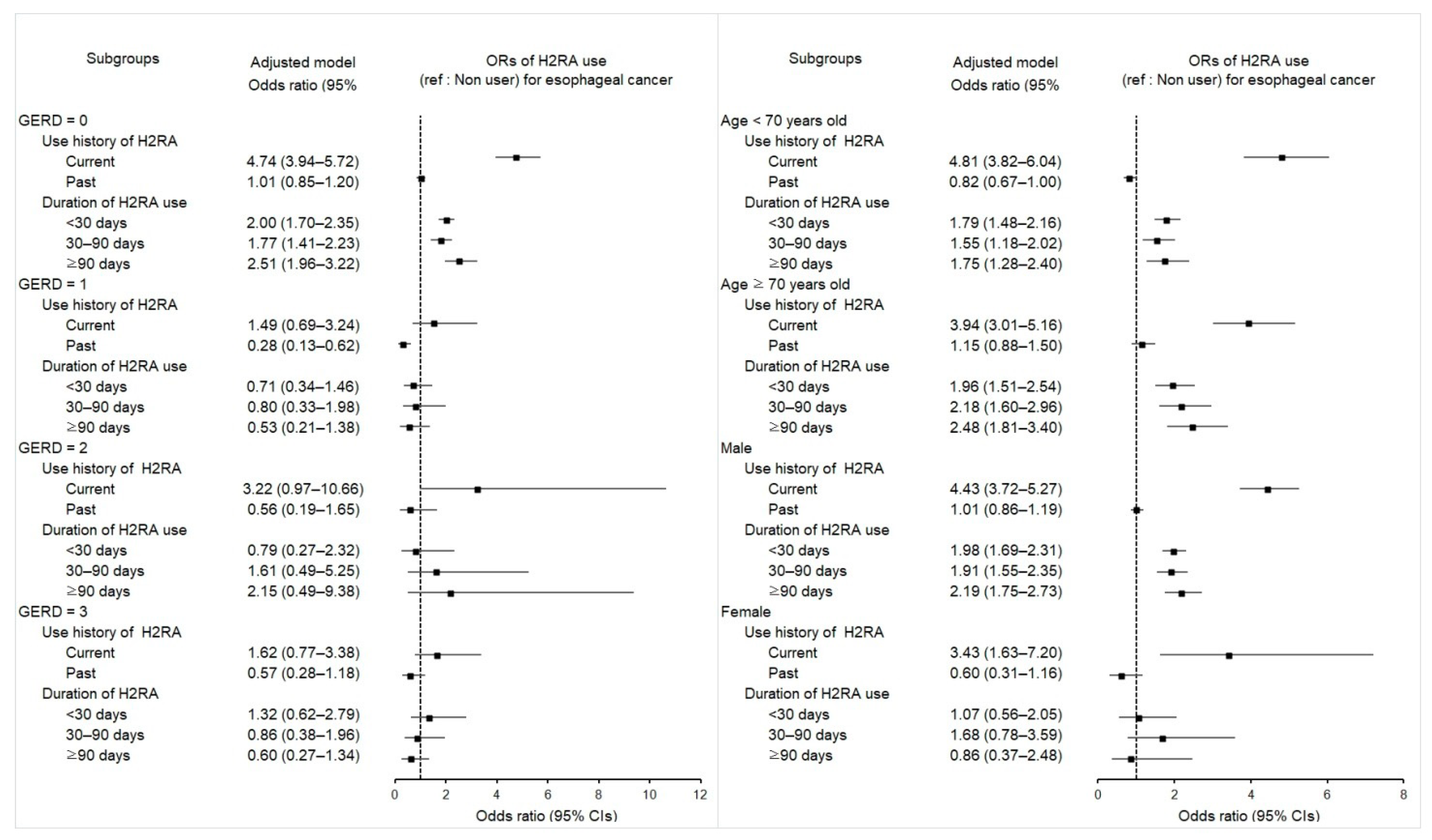
2.3. Associations between Previous H2RA Use and Its Duration and Esophageal Cancer
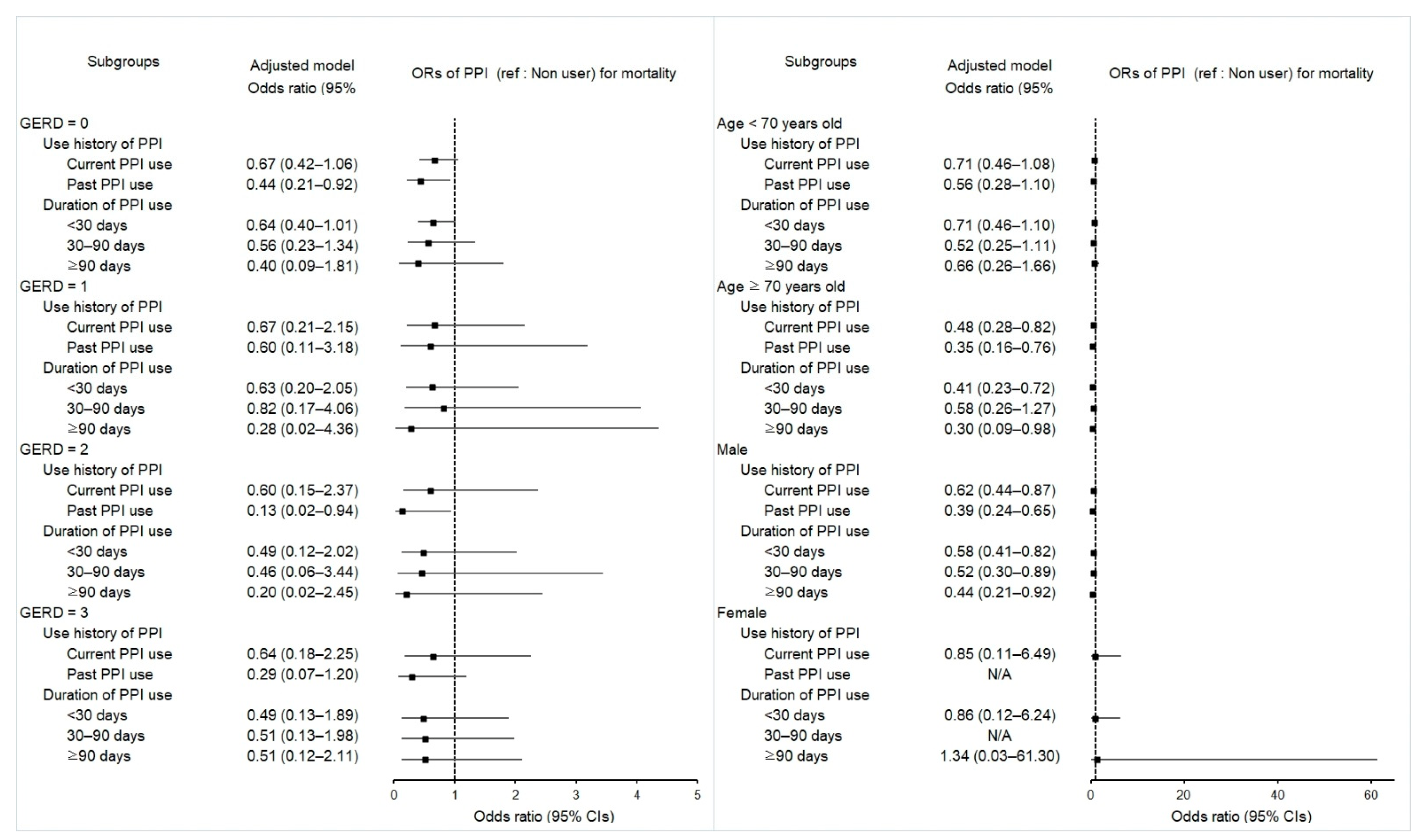
2.4. Associations between Mortality in Esophageal Cancer Patients and Use of PPI
| Characteristics | Deceased Pts | Survived Pts | Odds Ratios for Mortality (95% Confidence Interval) | |||
|---|---|---|---|---|---|---|
| Exposure/Total (%) | Exposure/Total (%) | Crude | p | Overlap Weighted Model † | p | |
| Total participants (n = 811) | ||||||
| Exposure history | ||||||
| Current | 151/470 (32.1) | 128/341 (37.5) | 0.70 (0.52–0.95) | 0.023 * | 0.62 (0.45–0.86) | 0.004 * |
| Past | 39/470 (8.3) | 46/341 (13.5) | 0.51 (0.32–0.81) | 0.004 * | 0.41 (0.25–0.67) | <0.001 * |
| Duration of PPI use (days) | ||||||
| <30 | 123/470 (26.2) | 108/341 (31.7) | 0.68 (0.49–0.94) | 0.019 * | 0.58 (0.41–0.80) | 0.001 * |
| 30–90 | 42/470 (8.9) | 41/341 (12.0) | 0.61 (0.38–0.98) | 0.040 * | 0.56 (0.34–0.94) | 0.029 * |
| ≥90 | 25/470 (5.3) | 25/341 (7.3) | 0.60 (0.33–1.07) | 0.084 | 0.48 (0.24–0.95) | 0.035 * |
| GERD = 0 (n = 507) | ||||||
| Exposure history | ||||||
| Current | 63/315 (20.0) | 39/192 (20.3) | 0.94 (0.60–1.48) | 0.790 | 0.67 (0.42–1.06) | 0.086 |
| Past | 15/315 (4.8) | 15/192 (7.8) | 0.58 (0.28–1.23) | 0.155 | 0.44 (0.21–0.92) | 0.030 * |
| Duration of PPI use (days) | ||||||
| <30 | 60/315 (19.0) | 39/192 (20.3) | 0.90 (0.57–1.41) | 0.635 | 0.64 (0.40–1.01) | 0.053 |
| 30–90 | 14/315 (4.4) | 12/192 (6.3) | 0.68 (0.31–1.51) | 0.343 | 0.56 (0.23–1.34) | 0.192 |
| ≥90 | 4/315 (1.3) | 3/192 (1.6) | 0.78 (0.17–3.52) | 0.743 | 0.40 (0.09–1.81) | 0.233 |
| GERD = 1 (n = 107) | ||||||
| Exposure history | ||||||
| Current | 23/50 (46.0) | 33/57 (57.9) | 0.58 (0.24–1.38) | 0.220 | 0.67 (0.21–2.15) | 0.499 |
| Past | 9/50 (18.0) | 9/57 (15.8) | 0.83 (0.26–2.63) | 0.756 | 0.60 (0.11–3.18) | 0.550 |
| Duration of PPI use (days) | ||||||
| <30 | 22/50 (44.0) | 31/57 (54.4) | 0.59 (0.25–1.42) | 0.240 | 0.63 (0.20–2.05) | 0.448 |
| 30–90 | 6/50 (12.0) | 9/57 (15.8) | 0.56 (0.16–1.92) | 0.353 | 0.82 (0.17–4.06) | 0.809 |
| ≥90 | 4/50 (8.0) | 2/57 (3.5) | 1.67 (0.27–10.39) | 0.585 | 0.28 (0.02–4.36) | 0.364 |
| GERD = 2 (n = 76) | ||||||
| Exposure history | ||||||
| Current | 25/42 (59.5) | 19/34 (55.9) | 0.71 (0.24–2.12) | 0.538 | 0.60 (0.15–2.37) | 0.465 |
| Past | 4/42 (9.5) | 8/34 (23.5) | 0.27 (0.06–1.22) | 0.089 | 0.13 (0.02–0.94) | 0.043 * |
| Duration of PPI use (days) | ||||||
| <30 | 21/42 (50.0) | 17/34 (50.0) | 0.67 (0.22–2.04) | 0.475 | 0.49 (0.12–2.02) | 0.320 |
| 30–90 | 6/42 (14.3) | 6/34 (17.6) | 0.54 (0.13–2.31) | 0.405 | 0.46 (0.06–3.44) | 0.450 |
| ≥90 | 2/42 (4.8) | 4/34 (11.8) | 0.27 (0.04–1.86) | 0.183 | 0.20 (0.02–2.45) | 0.210 |
| GERD ≥ 3 (n = 121) | ||||||
| Exposure history | ||||||
