The Boron Advantage: The Evolution and Diversification of Boron’s Applications in Medicinal Chemistry
Abstract
:1. Introduction
2. Boron Medicinal Chemistry
2.1. The Early History of Boron Medicinal Agents
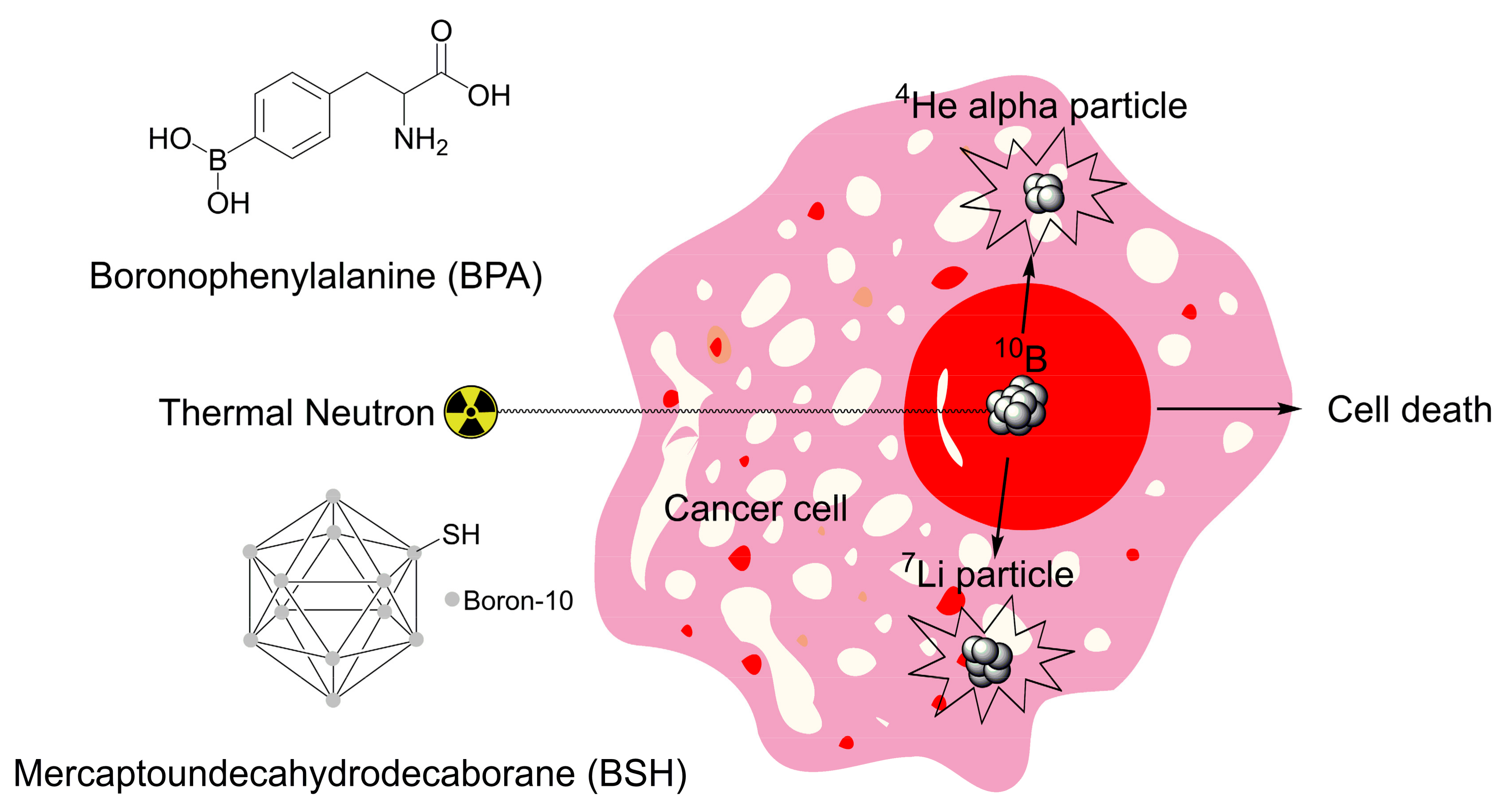
2.2. Boron Neutron Capture Therapy
2.3. Diazaborines
2.4. Boronic Acids
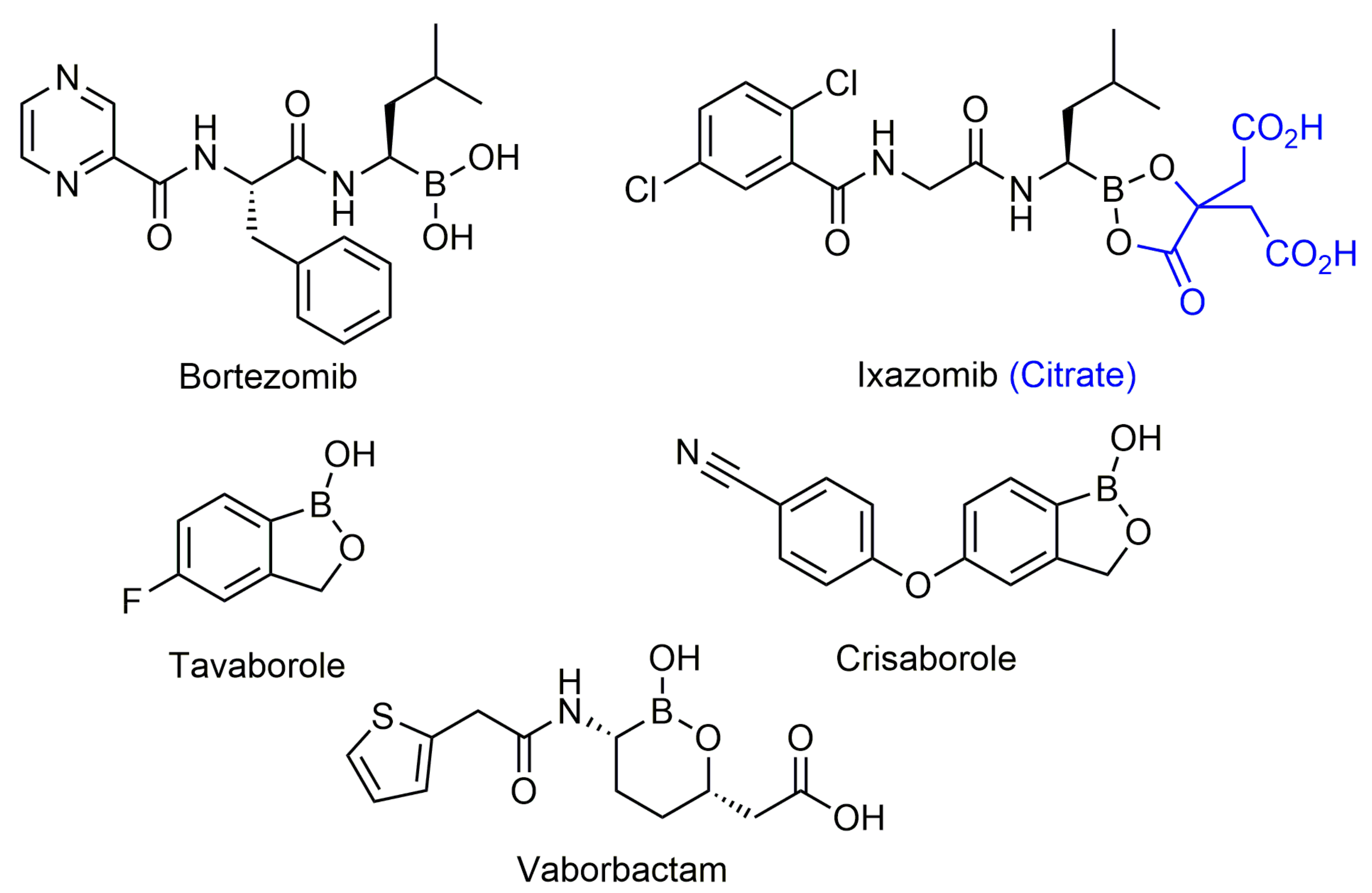
2.4.1. Peptidic Boronic Acids
General Information and History
Bortezomib and Ixazomib
Recent Studies on Proteasome Inhibitors
Other Applications of Peptidic Boronic Acids
Peptidic Boronic Acids in Clinical Trials
2.4.2. Benzoxaboroles
General Information and History
Tavaborole and Crisaborole
Benzoxaboroles as Antitubercular, Antitrypanosomal, and Antimalarial Agents
Miscellaneous Applications of Benzoxaboroles
Benzoxaboroles in Clinical Trials
2.4.3. Other Boronic Acids
Cyclic Boronic Acids as β-Lactamase Inhibitors
Vaborbactam, Taniborbactam and QPX7728
Additional Therapeutic Applications of Boronic Acids
2.5. Boron Clusters
2.5.1. General Information and Chemistry of Boron Clusters
2.5.2. The History of Boron Clusters
2.5.3. Boron Clusters in Novel BNCT Delivery Applications
2.5.4. Carboranes as Steroidal Agonists, Antagonists and Modulators
2.5.5. Therapeutic Applications of Carborane-Modified Nucleosides
2.5.6. Carboranes as Anti-Inflammatory and Anticancer Agents
2.5.7. Other Novel Applications of Boron Clusters
2.6. Boron as a Tool to Enhance Drug Efficacy, Delivery, and Targeting
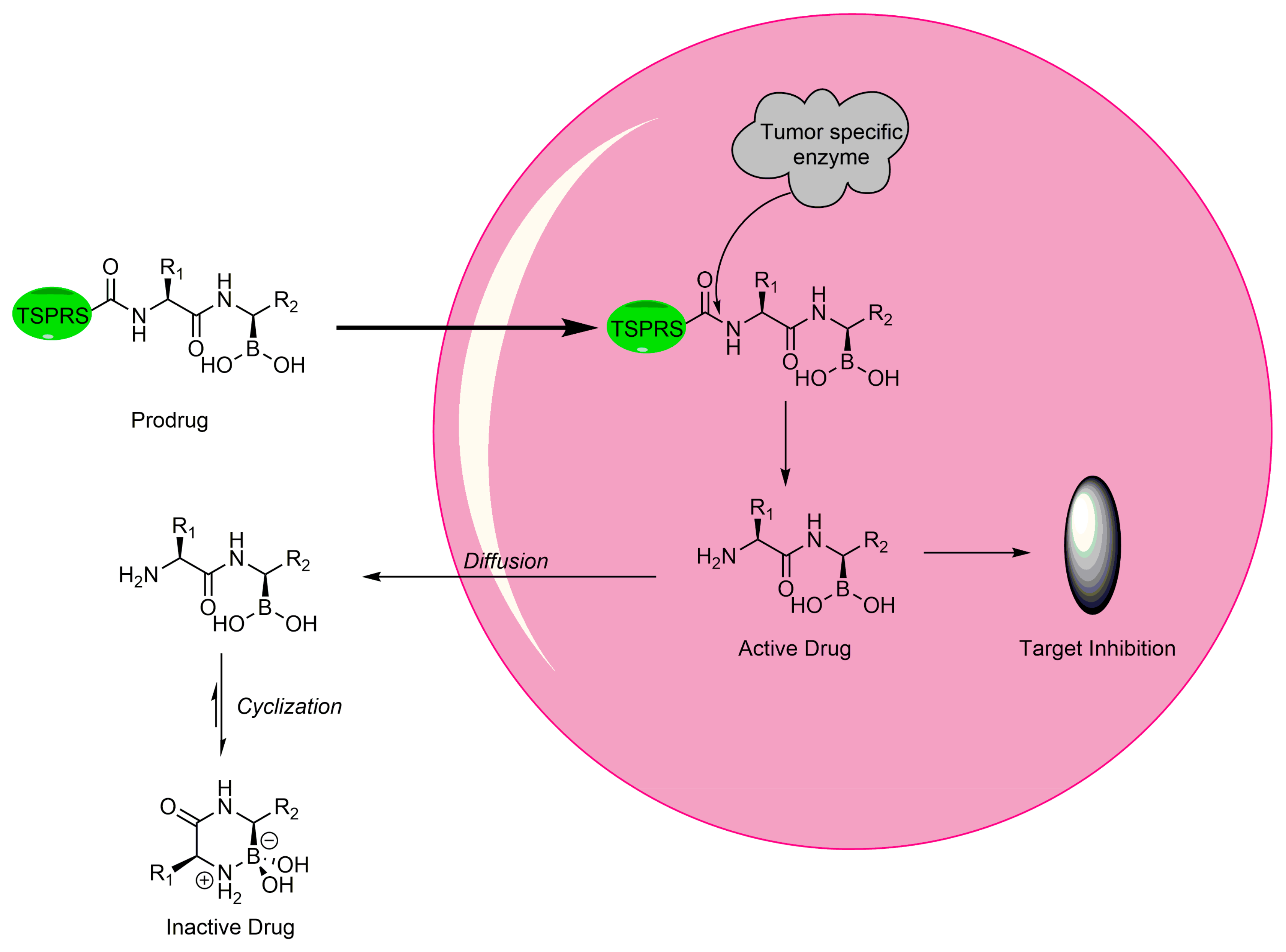
2.6.1. Boron as Protecting Group in Prodrugs
2.6.2. Prodrug-Modified Boron Compounds
2.6.3. Boron in Softdrugs
2.6.4. Boron in Micelle Formation as Nanocarriers
2.6.5. Phenylboronic Acid Nanoparticles
2.6.6. Boron in Dendrimers and Porphyrins
2.6.7. Utilizing Boron-Carbohydrate Chemistry for Drug Delivery
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hey-Hawkins, E.; Vinas-Teixidor, C. (Eds.) Boron-Based Compounds: Potential and Emerging Applications in Medicine; John Wiley & Sons, Ltd.: Oxford, UK, 2018. [Google Scholar]
- Hosmane, N.S.; Eagling, R. Handbook of Boron Science; World Scientific (Europe): Hackensack, NJ, USA, 2018; ISBN 978-1-78634-447-2. [Google Scholar]
- Leśnikowski, Z.J. Challenges and Opportunities for the Application of Boron Clusters in Drug Design. J. Med. Chem. 2016, 59, 7738–7758. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.J.; Ding, C.Z.; Akama, T.; Zhang, Y.; Hernandez, V.; Xia, Y. Therapeutic potential of boron-containing compounds. Future Med. Chem. 2009, 1, 1275–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, S.; Gao, P.; Sun, L.; Kang, D.; Kongsted, J.; Poongavanam, V.; Zhan, P.; Liu, X. Recent developments in the medicinal chemistry of single boron atom-containing compounds. Acta Pharm. Sin. B 2021, 11, 3035–3059. [Google Scholar] [CrossRef] [PubMed]
- Leśnikowski, Z.J. Recent developments with boron as a platform for novel drug design. Expert Opin. Drug Discov. 2016, 11, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.H.; Meacham, S.L. Growing Evidence for Human Health Benefits of Boron. Evid.-Based Complement. Altern. Med. 2011, 16, 169–180. [Google Scholar] [CrossRef]
