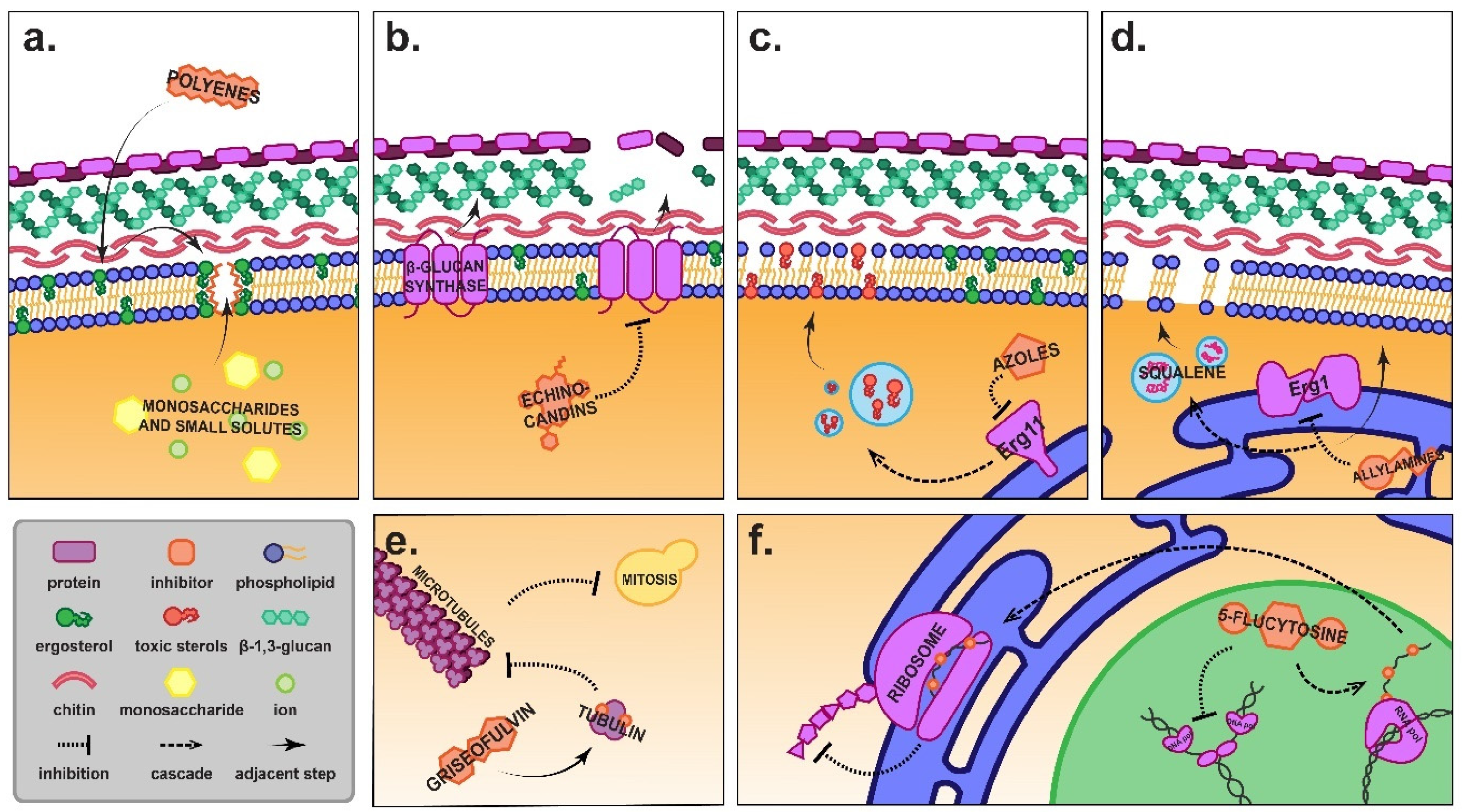
Augmenting Azoles with Drug Synergy to Expand the Antifungal Toolbox
Abstract
1. Introduction
1.1. The Burden of Fungal Disease
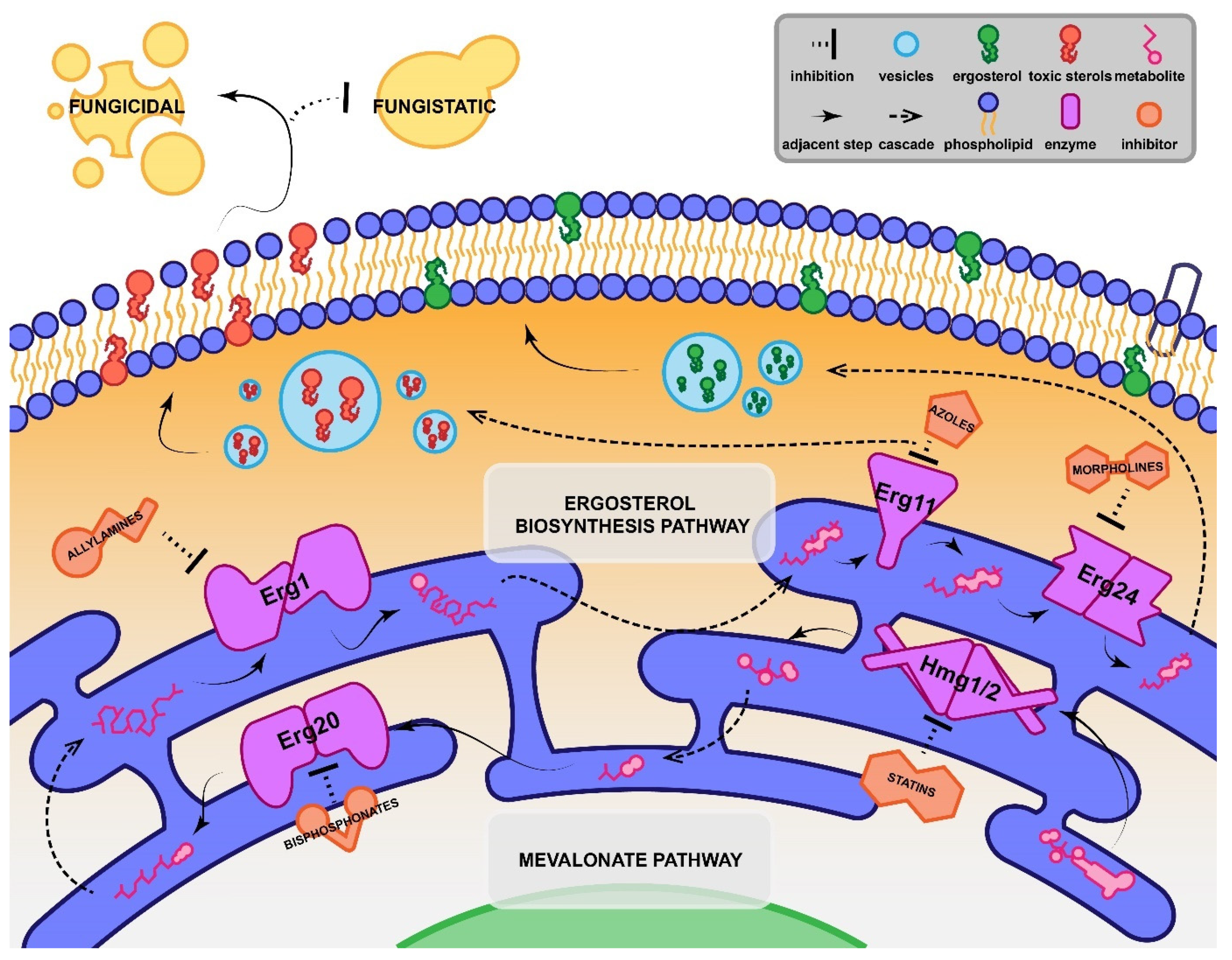
1.2. Azole Antifungals
| Class | Application | Azole | Brand | Mycosis | Notes | Ref. |
|---|---|---|---|---|---|---|
| Imidazole | Topical | butoconazole | Gynazole-1, Mycelex-3 | uncomplicated and recurrent vaginal candidiasis | [65] | |
| climbazole | Squaphane, Pitiren | dandruff and seborrhoeic dermatitis caused by Malassezia sp. | [66] | |||
| clotrimazole † | Lotrimin | oral and vaginal candidiasis, and tinea versicolor, cruris and pedis | WHO Essential Medicine | [66,67] | ||
| eberconazole | Ebernet | cutaneous candidiasis and dermatophytosis | Approved in EU in 2015 | [68] | ||
| econazole | Spectrazole, Ecostatin | tinea pedis and cruris, vaginal candidiasis | Also repels clothes moths | [69] | ||
| flutrimazole | Flusporan, Topiderm | cutaneous dermatophytosis including tinea pedis | [70] | |||
| isoconazole | Icaden, Travogen | tinea pedis and vaginal candidiasis | Effective against Gram-positive bacteria | [71] | ||
| ketoconazole † | Nizoral | seborrhoeic dermatitis, dandruff, tinea and cutaneous candidiasis | Also systemic | [72] | ||
| luliconazole | Luzu | tinea pedis and cruris and other dermatophytoses | FDA-approved in 2013 | [73] | ||
| miconazole † | Monistat, Desenex | dermatophytosis and cutaneous, oral and vaginal candidiasis | WHO Essential Medicine | [74] | ||
| oxiconazole | Oxistat, Oxizole | dermatophytoses and cutaneous candidiasis | [75] | |||
| sertaconazole | Ertaczo, Dermofix | tinea pedis and vaginal candidiasis | Also anti-inflammatory and anti-pruritic | [76,77] | ||
| sulconazole | Exelderm | dermatophytoses | Also anti-carpet beetle | [78,79] | ||
| tioconazole | Vagistat-1 | onychomycosis, dermatophytoses and vaginal candidiasis | Also called thioconazole | [80] | ||
| Systemic | ketoconazole | Nizoral (oral) | mycoses caused by Candida, Histoplasma and Coccidioides | Systemic use for extreme cases only | [81] | |
| Triazole | Topical | efinaconazole | Jublia, Clenafin | onychomycosis | Low cure rate, but higher than other drugs | [82] |
| fluconazole † | Diflucan | dermatophytoses and cutaneous candidiasis | WHO Essential Medicine, more commonly systemic | [21] | ||
| terconazole | Terazol | acute and chronic vaginal candidiasis | [83] | |||
| Systemic | fluconazole † | Diflucan | candidiasis, cryptococcosis, histoplasmosis, blastomycosis | WHO Essential Medicine, oral or intravenous | [21] | |
| fosfluconazole | Prodif | prophylaxis in the immunocompromised | Fluconazole prodrug | [84,85] | ||
| fosravuconazole | Nailin | onychomycosis | Ravuconazole prodrug | [86] | ||
| isavuconazonium | Cresemba | mucormycosis and invasive aspergillosis | Isavuconazole prodrug | [35,87] | ||
| itraconazole † | Sporanox, Orungal | aspergillosis, histoplasmosis, coccidioidomycosis and blastomycosis | WHO Essential Medicine | [48,88] | ||
| posaconazole | Noxafil, Posanol | invasive candidiasis, aspergilosis, mucormycosis and scedosporiosis | FDA-approved in 2006 | [89] | ||
| voriconazole † | Vfend | aspergillosis, candidiasis, penicilliosis, histoplasmosis and fusariosis | WHO Essential Medicine | [90] |
1.3. Antimicrobial Synergy
1.4. Aims and Scope of This Review
| Azole Synergy | Synergy in % Strains Tested | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Category | Synergist | Fluconazole | Itraconazole | Voriconazole | Isavuconazole | Posaconazole | Ketoconazole | Miconazole | C. albicans | AR Candida | A. fumigatus | AR Aspergillus | Dermatophytes | Other | Notes 1 | Ref. |
| Antimicrobials | ||||||||||||||||
| Antifungals | terbinafine | Incl. Scedosporium sp. and Pythium sp. | [104,105,106,107,108,109,110,111,112] | |||||||||||||
| caspofungin | Dep. on mechanism of resistance | [113,114,115,116] | ||||||||||||||
| anidulafungin | [114,117,118] | |||||||||||||||
| micafungin | Incl. C. auris | [115,116,118] | ||||||||||||||
| natamycin | Incl. Fusarium sp. | [119] | ||||||||||||||
| ciclopirox | [120] | |||||||||||||||
| flucytosine | Incl. C. auris | [121] | ||||||||||||||
| voriconazole | Limited synergy in the Mucorales | [122] | ||||||||||||||
| amorolfine | Onychomycosis clinical trials | [123,124,125,126] | ||||||||||||||
| K20 | Also clotrimazole, incl. C. neoformans | [127] | ||||||||||||||
| oxadiazole pept. | [128] | |||||||||||||||
| Antibacterials | sulfamethoxazole | Incl. C. auris | [129,130] | |||||||||||||
| sulfa- antibiotics | Some effective vs. biofilms and in vivo | [130] | ||||||||||||||
| doxycycline | Effective against biofilms and in vivo | [131,132,133] | ||||||||||||||
| tigecycolline | Incl. Fusarium sp., limited anti-biofilm | [132,134,135] | ||||||||||||||
| minocycline | Incl. C. neoformans, Scedosporium sp. | [133,136,137,138,139] | ||||||||||||||
| gentamicin | Effective against biofilms | [140] | ||||||||||||||
| linezolid | Little synergy, but reduced dosage | [141] | ||||||||||||||
| polymyxin B | Incl. Rhodotorula and Lichtheimia sp. | [142] | ||||||||||||||
| colistin | Incl. C. auris | [96,143,144] | ||||||||||||||
| Antiparasitics | pyrvinium pam. | [145,146] | ||||||||||||||
| chloroquine | [147] | |||||||||||||||
| artemisinins | Effective against biofilms | [146] | ||||||||||||||
| INK128 | Incl. Fusarium and Exophiala sp. | [148] | ||||||||||||||
| mefloquine | Incl. C. neoformans | [149] | ||||||||||||||
| Antivirals | saquinavir | Incl. Histoplasma capsulatum | [150] | |||||||||||||
| ritonavir | Incl. Histoplasma capsulatum | [150] | ||||||||||||||
| adamantanamine | Switch from fungistatic to fungicidal | [151] | ||||||||||||||
| ribavirin | Effective against biofilms and in vivo | [152] | ||||||||||||||
| lopinavir | Incl. C. auris | [153] | ||||||||||||||
| Efflux Inhibitors | ||||||||||||||||
| Calcium Inhibitors | tetrandrine | Effective in vivo | [154,155,156,157] | |||||||||||||
| verapamil | Dep. on mechanism of resistance | [158,159] | ||||||||||||||
| Other | eucalyptal D | Natural product | [160] | |||||||||||||
| dodenoic acid | [161] | |||||||||||||||
| azoffluxin | Incl. C. auris | [162] | ||||||||||||||
| ospemifeme | Incl. C. neoformans and C. auris | [163] | ||||||||||||||
| phialocephalarin | [164] | |||||||||||||||
| palmarumycin P3 | [164] | |||||||||||||||
| geraniol | Effective in vivo, natural product | [165] | ||||||||||||||
| Repurposed Drugs | ||||||||||||||||
| Statins | lovastatin | Incl. Rhizopus sp. | [166,167,168] | |||||||||||||
| atorvastatin | Incl. Rhizopus sp., Cryptococcus sp. | [166,167,169] | ||||||||||||||
| fluvastatin | Incl. Rhizopus sp. | [166,167] | ||||||||||||||
| simvastatin | Incl. Rhizopus sp., Cryptococcus sp. | [166,167,170] | ||||||||||||||
| pitavastatin | [171] | |||||||||||||||
| Bisphosphonates | risedronate | Incl. Cryptococcus sp. | [172] | |||||||||||||
| alendronate | Incl. Cryptococcus sp. | [172] | ||||||||||||||
| zoledronate | Incl. Cryptococcus sp. | [172] | ||||||||||||||
| Immunomodulators | promethazine | [173,174] | ||||||||||||||
| terfenadine | Effective against biofilms | [175] | ||||||||||||||
| ebastine | Effective against biofilms | [175] | ||||||||||||||
| dexamethasone | Effective against biofilms | [176] | ||||||||||||||
| budesonide | Effective in vivo | [177] | ||||||||||||||
| methotrexate | [178] | |||||||||||||||
| Psychoactives | bromperidol | [179] | ||||||||||||||
| fluoxetine | Effective in vivo, C. albicans only | [180] | ||||||||||||||
| haloperidol | [173] | |||||||||||||||
| sertraline | Incl. Trichosporon asahii | [181] | ||||||||||||||
| Calcineurin Inhibitors | cyclosporine | Incl. S. cerevisiae | [182,183,184,185,186,187] | |||||||||||||
| tacrolimus | Incl. S. cerevisiae | [183,188,189,190,191,192,193] | ||||||||||||||
| Other | PPIs | Proton pump inhibitors | [194] | |||||||||||||
| geldanamycin | [195] | |||||||||||||||
| ponatinib | Incl. C. neoformans | [196] | ||||||||||||||
| HSP990 | Incl. C. neoformans | [197] | ||||||||||||||
| givinostat | [198] | |||||||||||||||
| lonafarnib | Incl. E. dermatitidis | [199] | ||||||||||||||
| isoquercitrin | [200] | |||||||||||||||
| EDTA | Incl. C. deuterogattii | [201] | ||||||||||||||
| D-penicillamine | Copper ion chelator | [202] | ||||||||||||||
| licofelone | Effective against biofilms | [203] | ||||||||||||||
| phenylbutyrate | [204] | |||||||||||||||
| 17-AAG | Incl. E. dermatitidis | [205] | ||||||||||||||
| ketamine | [206] | |||||||||||||||
| ibuprofen | [207] | |||||||||||||||
| chlorhexidine | Incl. C. auris | [208] | ||||||||||||||
| ganetespib | [209] | |||||||||||||||
| HMA | Incl. Cryptococcus sp. | [210] | ||||||||||||||
| Natural Products | ||||||||||||||||
| Ess. Oil Extracts | thymol | [211] | ||||||||||||||
| carvacrol | Effective against biofilms of C. auris | [211,212] | ||||||||||||||
| acetophenone | [213] | |||||||||||||||
| osthole | [214] | |||||||||||||||
| houttuyfonate | [215] | |||||||||||||||
| menthol | [216] | |||||||||||||||
| tyrosol | Effective against biofilms | [217] | ||||||||||||||
| allyl isothiocyan. | Effective against biofilms | [218] | ||||||||||||||
| butylphthalide | Effective against biofilms | [219] | ||||||||||||||
| glabridin | [220] | |||||||||||||||
| chito-oligosacch. | [221] | |||||||||||||||
| oridonin | [222] | |||||||||||||||
| Crude Ess. Oils | sea-buckthorn | [223] | ||||||||||||||
| guava leaf | [224] | |||||||||||||||
| frankincense | [225] | |||||||||||||||
| TTO | [226] | |||||||||||||||
| Alkaloids | berberine | Incl. S. cerevisiae and T. marneffei | [227,228,229,230,231,232] | |||||||||||||
| palmatine | Effective against biofilms | [233,234] | ||||||||||||||
| harmine | [235] | |||||||||||||||
| Other Terpenoids | guttiferone | [236] | ||||||||||||||
| farnesol | Effective against C. auris biofilms | [237] | ||||||||||||||
| asiatic acid | Effective in vivo | [238] | ||||||||||||||
| Other Phenols | magnolol | [239] | ||||||||||||||
| diorcinol | Extreme decrease in required dosage | [240] | ||||||||||||||
| proanthocyan. | [241] | |||||||||||||||
| epigallocatechin | Effective against biofilms | [242] | ||||||||||||||
| asarone | Also clotrimazole | [243] | ||||||||||||||
| pyrogallol | [244] | |||||||||||||||
| Peptides | lactoferrin | Other incl. Cryptococcus sp. | [201,245] | |||||||||||||
| beauvericin | [246] | |||||||||||||||
| Novel Compounds | ||||||||||||||||
| ATTAF-1 and -2 | Novel azole derivatives | [247] | ||||||||||||||
| 31 and 42 | Novel azole derivatives | [248] | ||||||||||||||
| 15 and 24 | Isoquinolone and phthalazinone deriv. | [249] | ||||||||||||||
| B-7b | Novel berberine derivative | [250] | ||||||||||||||
| LQFM-79-81 | Novel guttiferone-A derivatives | [236] | ||||||||||||||
| phenylpentanol | Novel phenylpentanol derivatives | [171] | ||||||||||||||
| AR-12 | Novel celecoxib derivative | [251] | ||||||||||||||
