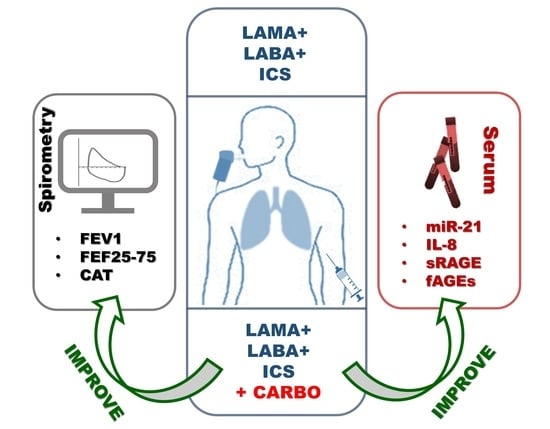
Carbocysteine Modifies Circulating miR-21, IL-8, sRAGE, and fAGEs Levels in Mild Acute Exacerbated COPD Patients: A Pilot Study
Abstract
:1. Introduction
2. Results
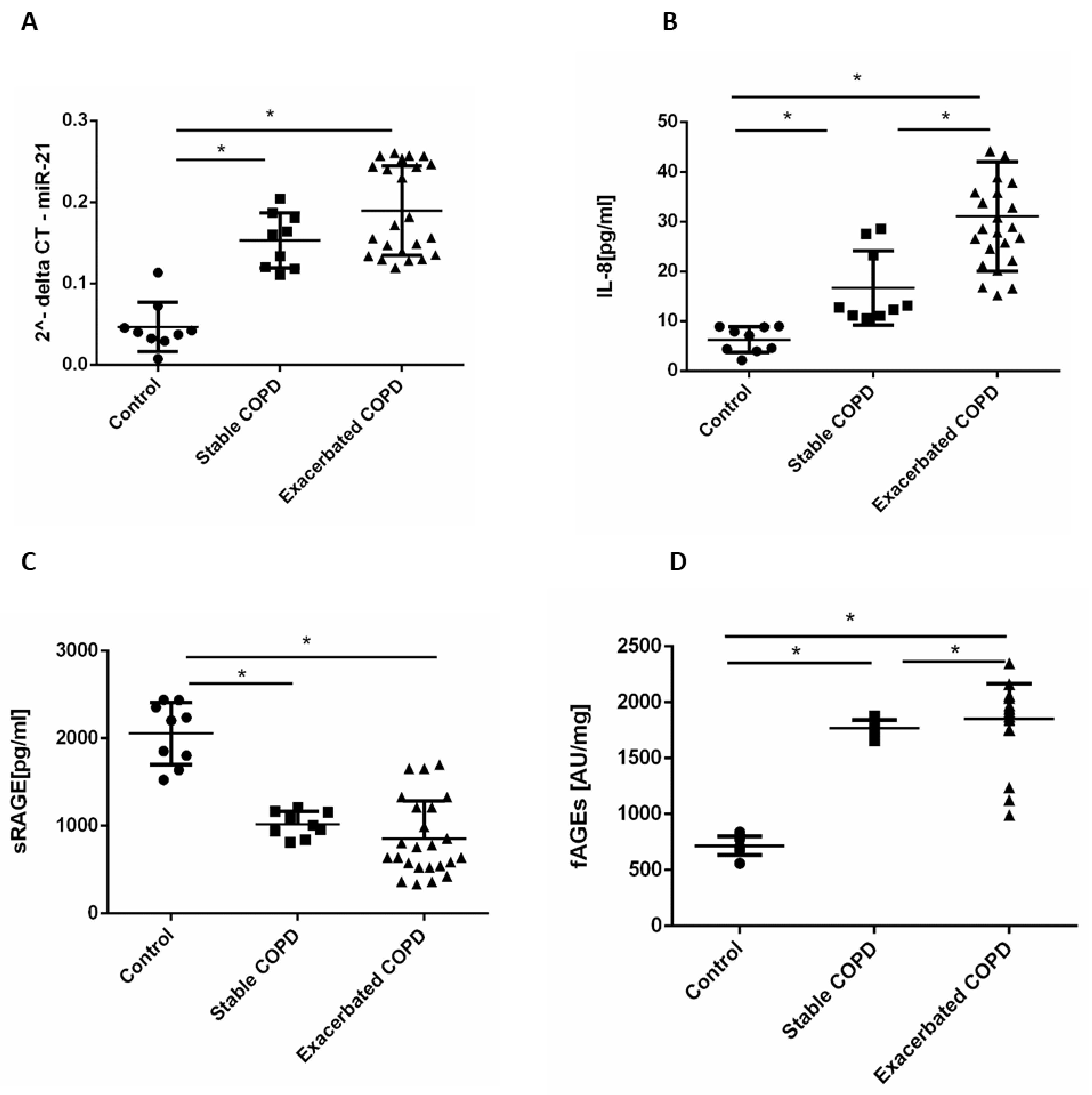
2.1. Stable and Exacerbated COPD Show Increased Levels of Circulating miR-21, IL-8 and fAGEs and Lower Levels of sRAGE Compared to Healthy Controls
2.2. In Vivo Effects of Carbocysteine on Symptoms and Functional Parameters in Mild Exacerbated COPD Patients
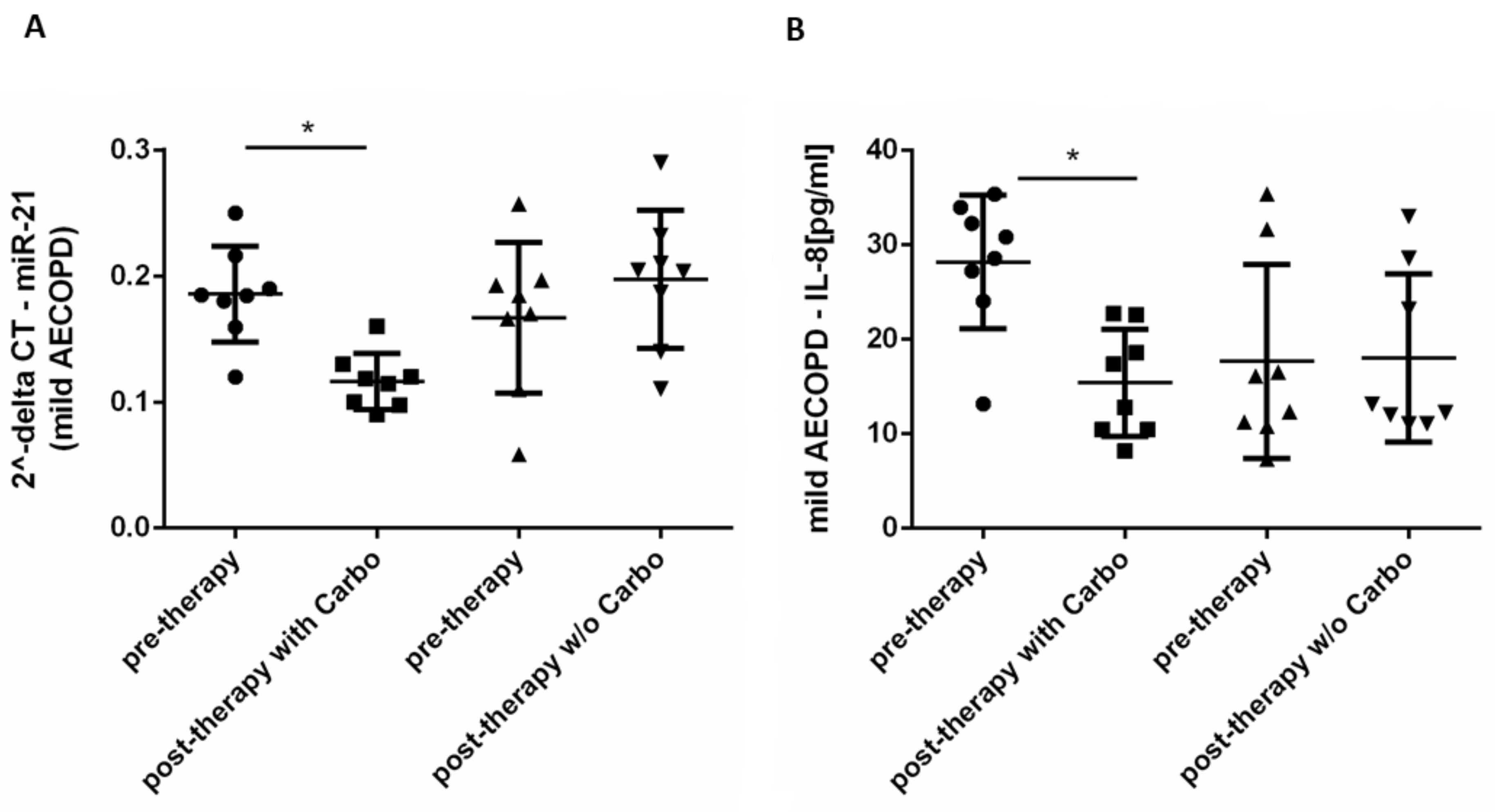
2.3. In Vivo Effects of Carbocysteine on Circulating miR-21 and IL-8 in Mild Exacerbated COPD Patients
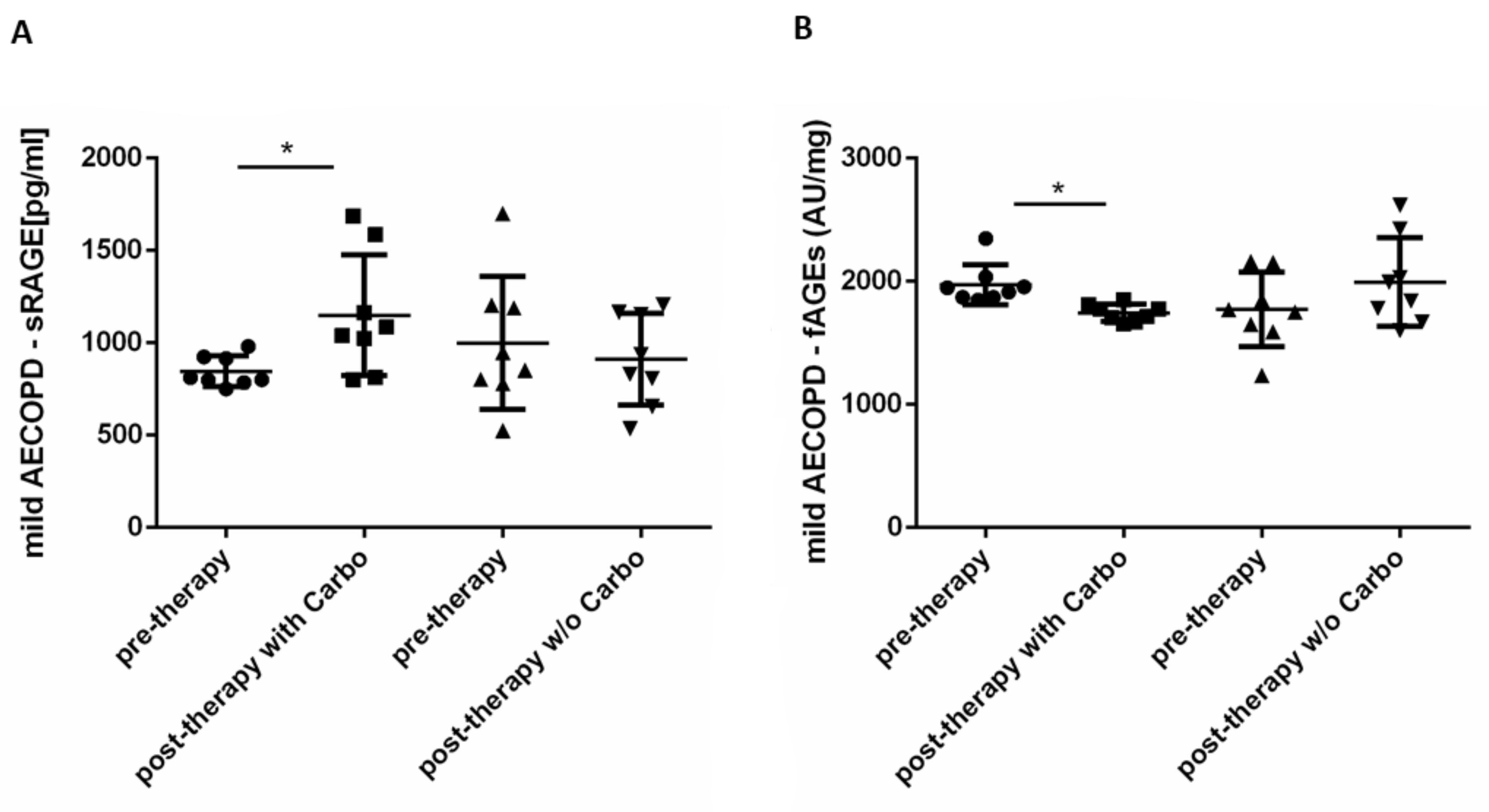
2.4. In Vivo Effects of Carbocysteine on Systemic sRAGE and fAGEs Levels in Mild Exacerbated COPD Patients
3. Discussion
4. Materials and Methods
4.1. Recruited Study Population
4.2. Study Design
4.3. Evaluation of Clinical and Functional Parameters
4.4. Evaluation of Serum miR-21
4.5. Measurement of IL-8 and sRAGE
4.6. Measurement of Fluorescent AGEs
4.7. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COPD | chronic obstructive pulmonary disease |
| AECOPD | acute exacerbation chronic obstructive pulmonary disease |
| Carbo | carbocysteine |
| miRNAs | microRNAs |
| CSE | cigarette smoke extract |
| fAGEs | fluorescent advanced glycation end products |
| sRAGE | soluble receptor AGE |
| CAT | COPD Assessment Test |
| FEV1 | Forced Expiratory Volume in 1 s |
| FEF25–75 | Forced Expiratory Flow |
References
- Singh, D.; Agusti, A.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Criner, G.J.; Frith, P.; Halpin, D.M.G.; Han, M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: The GOLD science committee report 2019. Eur. Respir. J. 2019, 53, 1900164. [Google Scholar] [CrossRef]