- Ocampo-Néstor, A.L.; Trujillo-Ferrara, J.G.; Abad-García, A.; Reyes-López, C.; Geninatti-Crich, S.; Soriano-Ursúa, M.A. Boron’s journey: Advances in the study and application of pharmacokinetics. Expert Opin. Ther. Pat. 2016, 27, 203–215. [Google Scholar] [CrossRef]
- Litovitz, T.L.; Klein-schwartz, W.; Oderda, G.M.; Schmitz, B.F. Clinical Manifestations of Toxicity in a Series of 784 Boric Acid Ingestions. Am. J. Emerg. Med. 1988, 6, 209–213. [Google Scholar] [CrossRef]
- Yang, F.; Zhu, M.; Zhang, J.; Zhou, H. Synthesis of biologically active boron-containing compounds. Med. Chem. Commun. 2018, 9, 201–211. [Google Scholar] [CrossRef]
- Hall, D.G. Structure, Properties, and Preparation of Boronic Acid Derivatives. Overview of Their Reactions and Applications, 1st ed.; Wiley-VCH: Weinheim, DE, USA, 2006; ISBN 3527309918. [Google Scholar]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef] [Green Version]
- Ciaravino, V.; Plattner, J.; Chanda, S. An Assessment of the Genetic Toxicology of Novel Boron-Containing Therapeutic Agents. Environ. Mol. Mutagen. 2013, 54, 338–346. [Google Scholar] [CrossRef]
- Woods, W.G. An Introduction to Boron: History, Sources, Uses, and Chemistry. Environ. Health Perspect. 1994, 102, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Issa, F.; Kassiou, M.; Rendina, L.M. Boron in Drug Discovery: Carboranes as Unique Pharmacophores in Biologically Active Compounds. Chem. Rev. 2011, 111, 5701–5722. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, M.F.; Maderna, A. Applications of Radiolabeled Boron Clusters to the Diagnosis and Treatment of Cancer. Chem. Rev. 1999, 99, 3421–3434. [Google Scholar] [CrossRef] [PubMed]
- Das, B.C.; Thapa, P.; Karki, R.; Schinke, C.; Das, S.; Kambhampati, S.; Banerjee, S.K.; Van Veldhuizen, P.; Verma, A.; Weiss, L.M.; et al. Boron chemicals in diagnosis and therapeutics. Future Med. Chem. 2013, 5, 653–676. [Google Scholar] [CrossRef] [Green Version]
- Ban, H.S.; Nakamura, H. Boron-based drug design. Chem. Rec. 2015, 15, 616–635. [Google Scholar] [CrossRef]
- Fein, M.M.; Bobinski, J.; Mayes, N.; Schwartz, N.; Cohen, M.S. Carboranes. I. The Preparation and Chemistry of 1-Isopropenylcarborane and its Derivatives (a New Family of Stable Clovoboranes). Inorg. Chem. 1963, 2, 1111–1115. [Google Scholar] [CrossRef]
- Wongthai, P.; Hagiwara, K.; Miyoshi, Y.; Wiriyasermkul, P.; Wei, L.; Ohgaki, R.; Kato, I.; Hamase, K.; Nagamori, S.; Kanai, Y. Boronophenylalanine, a boron delivery agent for boron neutron capture therapy, is transported by ATB0,+, LAT1 and LAT2. Cancer Sci. 2015, 106, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, M. Boron neutron capture therapy (BNCT): A unique role in radiotherapy with a view to entering the accelerator-based BNCT era. Int. J. Clin. Oncol. 2020, 25, 43–50. [Google Scholar] [CrossRef]
- Baldock, C.; De Boer, G.J.; Rafferty, J.B.; Stuitje, A.R.; Rice, D.W. Mechanism of action of diazaborines. Biochem. Pharmacol. 1998, 55, 1541–1549. [Google Scholar] [CrossRef]
- Bailey, P.J.; Cousins, G.; Snow, G.A.; White, A.J. Boron-Containing Antibacterial Agents: Effects on Growth and Morphology of Bacteria Under Various Culture Conditions. Antimicrob. Agents Chemother. 1980, 17, 549–553. [Google Scholar] [CrossRef] [Green Version]