| SCY-078 | Glucan synthase inhibitor | [252] | ||||||||||||||
| DIBI | Ion chelator | [253] | ||||||||||||||
| 1 – 34c | Novel caffeic acid derivative | [254] | ||||||||||||||
| chalcones | [255] | |||||||||||||||
| AT406 | IAP Inhibitor, incl. E. dermatitidis | [256] | ||||||||||||||
| B2 | Piperidone derivative, incl. C. neoformans | [257] | ||||||||||||||
| H1-J10 | Novel HSP90/HDAC inhibitors | [258] | ||||||||||||||
| L1-C2 | Novel lipopeptides | [259] | ||||||||||||||
| AZD8055 | Novel TOR inhibitor | [260] | ||||||||||||||
| KEY: | extremely strong synergy | synergistic in all strains tested | ||||||||||||||
| strong synergy | synergistic in >20% strains tested | |||||||||||||||
| weak synergy | synergistic in <20% strains tested | |||||||||||||||
| borderline synergy | not synergistic in any strains tested | |||||||||||||||
2. Synergy between Azoles and Currently Available Antimicrobials
2.1. Azole-Antifungal Synergy
2.2. Azole–Antibacterial Synergy
2.3. Azole–Antiparasitic Synergy
2.4. Azole–Antiviral Synergy
3. Active Efflux Modulators
4. Repurposing Other Pharmaceuticals
4.1. Statins
4.2. Bisphosphonates
4.3. Repurposing Miscellaneous Pharmaceuticals

5. Azole Synergy with Natural Products
6. Azole Synergy with Novel Compounds
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Enoch, D.A.; Yang, H.; Aliyu, S.H.; Micallef, C. The Changing Epidemiology of Invasive Fungal Infections. Methods Mol. Biol. 2017, 1508, 17–65. [Google Scholar] [CrossRef] [PubMed]
- Bongomin, F.; Gago, S.; Oladele, R.; Denning, D. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Schelenz, S.; Barnes, R.A.; Kibbler, C.C.; Jones, B.L.; Denning, D.W. Standards of Care for Patients with Invasive Fungal Infections within the United Kingdom: A National Audit. J. Infect. 2009, 58, 145–153. [Google Scholar] [CrossRef]
- Bitar, D.; Lortholary, O.; le Strat, Y.; Nicolau, J.; Coignard, B.; Tattevin, P.; Che, D.; Dromer, F. Population-Based Analysis of Invasive Fungal Infections, France, 2001-2010. Emerg. Infect. Dis. 2014, 20, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.R.; Pinner, R.W.; Hajjeh, R.A.; Brandt, M.E.; Reingold, A.L. The Epidemiological Features of Invasive Mycotic Infections in the San Francisco Bay Area, 1992-1993: Results of Population-Based Laboratory Active Surveillance. Clin. Infect. Dis. 1998, 27, 1138–1150. [Google Scholar] [CrossRef] [PubMed]
- Chapman, B.; Slavin, M.; Marriott, D.; Halliday, C.; Kidd, S.E.; Arthur, I.; Bak, N.; Heath, C.; Kennedy, K.; Morrissey, C.O.; et al. Changing Epidemiology of Candidaemia in Australia. J. Antimicrob. Chemother. 2017, 72, 1103–1108. [Google Scholar] [CrossRef]
- Tsay, S.V.; Mu, Y.; Williams, S.; Epson, E.; Nadle, J.; Bamberg, W.M.; Barter, D.M.; Johnston, H.L.; Farley, M.M.; Harb, S.; et al. Burden of Candidemia in the United States, 2017. Clin. Infect. Dis. 2020, 71, E449–E453. [Google Scholar] [CrossRef]
- Astvad, K.M.T.; Johansen, H.K.; Røder, B.L.; Rosenvinge, F.S.; Knudsen, J.D.; Lemming, L.; Schønheyder, H.C.; Hare, R.K.; Kristensen, L.; Nielsen, L.; et al. Update from a 12-Year Nationwide Fungemia Surveillance: Increasing Intrinsic and Acquired Resistance Causes Concern. J. Clin. Microbiol. 2018, 56, e01564-17. [Google Scholar] [CrossRef]
- Eyre, D.W.; Sheppard, A.E.; Madder, H.; Moir, I.; Moroney, R.; Quan, T.P.; Griffiths, D.; George, S.; Butcher, L.; Morgan, M.; et al. A Candida auris Outbreak and Its Control in an Intensive Care Setting. N. Engl. J. Med. 2018, 379, 1322–1331. [Google Scholar] [CrossRef]
- Ruiz-Gaitán, A.; Moret, A.M.; Tasias-Pitarch, M.; Aleixandre-López, A.I.; Martínez-Morel, H.; Calabuig, E.; Salavert-Lletí, M.; Ramírez, P.; López-Hontangas, J.L.; Hagen, F.; et al. An Outbreak Due to Candida auris with Prolonged Colonisation and Candidaemia in a Tertiary Care European Hospital. Mycoses 2018, 61, 498–505. [Google Scholar] [CrossRef]
- Schelenz, S.; Hagen, F.; Rhodes, J.L.; Abdolrasouli, A.; Chowdhary, A.; Hall, A.; Ryan, L.; Shackleton, J.; Trimlett, R.; Meis, J.F.; et al. First Hospital Outbreak of the Globally Emerging Candida auris in a European Hospital. Antimicrob. Resist. Infect. Control. 2016, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Prestel, C.; Anderson, E.; Forsberg, K.; Lyman, M.; de Perio, M.A.; Kuhar, D.; Edwards, K.; Rivera, M.; Shugart, A.; Walters, M.; et al. Candida auris Outbreak in a COVID-19 Specialty Care Unit—Florida, July–August 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 56–57. [Google Scholar] [CrossRef] [PubMed]
- Douglas, L.J. Candida Biofilms and Their Role in Infection. Trends Microbiol. 2003, 11, 30–36. [Google Scholar] [CrossRef]
- Seebacher, C.; Bouchara, J.-P.; Mignon, B. Updates on the Epidemiology of Dermatophyte Infections. Mycopathologia 2008, 166, 335–352. [Google Scholar] [CrossRef]
- Gupta, A.K.; Paquet, M.; Simpson, F.C. Therapies for the Treatment of Onychomycosis. Clin. Dermatol. 2013, 31, 544–554. [Google Scholar] [CrossRef]
- Kirchhoff, L.; Olsowski, M.; Rath, P.-M.; Steinmann, J. Exophiala dermatitidis: Key Issues of an Opportunistic Fungal Pathogen. Virulence 2019, 10, 984–998. [Google Scholar] [CrossRef]
- Cafarchia, C.; Iatta, R.; Immediato, D.; Puttilli, M.R.; Otranto, D. Azole Susceptibility of Malassezia pachydermatis and Malassezia furfur and Tentative Epidemiological Cut-off Values. Med. Mycol. 2015, 53, 743–748. [Google Scholar] [CrossRef]
- Iatta, R.; Puttilli, M.R.; Immediato, D.; Otranto, D.; Cafarchia, C. The Role of Drug Efflux Pumps in Malassezia pachydermatis and Malassezia furfur Defence against Azoles. Mycoses 2017, 60, 178–182. [Google Scholar] [CrossRef]
- Moen, M.D.; Lyseng-Williamson, K.A.; Scott, L.J. Liposomal Amphotericin B. Drugs 2009, 69, 361–392. [Google Scholar] [CrossRef]
- Kneale, M.; Bartholomew, J.S.; Davies, E.; Denning, D.W. Global Access to Antifungal Therapy and Its Variable Cost. J. Antimicrob. Chemother. 2016, 71, 3599–3606. [Google Scholar] [CrossRef]
- Brammer, K.W.; Farrow, P.R.; Faulkner, J.K. Pharmacokinetics and Tissue Penetration of Fluconazole in Humans. Clin. Infect. Dis. 1990, 12, S318–S326. [Google Scholar] [CrossRef] [PubMed]
- Verweij, P.E.; Snelders, E.; Kema, G.H.; Mellado, E.; Melchers, W.J. Azole Resistance in Aspergillus fumigatus: A Side-Effect of Environmental Fungicide Use? Lancet Infect. Dis. 2009, 9, 789–795. [Google Scholar] [CrossRef]
- Goldman, M.; Cloud, G.A.; Smedema, M.; Lemonte, A.; Connolly, P.; Mckinsey, D.S.; Kauffman, C.A.; Moskovitz, B.; Wheat, L.J.; Flanigan, C.; et al. Does Long-Term Itraconazole Prophylaxis Result in In Vitro Azole Resistance in Mucosal Candida albicans Isolates from Persons with Advanced Human Immunodeficiency Virus Infection? Antimicrob. Agents Chemother. 2000, 44, 1585–1587. [Google Scholar] [CrossRef]
- Ruggero, M.A.; Topal, J.E. Development of Echinocandin-Resistant Candida albicans Candidemia Following Brief Prophylactic Exposure to Micafungin Therapy. Transpl. Infect. Dis. 2014, 16, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Bastos, R.W.; Carneiro, H.C.S.; Oliveira, L.V.N.; Rocha, K.M.; Freitas, G.J.C.; Costa, M.C.; Magalhães, T.F.F.; Carvalho, V.S.D.; Rocha, C.E.; Ferreira, G.F.; et al. Environmental Triazole Induces Cross-Resistance to Clinical Drugs and Affects Morphophysiology and Virulence of Cryptococcus gattii and C. neoformans. Antimicrob. Agents Chemother. 2018, 62, e01179-17. [Google Scholar] [CrossRef]
- Bamba, S.; Lortholary, O.; Sawadogo, A.; Millogo, A.; Guiguemdé, R.T.; Bretagne, S. Decreasing Incidence of Cryptococcal Meningitis in West Africa in the Era of Highly Active Antiretroviral Therapy. AIDS 2012, 26, 1039–1041. [Google Scholar] [CrossRef]
- D’Arminio Monforte, A.; Sabin, C.A.; Phillips, A.; Sterne, J.; May, M.; Justice, A.; Dabis, F.; Grabar, S.; Ledergerber, B.; Gill, J.; et al. The Changing Incidence of AIDS Events in Patients Receiving Highly Active Antiretroviral Therapy. Arch. Intern. Med. 2005, 165, 416–423. [Google Scholar] [CrossRef]
- Bennett, J.E.; Izumikawa, K.; Marr, K.A. Mechanism of Increased Fluconazole Resistance in Candida glabrata during Prophylaxis. Antimicrob. Agents Chemother. 2004, 48, 1773–1777. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Silva, S.; Henriques, M. Candida glabrata: A Review of Its Features and Resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 673–688. [Google Scholar] [CrossRef]
- Chowdhary, A.; Sharma, C.; Meis, J.F. Candida auris: A Rapidly Emerging Cause of Hospital-Acquired Multidrug-Resistant Fungal Infections Globally. PLOS Pathog. 2017, 13, e1006290. [Google Scholar] [CrossRef]
- Denning, D.W.; Bromley, M. How to Bolster the Antifungal Pipeline. Science (1979) 2015, 347, 1414–1416. [Google Scholar] [CrossRef] [PubMed]
- Odds, F.C.; Brown, A.J.P.; Gow, N.A.R. Antifungal Agents: Mechanisms of Action. Trends Microbiol. 2003, 11, 272–279. [Google Scholar] [CrossRef]
- Allen, D.; Wilson, D.; Drew, R.; Perfect, J. Azole Antifungals: 35 Years of Invasive Fungal Infection Management. Expert Rev. Anti-Infect. Ther. 2015, 13, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Andes, D.; Kovanda, L.; Desai, A.; Kitt, T.; Zhao, M.; Walsh, T.J. Isavuconazole Concentration in Real-World Practice: Consistency with Results from Clinical Trials. Antimicrob. Agents Chemother. 2018, 62, e00585-18. [Google Scholar] [CrossRef] [PubMed]
- Miceli, M.H.; Kauffman, C.A. Isavuconazole: A New Broad-Spectrum Triazole Antifungal Agent. Clin. Infect. Dis. 2015, 61, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Brand, S.R.; Degenhardt, T.P.; Person, K.; Sobel, J.D.; Nyirjesy, P.; Schotzinger, R.J.; Tavakkol, A. A Phase 2, Randomized, Double-Blind, Placebo-Controlled, Dose-Ranging Study to Evaluate the Efficacy and Safety of Orally Administered VT-1161 in the Treatment of Recurrent Vulvovaginal Candidiasis. Am. J. Obstet. Gynecol. 2018, 218, 624.e1–624.e9. [Google Scholar] [CrossRef]
- Wiederhold, N.P.; Xu, X.; Wang, A.; Najvar, L.K.; Garvey, E.P.; Ottinger, E.A.; Alimardanov, A.; Cradock, J.; Behnke, M.; Hoekstra, W.J.; et al. In Vivo Efficacy of VT-1129 against Experimental Cryptococcal Meningitis with the Use of a Loading Dose-Maintenance Dose Administration Strategy. Antimicrob. Agents Chemother. 2018, 62, e01315-18. [Google Scholar] [CrossRef]
- Monk, B.C.; Keniya, M.V. Roles for Structural Biology in the Discovery of Drugs and Agrochemicals Targeting Sterol 14α-Demethylases. J. Fungi 2021, 7, 67. [Google Scholar] [CrossRef]
- Hargrove, T.Y.; Garvey, E.P.; Hoekstra, W.J.; Yates, C.M.; Wawrzak, Z.; Rachakonda, G.; Villalta, F.; Lepesheva, G.I. Crystal Structure of the New Investigational Drug Candidate VT-1598 in Complex with Aspergillus fumigatus Sterol 14α-Demethylase Provides Insights into Its Broad-Spectrum Antifungal Activity. Antimicrob. Agents Chemother. 2017, 61, e00570-17. [Google Scholar] [CrossRef]
- Antifungal Drugs Market Size 2019|Demand|Industry Forecast. Available online: https://www.reportsanddata.com/report-detail/antifungal-drugs-market (accessed on 8 March 2021).