| Current | 40/63 (63.5) | 37/58 (63.8) | 0.63 (0.22–1.77) | 0.382 | 0.64 (0.18–2.25) | 0.490 |
| Past | 11/63 (17.5) | 14/58 (24.1) | 0.46 (0.14–1.56) | 0.211 | 0.29 (0.07–1.20) | 0.088 |
| Duration of PPI use (days) | ||||||
| <30 | 20/63 (31.7) | 21/58 (36.2) | 0.56 (0.18–1.69) | 0.302 | 0.49 (0.13–1.89) | 0.302 |
| 30–90 | 16/63 (25.4) | 14/58 (24.1) | 0.67 (0.21–2.16) | 0.499 | 0.51 (0.13–1.98) | 0.329 |
| ≥90 | 15/63 (23.8) | 16/58 (27.6) | 0.55 (0.17–1.76) | 0.311 | 0.51 (0.12–2.11) | 0.350 |
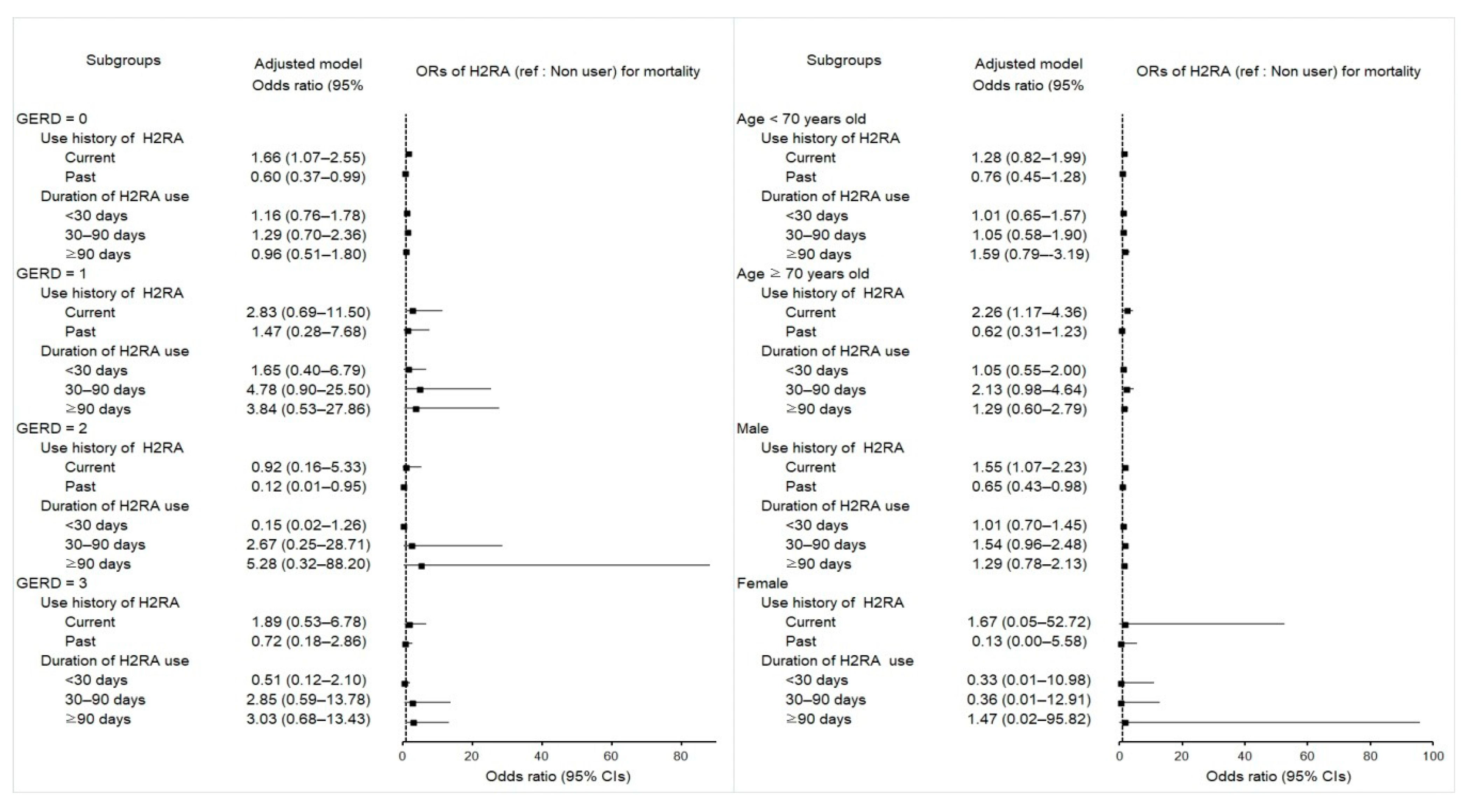
2.5. Associations between Mortality in Esophageal Cancer Patients and Use of H2RA
| Characteristics | Deceased Pts | Survived Pts | Odds Ratios for Mortality (95% Confidence Interval) | |||
|---|---|---|---|---|---|---|
| Exposure/Total (%) | Exposure/Total (%) | Crude | p | Overlap Weighted Model † | p | |
| Total participants (n = 811) | ||||||
| Exposure history | ||||||
| Current | 257/470 (54.7) | 130/341 (38.1) | 1.66 (1.17–2.34) | 0.004 * | 1.60 (1.12–2.28) | 0.010 * |
| Past | 102/470 (21.7) | 118/341 (34.6) | 0.72 (0.49–1.06) | 0.098 | 0.64 (0.43–0.96) | 0.030 * |
| Duration of H2RA use (days) | ||||||
| <30 | 191/470 (40.6) | 153/341 (44.9) | 1.05 (0.74–1.48) | 0.800 | 1.02 (0.71–1.45) | 0.924 |
| 30–90 | 94/470 (20.0) | 50/341 (14.7) | 1.58 (1.01–2.45) | 0.043 * | 1.49 (0.94–2.34) | 0.087 |
| ≥90 | 74/470 (15.7) | 45/341 (13.2) | 1.38 (0.87–2.19) | 0.174 | 1.37 (0.84–2.22) | 0.207 |
| GERD = 0 (n = 507) | ||||||
| Exposure history | ||||||
| Current | 164/315 (52.1) | 66/192 (34.4) | 1.84 (1.19–2.84) | 0.006 * | 1.66 (1.07–2.55) | 0.022 * |
| Past | 66/315 (21.0) | 63/192 (32.8) | 0.78 (0.48–1.25) | 0.296 | 0.60 (0.37–0.99) | 0.047 * |
| Duration of H2RA use (days) | ||||||
| <30 | 138/315 (43.8) | 85/192 (44.3) | 1.20 (0.79–1.84) | 0.392 | 1.16 (0.76–1.78) | 0.481 |
| 30–90 | 50/315 (15.9) | 22/192 (11.5) | 1.68 (0.93–3.06) | 0.088 | 1.29 (0.70–2.36) | 0.417 |
| ≥90 | 42/315 (13.3) | 22/192 (11.5) | 1.41 (0.77–2.60) | 0.265 | 0.96 (0.51–1.80) | 0.896 |
| GERD = 1 (n = 107) | ||||||
| Exposure history | ||||||
| Current | 30/50 (60.0) | 25/57 (43.9) | 2.40 (0.88–6.53) | 0.087 | 2.83 (0.69–11.50) | 0.147 |
| Past | 12/50 (24.0) | 16/57 (28.1) | 1.50 (0.48–4.65) | 0.483 | 1.47 (0.28–7.68) | 0.645 |
| Duration of H2RA use (days) | ||||||
| <30 | 24/50 (48.0) | 26/57 (45.6) | 1.85 (0.67–5.09) | 0.236 | 1.65 (0.40–6.79) | 0.490 |
| 30–90 | 12/50 (24.0) | 8/57 (14.0) | 3.0(0.87–10.29) | 0.081 | 4.78 (0.90–25.50) | 0.067 |
| ≥90 | 6/50 (12.0) | 7/57 (12.3) | 1.71 (0.43–6.83) | 0.445 | 3.84 (0.53–27.86) | 0.183 |
| GERD = 2 (n = 76) | ||||||
| Exposure history | ||||||
| Current | 27/42 (64.3) | 14/34 (41.2) | 1.07 (0.30–3.81) | 0.915 | 0.92 (0.16–5.33) | 0.924 |