- Högenauer, G.; Woisetschläger, M. A diazaborine derivative inhibits lipopolysaccharide biosynthesis. Nature 1981, 293, 662–664. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.; Turnowsky, F.; Högenauer, G.; Schütze, E. Effect of a diazaborine derivative (Sa 84.474) on the virulence of Escherichia coli. J. Antimicrob. Chemother. 1987, 20, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Jordan, C.A.; Sandoval, B.A.; Serobyan, M.V.; Gilling, D.H.; Groziak, M.P.; Xu, H.H.; Vey, J.L. Crystallographic insights into the structure-activity relationships of diazaborine enoyl-ACP reductase inhibitors. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2015, 71, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.C.; Franzblau, S.G.; Martin’, A.R. Syntheses and Evaluation of Benzodiazaborine Compounds Against M. Tuberculosis H37Rv in Vitro. Bioorg. Med. Chem. Lett. 1998, 8, 843–846. [Google Scholar] [CrossRef]
- Xia, Y.; Zhou, Y.; Carter, D.S.; McNeil, M.B.; Choi, W.; Halladay, J.; Berry, P.W.; Mao, W.; Hernandez, V.; O’Malley, T.; et al. Discovery of a cofactor-independent inhibitor of Mycobacterium tuberculosis InhA. Life Sci. Alliance 2018, 1, e201800025. [Google Scholar] [CrossRef] [Green Version]
- Robertson, G.T.; Ektnitphong, V.A.; Scherman, M.S.; McNeil, M.B.; Dennison, D.; Korkegian, A.; Smith, A.J.; Halladay, J.; Carter, D.S.; Xia, Y.; et al. Efficacy and Improved Resistance Potential of a Cofactor-Independent InhA Inhibitor of Mycobacterium tuberculosis in the C3HeB/FeJ Mouse Model. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [Green Version]
- Jungwirth, H.; Wendler, F.; Platzer, B.; Bergler, H.; Högenauer, G. Diazaborine resistance in yeast involves the efflux pumps Ycf1p and Flr1p and is enhanced by a gain-of-function allele of gene YAP1. Eur. J. Biochem. 2000, 267, 4809–4816. [Google Scholar] [CrossRef] [Green Version]
- Jungwirth, H.; Bergler, H.; Högenauer, G. Diazaborine Treatment of Baker’s Yeast Results in Stabilization of Aberrant mRNAs. J. Biol. Chem. 2001, 276, 36419–36424. [Google Scholar] [CrossRef] [Green Version]
- Loibl, M.; Klein, I.; Prattes, M.; Schmidt, C.; Kappel, L.; Zisser, G.; Gungl, A.; Krieger, E.; Pertschy, B.; Bergler, H. The drug diazaborine blocks ribosome biogenesis by inhibiting the AAA-ATPase drg1. J. Biol. Chem. 2014, 289, 3913–3922. [Google Scholar] [CrossRef] [Green Version]
- Kettner, C.A.; Shenvi, A.B. Inhibition of the serine proteases leukocyte elastase, pancreatic elastase, cathepsin G, and chymotrypsin by peptide boronic acids. J. Biol. Chem. 1984, 259, 15106–15114. [Google Scholar] [CrossRef]
- Kato, Y.; Kido, H.; Fukusen, N.; Katunuma, N. Peptide boronic acids, substrate analogs, inhibit chymase, and histamine release from rat mast cells. J. Biochem. 1988, 103, 820–822. [Google Scholar] [CrossRef] [PubMed]
- Tapparelli, C.; Metternich, R.; Ehrhardt, C.; Zurini, M.; Claeson, G.; Scully, M.F.; Stone, S.R. In vitro and in vivo characterization of a neutral boron-containing thrombin inhibitor. J. Biol. Chem. 1993, 268, 4734–4741. [Google Scholar] [CrossRef]