- Ko, F.W.; Chan, K.P.; Hui, D.S.; Goddard, J.R.; Shaw, J.G.; Reid, D.W.; Yang, I.A. Acute exacerbation of COPD. Respirology 2016, 21, 1152–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anthonisen, N.R.; Manfreda, J.; Warren, C.P.; Hershfield, E.S.; Harding, G.K.; Nelson, N.A. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann. Intern. Med. 1987, 106, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; MacNee, W.; Martinez, F.J.; Rabe, K.F.; Franciosi, L.G.; Barnes, P.J.; Brusasco, V.; Burge, P.S.; Calverley, P.M.; Celli, B.R.; et al. Outcomes for COPD pharmacological trials: From lung function to biomarkers. Eur. Respir. J. 2008, 31, 416–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grandjean, E.; Berthet, P.; Ruffmann, R.; Leuenberger, P. Cost-effectiveness analysis of oral N-acetylcysteine as a preventive treatment in chronic bronchitis. Pharmacol. Res. 2000, 42, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Paone, G.; Lanata, L.; Saibene, F.; Toti, S.; Palermo, P.; Graziani, C.; Flore, M.C.; Ramaccia, M.; Puglisi, G. A prospective study of the effects of carbocysteine lysine salt on frequency of exacerbations in COPD patients treated with or without inhaled steroids. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6727–6735. [Google Scholar] [CrossRef]
- Esposito, A.; Valentino, M.R.; Bruzzese, D.; Bocchino, M.; Ponticiello, A.; Stanziola, A.; Sanduzzi, A. Effect of CArbocisteine in Prevention of exaceRbation of chronic obstructive pulmonary disease (CAPRI study): An observational study. Pulm. Pharmacol. Ther. 2016, 37, 85–88. [Google Scholar] [CrossRef] [Green Version]
- Salimian, J.; Mirzaei, H.; Moridikia, A.; Harchegani, A.B.; Sahebkar, A.; Salehi, H. Chronic obstructive pulmonary disease: MicroRNAs and exosomes as new diagnostic and therapeutic biomarkers. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2018, 23, 27. [Google Scholar] [CrossRef] [PubMed]
- Kundaktepe, B.P.; Sozer, V.; Papila, C.; Durmus, S.; Kocael, P.C.; Simsek, G.; Gelisgen, R.; Zengin, K.; Ulualp, K.; Uzun, H. Associations Between miRNAs and Two Different Cancers: Breast and Colon. Cancer Manag. Res. 2020, 12, 871–879. [Google Scholar] [CrossRef] [Green Version]
