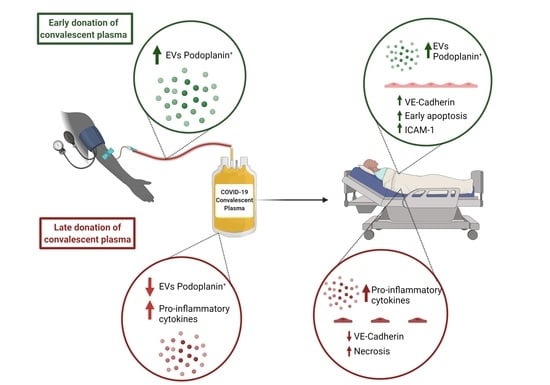
Use of Early Donated COVID-19 Convalescent Plasma Is Optimal to Preserve the Integrity of Lymphatic Endothelial Cells
Abstract
:1. Introduction
2. Results
2.1. Characteristics of COVID-19 Convalescent Plasma Donors
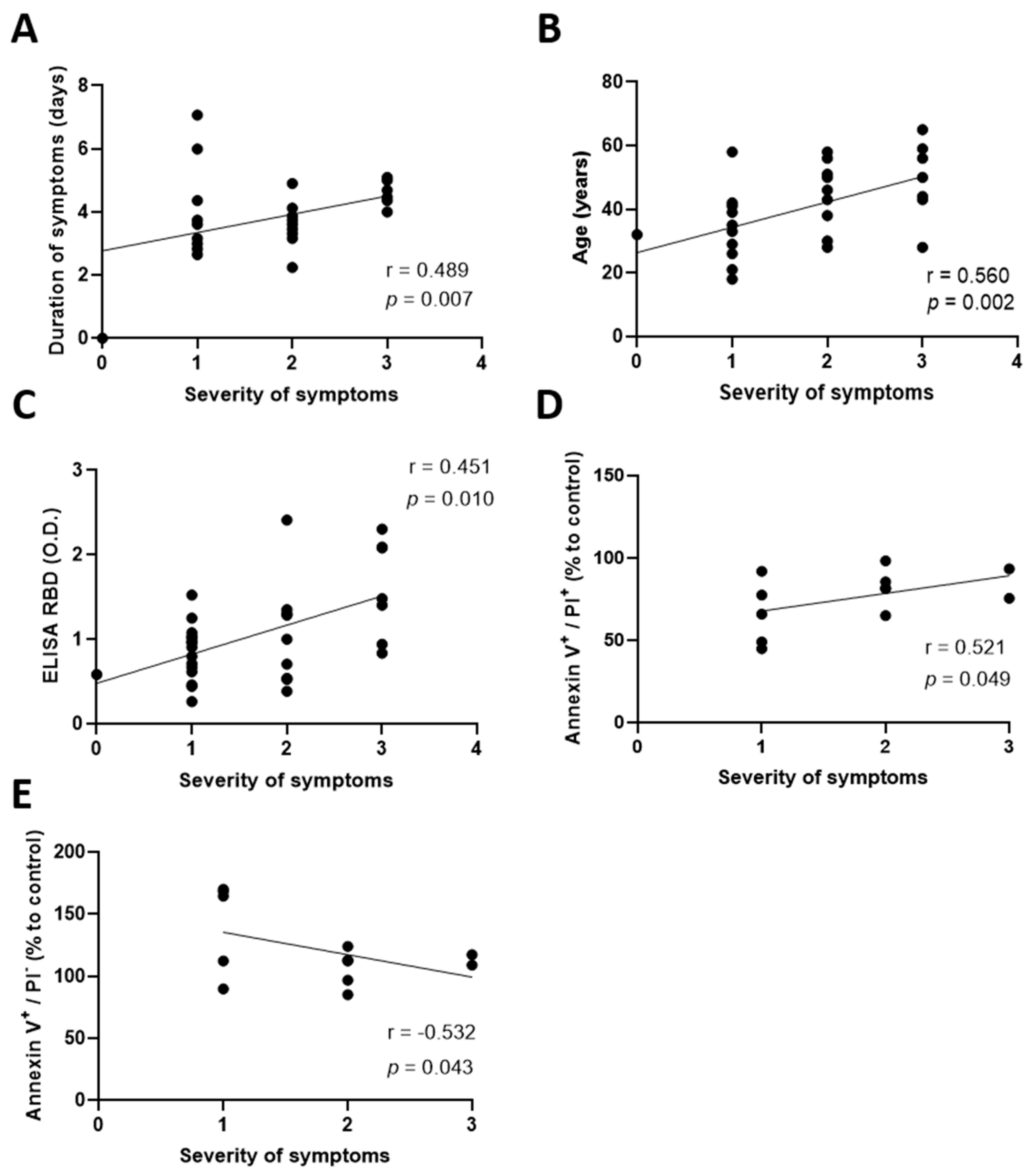
2.2. Elevated Antibody Concentrations and Prolonged Symptoms Are Detrimental for the Lymphatic Endothelium Integrity
2.3. COVID-19 Convalescent Plasma from Patients Experiencing Severe Symptoms Induces Cellular Necrosis
2.4. An Early Donation Could Help Maintain the Integrity of the Lymphatic Endothelium
2.5. Late Donations of COVID-19 Convalescent Plasma Contain Elevated Pro-Inflammatory Cytokines Levels
2.6. Extracellular Vesicles Derived from Human Lymphatic Endothelial Cells Are More Abundant in Early Donated Plasma
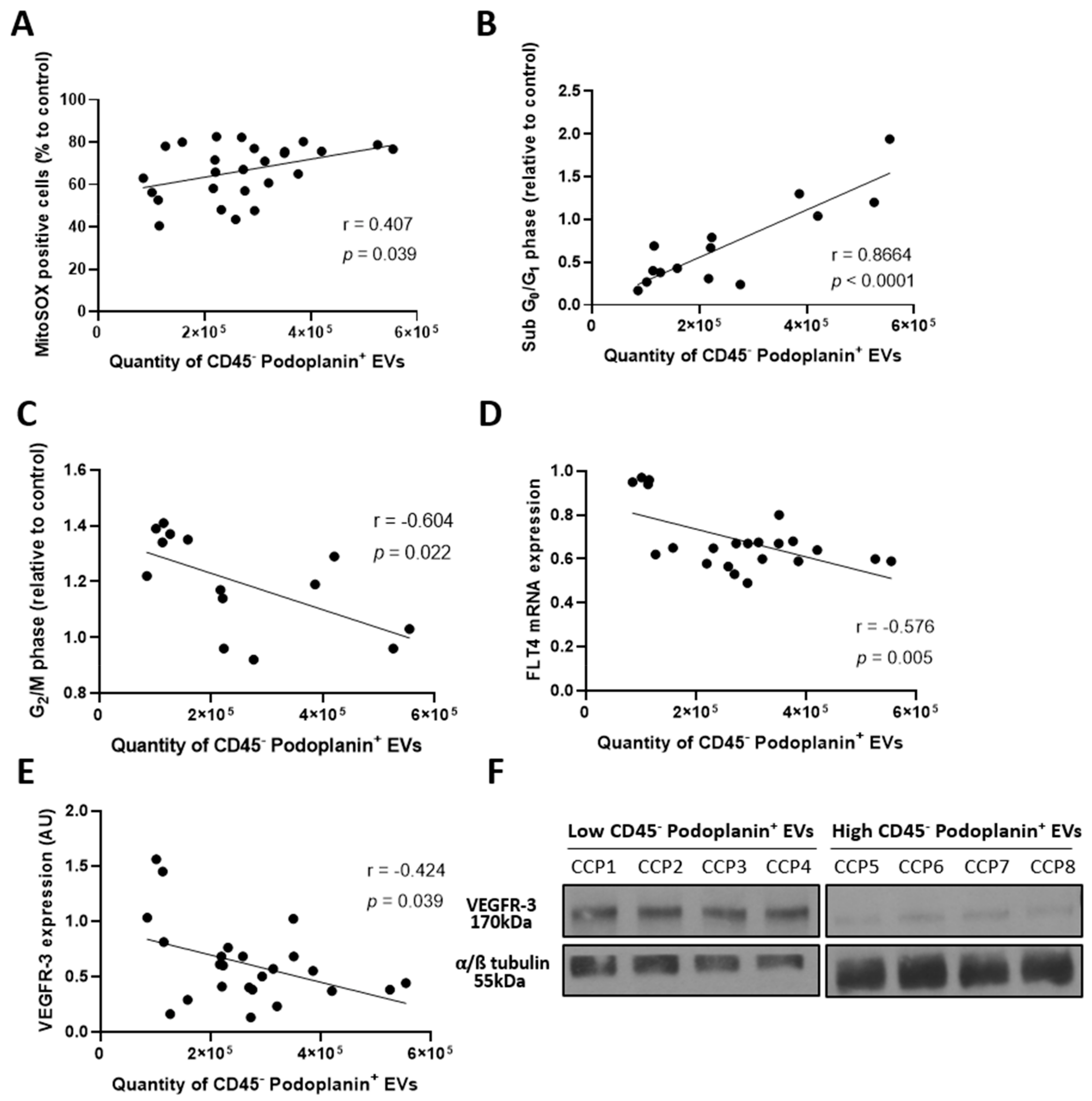
2.7. Elevated Plasma Extracellular Vesicles Derived from Human Lymphatic Endothelial Cells Correlates with Enhanced Lymphatic Endothelium Integrity
2.8. Secretion of Extracellular Vesicles Derived from Human Lymphatic Endothelial Cells Reflects an Alteration of the Lymphatic Endothelium
3. Discussion
4. Materials and Methods
4.1. Collection of COVID-19 Convalescent Plasma
4.2. Cell Culture
4.3. Production of Reactive Oxygen Species
4.4. Measurement of Cell Viability
4.5. Assessment of Cell-Cycle Distribution
4.6. Quantification of Receptor-Binding-Domain Antibodies
4.7. Quantification of Plasma Cytokines
4.8. Analyses of Extracellular Vesicles in Plasma and Lymphatic Endothelial Cell Supernatant
4.9. Total Cholesterol
4.10. Messenger RNA Analysis by RT-qPCR
4.11. Immunofluorescence
4.12. Transwell Permeability Assay
4.13. Immunoblotting
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World Health Organization Coronavirus Disease (COVID-19) Dashboard; World Health Organization: Geneve, Switzerland, 2020. [Google Scholar]
- Del Rio, C.; Collins, L.F.; Malani, P. Long-term Health Consequences of COVID-19. JAMA 2020, 324, 1723–1724. [Google Scholar] [CrossRef] [PubMed]
- Amraei, R.; Rahimi, N. COVID-19, Renin-Angiotensin System and Endothelial Dysfunction. Cells 2020, 9, 1652. [Google Scholar] [CrossRef] [PubMed]
- Rauch, A.; Dupont, A.; Goutay, J.; Caplan, M.; Staessens, S.; Moussa, M.; Jeanpierre, E.; Corseaux, D.; Lefevre, G.; Lassalle, F.; et al. Endotheliopathy is induced by plasma from critically-ill patients and associated with organ failure in severe COVID-19. Circulation 2020, 142, 1881–1884. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Chioh, F.W.J.; Fong, S.-W.; Young, B.E.; Wu, K.-X.; Siau, A.; Krishnan, S.; Chan, Y.-H.; Carissimo, G.; Teo, L.L.Y.; Gao, F.; et al. Convalescent COVID-19 patients are susceptible to endothelial dysfunction due to persistent immune activation. eLife 2021, 10, e64909. [Google Scholar] [CrossRef] [PubMed]
