One-Year Outcome of Combination Therapy with Full or Reduced Photodynamic Therapy and One Anti-Vascular Endothelial Growth Factor in Pachychoroid Neovasculopathy
Abstract
:1. Introduction
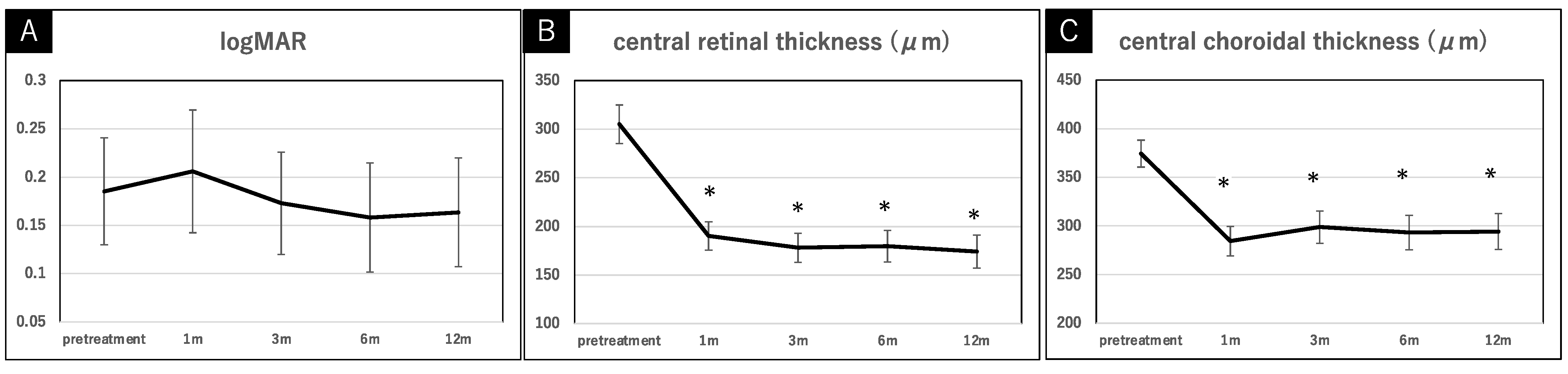
2. Results
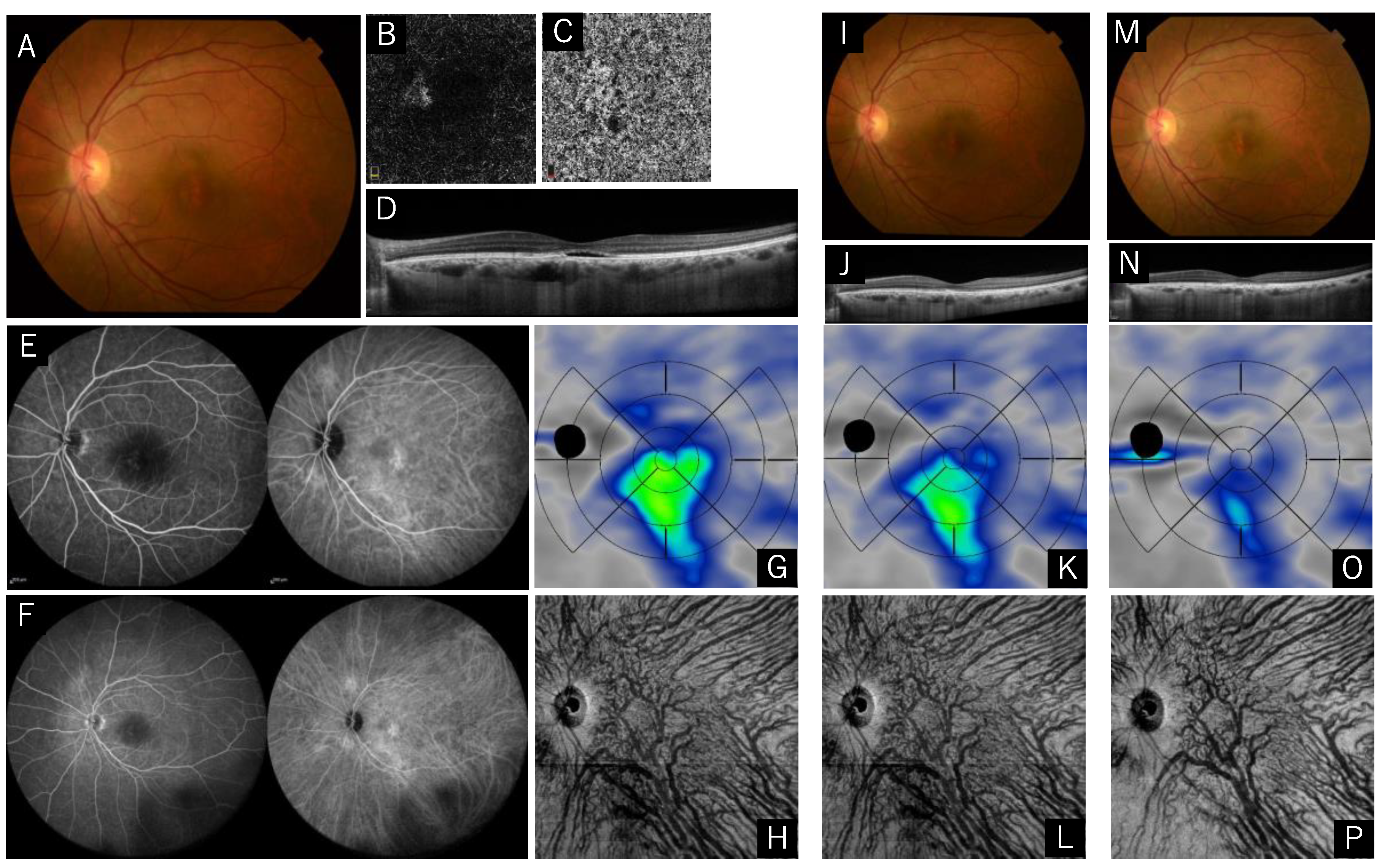
2.1. Case Presentations
Case 1: A 58-Year-Old Male (Reduced Treatment, no Recurrence)
Case 2: A 72-Year-Old Male (Full Treatment, Recurrent at Five Months after the Treatment)
3. Discussion
4. Materials and Methods
4.1. Population
4.2. Treatment Conditions
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PNV | pachychoroid neovasculopathy |
| PCV | polypoidal choroidal neovasculopathy |
| AMD | age-related macular disease |
| PDT | photodynamic therapy |
| VEGF | vascular endothelial growth factor |
| logMAR | logarithm of the minimum angle of resolution |
| CRT | central retinal thickness |
| CCT | central choroidal thickness |
| RPE | retinal pigment epithelium |
| OCT | optical coherence tomography |
| FA | fluorescein angiography |
| ICGA | indocyanine green angiography |
| MNV | macular neovascularization |
| BCVA | best corrected visual acuity |
References
- Yanagi, Y. Pachychoroid disease: A new perspective on exudative maculopathy. Jpn. J. Ophthalmol. 2020, 64, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Hoshino, J.; Mukai, R.; Nakamura, K.; Kikuchi, Y.; Kishi, S.; Akiyama, H. Vortex Vein Anastomosis at the Watershed in Pachychoroid Spectrum Diseases. Ophthalmol. Retin. 2020, 4, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Ledesma-Gil, G.; Cheung, C.M.G. Intervortex venous anastomosis in pachychoroid-related disorders. Retina 2020, 41, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Phasukkijwatana, N.; Freund, K.B.; Dolz-Marco, R.; Al-Sheikh, M.; Keane, P.A.; Egan, C.; Randhawa, S.; Stewart, J.M.; Liu, Q.; Hunyor, A.P.; et al. Peripapillary pachychoroid syndrome. Retina 2018, 38, 1652–1667. [Google Scholar] [CrossRef]
- Chung, H.; Byeon, S.H.; Freund, K.B. Focal Choroidal Excavation and Its Association with Pachychoroid Spectrum Disorders: A Review of the Literature and Multimodal Imaginig Findings. Retina 2017, 37, 199–221. [Google Scholar] [CrossRef]
- Miyake, M.; Ooto, S.; Yamashiro, K.; Takahashi, A.; Yoshikawa, M.; Akagi-Kurashige, Y.; Ueda-Arakawa, N.; Oishi, A.; Nakanishi, H.; Tamura, H.; et al. Pachychoroid neovasculopathy and age-related macular degeneration. Sci. Rep. 2015, 5, 16204. [Google Scholar] [CrossRef] [Green Version]