- Watabe, S.; Kikuchi, Y.; Morita, S.; Komura, D.; Numakura, S.; Kumagai-Togashi, A.; Watanabe, M.; Matsutani, N.; Kawamura, M.; Yasuda, M.; et al. Clinicopathological significance of microRNA-21 in extracellular vesicles of pleural lavage fluid of lung adenocarcinoma and its functions inducing the mesothelial to mesenchymal transition. Cancer Med. 2020, 9, 2879–2890. [Google Scholar] [CrossRef]
- Liu, B.; Wei, H.; Lan, M.; Jia, N.; Liu, J.; Zhang, M. MicroRNA-21 mediates the protective effects of salidroside against hypoxia/reoxygenation-induced myocardial oxidative stress and inflammatory response. Exp. Ther. Med. 2020, 19, 1655–1664. [Google Scholar] [CrossRef]
- Sheedy, F.J. Turning 21: Induction of miR-21 as a Key Switch in the Inflammatory Response. Front. Immunol. 2015, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- Fabbri, L.M.; Rabe, K.F. From COPD to chronic systemic inflammatory syndrome? Lancet 2007, 370, 797–799. [Google Scholar] [CrossRef]
- He, S.; Li, L.; Sun, S.; Zeng, Z.; Lu, J.; Xie, L. A Novel Murine Chronic Obstructive Pulmonary Disease Model and the Pathogenic Role of MicroRNA-21. Front. Physiol. 2018, 9, 503. [Google Scholar] [CrossRef]
- Pace, E.; Di Vincenzo, S.; Di Salvo, E.; Genovese, S.; Dino, P.; Sangiorgi, C.; Ferraro, M.; Gangemi, S. MiR-21 upregulation increases IL-8 expression and tumorigenesis program in airway epithelial cells exposed to cigarette smoke. J. Cell. Physiol. 2019, 234, 22183–22194. [Google Scholar] [CrossRef] [PubMed]
- Pace, E.; Giarratano, A.; Ferraro, M.; Bruno, A.; Siena, L.; Mangione, S.; Johnson, M.; Gjomarkaj, M. TLR4 upregulation underpins airway neutrophilia in smokers with chronic obstructive pulmonary disease and acute respiratory failure. Hum. Immunol. 2011, 72, 54–62. [Google Scholar] [CrossRef]
