Quercetin and Quercitrin from Agrimonia pilosa Ledeb Inhibit the Migration and Invasion of Colon Cancer Cells through the JNK Signaling Pathway
Abstract
:1. Introduction
2. Results
2.1. Fraction 4 from A. pilosa Inhibits the Migration of Colon Cancer Cells
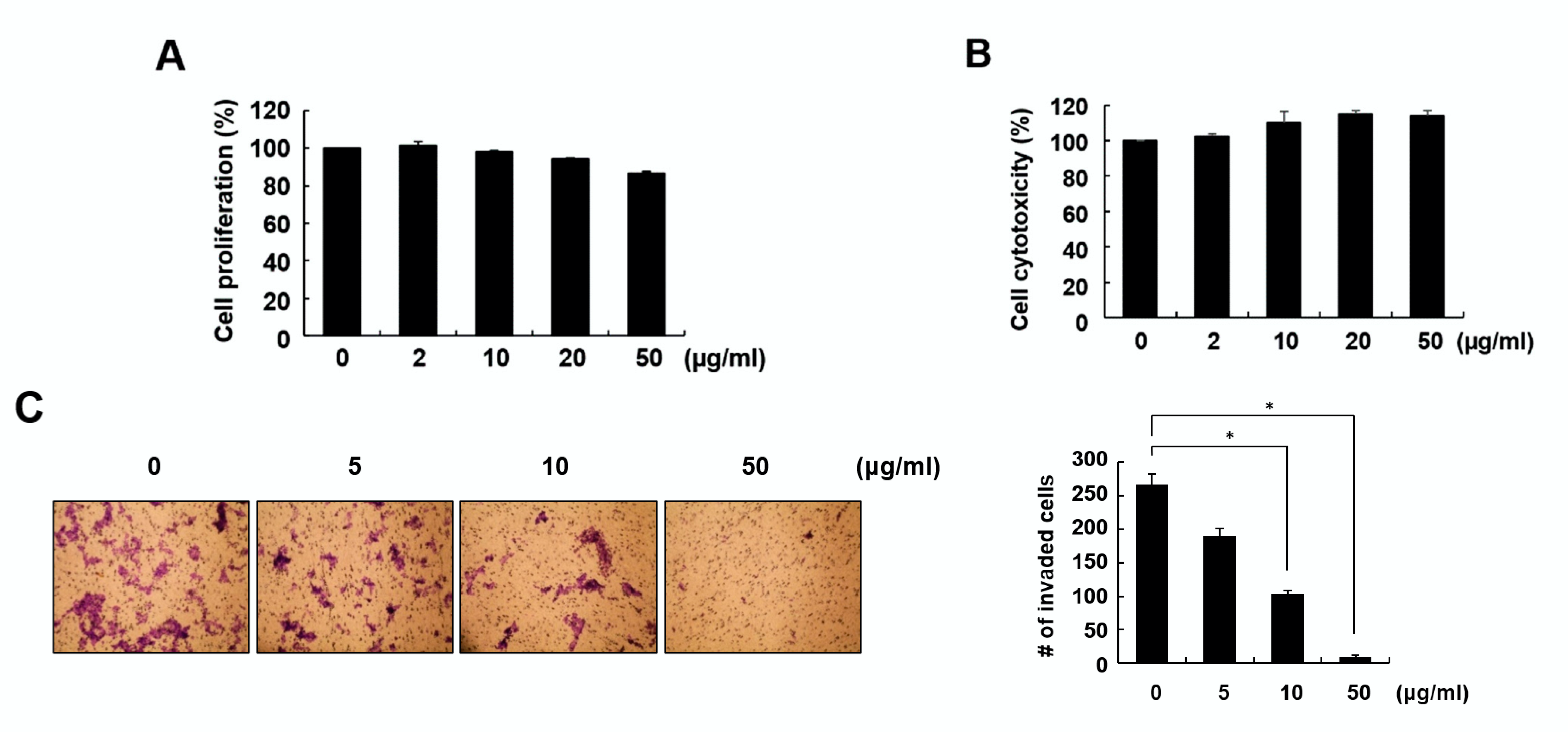
2.2. Fraction 4 from A. pilosa Inhibited Cell Invasion and Colony Formation
2.3. Identification of Quercetin and Quercitrin from Fraction 4 by UPLC-Q-TOF-HRMS
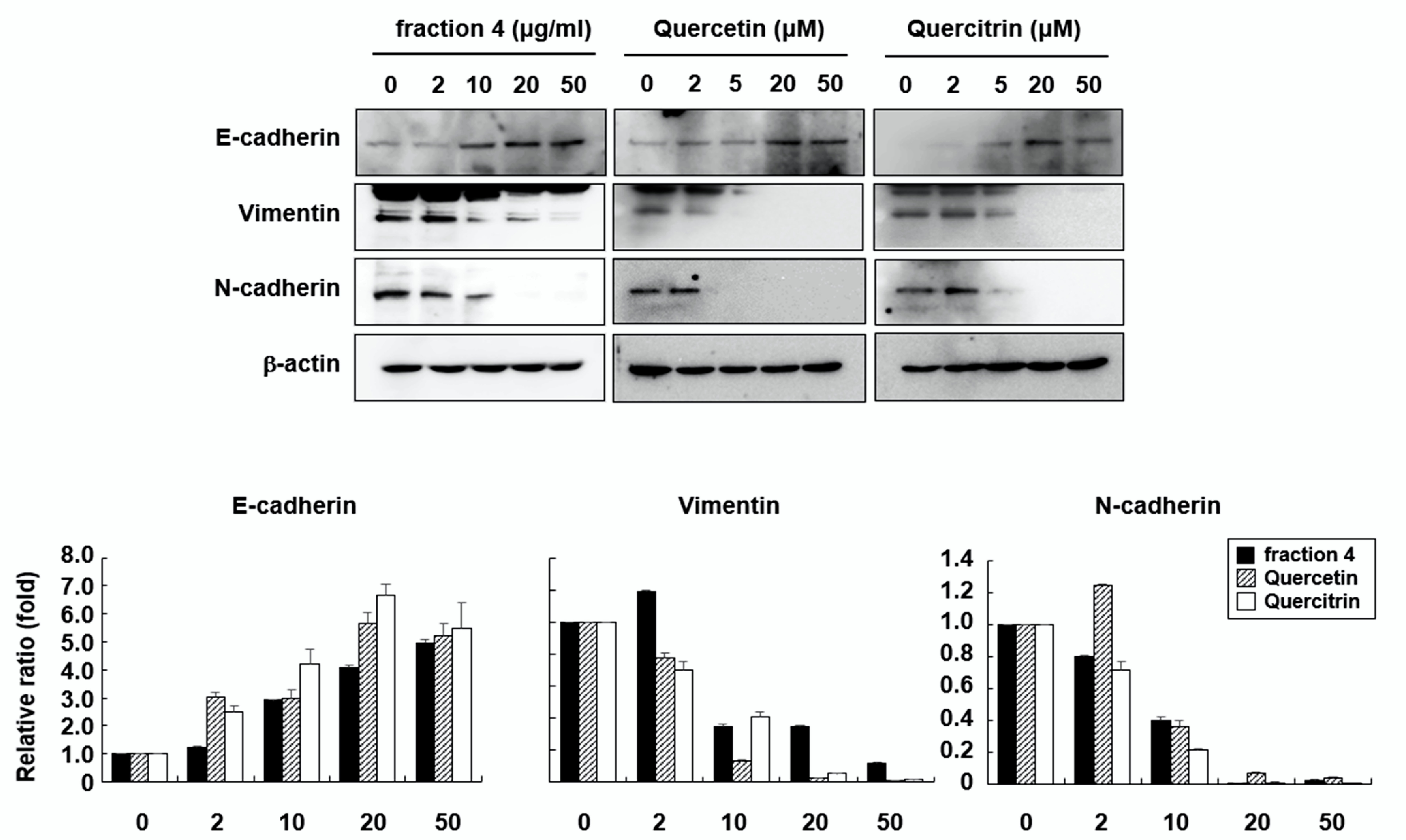
2.4. Quercetin and Quercitrin Induce Mesenchymal-to Epithelial Transition (MET)
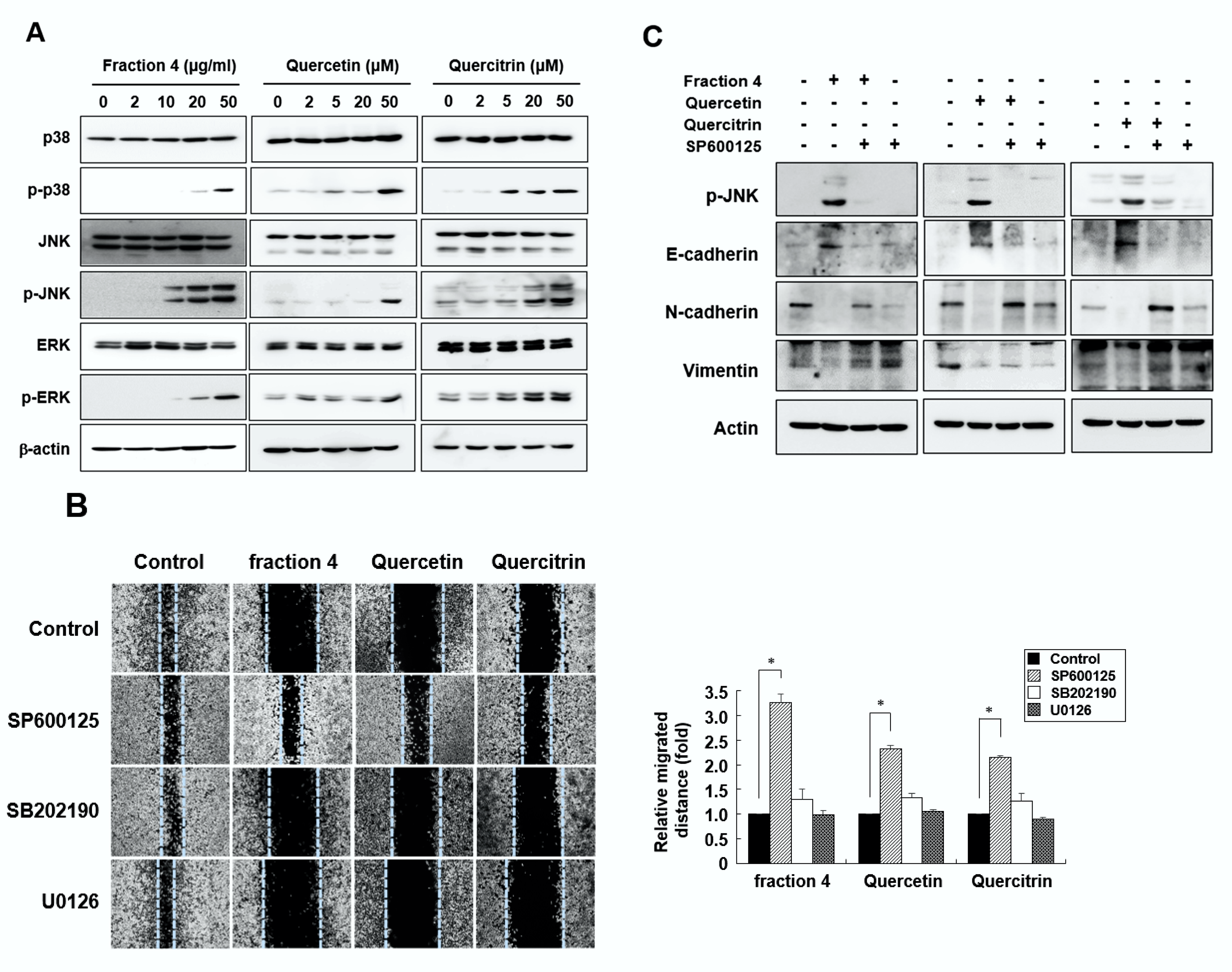
2.5. Quercetin and Quercitrin Inhibit Cell Migration through the JNK Signaling Pathway
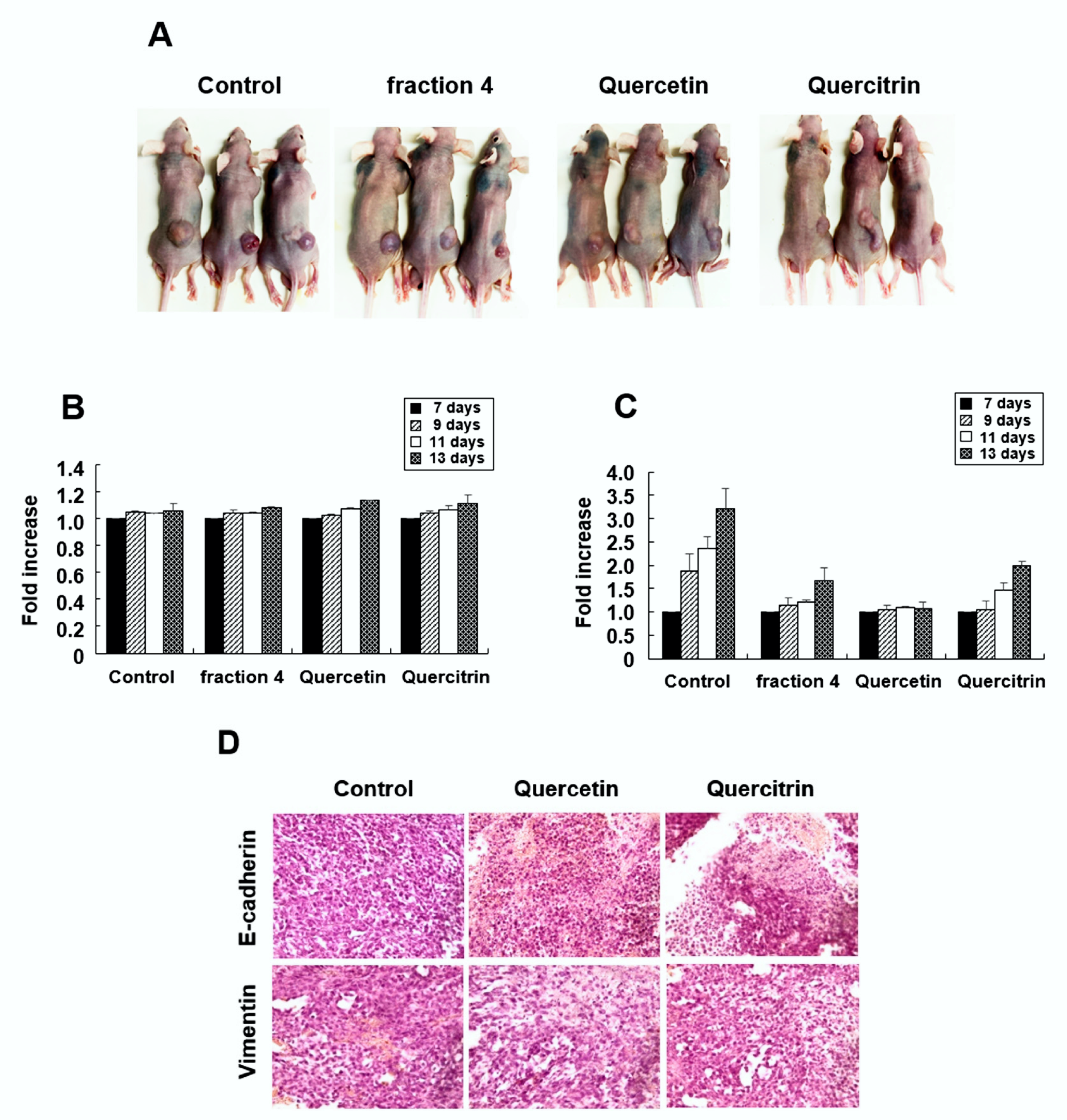
2.6. Quercetin and Quercitrin Inhibit the Growth of Tumors In Vivo
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Wound Healing Assay
4.3. Cell Proliferation and Cytotoxicity Assay
4.4. Cell Invasion Assay
4.5. Colony Formation Assay
4.6. Plant Material, Extraction, Fractionation, and Identification of Quercetin and Quercitrin
4.7. Western Blot Analysis
4.8. Nude Mouse Xenograft Assay
4.9. Immunohistochemistry
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Kloor, M.; Pox, C.P. Colorectal Cancer. Lancet 2014, 383, 1490–1502. [Google Scholar] [CrossRef]
