Tumor Necrosis Factor-α Mediates Lung Injury in the Early Phase of Endotoxemia
Abstract
1. Introduction
2. Results
2.1. Effects of KCF18 and SEM18 on Inhibiting Endotoxemia-Induced Cytokine Upregulations in Plasma
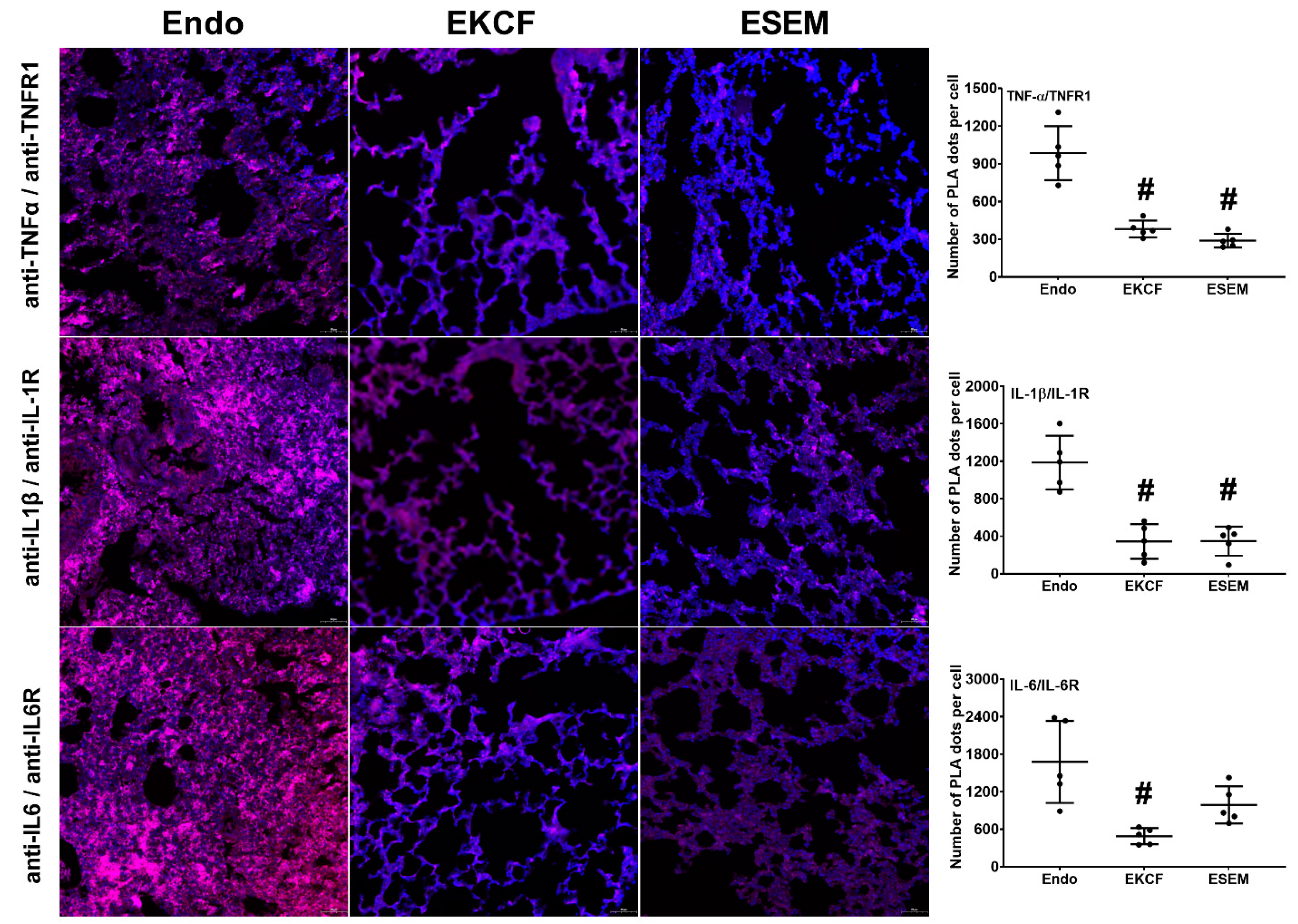
2.2. Effects of KCF18 and SEM18 on Inhibiting Endotoxemia-Induced Cytokine-Receptor Binding in Lungs
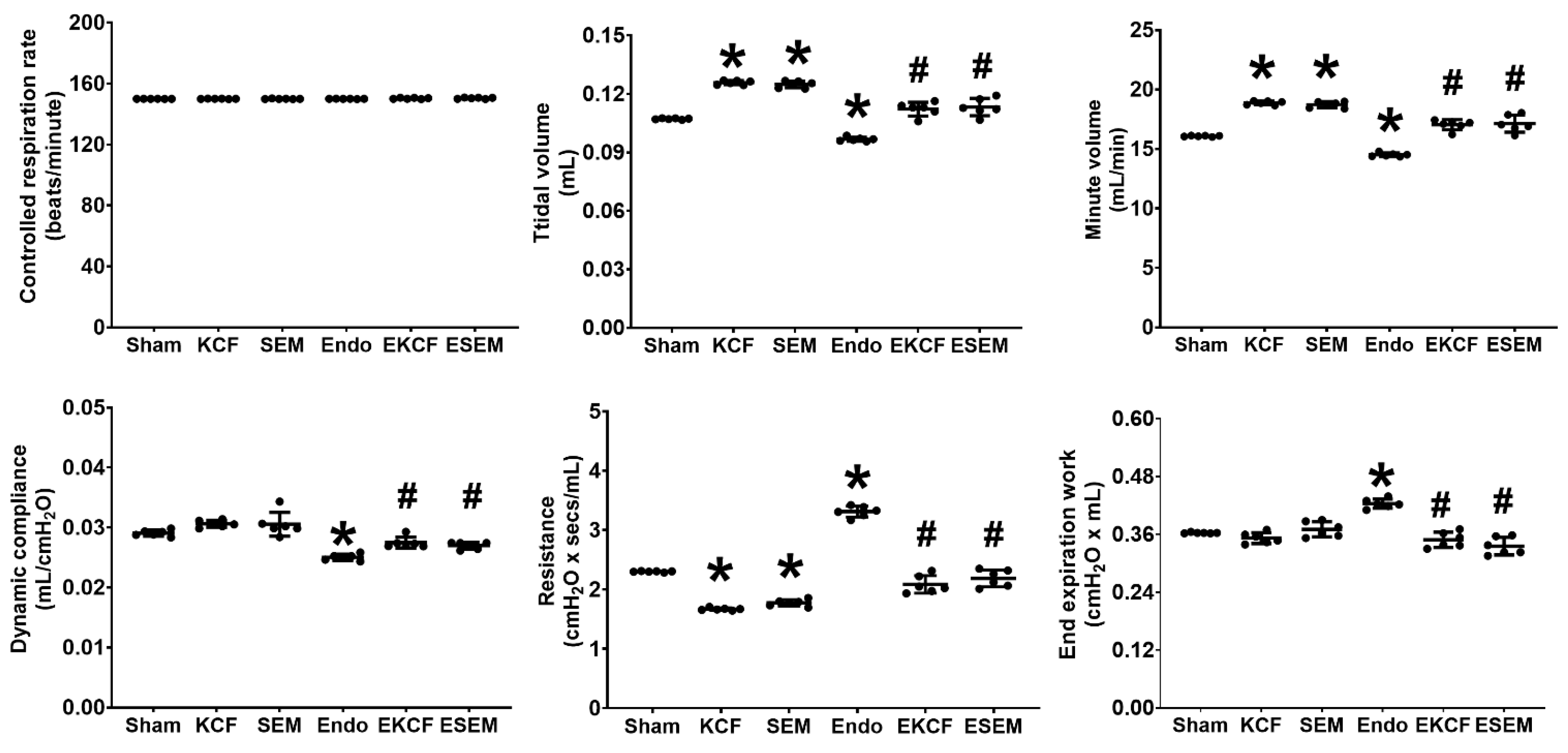
2.3. Effects of KCF18 and SEM18 on Alleviating Endotoxemia-Induced Lung Function Alterations
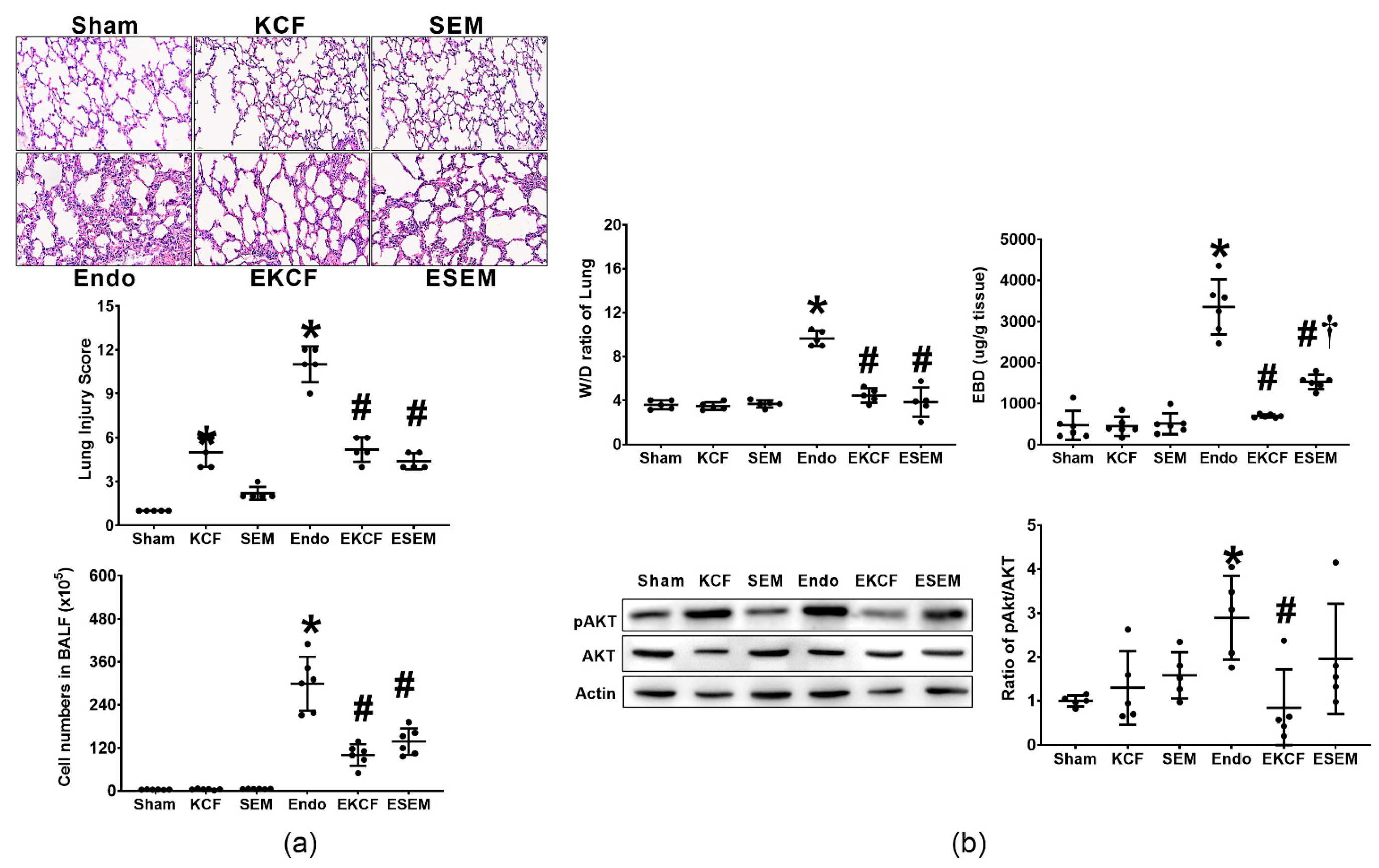
2.4. Effects of KCF18 and SEM18 on Alleviating Endotoxemia-Induced Lung Injury
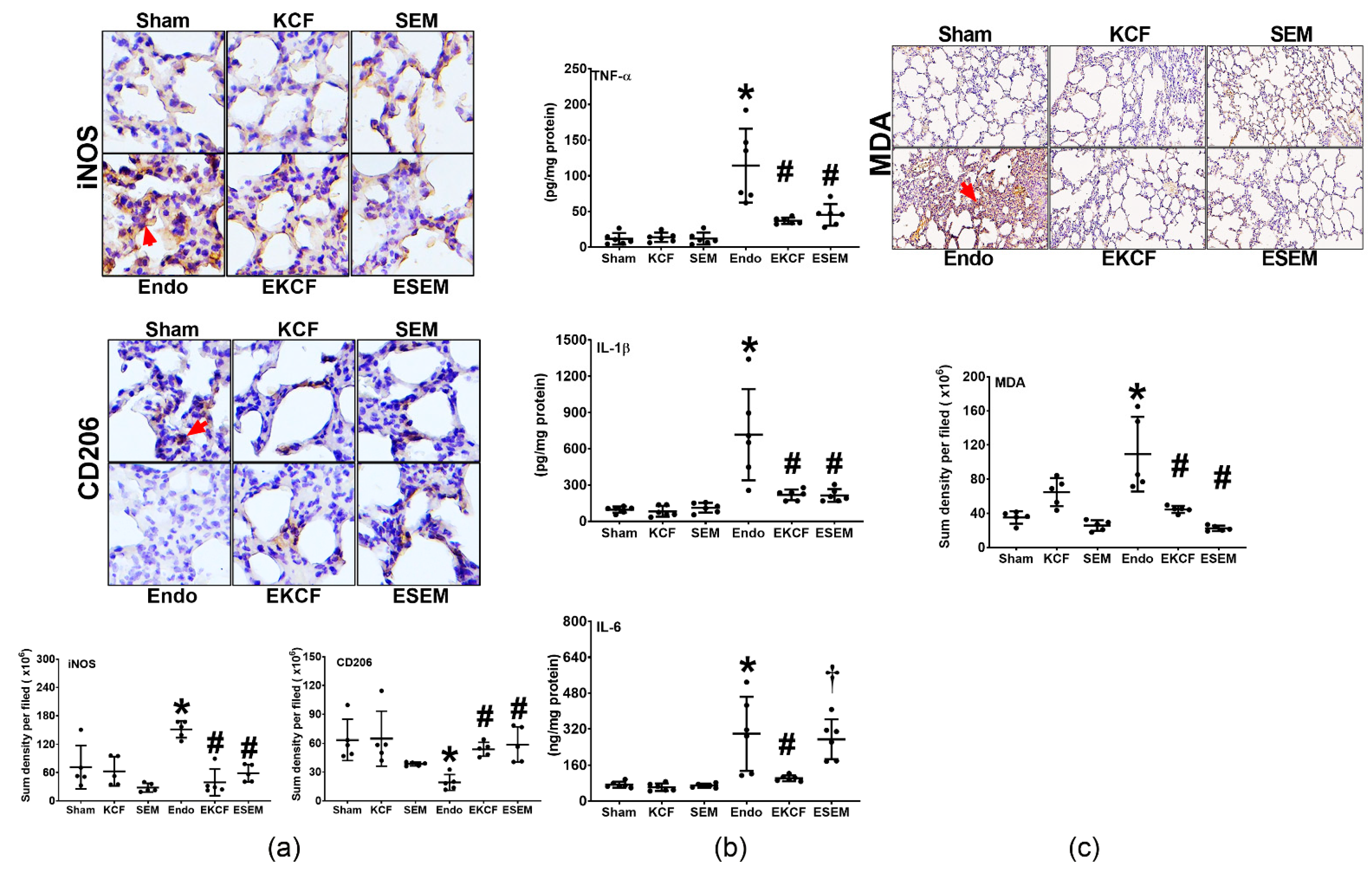
2.5. Effects of KCF18 and SEM18 on Alleviating Endotoxemia-Induced Lung Inflammation and Oxidation
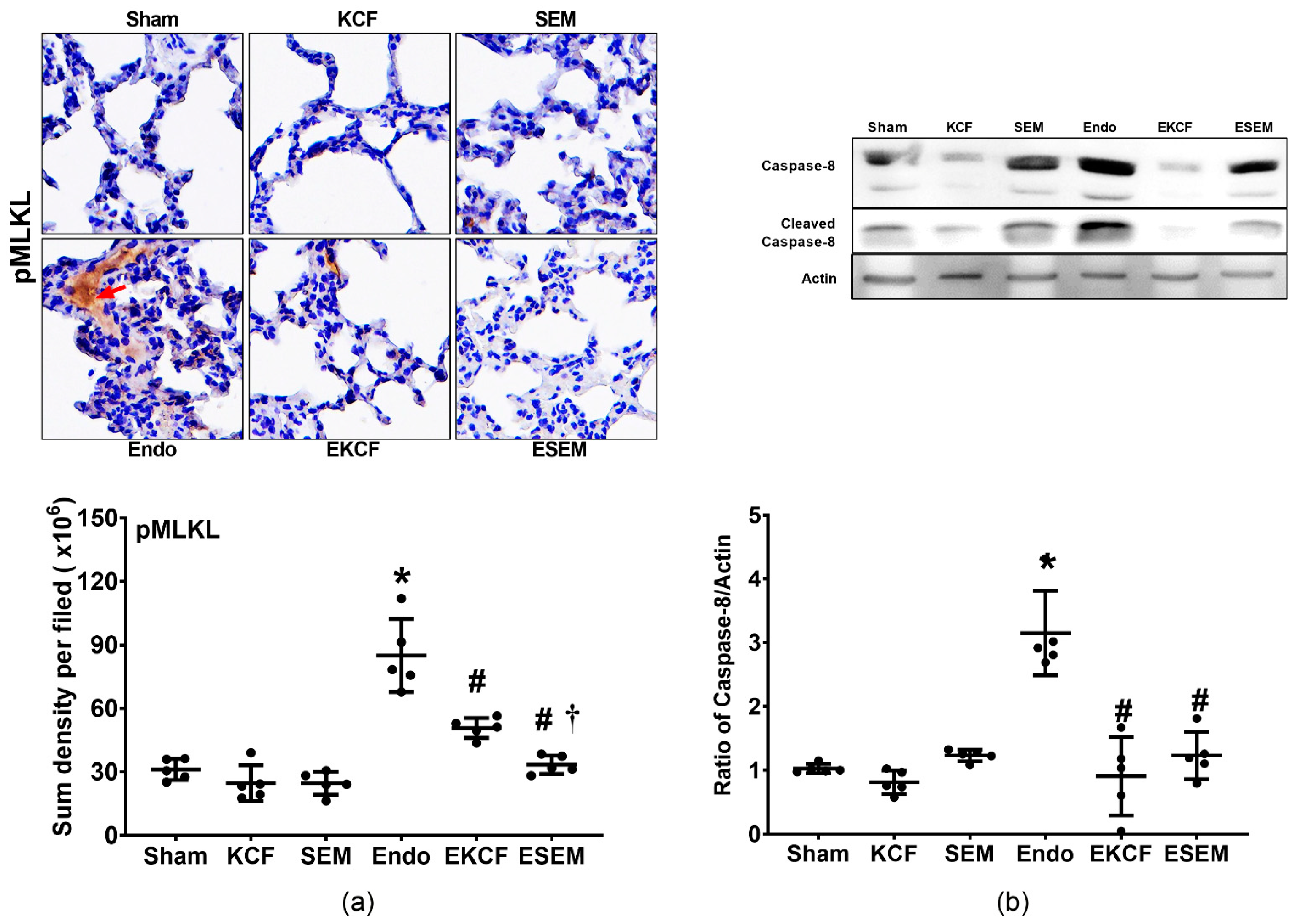
2.6. Effects of KCF18 and SEM18 on Alleviating Endotoxemia-Induced Lung Necroptosis
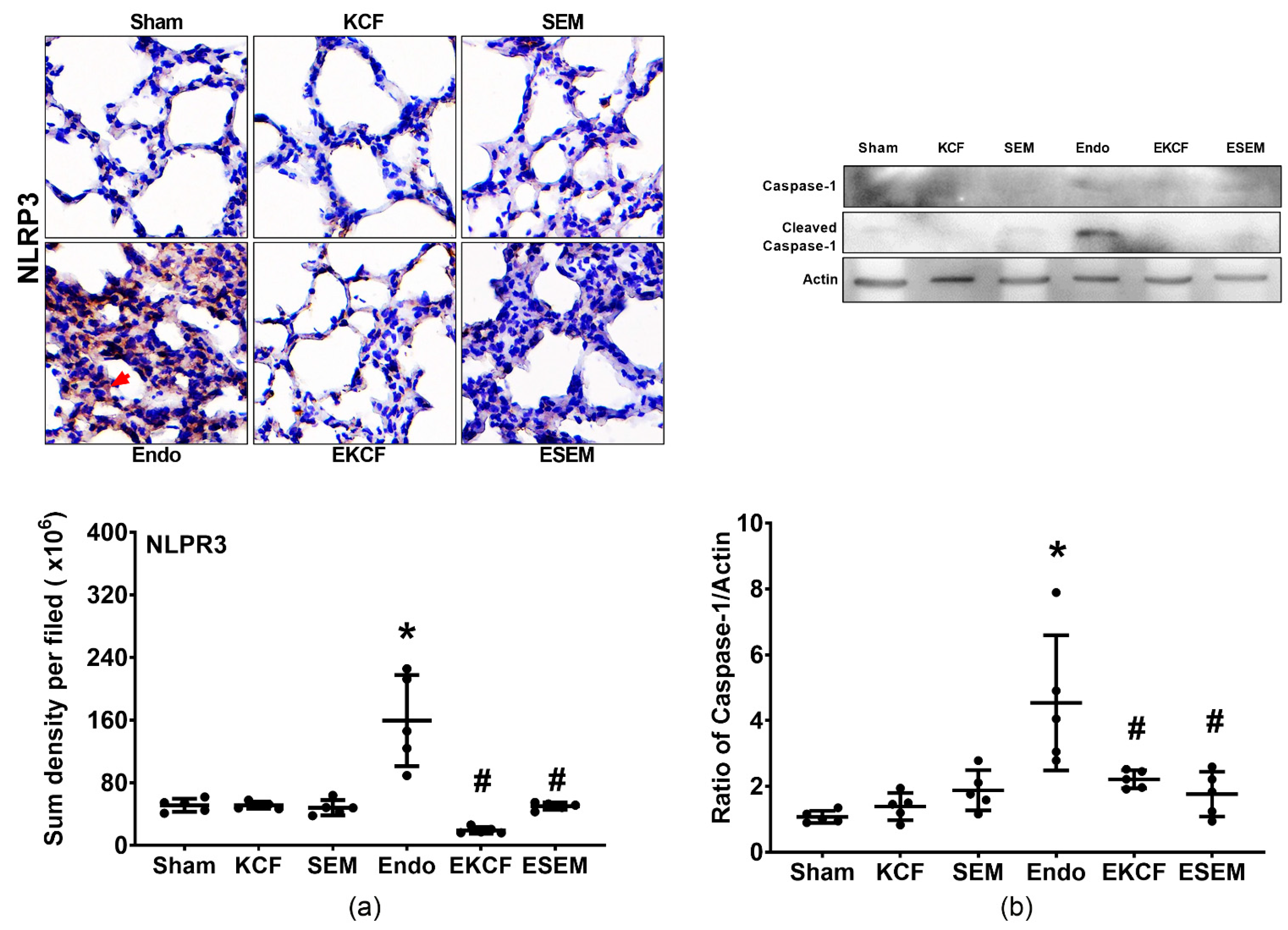
2.7. Effects of KCF18 and SEM18 on Alleviating Endotoxemia-Induced Lung Pyroptosis
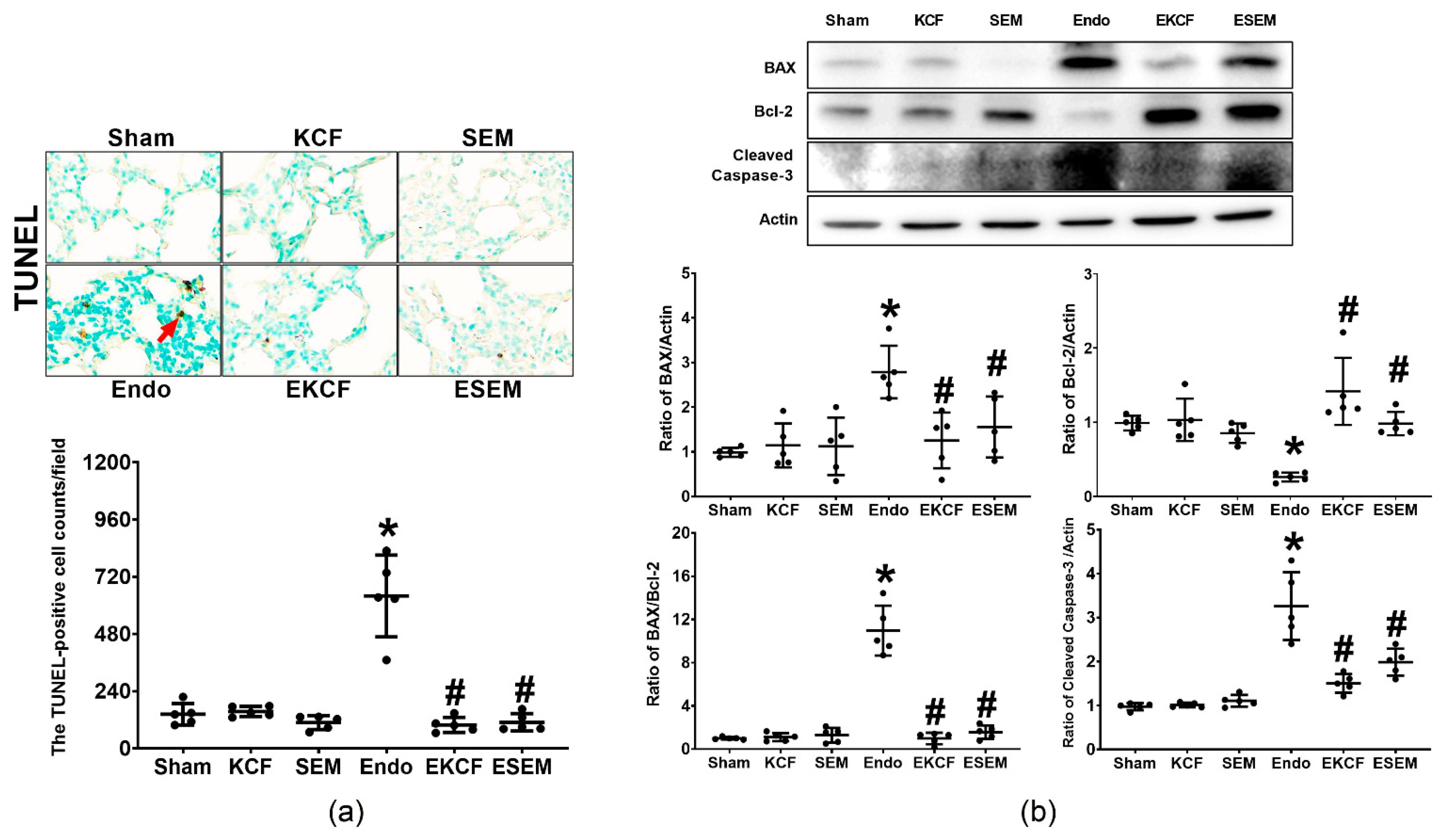
2.8. Effects of KCF18 and SEM18 on Alleviating Endotoxemia-Induced Lung Apoptosis
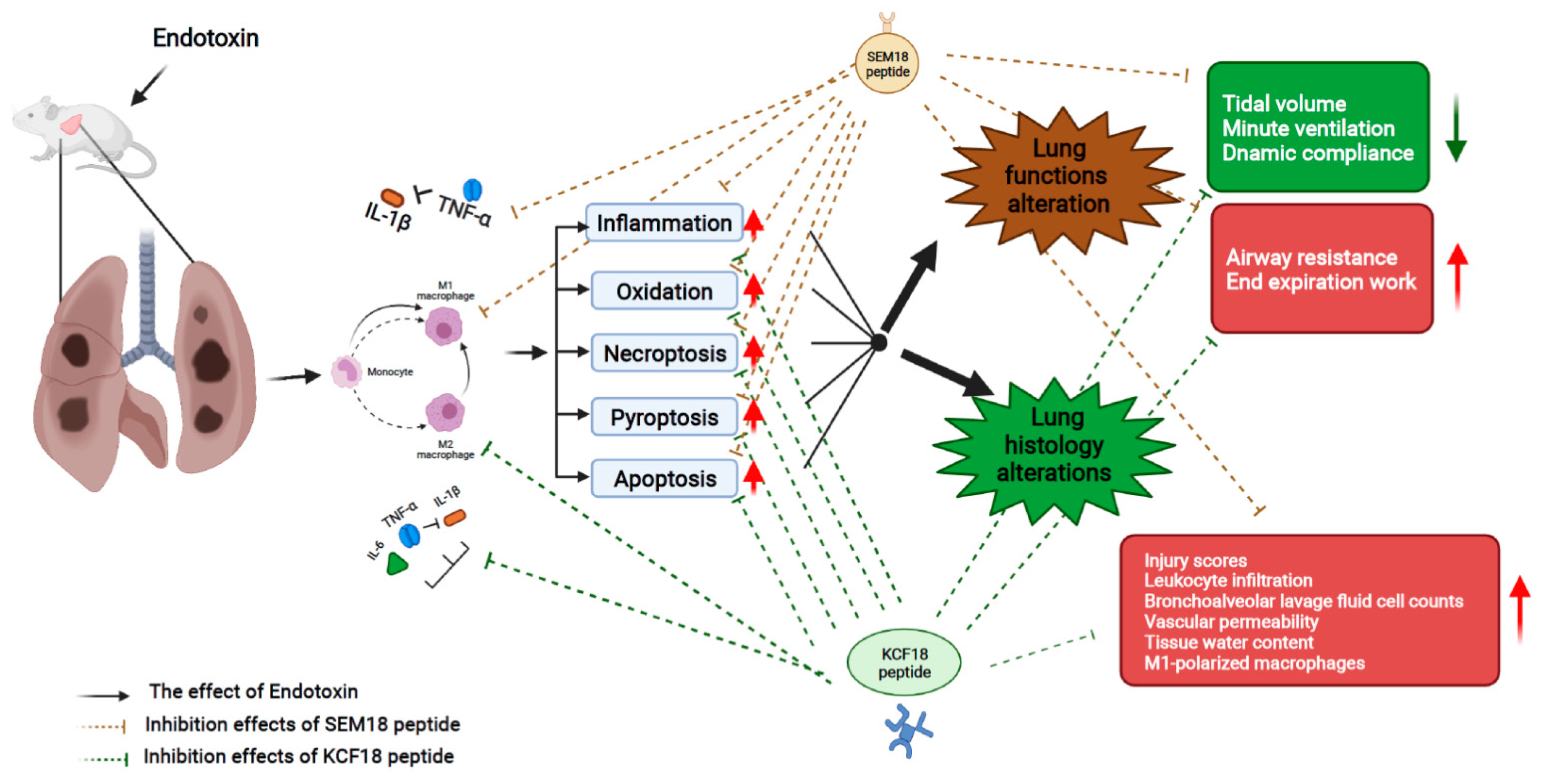
3. Discussion
4. Materials and Methods
4.1. Study Animals
4.2. Designs and Syntheses of Peptides
4.3. Monomicrobial Sepsis Model of Endotoxemia and Peptide Therapy
4.4. Lung function Assay
4.5. BALF Collection and Assay
4.6. Lung Tissue and Plasma Samples Harvesting and W/D Ratio Assay