- Joyner, M.J.; Carter, R.E.; Senefeld, J.W.; Klassen, S.A.; Mills, J.R.; Johnson, P.W.; Theel, E.S.; Wiggins, C.C.; Bruno, K.A.; Klompas, A.M.; et al. Convalescent Plasma Antibody Levels and the Risk of Death from Covid-19. N. Engl. J. Med. 2021, 384, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- RECOVERY Collaborative Group. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): A randomised controlled, open-label, platform trial. Lancet 2021, 397, 2049–2059. [Google Scholar] [CrossRef]
- Writing Committee for the REMAP-CAP Investigators. Effect of Convalescent Plasma on Organ Support–Free Days in Critically Ill Patients with COVID-19: A Randomized Clinical Trial. JAMA 2021, 326, 1690–1702. [Google Scholar] [CrossRef]
- Bégin, P.; Callum, J.; Jamula, E.; Cook, R.; Heddle, N.M.; Tinmouth, A.; Zeller, M.P.; Beaudoin-Bussières, G.; Amorim, L.; Bazin, R.; et al. Convalescent plasma for hospitalized patients with COVID-19: An open-label, randomized controlled trial. Nat. Med. 2021, 27, 2012–2024. [Google Scholar] [CrossRef]
- Piechotta, V.; Iannizzi, C.; Chai, K.L.; Valk, S.J.; Kimber, C.; Dorando, E.; Monsef, I.; Wood, E.M.; Lamikanra, A.A.; Roberts, D.J.; et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: A living systematic review. Cochrane Database Syst. Rev. 2021, 5, Cd013600. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.J.; Gebo, K.A.; Shoham, S.; Bloch, E.M.; Lau, B.; Shenoy, A.G.; Mosnaim, G.S.; Gniadek, T.J.; Fukuta, Y.; Patel, B.; et al. Randomized Controlled Trial of Early Outpatient COVID-19 Treatment with High-Titer Convalescent Plasma. medRxiv 2021, 21267485. [Google Scholar] [CrossRef]
- Milasan, A.; Smaani, A.; Martel, C. Early rescue of lymphatic function limits atherosclerosis progression in Ldlr mice. Atherosclerosis 2019, 283, 106–119. [Google Scholar] [CrossRef] [Green Version]
- Tammela, T.; Alitalo, K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell 2010, 140, 460–476. [Google Scholar] [CrossRef] [Green Version]
