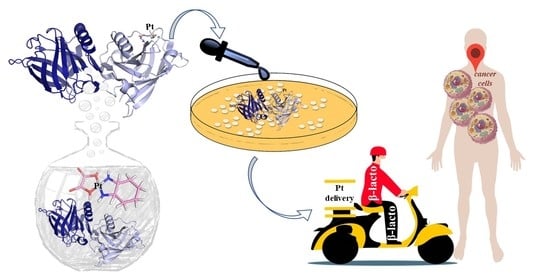
Protein-Based Delivery Systems for Anticancer Metallodrugs: Structure and Biological Activity of the Oxaliplatin/β-Lactoglobulin Adduct
Abstract
:Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kontopidis, G.; Holt, C.; Sawyer, L. β-Lactoglobulin: Binding properties, structure, and function. J Dairy Sci. 2004, 87, 785–796. [Google Scholar] [CrossRef] [Green Version]
- Dufour, E.; Genot, C.; Haertlé, T. β-lactoglobulin binding properties during its folding changes studied by fluorescence spec-troscopy. Protein Structure and Molecular Enzymology. Biochim. Biophys Acta 1994, 1205, 105–112. [Google Scholar] [CrossRef]
- Ko, S.; Gunasekaran, S. Preparation of sub-100-nm beta-lactoglobulin (BLG) nanoparticles. J. Microencapsul. 2006, 23, 887–898. [Google Scholar] [CrossRef]
- Teng, Z.; Luo, Y.; Li, Y.; Wang, Q. Cationic beta-lactoglobulin nanoparticles as a bioavailability enhancer: Effect of surface properties and size on the transport and delivery in vitro. Food Chem. 2016, 204, 391–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, Z.; Li, Y.; Niu, Y.; Xu, Y.; Yu, L.; Wang, Q. Cationic β-lactoglobulin nanoparticles as a bioavailability enhancer: Com-parison between ethylenediamine and polyethyleneimine as cationizers. Food Chem. 2014, 159, 333–342. [Google Scholar] [CrossRef]