4.7. PLA Method for Cytokine/Receptor Binding Analysis
4.8. Histological Analysis for Lung Injury Assay
4.9. EBD Extravasation Assay for Vascular Permeability Analysis
4.10. ELISA Method for Cytokine Analysis
4.11. Immunohistochemistry Staining Assay for Inflammation, Oxidation, Necroptosis, and Pyroptosis Analyses
4.12. TUNEL Method for Apoptosis Analysis
4.13. Immunoblotting Assay for Akt, Necroptosis, Pyroptosis, and Apoptosis Analyses
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, Y.M.; Luan, Y.Y.; Zhang, Q.H.; Sheng, Z.Y. Pathophysiological aspects of sepsis: An overview. Methods Mol. Biol. 2015, 1237, 5–15. [Google Scholar] [CrossRef]
- Herzum, I.; Renz, H. Inflammatory markers in SIRS, sepsis and septic shock. Curr. Med. Chem. 2008, 15, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, T.S.; Christman, J.W. The role of nuclear factor-kappa B in cytokine gene regulation. Am. J. Respir. Cell Mol. Biol. 1997, 17, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Hesse, D.G.; Tracey, K.J.; Fong, Y.; Manogue, K.R.; Palladino, M.A., Jr.; Cerami, A.; Shires, G.T.; Lowry, S.F. Cytokine appearance in human endotoxemia and primate bacteremia. Surg. Gynecol. Obstet. 1988, 166, 147–153. [Google Scholar] [PubMed]
- Bozza, F.A.; Salluh, J.I.; Japiassu, A.M.; Soares, M.; Assis, E.F.; Gomes, R.N.; Bozza, M.T.; Castro-Faria-Neto, H.C.; Bozza, P.T. Cytokine profiles as markers of disease severity in sepsis: A multiplex analysis. Crit. Care 2007, 11, R49. [Google Scholar] [CrossRef]
