Lineage Differentiation Potential of Different Sources of Mesenchymal Stem Cells for Osteoarthritis Knee
Abstract
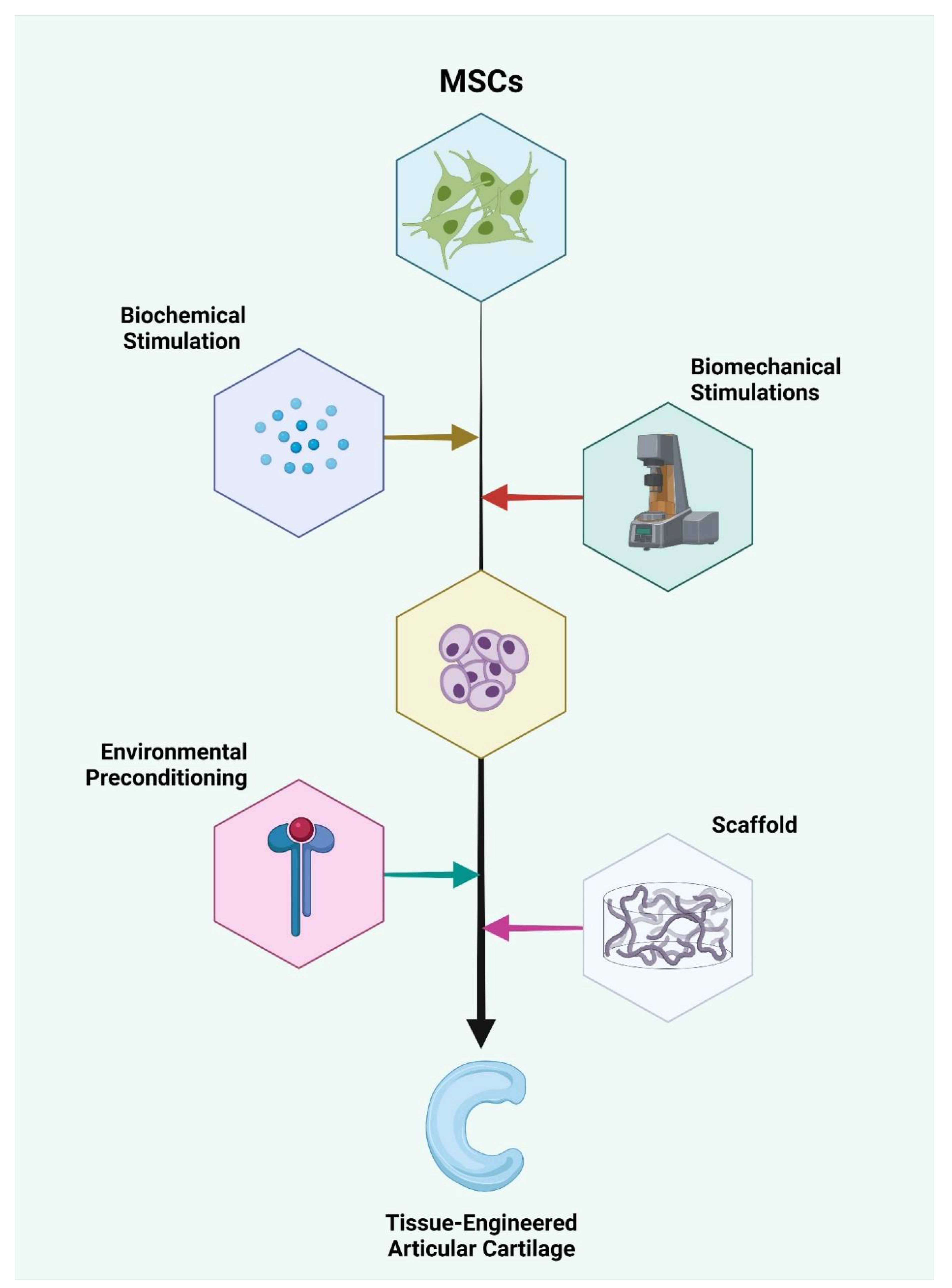
1. Introduction
2. Differentiation Potential of MSCs
3. Bone Marrow-Derived MSCs (BM-MSCs)
4. Adipose Tissue-Derived MSCs (AD-MSCs)
5. Hematopoietic Stem Cells (HSCs)
6. Placental Derived MSCs (Pl-MSCs)
7. Amniotic Fluid-Derived MSCs (Af-MSCs)
8. Peripheral Blood-Derived MSCs (PB-MSCs)
9. Synovium-Derived MSCs (Sy-MSCs)
10. Dental Tissue-Derived MSCs (D-MSCs)
11. Periosteum-Derived MSCs (P-MSCs)
12. Endometrium-Derived MSCs (En-MSCs)
13. Induced Pluripotent Stem Cells (iPSCs)
14. Comparison of Lineage Differentiation of Various Sources of MSCs
15. Challenges in the Source of MSC Identification
16. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dzobo, K.; Thomford, N.E.; Senthebane, D.A.; Shipanga, H.; Rowe, A.; Dandara, C.; Pillay, M.; Motaung, K.S.C.M. Advances in Regenerative Medicine and Tissue Engineering: Innovation and Transformation of Medicine. Stem Cells Int. 2018, 2018, e2495848. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, I.F.; Pereira, H.; de Girolamo, L.; Cucchiarini, M.; Espregueira-Mendes, J.; Reis, R.L.; Oliveira, J.M. Orthopaedic Regenerative Tissue Engineering En Route to the Holy Grail: Disequilibrium between the Demand and the Supply in the Operating Room. J. Exp. Orthop. 2018, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Tatara, A.M.; Mikos, A.G. Tissue Engineering in Orthopaedics. J. Bone Jt. Surg. Am. 2016, 98, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, R.; Spohn, G.; Baer, P.C. Mesenchymal Stem/Stromal Cells in Regenerative Medicine: Can Preconditioning Strategies Improve Therapeutic Efficacy? Transfus. Med. Hemother. 2016, 43, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Christ, G.J.; Saul, J.M.; Furth, M.E.; Andersson, K.-E. The Pharmacology of Regenerative Medicine. Pharm. Rev. 2013, 65, 1091–1133. [Google Scholar] [CrossRef] [PubMed]
- Arvidson, K.; Abdallah, B.M.; Applegate, L.A.; Baldini, N.; Cenni, E.; Gomez-Barrena, E.; Granchi, D.; Kassem, M.; Konttinen, Y.T.; Mustafa, K.; et al. Bone Regeneration and Stem Cells. J. Cell Mol. Med. 2011, 15, 718–746. [Google Scholar] [CrossRef]
- Karuppal, R. Current Concepts in the Articular Cartilage Repair and Regeneration. J. Orthop. 2017, 14, A1–A3. [Google Scholar] [CrossRef]
- Nam, Y.; Rim, Y.A.; Lee, J.; Ju, J.H. Current Therapeutic Strategies for Stem Cell-Based Cartilage Regeneration. Stem Cells Int 2018, 2018, 8490489. [Google Scholar] [CrossRef]
- Gugjoo, M.B.; Amarpal; Sharma, G.T.; Aithal, H.P.; Kinjavdekar, P. Cartilage Tissue Engineering: Role of Mesenchymal Stem Cells along with Growth Factors & Scaffolds. Indian J. Med. Res. 2016, 144, 339. [Google Scholar] [CrossRef]
- Goldberg, A.; Mitchell, K.; Soans, J.; Kim, L.; Zaidi, R. The Use of Mesenchymal Stem Cells for Cartilage Repair and Regeneration: A Systematic Review. J. Orthop. Surg. Res. 2017, 12, 39. [Google Scholar] [CrossRef]
- Muthu, S.; Jeyaraman, M.; Jain, R.; Gulati, A.; Jeyaraman, N.; Prajwal, G.S.; Mishra, P.C. Accentuating the Sources of Mesenchymal Stem Cells as Cellular Therapy for Osteoarthritis Knees—A Panoramic Review. Stem Cell Investig. 2021, 8, 13. [Google Scholar] [CrossRef]
- Jeyaraman, M.; Muthu, S.; Ganie, P.A. Does the Source of Mesenchymal Stem Cell Have an Effect in the Management of Osteoarthritis of the Knee? Meta-Analysis of Randomized Controlled Trials. Cartilage 2020, 13, 1532S–1547S. [Google Scholar] [CrossRef]
- Chen, Y.; Shao, J.-Z.; Xiang, L.-X.; Dong, X.-J.; Zhang, G.-R. Mesenchymal Stem Cells: A Promising Candidate in Regenerative Medicine. Int. J. Biochem. Cell Biol. 2008, 40, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef]
- Rohban, R.; Pieber, T.R. Mesenchymal Stem and Progenitor Cells in Regeneration: Tissue Specificity and Regenerative Potential. Stem Cells Int. 2017, 2017. [Google Scholar] [CrossRef]
- Salgado, A.J.; Oliveira, J.M.; Martins, A.; Teixeira, F.G.; Silva, N.A.; Neves, N.M.; Sousa, N.; Reis, R.L. Tissue Engineering and Regenerative Medicine: Past, Present, and Future. Int. Rev. Neurobiol. 2013, 108, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Chiu, S.M.; Motan, D.A.L.; Zhang, Z.; Chen, L.; Ji, H.-L.; Tse, H.-F.; Fu, Q.-L.; Lian, Q. Mesenchymal Stem Cells and Immunomodulation: Current Status and Future Prospects. Cell Death Dis. 2016, 7, e2062. [Google Scholar] [CrossRef] [PubMed]
- Wosen, J.E.; Mukhopadhyay, D.; Macaubas, C.; Mellins, E.D. Epithelial MHC Class II Expression and Its Role in Antigen Presentation in the Gastrointestinal and Respiratory Tracts. Front. Immunol. 2018, 9, 2144. [Google Scholar] [CrossRef]
- Ryan, J.M.; Barry, F.P.; Murphy, J.M.; Mahon, B.P. Mesenchymal Stem Cells Avoid Allogeneic Rejection. J. Inflamm. 2005, 2, 8. [Google Scholar] [CrossRef]
- Schnabel, L.V.; Pezzanite, L.M.; Antczak, D.F.; Felippe, M.J.B.; Fortier, L.A. Equine Bone Marrow-Derived Mesenchymal Stromal Cells Are Heterogeneous in MHC Class II Expression and Capable of Inciting an Immune Response in Vitro. Stem Cell Res. Ther. 2014, 5, 13. [Google Scholar] [CrossRef]