- Siegel, R.; DeSantis, C.; Virgo, K.; Stein, K.; Mariotto, A.; Smith, T.; Cooper, D.; Gansler, T.; Lerro, C.; Fedewa, S.; et al. Cancer Treatment and Survivorship Statistics, 2012. CA Cancer J. Clin. 2012, 62, 220–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, Y.; Cong, Y.-S.; Zhou, J. Implications of Telomerase Reverse Transcriptase in Tumor Metastasis. BMB Rep. 2020, 53, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.; Datta, P.K. Regulation of EMT in Colorectal Cancer: A Culprit in Metastasis. Cancers 2017, 9, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Li, W. Epithelial-Mesenchymal Transition in Human Cancer: Comprehensive Reprogramming of Metabolism, Epigenetics, and Differentiation. Pharmacol. Ther. 2015, 150, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The Basics of Epithelial-Mesenchymal Transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jie, X.-X.; Zhang, X.-Y.; Xu, C.-J. Epithelial-to-Mesenchymal Transition, Circulating Tumor Cells and Cancer Metastasis: Mechanisms and Clinical Applications. Oncotarget 2017, 8, 81558–81571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.B.; Hwang, S.H.; Suh, H.-W.; Lim, S.S. Phytochemical Analysis of Agrimonia pilosa Ledeb, Its Antioxidant Activity and Aldose Reductase Inhibitory Potential. Int. J. Mol. Sci. 2017, 18, 379. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.Y.; Yu, Q.-M.; Kong, H.-J.; Lee, J.-Y.; Yang, K.-M.; Seo, J.