- Vistoli, G.; De Maddis, D.; Cipak, A.; Zarkovic, N.; Carini, M.; Aldini, G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): An overview of their mechanisms of formation. Free Radic. Res. 2013, 47 (Suppl. 1), 3–27. [Google Scholar] [CrossRef] [Green Version]
- Allegra, A.; Musolino, C.; Pace, E.; Innao, V.; Di Salvo, E.; Ferraro, M.; Casciaro, M.; Spatari, G.; Tartarisco, G.; Allegra, A.G.; et al. Evaluation of the AGE/sRAGE Axis in Patients with Multiple Myeloma. Antioxidants 2019, 8, 55. [Google Scholar] [CrossRef] [Green Version]
- Oczypok, E.A.; Perkins, T.N.; Oury, T.D. All the “RAGE” in lung disease: The receptor for advanced glycation endproducts (RAGE) is a major mediator of pulmonary inflammatory responses. Paediatr. Respir. Rev. 2017, 23, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Yonekura, H.; Yamagishi, S.; Fujimori, H.; Yamamoto, Y.; Yamamoto, H. The receptor for advanced glycation end products is induced by the glycation products themselves and tumor necrosis factor-alpha through nuclear factor-kappa B, and by 17beta-estradiol through Sp-1 in human vascular endothelial cells. J. Biol. Chem. 2000, 275, 25781–25790. [Google Scholar] [CrossRef] [Green Version]
- Gopal, P.; Reynaert, N.L.; Scheijen, J.L.; Schalkwijk, C.G.; Franssen, F.M.; Wouters, E.F.; Rutten, E.P. Association of plasma sRAGE, but not esRAGE with lung function impairment in COPD. Respir. Res. 2014, 15, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, D.J.; Yerkovich, S.T.; Towers, M.A.; Carroll, M.L.; Thomas, R.; Upham, J.W. Reduced soluble receptor for advanced glycation end-products in COPD. Eur. Respir. J. 2011, 37, 516–522. [Google Scholar] [CrossRef] [Green Version]
- Izushi, Y.; Teshigawara, K.; Liu, K.; Wang, D.; Wake, H.; Takata, K.; Yoshino, T.; Takahashi, H.K.; Mori, S.; Nishibori, M. Soluble form of the receptor for advanced glycation end-products attenuates inflammatory pathogenesis in a rat model of lipopolysaccharide-induced lung injury. J. Pharmacol. Sci. 