- Shafaei, Z.; Ghalandari, B.; Vaseghi, A.; Divsalar, A.; Haertlé, T.; Saboury, A.A.; Sawyer, L. β-Lactoglobulin: An efficient nanocarrier for advanced delivery systems. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1685–1692. [Google Scholar] [CrossRef]
- Ghalandari, B.; Divsalar, A.; Saboury, A.A.; Parivar, K. β-Lactoglobulin nanoparticle as a chemotherapy agent carrier for oral drug delivery system. J. Iran. Chem. Soc. 2014, 12, 613–619. [Google Scholar] [CrossRef]
- Leilabadi-Asl, A.; Divsalar, A.; Saboury, A.A.; Parivar, K. Probing the interaction of two chemotherapeutic drugs of oxali-palladium and 5-fluorouracil simultaneously with milk carrier protein of β-lactoglobulin. Int. J. Biol. Macrom. 2018, 112, 422–432. [Google Scholar] [CrossRef]
- Brauckmann, C.; Wehe, C.A.; Kieshauer, M.; Lanvers-Kaminsky, C.; Sperling, M.; Karst, U. The interaction of platinum-based drugs with native biologically relevant proteins. Anal. Bioanal. Chem. 2013, 405, 1855–1864. [Google Scholar] [CrossRef]