- Schwager, S.; Detmar, M. Inflammation and Lymphatic Function. Front. Immunol. 2019, 10, 308. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Garner, R.; Salehi, S.; La Rocca, M.; Duncan, D. Association between ABO blood types and coronavirus disease 2019 (COVID-19), genetic associations, and underlying molecular mechanisms: A literature review of 23 studies. Ann. Hematol. 2021, 100, 1123–1132. [Google Scholar] [CrossRef]
- Hariyanto, T.I.; Kurniawan, A. Dyslipidemia is associated with severe coronavirus disease 2019 (COVID-19) infection. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1463–1465. [Google Scholar] [CrossRef]
- De Candia, P.; Prattichizzo, F.; Garavelli, S.; La Grotta, R.; De Rosa, A.; Pontarelli, A.; Parrella, R.; Ceriello, A.; Matarese, G. Effect of time and titer in convalescent plasma therapy for COVID-19. iScience 2021, 24, 102898. [Google Scholar] [CrossRef]
- Figueiredo-Campos, P.; Blankenhaus, B.; Mota, C.; Gomes, A.; Serrano, M.; Ariotti, S.; Costa, C.; Nunes-Cabaço, H.; Mendes, A.M.; Gaspar, P.; et al. Seroprevalence of anti-SARS-CoV-2 antibodies in COVID-19 patients and healthy volunteers up to 6 months post disease onset. Eur. J. Immunol. 2020, 50, 2025–2040. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Laforge, M.; Elbim, C.; Frère, C.; Hémadi, M.; Massaad, C.; Nuss, P.; Benoliel, J.-J.; Becker, C. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immunol. 2020, 20, 515–516. [Google Scholar] [CrossRef]
- Casadevall, A.; Joyner, M.J.; Pirofski, L.A. SARS-CoV-2 viral load and antibody responses: The case for convalescent plasma therapy. J. Clin. Investig. 2020, 130, 5112–5114. [Google Scholar] [CrossRef]
- Gallo Marin, B.; Aghagoli, G.; Lavine, K.; Yang, L.; Siff, E.J.; Chiang, S.S.; Salazar-Mather, T.P.; Dumenco, L.; Savaria, M.C.; Aung, S.N.; et al. Predictors of COVID-19 severity: A literature review. Rev. Med. Virol. 2021, 31, e2146. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Sang, L.; Ye, F.; Ruan, S.; Zhong, B.; Song, T.; Alshukairi, A.N.; Chen, R.; Zhang, Z.; et al. Kinetics of viral load and antibody response in relation to COVID-19 severity. J. Clin. Investig. 2020, 130, 5235–5244. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; Lam, E.C.; Astudillo, M.G.; Yang, D.; Miller, T.E.; Feldman, J.; Hauser, B.M.; Caradonna, T.M.; Clayton, K.L.; Nitido, A.D.; et al. COVID-19-neutralizing antibodies predict disease severity and survival. Cell 2021, 184, 476–488.e411. [Google Scholar] [CrossRef]
- Beaudoin-Bussières, G.; Laumaea, A.; Anand, S.P.; Prévost, J.; Gasser, R.; Goyette, G.; Medjahed, H.; Perreault, J.; Tremblay, T.; Lewin, A.; et al. Decline of Humoral Responses against SARS-CoV-2 Spike in Convalescent Individuals. mBio 2020, 11, e02590-20. [Google Scholar] [CrossRef]