- Miki, A.; Kusuhara, S.; Otsuji, T.; Kawashima, Y.; Miki, K.; Imai, H.; Nakamura, M.; Tsujikawa, A. Photodynamic therapy combined with anti-vascular endothelial growth factor therapy for pachychoroid neovasculopathy. PLoS ONE 2021, 16, e0248760. [Google Scholar] [CrossRef]
- Matsumoto, H.; Mukai, R.; Kikuchi, Y.; Morimoto, M.; Akiyama, H. One-year outcomes of half-fluence photodynamic therapy combined with intravitreal injection of aflibercept for pachychoroid neovasculopathy without polypoidal lesions. Jpn. J. Ophthalmol. 2020, 64, 203–209. [Google Scholar] [CrossRef]
- Koh, A.; Lai, T.; Takahashi, K.; Wong, T.Y.; Chen, L.-J.; Ruamviboonsuk, P.; Tan, C.S.; Feller, C.; Margaron, P.; Lim, T.H.; et al. Efficacy and Safety of Ranibizumab with or without Verteporfin Photodynamic Therapy for Polypoidal Choroidal Vasculopathy: A Randomized Clinical Trial. JAMA Ophthalmol. 2017, 135, 1206–1213. [Google Scholar] [CrossRef]
- Kim, J.H.; Chang, Y.S.; Kim, J.W.; Kim, C.G.; Lee, D.W. Submacular hemorrhage and grape-like polyp clusters: Factors associated with reactivation of the lesion in polypoidal choroidal vasculopathy. Eye 2017, 31, 1678–1684. [Google Scholar] [CrossRef]
- Kang, H.M.; Koh, H.J.; Lee, S.C. Baseline polyp size as a potential predictive factor for recurrence of polypoidal choroidal vasculopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 254, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.K.; Kim, K.S.; Kim, W.; Lee, S.B.; Jeon, S. Responses to Photodynamic Therapy in Patients With Polypoidal Choroidal Vasculopathy Consisting of Polyps Resembling Grape Clusters. Am. J. Ophthalmol. 2012, 154, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, Y.; Ting, D.S.W.; Ng, W.Y.; Lee, S.Y.; Mathur, R.; Chan, C.M.; Yeo, I.; Wong, T.Y.; Cheung, C.M.G. Choroidal vascular hyperpermeability as a predictor of treatment response for polypoidal choroidal vasculopathy. Retina 2018, 38, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.Y.Y.; Ho, C.P.S. Current management strategy of polypoidal choroidal vasculopathy. Indian J. Ophthalmol. 2018, 66, 1727–1735. [Google Scholar] [CrossRef]
- Jung, B.J.; Kim, J.Y.; Lee, J.H.; Baek, J.; Lee, K.; Lee, W.K. Intravitreal aflibercept and ranibizumab for pachychoroid neovasculopathy. Sci. Rep. 2019, 9, 2055. [Google Scholar] [CrossRef]
- Hikichi, T.; Kubo, N.; Yamauchi, M. One-year comparison of anti-vascular endothelial growth factor and half-dose photodynamic therapies for pachychoroid neovasculopathy. Eye 2021, 35, 3367–3375. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, W.K. One-Year results of adjunctive photodynamic therapy for type 1 neovascularization associated with thickened choroid. Retina 2016, 36, 889–895. [Google Scholar] [CrossRef]