- Global Antifungal Drugs Market Report 2020|Orbis Research. Available online: https://www.orbisresearch.com/reports/index/global-antifungal-drugs-market-report-2020 (accessed on 8 March 2021).
- Zonios, D.; Yamazaki, H.; Murayama, N.; Natarajan, V.; Palmore, T.; Childs, R.; Skinner, J.; Bennett, J.E. Voriconazole Metabolism, Toxicity, and the Effect of Cytochrome P450 2C19 Genotype. J. Infect. Dis. 2014, 209, 1941–1948. [Google Scholar] [CrossRef]
- Mann, P.A.; McNicholas, P.M.; Chau, A.S.; Patel, R.; Mendrick, C.; Ullmann, A.J.; Cornely, O.A.; Patino, H.; Black, T.A. Impact of Antifungal Prophylaxis on Colonization and Azole Susceptibility of Candida Species. Antimicrob. Agents Chemother. 2009, 53, 5026–5034. [Google Scholar] [CrossRef] [PubMed]
- Chaabane, F.; Graf, A.; Jequier, L.; Coste, A.T. Review on Antifungal Resistance Mechanisms in the Emerging Pathogen Candida auris. Front. Microbiol. 2019, 10, 2788. [Google Scholar] [CrossRef]
- Brajtburg, J.; Powderly, W.G.; Kobayashi, G.S.; Medoff, G. Amphotericin B: Current Understanding of Mechanisms of Action. Antimicrob. Agents Chemother. 1990, 34, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Perlin, D.S. Current Perspectives on Echinocandin Class Drugs. Future Microbiol. 2011, 6, 441–457. [Google Scholar] [CrossRef] [PubMed]
- Georgopapadakou, N.H. Antifungals: Mechanism of Action and Resistance, Established and Novel Drugs. Curr. Opin. Microbiol. 1998, 1, 547–557. [Google Scholar] [CrossRef]
- Leyden, J. Pharmacokinetics and Pharmacology of Terbinafine and Itraconazole. J. Am. Acad. Dermatol. 1998, 38, S42–S47. [Google Scholar] [CrossRef]
- de Carli, L.; Larizza, L. Griseofulvin. Mutat. Res./Rev. Genet. Toxicol. 1988, 195, 91–126. [Google Scholar] [CrossRef]
- Vermes, A. Flucytosine: A Review of Its Pharmacology, Clinical Indications, Pharmacokinetics, Toxicity and Drug Interactions. J. Antimicrob. Chemother. 2000, 46, 171–179. [Google Scholar] [CrossRef]
- Heilmann, C.J.; Schneider, S.; Barker, K.S.; Rogers, P.D.; Morschhäuser, J. An A643T Mutation in the Transcription Factor Upc2p Causes Constitutive ERG11 Upregulation and Increased Fluconazole Resistance in Candida albicans. Antimicrob. Agents Chemother. 2010, 54, 353–359. [Google Scholar] [CrossRef]
- Holmes, A.R.; Cardno, T.S.; Strouse, J.J.; Ivnitski-Steele, I.; Keniya, M.V.; Lackovic, K.; Monk, B.C.; Sklar, L.A.; Cannon, R.D. Targeting Efflux Pumps to Overcome Antifungal Drug Resistance. Future Med. Chem. 2016, 8, 1485–1501. [Google Scholar] [CrossRef]
- Cannon, R.D.; Lamping, E.; Holmes, A.R.; Niimi, K.; Baret, P.V.; Keniya, M.V.; Tanabe, K.; Niimi, M.; Goffeau, A.; Monk, B.C. Efflux-Mediated Antifungal Drug Resistance. Clin. Microbiol. Rev. 2009, 22, 291–321. [Google Scholar] [CrossRef] [PubMed]
- Lamping, E.; Monk, B.C.; Niimi, K.; Holmes, A.R.; Tsao, S.; Tanabe, K.; Niimi, M.; Uehara, Y.; Cannon, R.D. Characterization of Three Classes of Membrane Proteins Involved in Fungal Azole Resistance by Functional Hyperexpression in Saccharomyces cerevisiae. Eukaryot. Cell 2007, 6, 1150–1165. [Google Scholar] [CrossRef] [PubMed]
- Selmecki, A.; Forche, A.; Berman, J. Aneuploidy and Isochromosome Formation in Drug-Resistant Candida albicans. Science (1979) 2006, 313, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Selmecki, A.M.; Dulmage, K.; Cowen, L.E.; Anderson, J.B.; Berman, J. Acquisition of Aneuploidy Provides Increased Fitness during the Evolution of Antifungal Drug Resistance. PLoS Genet. 2009, 5, e1000705. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.R.; Frade, J.P.; Etienne, K.A.; Pfaller, M.A.; Diekema, D.J.; Balajee, S.A. Azole Resistance in Aspergillus fumigatus Isolates from the ARTEMIS Global Surveillance Study Is Primarily Due to the TR/L98H Mutation in the Cyp51A Gene. Antimicrob. Agents Chemother. 2011, 55, 4465–4468. [Google Scholar] [CrossRef]
- Gonzalez-Jimenez, I.; Lucio, J.; Amich, J.; Cuesta, I.; Arroyo, R.S.; Alcazar-Fuoli, L.; Mellado, E. A CYP51b Mutation Contributes to Azole Resistance in Aspergillus fumigatus. J. Fungi 2020, 6, 315. [Google Scholar] [CrossRef]
- Leonardelli, F.; Macedo, D.; Dudiuk, C.; Cabeza, M.S.; Gamarra, S.; Garcia-Effron, G. Aspergillus fumigatus Intrinsic Fluconazole Resistance Is Due to the Naturally Occurring T301I Substitution in Cyp51Ap. Antimicrob. Agents Chemother. 2016, 60, 5420–5426. [Google Scholar] [CrossRef]
- Vermitsky, J.P.; Edlind, T.D. Azole Resistance in Candida glabrata: Coordinate Upregulation of Multidrug Transporters and Evidence for a Pdr1-like Transcription Factor. Antimicrob. Agents Chemother. 2004, 48, 3773–3781. [Google Scholar] [CrossRef]
- Hagiwara, D.; Miura, D.; Shimizu, K.; Paul, S.; Ohba, A.; Gonoi, T.; Watanabe, A.; Kamei, K.; Shintani, T.; Moye-Rowley, W.S.; et al. A Novel Zn2-Cys6 Transcription Factor AtrR Plays a Key Role in an Azole Resistance Mechanism of Aspergillus fumigatus by Co-Regulating Cyp51A and Cdr1B Expressions. PLoS Pathog. 2017, 13, e1006096. [Google Scholar] [CrossRef]
- Keniya, M.V.; Sabherwal, M.; Wilson, R.K.; Woods, M.A.; Sagatova, A.A.; Tyndall, J.D.A.; Monk, B.C. Crystal Structures of Full-Length Lanosterol 14α-Demethylases of Prominent Fungal Pathogens Candida albicans and Candida glabrata Provide Tools for Antifungal Discovery. Antimicrob. Agents Chemother. 2018, 62, e01134-18. [Google Scholar] [CrossRef]
- Sheng, C.; Miao, Z.; Ji, H.; Yao, J.; Wang, W.; Che, X.; Dong, G.; Lü, J.; Guo, W.; Zhang, W. Three-Dimensional Model of Lanosterol 14α-Demethylase from Cryptococcus neoformans: Active-Site Characterization and Insights into Azole Binding. Antimicrob. Agents Chemother. 2009, 53, 3487–3495. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.E.; Kontoyiannis, D.P. Rationale for Combination Antifungal Therapy. Pharmacotherapy 2001, 21, 149S–164S. [Google Scholar] [CrossRef] [PubMed]
- Droegemueller, W.; Adamson, D.G.; Brown, D.; Cibley, L.; Fleury, F.; Lepage, M.E.; Henzl, M.R. Three-Day Treatment with Butoconazole Nitrate for Vulvovaginal Candidiasis. Obstet. Gynecol. 1984, 64, 530–534. [Google Scholar] [PubMed]
- Schmidt, A. In Vitro Activity of Climbazole, Clotrimazole and Silver-Sulphadiazine against Isolates of Malassezia pachydermatis. J. Vet. Med. Ser. B 1997, 44, 193–197. [Google Scholar] [CrossRef]
- Mendling, W.; Plempel, M. Vaginal Secretion Levels after 6 Days, 3 Days and 1 Day of Treatment with 100, 200 and 500 Mg Vaginal Tablets of Clotrimazole and Their Therapeutic Efficacy. Chemotherapy 1982, 28, 43–47. [Google Scholar] [CrossRef]
- Fernández-Torres, B.; Inza, I.; Guarro, J. In Vitro Activities of the New Antifungal Drug Eberconazole and Three Other Topical Agents against 200 Strains of Dermatophytes. J. Clin. Microbiol. 2003, 41, 5209–5211. [Google Scholar] [CrossRef]
- Thienpont, D.; van Cutsem, J.; van Nueten, J.M. Biological and Toxicological Properties of Econazole, a Broad Spectrum Antimycotic. Arzneim.-Forsch./Drug Res. 1975, 25, 224–230. [Google Scholar]
- Gerven, F.; van Odds, F.C. The Anti-Malassezia furfur Activity In Vitro and in Experimental Dermatitis of Six Imidazole Antifungal Agents: Bifonazole, Clotrimazole, Flutrimazole, Ketoconazole, Miconazole and Sertaconazole. Mycoses 1995, 38, 389–393. [Google Scholar] [CrossRef]
- Veraldi, S. Isoconazole Nitrate: A Unique Broad-Spectrum Antimicrobial Azole Effective in the Treatment of Dermatomycoses, Both as Monotherapy and in Combination with Corticosteroids. Mycoses 2013, 56, 3–15. [Google Scholar] [CrossRef]
- Green, C.A.; Farr, P.M.; Shuster, S. Treatment of Seborrhoeic Dermatitis with Ketoconazole: II. Response of Seborrhoeic Dermatitis of the Face, Scalp and Trunk to Topical Ketoconazole. Br. J. Dermatol. 1987, 116, 217–221. [Google Scholar] [CrossRef]
- Khanna, D.; Bharti, S. Luliconazole for the Treatment of Fungal Infections: An Evidence-Based Review. Core Evid. 2014, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- van Cutsem, J.M.; Thienpont, D. Miconazole, a Broad-Spectrum Antimycotic Agent with Antibacterial Activity. Chemotherapy 1972, 17, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Polak, A. Oxiconazole, a New Imidazole Derivative. Evaluation of Antifungal Activity In Vitro and In Vivo. Arzneim.-Forsch./Drug Res. 1982, 32, 17–24. [Google Scholar]
- Liebel, F.; Lyte, P.; Garay, M.; Babad, J.; Southall, M.D. Anti-Inflammatory and Anti-Itch Activity of Sertaconazole Nitrate. Arch. Dermatol. Res. 2006, 298, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Muñoz, A.J.; Giusiano, G.; Ezkurra, P.A.; Quindós, G. Sertaconazole: Updated Review of a Topical Antifungal Agent. Expert Rev. Anti-Infect. Ther. 2005, 3, 333–342. [Google Scholar] [CrossRef]
- Benfield, P.; Stephen, P. Sulconazole: A Review of Its Antimicrobial Activity and Therapeutic Use in Superficial Dermatomycoses. Drugs 1988, 35, 143–153. [Google Scholar] [CrossRef]
- Sunderland, M.R.; Cruickshank, R.H.; Leighs, S.J. The Efficacy of Antifungal Azole and Antiprotozoal Compounds in Protection of Wool from Keratin-Digesting Insect Larvae. Text. Res. J. 2014, 84, 924–931. [Google Scholar] [CrossRef]
- Jevons, S.; Gymer, G.E.; Brammer, K.W.; Cox, D.A.; Leeming, M.R. Antifungal Activity of Tioconazole (UK-20,349), a New Imidazole Derivative. Antimicrob. Agents Chemother. 1979, 15, 597–602. [Google Scholar] [CrossRef]
- Dismukes, W.E.; Stamm, A.M.; Graybill, J.R.; Craven, P.C.; Stevens, D.A.; Stiller, R.L.; Sarosi, G.A.; Medoff, G.; Gregg, C.R.; Gallis, H.A.; et al. Treatment of Systemic Mycoses with Ketoconazole: Emphasis on Toxicity and Clinical Response in 52 Patients. National Institute of Allergy and Infectious Diseases Collaborative Antifungal Study. Ann. Intern. Med. 1983, 98, 13–20. [Google Scholar] [CrossRef]
- Elewski, B.E.; Rich, P.; Pollak, R.; Pariser, D.M.; Watanabe, S.; Senda, H.; Ieda, C.; Smith, K.; Pillai, R.; Ramakrishna, T.; et al. Efinaconazole 10% Solution in the Treatment of Toenail Onychomycosis: Two Phase III Multicenter, Randomized, Double-Blind Studies. J. Am. Acad. Dermatol. 2013, 68, 600–608. [Google Scholar] [CrossRef]
- van Cutsem, J.; van Gerven, F.; Zaman, R.; Janssen, P.A.J. Terconazole—A New Broad-Spectrum Antifungal. Chemotherapy 1983, 29, 322–331. [Google Scholar] [CrossRef]
- Sobue, S.; Tan, K.; Layton, G.; Eve, M.; Sanderson, J.B. Pharmacokinetics of Fosfluconazole and Fluconazole Following Multiple Intravenous Administration of Fosfluconazole in Healthy Male Volunteers. Br. J. Clin. Pharmacol. 2004, 58, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, D.; Nakamura, T.; Shigematsu, R.; Matsui, M.; Araki, S.; Kubo, K.; Sato, H.; Shirahata, A. Fosfluconazole for Antifungal Prophylaxis in Very Low Birth Weight Infants. Int. J. Pediatr. 2009, 2009, 274768. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Tsubouchi, I.; Okubo, A. Efficacy and Safety of Fosravuconazole L-Lysine Ethanolate, a Novel Oral Triazole Antifungal Agent, for the Treatment of Onychomycosis: A Multicenter, Double-Blind, Randomized Phase III Study. J. Dermatol. 2018, 45, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Marty, F.M.; Ostrosky-Zeichner, L.; Cornely, O.A.; Mullane, K.M.; Perfect, J.R.; Thompson, G.R.; Alangaden, G.J.; Brown, J.M.; Fredricks, D.N.; Heinz, W.J.; et al. Isavuconazole Treatment for Mucormycosis: A Single-Arm Open-Label Trial and Case-Control Analysis. Lancet Infect. Dis. 2016, 16, 828–837. [Google Scholar] [CrossRef]
- Odds, F.C. Itraconazole—A New Oral Antifungal Agent with a Very Broad Spectrum of Activity in Superficial and Systemic Mycoses. J. Dermatol. Sci. 1993, 5, 65–72. [Google Scholar] [CrossRef]
- Torres, H.A.; Hachem, R.Y.; Chemaly, R.F.; Kontoyiannis, D.P.; Raad, I.I. Posaconazole: A Broad-Spectrum Triazole Antifungal. Lancet Infect. Dis. 2005, 5, 775–785. [Google Scholar] [CrossRef]
- Hoffman, H.L.; Rathbun, R.C. Review of the Safety and Efficacy of Voriconazole. Expert Opin. Investig. Drugs 2002, 11, 409–429. [Google Scholar] [CrossRef]
- BioCentury—Abasol Abafungin Regulatory Update. Available online: https://www.biocentury.com/article/126706/abasol-abafungin-regulatory-update (accessed on 16 March 2021).