- Gogos, C.A.; Drosou, E.; Bassaris, H.P.; Skoutelis, A. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: A marker for prognosis and future therapeutic options. J. Infect Dis. 2000, 181, 176–180. [Google Scholar] [CrossRef]
- Schulte, W.; Bernhagen, J.; Bucala, R. Cytokines in sepsis: Potent immunoregulators and potential therapeutic targets—An updated view. Mediat. Inflamm. 2013, 2013, 165974. [Google Scholar] [CrossRef]
- Parameswaran, N.; Patial, S. Tumor necrosis factor-α signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef]
- Sheikh, M.S.; Huang, Y. Death receptor activation complexes: It takes two to activate TNF receptor 1. Cell Cycle 2003, 2, 550–552. [Google Scholar] [CrossRef]
- Challa, S.; Chan, F.K. Going up in flames: Necrotic cell injury and inflammatory diseases. C. Mol. Life Sci. 2010, 67, 3241–3253. [Google Scholar] [CrossRef]
- Wang, S.; El-Deiry, W.S. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene 2003, 22, 8628–8633. [Google Scholar] [CrossRef] [PubMed]
- Conos, S.A.; Chen, K.W.; De Nardo, D.; Hara, H.; Whitehead, L.; Núñez, G.; Masters, S.L.; Murphy, J.M.; Schroder, K.; Vaux, D.L.; et al. Active MLKL triggers the NLRP3 inflammasome in a cell-intrinsic manner. Proc. Natl. Acad. Sci. USA 2017, 114, E961–E969. [Google Scholar] [CrossRef] [PubMed]