- Stolzing, A.; Jones, E.; McGonagle, D.; Scutt, A. Age-Related Changes in Human Bone Marrow-Derived Mesenchymal Stem Cells: Consequences for Cell Therapies. Mech. Ageing Dev. 2008, 129, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Marędziak, M.; Marycz, K.; Tomaszewski, K.A.; Kornicka, K.; Henry, B.M. The Influence of Aging on the Regenerative Potential of Human Adipose Derived Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, e2152435. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-H.K.; Ogando, C.R.; Wang See, C.; Chang, T.-Y.; Barabino, G.A. Changes in Phenotype and Differentiation Potential of Human Mesenchymal Stem Cells Aging in Vitro. Stem Cell Res. Ther. 2018, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Kasper, G.; Mao, L.; Geissler, S.; Draycheva, A.; Trippens, J.; Kühnisch, J.; Tschirschmann, M.; Kaspar, K.; Perka, C.; Duda, G.N.; et al. Insights into Mesenchymal Stem Cell Aging: Involvement of Antioxidant Defense and Actin Cytoskeleton. Stem Cells 2009, 27, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.O.; Yue, R.; Murphy, M.M.; Peyer, J.G.; Morrison, S.J. Leptin-Receptor-Expressing Mesenchymal Stromal Cells Represent the Main Source of Bone Formed by Adult Bone Marrow. Cell Stem Cell 2014, 15, 154–168. [Google Scholar] [CrossRef]
- Nakamura, T.; Shiojima, S.; Hirai, Y.; Iwama, T.; Tsuruzoe, N.; Hirasawa, A.; Katsuma, S.; Tsujimoto, G. Temporal Gene Expression Changes during Adipogenesis in Human Mesenchymal Stem Cells. Biochem. Biophys. Res. Commun. 2003, 303, 306–312. [Google Scholar] [CrossRef]
- Solchaga, L.A.; Penick, K.J.; Welter, J.F. Chondrogenic Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells: Tips and Tricks. Methods Mol. Biol. 2011, 698, 253–278. [Google Scholar] [CrossRef]
- Stromps, J.-P.; Paul, N.E.; Rath, B.; Nourbakhsh, M.; Bernhagen, J.; Pallua, N. Chondrogenic Differentiation of Human Adipose-Derived Stem Cells: A New Path in Articular Cartilage Defect Management? BioMed Res. Int. 2014, 2014, e740926. [Google Scholar] [CrossRef]
- Birmingham, E.; Niebur, G.L.; McHugh, P.E.; Shaw, G.; Barry, F.P.; McNamara, L.M. Osteogenic Differentiation of Mesenchymal Stem Cells Is Regulated by Osteocyte and Osteoblast Cells in a Simplified Bone Niche. Eur. Cell Mater. 2012, 23, 13–27. [Google Scholar] [CrossRef]
- Mohamed-Ahmed, S.; Fristad, I.; Lie, S.A.; Suliman, S.; Mustafa, K.; Vindenes, H.; Idris, S.B. Adipose-Derived and Bone Marrow Mesenchymal Stem Cells: A Donor-Matched Comparison. Stem Cell Res. Ther. 2018, 9, 168. [Google Scholar] [CrossRef]
- Kurenkova, A.D.; Medvedeva, E.V.; Newton, P.T.; Chagin, A.S. Niches for Skeletal Stem Cells of Mesenchymal Origin. Front. Cell Dev. Biol. 2020, 8, 592. [Google Scholar] [CrossRef] [PubMed]
- Woo, D.-H.; Hwang, H.S.; Shim, J.H. Comparison of Adult Stem Cells Derived from Multiple Stem Cell Niches. Biotechnol. Lett. 2016, 38, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Muruganandan, S.; Roman, A.A.; Sinal, C.J. Adipocyte Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells: Cross Talk with the Osteoblastogenic Program. Cell Mol. Life Sci. 2009, 66, 236–253. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Li, Q.; Luo, S.; Liu, Z.; Luo, D.; Zhang, B.; Zhang, D.; Rao, P.; Xiao, J. PPARγ and Wnt Signaling in Adipogenic and Osteogenic Differentiation of Mesenchymal Stem Cells. Curr. Stem Cell Res. 2016, 11, 216–225. [Google Scholar] [CrossRef]
- Park, S.-H.; Sim, W.Y.; Min, B.-H.; Yang, S.S.; Khademhosseini, A.; Kaplan, D.L. Chip-Based Comparison of the Osteogenesis of Human Bone Marrow- and Adipose Tissue-Derived Mesenchymal Stem Cells under Mechanical Stimulation. PLoS ONE 2012, 7, e46689. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, P.; Kornacker, M.; Mehlhorn, A.; Seckinger, A.; Vohrer, J.; Schmal, H.; Kasten, P.; Eckstein, V.; Südkamp, N.P.; Krause, U. Comparison of Immunological Properties of Bone Marrow Stromal Cells and Adipose Tissue-Derived Stem Cells before and after Osteogenic Differentiation in Vitro. Tissue Eng. 2007, 13, 111–121. [Google Scholar] [CrossRef]
- Guneta, V.; Tan, N.S.; Chan, S.K.J.; Tanavde, V.; Lim, T.C.; Wong, T.C.M.; Choong, C. Comparative Study of Adipose-Derived Stem Cells and Bone Marrow-Derived Stem Cells in Similar Microenvironmental Conditions. Exp. Cell Res. 2016, 348, 155–164. [Google Scholar] [CrossRef]
- Strioga, M.; Viswanathan, S.; Darinskas, A.; Slaby, O.; Michalek, J. Same or Not the Same? Comparison of Adipose Tissue-Derived versus Bone Marrow-Derived Mesenchymal Stem and Stromal Cells. Stem Cells Dev. 2012, 21, 2724–2752. [Google Scholar] [CrossRef]
- Hennig, T.; Lorenz, H.; Thiel, A.; Goetzke, K.; Dickhut, A.; Geiger, F.; Richter, W. Reduced Chondrogenic Potential of Adipose Tissue Derived Stromal Cells Correlates with an Altered TGFβ Receptor and BMP Profile and Is Overcome by BMP-6. J. Cell. Physiol. 2007, 211, 682–691. [Google Scholar] [CrossRef]