- Odds, F.C. Synergy, Antagonism, and What the Chequerboard Puts between Them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Xu, X.; Xu, L.; Yuan, G.; Wang, Y.; Qu, Y.; Zhou, M. Synergistic Combination of Two Antimicrobial Agents Closing Each Other’s Mutant Selection Windows to Prevent Antimicrobial Resistance. Sci. Rep. 2018, 8, 7237. [Google Scholar] [CrossRef]
- Coates, A.R.M.; Hu, Y.; Holt, J.; Yeh, P. Antibiotic Combination Therapy against Resistant Bacterial Infections: Synergy, Rejuvenation and Resistance Reduction. Expert Rev. Anti-Infect. Ther. 2020, 18, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Zubko, E.I.; Zubko, M.K. Co-Operative Inhibitory Effects of Hydrogen Peroxide and Iodine against Bacterial and Yeast Species. BMC Res. Notes 2013, 6, 272. [Google Scholar] [CrossRef] [PubMed]
- Bibi, M.; Murphy, S.; Benhamou, R.I.; Rosenberg, A.; Ulman, A.; Bicanic, T.; Fridman, M.; Berman, J. Combining Colistin and Fluconazole Synergistically Increases Fungal Membrane Permeability and Antifungal Cidality. ACS Infect. Dis. 2021, 7, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Trickey, A.; May, M.T.; Vehreschild, J.J.; Obel, N.; Gill, M.J.; Crane, H.M.; Boesecke, C.; Patterson, S.; Grabar, S.; Cazanave, C.; et al. Survival of HIV-Positive Patients Starting Antiretroviral Therapy between 1996 and 2013: A Collaborative Analysis of Cohort Studies. Lancet HIV 2017, 4, e349–e356. [Google Scholar] [CrossRef]
- Bell, A. Antimalarial Drug Synergism and Antagonism: Mechanistic and Clinical Significance. FEMS Microbiol. Lett. 2005, 253, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Hitchings, G.H. Mechanism of Action of Trimethoprim-Sulfamethoxazole. J. Infect. Dis. 1973, 128, S433–S436. [Google Scholar] [CrossRef]
- Bennett, J.E.; Dismukes, W.E.; Duma, R.J.; Medoff, G.; Sande, M.A.; Gallis, H.; Leonard, J.; Fields, B.T.; Bradshaw, M.; Haywood, H.; et al. A Comparison of Amphotericin B Alone and Combined with Flucytosine in the Treatment of Cryptoccal Meningitis. N. Engl. J. Med. 1979, 301, 126–131. [Google Scholar] [CrossRef]
- Stamm, A.M.; Diasio, R.B.; Dismukes, W.E.; Shadomy, S.; Cloud, G.A.; Bowles, C.A.; Karam, G.H.; Espinel-Ingroff, A. Toxicity of Amphotericin B plus Flucytosine in 194 Patients with Cryptococcal Meningitis. Am. J. Med. 1987, 83, 236–242. [Google Scholar] [CrossRef]
- Campitelli, M.; Zeineddine, N.; Samaha, G.; Maslak, S. Combination Antifungal Therapy: A Review of Current Data. J. Clin. Med. Res. 2017, 9, 451–456. [Google Scholar] [CrossRef]
- Johnson, M.D.; MacDougall, C.; Ostrosky-Zeichner, L.; Perfect, J.R.; Rex, J.H. Combination Antifungal Therapy. Antimicrob. Agents Chemother. 2004, 48, 693–715. [Google Scholar] [CrossRef]
- Weig, M.; Müller, F.M.C. Synergism of Voriconazole and Terbinafine against Candida albicans Isolates from Human Immunodeficiency Virus-Infected Patients with Oropharyngeal Candidiasis. Antimicrob. Agents Chemother. 2001, 45, 966–968. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.A.; Elewski, B. Successful Treatment of Fluconazole-Resistant Oropharyngeal Candidiasis by a Combination of Fluconazole and Terbinafine. Clin. Diagn. Lab. Immunol. 1999, 6, 921–923. [Google Scholar] [CrossRef] [PubMed]
- Perea, S.; Gonzalez, G.; Fothergill, A.W.; Sutton, D.A.; Rinaldi, M.G. In Vitro Activities of Terbinafine in Combination with Fluconazole, Itraconazole, Voriconazole, and Posaconazole against Clinical Isolates of Candida glabrata with Decreased Susceptibility to Azoles. J. Clin. Microbiol. 2002, 40, 1831–1833. [Google Scholar] [CrossRef]
- Cantón, E.; Pemân, J.; Gobernado, M.; Viudes, A.; Espinel-Ingroff, A. Synergistic Activities of Fluconazole and Voriconazole with Terbinafine against Four Candida Species Determined by Checkerboard, Time-Kill, and Etest Methods. Antimicrob. Agents Chemother. 2005, 49, 1593–1596. [Google Scholar] [CrossRef] [PubMed]
- Meletiadis, J.; Mouton, J.W.; Rodriguez-Tudela, J.L.; Meis, J.F.G.M.; Verweij, P.E. In Vitro Interaction of Terbinafine with Itraconazole against Clinical Isolates of Scedosporium prolificans. Antimicrob. Agents Chemother. 2000, 44, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Meletiadis, J.; Mouton, J.W.; Meis, J.F.G.M.; Verweij, P.E. In Vitro Drug Interaction Modeling of Combinations of Azoles with Terbinafine against Clinical Scedosporium prolificans Isolates. Antimicrob. Agents Chemother. 2003, 47, 106–117. [Google Scholar] [CrossRef]
- Ryder, N.S.; Leitner, I. Synergistic Interaction of Terbinafine with Triazoles or Amphotericin B against Aspergillus Species. Med. Mycol. 2001, 39, 91–95. [Google Scholar] [CrossRef]
- Bidaud, A.L.; Schwarz, P.; Chowdhary, A.; Dannaoui, E. In Vitro Antifungal Combination of Terbinafine with Itraconazole against Isolates of Trichophyton Species. Antimicrob. Agents Chemother. 2021, 66, e0144921. [Google Scholar] [CrossRef]
- Barchiesi, F.; Di Francesco, L.F.; Scalise, G. In Vitro Activities of Terbinafine in Combination with Fluconazole and Itraconazole against Isolates of Candida albicans with Reduced Susceptibility to Azoles. Antimicrob. Agents Chemother. 1997, 41, 1812–1814. [Google Scholar] [CrossRef]
- Mavridou, E.; Meletiadis, J.; Rijs, A.; Mouton, J.W.; Verweij, P.E. The Strength of Synergistic Interaction between Posaconazole and Caspofungin Depends on the Underlying Azole Resistance Mechanism of Aspergillus fumigatus. Antimicrob. Agents Chemother. 2015, 59, 1738–1744. [Google Scholar] [CrossRef][Green Version]
- Buil, J.B.; Brüggemann, R.J.M.; Denardi, L.B.; Melchers, W.J.G.; Verweij, P.E. In Vitro Interaction of Isavuconazole and Anidulafungin against Azole-Susceptible and Azole-Resistant Aspergillus fumigatus Isolates. J. Antimicrob. Chemother. 2020, 75, 2582–2586. [Google Scholar] [CrossRef] [PubMed]
- Fakhim, H.; Chowdhary, A.; Prakash, A.; Vaezi, A.; Dannaoui, E.; Meis, J.F.; Badali, H. In Vitro Interactions of Echinocandins with Triazoles against Multidrug-Resistant Candida auris. Antimicrob. Agents Chemother. 2017, 61, e01056-17. [Google Scholar] [CrossRef] [PubMed]
- Fakhim, H.; Vaezi, A.; Dannaoui, E.; Sharma, C.; Mousavi, B.; Chowdhary, A.; Meis, J.F.; Badali, H. In Vitro Combination of Voriconazole with Micafungin against Azole-Resistant Clinical Isolates of Aspergillus fumigatus from Different Geographical Regions. Diagn. Microbiol. Infect. Dis. 2018, 91, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Messer, S.A.; Deshpande, L.M.; Rhomberg, P.R.; Utt, E.A.; Castanheira, M. Evaluation of Synergistic Activity of Isavuconazole or Voriconazole plus Anidulafungin and the Occurrence and Genetic Characterization of Candida auris Detected in a Surveillance Program. Antimicrob. Agents Chemother. 2021, 65, e02031-20. [Google Scholar] [CrossRef]
- Caballero, U.; Kim, S.; Eraso, E.; Quindós, G.; Vozmediano, V.; Schmidt, S.; Jauregizar, N. In Vitro Synergistic Interactions of Isavuconazole and Echinocandins against Candida auris. Antibiotics 2021, 10, 355. [Google Scholar] [CrossRef]
- Sradhanjali, S.; Yein, B.; Sharma, S.; Das, S. In Vitro Synergy of Natamycin and Voriconazole against Clinical Isolates of Fusarium, Candida, Aspergillus and Curvularia spp. Br. J. Ophthalmol. 2018, 102, 142–145. [Google Scholar] [CrossRef]
- Gupta, A.K.; Kohli, Y. In Vitro Susceptibility Testing of Ciclopirox, Terbinafine, Ketoconazole and Itraconazole against Dermatophytes and Nondermatophytes, and In Vitro Evaluation of Combination Antifungal Activity. Br. J. Dermatol. 2003, 149, 296–305. [Google Scholar] [CrossRef]
- Bidaud, A.L.; Botterel, F.; Chowdhary, A.; Dannaouia, E. In Vitro Antifungal Combination of Flucytosine with Amphotericin B, Voriconazole, or Micafungin against Candida auris Shows No Antagonism. Antimicrob. Agents Chemother. 2019, 63, e01393-19. [Google Scholar] [CrossRef]
- Macedo, D.; Leonardelli, F.; Dudiuk, C.; Vitale, R.G.; del Valle, E.; Giusiano, G.; Gamarra, S.; Garcia-Effron, G. In Vitro and In Vivo Evaluation of Voriconazole-Containing Antifungal Combinations against Mucorales Using a Galleria mellonella Model of Mucormycosis. J. Fungi 2019, 5, 5. [Google Scholar] [CrossRef]
- Polak, A. Combination of Amorolfine with Various Antifungal Drugs in Dermatophytosis. Mycoses 2009, 36, 43–49. [Google Scholar] [CrossRef]
- Tamura, T.; Asahara, M.; Yamamoto, M.; Yamaura, M.; Matsumura, M.; Goto, K.; Rezaei-Matehkolaei, A.; Mirhendi, H.; Makimura, M.; Makimura, K. In Vitro Susceptibility of Dermatomycoses Agents to Six Antifungal Drugs and Evaluation by Fractional Inhibitory Concentration Index of Combined Effects of Amorolfine and Itraconazole in Dermatophytes. Microbiol. Immunol. 2014, 58, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lecha, M. Amorolfine and Itraconazole Combination for Severe Toenail Onychomycosis; Results of an Open Randomized Trial in Spain. Br. J. Dermatol. 2008, 145, 21–26. [Google Scholar] [CrossRef]
- Laurent, A.; Monod, M. Production of Trichophyton rubrum Microspores in Large Quantities and Its Application to Evaluate Amorolfine/Azole Compound Interactions In Vitro. Mycoses 2017, 60, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.K.; Grilley, M.; Anderson, T.; Dhiman, C.; Oblad, J.; Chang, C.-W.T.; Sorensen, K.N.; Takemoto, J.Y. In Vitro Antifungal Synergy between Amphiphilic Aminoglycoside K20 and Azoles against Candida Species and Cryptococcus neoformans. Med. Mycol. 2015, 53, 837–844. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Revie, N.M.; Robbins, N.; Whitesell, L.; Frost, J.R.; Appavoo, S.D.; Yudin, A.K.; Cowen, L.E. Oxadiazole-Containing Macrocyclic Peptides Potentiate Azole Activity against Pathogenic Candida Species. mSphere 2020, 5, e00256-20. [Google Scholar] [CrossRef]
- Eldesouky, H.E.; Li, X.; Abutaleb, N.S.; Mohammad, H.; Seleem, M.N. Synergistic Interactions of Sulfamethoxazole and Azole Antifungal Drugs against Emerging Multidrug-Resistant Candida auris. Int. J. Antimicrob. Agents 2018, 52, 754–761. [Google Scholar] [CrossRef]
- Eldesouky, H.E.; Mayhoub, A.; Hazbun, T.R.; Seleema, M.N. Reversal of Azole Resistance in Candida albicans by Sulfa Antibacterial Drugs. Antimicrob. Agents Chemother. 2018, 62, e00701-17. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, C.; Lu, C.; Liu, P.; Li, Y.; Li, H.; Sun, S. Synergistic Effect of Doxycycline and Fluconazole against Candida albicans Biofilms and the Impact of Calcium Channel Blockers. FEMS Yeast Res. 2013, 13, 453–462. [Google Scholar] [CrossRef]
- Hooper, R.W.; Ashcraft, D.S.; Pankey, G.A. In Vitro Synergy with Fluconazole plus Doxycycline or Tigecycline against Clinical Candida glabrata Isolates. Med. Mycol. 2019, 57, 122–126. [Google Scholar] [CrossRef]
- Gu, W.; Yu, Q.; Yu, C.; Sun, S. In Vivo Activity of Fluconazole/Tetracycline Combinations in Galleria mellonella with Resistant Candida albicans Infection. J. Glob. Antimicrob. Resist. 2018, 13, 74–80. [Google Scholar] [CrossRef]
- Venturini, T.P.; Rossato, L.; Chassot, F.; Keller, J.T.; Piasentin, F.B.; Santurio, J.M.; Alves, S.H. In Vitro Synergistic Combinations of Pentamidine, Polymyxin B, Tigecycline and Tobramycin with Antifungal Agents against Fusarium spp. J. Med. Microbiol. 2016, 65, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Hacioglu, M.; Tan, A.S.B.; Dosler, S.; Inan, N.; Otuk, G. In Vitro Activities of Antifungals Alone and in Combination with Tigecycline against Candida albicans Biofilms. PeerJ 2018, 2018, e5263. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Sun, Y.; Yuan, M.; Li, M.; Zeng, T. In Vitro and In Vivo Study on the Synergistic Effect of Minocycline and Azoles against Pathogenic Fungi. Antimicrob. Agents Chemother. 2020, 64, e00290-20. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Jiang, S.; Tan, L.; Shi, H.; Yang, L.; Sun, Y.; Wang, X. Antifungal Activity of Minocycline and Azoles Against Fluconazole-Resistant Candida Species. Front. Microbiol. 2021, 12, 1185. [Google Scholar] [CrossRef]