- Anand, S.P.; Prévost, J.; Nayrac, M.; Beaudoin-Bussières, G.; Benlarbi, M.; Gasser, R.; Brassard, N.; Laumaea, A.; Gong, S.Y.; Bourassa, C.; et al. Longitudinal analysis of humoral immunity against SARS-CoV-2 Spike in convalescent individuals up to 8 months post-symptom onset. Cell Rep. Med. 2021, 2, 100290. [Google Scholar] [CrossRef] [PubMed]
- Huggenberger, R.; Ullmann, S.; Proulx, S.T.; Pytowski, B.; Alitalo, K.; Detmar, M. Stimulation of lymphangiogenesis via VEGFR-3 inhibits chronic skin inflammation. J. Exp. Med. 2010, 207, 2255–2269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kajiya, K.; Sawane, M.; Huggenberger, R.; Detmar, M. Activation of the VEGFR-3 Pathway by VEGF-C Attenuates UVB-Induced Edema Formation and Skin Inflammation by Promoting Lymphangiogenesis. J. Investig. Dermatol. 2009, 129, 1292–1298. [Google Scholar] [CrossRef] [Green Version]
- Milasan, A.; Farhat, M.; Martel, C. Extracellular Vesicles as Potential Prognostic Markers of Lymphatic Dysfunction. Front. Physiol. 2020, 11, 476. [Google Scholar] [CrossRef] [PubMed]
- Milasan, A.; Tessandier, N.; Tan, S.; Brisson, A.; Boilard, E.; Martel, C. Extracellular vesicles are present in mouse lymph and their level differs in atherosclerosis. J. Extracell. Vesicles 2016, 5, 31427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsiao, C.-J.; Hsiao, G.; Chen, W.-L.; Wang, S.-W.; Chiang, C.-P.; Liu, L.-Y.; Guh, J.-H.; Lee, T.-H.; Chung, C.-L. Cephalochromin Induces G0/G1 Cell Cycle Arrest and Apoptosis in A549 Human Non-Small-Cell Lung Cancer Cells by Inflicting Mitochondrial Disruption. J. Nat. Prod. 2014, 77, 758–765. [Google Scholar] [CrossRef]
- Ohi, R.; Gould, K.L. Regulating the onset of mitosis. Curr. Opin. Cell Biol. 1999, 11, 267–273. [Google Scholar] [CrossRef]
- Huggenberger, R.; Siddiqui, S.S.; Brander, D.; Ullmann, S.; Zimmermann, K.; Antsiferova, M.; Werner, S.; Alitalo, K.; Detmar, M. An important role of lymphatic vessel activation in limiting acute inflammation. Blood 2011, 117, 4667–4678. [Google Scholar] [CrossRef] [Green Version]
- Jurisic, G.; Sundberg, J.P.; Detmar, M. Blockade of VEGF receptor-3 aggravates inflammatory bowel disease and lymphatic vessel enlargement. Inflamm. Bowel. Dis. 2013, 19, 1983–1989. [Google Scholar] [CrossRef] [Green Version]
- Reed, H.O.; Wang, L.; Sonett, J.; Chen, M.; Yang, J.; Li, L.; Aradi, P.; Jakus, Z.; D’Armiento, J.; Hancock, W.W.; et al. Lymphatic impairment leads to pulmonary tertiary lymphoid organ formation and alveolar damage. J. Clin. Investig. 2019, 129, 2514–2526. [Google Scholar] [CrossRef]