- Brauckmann, C.; Faber, H.; Lanvers-Kaminsky, C.; Sperling, M.; Karst, U. Influence of cimetidine and its metabolites on cis-platin-investigation of adduct formation by means of electrochemistry/liquid chromatography/electrospray mass spectrometry. J. Chromatogr. A 2013, 1279, 49–57. [Google Scholar] [CrossRef]
- Ghalandari, B.; Divsalar, A.; Eslami-Moghadam, M.; Saboury, A.A.; Haertlé, T.; Amanlou, M.; Parivar, K. Probing of the Interaction between β-Lactoglobulin and the Anticancer Drug Oxaliplatin. Appl. Biochem. Biotechnol. 2015, 175, 974–987. [Google Scholar] [CrossRef] [PubMed]
- Chanphai, P.; Bariyanga, J.; Bérubé, G.; Tajmir-Riahi, H.A. Complexation of cis-Pt and trans-Pt(NH3)2Cl2 with serum proteins: A potential application for drug delivery. J. Biomol. Struct. Dyn. 2020, 38, 2777–2783. [Google Scholar] [CrossRef] [PubMed]
- Izadi, Z.; Divsalar, A.; Saboury, A.A.; Sawyer, L. β-lactoglobulin-pectin Nanoparticle-based Oral Drug Delivery System for Potential Treatment of Colon Cancer. Chem. Biol. Drug Des. 2016, 88, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Siddik, Z.H. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene 2003, 22, 7265–7279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef]