- Tan, L.; Shi, H.; Chen, M.; Wang, Z.; Yao, Z.; Sun, Y. In Vitro Synergistic Effect of Minocycline Combined with Antifungals against Cryptococcus neoformans. J. Med. Mycol. 2022, 32, 101227. [Google Scholar] [CrossRef]
- Yang, F.; Sun, Y.; Lu, Q. The Synergistic Effect of Minocycline and Azole Antifungal Drugs against Scedosporium and Lomentospora Species. BMC Microbiol. 2022, 22, 21. [Google Scholar] [CrossRef]
- Lu, M.; Yu, C.; Cui, X.; Shi, J.; Yuan, L.; Sun, S. Gentamicin Synergises with Azoles against Drug-Resistant Candida albicans. Int. J. Antimicrob. Agents 2018, 51, 107–114. [Google Scholar] [CrossRef]
- Lu, M.; Yang, X.; Yu, C.; Gong, Y.; Yuan, L.; Hao, L.; Sun, S. Linezolid in Combination With Azoles Induced Synergistic Effects Against Candida albicans and Protected Galleria mellonella Against Experimental Candidiasis. Front. Microbiol. 2019, 9, 3142. [Google Scholar] [CrossRef]
- Yousfi, H.; Ranque, S.; Rolain, J.M.; Bittar, F. In Vitro Polymyxin Activity against Clinical Multidrug-Resistant Fungi. Antimicrob. Resist. Infect. Control 2019, 8, 66. [Google Scholar] [CrossRef]
- Schwarz, P.; Djenontin, E.; Dannaoui, E. Colistin and Isavuconazole Interact Synergistically In Vitro against Aspergillus nidulans and Aspergillus niger. Microorganisms 2020, 8, 1447. [Google Scholar] [CrossRef]
- Schwarz, P.; Bidaud, A.-L.; Dannaoui, E. In Vitro Synergy of Isavuconazole in Combination with Colistin against Candida auris. Sci. Rep. 2020, 10, 21448. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Sun, Y.; He, C.; Zeng, T.; Li, M. Synergy between Pyrvinium Pamoate and Azoles against Exophiala dermatitidis. Antimicrob. Agents Chemother. 2018, 62, e02361-17. [Google Scholar] [CrossRef] [PubMed]
- de Cremer, K.; Lanckacker, E.; Cools, T.L.; Bax, M.; de Brucker, K.; Cos, P.; Cammue, B.P.A.; Thevissen, K. Artemisinins, New Miconazole Potentiators Resulting in Increased Activity against Candida albicans Biofilms. Antimicrob. Agents Chemother. 2015, 59, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wan, Z.; Liu, W.; Li, R. Synergistic Activity of Chloroquine with Fluconazole against Fluconazole-Resistant Isolates of Candida Species. Antimicrob. Agents Chemother. 2015, 59, 1365–1369. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Sun, Y.; He, C.; Li, M.; Zeng, T.; Lu, Q. INK128 Exhibits Synergy with Azoles against Exophiala spp. and Fusarium spp. Front. Microbiol. 2016, 7, 1658. [Google Scholar] [CrossRef]
- Montoya, M.C.; Beattie, S.; Alden, K.M.; Krysan, D.J. Derivatives of the Antimalarial Drug Mefloquine Are Broad-Spectrum Antifungal Molecules with Activity against Drug-Resistant Clinical Isolates. Antimicrob. Agents Chemother. 2020, 64, e02331-19. [Google Scholar] [CrossRef]
- Brilhante, R.S.N.; Caetano, É.P.; Riello, G.B.; de M. Guedes, G.M.; de Souza Collares Maia Castelo-Branco, D.; Fechine, M.A.B.; de Oliveira, J.S.; de Camargo, Z.P.; de Mesquita, J.R.L.; Monteiro, A.J.; et al. Antiretroviral Drugs Saquinavir and Ritonavir Reduce Inhibitory Concentration Values of Itraconazole against Histoplasma capsulatum Strains In Vitro. Braz. J. Infect. Dis. 2016, 20, 155–159. [Google Scholar] [CrossRef]
- LaFleur, M.D.; Sun, L.; Lister, I.; Keating, J.; Nantel, A.; Long, L.; Ghannoum, M.; North, J.; Lee, R.E.; Coleman, K.; et al. Potentiation of Azole Antifungals by 2-Adamantanamine. Antimicrob. Agents Chemother. 2013, 57, 3585–3592. [Google Scholar] [CrossRef]
- Zhang, M.; Yan, H.; Lu, M.; Wang, D.; Sun, S. Antifungal Activity of Ribavirin Used Alone or in Combination with Fluconazole against Candida albicans Is Mediated by Reduced Virulence. Int. J. Antimicrob. Agents 2020, 55, 105804. [Google Scholar] [CrossRef]
- Eldesouky, H.E.; Salama, E.A.; Lanman, N.A.; Hazbun, T.R.; Seleem, M.N. Potent Synergistic Interactions between Lopinavir and Azole Antifungal Drugs against Emerging Multidrug-Resistant Candida auris. Antimicrob. Agents Chemother. 2020, 65, e00684-20. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Liu, W.D.; Shen, Y.N.; Li, D.M.; Zhu, K.J.; Zhang, H. The Efflux Pump Inhibitor Tetrandrine Exhibits Synergism with Fluconazole or Voriconazole against Candida parapsilosis. Mol. Biol. Rep. 2019, 46, 5867–5874. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-X.; Song, Y.-J.; Jiang, L.; Zhao, Y.-J.; Guo, H.; Li, D.-M.; Zhu, K.-J.; Zhang, H. Synergistic Effects of Tetrandrine with Posaconazole Against Aspergillus fumigatus. Microb. Drug Resist. 2017, 23, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Li, S.; Gao, A.; Zhu, K.; Zhang, H. Tetrandrine Enhances the Antifungal Activity of Fluconazole in a Murine Model of Disseminated Candidiasis. Phytomedicine 2018, 46, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Li, S.X.; Song, Y.J.; Zhang, L.L.; Shi, J.P.; Ma, Z.L.; Guo, H.; Dong, H.Y.; Li, Y.M.; Zhang, H. An In Vitro and In Vivo Study on the Synergistic Effect and Mechanism of Itraconazole or Voriconazole Alone and in Combination with Tetrandrine against Aspergillus fumigatus. J. Med. Microbiol. 2015, 64, 1008–1020. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Zhang, Z.; Chen, P.; Long, N.; Lu, L.; Sang, H. In Vitro and In Vivo Efficacy of a Synergistic Combination of Itraconazole and Verapamil Against Aspergillus fumigatus. Front. Microbiol. 2019, 10, 1266. [Google Scholar] [CrossRef]
- Afeltra, J.; Vitale, R.G.; Mouton, J.W.; Verweij, P.E. Potent Synergistic In Vitro Interaction between Nonantimicrobial Membrane-Active Compounds and Itraconazole against Clinical Isolates of Aspergillus fumigatus Resistant to Itraconazole. Antimicrob. Agents Chemother. 2004, 48, 1335–1343. [Google Scholar] [CrossRef]
- Xu, J.; Liu, R.; Sun, F.; An, L.; Shang, Z.; Kong, L.; Yang, M. Eucalyptal D Enhances the Antifungal Effect of Fluconazole on Fluconazole-Resistant Candida albicans by Competitively Inhibiting Efflux Pump. Front. Cell. Infect. Microbiol. 2019, 9, 211. [Google Scholar] [CrossRef]
- Yang, D.L.; Hu, Y.L.; Yin, Z.X.; Zeng, G.S.; Li, D.; Zhang, Y.Q.; Xu, Z.H.; Guan, X.M.; Weng, L.X.; Wang, L.H. Cis-2-Dodecenoic Acid Mediates Its Synergistic Effect with Triazoles by Interfering with Efflux Pumps in Fluconazole-Resistant Candida albicans. Biomed. Environ. Sci. 2019, 32, 199–209. [Google Scholar] [CrossRef]
- Iyer, K.R.; Camara, K.; Daniel-Ivad, M.; Trilles, R.; Pimentel-Elardo, S.M.; Fossen, J.L.; Marchillo, K.; Liu, Z.; Singh, S.; Muñoz, J.F.; et al. An Oxindole Efflux Inhibitor Potentiates Azoles and Impairs Virulence in the Fungal Pathogen Candida auris. Nat. Commun. 2020, 11, 6429. [Google Scholar] [CrossRef]
- Eldesouky, H.E.; Salama, E.A.; Hazbun, T.R.; Mayhoub, A.S.; Seleem, M.N. Ospemifene Displays Broad-Spectrum Synergistic Interactions with Itraconazole through Potent Interference with Fungal Efflux Activities. Sci. Rep. 2020, 10, 6089. [Google Scholar] [CrossRef]
- Xie, F.; Chang, W.; Zhang, M.; Li, Y.; Li, W.; Shi, H.; Zheng, S.; Lou, H. Quinone Derivatives Isolated from the Endolichenic Fungus Phialocephala fortinii Are Mdr1 Modulators That Combat Azole Resistance in Candida albicans. Sci. Rep. 2016, 6, 33687. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Fatima, Z.; Ahmad, K.; Hameed, S. Fungicidal Action of Geraniol against Candida albicans Is Potentiated by Abrogated CaCdr1p Drug Efflux and Fluconazole Synergism. PLoS ONE 2018, 13, e0203079. [Google Scholar] [CrossRef] [PubMed]
- Nyilasi, I.; Kocsubé, S.; Krizsán, K.; Galgóczy, L.; Pesti, M.; Papp, T.; Vágvölgyi, C. In Vitro Synergistic Interactions of the Effects of Various Statins and Azoles against Some Clinically Important Fungi. FEMS Microbiol. Lett. 2010, 307, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Nyilasi, I.; Kocsubé, S.; Krizsán, K.; Galgóczy, L.; Papp, T.; Pesti, M.; Nagy, K.; Vágvölgyi, C. Susceptibility of Clinically Important Dermatophytes against Statins and Different Statin-Antifungal Combinations. Med. Mycol. 2013, 52, 140–148. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, H.; Zhou, X.; Luo, H.; Tang, F.; Yang, J.; Alterovitz, G.; Cheng, L.; Ren, B. Lovastatin Synergizes with Itraconazole against Planktonic Cells and Biofilms of Candida albicans through the Regulation on Ergosterol Biosynthesis Pathway. Appl. Microbiol. Biotechnol. 2018, 102, 5255–5264. [Google Scholar] [CrossRef] [PubMed]
- de Q. Ribeiro, N.; Costa, M.C.; Magalhães, T.F.F.; Carneiro, H.C.S.; Oliveira, L.V.; Fontes, A.C.L.; Santos, J.R.A.; Ferreira, G.F.; de S. Araujo, G.R.; Alves, V.; et al. Atorvastatin as a Promising Anticryptococcal Agent. Int. J. Antimicrob. Agents 2017, 49, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Brilhante, R.S.; Caetano, E.P.; Oliveira, J.S.; Castelo-Branco, D.; Souza, E.R.; Alencar, L.P.; Cordeiro, R.; Bandeira, T.; Sidrim, J.J.; Rocha, M.F. Simvastatin Inhibits Planktonic Cells and Biofilms of Candida and Cryptococcus Species. Braz. J. Infect. Dis. 2015, 19, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Eldesouky, H.E.; Salama, E.A.; Li, X.; Hazbun, T.R.; Mayhoub, A.S.; Seleem, M.N. Repurposing Approach Identifies Pitavastatin as a Potent Azole Chemosensitizing Agent Effective against Azole-Resistant Candida Species. Sci. Rep. 2020, 10, 7525. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.; Campbell, L.; Ky, D.; Hibbs, D.; Carter, D. The Antifungal and Synergistic Effect of Bisphosphonates in Cryptococcus. Antimicrob. Agents Chemother. 2021, 65, e01753-20. [Google Scholar] [CrossRef]
- Aneke, C.I.; Rhimi, W.; Otranto, D.; Cafarchia, C. Synergistic Effects of Efflux Pump Modulators on the Azole Antifungal Susceptibility of Microsporum canis. Mycopathologia 2020, 185, 279–288. [Google Scholar] [CrossRef]
- Brilhante, R.S.N.; de Oliveira, J.S.; de Jesus Evangelista, A.J.; Pereira, V.S.; Alencar, L.P.; de Souza Collares Maia Castelo-Branco, D.; Câmara, L.M.C.; de Lima-Neto, R.G.; de A. Cordeiro, R.; Sidrim, J.J.C.; et al. In Vitro Effects of Promethazine on Cell Morphology and Structure and Mitochondrial Activity of Azole-Resistant Candida tropicalis. Med. Mycol. 2017, 56, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.K.; Garneau-Tsodikova, S. Synergistic Combinations of Azoles and Antihistamines against Candida Species In Vitro. Med. Mycol. 2019, 57, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wang, D.; Yu, C.; Huang, X.; Li, X.; Sun, S. Strong Synergism of Dexamethasone in Combination with Fluconazole against Resistant Candida albicans Mediated by Inhibiting Drug Efflux and Reducing Virulence. Int. J. Antimicrob. Agents 2017, 50, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, C.; Huang, X.; Sun, S. Synergistic Effects and Mechanisms of Budesonide in Combination with Fluconazole against Resistant Candida albicans. PLoS ONE 2016, 11, e0168936. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Gao, L.; Yu, P.; Kosgey, J.C.; Jia, L.; Fang, Y.; Xiong, J.; Zhang, F. In Vitro Synergy of Azole Antifungals and Methotrexate against Candida albicans. Life Sci. 2019, 235, 116827. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, S.Y.L.; Garzan, A.; Dennis, E.K.; Shrestha, S.K.; Garneau-Tsodikova, S. Repurposing Antipsychotic Drugs into Antifungal Agents: Synergistic Combinations of Azoles and Bromperidol Derivatives in the Treatment of Various Fungal Infections. Eur. J. Med. Chem. 2017, 139, 12–21. [Google Scholar] [CrossRef]
- Gu, W.; Guo, D.; Zhang, L.; Xu, D.; Sun, S. The Synergistic Effect of Azoles and Fluoxetine against Resistant Candida albicans Strains Is Attributed to Attenuating Fungal Virulence. Antimicrob. Agents Chemother. 2016, 60, 6179–6188. [Google Scholar] [CrossRef]
- Cong, L.; Liao, Y.; Yang, S.; Yang, R. In Vitro Antifungal Activity of Sertraline and Synergistic Effects in Combination with Antifungal Drugs against Planktonic Forms and Biofilms of Clinical Trichosporon asahii Isolates. PLoS ONE 2016, 11, e0167903. [Google Scholar] [CrossRef]