- Tian, Z.Q.; Brown, B.B.; Mack, D.P.; Hutton, C.A.; Bartlett, P.A. Potentially macrocyclic peptidyl boronic acids as chymotrypsin inhibitors. J. Org. Chem. 1997, 62, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.; Palombella, V.J.; Elliott, P.J. Proteasome inhibition: A new strategy in cancer treatment. Investig. New Drugs 2000, 18, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Touchet, S.; Carreaux, F.; Carboni, B.; Bouillon, A.; Boucher, J.L. Aminoboronic acids and esters: From synthetic challenges to the discovery of unique classes of enzyme inhibitors. Chem. Soc. Rev. 2011, 40, 3895–3914. [Google Scholar] [CrossRef]
- Draganov, A.; Wang, D.; Wang, B. The Future of Boron in Medicinal Chemistry: Therapeutic and Diagnostic Applications. Top. Med. Chem. 2014, 17, 1–28. [Google Scholar] [CrossRef]
- Larocca, A.; Mina, R.; Offidani, M.; Marina, A.; Ledda, A.; Patriarca, F.; Evangelista, A.; Benevolo, G.; Oddolo, D.; Innao, V.; et al. First-line therapy with either bortezomib-melphalan-prednisone or lenalidomide-dexamethasone followed by lenalidomide for transplant-ineligible multiple myeloma patients: A pooled analysis of two randomized trials. Haematologica 2020, 105, 1074–1080. [Google Scholar] [CrossRef] [Green Version]
- Mereddy, G.R.; Chakradhar, A.; Rutkoski, R.M.; Jonnalagadda, S.C. Benzoboroxoles: Synthesis and applications in medicinal chemistry. J. Organomet. Chem. 2018, 865, 12–22. [Google Scholar] [CrossRef]
- Paramore, A.; Frantz, S. Fresh from the pipeline: Bortezomib. Nat. Rev. Cancer 2003, 2, 556–557. [Google Scholar] [CrossRef]
- Offidani, M.; Corvatta, L.; Caraffa, P.; Gentili, S.; Maracci, L.; Leoni, P. An evidence-based review of ixazomib citrate and its potential in the treatment of newly diagnosed multiple myeloma. Onco. Targets. Ther. 2014, 7, 1793–1800. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Yao, S.; Xu, B.; Ge, Z.; Cui, J.; Cheng, T.; Li, R. Design, synthesis and biological evaluation of tripeptide boronic acid proteasome inhibitors. Bioorg. Med. Chem. 2009, 17, 6851–6861. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Momose, I.; Abe, M.; Abe, H.; Sawa, R.; Umezawa, Y.; Ikeda, D.; Takahashi, Y.; Akamatsu, Y. Synthesis of boronic acid derivatives of tyropeptin: Proteasome inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 2343–2345. [Google Scholar] [CrossRef] [PubMed]
- Momose, I.; Abe, H.; Watanabe, T.; Ohba, S.I.; Yamazaki, K.; Dan, S.; Yamori, T.; Masuda, T.; Nomoto, A. Antitumor effects of tyropeptin-boronic acid derivatives: New proteasome inhibitors. Cancer Sci. 2014, 105, 1609–1615. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Lei, M.; Wu, W.; Feng, H.; Wang, J.; Chen, S.; Zhu, Y.; Hu, S.; Liu, Z.; Jiang, C. Design, synthesis and docking studies of novel dipeptidyl boronic acid proteasome inhibitors constructed from αα- and αβ-amino acids. Bioorg. Med. Chem. Lett. 2016, 26, 1958–1962. [Google Scholar] [CrossRef]
- Han, L.Q.; Yuan, X.; Wu, X.Y.; Li, R.D.; Xu, B.; Cheng, Q.; Liu, Z.M.; Zhou, T.Y.; An, H.Y.; Wang, X.; et al. Urea-containing peptide boronic acids as potent proteasome inhibitors. Eur. J. Med. Chem. 2017, 125, 925–939. [Google Scholar] [CrossRef]
- Nitsche, C.; Zhang, L.; Weigel, L.F.; Schilz, J.; Graf, D.; Bartenschlager, R.; Hilgenfeld, R.; Klein, C.D. Peptide-Boronic Acid Inhibitors of Flaviviral Proteases: Medicinal Chemistry and Structural Biology. J. Med. Chem. 2017, 60, 511–516. [Google Scholar] [CrossRef]