- Pachón-Peña, G.; Yu, G.; Tucker, A.; Wu, X.; Vendrell, J.; Bunnell, B.A.; Gimble, J.M. Stromal Stem Cells from Adipose Tissue and Bone Marrow of Age-Matched Female Donors Display Distinct Immunophenotypic Profiles. J. Cell Physiol. 2011, 226, 843–851. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Sekiya, I.; Yagishita, K.; Muneta, T. Comparison of Human Stem Cells Derived from Various Mesenchymal Tissues: Superiority of Synovium as a Cell Source. Arthritis Rheum. 2005, 52, 2521–2529. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Challen, G.A.; Sirin, O.; Lin, K.K.-Y.; Goodell, M.A. Hematopoietic Stem Cell Characterization and Isolation. Methods Mol. Biol. 2011, 750, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Sharkis, S.J.; Collector, M.I.; Barber, J.P.; Vala, M.S.; Jones, R.J. Phenotypic and Functional Characterization of the Hematopoietic Stem Cell. Stem Cells 1997, 15 (Suppl. 1), 41–44. [Google Scholar] [CrossRef]
- Liang, Y.; Van Zant, G.; Szilvassy, S.J. Effects of Aging on the Homing and Engraftment of Murine Hematopoietic Stem and Progenitor Cells. Blood 2005, 106, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Srour, E.F.; Jetmore, A.; Wolber, F.M.; Plett, P.A.; Abonour, R.; Yoder, M.C.; Orschell-Traycoff, C.M. Homing, Cell Cycle Kinetics and Fate of Transplanted Hematopoietic Stem Cells. Leukemia 2001, 15, 1681–1684. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mehrotra, M.; Williams, C.R.; Ogawa, M.; LaRue, A.C. Hematopoietic Stem Cells Give Rise to Osteo-Chondrogenic Cells. Blood Cells Mol. Dis. 2013, 50, 41–49. [Google Scholar] [CrossRef][Green Version]
- Bethel, M.; Srour, E.F.; Kacena, M.A. Hematopoietic Cell Regulation of Osteoblast Proliferation and Differentiation. Curr. Osteoporos. Rep. 2011, 9, 96–102. [Google Scholar] [CrossRef][Green Version]
- Lévesque, J.-P.; Helwani, F.M.; Winkler, I.G. The Endosteal ‘Osteoblastic’ Niche and Its Role in Hematopoietic Stem Cell Homing and Mobilization. Leukemia 2010, 24, 1979–1992. [Google Scholar] [CrossRef]
- Staudt, N.D.; Maurer, A.; Spring, B.; Kalbacher, H.; Aicher, W.K.; Klein, G. Processing of CXCL12 by Different Osteoblast-Secreted Cathepsins. Stem Cells Dev. 2012, 21, 1924–1935. [Google Scholar] [CrossRef]
- Chotinantakul, K.; Leeanansaksiri, W. Hematopoietic Stem Cell Development, Niches, and Signaling Pathways. Bone Marrow Res. 2012, 2012, 270425. [Google Scholar] [CrossRef]
- Wang, H.; Leng, Y.; Gong, Y. Bone Marrow Fat and Hematopoiesis. Front. Endocrinol. 2018, 9, 694. [Google Scholar] [CrossRef] [PubMed]
- Sera, Y.; LaRue, A.C.; Moussa, O.; Mehrotra, M.; Duncan, J.D.; Williams, C.R.; Nishimoto, E.; Schulte, B.A.; Watson, P.M.; Watson, D.K.; et al. Hematopoietic Stem Cell Origin of Adipocytes. Exp. Hematol. 2009, 37, 1108–1120. [Google Scholar] [CrossRef] [PubMed]
- Gavin, K.M.; Majka, S.M.; Kohrt, W.M.; Miller, H.L.; Sullivan, T.M.; Klemm, D.J. Hematopoietic-to-Mesenchymal Transition of Adipose Tissue Macrophages Is Regulated by Integrin Β1 and Fabricated Fibrin Matrices. Adipocyte 2017, 6, 234–249. [Google Scholar] [CrossRef]
- Siddesh, S.E.; Gowda, D.M.; Jain, R.; Gulati, A.; Patil, G.S.; Anudeep, T.C.; Jeyaraman, N.; Muthu, S.; Jeyaraman, M. Placenta-Derived Mesenchymal Stem Cells (P-MSCs) for COVID-19 Pneumonia—A Regenerative Dogma. Stem Cell Investig. 2021, 8, 3. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, R.; Zou, Q.; Chen, Y.; Zhou, M.; Li, X.; Ran, R.; Chen, Q. Comparison of the Biological Characteristics of Mesenchymal Stem Cells Derived from the Human Placenta and Umbilical Cord. Sci. Rep. 2018, 8, 5014. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, Y.; Zhang, Y.; Hao, G.; Liu, T.; Wang, L.; Yang, T.; Wang, Q.; Zhang, G.; Wei, J.; et al. Placental Mesenchymal Stem Cells of Fetal and Maternal Origins Demonstrate Different Therapeutic Potentials. Stem Cell Res. 2014, 5, 48. [Google Scholar] [CrossRef]
- Parolini, O.; Alviano, F.; Bagnara, G.P.; Bilic, G.; Bühring, H.-J.; Evangelista, M.; Hennerbichler, S.; Liu, B.; Magatti, M.; Mao, N.; et al. Concise Review: Isolation and Characterization of Cells from Human Term Placenta: Outcome of the First International Workshop on Placenta Derived Stem Cells. Stem Cells 2008, 26, 300–311. [Google Scholar] [CrossRef]
- Can, A.; Karahuseyinoglu, S. Concise Review: Human Umbilical Cord Stroma with Regard to the Source of Fetus-Derived Stem Cells. Stem Cells 2007, 25, 2886–2895. [Google Scholar] [CrossRef]
- Portmann-Lanz, C.B.; Schoeberlein, A.; Huber, A.; Sager, R.; Malek, A.; Holzgreve, W.; Surbek, D.V. Placental Mesenchymal Stem Cells as Potential Autologous Graft for Pre- and Perinatal Neuroregeneration. Am. J. Obs. Gynecol. 2006, 194, 664–673. [Google Scholar] [CrossRef]
- Paldino, E.; Cenciarelli, C.; Giampaolo, A.; Milazzo, L.; Pescatori, M.; Hassan, H.J.; Casalbore, P. Induction of Dopaminergic Neurons from Human Wharton’s Jelly Mesenchymal Stem Cell by Forskolin. J. Cell Physiol. 2014, 229, 232–244. [Google Scholar] [CrossRef]