- Kitajima, Y.; Maruyama-Inoue, M.; Ito, A.; Sato, S.; Inoue, T.; Yamane, S.; Kadonosono, K. One-year outcome of combination therapy with intravitreal anti-vascular endothelial growth factor and photodynamic therapy in patients with pachychoroid neovasculopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 1279–1285. [Google Scholar] [CrossRef]
- Chan, W.-M.; Lam, D.S.; Lai, T.; Yuen, K.S.; Liu, D.T.; Chan, C.K.; Chen, W.-Q. Treatment of choroidal neovascularization in central serous chorioretinopathy by photodynamic therapy with verteporfin. Am. J. Ophthalmol. 2003, 136, 836–845. [Google Scholar] [CrossRef]
- Warrow, D.J.; Hoang, Q.V.; Freund, K.B. Pachychoroid pigment epitheliopathy. Retina 2013, 33, 1659–1672. [Google Scholar] [CrossRef]
- Fung, A.T.; Yannuzzi, L.A.; Freund, K. Type 1 (sub-retinal pigment epithelial) neovascularization in central serous chorioretinopathy masquerading as neovascular age-related macular degeneration. Retina 2012, 32, 1829–1837. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.E.; Freund, K.B. Pachychoroid Neovasculopathy. Retina 2015, 35, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.-M.; Lam, D.S.C.; Lai, T.Y.Y.; Tam, B.S.M.; Liu, D.T.L.; Chan, C.K.M. Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: A novel treatment at the primary disease level. Br. J. Ophthalmol. 2003, 87, 1453–1458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlötzer-Schrehardt, U.; Viestenz, A.; Naumann, G.O.H.; Laqua, H.; Michels, S.; Schmidt-Erfurth, U. Dose-related structural effects of photodynamic therapy on choroidal and retinal structures of human eyes. Graefe’s Arch. Clin. Exp. Ophthalmol. 2002, 240, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Erfurth, U.; Laqua, H.; Schlötzer-Schrehard, U.; Viestenz, A.; Naumann, G.O.H. Histopathological changes following photodynamic therapy in human eyes. Arch. Ophthalmol. 2002, 120, 835–844. [Google Scholar] [PubMed]
- Piccolino, F.C.; Eandi, C.M.; Ventre, L.; De La Longrais, R.C.R.; Grignolo, F. Photodynamic Therapy for Chronic Central Serous Chorioretinopathy. Retina 2003, 23, 752–763. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.Y.; Chan, W.-M.; Lam, D.S. Transient reduction in retinal function revealed by multifocal electroretinogram after photodynamic therapy. Am. J. Ophthalmol. 2004, 137, 826–833. [Google Scholar] [CrossRef]