- Cheng, Y.; Wong, R.; Soo, Y.O.; Wong, W.S.; Lee, C.K.; Ng, M.H.; Chan, P.; Wong, K.C.; Leung, C.B.; Cheng, G. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur. J. Clin. Microbiol. Infect. Dis. 2005, 24, 44–46. [Google Scholar] [CrossRef]
- Zhang, J.S.; Chen, J.T.; Liu, Y.X.; Zhang, Z.S.; Gao, H.; Liu, Y.; Wang, X.; Ning, Y.; Liu, Y.F.; Gao, Q.; et al. A serological survey on neutralizing antibody titer of SARS convalescent sera. J. Med. Virol. 2005, 77, 147–150. [Google Scholar] [CrossRef]
- Van Griensven, J.; Edwards, T.; de Lamballerie, X.; Semple, M.G.; Gallian, P.; Baize, S.; Horby, P.W.; Raoul, H.; Magassouba, N.; Antierens, A.; et al. Evaluation of Convalescent Plasma for Ebola Virus Disease in Guinea. N. Engl. J. Med. 2016, 374, 33–42. [Google Scholar] [CrossRef]
- Frame, J.D.; Verbrugge, G.P.; Gill, R.G.; Pinneo, L. The use of Lassa fever convalescent plasma in Nigeria. Trans. R. Soc. Trop. Med. Hyg. 1984, 78, 319–324. [Google Scholar] [CrossRef]
- Russell, P.S.; Hong, J.; Windsor, J.A.; Itkin, M.; Phillips, A.R.J. Renal Lymphatics: Anatomy, Physiology, and Clinical Implications. Front. Physiol. 2019, 10, 251. [Google Scholar] [CrossRef]
- Estaquier, J.; Vallette, F.; Vayssiere, J.L.; Mignotte, B. The mitochondrial pathways of apoptosis. Adv. Exp. Med. Biol. 2012, 942, 157–183. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, G.; Zhang, P.; Fan, J. Programmed cell death and its role in inflammation. Mil. Med. Res. 2015, 2, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libster, R.; Pérez Marc, G.; Wappner, D.; Coviello, S.; Bianchi, A.; Braem, V.; Esteban, I.; Caballero, M.T.; Wood, C.; Berrueta, M.; et al. Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults. N. Engl. J. Med. 2021, 384, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Korley, F.K.; Durkalski-Mauldin, V.; Yeatts, S.D.; Schulman, K.; Davenport, R.D.; Dumont, L.J.; El Kassar, N.; Foster, L.D.; Hah, J.M.; Jaiswal, S.; et al. Early Convalescent Plasma for High-Risk Outpatients with Covid-19. N. Engl. J. Med. 2021, 385, 1951–1960. [Google Scholar] [CrossRef] [PubMed]