2016, 130, 226–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapey, E.; Bafadhel, M.; Bolton, C.E.; Wilkinson, T.; Hurst, J.R.; Quint, J.K. Building toolkits for COPD exacerbations: Lessons from the past and present. Thorax 2019, 74, 898–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpagnano, G.E.; Resta, O.; Foschino-Barbaro, M.P.; Spanevello, A.; Stefano, A.; Di Gioia, G.; Serviddio, G.; Gramiccioni, E. Exhaled Interleukine-6 and 8-isoprostane in chronic obstructive pulmonary disease: Effect of carbocysteine lysine salt monohydrate (SCMC-Lys). Eur. J. Pharmacol. 2004, 505, 169–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.P.; Kang, J.; Huang, S.G.; Chen, P.; Yao, W.Z.; Yang, L.; Bai, C.X.; Wang, C.Z.; Wang, C.; Chen, B.Y.; et al. Effect of carbocisteine on acute exacerbation of chronic obstructive pulmonary disease (PEACE Study): A randomised placebo-controlled study. Lancet 2008, 371, 2013–2018. [Google Scholar] [CrossRef]
- Simon, M.R.; Chinchilli, V.M.; Phillips, B.R.; Sorkness, C.A.; Lemanske, R.F., Jr.; Szefler, S.J.; Taussig, L.; Bacharier, L.B.; Morgan, W.; Childhood Asthma, R.; et al. Forced expiratory flow between 25% and 75% of vital capacity and FEV1/forced vital capacity ratio in relation to clinical and physiological parameters in asthmatic children with normal FEV1 values. J. Allergy Clin. Immunol. 2010, 126, 527–534.e8. [Google Scholar] [CrossRef] [Green Version]
- Aaron, S.D. Mucolytics for COPD: Negotiating a slippery slope towards proof of efficacy. Eur. Respir. J. 2017, 50, 1701465. [Google Scholar] [CrossRef] [PubMed]
- De Smet, E.G.; Mestdagh, P.; Vandesompele, J.; Brusselle, G.G.; Bracke, K.R. Non-coding RNAs in the pathogenesis of COPD. Thorax 2015, 70, 782–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conickx, G.; Avila Cobos, F.; van den Berge, M.; Faiz, A.; Timens, W.; Hiemstra, P.S.; Joos, G.F.; Brusselle, G.G.; Mestdagh, P.; Bracke, K.R. microRNA profiling in lung tissue and bronchoalveolar lavage of cigarette smoke-exposed mice and in COPD patients: A translational approach. Sci. Rep. 2017, 7, 12871. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.; Wu, M.; Lin, H.; Liu, C.; Yang, H.; Zhan, J.; Sun, S. An increased ratio of serum miR-21 to miR-181a levels is associated with the early pathogenic process of chronic obstructive pulmonary disease in asymptomatic heavy smokers. Mol. Biosyst. 2014, 10, 1072–1081. [Google Scholar] [CrossRef]
- Pace, E.; Ferraro, M.; Siena, L.; Melis, M.; Montalbano, A.M.; Johnson, M.; Bonsignore, M.R.; Bonsignore, G.; Gjomarkaj, M. Cigarette smoke increases Toll-like receptor 4 and modifies lipopolysaccharide-mediated responses in airway epithelial cells. Immunology 2008, 124, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bai, C. The Significance of Serum Interleukin-8 in Acute Exacerbations of Chronic Obstructive Pulmonary Disease. Tanaffos 2018, 17, 13–21. [Google Scholar] [PubMed]
- Crisafulli, E.; Torres, A.; Huerta, A.; Guerrero, M.; Gabarrus, A.; Gimeno, A.; Martinez, R.; Soler, N.; Fernandez, L.; Wedzicha, J.A.; et al. Predicting In-Hospital Treatment Failure (</=7 days) in Patients with COPD Exacerbation Using Antibiotics and Systemic Steroids. COPD 2016, 13, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Hoonhorst, S.J.; Lo Tam Loi, A.T.; Pouwels, S.D.; Faiz, A.; Telenga, E.D.; van den Berge, M.; Koenderman, L.; Lammers, J.W.; Boezen, H.M.; van Oosterhout, A.J.; et al. Advanced glycation endproducts and their receptor in different body compartments in COPD. Respir. Res. 2016, 17, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downs, C.A.; Johnson, N.M.; Tsaprailis, G.; Helms, M.N. RAGE-induced changes in the proteome of alveolar epithelial cells. J. Proteom. 2018, 177, 11–20. [Google Scholar] [CrossRef]