- Jia, W.; Zhang, H.; Li, C.; Li, G.; Liu, X.; Wei, J. The Calcineruin Inhibitor Cyclosporine a Synergistically Enhances the Susceptibility of Candida albicans Biofilms to Fluconazole by Multiple Mechanisms. BMC Microbiol. 2016, 16, 113. [Google Scholar] [CrossRef]
- Tome, M.; Zupan, J.; Tomičić, Z.; Matos, T.; Raspor, P. Synergistic and Antagonistic Effects of Immunomodulatory Drugs on the Action of Antifungals against Candida glabrata and Saccharomyces cerevisiae. PeerJ 2018, 2018, e4999. [Google Scholar] [CrossRef]
- Onyewu, C.; Blankenship, J.R.; Del Poeta, M.; Heitman, J. Ergosterol Biosynthesis Inhibitors Become Fungicidal When Combined with Calcineurin Inhibitors against Candida albicans, Candida glabrata, and Candida krusei. Antimicrob. Agents Chemother. 2003, 47, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Uppuluri, P.; Nett, J.; Heitman, J.; Andes, D. Synergistic Effect of Calcineurin Inhibitors and Fluconazole against Candida albicans Biofilms. Antimicrob. Agents Chemother. 2008, 52, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, W.J.; Schell, W.A.; Blankenship, J.R.; Onyewu, C.; Heitman, J.; Perfect, J.R. In Vitro Interactions between Antifungals and Immunosuppressants against Aspergillus fumigatus. Antimicrob. Agents Chemother. 2004, 48, 1664–1669. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, O.; Moreillon, P.; Glauser, M.P.; Bille, J.; Sanglard, D. Potent Synergism of the Combination of Fluconazole and Cyclosporine in Candida albicans. Antimicrob. Agents Chemother. 2000, 44, 2373–2381. [Google Scholar] [CrossRef]
- Gao, L.; Sun, Y.; He, C.; Zeng, T.; Li, M. Synergistic Effects of Tacrolimus and Azoles against Exophiala dermatitidis. Antimicrob. Agents Chemother. 2017, 61, e00948-17. [Google Scholar] [CrossRef]
- Gao, L.; Sun, Y. In Vitro Interactions of Antifungal Agents and Tacrolimus against Aspergillus Biofilms. Antimicrob. Agents Chemother. 2015, 59, 7097–7099. [Google Scholar] [CrossRef]
- Denardi, L.B.; Mario, D.A.N.; Loreto, É.S.; Santurio, J.M.; Alves, S.H. Synergistic Effects of Tacrolimus and Azole Antifungal Compounds in Fluconazole-Susceptible and Fluconazole-Resistant Candida glabrata Isolates. Braz. J. Microbiol. 2015, 46, 125–129. [Google Scholar] [CrossRef]
- Kubiça, T.F.; Denardi, L.B.; Azevedo, M.I.; Oliveira, V.; Severo, L.C.; Santurio, J.M.; Alves, S.H. Antifungal Activities of Tacrolimus in Combination with Antifungal Agents against Fluconazole-Susceptible and Fluconazole-Resistant Trichosporon asahii Isolates. Braz. J. Infect. Dis. 2016, 20, 539–545. [Google Scholar] [CrossRef]
- Borba-Santos, L.P.; Reis de Sá, L.F.; Ramos, J.A.; Rodrigues, A.M.; de Camargo, Z.P.; Rozental, S.; Ferreira-Pereira, A. Tacrolimus Increases the Effectiveness of Itraconazole and Fluconazole against Sporothrix spp. Front. Microbiol. 2017, 8, 1759. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, J.; Yang, L.; He, Y. Tacrolimus, Not Triamcinolone Acetonide, Interacts Synergistically with Itraconazole, Terbinafine, Bifonazole, and Amorolfine against Clinical Dermatophyte Isolates. J. De Mycol. Med. 2018, 28, 612–616. [Google Scholar] [CrossRef]
- Lu, M.; Yan, H.; Yu, C.; Yuan, L.; Sun, S. Proton Pump Inhibitors Act Synergistically with Fluconazole against Resistant Candida albicans. Sci. Rep. 2020, 10, 498. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Zhang, J.; Zhuge, Y.; Xu, K.; Liu, J.; Wang, J.; Li, L.; Chu, M. Synergistic Effects of Geldanamycin with Fluconazole Are Associated with Reactive Oxygen Species in Candida tropicalis Resistant to Azoles and Amphotericin B. Free Radic. Res. 2019, 53, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Jiang, T.; Zhou, J.; Mei, Y.; Li, J.; Tan, J.; Wei, L.; Li, J.; Peng, Y.; Chen, C.; et al. Repurposing the FDA-Approved Anticancer Agent Ponatinib as a Fluconazole Potentiator by Suppression of Multidrug Efflux and Pma1 Expression in a Broad Spectrum of Yeast Species. Microb. Biotechnol. 2022, 15, 482–498. [Google Scholar] [CrossRef] [PubMed]
- Li, L.P.; An, M.M.; Shen, H.; Huang, X.; Yao, X.; Liu, J.; Zhu, F.; Zhang, S.Q.; Chen, S.M.; He, L.J.; et al. The Non-Geldanamycin Hsp90 Inhibitors Enhanced the Antifungal Activity of Fluconazole. Am. J. Transl. Res. 2015, 7, 2589–2602. [Google Scholar]
- Sun, Y.; Gao, L.; He, C.; Wu, Q.; Li, M.; Zeng, T. Givinostat Exhibits In Vitro Synergy with Posaconazole against Aspergillus spp. Med. Mycol. 2016, 55, myw131. [Google Scholar] [CrossRef][Green Version]
- Qiao, J.; Sun, Y.; Gao, L.; He, C.; Zheng, W. Lonafarnib Synergizes with Azoles against Aspergillus spp. and Exophiala spp. Med. Mycol. 2018, 56, 452–457. [Google Scholar] [CrossRef]
- Kim, S.; Woo, E.-R.; Lee, D.G. Synergistic Antifungal Activity of Isoquercitrin: Apoptosis and Membrane Permeabilization Related to Reactive Oxygen Species in Candida albicans. IUBMB Life 2019, 71, 283–292. [Google Scholar] [CrossRef]
- Lai, Y.-W.; Campbell, L.T.; Wilkins, M.R.; Pang, C.N.I.; Chen, S.; Carter, D.A. Synergy and Antagonism between Iron Chelators and Antifungal Drugs in Cryptococcus. Int. J. Antimicrob. Agents 2016, 48, 388–394. [Google Scholar] [CrossRef]
- Li, Y.; Jiao, P.; Li, Y.; Gong, Y.; Chen, X.; Sun, S. The Synergistic Antifungal Effect and Potential Mechanism of D-Penicillamine Combined With Fluconazole Against Candida albicans. Front. Microbiol. 2019, 10, 2853. [Google Scholar] [CrossRef]
- Liu, X.; Li, T.; Wang, D.; Yang, Y.; Sun, W.; Liu, J.; Sun, S. Synergistic Antifungal Effect of Fluconazole Combined with Licofelone against Resistant Candida albicans. Front. Microbiol. 2017, 8, 2101. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, L.; Lu, X.; Feng, L.; Sun, S. The Synergistic Antifungal Effects of Sodium Phenylbutyrate Combined with Azoles against Candida albicans via the Regulation of the Ras–CAMP–PKA Signalling Pathway and Virulence. Can. J. Microbiol. 2019, 65, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Sun, Y.; He, C.; Li, M.; Zeng, T. In Vitro Interactions between 17-AAG and Azoles against Exophiala dermatitidis. Mycoses 2018, 61, 853–856. [Google Scholar] [CrossRef] [PubMed]
- de Andrade Neto, J.B.; da Silva, C.R.; Barroso, F.D.; do Amaral Valente Sá, L.G.; de Sousa Campos, R.; S Aires do Nascimento, F.B.; Sampaio, L.S.; da Silva, A.R.; da Silva, L.J.; de Sá Carneiro, I.; et al. Synergistic Effects of Ketamine and Azole Derivatives on Candida Spp. Resistance to Fluconazole. Future Microbiol. 2020, 15, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Ahangarkani, F.; Khodavaisy, S.; Mahmoudi, S.; Shokohi, T.; Rezai, M.S.; Fakhim, H.; Dannaoui, E.; Faraji, S.; Chowdhary, A.; Meis, J.F.; et al. Indifferent Effect of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) Combined with Fluconazole against Multidrug-Resistant Candida auris. Curr. Med. Mycol. 2019, 5, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Wang, Y.; Xi, Y.; Yang, Z.; Zhang, H.; Ge, X. Activity of Chlorhexidine Acetate in Combination with Fluconazole against Suspensions and Biofilms of Candida auris. J. Infect. Chemother. 2022, 28, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Tu, J.; Sheng, C.; Chen, X.; Liu, N. Effects of Hsp90 Inhibitor Ganetespib on Inhibition of Azole-Resistant Candida albicans. Front. Microbiol. 2021, 12, 1280. [Google Scholar] [CrossRef]
- Vu, K.; Blumwald, E.; Gelli, A. The Antifungal Activity of HMA, an Amiloride Analog and Inhibitor of Na+/H+ Exchangers. Front. Microbiol. 2021, 12, 1055. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, A.; Manzoor, N. Reversal of Efflux Mediated Antifungal Resistance Underlies Synergistic Activity of Two Monoterpenes with Fluconazole. Eur. J. Pharm. Sci. 2013, 48, 80–86. [Google Scholar] [CrossRef]
- Shaban, S.; Patel, M.; Ahmad, A. Improved Efficacy of Antifungal Drugs in Combination with Monoterpene Phenols against Candida auris. Sci. Rep. 2020, 10, 1162. [Google Scholar] [CrossRef]
- de Aguiar, F.L.L.; de Morais, S.M.; dos Santos, H.S.; Albuquerque, M.R.J.R.; Bandeira, P.N.; de Brito, E.H.S.; Rocha, M.F.G.; dos Santos Fontenelle, R.O. Antifungal Activity and Synergistic Effect of Acetophenones Isolated from Species Croton against Dermatophytes and Yeasts. J. Med. Plants Res. 2016, 10, 216–222. [Google Scholar] [CrossRef]
- Li, D.D.; Chai, D.; Huang, X.W.; Guan, S.X.; Du, J.; Zhang, H.Y.; Sun, Y.; Jiang, Y.Y. Potent In Vitro Synergism of Fluconazole and Osthole against Fluconazole-Resistant Candida albicans. Antimicrob. Agents Chemother. 2017, 61, e00436-17. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Cui, Y.; Zhang, M.; Wang, T.; Wu, D.; Wang, C. Synergistic In Vitro Activity of Sodium Houttuyfonate with Fluconazole against Clinical Candida albicans Strains under Planktonic Growing Conditions. Pharm. Biol. 2017, 55, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Sharifzadeh, A.; Khosravi, A.R.; Shokri, H.; Tari, P.S. Synergistic Anticandidal Activity of Menthol in Combination with Itraconazole and Nystatin against Clinical Candida glabrata and Candida krusei Isolates. Microb. Pathog. 2017, 107, 390–396. [Google Scholar] [CrossRef] [PubMed]
- de A. Cordeiro, R.; Teixeira, C.E.C.; Brilhante, R.S.N.; Castelo-Branco, D.S.C.M.; Alencar, L.P.; de Oliveira, J.S.; Monteiro, A.J.; Bandeira, T.J.P.G.; Sidrim, J.J.C.; Moreira, J.L.B.; et al. Exogenous Tyrosol Inhibits Planktonic Cells and Biofilms of Candida Species and Enhances Their Susceptibility to Antifungals. FEMS Yeast Res. 2015, 15, 12. [Google Scholar] [CrossRef]
- Raut, J.S.; Bansode, B.S.; Jadhav, A.K.; Karuppayil, S.M. Activity of Allyl Isothiocyanate and Its Synergy with Fluconazole against Candida albicans Biofilms. J. Microbiol. Biotechnol. 2017, 27, 685–693. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, W.; Huang, X.; Hao, L.; Li, Y.; Sun, S. Antifungal Activity and Potential Mechanism of N-Butylphthalide Alone and in Combination with Fluconazole against Candida albicans. Front. Microbiol. 2019, 10, 1461. [Google Scholar] [CrossRef]
- Nabili, M.; Aslani, N.; Shokohi, T.; Hedayati, M.T.; Hassanmoghadam, F.; Moazeni, M. In Vitro Interaction between Glabridin and Voriconazole against Aspergillus fumigatus Isolates. Rev. Iberoam. De Micol. 2021, 38, 145–147. [Google Scholar] [CrossRef]
- Ganan, M.; Lorentzen, S.B.; Gaustad, P.; Sørlie, M. Synergistic Antifungal Activity of Chito-Oligosaccharides and Commercial Antifungals on Biofilms of Clinical Candida Isolates. J. Fungi 2021, 7, 718. [Google Scholar] [CrossRef]
- Chen, H.; Li, H.; Duan, C.; Song, C.; Peng, Z.; Shi, W. Reversal of Azole Resistance in Candida albicans by Oridonin. J. Glob. Antimicrob. Resist. 2021, 24, 296–302. [Google Scholar] [CrossRef]
- Sadowska, B.; Budzyńska, A.; Stochmal, A.; Żuchowski, J.; Różalska, B. Novel Properties of Hippophae Rhamnoides L. Twig and Leaf Extracts—Anti-Virulence Action and Synergy with Antifungals Studied In Vitro on Candida Spp. Model. Microb. Pathog. 2017, 107, 372–379. [Google Scholar] [CrossRef]
- da Gabriella, G.; Pippi, B.; Dalla Lana, D.F.; Amaral, A.P.S.; Teixeira, M.L.; de Souza, K.C.B.; Fuentefria, A.M. Reversal of Fluconazole Resistance Induced by a Synergistic Effect with Acca sellowiana in Candida glabrata Strains. Pharm. Biol. 2016, 54, 2410–2419. [Google Scholar] [CrossRef]
- Sadhasivam, S.; Palanivel, S.; Ghosh, S. Synergistic Antimicrobial Activity of Boswellia serrata Roxb. Ex Colebr. (Burseraceae) Essential Oil with Various Azoles against Pathogens Associated with Skin, Scalp and Nail Infections. Lett. Appl. Microbiol. 2016, 63, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Roana, J.; Mandras, N.; Scalas, D.; Campagna, P.; Tullio, V. Antifungal Activity of Melaleuca alternifolia Essential Oil (TTO) and Its Synergy with Itraconazole or Ketoconazole against Trichophyton rubrum. Molecules 2021, 26, 461. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, Q.; Ma, K.; Shi, P.; Liu, W.; Huang, Z. Fluconazole Inhibits Cellular Ergosterol Synthesis to Confer Synergism with Berberine against Yeast Cells. J. Glob. Antimicrob. Resist. 2018, 13, 125–130. [Google Scholar] [CrossRef]