| Past | 6/42 (14.3) | 15/34 (44.1) | 0.22 (0.05–0.94) | 0.042 * | 0.12 (0.01–0.95) | 0.044 * |
| Duration of H2RA use (days) | ||||||
| <30 | 14/42 (33.3) | 15/34 (44.1) | 0.52 (0.14–1.93) | 0.327 | 0.15 (0.02–1.26) | 0.081 |
| 30–90 | 12/42 (28.6) | 10/34 (29.4) | 0.67 (0.17–2.65) | 0.564 | 2.67 (0.25–28.71) | 0.419 |
| ≥90 | 7/42 (16.7) | 4/34 (11.8) | 0.97 (0.19–5.03) | 0.973 | 5.28 (0.32–88.20) | 0.247 |
| GERD ≥ 3 (n = 121) | ||||||
| Exposure history | ||||||
| Current | 36/63 (57.1) | 25/58 (43.1) | 1.44 (0.50–4.14) | 0.498 | 1.89 (0.53–6.78) | 0.327 |
| Past | 18/63 (28.6) | 24/58 (41.4) | 0.75 (0.25–2.27) | 0.611 | 0.72 (0.18–2.86) | 0.645 |
| Duration of H2RA use (days) | ||||||
| <30 | 15/63 (23.8) | 27/58 (46.6) | 0.56 (0.18–1.70) | 0.303 | 0.51 (0.12–2.10) | 0.349 |
| 30–90 | 20/63 (31.7) | 10/58 (17.2) | 2.00 (0.60–6.61) | 0.256 | 2.85 (0.59–13.78) | 0.193 |
| ≥90 | 19/63 (30.2) | 12/58 (20.7) | 1.58 (0.49–5.12) | 0.443 | 3.03 (0.68–13.43) | 0.145 |
3. Discussion
4. Materials and Methods
4.1. Study Population and Participant Selection
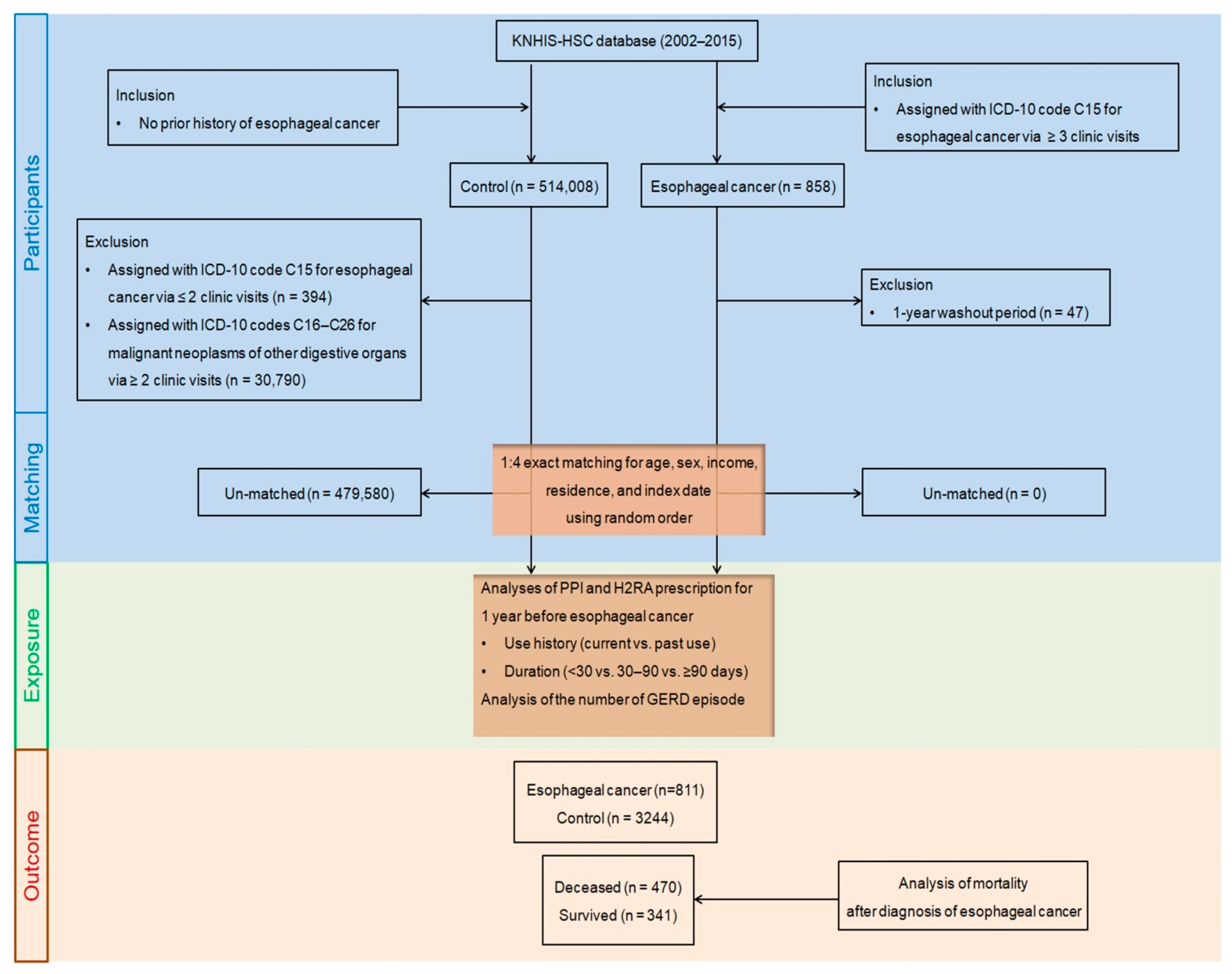
4.2. Exposures (PPIs and H2RAs)
4.3. Outcome (Esophageal Cancer)
4.4. Covariates
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y. Epidemiology of esophageal cancer. World J. Gastroenterol. 2013, 19, 5598–5606. [Google Scholar] [CrossRef] [PubMed]
- Soroush, A.; Etemadi, A.; Abrams, J.A. Non-Acid Fluid Exposure and Esophageal Squamous Cell Carcinoma. Dig. Dis. Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Kwon, M.J.; Ra, Y.J.; Lee, H.S.; Kim, H.S.; Nam, E.S.; Cho, S.J.; Park, H.-R.; Min, S.K.; Seo, J.; et al. Significance of druggable targets (PD-L1, KRAS, BRAF, PIK3CA, MSI, and HPV) on curatively resected esophageal squamous cell carcinoma. Diagn. Pathol. 2020, 15, 126. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.-K.; Tae, C.H.; Song, K.H.; Kang, S.J.; Park, J.K.; Gong, E.J.; Shin, J.E.; Lim, H.C.; Kil Lee, S.; Jung, D.H.; et al. 2020 Seoul Consensus on the Diagnosis and Management of Gastroesophageal Reflux Disease. J. Neurogastroenterol. Motil. 2021, 27, 453–481. [Google Scholar] [CrossRef] [PubMed]
- Corley, D.A.; Mehtani, K.; Quesenberry, C.; Zhao, W.; de Boer, J.; Weiss, N.S. Impact of Endoscopic Surveillance on Mortality from Barrett’s Esophagus–Associated Esophageal Adenocarcinomas. Gastroenterology 2013, 145, 312–319.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heath, E.I.; Limburg, P.J.; Hawk, E.T.; Forastiere, A.A. Adenocarcinoma of the esophagus: Risk factors and prevention. Oncology 2000, 14, 507–514. [Google Scholar]
- Wani, S.; Falk, G.; Hall, M.; Gaddam, S.; Wang, A.; Gupta, N.; Singh, M.; Singh, V.; Chuang, K.; Boolchand, V.; et al. Patients with Nondysplastic Barrett’s Esophagus Have Low Risks for Developing Dysplasia or Esophageal Adenocarcinoma. Clin. Gastroenterol. Hepatol. 2011, 9, 220–227.e1. [Google Scholar] [CrossRef]