- Hirami, Y.; Tsujikawa, A.; Otani, A.; Yodoi, Y.; Aikawa, H.; Mandai, M.; Yoshimura, N. Hemorrhagic complications after photodynamic therapy for polypoidal choroidal vasculopathy. Retina 2007, 27, 335–341. [Google Scholar] [CrossRef]
- Gomi, F.; Sawa, M.; Wakabayashi, T.; Sasamoto, Y.; Suzuki, M.; Tsujikawa, M. Efficacy of Intravitreal Bevacizumab Combined With Photodynamic Therapy for Polypoidal Choroidal Vasculopathy. Am. J. Ophthalmol. 2010, 150, 48–54. [Google Scholar] [CrossRef]
- Ruamviboonsuk, P.; Tadarati, M.; Vanichvaranont, S.; Hanutsaha, P.; Pokawattana, N. Photodynamic therapy combined with ranibizumab for polypoidal choroidal vasculopathy: Results of a 1-year preliminary study. Br. J. Ophthalmol. 2010, 94, 1045–1051. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Schlo¨tzer-Schrehard, U.; Cursiefen, C.; Michels, S.; Beckendorf, A.; Naumann, G.O.H. Influence of Photodynamic Therapy on Expression of Vascular Endothelial Growth Factor (VEGF), VEGF Receptor 3, and Pigment Epithelium–Derived Factor. Investig. Opthalmology Vis. Sci. 2003, 44, 4473–4480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smretschnig, E.; Hagen, S.; Glittenberg, C.; Ristl, R.; Krebs, I.; Binder, S.; Ansari-Shahrezaei, S. Intravitreal anti-vascular endothelial growth factor combined with half-fluence photodynamic therapy for choroidal neovascularization in chronic central serous chorioretinopathy. Eye 2016, 30, 805–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huynh, E.; Chandrasekera, E.; Bukowska, D.; McLenachan, S.; Mackey, D.A.; Chen, F.K. Past, Present, and Future Concepts of the Choroidal Scleral Interface Morphology on Optical Coherence Tomography. Asia-Pac. J. Ophthalmol. 2017, 6, 94–103. [Google Scholar] [CrossRef]
- Sato-Akushichi, M.; Ono, S.; Klose, G.; Song, Y. Choroidal Volume Evaluation after Photodynamic Therapy Using New Optical Coherence Tomography Imaging Algorithm. Pharmaceuticals 2021, 14, 1140. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| All (n = 29) | Full-Treatment Group (n = 16) | Reduced-Treatment Group (n = 13) | p-Value | |
|---|---|---|---|---|
| age (years, (SD)) | 68.3 (8.5) | 68.3 (10.2) | 68.5 (5.7) | 0.510 1 |
| sex Male/Female | 25/4 | 14/2 | 11/2 | 1.000 2 |
| logMAR (average, (SD)) | 0.19 (0.29) | 0.18 (0.25) | 0.19 (0.34) | 0.774 1 |
| central retinal thickness (μm) | 305.2 (104.8) | 283.1 (80.7) | 332.4 (123.0) | 0.245 1 |
| central choroidal thickness (μm) | 374.4 (73.2) | 393.8 (67.1) | 350.7 (73.5) | 0.203 1 |