- Xie, S.C.; Gillett, D.L.; Spillman, N.J.; Tsu, C.; Luth, M.R.; Ottilie, S.; Duffy, S.; Gould, A.E.; Hales, P.; Seager, B.A.; et al. Target Validation and Identification of Novel Boronate Inhibitors of the Plasmodium falciparum Proteasome. J. Med. Chem. 2018, 61, 10053–10066. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Bryson, D.I.; Crumpton, J.B.; Wynn, J.; Santos, W.L. Branched peptide boronic acids (BPBAs): A novel mode of binding towards RNA. Chem. Commun. 2013, 49, 2436–2438. [Google Scholar] [CrossRef] [Green Version]
- Wynn, J.E.; Zhang, W.; Tebit, D.M.; Gray, L.R.; Hammarskjold, M.L.; Rekosh, D.; Santos, W.L. Characterization and in vitro activity of a branched peptide boronic acid that interacts with HIV-1 RRE RNA. Bioorg. Med. Chem. 2016, 24, 3947–3952. [Google Scholar] [CrossRef] [Green Version]
- Wynn, J.E.; Zhang, W.; Falkinham, J.O.; Santos, W.L. Branched Peptides: Acridine and Boronic Acid Derivatives as Antimicrobial Agents. ACS Med. Chem. Lett. 2017, 8, 820–823. [Google Scholar] [CrossRef]
- Shimizu, K.; Maruyama, M.; Yasui, Y.; Minegishi, H.; Ban, H.S.; Nakamura, H. Boron-containing phenoxyacetanilide derivatives as hypoxia-inducible factor (HIF)-1α inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 1453–1456. [Google Scholar] [CrossRef] [PubMed]
- Boloor, A.; Hanway, D.; Joshi, M.; Winn, D.T.; Mendez, G.; Walls, M.; Wei, P.; Qian, F.; Zhang, X.; Zhang, Y.; et al. Synthesis and antiviral activity of HCV NS3/4A peptidomimetic boronic acid inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 5708–5711. [Google Scholar] [CrossRef] [PubMed]
- Gorovoy, A.S.; Gozhina, O.V.; Svendsen, J.S.; Domorad, A.A.; Tetz, G.V.; Tetz, V.V.; Lejon, T. Boron-Containing Peptidomimetics—A Novel Class of Selective Anti-tubercular Drugs. Chem. Biol. Drug Des. 2013, 81, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Pechenov, A.; Stefanova, M.E.; Nicholas, R.A.; Peddi, S.; Gutheil, W.G. Potential transition state analogue inhibitors for the penicillin-binding proteins. Biochemistry 2003, 42, 579–588. [Google Scholar] [CrossRef]
- Suzuki, N.; Suzuki, T.; Ota, Y.; Nakano, T.; Kurihara, M.; Okuda, H.; Yamori, T.; Tsumoto, H.; Nakagawa, H.; Miyata, N. Design, synthesis, and biological activity of boronic acid-based histone deacetylase inhibitors. J. Med. Chem. 2009, 52, 2909–2922. [Google Scholar] [CrossRef]
- Albers, H.M.H.G.; Dong, A.; Van Meeteren, L.A.; Egan, D.A.; Sunkara, M.; Van Tilburg, E.W.; Schuurman, K.; Van Tellingen, O.; Morris, A.J.; Smyth, S.S.; et al. Boronic acid-based inhibitor of autotaxin reveals rapid turnover of LPA in the circulation. Proc. Natl. Acad. Sci. USA 2010, 107, 7257–7262. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Soria, G.; Gonzalez-Galvez, G.; Argoud, G.M.; Gerstman, M.; Littlejohn, T.W.; Schwartz, S.L.; O’Farrell, A.M.; Li, X.; Cherrington, J.M.; Bennett, C.; et al. The dipeptidyl peptidase-4 inhibitor PHX1149 improves blood glucose control in patients with type 2 diabetes mellitus. Diabetes, Obes. Metab. 2008, 10, 293–300. [Google Scholar] [CrossRef]
- Phenomix Corporation—The 2011 Biotech Graveyard. Available online: https://www.fiercebiotech.com/special-report/phenomix-corporation-2011-biotech-graveyard (accessed on 12 February 2022).