- Kim, M.J.; Shin, K.S.; Jeon, J.H.; Lee, D.R.; Shim, S.H.; Kim, J.K.; Cha, D.-H.; Yoon, T.K.; Kim, G.J. Human Chorionic-Plate-Derived Mesenchymal Stem Cells and Wharton’s Jelly-Derived Mesenchymal Stem Cells: A Comparative Analysis of Their Potential as Placenta-Derived Stem Cells. Cell Tissue Res. 2011, 346, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Quirici, N.; Soligo, D.; Bossolasco, P.; Servida, F.; Lumini, C.; Deliliers, G.L. Isolation of Bone Marrow Mesenchymal Stem Cells by Anti-Nerve Growth Factor Receptor Antibodies. Exp. Hematol. 2002, 30, 783–791. [Google Scholar] [CrossRef]
- Youssef, A.; Han, V.K.M. Regulation of Osteogenic Differentiation of Placental-Derived Mesenchymal Stem Cells by Insulin-Like Growth Factors and Low Oxygen Tension. Stem Cells Int. 2017, 2017, e4576327. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.N.; Moschidou, D.; Abdulrazzak, H.; Kalirai, B.S.; Vanleene, M.; Osatis, S.; Shefelbine, S.J.; Horwood, N.J.; Marenzana, M.; De Coppi, P.; et al. Potential of Human Fetal Chorionic Stem Cells for the Treatment of Osteogenesis Imperfecta. Stem Cells Dev. 2014, 23, 262–276. [Google Scholar] [CrossRef]
- Hsu, S.; Huang, T.-B.; Cheng, S.-J.; Weng, S.-Y.; Tsai, C.-L.; Tseng, C.-S.; Chen, D.C.; Liu, T.-Y.; Fu, K.-Y.; Yen, B.L. Chondrogenesis from Human Placenta-Derived Mesenchymal Stem Cells in Three-Dimensional Scaffolds for Cartilage Tissue Engineering. Tissue Eng. Part. A 2011, 17, 1549–1560. [Google Scholar] [CrossRef]
- Noh, Y.K.; Du, P.; Dos Santos Da Costa, A.; Park, K. Induction of Chondrogenesis of Human Placenta-Derived Mesenchymal Stem Cells via Heparin-Grafted Human Fibroblast Derived Matrix. Biomater. Res. 2018, 22, 12. [Google Scholar] [CrossRef]
- Park, Y.-B.; Seo, S.; Kim, J.-A.; Heo, J.-C.; Lim, Y.-C.; Ha, C.-W. Effect of Chondrocyte-Derived Early Extracellular Matrix on Chondrogenesis of Placenta-Derived Mesenchymal Stem Cells. Biomed. Mater. 2015, 10, 035014. [Google Scholar] [CrossRef]
- Spitzhorn, L.-S.; Rahman, M.S.; Schwindt, L.; Ho, H.-T.; Wruck, W.; Bohndorf, M.; Wehrmeyer, S.; Ncube, A.; Beyer, I.; Hagenbeck, C.; et al. Isolation and Molecular Characterization of Amniotic Fluid-Derived Mesenchymal Stem Cells Obtained from Caesarean Sections. Stem Cells Int. 2017, 2017, e5932706. [Google Scholar] [CrossRef]
- Simoni, G.; Colognato, R. The Amniotic Fluid-Derived Cells: The Biomedical Challenge for the Third Millennium. J. Prenat. Med. 2009, 3, 34–36. [Google Scholar]
- Miranda-Sayago, J.M.; Fernández-Arcas, N.; Benito, C.; Reyes-Engel, A.; Carrera, J.; Alonso, A. Lifespan of Human Amniotic Fluid-Derived Multipotent Mesenchymal Stromal Cells. Cytotherapy 2011, 13, 572–581. [Google Scholar] [CrossRef]
- Loukogeorgakis, S.P.; Coppi, P.D. Concise Review: Amniotic Fluid Stem Cells: The Known, the Unknown, and Potential Regenerative Medicine Applications. Stem Cells 2017, 35, 1663–1673. [Google Scholar] [CrossRef]
- Moorefield, E.C.; McKee, E.E.; Solchaga, L.; Orlando, G.; Yoo, J.J.; Walker, S.; Furth, M.E.; Bishop, C.E. Cloned, CD117 Selected Human Amniotic Fluid Stem Cells Are Capable of Modulating the Immune Response. PLoS ONE 2011, 6, e26535. [Google Scholar] [CrossRef] [PubMed]
- Kolambkar, Y.M.; Peister, A.; Soker, S.; Atala, A.; Guldberg, R.E. Chondrogenic Differentiation of Amniotic Fluid-Derived Stem Cells. J. Mol. Hist. 2007, 38, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Zuliani, C.C.; Damas, I.I.; Andrade, K.C.; Westin, C.B.; Moraes, Â.M.; Coimbra, I.B. Chondrogenesis of Human Amniotic Fluid Stem Cells in Chitosan-Xanthan Scaffold for Cartilage Tissue Engineering. Sci. Rep. 2021, 11, 3063. [Google Scholar] [CrossRef]
- Pipino, C.; Tomo, P.D.; Mandatori, D.; Cianci, E.; Lanuti, P.; Cutrona, M.B.; Penolazzi, L.; Pierdomenico, L.; Lambertini, E.; Antonucci, I.; et al. Calcium Sensing Receptor Activation by Calcimimetic R-568 in Human Amniotic Fluid Mesenchymal Stem Cells: Correlation with Osteogenic Differentiation. Stem Cells Dev. 2014, 23, 2959–2971. [Google Scholar] [CrossRef]
- D’Alimonte, I.; Lannutti, A.; Pipino, C.; Di Tomo, P.; Pierdomenico, L.; Cianci, E.; Antonucci, I.; Marchisio, M.; Romano, M.; Stuppia, L.; et al. Wnt Signaling Behaves as a “Master Regulator” in the Osteogenic and Adipogenic Commitment of Human Amniotic Fluid Mesenchymal Stem Cells. Stem Cell Rev. Rep. 2013, 9, 642–654. [Google Scholar] [CrossRef]
- Aziz, S.G.-G.; Pashaei-Asl, F.; Fardyazar, Z.; Pashaiasl, M. Isolation, Characterization, Cryopreservation of Human Amniotic Stem Cells and Differentiation to Osteogenic and Adipogenic Cells. PLoS ONE 2016, 11, e0158281. [Google Scholar] [CrossRef]
- Trohatou, O.; Zagoura, D.; Orfanos, N.K.; Pappa, K.I.; Marinos, E.; Anagnou, N.P.; Roubelakis, M.G. MiR-26a Mediates Adipogenesis of Amniotic Fluid Mesenchymal Stem/Stromal Cells via PTEN, Cyclin E1, and CDK6. Stem Cells Dev. 2017, 26, 482–494. [Google Scholar] [CrossRef]
- Kassis, I.; Zangi, L.; Rivkin, R.; Levdansky, L.; Samuel, S.; Marx, G.; Gorodetsky, R. Isolation of Mesenchymal Stem Cells from G-CSF-Mobilized Human Peripheral Blood Using Fibrin Microbeads. Bone Marrow Transpl. 2006, 37, 967–976. [Google Scholar] [CrossRef]