| past treatment history (%, (n)) | 31.0 (9) | 43.8 (7) | 13.3 (2) | 0.130 2 |
| diabetes mellitus (%, (n)) | 10.3 (3) | 6.3 (1) | 15.4 (2) | 0.573 2 |
| hypertension (%, (n)) | 44.8 (13) | 43.8 (7) | 46.2 (6) | 1.000 2 |
| smoking history (%, (n)) | 65.5 (19) | 75.0 (12) | 53.8 (7) | 0.190 2 |
| spot size (μm) | 4703.4 (1202.7) | 4656.3 (1033.8) | 4761.5 (1380.4) | 0.982 1 |
| All (n = 29) | Full-Treatment Group (n = 16) | Reduced-Treatment Group (n = 13) | |||
|---|---|---|---|---|---|
| no recurrence (%, (n)) | 75.9 (22) | 68.8 (11) | 84.6 (11) | ||
| anti-VEGF | 0.28 (0.58) | 0.31 (0.58) | 0.23 (0.58) | ||
| past treatment history | + (n = 9) | + (n = 7) | − (n = 9) | + (n = 2) | − (n = 11) |
| no recurrence (%, (n)) | 66.7 (6) | 57.1 (4) | 77.8 (7) | 100.0 (2) | 81.8 (9) |
| anti-VEGF | 0.22 (0.42) | 0.29 (0.45) | 0.33 (0.67) | 0 (0) | 0.27 (0.62) |
| All (n = 29) | Ranibizumab Group (n = 24) | Aflibercept Group (n = 5) | |||
|---|---|---|---|---|---|
| no recurrence (%, (n)) | 75.9 (22) | 79.2 (19) | 60.0 (3) | ||
| past treatment history | + (n = 9) | + (n = 7) | − (n = 17) | + (n = 2) | − (n = 3) |
| no recurrence (%, (n)) | 66.7 (6) | 71.5 (5) | 82.4 (14) | 50.0 (1) | 66.7 (2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato-Akushichi, M.; Ono, S.; Taneda, T.; Klose, G.; Sasamori, A.; Song, Y. One-Year Outcome of Combination Therapy with Full or Reduced Photodynamic Therapy and One Anti-Vascular Endothelial Growth Factor in Pachychoroid Neovasculopathy. Pharmaceuticals 2022, 15, 483. https://doi.org/10.3390/ph15040483
Sato-Akushichi M, Ono S, Taneda T, Klose G, Sasamori A, Song Y. One-Year Outcome of Combination Therapy with Full or Reduced Photodynamic Therapy and One Anti-Vascular Endothelial Growth Factor in Pachychoroid Neovasculopathy. Pharmaceuticals. 2022; 15(4):483. https://doi.org/10.3390/ph15040483
Chicago/Turabian StyleSato-Akushichi, Miki, Shinji Ono, Tatsuro Taneda, Gerd Klose, Asuka Sasamori, and Youngseok Song. 2022. "One-Year Outcome of Combination Therapy with Full or Reduced Photodynamic Therapy and One Anti-Vascular Endothelial Growth Factor in Pachychoroid Neovasculopathy" Pharmaceuticals 15, no. 4: 483. https://doi.org/10.3390/ph15040483
APA StyleSato-Akushichi, M., Ono, S., Taneda, T., Klose, G., Sasamori, A., & Song, Y. (2022). One-Year Outcome of Combination Therapy with Full or Reduced Photodynamic Therapy and One Anti-Vascular Endothelial Growth Factor in Pachychoroid Neovasculopathy. Pharmaceuticals, 15(4), 483. https://doi.org/10.3390/ph15040483