- Wu, J.C.Y. Gastroesophageal reflux disease: An Asian perspective. J. Gastroenterol. Hepatol. 2008, 23, 1785–1793. [Google Scholar] [CrossRef]
- Celebi, A.; Aydin, D.; Kocaman, O.; Konduk, B.T.; Senturk, O.; Hulagu, S. Comparison of the effects of esomeprazole 40 mg, rabeprazole 20 mg, lansoprazole 30 mg, and pantoprazole 40 mg on intragastrıc pH in extensive metabolizer patients with gastroesophageal reflux disease. Turk. J. Gastroenterol. 2020, 27, 408–414. [Google Scholar] [CrossRef]
- Hvid-Jensen, F.; Pedersen, L.; Funch-Jensen, P.; Drewes, A.M. Proton pump inhibitor use may not prevent high-grade dysplasia and oesophageal adenocarcinoma in Barrett’s oesophagus: A nationwide study of 9883 patients. Aliment. Pharmacol. Ther. 2014, 39, 984–991. [Google Scholar] [CrossRef]
- Brusselaers, N.; Engstrand, L.; Lagergren, J. Maintenance proton pump inhibition therapy and risk of oesophageal cancer. Cancer Epidemiol. 2018, 53, 172–177. [Google Scholar] [CrossRef]
- Chu, M.P.; Hecht, J.R.; Slamon, D.; Wainberg, Z.A.; Bang, Y.-J.; Hoff, P.M.; Sobrero, A.; Qin, S.; Afenjar, K.; Houe, V.; et al. Association of Proton Pump Inhibitors and Capecitabine Efficacy in Advanced Gastroesophageal Cancer: Secondary Analysis of the TRIO-013/LOGiC Randomized Clinical Trial. JAMA Oncol. 2017, 3, 767–773. [Google Scholar] [CrossRef]
- Lai, S.-W.; Liao, K.-F.; Lai, H.-C.; Muo, C.-H.; Sung, F.-C. Atorvastatin correlates with decreased risk of esophageal cancer: A population-based case–control study from Taiwan. Libyan J. Med. 2012, 7, 18830–18834. [Google Scholar] [CrossRef]
- Rodríguez, L.A.G.; Lagergren, J.; Lindblad, M. Gastric acid suppression and risk of oesophageal and gastric adenocarcinoma: A nested case control study in the UK. Gut 2006, 55, 1538–1544. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.P.; Tazare, J.R.; Williamson, E.; Mansfield, K.E.; Evans, S.J.; Tomlinson, L.A.; Bhaskaran, K.; Smeeth, L.; Wing, K.; Douglas, I.J. Proton pump inhibitors and risk of all-cause and cause-specific mortality: A cohort study. Br. J. Clin. Pharmacol. 2021, 87, 3150–3161. [Google Scholar] [CrossRef]
- Lee, Y.; Urbanska, A.M.; Hayakawa, Y.; Wang, H.; Au, A.S.; Luna, A.M.; Chang, W.; Jin, G.; Bhagat, G.; Abrams, J.A.; et al. Gastrin stimulates a cholecystokinin-2-receptor-expressing cardia progenitor cell and promotes progression of Barrett’s-like esophagus. Oncotarget 2016, 8, 203–214. [Google Scholar] [CrossRef] [Green Version]
- Namazi, M.R.; Jowkar, F. A succinct review of the general and immunological pharmacologic effects of proton pump inhibitors. J. Clin. Pharm. Ther. 2008, 33, 215–217. [Google Scholar] [CrossRef]