- Sanderson, B.J.; Ferguson, L.R.; Denny, W.A. Mutagenic and carcinogenic properties of platinum-based anticancer drugs. Mutat. Res. Mol. Mech. Mutagen. 1996, 355, 59–70. [Google Scholar] [CrossRef]
- Rixe, O.; Ortuzar, W.; Alvarez, M.; Parker, R.; Reed, E.; Paull, K.; Fojo, T. Oxaliplatin, tetraplatin, cisplatin, and carboplatin: Spectrum of activity in drug-resistant cell lines and in the cell lines of the National Cancer Institute’s Anticancer Drug Screen panel. Biochem. Pharmacol. 1996, 52, 1855–1865. [Google Scholar] [CrossRef]
- Balasco, N.; Ferraro, G.; Loreto, D.; Iacobucci, I.; Monti, M.; Merlino, A. Cisplatin binding to β-lactoglobulin: A structural study. Dalton Trans. 2020, 49, 12450–12457. [Google Scholar] [CrossRef]
- Raymond, E.; Chaney, S.G.; Taamma, A.; Cvitkovic, E. Oxaliplatin: A review of preclinical and clinical studies. Ann. Oncol. 1998, 9, 1053–1071. [Google Scholar] [CrossRef]
- Almeida, G.M.; Duarte, T.L.; Steward, W.P.; Jones, G.D. Detection of oxaliplatin-induced DNA crosslinks in vitro and in cancer patients using the alkaline comet assay. DNA Repair 2006, 5, 219–225. [Google Scholar] [CrossRef]