- Ouryazdanpanah, N.; Dabiri, S.; Derakhshani, A.; Vahidi, R.; Farsinejad, A. Peripheral Blood-Derived Mesenchymal Stem Cells: Growth Factor-Free Isolation, Molecular Characterization and Differentiation. Iran. J. Pathol. 2018, 13, 461–466. [Google Scholar]
- Chong, P.-P.; Selvaratnam, L.; Abbas, A.A.; Kamarul, T. Human Peripheral Blood Derived Mesenchymal Stem Cells Demonstrate Similar Characteristics and Chondrogenic Differentiation Potential to Bone Marrow Derived Mesenchymal Stem Cells. J. Orthop. Res. 2012, 30, 634–642. [Google Scholar] [CrossRef]
- Ab Kadir, R.; Zainal Ariffin, S.H.; Megat Abdul Wahab, R.; Kermani, S.; Senafi, S. Characterization of Mononucleated Human Peripheral Blood Cells. Sci. World J. 2012, 2012, e843843. [Google Scholar] [CrossRef]
- Li, S.; Huang, K.-J.; Wu, J.-C.; Hu, M.S.; Sanyal, M.; Hu, M.; Longaker, M.T.; Lorenz, H.P. Peripheral Blood-Derived Mesenchymal Stem Cells: Candidate Cells Responsible for Healing Critical-Sized Calvarial Bone Defects. Stem Cells Transl. Med. 2015, 4, 359–368. [Google Scholar] [CrossRef]
- Lotfy, A.; El-Sherbiny, Y.M.; Cuthbert, R.; Jones, E.; Badawy, A. Comparative Study of Biological Characteristics of Mesenchymal Stem Cells Isolated from Mouse Bone Marrow and Peripheral Blood. Biomed. Rep. 2019, 11, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Haleem, A.M.; Singergy, A.A.E.; Sabry, D.; Atta, H.M.; Rashed, L.A.; Chu, C.R.; El Shewy, M.T.; Azzam, A.; Abdel Aziz, M.T. The Clinical Use of Human Culture-Expanded Autologous Bone Marrow Mesenchymal Stem Cells Transplanted on Platelet-Rich Fibrin Glue in the Treatment of Articular Cartilage Defects: A Pilot Study and Preliminary Results. Cartilage 2010, 1, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Valenti, M.T.; Carbonare, L.D.; Donatelli, L.; Bertoldo, F.; Zanatta, M.; Lo Cascio, V. Gene Expression Analysis in Osteoblastic Differentiation from Peripheral Blood Mesenchymal Stem Cells. Bone 2008, 43, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-R.; Yan, X.; Yuan, F.-Z.; Ye, J.; Xu, B.-B.; Zhou, Z.-X.; Mao, Z.-M.; Guan, J.; Song, Y.-F.; Sun, Z.-W.; et al. The Use of Peripheral Blood-Derived Stem Cells for Cartilage Repair and Regeneration In Vivo: A Review. Front. Pharm. 2020, 11, 404. [Google Scholar] [CrossRef] [PubMed]
- Lyahyai, J.; Mediano, D.R.; Ranera, B.; Sanz, A.; Remacha, A.R.; Bolea, R.; Zaragoza, P.; Rodellar, C.; Martín-Burriel, I. Isolation and Characterization of Ovine Mesenchymal Stem Cells Derived from Peripheral Blood. BMC Vet. Res. 2012, 8, 169. [Google Scholar] [CrossRef] [PubMed]
- Spaas, J.H.; De Schauwer, C.; Cornillie, P.; Meyer, E.; Van Soom, A.; Van de Walle, G.R. Culture and Characterisation of Equine Peripheral Blood Mesenchymal Stromal Cells. Vet. J. 2013, 195, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.-L.; Zhang, J.-Y.; Fu, X.; Duan, X.-N.; Leung, K.K.M.; Jia, Z.-Q.; Wang, W.-P.; Zhou, C.-Y.; Yu, J.-K. Comparative Study of the Biological Characteristics of Mesenchymal Stem Cells from Bone Marrow and Peripheral Blood of Rats. Tissue Eng. Part A 2012, 18, 1793–1803. [Google Scholar] [CrossRef]
- Wu, G.; Pan, M.; Wang, X.; Wen, J.; Cao, S.; Li, Z.; Li, Y.; Qian, C.; Liu, Z.; Wu, W.; et al. Osteogenesis of Peripheral Blood Mesenchymal Stem Cells in Self Assembling Peptide Nanofiber for Healing Critical Size Calvarial Bony Defect. Sci. Rep. 2015, 5, 16681. [Google Scholar] [CrossRef]
- Li, N.; Gao, J.; Mi, L.; Zhang, G.; Zhang, L.; Zhang, N.; Huo, R.; Hu, J.; Xu, K. Synovial Membrane Mesenchymal Stem Cells: Past Life, Current Situation, and Application in Bone and Joint Diseases. Stem Cell Res. Ther. 2020, 11, 381. [Google Scholar] [CrossRef]
- Greif, D.N.; Kouroupis, D.; Murdock, C.J.; Griswold, A.J.; Kaplan, L.D.; Best, T.M.; Correa, D. Infrapatellar Fat Pad/Synovium Complex in Early-Stage Knee Osteoarthritis: Potential New Target and Source of Therapeutic Mesenchymal Stem/Stromal Cells. Front. Bioeng. Biotechnol. 2020, 8, 860. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraman, M.; Muthu, S.; Jeyaraman, N.; Ranjan, R.; Jha, S.K.; Mishra, P. Synovium Derived Mesenchymal Stromal Cells (Sy-MSCs): A Promising Therapeutic Paradigm in the Management of Knee Osteoarthritis. Indian J. Orthop. 2021, 56, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Varshney, R.R.; Ren, L.; Cai, D.; Wang, D.-A. Synovium-Derived Mesenchymal Stem Cells: A New Cell Source for Musculoskeletal Regeneration. Tissue Eng. Part. B Rev. 2009, 15, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Gale, A.L.; Linardi, R.L.; McClung, G.; Mammone, R.M.; Ortved, K.F. Comparison of the Chondrogenic Differentiation Potential of Equine Synovial Membrane-Derived and Bone Marrow-Derived Mesenchymal Stem Cells. Front. Vet. Sci. 2019, 6, 178. [Google Scholar] [CrossRef]
- Pei, M.; He, F.; Vunjak-Novakovic, G. Synovium-Derived Stem Cell-Based Chondrogenesis. Differentiation 2008, 76, 1044–1056. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, S.; Jiang, Q.; Pei, M. Determinants of Stem Cell Lineage Differentiation toward Chondrogenesis versus Adipogenesis. Cell Mol. Life Sci. 2019, 76, 1653–1680. [Google Scholar] [CrossRef]