- Joosten, L.A.; Netea, M.G.; Dinarello, C.A. Interleukin-1β in innate inflammation, autophagy and immunity. Semin. Immunol. 2013, 25, 416–424. [Google Scholar] [CrossRef]
- Kuno, K.; Matsushima, K. The IL-1 receptor signaling pathway. J. Leukoc. Biol. 1994, 56, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Ran, S.; Huang, J.; Liu, B.; Gu, S.; Jiang, W.; Liang, J. Enterococcus Faecalis activates NLRP3 inflammasomes leading to increased interleukin-1 beta secretion and pyroptosis of THP-1 macrophages. Microb. Pathog. 2021, 154, 104761. [Google Scholar] [CrossRef]
- Shalaby, M.R.; Waage, A.; Aarden, L.; Espevik, T. Endotoxin, tumour necrosis factor-α and interleukin 1 induce interleukin 6 production in vivo. Clin. Immunol. Immunopathol. 1989, 53, 488–498. [Google Scholar] [CrossRef]
- Uciechowski, P.; Dempke, W.C.M. Interleukin-6: A Masterplayer in the Cytokine Network. Oncology 2020, 98, 131–137. [Google Scholar] [CrossRef]
- Zegeye, M.M.; Lindkvist, M.; Fälker, K.; Kumawat, A.K.; Paramel, G.; Grenegård, M.; Sirsjö, A.; Ljungberg, L.U. Activation of the JAK/STAT3 and PI3K/AKT pathways are crucial for IL-6 trans-signaling-mediated pro-inflammatory response in human vascular endothelial cells. Cell Commun. Signal. 2018, 16, 55. [Google Scholar] [CrossRef]
- Hamesch, K.; Borkham-Kamphorst, E.; Strnad, P.; Weiskirchen, R. Lipopolysaccharide-induced inflammatory liver injury in mice. Lab. Anim. 2015, 49, 37–46. [Google Scholar] [CrossRef]