- Bogliolo, S.; Cassani, C.; Gardella, B.; Musacchi, V.; Babilonti, L.; Venturini, P.-L.; Ferrero, S.; Spinillo, A. Oxaliplatin for the treatment of ovarian cancer. Expert Opin. Investig. Drugs 2015, 24, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Loreto, D.; Ferraro, G.; Merlino, A. Protein-metallodrugs interactions: Effects on the overall protein structure and characteri-zation of Au, Ru and Pt binding sites. Int. J. Biol. Macrom. 2020, 163, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Messori, L.; Marzo, T.; Merlino, A. Interactions of carboplatin and oxaliplatin with proteins: Insights from X-ray structures and mass spectrometry studies of their ribonuclease A adducts. J. Inorg. Biochem. 2015, 153, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Marzo, T.; Ferraro, G.; Cucci, L.M.; Pratesi, A.; Hansson, O.; Satriano, C.; Merlino, A.; La Mendola, D. Oxaliplatin inhibits angiogenin proliferative and cell migration effects in prostate cancer cells. J. Inorg. Biochem. 2022, 226, 111657. [Google Scholar] [CrossRef] [PubMed]
- Merlino, A. Recent advances in protein metalation: Structural studies. Chem. Commun. 2021, 57, 1295–1307. [Google Scholar] [CrossRef]
- Messori, L.; Marzo, T.; Merlino, A. The X-ray structure of the complex formed in the reaction between oxaliplatin and lysozyme. Chem. Commun. 2014, 50, 8360–8362. [Google Scholar] [CrossRef]