- Zha, K.; Sun, Z.; Yang, Y.; Chen, M.; Gao, C.; Fu, L.; Li, H.; Sui, X.; Guo, Q.; Liu, S. Recent Developed Strategies for Enhancing Chondrogenic Differentiation of MSC: Impact on MSC-Based Therapy for Cartilage Regeneration. Stem Cells Int. 2021, 2021, 8830834. [Google Scholar] [CrossRef]
- Sahu, N.; Budhiraja, G.; Subramanian, A. Preconditioning of Mesenchymal Stromal Cells with Low-Intensity Ultrasound: Influence on Chondrogenesis and Directed SOX9 Signaling Pathways. Stem Cell Res. Ther. 2020, 11, 6. [Google Scholar] [CrossRef]
- Skeletal Muscle Repair by Adult Human Mesenchymal Stem Cells from Synovial Membrane. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2173757/ (accessed on 24 August 2021).
- Mizuno, M.; Katano, H.; Mabuchi, Y.; Ogata, Y.; Ichinose, S.; Fujii, S.; Otabe, K.; Komori, K.; Ozeki, N.; Koga, H.; et al. Specific Markers and Properties of Synovial Mesenchymal Stem Cells in the Surface, Stromal, and Perivascular Regions. Stem Cell Res. 2018, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Bami, M.; Sarlikiotis, T.; Milonaki, M.; Vikentiou, M.; Konsta, E.; Kapsimali, V.; Pappa, V.; Koulalis, D.; Johnson, E.O.; Soucacos, P.N. Superiority of Synovial Membrane Mesenchymal Stem Cells in Chondrogenesis, Osteogenesis, Myogenesis and Tenogenesis in a Rabbit Model. Injury 2020, 51, 2855–2865. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, K.; Matsukura, Y.; Muneta, T.; Ozeki, N.; Mizuno, M.; Katano, H.; Sekiya, I. Fibrous Synovium Releases Higher Numbers of Mesenchymal Stem Cells Than Adipose Synovium in a Suspended Synovium Culture Model. Arthrosc. J. Arthrosc. Relat. Surg. 2017, 33, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Yasui, T.; Mabuchi, Y.; Morikawa, S.; Onizawa, K.; Akazawa, C.; Nakagawa, T.; Okano, H.; Matsuzaki, Y. Isolation of Dental Pulp Stem Cells with High Osteogenic Potential. Inflamm. Regen. 2017, 37, 8. [Google Scholar] [CrossRef]
- Mortada, I.; Mortada, R. Dental Pulp Stem Cells and Osteogenesis: An Update. Cytotechnology 2018, 70, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, N. Characterisation of Dental Pulp Stem Cells: A New Horizon for Tissue Regeneration? Arch. Oral Biol. 2012, 57, 1439–1458. [Google Scholar] [CrossRef]
- Karaöz, E.; Doğan, B.N.; Aksoy, A.; Gacar, G.; Akyüz, S.; Ayhan, S.; Genç, Z.S.; Yürüker, S.; Duruksu, G.; Demircan, P.C.; et al. Isolation and in Vitro Characterisation of Dental Pulp Stem Cells from Natal Teeth. Histochem. Cell Biol. 2010, 133, 95–112. [Google Scholar] [CrossRef]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.W.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem Cell Properties of Human Dental Pulp Stem Cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Jacek, P.; Szustak, M.; Kubiak, K.; Gendaszewska-Darmach, E.; Ludwicka, K.; Bielecki, S. Scaffolds for Chondrogenic Cells Cultivation Prepared from Bacterial Cellulose with Relaxed Fibers Structure Induced Genetically. Nanomaterials 2018, 8, 1066. [Google Scholar] [CrossRef]
- Mata, M.; Milian, L.; Oliver, M.; Zurriaga, J.; Sancho-Tello, M.; de Llano, J.J.M.; Carda, C. In Vivo Articular Cartilage Regeneration Using Human Dental Pulp Stem Cells Cultured in an Alginate Scaffold: A Preliminary Study. Stem Cells Int. 2017, 2017, e8309256. [Google Scholar] [CrossRef]
- Yang, H.; Cao, Y.; Zhang, J.; Liang, Y.; Su, X.; Zhang, C.; Liu, H.; Han, X.; Ge, L.; Fan, Z. DLX5 and HOXC8 Enhance the Chondrogenic Differentiation Potential of Stem Cells from Apical Papilla via LINC01013. Stem Cell Res. 2020, 11, 271. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yuan, S.; Sun, J.; Gong, Y.; Liu, S.; Guo, R.; He, W.; Kang, P.; Li, R. Inhibitory Effect of the TSG-6 on the BMP-4/Smad Signaling Pathway and Odonto/Osteogenic Differentiation of Dental Pulp Stem Cells. Biomed. Pharmacother. 2020, 128, 110266. [Google Scholar] [CrossRef] [PubMed]
- Amir, L.R.; Suniarti, D.F.; Utami, S.; Abbas, B. Chitosan as a Potential Osteogenic Factor Compared with Dexamethasone in Cultured Macaque Dental Pulp Stromal Cells. Cell Tissue Res. 2014, 358, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Robey, P.G.; Gronthos, S. Comparison of Human Dental Pulp and Bone Marrow Stromal Stem Cells by CDNA Microarray Analysis. Bone 2001, 29, 532–539. [Google Scholar] [CrossRef]
- Tabatabaei, F.S.; Torshabi, M. In Vitro Proliferation and Osteogenic Differentiation of Endometrial Stem Cells and Dental Pulp Stem Cells. Cell Tissue Bank 2017, 18, 239–247. [Google Scholar] [CrossRef]
- Laino, G.; d’Aquino, R.; Graziano, A.; Lanza, V.; Carinci, F.; Naro, F.; Pirozzi, G.; Papaccio, G. A New Population of Human Adult Dental Pulp Stem Cells: A Useful Source of Living Autologous Fibrous Bone Tissue (LAB). J. Bone Min. Res. 2005, 20, 1394–1402. [Google Scholar] [CrossRef]