- Study of Dutogliptin in Combination with Filgrastim in Post-Myocardial Infarction—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03486080 (accessed on 12 February 2022).
- Talabostat and Pembrolizumab for the Treatment of Advanced Solid Cancers—Tabular View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04171219 (accessed on 12 February 2022).
- Momose, I.; Kawada, M. The therapeutic potential of microbial proteasome inhibitors. Int. Immunopharmacol. 2016, 37, 23–30. [Google Scholar] [CrossRef]
- Fernandes, G.F.S.; Denny, W.A.; Dos Santos, J.L. Boron in drug design: Recent advances in the development of new therapeutic agents. Eur. J. Med. Chem. 2019, 179, 791–804. [Google Scholar] [CrossRef]
- Liu, C.T.; Tomsho, J.W.; Benkovic, S.J. The unique chemistry of benzoxaboroles: Current and emerging applications in biotechnology and therapeutic treatments. Bioorg. Med. Chem. 2014, 22, 4462–4473. [Google Scholar] [CrossRef]
- Nocentini, A.; Supuran, C.T.; Winum, J.-Y.Y. Benzoxaborole compounds for therapeutic uses: A patent review (2010–2018). Expert Opin. Ther. Pat. 2018, 28, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Hall, S.; Zane, L.T.; Lipner, S.R.; Rich, P. Evaluation of the efficacy and safety of tavaborole topical solution, 5%, in the treatment of onychomycosis of the toenail in adults: A pooled analysis of an 8-week, post-study follow-up from two randomized phase 3 studies. J. Dermatolog. Treat. 2018, 29, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Rock, F.L.; Mao, W.; Yaremchuk, A.; Tukalo, M.; Crepin, T.; Zhou, H.; Zhang, Y.; Hernandez, V.; Akama, T.; Baker, J.; et al. An antifungal agent inhibits an aminoacyl-tRNS synthetase by trapping tRNA in the Editing Site. Science 2007, 316, 1759–1761. [Google Scholar] [CrossRef] [PubMed]
- Sonoiki, E.; Palencia, A.; Guo, D.; Ahyong, V.; Dong, C.; Li, X.; Hernandez, V.S.; Zhang, Y.; Choi, W.; Gut, J.; et al. Antimalarial Benzoxaboroles Target Plasmodium falciparum Leucyl-tRNA Synthetase. Antimicrob. Agents Chemother. 2016, 60, 4886–4895. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Hernandez, V.; Rock, F.L.; Choi, W.; Mak, Y.S.L.; Mohan, M.; Mao, W.; Zhou, Y.; Easom, E.E.; Plattner, J.J.; et al. Discovery of a Potent and Specific M. tuberculosis Leucyl-tRNA Synthetase Inhibitor: (S)-3-(Aminomethyl)-4-chloro-7-(2-hydroxyethoxy)benzo[c][1,2]oxaborol-1(3H)-ol (GSK656). J. Med. Chem. 2017, 60, 8011–8026. [Google Scholar] [CrossRef]
- Fischer, A. FDA Approves Eucrisa for Eczema|FDA. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-eucrisa-eczema (accessed on 12 February 2022).
- Akama, T.; Baker, S.J.; Zhang, Y.K.; Hernandez, V.; Zhou, H.; Sanders, V.; Freund, Y.; Kimura, R.; Maples, K.R.; Plattner, J.J. Discovery and structure-activity study of a novel benzoxaborole anti-inflammatory agent (AN2728) for the potential topical treatment of psoriasis and atopic dermatitis. Bioorg. Med. Chem. Lett. 2009, 19, 2129–2132. [Google Scholar] [CrossRef]
- An Early Bactericidal Activity, Safety and Tolerability of GSK3036656 in Subjects With Drug-sensitive Pulmonary Tuberculosis—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03557281 (accessed on 12 February 2022).