- Teijeira, A.; Hunter, M.C.; Russo, E.; Proulx, S.T.; Frei, T.; Debes, G.F.; Coles, M.; Melero, I.; Detmar, M.; Rouzaut, A.; et al. T Cell Migration from Inflamed Skin to Draining Lymph Nodes Requires Intralymphatic Crawling Supported by ICAM-1/LFA-1 Interactions. Cell Rep. 2017, 18, 857–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cromer, W.E.; Zawieja, S.D.; Tharakan, B.; Childs, E.W.; Newell, M.K.; Zawieja, D.C. The effects of inflammatory cytokines on lymphatic endothelial barrier function. Angiogenesis 2014, 17, 395–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jannaway, M.; Scallan, J.P. VE-Cadherin and Vesicles Differentially Regulate Lymphatic Vascular Permeability to Solutes of Various Sizes. Front. Physiol. 2021, 12, 687563. [Google Scholar] [CrossRef]
- Wirz, O.F.; Röltgen, K.; Stevens, B.A.; Pandey, S.; Sahoo, M.K.; Tolentino, L.; Verghese, M.; Nguyen, K.; Hunter, M.; Snow, T.T.; et al. Use of Outpatient-Derived COVID-19 Convalescent Plasma in COVID-19 Patients Before Seroconversion. Front. Immunol. 2021, 12, 739037. [Google Scholar] [CrossRef]
- Bonny, T.S.; Patel, E.U.; Zhu, X.; Bloch, E.M.; Grabowski, M.K.; Abraham, A.G.; Littlefield, K.; Shrestha, R.; Benner, S.E.; Laeyendecker, O.; et al. Cytokine and Chemokine Levels in Coronavirus Disease 2019 Convalescent Plasma. Open Forum. Infect. Dis. 2021, 8, ofaa574. [Google Scholar] [CrossRef]
- Barry, O.P.; Praticò, D.; Savani, R.C.; FitzGerald, G.A. Modulation of monocyte-endothelial cell interactions by platelet microparticles. J. Clin. Investig. 1998, 102, 136–144. [Google Scholar] [CrossRef]
- Rosell, A.; Havervall, S.; Meijenfeldt, F.V.; Hisada, Y.; Aguilera, K.; Grover, S.P.; Lisman, T.; Mackman, N.; Thålin, C. Patients with COVID-19 Have Elevated Levels of Circulating Extracellular Vesicle Tissue Factor Activity That Is Associated with Severity and Mortality—Brief Report. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 878–882. [Google Scholar] [CrossRef]
- Krishnamachary, B.; Cook, C.; Kumar, A.; Spikes, L.; Chalise, P.; Dhillon, N.K. Extracellular vesicle-mediated endothelial apoptosis and EV-associated proteins correlate with COVID-19 disease severity. J. Extracell. Vesicles 2021, 10, e12117. [Google Scholar] [CrossRef]
- Troyer, Z.; Alhusaini, N.; Tabler, C.O.; Sweet, T.; de Carvalho, K.I.L.; Schlatzer, D.M.; Carias, L.; King, C.L.; Matreyek, K.; Tilton, J.C. Extracellular vesicles carry SARS-CoV-2 spike protein and serve as decoys for neutralizing antibodies. J. Extracell. Vesicles 2021, 10, e12112. [Google Scholar] [CrossRef]
- Askenase, P.W. COVID-19 therapy with mesenchymal stromal cells (MSC) and convalescent plasma must consider exosome involvement: Do the exosomes in convalescent plasma antagonize the weak immune antibodies? J. Extracell. Vesicles 2020, 10, e12004. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [Green Version]