-S. Antioxidant and Anti-Inflammatory Activities of Agrimonia pilosa Ledeb. Extract. Evid. Based Complement. Altern. Med. 2020, 2020, 8571207. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Zhou, S.; Ding, G.X.; Kim, J.H.; Hong, M.H.; Shin, Y.-C.; Kim, G.J.; Ko, S.-G. Antihyperglycemic Activity of Herb Extracts on Streptozotocin-Induced Diabetic Rats. Biosci. Biotechnol. Biochem. 2006, 70, 2556–2559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, X.; Song, S.; Wang, J.; Zhang, Q.; Qiu, F.; Zhao, F. Tiliroside, the Major Component of Agrimonia pilosa Ledeb Ethanol Extract, Inhibits MAPK/JNK/p38-Mediated Inflammation in Lipopolysaccharide-Activated RAW 264.7 Macrophages. Exp. Ther. Med. 2016, 12, 499–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koshiura, R.; Miyamoto, K.; Ikeya, Y.; Taguchi, H. Antitumor Activity of Methanol Extract from Roots of Agrimonia pilosa Ledeb. Jpn. J. Pharmacol. 1985, 38, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nho, K.J.; Chun, J.M.; Kim, H.K. Agrimonia pilosa Ethanol Extract Induces Apoptotic Cell Death in HepG2 Cells. J. Ethnopharmacol. 2011, 138, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Deng, H.; Jin, S.; Ma, X.; Zha, K.; Xie, M. The Isolation, Structural Characterization and anti-Osteosarcoma Activity of a Water Soluble Polysaccharide from Agrimonia pilosa. Carbohydr. Polym. 