- D’Aquino, R.; Graziano, A.; Sampaolesi, M.; Laino, G.; Pirozzi, G.; De Rosa, A.; Papaccio, G. Human Postnatal Dental Pulp Cells Co-Differentiate into Osteoblasts and Endotheliocytes: A Pivotal Synergy Leading to Adult Bone Tissue Formation. Cell Death Differ. 2007, 14, 1162–1171. [Google Scholar] [CrossRef]
- D’Aquino, R.; De Rosa, A.; Lanza, V.; Tirino, V.; Laino, L.; Graziano, A.; Desiderio, V.; Laino, G.; Papaccio, G. Human Mandible Bone Defect Repair by the Grafting of Dental Pulp Stem/Progenitor Cells and Collagen Sponge Biocomplexes. Eur. Cell Mater. 2009, 18, 75–83. [Google Scholar] [CrossRef]
- Colnot, C.; Zhang, X.; Knothe Tate, M.L. Current Insights on the Regenerative Potential of the Periosteum: Molecular, Cellular, and Endogenous Engineering Approaches. J. Orthop. Res. 2012, 30, 1869–1878. [Google Scholar] [CrossRef]
- Chang, H.; Knothe Tate, M.L. Concise Review: The Periosteum: Tapping into a Reservoir of Clinically Useful Progenitor Cells. Stem Cells Transl. Med. 2012, 1, 480–491. [Google Scholar] [CrossRef]
- Moore, E.R.; Zhu, Y.X.; Ryu, H.S.; Jacobs, C.R. Periosteal Progenitors Contribute to Load-Induced Bone Formation in Adult Mice and Require Primary Cilia to Sense Mechanical Stimulation. Stem Cell Res. Ther. 2018, 9, 190. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Fateh, A.; Salem, D.M.; Intini, G. Periosteum. J. Dent. Res. 2014, 93, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Fitzsimmons, J.S.; Sanyal, A.; Mello, M.A.; Mukherjee, N.; O’Driscoll, S.W. Localization of Chondrocyte Precursors in Periosteum. Osteoarthr. Cartil. 2001, 9, 215–223. [Google Scholar] [CrossRef]
- Duchamp de Lageneste, O.; Julien, A.; Abou-Khalil, R.; Frangi, G.; Carvalho, C.; Cagnard, N.; Cordier, C.; Conway, S.J.; Colnot, C. Periosteum Contains Skeletal Stem Cells with High Bone Regenerative Potential Controlled by Periostin. Nat. Commun. 2018, 9, 773. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Nakata, H.; Yamamoto, M.; Miyasaka, M.; Kasugai, S.; Kuroda, S. Osteogenic Potential of Mouse Periosteum-Derived Cells Sorted for CD90 In Vitro and In Vivo. Stem Cells Transl. Med. 2016, 5, 227–234. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Lim, S.-M.; Shin, H.-C.; Lee, C.-W.; Kim, S.-L.; Kim, D.-I. Chondrogenesis of Human Periosteum-Derived Progenitor Cells in Atelocollagen. Biotechnol. Lett. 2007, 29, 323–329. [Google Scholar] [CrossRef]
- De Bari, C.; Dell’Accio, F.; Luyten, F.P. Human Periosteum-Derived Cells Maintain Phenotypic Stability and Chondrogenic Potential throughout Expansion Regardless of Donor Age. Arthritis Rheum. 2001, 44, 85–95. [Google Scholar] [CrossRef]
- Zuo, W.; Xie, B.; Li, C.; Yan, Y.; Zhang, Y.; Liu, W.; Huang, J.; Chen, D. The Clinical Applications of Endometrial Mesenchymal Stem Cells. Biopreserv. Biobank 2018, 16, 158–164. [Google Scholar] [CrossRef]
- Patel, A.N.; Park, E.; Kuzman, M.; Benetti, F.; Silva, F.J.; Allickson, J.G. Multipotent Menstrual Blood Stromal Stem Cells: Isolation, Characterization, and Differentiation. Cell Transpl. 2008, 17, 303–311. [Google Scholar] [CrossRef]
- Musina, R.A.; Belyavski, A.V.; Tarusova, O.V.; Solovyova, E.V.; Sukhikh, G.T. Endometrial Mesenchymal Stem Cells Isolated from the Menstrual Blood. Bull. Exp. Biol. Med. 2008, 145, 539–543. [Google Scholar] [CrossRef]
- Chan, R.W.S.; Schwab, K.E.; Gargett, C.E. Clonogenicity of Human Endometrial Epithelial and Stromal Cells. Biol. Reprod. 2004, 70, 1738–1750. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, L.; Hufnagel, D.; Taylor, H.S. The Endometrium as a Source of Mesenchymal Stem Cells for Regenerative Medicine. Biol. Reprod. 2015, 92, 138. [Google Scholar] [CrossRef] [PubMed]
- Gargett, C.E.; Schwab, K.E.; Deane, J.A. Endometrial Stem/Progenitor Cells: The First 10 Years. Hum. Reprod. Update 2016, 22, 137–163. [Google Scholar] [CrossRef]
- Allickson, J.G.; Sanchez, A.; Yefimenko, N.; Borlongan, C.V.; Sanberg, P.R. Recent Studies Assessing the Proliferative Capability of a Novel Adult Stem Cell Identified in Menstrual Blood. Open Stem Cell J. 2011, 3, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Darzi, S.; Zarnani, A.H.; Jeddi-Tehrani, M.; Entezami, K.; Mirzadegan, E.; Akhondi, M.M.; Talebi, S.; Khanmohammadi, M.; Kazemnejad, S. Osteogenic Differentiation of Stem Cells Derived from Menstrual Blood versus Bone Marrow in the Presence of Human Platelet Releasate. Tissue Eng. Part A 2012, 18, 1720–1728. [Google Scholar] [CrossRef] [PubMed]
- Rink, B.E.; Amilon, K.R.; Esteves, C.L.; French, H.M.; Watson, E.; Aurich, C.; Donadeu, F.X. Isolation and Characterization of Equine Endometrial Mesenchymal Stromal Cells. Stem Cell Res. Ther. 2017, 8, 166. [Google Scholar] [CrossRef] [PubMed]
- Wolff, E.F.; Wolff, A.B.; Du, H.; Taylor, H.S. Demonstration of Multipotent Stem Cells in the Adult Human Endometrium by In Vitro Chondrogenesis. Reprod. Sci. 2007, 14, 524–533. [Google Scholar] [CrossRef]
- Embryonic and Induced Pluripotent Stem Cell Markers Research Areas: R&D Systems. Available online: https://www.rndsystems.com/research-area/embryonic-and-induced-pluripotent-stem-cell-markers (accessed on 10 March 2022).