- Li, D.D.; Xu, Y.; Zhang, D.Z.; Quan, H.; Mylonakis, E.; Hu, D.D.; Li, M.B.; Zhao, L.X.; Zhu, L.H.; Wang, Y.; et al. Fluconazole Assists Berberine to Kill Fluconazole-Resistant Candida albicans. Antimicrob. Agents Chemother. 2013, 57, 6016–6027. [Google Scholar] [CrossRef]
- Wei, G.X.; Xu, X.; Wu, C.D. In Vitro Synergism between Berberine and Miconazole against Planktonic and Biofilm Candida Cultures. Arch. Oral Biol. 2011, 56, 565–572. [Google Scholar] [CrossRef]
- Iwazaki, R.S.; Endo, E.H.; Ueda-Nakamura, T.; Nakamura, C.V.; Garcia, L.B.; Filho, B.P.D. In Vitro Antifungal Activity of the Berberine and Its Synergism with Fluconazole. Int. J. Gen. Mol. Microbiol. 2010, 97, 201–205. [Google Scholar] [CrossRef]
- Quan, H.; Cao, Y.Y.; Xu, Z.; Zhao, J.X.; Gao, P.H.; Qin, X.F.; Jiang, Y.Y. Potent In Vitro Synergism of Fluconazole and Berberine Chloride against Clinical Isolates of Candida albicans Resistant to Fluconazole. Antimicrob. Agents Chemother. 2006, 50, 1096–1099. [Google Scholar] [CrossRef]
- Luo, H.; Pan, K.-S.; Luo, X.-L.; Zheng, D.-Y.; Andrianopoulos, A.; Wen, L.-M.; Zheng, Y.-Q.; Guo, J.; Huang, C.-Y.; Li, X.-Y.; et al. In Vitro Susceptibility of Berberine Combined with Antifungal Agents Against the Yeast Form of Talaromyces marneffei. Mycopathologia 2019, 184, 295–301. [Google Scholar] [CrossRef]
- Wang, T.; Shao, J.; Da, W.; Li, Q.; Shi, G.; Wu, D.; Wang, C. Strong Synergism of Palmatine and Fluconazole/Itraconazole Against Planktonic and Biofilm Cells of Candida Species and Efflux-Associated Antifungal Mechanism. Front. Microbiol. 2018, 9, 2892. [Google Scholar] [CrossRef]
- Campos, R.D.; da Silva, C.R.; de Andrade Neto, J.B.; Sampaio, L.S.; Aires do Nascimento, F.B.S.; de Moraes, M.O.; Cavalcanti, B.C.; Magalhães, H.I.F.; Gomes, A.O.; Lobo, M.D.P.; et al. Antifungal Activity of Palmatine against Strains of Candida Spp. Resistant to Azoles in Planktonic Cells and Biofilm. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3657–3669. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Gao, Y.; Hao, L. Synergistic Effects and Mechanisms of Combined Treatment With Harmine Hydrochloride and Azoles for Resistant Candida albicans. Front. Microbiol. 2019, 10, 2295. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro de Carvalho, R.; Chaves Silva, N.; Cusinato, M.; Tranches Dias, K.S.; dos Santos, M.H.; Viegas Junior, C.; Silva, G.; Tranches Dias, A.L. Promising Synergistic Activity of Fluconazole with Bioactive Guttiferone-A and Derivatives against Non-albicans Candida Species. J. De Mycol. Med. 2018, 28, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Nagy, F.; Vitális, E.; Jakab, Á.; Borman, A.M.; Forgács, L.; Tóth, Z.; Majoros, L.; Kovács, R. In Vitro and In Vivo Effect of Exogenous Farnesol Exposure Against Candida auris. Front. Microbiol. 2020, 11, 957. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, C.; Zhao, X.; Wang, D.; Liu, Y.; Sun, S. Antifungal Activity and Potential Mechanism of Asiatic Acid Alone and in Combination with Fluconazole against Candida albicans. Biomed. Pharmacother. 2021, 139, 111568. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-M.; Liao, K.; Liang, S.; Yu, P.-H.; Wang, D.-Y. Synergistic Activity of Magnolol with Azoles and Its Possible Antifungal Mechanism against Candida albicans. J. Appl. Microbiol. 2015, 118, 826–838. [Google Scholar] [CrossRef]
- Li, Y.; Chang, W.; Zhang, M.; Li, X.; Jiao, Y.; Lou, H. Synergistic and Drug-Resistant Reversing Effects of Diorcinol D Combined with Fluconazole against Candida albicans. FEMS Yeast Res. 2015, 15, 1. [Google Scholar] [CrossRef]
- Moraes, R.C.; Carvalho, A.R.; Lana, A.J.D.; Kaiser, S.; Pippi, B.; Fuentefria, A.M.; Ortega, G.G. In Vitro Synergism of a Water Insoluble Fraction of Uncaria tomentosa Combined with Fluconazole and Terbinafine against Resistant Non-Candida albicans Isolates. Pharm. Biol. 2017, 55, 406–415. [Google Scholar] [CrossRef]
- Behbehani, J.M.; Irshad, M.; Shreaz, S.; Karched, M. Synergistic Effects of Tea Polyphenol Epigallocatechin 3-O-Gallate and Azole Drugs against Oral Candida Isolates. J. De Mycol. Med. 2019, 29, 158–167. [Google Scholar] [CrossRef]
- Kumar, S.N.; Aravind, S.R.; Sreelekha, T.T.; Jacob, J.; Kumar, B.S.D. Asarones from Acorus calamus in Combination with Azoles and Amphotericin B: A Novel Synergistic Combination to Compete Against Human Pathogenic Candida Species In Vitro. Appl. Biochem. Biotechnol. 2015, 175, 3683–3695. [Google Scholar] [CrossRef]
- Yao, D.; Zhang, G.; Chen, W.; Chen, J.; Li, Z.; Zheng, X.; Yin, H.; Hu, X. Pyrogallol and Fluconazole Interact Synergistically In Vitro against Candida glabrata through an Efflux-Associated Mechanism. Antimicrob. Agents Chemother. 2021, 65, e0010021. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Kakeya, H.; Miyazaki, T.; Izumikawa, K.; Yanagihara, K.; Ohno, H.; Yamamoto, Y.; Tashiro, T.; Kohno, S. Synergistic Antifungal Effect of Lactoferrin with Azole Antifungals against Candida albicans and a Proposal for a New Treatment Method for Invasive Candidiasis. Jpn. J. Infect. Dis. 2011, 64, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Shekhar-Guturja, T.; Tebung, W.A.; Mount, H.; Liu, N.; Köhler, J.R.; Whiteway, M.; Cowen, L.E. Beauvericin Potentiates Azole Activity via Inhibition of Multidrug Efflux, Blocks Candida albicans Morphogenesis, and Is Effluxed via Yor1 and Circuitry Controlled by Zcf29. Antimicrob. Agents Chemother. 2016, 60, 7468–7480. [Google Scholar] [CrossRef] [PubMed]
- Fakhim, H.; Emami, S.; Vaezi, A.; Hashemi, S.M.; Faeli, L.; Diba, K.; Dannaoui, E.; Badali, H. In Vitro Activities of Novel Azole Compounds ATTAF-1 and ATTAF-2 against Fluconazole-Susceptible and-Resistant Isolates of Candida Species. Antimicrob. Agents Chemother. 2017, 61, e01106-16. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Liu, C.; Liu, J.; Wang, Y.; Li, J.; Xiang, M. Anti-Candida Activity of New Azole Derivatives Alone and in Combination with Fluconazole. Mycopathologia 2015, 180, 203–207. [Google Scholar] [CrossRef]
- Mood, A.D.; Premachandra, I.D.U.A.; Hiew, S.; Wang, F.; Scott, K.A.; Oldenhuis, N.J.; Liu, H.; Van Vranken, D.L. Potent Antifungal Synergy of Phthalazinone and Isoquinolones with Azoles Against Candida albicans. ACS Med. Chem. Lett. 2017, 8, 168–173. [Google Scholar] [CrossRef]
- Li, L.P.; Liu, W.; Liu, H.; Zhu, F.; Zhang, D.Z.; Shen, H.; Xu, Z.; Qi, Y.P.; Zhang, S.Q.; Chen, S.M.; et al. Synergistic Antifungal Activity of Berberine Derivative B-7b and Fluconazole. PLoS ONE 2015, 10, e0126393. [Google Scholar] [CrossRef][Green Version]
- Koselny, K.; Green, J.; DiDone, L.; Halterman, J.P.; Fothergill, A.W.; Wiederhold, N.P.; Patterson, T.F.; Cushion, M.T.; Rappelye, C.; Wellington, M.; et al. The Celecoxib Derivative AR-12 Has Broad-Spectrum Antifungal Activity In Vitro and Improves the Activity of Fluconazole in a Murine Model of Cryptococcosis. Antimicrob. Agents Chemother. 2016, 60, 7115–7127. [Google Scholar] [CrossRef]
- Ghannoum, M.; Long, L.; Larkin, E.L.; Isham, N.; Sherif, R.; Borroto-Esoda, K.; Barat, S.; Angulo, D. Evaluation of the Antifungal Activity of the Novel Oral Glucan Synthase Inhibitor SCY-078, Singly and in Combination, for the Treatment of Invasive Aspergillosis. Antimicrob. Agents Chemother. 2018, 62, e00244-18. [Google Scholar] [CrossRef]
- Savage, K.A.; del Carmen Parquet, M.; Allan, D.S.; Davidson, R.J.; Holbein, B.E.; Lilly, E.A.; Fidel, P.L. Iron Restriction to Clinical Isolates of Candida albicans by the Novel Chelator Dibi Inhibits Growth and Increases Sensitivity to Azoles In Vitro and In Vivo in a Murine Model of Experimental Vaginitis. Antimicrob. Agents Chemother. 2018, 62, e02576-17. [Google Scholar] [CrossRef]
- Dai, L.; Zang, C.; Tian, S.; Liu, W.; Tan, S.; Cai, Z.; Ni, T.; An, M.; Li, R.; Gao, Y.; et al. Design, Synthesis, and Evaluation of Caffeic Acid Amides as Synergists to Sensitize Fluconazole-Resistant Candida albicans to Fluconazole. Bioorganic Med. Chem. Lett. 2015, 25, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Dong, H.H.; Zhao, F.; Wang, J.; Yan, F.; Jiang, Y.Y.; Jin, Y.S. The Synthesis and Synergistic Antifungal Effects of Chalcones against Drug Resistant Candida albicans. Bioorganic Med. Chem. Lett. 2016, 26, 3098–3102. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gao, L.; He, C.; Li, M.; Zeng, T. In Vitro Interactions between IAP Antagonist AT406 and Azoles against Planktonic Cells and Biofilms of Pathogenic Fungi Candida albicans and Exophiala dermatitidis. Med. Mycol. 2018, 56, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Tu, J.; Ji, C.; Li, Z.; Han, G.; Liu, N.; Li, J.; Sheng, C. Discovery of Piperidol Derivatives for Combinational Treatment of Azole-Resistant Candidiasis. ACS Infect. Dis. 2021, 7, 650–660. [Google Scholar] [CrossRef]
- Li, C.; Tu, J.; Han, G.; Liu, N.; Sheng, C. Heat Shock Protein 90 (Hsp90)/Histone Deacetylase (HDAC) Dual Inhibitors for the Treatment of Azoles-Resistant Candida albicans. Eur. J. Med. Chem. 2022, 227, 113961. [Google Scholar] [CrossRef]
- Czechowicz, P.; Neubauer, D.; Nowicka, J.; Kamysz, W.; Gościniak, G. Antifungal Activity of Linear and Disulfide-Cyclized Ultrashort Cationic Lipopeptides Alone and in Combination with Fluconazole against Vulvovaginal Candida spp. Pharmaceutics 2021, 13, 1589. [Google Scholar] [CrossRef]
- Sun, Y.; Tan, L.; Yao, Z.; Gao, L.; Yang, J.; Zeng, T. In Vitro and In Vivo Interactions of TOR Inhibitor AZD8055 and Azoles against Pathogenic Fungi. Microbiol. Spectr. 2022, 10, e0200721. [Google Scholar] [CrossRef]
- Chang, Y.-L.; Yu, S.-J.; Heitman, J.; Wellington, M.; Chen, Y.-L. New Facets of Antifungal Therapy. Virulence 2017, 8, 222–236. [Google Scholar] [CrossRef]
- Cavalheiro, A.S.; Maboni, G.; de Azevedo, M.I.; Argenta, J.S.; Pereira, D.I.B.; Spader, T.B.; Alves, S.H.; Santurio, J.M. In Vitro Activity of Terbinafine Combined with Caspofungin and Azoles against Pythium insidiosum. Antimicrob. Agents Chemother. 2009, 53, 2136–2138. [Google Scholar] [CrossRef]
- Argenta, J.S.; Santurio, J.M.; Alves, S.H.; Pereira, D.I.B.; Cavalheiro, A.S.; Spanamberg, A.; Ferreiro, L. In Vitro Activities of Voriconazole, Itraconazole, and Terbinafine Alone or in Combination against Pythium insidiosum Isolates from Brazil. Antimicrob. Agents Chemother. 2008, 52, 767–769. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed]
- Kordalewska, M.; Lee, A.; Park, S.; Berrio, I.; Chowdhary, A.; Zhao, Y.; Perlin, D.S. Understanding Echinocandin Resistance in the Emerging Pathogen Candida auris. Antimicrob. Agents Chemother. 2018, 62, e00238-18. [Google Scholar] [CrossRef] [PubMed]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to Azoles and Echinocandins Worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.K.; Chang, C.-W.T.; Meissner, N.; Oblad, J.; Shrestha, J.P.; Sorensen, K.N.; Grilley, M.M.; Takemoto, J.Y. Antifungal Amphiphilic Aminoglycoside K20: Bioactivities and Mechanism of Action. Front. Microbiol. 2014, 5, 671. [Google Scholar] [CrossRef] [PubMed]
- Pichler, H.; Gaigg, B.; Hrastnik, C.; Achleitner, G.; Kohlwein, S.D.; Zellnig, G.; Perktold, A.; Daum, G. A Subfraction of the Yeast Endoplasmic Reticulum Associates with the Plasma Membrane and Has a High Capacity to Synthesize Lipids. Eur. J. Biochem. 2001, 268, 2351–2361. [Google Scholar] [CrossRef]
- Istvan, E.S.; Deisenhofer, J. Structural Mechanism for Statin Inhibition of HMG-CoA Reductase. Science (1979) 2001, 292, 1160–1164. [Google Scholar] [CrossRef]