- Sukkar, M.B.; Wood, L.G.; Tooze, M.; Simpson, J.L.; McDonald, V.M.; Gibson, P.G.; Wark, P.A. Soluble RAGE is deficient in neutrophilic asthma and COPD. Eur. Respir. J. 2012, 39, 721–729. [Google Scholar] [CrossRef]
- Cockayne, D.A.; Cheng, D.T.; Waschki, B.; Sridhar, S.; Ravindran, P.; Hilton, H.; Kourteva, G.; Bitter, H.; Pillai, S.G.; Visvanathan, S.; et al. Systemic biomarkers of neutrophilic inflammation, tissue injury and repair in COPD patients with differing levels of disease severity. PLoS ONE 2012, 7, e38629. [Google Scholar] [CrossRef]
- Pace, E.; Ferraro, M.; Di Vincenzo, S.; Cipollina, C.; Gerbino, S.; Cigna, D.; Caputo, V.; Balsamo, R.; Lanata, L.; Gjomarkaj, M. Comparative cytoprotective effects of carbocysteine and fluticasone propionate in cigarette smoke extract-stimulated bronchial epithelial cells. Cell Stress Chaperones 2013, 18, 733–743. [Google Scholar] [CrossRef] [Green Version]
- Pace, E.; Di Vincenzo, S.; Ferraro, M.; Siena, L.; Chiappara, G.; Dino, P.; Vitulo, P.; Bertani, A.; Saibene, F.; Lanata, L.; et al. Effects of Carbocysteine and Beclomethasone on Histone Acetylation/Deacetylation Processes in Cigarette Smoke Exposed Bronchial Epithelial Cells. J. Cell. Physiol. 2017, 232, 2851–2859. [Google Scholar] [CrossRef]
- Aaron, S.D.; Donaldson, G.C.; Whitmore, G.A.; Hurst, J.R.; Ramsay, T.; Wedzicha, J.A. Time course and pattern of COPD exacerbation onset. Thorax 2012, 67, 238–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zilin, S.; Naifeng, L.; Bicheng, L.; Jiping, W. The determination of AGE-peptides by flow injection assay, a practical marker of diabetic nephropathy. Clin. Chim. Acta Int. J. Clin. Chem. 2001, 313, 69–75. [Google Scholar] [CrossRef]
- Villa, M.; Parravano, M.; Micheli, A.; Gaddini, L.; Matteucci, A.; Mallozzi, C.; Facchiano, F.; Malchiodi-Albedi, F.; Pricci, F. A quick, simple method for detecting circulating fluorescent advanced glycation end-products: Correlation with in vitro and in vivo non-enzymatic glycation. Metab. Clin. Exp. 2017, 71, 64–69. [Google Scholar] [CrossRef] [PubMed]