- Simeone, P.; Bologna, G.; Lanuti, P.; Pierdomenico, L.; Guagnano, M.T.; Pieragostino, D.; Del Boccio, P.; Vergara, D.; Marchisio, M.; Miscia, S.; et al. Extracellular Vesicles as Signaling Mediators and Disease Biomarkers across Biological Barriers. Int. J. Mol. Sci. 2020, 21, 2514. [Google Scholar] [CrossRef] [Green Version]
- Breslin, J.W.; Gaudreault, N.; Watson, K.D.; Reynoso, R.; Yuan, S.Y.; Wu, M.H. Vascular endothelial growth factor-C stimulates the lymphatic pump by a VEGF receptor-3-dependent mechanism. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H709–H718. [Google Scholar] [CrossRef] [Green Version]
- Riccardi, C.; Nicoletti, I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat. Protoc. 2006, 1, 1458–1461. [Google Scholar] [CrossRef]
- Perreault, J.; Tremblay, T.; Fournier, M.-J.; Drouin, M.; Beaudoin-Bussières, G.; Prévost, J.; Lewin, A.; Bégin, P.; Finzi, A.; Bazin, R. Waning of SARS-CoV-2 RBD antibodies in longitudinal convalescent plasma samples within 4 months after symptom onset. Blood 2020, 136, 2588–2591. [Google Scholar] [CrossRef]
- Morales-Kastresana, A.; Telford, B.; Musich, T.A.; McKinnon, K.; Clayborne, C.; Braig, Z.; Rosner, A.; Demberg, T.; Watson, D.C.; Karpova, T.S.; et al. Labeling Extracellular Vesicles for Nanoscale Flow Cytometry. Sci. Rep. 2017, 7, 1878. [Google Scholar] [CrossRef] [PubMed]
- Vachon, L.; Smaani, A.; Tessier, N.; Jean, G.; Demers, A.; Milasan, A.; Ardo, N.; Jarry, S.; Villeneuve, L.; Alikashani, A.; et al. Downregulation of low-density lipoprotein receptor mRNA in lymphatic endothelial cells impairs lymphatic function through changes in intracellular lipids. Theranostics 2021, 12, 1440–1458. [Google Scholar] [CrossRef] [PubMed]
- Poncelet, P.; Robert, S.; Bailly, N.; Garnache-Ottou, F.; Bouriche, T.; Devalet, B.; Segatchian, J.H.; Saas, P.; Mullier, F. Tips and tricks for flow cytometry-based analysis and counting of microparticles. Transfus. Apher. Sci. 2015, 53, 110–126. [Google Scholar] [CrossRef] [PubMed]
- Poncelet, P.; Robert, S.; Bouriche, T.; Bez, J.; Lacroix, R.; Dignat-George, F. Standardized counting of circulating platelet microparticles using currently available flow cytometers and scatter-based triggering: Forward or side scatter? Cytom. A 2016, 89, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Beuthan, J.; Minet, O.; Helfmann, J.; Herrig, M.; Muller, G. The spatial variation of the refractive index in biological cells. Phys. Med. Biol. 1996, 41, 369–382. [Google Scholar] [CrossRef] [PubMed]