2018, 187, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.Y.; Kim, M.-M. The Inhibitory Effect of Agrimonia pilosa Methanolic Extract on Matrix Metalloproteinases in HT1080 Cells. J. Food Biochem. 2021, 45, e13894. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.A.; Huang, R.Y.-J.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef] [Green Version]
- Pearlman, R.L.; Montes de Oca, M.K.; Pal, H.C.; Afaq, F. Potential Therapeutic Targets of Epithelial-Mesenchymal Transition in Melanoma. Cancer Lett. 2017, 391, 125–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabasum, S.; Singh, R.P. Fisetin Suppresses Migration, Invasion and Stem-Cell-Like Phenotype of Human Non-Small Cell Lung Carcinoma Cells via Attenuation of Epithelial to Mesenchymal Transition. Chem. Biol. Interact. 2019, 303, 14–21. [Google Scholar] [CrossRef]
- Shankar, S.; Ganapathy, S.; Hingorani, S.R.; Srivastava, R.K. EGCG Inhibits Growth, Invasion, Angiogenesis and Metastasis of Pancreatic Cancer. Front. Biosci. 2008, 13, 440–452. [Google Scholar] [CrossRef] [Green Version]
- Chang, W.-S.; Tsai, C.-W.; Yang, J.-S.; Hsu, Y.-M.; Shih, L.-C.; Chiu, H.-Y.; Bau, D.-T.; Tsai, F.-J. Resveratrol Inhibited the Metastatic Behaviors of Cisplatin-Resistant Human Oral Cancer Cells via Phosphorylation of ERK/p-38 and Suppression of MMP-2/9. J. Food Biochem. 2021, 45, e13666. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Feng, Q.; Zhang, H.; Liu, Q.; Gong, J. Curcumin Enhances Drug Sensitivity of Gemcitabine-Resistant Lung Cancer Cells and Inhibits Metastasis. Pharmazie 2021, 76, 538–543. [Google Scholar] [PubMed]