- Adami, H.-O.; Andersen, I.T.; Heide-Jørgensen, U.; Chang, E.T.; Nørgaard, M.; Sørensen, H.T. Ranitidine Use and Risk of Upper Gastrointestinal Cancers. Cancer Epidemiol. Biomark. Prev. 2021, 30, 2302–2308. [Google Scholar] [CrossRef]
- Tan, M.C.; El-Serag, H.B.; Yu, X.; Thrift, A.P. Acid suppression medications reduce risk of oesophageal adenocarcinoma in Barrett’s oesophagus: A nested case-control study in US male veterans. Aliment. Pharmacol. Ther. 2018, 48, 469–477. [Google Scholar] [CrossRef] [Green Version]
- Tvingsholm, S.A.; Dehlendorff, C.; Østerlind, K.; Friis, S.; Jäättelä, M. Proton pump inhibitor use and cancer mortality. Int. J. Cancer 2018, 143, 1315–1326. [Google Scholar] [CrossRef] [Green Version]
- Hillman, L.C.; Chiragakis, L.; Shadbolt, B.; Kaye, G.L.; Clarke, A.C. Proton-pump inhibitor therapy and the development of dysplasia in patients with Barrett’s oesophagus. Med. J. Aust. 2004, 180, 387–391. [Google Scholar] [CrossRef]
- Aydin, Y.; Akin, H. Letter: Proton pump inhibitor usage still seems to reduce the risk of high-grade dysplasia and/or oesophageal adenocarcinoma in Barrett’s oesophagus. Aliment. Pharmacol. Ther. 2014, 40, 859–860. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Garg, S.K.; Singh, P.P.; Iyer, P.G.; El-Serag, H.B. Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett’s oesophagus: A systematic review and meta-analysis. Gut 2013, 63, 1229–1237. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.M.; Richardson, P.; El–Serag, H.B. Medications (NSAIDs, Statins, Proton Pump Inhibitors) and the Risk of Esophageal Adenocarcinoma in Patients with Barrett’s Esophagus. Gastroenterology 2010, 138, 2260–2266. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Sun, T.-T.; Hong, J.; Fang, J.-Y.; Xiong, H.; Meltzer, S.J. Proton Pump Inhibitors Do Not Reduce the Risk of Esophageal Adenocarcinoma in Patients with Barrett’s Esophagus: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0169691. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Sun, C.; Wu, Y.; Chen, X.; Kailas, S.; Karadsheh, Z.; Li, G.; Guo, Z.; Yang, H.; Hu, L.; et al. Do proton pump inhibitors prevent Barrett’s esophagus progression to high-grade dysplasia and esophageal adenocarcinoma? An updated meta-analysis. J. Cancer Res. Clin. Oncol. 2021, 147, 2681–2691. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Aguirre, T.V.; Davis, S.; Kuebeler, M.; Bhattacharyya, A.; Sampliner, R.E. Proton Pump Inhibitors Are Associated with Reduced Incidence of Dysplasia in Barrett’s Esophagus. Am. J. Gastroenterol. 2004, 99, 1877–1883. [Google Scholar] [CrossRef] [PubMed]
- Chueca, E.; Apostolova, N.; Esplugues, J.V.; García-González, M.A.; Lanas, A.; Piazuelo, E. Proton Pump Inhibitors Display Antitumor Effects in Barrett’s Adenocarcinoma Cells. Front. Pharmacol. 2016, 7, 452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McColl, K.E.; Kennerley, P. Proton pump inhibitors—Differences emerge in hepatic metabolism. Dig. Liver Dis. 2002, 34, 461–467. [Google Scholar] [CrossRef]