| Correlation Coefficient miR-21 (2^-deltaCT) | p Values | |
|---|---|---|
| FEV1(l) | −0.7769406 | 0.031 |
| FEV1(%) | −0.8189638 | 0.019 |
| FEF 25–75(l) | −0.6835159 | 0.078 |
| FEF 25–75(%) | −0.718366 | 0.055 |
| Controls | Stable COPD | Exacerbated COPD | |
|---|---|---|---|
| N | 9 | 9 | 24 |
| Age | 50 ± 10 | 62 ± 8 | 58 ± 18 |
| Gender (M/F) | 4/5 | 5/4 | 12/12 |
| pack/year | - | 35 ± 10.31 | 40.7 ± 11.86 |
| FEV1(%predicted) | 106.22 ± 12.21 | 57.50 ± 11.03 | 48.31 ± 16.77 |
| FEF25–75(%predicted) | 101.78 ± 12.34 | 61.73 ± 10.43 | 50.24 ± 17.09 |
| GOLD | 2 | 2–3 | |
| BMI | 27.81 ± 3.11 | 28.05 ± 2.26 | 28.51 ± 6.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraro, M.; Di Vincenzo, S.; Sangiorgi, C.; Leto Barone, S.; Gangemi, S.; Lanata, L.; Pace, E. Carbocysteine Modifies Circulating miR-21, IL-8, sRAGE, and fAGEs Levels in Mild Acute Exacerbated COPD Patients: A Pilot Study. Pharmaceuticals 2022, 15, 218. https://doi.org/10.3390/ph15020218
Ferraro M, Di Vincenzo S, Sangiorgi C, Leto Barone S, Gangemi S, Lanata L, Pace E. Carbocysteine Modifies Circulating miR-21, IL-8, sRAGE, and fAGEs Levels in Mild Acute Exacerbated COPD Patients: A Pilot Study. Pharmaceuticals. 2022; 15(2):218. https://doi.org/10.3390/ph15020218
Chicago/Turabian StyleFerraro, Maria, Serena Di Vincenzo, Claudia Sangiorgi, Stefania Leto Barone, Sebastiano Gangemi, Luigi Lanata, and Elisabetta Pace. 2022. "Carbocysteine Modifies Circulating miR-21, IL-8, sRAGE, and fAGEs Levels in Mild Acute Exacerbated COPD Patients: A Pilot Study" Pharmaceuticals 15, no. 2: 218. https://doi.org/10.3390/ph15020218
APA StyleFerraro, M., Di Vincenzo, S., Sangiorgi, C., Leto Barone, S., Gangemi, S., Lanata, L., & Pace, E. (2022). Carbocysteine Modifies Circulating miR-21, IL-8, sRAGE, and fAGEs Levels in Mild Acute Exacerbated COPD Patients: A Pilot Study. Pharmaceuticals, 15(2), 218. https://doi.org/10.3390/ph15020218