- Brito, A.F.; Ribeiro, M.; Abrantes, A.M.; Pires, A.S.; Teixo, R.J.; Tralhão, J.G.; Botelho, M.F. Quercetin in Cancer Treatment, Alone or in Combination with Conventional Therapeutics? Curr. Med. Chem. 2015, 22, 3025–3039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Zhang, J.; Wang, Y.; Liang, X.; Wusiman, Z.; Yin, Y.; Shen, Q. Synergistic Inhibition of Migration and Invasion of Breast Cancer Cells by Dual Docetaxel/Quercetin-Loaded Nanoparticles via Akt/MMP-9 Pathway. Int. J. Pharm. 2017, 523, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.; Hong, W.; Fan, P.; Qian, D.; Zhu, J.; Bai, B. Quercetin Inhibits Cell Migration and Invasion in Human Osteosarcoma Cells. Cell. Physiol. Biochem. 2017, 43, 553–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhi, K.; Li, M.; Bai, J.; Wu, Y.; Zhou, S.; Zhang, X.; Qu, L. Quercitrin Treatment Protects Endothelial Progenitor Cells from Oxidative Damage via Inducing Autophagy through Extracellular Signal-Regulated Kinase. Angiogenesis 2016, 19, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Cincin, Z.B.; Unlu, M.; Kiran, B.; Bireller, E.S.; Baran, Y.; Cakmakoglu, B. Molecular Mechanisms of Quercitrin-Induced