- Shi, S.; Klotz, U. Proton pump inhibitors: An update of their clinical use and pharmacokinetics. Eur. J. Clin. Pharmacol. 2008, 64, 935–951. [Google Scholar] [CrossRef]
- Kastelein, F.; Spaander, M.C.; Steyerberg, E.W.; Biermann, K.; Valkhoff, V.E.; Kuipers, E.J.; Bruno, M.J.; ProBar Study, G. Proton Pump Inhibitors Reduce the Risk of Neoplastic Progression in Patients with Barrett’s Esophagus. Clin. Gastroenterol. Hepatol. 2013, 11, 382–388. [Google Scholar] [CrossRef]
- Thota, P.N.; Hajifathalian, K.; Benjamin, T.; Runkana, A.; Lopez, R.; Sanaka, M.R. Lack of incremental effect of histamine receptor antagonists over proton pump inhibitors on the risk of neoplastic progression in patients with Barrett’s esophagus: A cohort study. J. Dig. Dis. 2017, 18, 143–150. [Google Scholar] [CrossRef]
- Lim, S.H.; Hong, M.; Ahn, S.; Choi, Y.-L.; Kim, K.-M.; Oh, D.; Ahn, Y.C.; Jung, S.-H.; Ahn, M.-J.; Park, K.; et al. Changes in tumour expression of programmed death-ligand 1 after neoadjuvant concurrent chemoradiotherapy in patients with squamous oesophageal cancer. Eur. J. Cancer 2015, 52, 1–9. [Google Scholar] [CrossRef]
- Udelnow, A.; Kreyes, A.; Ellinger, S.; Landfester, K.; Walther, P.; Klapperstueck, T.; Wohlrab, J.; Henne-Bruns, D.; Knippschild, U.; Würl, P. Omeprazole Inhibits Proliferation and Modulates Autophagy in Pancreatic Cancer Cells. PLoS ONE 2011, 6, e20143. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Zaguilan, R.; Lynch, R.M.; Martinez, G.M.; Gillies, R.J. Vacuolar-type H(+)-ATPases are functionally expressed in plasma membranes of human tumor cells. Am. J. Physiol. Physiol. 1993, 265, C1015–C1029. [Google Scholar] [CrossRef]
- Neumann, J.; Binter, M.B.; Fehse, C.; Marušáková, M.; Büxel, M.L.; Kirchhefer, U.; Hofmann, B.; Gergs, U. Amitriptyline functionally antagonizes cardiac H2 histamine receptors in transgenic mice and human atria. Naunyn-Schmiedebergs Arch. Exp. Pathol. Pharmakol. 2021, 394, 1251–1262. [Google Scholar] [CrossRef]
- Neumann, J.; Kirchhefer, U.; Dhein, S.; Hofmann, B.; Gergs, U. The Roles of Cardiovascular H2-Histamine Receptors Under Normal and Pathophysiological Conditions. Front. Pharmacol. 2021, 12, 732842. [Google Scholar] [CrossRef]
- Min, C.; Bang, W.J.; Kim, M.; Oh, D.J.; Choi, H.G. Rheumatoid arthritis and neurodegenerative dementia: A nested case-control study and a follow-up study using a national sample cohort. Clin. Rheumatol. 2020, 39, 159–166. [Google Scholar] [CrossRef]