- Russell, J.A.; Rush, B.; Boyd, J. Pathophysiology of Septic Shock. Crit. Care Clin. 2018, 34, 43–61. [Google Scholar] [CrossRef]
- Jiang, S.J.; Tsai, P.I.; Peng, S.Y.; Chang, C.C.; Chung, Y.; Tsao, H.H.; Huang, H.T.; Chen, S.Y.; Hsu, H.J. A potential peptide derived from cytokine receptors can bind proinflammatory cytokines as a therapeutic strategy for anti-inflammation. Sci. Rep. 2019, 9, 2317. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.J.; Chang, C.Y.; Chiang, M.; Le, V.L.; Hsu, H.J.; Huang, C.J. Simultaneous Inhibition of Three Major Cytokines and Its Therapeutic Effects: A Peptide-Based Novel Therapy against Endotoxemia in Mice. J. Pers. Med. 2021, 11, 436. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Hsu, H.J.; Foo, J.; Shih, H.J.; Huang, C.J. Peptide-Based TNF-α-Binding Decoy Therapy Mitigates Lipopolysaccharide-Induced Liver Injury in Mice. Pharmaceuticals 2020, 13, 280. [Google Scholar] [CrossRef] [PubMed]
- Remick, D.G.; Newcomb, D.E.; Bolgos, G.L.; Call, D.R. Comparison of the mortality and inflammatory response of two models of sepsis: Lipopolysaccharide vs. cecal ligation and puncture. Shock 2000, 13, 110–116. [Google Scholar] [CrossRef]
- Kroemer, R.T. Structure-based drug design: Docking and scoring. Curr. Protein Pept. Sci. 2007, 8, 312–328. [Google Scholar] [CrossRef]