Apoptosis in Non-Small Cell Lung Cancer. Arch. Med. Res. 2014, 45, 445–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Wang, Z.; Li, S.; Xin, Q.; Yuan, M.; Li, H.; Song, X.; Gao, H.; Pervaiz, N.; Sun, X.; et al. Quercetin Inhibits the Migration and Invasion of HCCLM3 Cells by Suppressing the Expression of p-Akt1, Matrix Metalloproteinase (MMP) MMP-2, and MMP-9. Med. Sci. Monit. 2018, 24, 2583–2589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, W.-W.; Hsu, S.-C.; Chueh, F.-S.; Chen, Y.-Y.; Yang, J.-S.; Lin, J.-P.; Lien, J.-C.; Tsai, C.-H.; Chung, J.-G. Quercetin Inhibits Migration and Invasion of SAS Human Oral Cancer Cells through Inhibition of NF-κB and Matrix Metalloproteinase-2/-9 Signaling Pathways. Anticancer Res. 2013, 33, 1941–1950. [Google Scholar] [PubMed]
- Lin, C.-W.; Hou, W.-C.; Shen, S.-C.; Juan, S.-H.; Ko, C.-H.; Wang, L.-M.; Chen, Y.-C. Quercetin Inhibition of Tumor Invasion via Suppressing PKCδ/ERK/AP-1-Dependent Matrix Metalloproteinase-9 Activation in Breast Carcinoma Cells. Carcinogenesis 2008, 29, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Song, Y.; Zhang, X. Quercetin Suppresses the Migration and Invasion in Human Colon Cancer Caco-2 Cells through Regulating Toll-Like Receptor 4/Nuclear Factor-Kappa B Pathway. Pharmacogn. Mag. 2016, 12, S237–S244. [Google Scholar] [PubMed] [Green Version]