- Kim, S.Y.; Min, C.; Oh, D.J.; Choi, H.G. Tobacco Smoking and Alcohol Consumption Are Related to Benign Parotid Tumor: A Nested Case-Control Study Using a National Health Screening Cohort. Clin. Exp. Otorhinolaryngol. 2019, 12, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; Min, C.; Oh, D.J.; Choi, H.G. Bidirectional Association Between GERD and Asthma: Two Longitudinal Follow-Up Studies Using a National Sample Cohort. J. Allergy Clin. Immunol. Pract. 2020, 8, 1005–1013.e9. [Google Scholar] [CrossRef]
- Kim, S.Y.; Oh, D.J.; Park, B.; Choi, H.G. Bell’s palsy and obesity, alcohol consumption and smoking: A nested case-control study using a national health screening cohort. Sci. Rep. 2020, 10, 4248. [Google Scholar] [CrossRef]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.-M.; Sundararajan, V. Updating and Validating the Charlson Comorbidity Index and Score for Risk Adjustment in Hospital Discharge Abstracts Using Data from 6 Countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Thomas, L.E. Addressing Extreme Propensity Scores via the Overlap Weights. Am. J. Epidemiol. 2019, 188, 250–257. [Google Scholar] [CrossRef] [Green Version]
- Thomas, L.E.; Li, F.; Pencina, M.J. Overlap Weighting: A Propensity Score Method That Mimics Attributes of a Randomized Clinical Trial. JAMA 2020, 323, 2417–2418. [Google Scholar] [CrossRef]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef] [Green Version]
| Characteristics | After PS Overlap Weighting Adjustment | After PS Overlap Weighting Adjustment in Esophageal Cancer Pts | ||||
|---|---|---|---|---|---|---|
| Esophageal Cancer | Control | SMD | Deceased pts | Survived pts | SMD | |
| Total participants (n, %) | 811 (100) | 3244 (100) | 341 (100) | 470 (100) | ||
| Age, % | 0.00 | 0.00 | ||||
| 40–44 | 0.21 | 0.21 | 0.10 | 0.10 | ||
| 45–49 | 1.54 | 1.54 | 1.47 | 1.47 | ||
| 50–54 | 6.25 | 6.25 | 6.51 | 6.51 | ||
| 55–59 | 10.90 | 10.90 | 11.59 | 11.59 | ||
| 60–64 | 15.43 | 15.43 | 15.94 | 15.94 | ||
| 65–69 | 22.77 | 22.77 | 22.12 | 22.12 | ||
| 70–74 | 20.56 | 20.56 | 20.90 | 20.90 | ||
| 75–79 | 15.14 | 15.14 | 14.52 | 14.52 | ||
| 80–84 | 5.59 | 5.59 | 5.21 | 5.21 | ||
| 85+ | 1.60 | 1.60 | 1.64 | 1.64 | ||
| Sex, % | 0.00 | 0.00 | ||||
| Male | 92.98 | 92.98 | 93.46 | 93.46 | ||
| Female | 7.02 | 7.02 | 6.54 | 6.54 | ||
| Income, % | 0.00 | 0.00 | ||||
| 1 (lowest) | 16.94 | 16.94 | 16.71 | 16.71 | ||
| 2 | 14.10 | 14.10 | 12.56 | 12.56 | ||
| 3 | 16.13 | 16.13 | 17.27 | 17.27 | ||
| 4 | 21.92 | 21.92 | 23.55 | 23.55 | ||
| 5 (highest) | 30.92 | 30.92 | 29.91 | 29.91 | ||
| Region of residence, % | 0.00 | 0.00 | ||||
| Urban | 38.42 | 38.42 | 39.55 | 39.55 | ||
| Rural | 61.58 | 61.58 | 60.45 | 60.45 | ||
| Obesity †, % | 0.00 | 0.00 | ||||
| Underweight | 5.82 | 5.82 | 5.95 | 5.95 | ||
| Normal | 46.86 | 46.86 | 51.63 | 51.63 | ||
| Overweight | 25.34 | 25.34 | 23.80 | 23.80 | ||
| Obese I | 21.00 | 21.00 | 18.02 | 18.02 | ||
| Obese II | 0.98 | 0.98 | 0.60 | 0.60 | ||