- Malaviya, R.; Laskin, J.D.; Laskin, D.L. Anti-TNFα therapy in inflammatory lung diseases. Pharmacol. Ther. 2017, 180, 90–98. [Google Scholar] [CrossRef]
- Monaco, C.; Nanchahal, J.; Taylor, P.; Feldmann, M. Anti-TNF therapy: Past, present and future. Int. Immunol. 2015, 27, 55–62. [Google Scholar] [CrossRef]
- Lv, S.; Han, M.; Yi, R.; Kwon, S.; Dai, C.; Wang, R. Anti-TNF-α therapy for patients with sepsis: A systematic meta-analysis. Int. J. Clin. Pract. 2014, 68, 520–528. [Google Scholar] [CrossRef]
- Weehuizen, T.A.; Lankelma, J.M.; De Jong, H.K.; De Boer, O.J.; Roelofs, J.J.; Day, N.P.; Gram, H.; De Vos, A.F.; Wiersinga, W.J. Therapeutic Administration of a Monoclonal Anti-Il-1β Antibody Protects Against Experimental Melioidosis. Shock 2016, 46, 566–574. [Google Scholar] [CrossRef][Green Version]
- Riedemann, N.C.; Neff, T.A.; Guo, R.F.; Bernacki, K.D.; Laudes, I.J.; Sarma, J.V.; Lambris, J.D.; Ward, P.A. Protective effects of IL-6 blockade in sepsis are linked to reduced C5a receptor expression. J. Immunol. 2003, 170, 503–507. [Google Scholar] [CrossRef]
- Gong, W.; Wen, H. Sepsis Induced by Cecal Ligation and Puncture. Methods Mol. Biol. 2019, 1960, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.Y.; Kao, M.C.; Lin, J.A.; Chen, T.Y.; Cheng, C.F.; Wong, C.S.; Tzeng, I.S.; Huang, C.J. Effects of MgSO4 on inhibiting Nod-like receptor protein 3 inflammasome involve decreasing intracellular calcium. J. Surg. Res. 2018, 221, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Aparici, M.; Gavaldà, A.; Ramos, I.; Carcasona, C.; Otal, R.; Fernández-Blanco, J.A.; Montero, J.L.; García, V.M.; López, R.; De Alba, J.; et al. In vitro and in vivo preclinical profile of abediterol (LAS100977), an inhaled long-acting β2-adrenoceptor agonist, compared with indacaterol, olodaterol and vilanterol. Eur. J. Pharmacol. 2016, 770, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Kao, M.C.; Yang, C.H.; Sheu, J.; Huang, C.J. Cepharanthine mitigates pro-inflammatory cytokine response in lung injury induced by hemorrhagic shock/resuscitation in rats. Cytokine 2015, 76, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, S.; Fredriksson, R.; Wallén-Mackenzie, Å. In Situ Proximity Ligation Assay (PLA). Methods Mol. Biol. 2015, 1318, 149–159. [Google Scholar] [CrossRef]
- Wick, M.J.; Harral, J.W.; Loomis, Z.L.; Dempsey, E.C. An Optimized Evans Blue Protocol to Assess Vascular Leak in the Mouse. J. Vis. Exp. 2018, 139, 57037. [Google Scholar] [CrossRef]
- Beljaars, L.; Schippers, M.; Reker-Smit, C.; Martinez, F.O.; Helming, L.; Poelstra, K.; Melgert, B.N. Hepatic Localization of Macrophage Phenotypes during Fibrogenesis and Resolution of Fibrosis in Mice and Humans. Front. Immunol. 2014, 5, 430. [Google Scholar] [CrossRef]
- Marnett, L.J. Lipid peroxidation-DNA damage by malondialdehyde. Mutat. Res. 1999, 424, 83–95. [Google Scholar] [CrossRef]