- Kim, S.-A.; Kim, Y.-C.; Kim, S.-W.; Lee, S.-H.; Min, J.-J.; Ahn, S.-G.; Yoon, J.-H. Antitumor activity of novel indirubin derivatives in rat tumor model. Clin. Cancer Res. 2007, 13, 253–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trinh, N.-T.; Nguyen, T.M.N.; Yook, J.-I.; Ahn, S.-G.; Kim, S.-A. Quercetin and Quercitrin from Agrimonia pilosa Ledeb Inhibit the Migration and Invasion of Colon Cancer Cells through the JNK Signaling Pathway. Pharmaceuticals 2022, 15, 364. https://doi.org/10.3390/ph15030364
Trinh N-T, Nguyen TMN, Yook J-I, Ahn S-G, Kim S-A. Quercetin and Quercitrin from Agrimonia pilosa Ledeb Inhibit the Migration and Invasion of Colon Cancer Cells through the JNK Signaling Pathway. Pharmaceuticals. 2022; 15(3):364. https://doi.org/10.3390/ph15030364
Chicago/Turabian StyleTrinh, Nguyet-Tran, Thi Minh Ngoc Nguyen, Jong-In Yook, Sang-Gun Ahn, and Soo-A Kim. 2022. "Quercetin and Quercitrin from Agrimonia pilosa Ledeb Inhibit the Migration and Invasion of Colon Cancer Cells through the JNK Signaling Pathway" Pharmaceuticals 15, no. 3: 364. https://doi.org/10.3390/ph15030364
APA StyleTrinh, N.-T., Nguyen, T. M. N., Yook, J.-I., Ahn, S.-G., & Kim, S.-A. (2022). Quercetin and Quercitrin from Agrimonia pilosa Ledeb Inhibit the Migration and Invasion of Colon Cancer Cells through the JNK Signaling Pathway. Pharmaceuticals, 15(3), 364. https://doi.org/10.3390/ph15030364