| Smoking status, % | 0.00 | 0.00 | ||||
| Nonsmoker | 42.92 | 42.92 | 38.91 | 38.91 | ||
| Past smoker | 22.50 | 22.50 | 21.92 | 21.92 | ||
| Current smoker | 34.58 | 34.58 | 39.17 | 39.17 | ||
| Alcohol consumption, % | 0.00 | 0.00 | ||||
| <1 time a week | 46.71 | 46.71 | 42.65 | 42.65 | ||
| ≥1 time a week | 53.29 | 53.29 | 57.35 | 57.35 | 0.00 | |
| SBP (Mean, SD) | 129.53 (14.48) | 129.53 (6.73) | 0.00 | 129.41 (10.10) | 129.41 (12.64) | 0.00 |
| DBP (Mean, SD) | 78.79 (8.57) | 78.79 (4.18) | 0.00 | 78.72 (5.75) | 78.72 (7.78) | 0.00 |
| Fasting blood glucose (Mean, SD) | 104.48 (22.57) | 104.48 (12.57) | 0.00 | 104.23 (15.74) | 104.23 (19.57) | 0.00 |
| Total cholesterol (Mean, SD) | 188.18 (30.98) | 188.18 (14.88) | 0.00 | 185.96 (21.90) | 185.96 (26.55) | 0.00 |
| CCI score (Mean, SD) | 2.38 (1.93) | 2.38 (1.05) | 0.00 | 3.14 (1.51) | 3.14 (1.79) | 0.00 |
| GERD episodes for 1 year before index date (Mean, SD) | 0.93 (1.48) | 0.93 (1.12) | 0.00 | 1.27 (1.38) | 1.27 (1.50) | 0.00 |
| Treatment type, % | 0.00 | |||||
| No records of treatment | - | - | 34.65 | 34.65 | ||
| Surgery only | - | - | 16.04 | 16.04 | ||
| Surgery + RT or CT | - | - | 49.31 | 49.31 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.G.; Lee, H.K.; Kang, H.S.; Lim, H.; Kim, J.-H.; Kim, J.H.; Kim, N.Y.; Cho, S.-J.; Nam, E.S.; Min, K.-W.; et al. Possible Association between the Use of Proton Pump Inhibitors and H2 Receptor Antagonists, and Esophageal Cancer: A Nested Case–Control Study Using a Korean National Health Screening Cohort. Pharmaceuticals 2022, 15, 517. https://doi.org/10.3390/ph15050517
Choi HG, Lee HK, Kang HS, Lim H, Kim J-H, Kim JH, Kim NY, Cho S-J, Nam ES, Min K-W, et al. Possible Association between the Use of Proton Pump Inhibitors and H2 Receptor Antagonists, and Esophageal Cancer: A Nested Case–Control Study Using a Korean National Health Screening Cohort. Pharmaceuticals. 2022; 15(5):517. https://doi.org/10.3390/ph15050517
Chicago/Turabian StyleChoi, Hyo Geun, Hong Kyu Lee, Ho Suk Kang, Hyun Lim, Joo-Hee Kim, Ji Hee Kim, Nan Young Kim, Seong-Jin Cho, Eun Sook Nam, Kyueng-Whan Min, and et al. 2022. "Possible Association between the Use of Proton Pump Inhibitors and H2 Receptor Antagonists, and Esophageal Cancer: A Nested Case–Control Study Using a Korean National Health Screening Cohort" Pharmaceuticals 15, no. 5: 517. https://doi.org/10.3390/ph15050517
APA StyleChoi, H. G., Lee, H. K., Kang, H. S., Lim, H., Kim, J.-H., Kim, J. H., Kim, N. Y., Cho, S.-J., Nam, E. S., Min, K.-W., & Kwon, M. J. (2022). Possible Association between the Use of Proton Pump Inhibitors and H2 Receptor Antagonists, and Esophageal Cancer: A Nested Case–Control Study Using a Korean National Health Screening Cohort. Pharmaceuticals, 15(5), 517. https://doi.org/10.3390/ph15050517