- Marasco, D.; Messori, L.; Marzo, T.; Merlino, A. Oxaliplatin vs. cisplatin: Competition experiments on their binding to lysozyme. Dalton Trans. 2015, 44, 10392–10398. [Google Scholar] [CrossRef] [Green Version]
- Belviso, B.D.; Galliani, A.; Lasorsa, A.; Mirabelli, V.; Caliandro, R.; Arnesano, F.; Natile, G. Oxaliplatin Binding to Human Copper Chaperone Atox1 and Protein Dimerization. Inorg. Chem. 2016, 55, 6563–6573. [Google Scholar] [CrossRef]
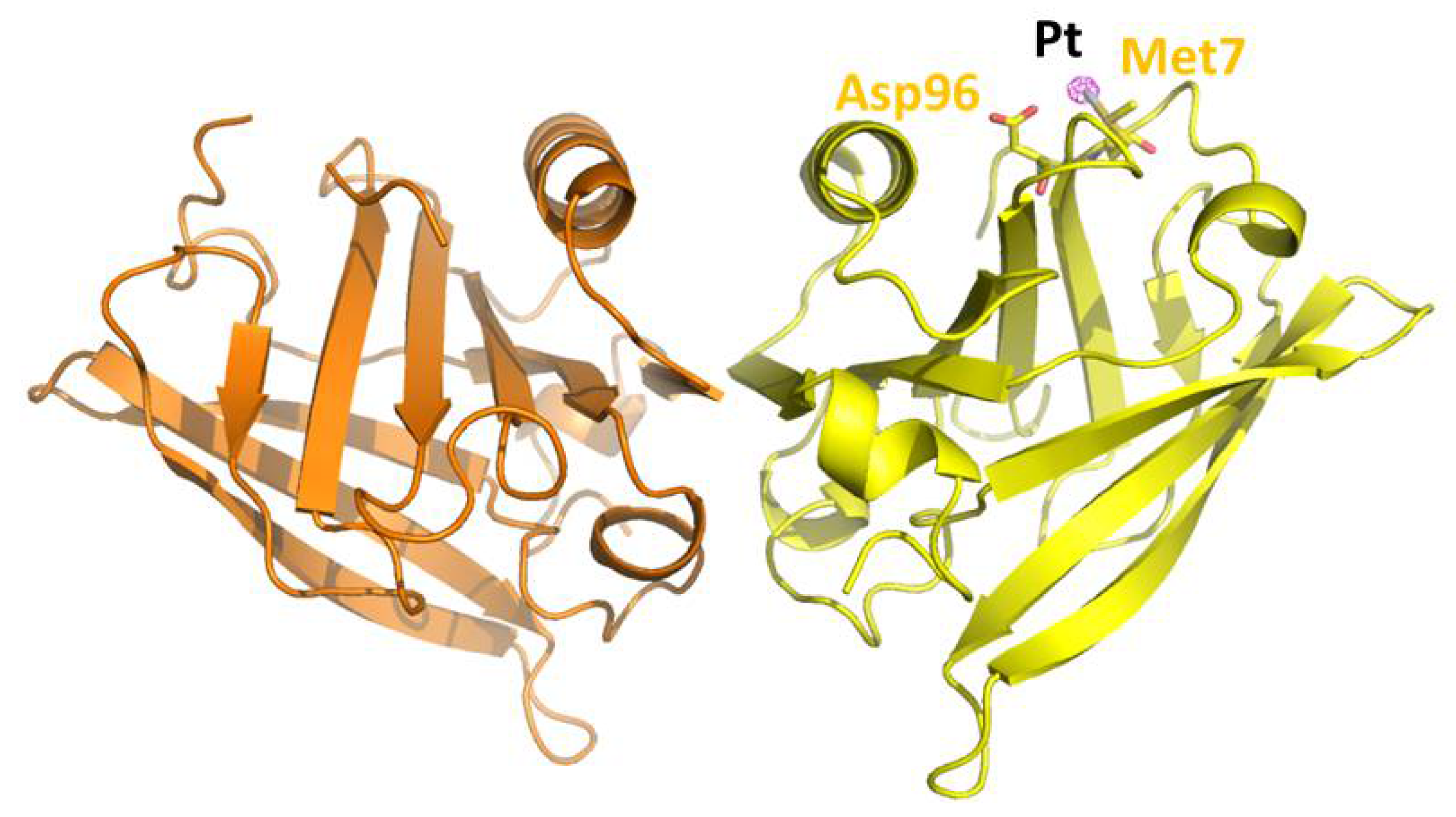
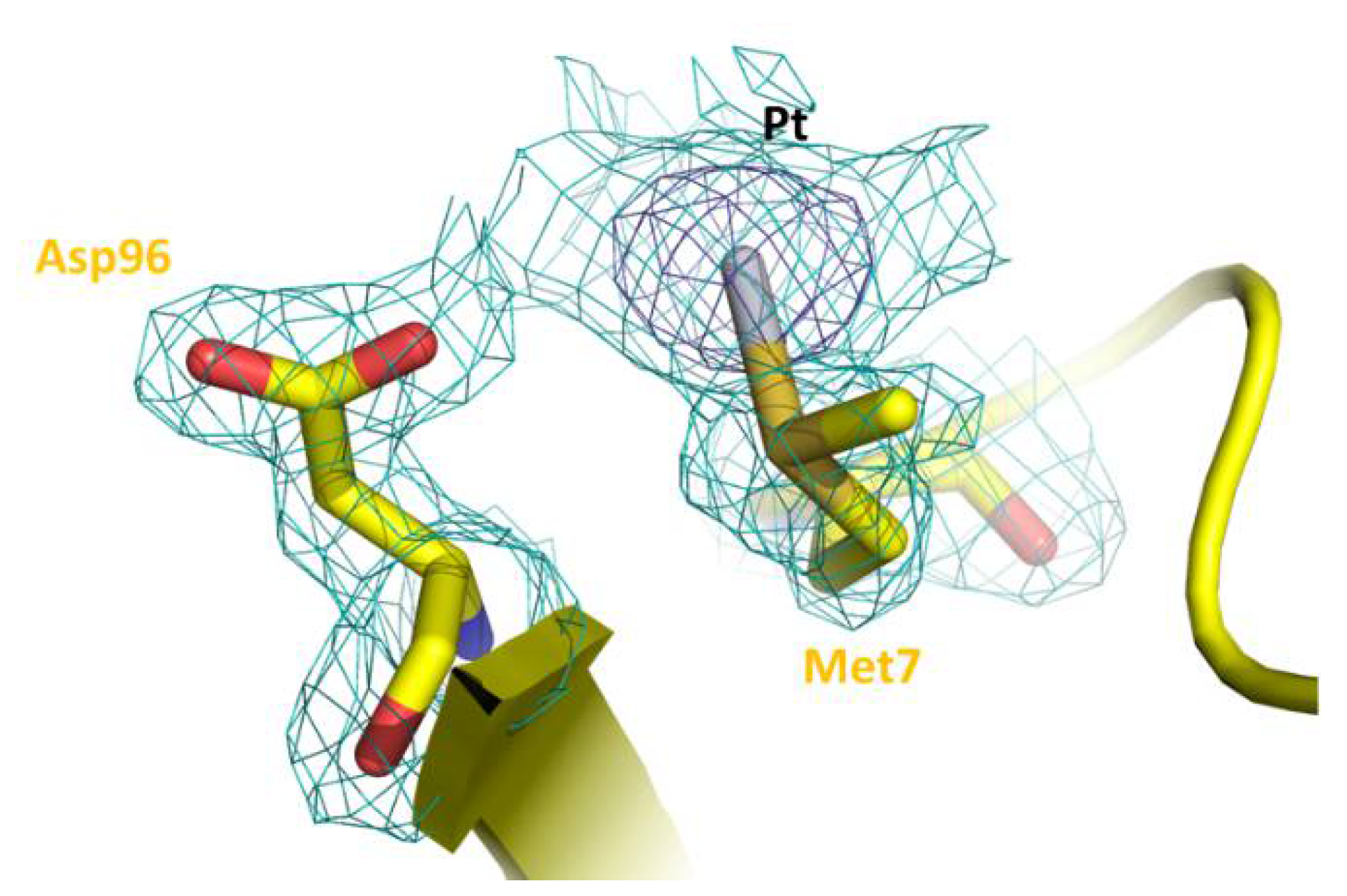
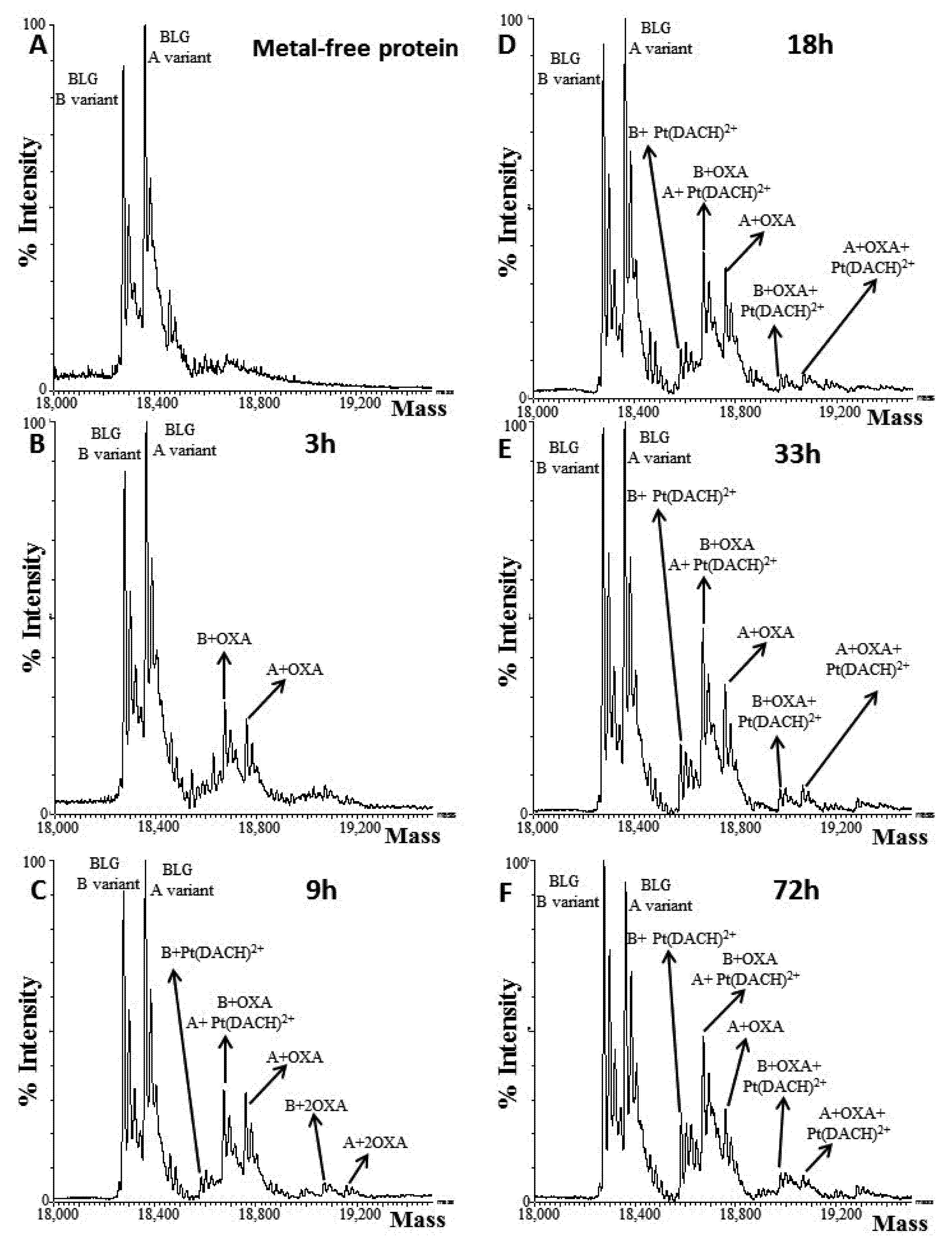
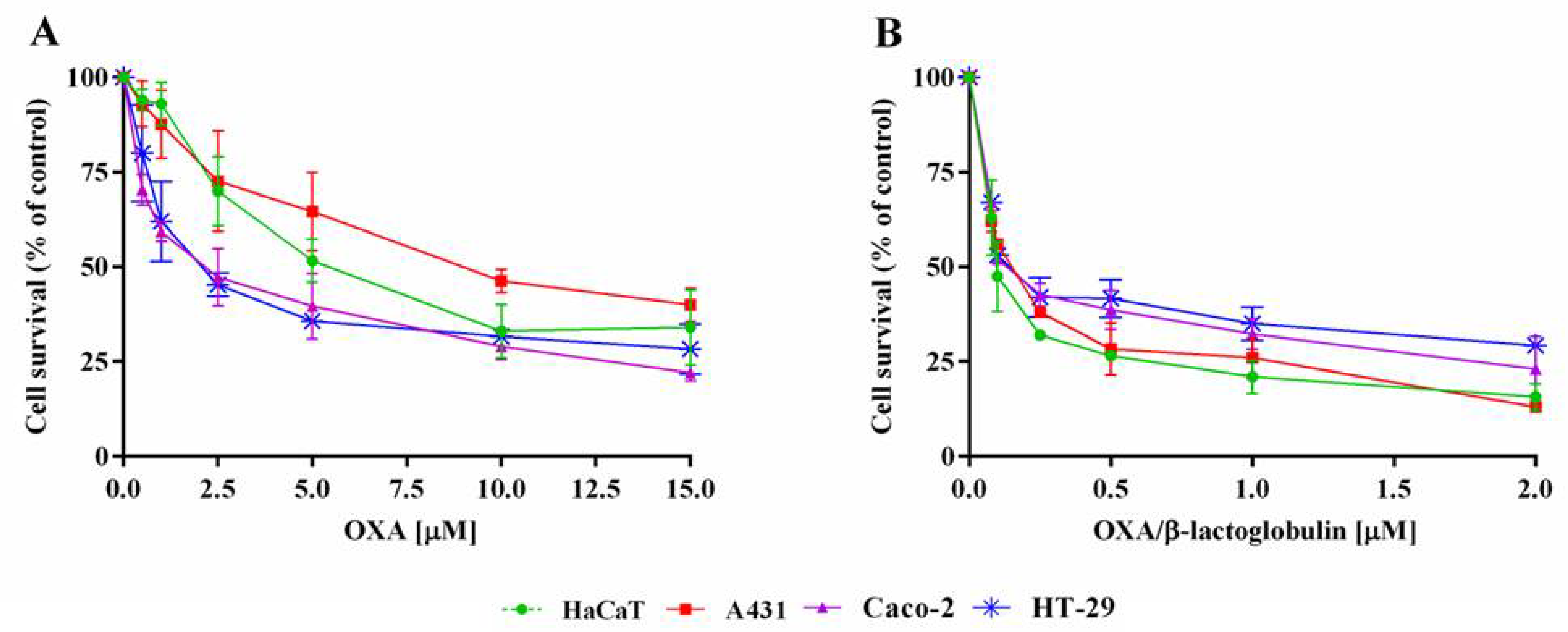
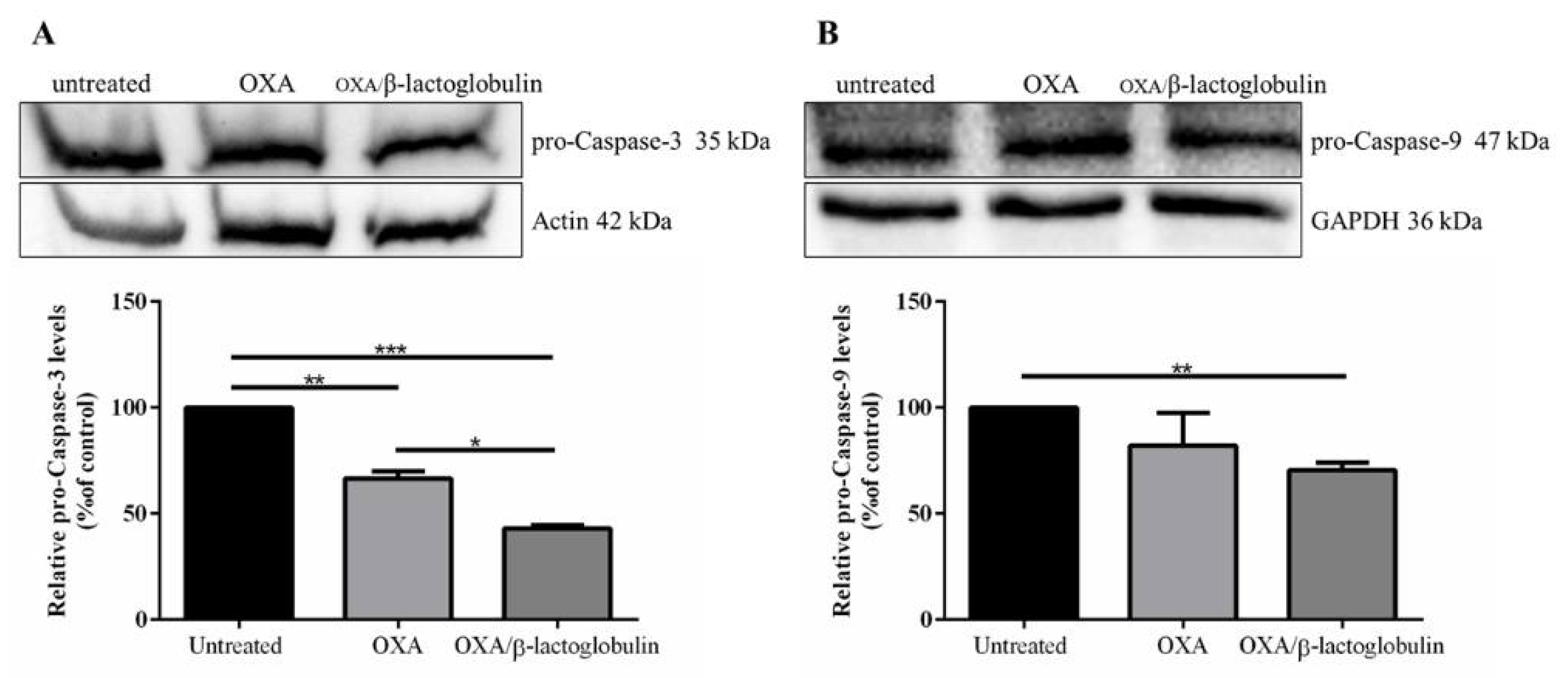
| A431 | HT-29 | Caco-2 | HaCaT | |
|---|---|---|---|---|
| OXA | 8.8 ± 1.1 | 2.1 ± 0.6 | 3.4 ± 1.1 | 7.3 ± 2.3 |
| OXA/β-lactoglobulin | 0.149 ± 0.001 | 0.066 ± 0.005 | 0.066 ± 0.001 | 0.110± 0.014 |
| β-lactoglobulin | >15 | >15 | >15 | >15 |
| CDDP | 7.3 ± 0.1 | 5.9 ± 1.6 | 9.2 ± 1.3 | 3.4 ± 1.0 |
| Compound | Pt content (10−9 µg/cell) |
|---|---|
| Control | N.D. |
| OXA | 4.00 ± 0.06 |