| Variables | |
|---|---|
| Age, years | 41 ± 14 |
| Female sex, n (%) | 17 (38%) |
| Duration of symptoms, days (IQR) | 14 (10–20) |
| Time of donation, days | 67 ± 25 |
| ABO Blood group, n (%) | |
| O | 18 (40) |
| A | 15 (33) |
| B | 5 (11) |
| AB | 7 (16) |
| Rhesus, n (%) | |
| Positive | 38 (84) |
| Negative | 7 (16) |
| Severity, n (%) | |
| Asymptomatic | 1 (2) |
| Mild | 12 (27) |
| Moderate | 9 (20) |
| Severe | 7 (16) |
| Loss of smell/taste, n (%) | |
| Yes | 20 (44) |
| No | 7 (16) |
| Unknown | 18 (40) |
| O.D RBD-antibody concentrations, AU (min, max) | 1.08 ± 0.54 (0.26, 2.41) |
| Total cholesterol, mg/dL | 188.5 ± 45.1 |
| CD235a+ (EVs/mL) | CD45+ (EVs/mL) | CD45− CLEC2+ (EVs/mL) | CD45− Podopanin+ (EVs/mL) | |||||
|---|---|---|---|---|---|---|---|---|
| Pearson (r) | p Value | Pearson (r) | p Value | Pearson (r) | p Value | Spearman (r) | p Value | |
| Early apoptosis | −0.026 | 0.899 | 0.464 | 0.017 * | 0.158 | 0.439 | 0.561 | 0.003 ** |
| Permeability | 0.019 | 0.954 | −0.596 | 0.041 * | −0.103 | 0.749 | −0.836 | 0.0007 *** |
| FLT4 mRNA | 0.322 | 0.144 | 0.078 | 0.729 | 0.292 | 0.187 | 0.616 | 0.002 ** |
| ICAM1 mRNA | 0.009 | 0.970 | 0.300 | 0.174 | −0.185 | 0.409 | 0.689 | 0.0004 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amri, N.; Bégin, R.; Tessier, N.; Vachon, L.; Villeneuve, L.; Bégin, P.; Bazin, R.; Loubaki, L.; Martel, C. Use of Early Donated COVID-19 Convalescent Plasma Is Optimal to Preserve the Integrity of Lymphatic Endothelial Cells. Pharmaceuticals 2022, 15, 365. https://doi.org/10.3390/ph15030365
Amri N, Bégin R, Tessier N, Vachon L, Villeneuve L, Bégin P, Bazin R, Loubaki L, Martel C. Use of Early Donated COVID-19 Convalescent Plasma Is Optimal to Preserve the Integrity of Lymphatic Endothelial Cells. Pharmaceuticals. 2022; 15(3):365. https://doi.org/10.3390/ph15030365
Chicago/Turabian StyleAmri, Nada, Rémi Bégin, Nolwenn Tessier, Laurent Vachon, Louis Villeneuve, Philippe Bégin, Renée Bazin, Lionel Loubaki, and Catherine Martel. 2022. "Use of Early Donated COVID-19 Convalescent Plasma Is Optimal to Preserve the Integrity of Lymphatic Endothelial Cells" Pharmaceuticals 15, no. 3: 365. https://doi.org/10.3390/ph15030365
APA StyleAmri, N., Bégin, R., Tessier, N., Vachon, L., Villeneuve, L., Bégin, P., Bazin, R., Loubaki, L., & Martel, C. (2022). Use of Early Donated COVID-19 Convalescent Plasma Is Optimal to Preserve the Integrity of Lymphatic Endothelial Cells. Pharmaceuticals, 15(3), 365. https://doi.org/10.3390/ph15030365