- Kang, R.; Zhou, Y.; Tan, S.; Zhou, G.; Aagaard, L.; Xie, L.; Bünger, C.; Bolund, L.; Luo, Y. Mesenchymal Stem Cells Derived from Human Induced Pluripotent Stem Cells Retain Adequate Osteogenicity and Chondrogenicity but Less Adipogenicity. Stem Cell Res. 2015, 6, 144. [Google Scholar] [CrossRef]
- Sheyn, D.; Ben-David, S.; Shapiro, G.; De Mel, S.; Bez, M.; Ornelas, L.; Sahabian, A.; Sareen, D.; Da, X.; Pelled, G.; et al. Human Induced Pluripotent Stem Cells Differentiate Into Functional Mesenchymal Stem Cells and Repair Bone Defects. Stem Cells Transl. Med. 2016, 5, 1447–1460. [Google Scholar] [CrossRef]
- Jeon, O.H.; Panicker, L.M.; Lu, Q.; Chae, J.J.; Feldman, R.A.; Elisseeff, J.H. Human IPSC-Derived Osteoblasts and Osteoclasts Together Promote Bone Regeneration in 3D Biomaterials. Sci. Rep. 2016, 6, 26761. [Google Scholar] [CrossRef]
- Kargozar, S.; Lotfibakhshaeish, N.; Ebrahimi-Barough, S.; Nazari, B.; Hill, R.G. Stimulation of Osteogenic Differentiation of Induced Pluripotent Stem Cells (IPSCs) Using Bioactive Glasses: An in Vitro Study. Front. Bioeng. Biotechnol. 2019, 7, 355. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Tong, X.; Huang, X.; Zhang, J.; Qin, H.; Hu, Q. Patient-Derived Human Induced Pluripotent Stem Cells From Gingival Fibroblasts Composited With Defined Nanohydroxyapatite/Chitosan/Gelatin Porous Scaffolds as Potential Bone Graft Substitutes. Stem Cells Transl. Med. 2016, 5, 95–105. [Google Scholar] [CrossRef]
- Ardeshirylajimi, A.; Soleimani, M. Enhanced Growth and Osteogenic Differentiation of Induced Pluripotent Stem Cells by Extremely Low-Frequency Electromagnetic Field. Cell Mol. Biol. 2015, 61, 36–41. [Google Scholar] [PubMed]
- Diederichs, S.; Klampfleuthner, F.A.M.; Moradi, B.; Richter, W. Chondral Differentiation of Induced Pluripotent Stem Cells Without Progression Into the Endochondral Pathway. Front. Cell Dev. Biol. 2019, 7, 270. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-H.; Wu, K.-C.; Ding, D.-C. Induced Pluripotent Stem Cell-Differentiated Chondrocytes Repair Cartilage Defect in a Rabbit Osteoarthritis Model. Stem Cells Int. 2020, 2020, e8867349. [Google Scholar] [CrossRef]
- Taura, D.; Noguchi, M.; Sone, M.; Hosoda, K.; Mori, E.; Okada, Y.; Takahashi, K.; Homma, K.; Oyamada, N.; Inuzuka, M.; et al. Adipogenic Differentiation of Human Induced Pluripotent Stem Cells: Comparison with That of Human Embryonic Stem Cells. FEBS Lett. 2009, 583, 1029–1033. [Google Scholar] [CrossRef]

| Source of MSCs | Osteogenesis | Chondrogenesis | Adipogenesis |
|---|---|---|---|
| BM-MSC | ++++ | +++ | + |
| AD-MSC | ++ | ++ | ++++ |
| HSC | + | +/− | + |
| Pl-MSC | +++ | ++ | ++ |
| Af-MSC | ++ | +++ | ++ |
| PB-MSC | ++ | +++ | ++ |
| Sy-MSC | +++ | ++++ | + |
| D-MSC | ++++ | +++ | + |
| P-MSC | ++++ | +++ | + |
| En-MSC | ++ | ++ | ++ |
| iPSC | +++ | +++ | +++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prajwal, G.S.; Jeyaraman, N.; Kanth V, K.; Jeyaraman, M.; Muthu, S.; Rajendran, S.N.S.; Rajendran, R.L.; Khanna, M.; Oh, E.J.; Choi, K.Y.; et al. Lineage Differentiation Potential of Different Sources of Mesenchymal Stem Cells for Osteoarthritis Knee. Pharmaceuticals 2022, 15, 386. https://doi.org/10.3390/ph15040386
Prajwal GS, Jeyaraman N, Kanth V K, Jeyaraman M, Muthu S, Rajendran SNS, Rajendran RL, Khanna M, Oh EJ, Choi KY, et al. Lineage Differentiation Potential of Different Sources of Mesenchymal Stem Cells for Osteoarthritis Knee. Pharmaceuticals. 2022; 15(4):386. https://doi.org/10.3390/ph15040386
Chicago/Turabian StylePrajwal, Gollahalli Shivashankar, Naveen Jeyaraman, Krishna Kanth V, Madhan Jeyaraman, Sathish Muthu, Sree Naga Sowndary Rajendran, Ramya Lakshmi Rajendran, Manish Khanna, Eun Jung Oh, Kang Young Choi, and et al. 2022. "Lineage Differentiation Potential of Different Sources of Mesenchymal Stem Cells for Osteoarthritis Knee" Pharmaceuticals 15, no. 4: 386. https://doi.org/10.3390/ph15040386
APA StylePrajwal, G. S., Jeyaraman, N., Kanth V, K., Jeyaraman, M., Muthu, S., Rajendran, S. N. S., Rajendran, R. L., Khanna, M., Oh, E. J., Choi, K. Y., Chung, H. Y., Ahn, B.-C., & Gangadaran, P. (2022). Lineage Differentiation Potential of Different Sources of Mesenchymal Stem Cells for Osteoarthritis Knee. Pharmaceuticals, 15(4), 386. https://doi.org/10.3390/ph15040386