- Tenero, D.; Derimanov, G.; Carlton, A.; Tonkyn, J.; Davies, M.; Cozens, S.; Gresham, S.; Gaudion, A.; Puri, A.; Muliaditan, M.; et al. First-time-in-human study and prediction of early bactericidal activity for GSK3036656, a potent leucyl-tRNA synthetase inhibitor for tuberculosis treatment. Antimicrob. Agents Chemother. 2019, 63, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Guy, C.S.; Murray, K.; Gibson, M.I.; Fullam, E. Dimeric benzoboroxoles for targeted activity against: Mycobacterium tuberculosis. Org. Biomol. Chem. 2019, 17, 9524–9528. [Google Scholar] [CrossRef] [Green Version]
- Wall, R.J.; Rico, E.; Lukac, I.; Zuccotto, F.; Elg, S.; Gilbert, I.H.; Freund, Y.; Alley, M.R.K.; Field, M.C.; Wyllie, S.; et al. Clinical and veterinary trypanocidal benzoxaboroles target CPSF3. Proc. Natl. Acad. Sci. USA 2018, 115, 9616–9621. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, R.T.; Nare, B.; Wring, S.A.; Orr, M.D.; Chen, D.; Sligar, J.M.; Jenks, M.X.; Noe, R.A.; Bowling, T.S.; Mercer, L.T.; et al. SCYX-7158, an Orally-Active Benzoxaborole for the Treatment of Stage 2 Human African Trypanosomiasis. PLoS Negl. Trop. Dis. 2011, 5, e1151. [Google Scholar] [CrossRef] [Green Version]
- Ding, D.; Meng, Q.; Gao, G.; Zhao, Y.; Wang, Q.; Nare, B.; Jacobs, R.; Rock, F.; Alley, M.R.K.; Plattner, J.J.; et al. Design, synthesis, and structure-activity relationship of trypanosoma brucei leucyl-tRNA synthetase inhibitors as antitrypanosomal agents. J. Med. Chem. 2011, 54, 1276–1287. [Google Scholar] [CrossRef] [PubMed]
- Prospective Study on Efficacy and Safety of Acoziborole (SCYX-7158) in Patients Infected by Human African Trypanosomiasis Due to T.b. Gambiense—Full Text View—ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03087955 (accessed on 12 February 2022).
- Gumbo, M.; Beteck, R.M.; Mandizvo, T.; Seldon, R.; Warner, D.F.; Hoppe, H.C.; Isaacs, M.; Laming, D.; Tam, C.C.; Cheng, L.W.; et al. Cinnamoyl-oxaborole amides: Synthesis and their in vitro biological activity. Molecules 2018, 23, 2038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, D.; Zhao, Y.; Meng, Q.; Xie, D.; Nare, B.; Chen, D.; Bacchi, C.J.; Yarlett, N.; Zhang, Y.K.; Hernandez, V.; et al. Discovery of novel benzoxaborole-based potent antitrypanosomal agents. ACS Med. Chem. Lett. 2010, 1, 165–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begolo, D.; Vincent, I.M.; Giordani, F.; Pöhner, I.; Witty, M.J.; Rowan, T.G.; Bengaly, Z.; Gillingwater, K.; Freund, Y.; Wade, R.C.; et al. The trypanocidal benzoxaborole AN7973 inhibits trypanosome mRNA processing. PLoS Pathog. 2018, 14, e1007315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonoiki, E.; Ng, C.L.; Lee, M.C.S.; Guo, D.; Zhang, Y.-K.; Zhou, Y.; Alley, M.R.K.; Ahyong, V.; Sanz, L.M.; Lafuente-Monasterio, M.J.; et al. A potent antimalarial benzoxaborole targets a Plasmodium falciparum cleavage and polyadenylation specificity factor homologue. Nat. Commun. 2017, 8, 14574. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, S. Recent development of leucyl-tRNA synthetase inhibitors as antimicrobial agents. Medchemcomm 2019, 10, 1329–1341. [Google Scholar] [CrossRef]
- Sindhe, K.M.V.; Wu, W.; Legac, J.; Zhang, Y.; Easom, E.E.; Cooper, R.A.; Plattner, J.J.; Freund, Y.R.; Derisi, J.L.; Rosenthal, P.J. Plasmodium falciparum Resistance to a Lead Benzoxaborole Due to Blocked Compound Activation and Altered Ubiquitination or Sumoylation. MBio 2020, 11, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-K.K.; Plattner, J.J.; Easom, E.E.; Jacobs, R.T.; Guo, D.; Freund, Y.R.; Berry, P.; Ciaravino, V.; Erve, J.C.L.; Rosenthal, P.J.; et al. Benzoxaborole Antimalarial Agents. Part 5. Lead Optimization of Novel Amide Pyrazinyloxy Benzoxaboroles and Identification of a Preclinical Candidate. J. Med. Chem. 2017, 60, 5889–5908. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, F.; Qiao, Z.; Zhu, M.; Zhou, H. Chalcone–benzoxaborole hybrids as novel anticancer agents. Bioorg. Med. Chem. Lett. 2016, 26, 5797–5801. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, J.; Hao, G.; Xin, W.; Yang, F.; Zhu, M.; Zhou, H. Design, synthesis, and structure-activity relationship of 7-propanamide benzoxaboroles as potent anticancer agents. J. Med. Chem. 2019, 62, 6765–6784. [Google Scholar] [CrossRef]
- Nocentini, A.; Cadoni, R.; Del Prete, S.; Capasso, C.; Dumy, P.; Gratteri, P.; Supuran, C.T.; Winum, J.Y. Benzoxaboroles as Efficient Inhibitors of the β-Carbonic Anhydrases from Pathogenic Fungi: Activity and Modeling Study. ACS Med. Chem. Lett. 2017, 8, 1194–1198. [Google Scholar] [CrossRef] [PubMed]
- Nocentini, A.; Cadoni, R.; Dumy, P.; Supuran, C.T.; Winum, J.Y. Carbonic anhydrases from Trypanosoma cruzi and Leishmania donovani chagasi are inhibited by benzoxaboroles. J. Enzyme Inhib. Med. Chem. 2018, 33, 286–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larcher, A.; Nocentini, A.; Supuran, C.T.; Winum, J.Y.; Van Der Lee, A.; Vasseur, J.J.; Laurencin, D.; Smietana, M. Bis-benzoxaboroles: Design, synthesis, and biological evaluation as carbonic anhydrase inhibitors. ACS Med. Chem. Lett. 2019, 10, 1205–1210. [Google Scholar] [CrossRef] [PubMed]