| OXA/β-lactoglobulin | 7.81 ± 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monti, D.M.; Loreto, D.; Iacobucci, I.; Ferraro, G.; Pratesi, A.; D’Elia, L.; Monti, M.; Merlino, A. Protein-Based Delivery Systems for Anticancer Metallodrugs: Structure and Biological Activity of the Oxaliplatin/β-Lactoglobulin Adduct. Pharmaceuticals 2022, 15, 425. https://doi.org/10.3390/ph15040425
Monti DM, Loreto D, Iacobucci I, Ferraro G, Pratesi A, D’Elia L, Monti M, Merlino A. Protein-Based Delivery Systems for Anticancer Metallodrugs: Structure and Biological Activity of the Oxaliplatin/β-Lactoglobulin Adduct. Pharmaceuticals. 2022; 15(4):425. https://doi.org/10.3390/ph15040425
Chicago/Turabian StyleMonti, Daria Maria, Domenico Loreto, Ilaria Iacobucci, Giarita Ferraro, Alessandro Pratesi, Luigi D’Elia, Maria Monti, and Antonello Merlino. 2022. "Protein-Based Delivery Systems for Anticancer Metallodrugs: Structure and Biological Activity of the Oxaliplatin/β-Lactoglobulin Adduct" Pharmaceuticals 15, no. 4: 425. https://doi.org/10.3390/ph15040425
APA StyleMonti, D. M., Loreto, D., Iacobucci, I., Ferraro, G., Pratesi, A., D’Elia, L., Monti, M., & Merlino, A. (2022). Protein-Based Delivery Systems for Anticancer Metallodrugs: Structure and Biological Activity of the Oxaliplatin/β-Lactoglobulin Adduct. Pharmaceuticals, 15(4), 425. https://doi.org/10.3390/ph15040425