- Wang, H.; Sun, L.; Su, L.; Rizo, J.; Liu, L.; Wang, L.F.; Wang, F.S.; Wang, X. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol. Cell 2014, 54, 133–146. [Google Scholar] [CrossRef]
- Bohlinger, I.; Leist, M.; Gantner, F.; Angermüller, S.; Tiegs, G.; Wendel, A. DNA fragmentation in mouse organs during endotoxic shock. Am. J. Pathol. 1996, 149, 1381–1393. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.-Y.; Chang, C.-Y.; Hsu, H.-J.; Shih, H.-J.; Huang, I.-T.; Patel, H.H.; Huang, C.-J. Tumor Necrosis Factor-α Mediates Lung Injury in the Early Phase of Endotoxemia. Pharmaceuticals 2022, 15, 287. https://doi.org/10.3390/ph15030287
Chen K-Y, Chang C-Y, Hsu H-J, Shih H-J, Huang I-T, Patel HH, Huang C-J. Tumor Necrosis Factor-α Mediates Lung Injury in the Early Phase of Endotoxemia. Pharmaceuticals. 2022; 15(3):287. https://doi.org/10.3390/ph15030287
Chicago/Turabian StyleChen, Kung-Yen, Chao-Yuan Chang, Hao-Jen Hsu, Hung-Jen Shih, I-Tao Huang, Hemal H. Patel, and Chun-Jen Huang. 2022. "Tumor Necrosis Factor-α Mediates Lung Injury in the Early Phase of Endotoxemia" Pharmaceuticals 15, no. 3: 287. https://doi.org/10.3390/ph15030287
APA StyleChen, K.-Y., Chang, C.-Y., Hsu, H.-J., Shih, H.-J., Huang, I.-T., Patel, H. H., & Huang, C.-J. (2022). Tumor Necrosis Factor-α Mediates Lung Injury in the Early Phase of Endotoxemia. Pharmaceuticals, 15(3), 287. https://doi.org/10.3390/ph15030287