- van Beek, E.; Pieterman, E.; Cohen, L.; Löwik, C.; Papapoulos, S. Farnesyl Pyrophosphate Synthase Is the Molecular Target of Nitrogen-Containing Bisphosphonates. Biochem. Biophys. Res. Commun. 1999, 264, 108–111. [Google Scholar] [CrossRef]
- Balfour, J.A.; Faulds, D. Terbinafine: A Review of Its Pharmacodynamic and Pharmacokinetic Properties, and Therapeutic Potential in Superficial Mycoses. Drugs 1992, 43, 259–284. [Google Scholar] [CrossRef]
- Polak, A. Preclinical Data and Mode of Action of Amorolfine. Dermatology 1992, 184, 3–7. [Google Scholar] [CrossRef]
- Mishima, E.; Maruyama, K.; Nakazawa, T.; Abe, T.; Ito, S. Acute Kidney Injury from Excessive Potentiation of Calcium-Channel Blocker via Synergistic CYP3A4 Inhibition by Clarithromycin Plus Voriconazole. Intern. Med. 2017, 56, 1687–1690. [Google Scholar] [CrossRef]
- Miyazaki, H.; Miyazaki, Y.; Geber, A.; Parkinson, T.; Hitchcock, C.; Falconer, D.J.; Ward, D.J.; Marsden, K.; Bennett, J.E. Fluconazole Resistance Associated with Drug Efflux and Increased Transcription of a Drug Transporter Gene, PDH1, in Candida glabrata. Antimicrob. Agents Chemother. 1998, 42, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Albertson, G.D.; Niimi, M.; Cannon, R.D.; Jenkinson, H.F. Multiple Efflux Mechanisms Are Involved in Candida albicans Fluconazole Resistance. Antimicrob. Agents Chemother. 1996, 40, 2835–2841. [Google Scholar] [CrossRef] [PubMed]
- Fraczek, M.G.; Bromley, M.; Buied, A.; Moore, C.B.; Rajendran, R.; Rautemaa, R.; Ramage, G.; Denning, D.W.; Bowyer, P. The Cdr1B Efflux Transporter Is Associated with Non-Cyp51a-Mediated Itraconazole Resistance in Aspergillus Fumigatus. J. Antimicrob. Chemother. 2013, 68, 1486–1496. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Rossi, N.M.; Peres, N.T.A.; Rossi, A. Antifungal Resistance Mechanisms in Dermatophytes. Mycopathologia 2008, 166, 369–383. [Google Scholar] [CrossRef]
- Webber, M.A. The Importance of Efflux Pumps in Bacterial Antibiotic Resistance. J. Antimicrob. Chemother. 2003, 51, 9–11. [Google Scholar] [CrossRef]
- Sanchez, C.P.; McLean, J.E.; Rohrbach, P.; Fidock, D.A.; Stein, W.D.; Lanzer, M. Evidence for a Pfcrt-Associated Chloroquine Efflux System in the Human Malarial Parasite Plasmodium falciparum. Biochemistry 2005, 44, 9862–9870. [Google Scholar] [CrossRef]
- Liu, S.; Yue, L.; Gu, W.; Li, X.; Zhang, L.; Sun, S. Synergistic Effect of Fluconazole and Calcium Channel Blockers against Resistant Candida albicans. PLoS ONE 2016, 11, e0150859. [Google Scholar] [CrossRef]
- Chang, W.; Liu, J.; Zhang, M.; Shi, H.; Zheng, S.; Jin, X.; Gao, Y.; Wang, S.; Ji, A.; Lou, H. Efflux Pump-Mediated Resistance to Antifungal Compounds Can Be Prevented by Conjugation with Triphenylphosphonium Cation. Nat. Commun. 2018, 9, 5102. [Google Scholar] [CrossRef]
- Miranda-Cadena, K.; Marcos-Arias, C.; Mateo, E.; Aguirre-Urizar, J.M.; Quindós, G.; Eraso, E. In Vitro Activities of Carvacrol, Cinnamaldehyde and Thymol against Candida Biofilms. Biomed. Pharmacother. 2021, 143, 112218. [Google Scholar] [CrossRef]
- Prasad, R.; Rawal, M.K. Efflux Pump Proteins in Antifungal Resistance. Front. Pharmacol. 2014, 5, 202. [Google Scholar] [CrossRef]
- Perfect, J.R. The Antifungal Pipeline: A Reality Check. Nat. Rev. Drug Discov. 2017, 16, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug Repurposing: Progress, Challenges and Recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Cabral, M.E.; Figueroa, L.I.C.; Fariña, J.I. Synergistic Antifungal Activity of Statin-Azole Associations as Witnessed by Saccharomyces cerevisiae- and Candida utilis-Bioassays and Ergosterol Quantification. Rev. Iberoam. De Micol. 2013, 30, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.D.; Panza, G.; Zaleski, A.; Taylor, B. Statin-Associated Side Effects. J. Am. Coll. Cardiol. 2016, 67, 2395–2410. [Google Scholar] [CrossRef]
- Shaukat, A.; Benekli, M.; Vladutiu, G.D.; Slack, J.L.; Wetzler, M.; Baer, M.R. Simvastatin–Fluconazole Causing Rhabdomyolysis. Ann. Pharmacother. 2003, 37, 1032–1035. [Google Scholar] [CrossRef]
- Drake, M.T.; Clarke, B.L.; Khosla, S. Bisphosphonates: Mechanism of Action and Role in Clinical Practice. Mayo Clin. Proc. 2008, 83, 1032–1045. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Mehrotra, B.; Rosenberg, T.J.; Engroff, S.L. Osteonecrosis of the Jaws Associated with the Use of Bisphosphonates: A Review of 63 Cases. J. Oral Maxillofac. Surg. 2004, 62, 527–534. [Google Scholar] [CrossRef]
- Cabillic, F.; Toutirais, O.; Lavoué, V.; de La Pintière, C.T.; Daniel, P.; Rioux-Leclerc, N.; Turlin, B.; Mönkkönen, H.; Mönkkönen, J.; Boudjema, K.; et al. Aminobisphosphonate-Pretreated Dendritic Cells Trigger Successful Vγ9Vδ2 T Cell Amplification for Immunotherapy in Advanced Cancer Patients. Cancer Immunol. Immunother. 2010, 59, 1611–1619. [Google Scholar] [CrossRef]
- Singh, N. Antifungal Prophylaxis for Solid Organ Transplant Recipients: Seeking Clarity amidst Controversy. Clin. Infect. Dis. 2000, 31, 545–553. [Google Scholar] [CrossRef]
- Shirazi, F.; Kontoyiannis, D.P. The Calcineurin Pathway Inhibitor Tacrolimus Enhances the In Vitro Activity of Azoles against Mucorales via Apoptosis. Eukaryot. Cell 2013, 12, 1225–1234. [Google Scholar] [CrossRef]
- Cyert, M.S. Calcineurin Signaling in Saccharomyces cerevisiae: How Yeast Go Crazy in Response to Stress. Biochem. Biophys. Res. Commun. 2003, 311, 1143–1150. [Google Scholar] [CrossRef]
- Tegos, G.P.; Haynes, M.; Jacob Strouse, J.; Md. T. Khan, M.; Bologa, C.G.; Oprea, T.I.; Sklar, L.A. Microbial Efflux Pump Inhibition: Tactics and Strategies. Curr. Pharm. Des. 2012, 17, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Stathopoulos-Gerontides, A.; Guo, J.J.; Cyert, M.S. Yeast Calcineurin Regulates Nuclear Localization of the Crz1p Transcription Factor through Dephosphorylation. Genes Dev. 1999, 13, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The Re-Emergence of Natural Products for Drug Discovery in the Genomics Era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Y.; Yan, L.; Liang, R.M.; di Dai, B.; Tang, R.J.; Gao, P.H.; Jiang, Y.Y. Proteomic Analysis Reveals a Synergistic Mechanism of Fluconazole and Berberine against Fluconazole-Resistant Candida albicans: Endogenous ROS Augmentation. J. Proteome Res. 2009, 8, 5296–5304. [Google Scholar] [CrossRef] [PubMed]
- Dangles, O. Antioxidant Activity of Plant Phenols: Chemical Mechanisms and Biological Significance. Curr. Org. Chem. 2012, 16, 692–714. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure-Activity Relationship) Models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef]
- Jothi, R.; Sangavi, R.; Kumar, P.; Pandian, S.K.; Gowrishankar, S. Catechol Thwarts Virulent Dimorphism in Candida albicans and Potentiates the Antifungal Efficacy of Azoles and Polyenes. Sci. Rep. 2021, 11, 21049. [Google Scholar] [CrossRef]
- Bahar, A.; Ren, D. Antimicrobial Peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Chen, J.; Zhang, M.; Tian, C.; Peng, X.; Li, G.; Chang, W.; Lou, H. Azole-Triphenylphosphonium Conjugates Combat Antifungal Resistance and Alleviate the Development of Drug-Resistance. Bioorganic Chem. 2021, 110, 104771. [Google Scholar] [CrossRef]
- Zhu, T.; Chen, X.; Li, C.; Tu, J.; Liu, N.; Xu, D.; Sheng, C. Lanosterol 14α-Demethylase (CYP51)/Histone Deacetylase (HDAC) Dual Inhibitors for Treatment of Candida tropicalis and Cryptococcus neoformans Infections. Eur. J. Med. Chem. 2021, 221, 113524. [Google Scholar] [CrossRef] [PubMed]
- Elias, R.; Basu, P.; Fridman, M. Fluconazole-COX Inhibitor Hybrids: A Dual-Acting Class of Antifungal Azoles. J. Med. Chem. 2022, 65, 2361–2373. [Google Scholar] [CrossRef] [PubMed]
- Uren, A.G.; Beilharz, T.; O’Connell, M.J.; Bugg, S.J.; van Driel, R.; Vaux, D.L.; Lithgow, T. Role for Yeast Inhibitor of Apoptosis (IAP)-like Proteins in Cell Division. Proc. Natl. Acad. Sci. USA 1999, 96, 10170–10175. [Google Scholar] [CrossRef] [PubMed]
- Scorzoni, L.; de Paula e Silva, A.C.A.; Marcos, C.M.; Assato, P.A.; de Melo, W.C.M.A.; de Oliveira, H.C.; Costa-Orlandi, C.B.; Mendes-Giannini, M.J.S.; Fusco-Almeida, A.M. Antifungal Therapy: New Advances in the Understanding and Treatment of Mycosis. Front. Microbiol. 2017, 8, 36. [Google Scholar] [CrossRef]
- Weng, J.; Wong, S.N.; Xu, X.; Xuan, B.; Wang, C.; Chen, R.; Sun, C.C.; Lakerveld, R.; Kwok, P.C.L.; Chow, S.F. Cocrystal Engineering of Itraconazole with Suberic Acid via Rotary Evaporation and Spray Drying. Cryst. Growth Des. 2019, 19, 2736–2745. [Google Scholar] [CrossRef]
| Source | ||||
|---|---|---|---|---|
| Type | Synergist | Common Name | Latin Name | Ref. |
| Essential Oil Extracts | thymol | thyme, ajwain, wild bergamot | Thymus vulgaris, Trachyspermum ammi, Monarda fistulosa | [211] |
| carvacrol | oregano, thyme, marjoram | Origanum vulgare, Thymus vulgaris, Origanum majorana | [211] | |
| acetophenone | apple, apricot, beef, cheese, croton | Malus domestica, Prunus armeniaca, Bos taurus, Croton sp. | [213] | |
| osthole | snowparsley, wild celery, shishiudo | Cnidium monnieri, Angelica archangelica, Angelica pubescens | [214] | |
| houttuyfonate | fish mint | Houttuynia cordata | [215] | |
| menthol | wild mint, peppermint | Mentha arvensis, Mentha piperita | [216] | |
| tyrosol | olive, argan | Olea europaea, Argania spinosa | [217] | |
| allyl isothiocyanate | mustard, radish, horseradish, wasabi | Sinapis alba, Raphanus raphanistrum, Armoracia rusticana | [218] | |
| butylphthalide | celery | Apium graveolens | [219] | |
| glabridin | liquorice | Glycyrrhiza glabra | [220] | |
| oridonin | blushred | Rabdosia rubescens | [222] | |
| Crude Essential Oils | sea-buckthorn | sea-buckthorn | Hippophae rhamnoides | [223] |
| guava leaf | guava, pineapple guava | Psidium guajava, Acca sellowiana | [224] | |
| frankincense | Indian frankincense | Boswellia serrata | [225] | |
| TTO | tea tree | Melaleuca alternifolia | [226] | |
| Alkaloids | berberine | barberry, tree turmeric, prickly poppy | Berberis vulgaris, Berberis aristate, Argemone mexicana | [228,229,230,231,232] |
| palmatine | Amur cork tree, yanhusuo | Phellodendron amurense, Corydalis yanhusuo | [233,234] | |
| harmine | wild rue, ayahuasca | Peganum harmala, Banisteriopsis caapi | [235] | |
| Other terpenoids | guttiferone | boarwood root | Symphonia globulifera | [236] |
| farnesol | plants, animals and fungi | [237] | ||
| Other phenols | magnolol | Chinese magnolia, southern magnolia | Magnolia officinalis, Magnolia grandiflora | [239] |
| diorcinol | fungal symbiont | Epichloe bromicola | [240] | |
| proanthocyanidin | pine bark, cranberries, grape seeds | Pinus sp., Vaccinium oxycoccus, Vitis labrusca | [241] | |
| epigallocatechin | black tea, white tea, green tea | Camellia sinensis | [242] | |
| asarone | sweet flag, wild ginger | Acorus calamus, Asarum sp. | [243] | |
| Peptides | lactoferrin | bovine and human (milk, mucous) | Bos taurus, Homo sapiens | [201,245] |
| beauvericin | white muscardine, Fusarium | Beauveria bassiana, Fusarium sp. | [246] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kane, A.; Carter, D.A. Augmenting Azoles with Drug Synergy to Expand the Antifungal Toolbox. Pharmaceuticals 2022, 15, 482. https://doi.org/10.3390/ph15040482
Kane A, Carter DA. Augmenting Azoles with Drug Synergy to Expand the Antifungal Toolbox. Pharmaceuticals. 2022; 15(4):482. https://doi.org/10.3390/ph15040482
Chicago/Turabian StyleKane, Aidan, and Dee A. Carter. 2022. "Augmenting Azoles with Drug Synergy to Expand the Antifungal Toolbox" Pharmaceuticals 15, no. 4: 482. https://doi.org/10.3390/ph15040482
APA StyleKane, A., & Carter, D. A. (2022). Augmenting Azoles with Drug Synergy to Expand the Antifungal Toolbox. Pharmaceuticals, 15(4), 482. https://doi.org/10.3390/ph15040482