- Van Bocxlaer, K.; Gaukel, E.; Hauser, D.; Park, S.H.; Schock, S.; Yardley, V.; Randolph, R.; Plattner, J.J.; Merchant, T.; Croft, S.L.; et al. Topical treatment for cutaneous leishmaniasis: Dermato-pharmacokinetic lead optimization of benzoxaboroles. Antimicrob. Agents Chemother. 2018, 62, e02419-17. [Google Scholar] [CrossRef] [Green Version]
- Lapa, G.B.; Mirchink, E.P.; Isakova, E.B.; Preobrazhenskaya, M.N. Two approaches to the use of benzo[c][1,2]oxaboroles as active fragments for synthetic transformation of clarithromycin. J. Enzyme Inhib. Med. Chem. 2017, 32, 452–456. [Google Scholar] [CrossRef]
- Adamczyk-Woźniak, A.; Borys, K.M.; Sporzyński, A. Recent Developments in the Chemistry and Biological Applications of Benzoxaboroles. Chem. Rev. 2015, 115, 5224–5247. [Google Scholar] [CrossRef]
- Akama, T.; Dong, C.; Virtucio, C.; Freund, Y.R.; Chen, D.; Orr, M.D.; Jacobs, R.T.; Zhang, Y.K.; Hernandez, V.; Liu, Y.; et al. Discovery and structure-activity relationships of 6-(benzoylamino) benzoxaboroles as orally active anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2013, 23, 5870–5873. [Google Scholar] [CrossRef]
- Akama, T.; Virtucio, C.; Dong, C.; Kimura, R.; Zhang, Y.K.; Nieman, J.A.; Sharma, R.; Lu, X.; Sales, M.; Singh, R.; et al. Structure-activity relationships of 6-(aminomethylphenoxy)-benzoxaborole derivatives as anti-inflammatory agent. Bioorg. Med. Chem. Lett. 2013, 23, 1680–1683. [Google Scholar] [CrossRef]
- Dong, C.; Virtucio, C.; Zemska, O.; Baltazar, G.; Zhou, Y.; Baia, D.; Jones-Iatauro, S.; Sexton, H.; Martin, S.; Dee, J.; et al. Treatment of Skin Inflammation with Benzoxaborole Phosphodiesterase Inhibitors: Selectivity, Cellular Activity, and Effect on Cytokines Associated with Skin Inflammation and Skin Architecture Changes. J. Pharmacol. Exp. Ther. 2016, 358, 413–422. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhang, Y.K.; Liu, Y.; Zhang, S.; Ding, C.Z.; Zhou, Y.; Plattner, J.J.; Baker, S.J.; Liu, L.; Bu, W.; et al. Synthesis of new acylsulfamoyl benzoxaboroles as potent inhibitors of HCV NS3 protease. Bioorg. Med. Chem. Lett. 2010, 20, 7493–7497. [Google Scholar] [CrossRef]
- Xia, Y.; Cao, K.; Zhou, Y.; Alley, M.R.K.; Rock, F.; Mohan, M.; Meewan, M.; Baker, S.J.; Lux, S.; Ding, C.Z.; et al. Synthesis and SAR of novel benzoxaboroles as a new class of β-lactamase inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 2533–2536. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Basak, S.; Li, Y.; Merino, J.; Iuliano, J.N.; Walker, S.G.; Tonge, P.J. Antibacterial Activity and Mode of Action of a Sulfonamide-Based Class of Oxaborole Leucyl-tRNA-Synthetase Inhibitors. ACS Infect. Dis. 2019, 5, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Lunde, C.S.; Stebbins, E.E.; Jumani, R.S.; Hasan, M.M.; Miller, P.; Barlow, J.; Freund, Y.R.; Berry, P.; Stefanakis, R.; Gut, J.; et al. Identification of a potent benzoxaborole drug candidate for treating cryptosporidiosis. Nat. Commun. 2019, 10, 2816. [Google Scholar] [CrossRef] [PubMed]
- Efficacy and Safety of AN2898 and AN2728 Topical Ointments to Treat Mild-to-Moderate Atopic Dermatitis—Study Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01301508 (accessed on 12 February 2022).
- Cumulative Irritation Test—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT00781664 (accessed on 12 February 2022).
- Mendes, R.E.; Alley, M.R.K.; Sader, H.S.; Biedenbach, D.J.; Jones, R.N. Potency and Spectrum of Activity of AN3365, a Novel Boron-Containing Protein Synthesis Inhibitor, Tested against Clinical Isolates of Enterobacteriaceae and Nonfermentative Gram-Negative Bacilli. Antimicrob. Agents Chemother. 2013, 57, 2849–2857. [Google Scholar] [CrossRef] [Green Version]
- Novel Drug Approvals for 2017|FDA. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2017 (accessed on 12 February 2022).
- Morandi, F.; Caselli, E.; Morandi, S.; Focia, P.J.; Blázquez, J.; Shoichet, B.K.; Prati, F. Nanomolar inhibitors of AmpC β-lactamase. J. Am. Chem. Soc. 2003, 125, 685–695. [Google Scholar] [CrossRef]
- Zhou, J.; Stapleton, P.; Haider, S.; Healy, J. Boronic acid inhibitors of the class A β-lactamase KPC-2. Bioorg. Med. Chem. 2018, 26, 2921–2927. [Google Scholar] [CrossRef]
- Crompton, I.E.; Cuthbert, B.K.; Lowe, G.; Waley, S.G. β-Lactamase inhibitors. The inhibition of serine β-lactamases by specific boronic acids. Biochem. J. 1988, 251, 453–459. [Google Scholar] [CrossRef]
- Weston, G.S.; Blázquez, J.; Baquero, F.; Shoichet, B.K. Structure-based enhancement of boronic acid-based inhibitors of AmpC β- lactamase. J. Med. Chem. 1998, 41, 4577–4586. [Google Scholar] [CrossRef]
- Ambler, R.P. The structure of beta-lactamases. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1980, 289, 321–331. [Google Scholar]
- Tondi, D.; Venturelli, A.; Bonnet, R.; Pozzi, C.; Shoichet, B.K.; Costi, M.P. Targeting class A and C serine β-lactamases with a broad-spectrum boronic acid derivative. J. Med. Chem. 2014, 57, 5449–5458. [Google Scholar] [CrossRef] [Green Version]
- Beesley, T.; Gascoyne, N.; Knott-Hunziker, V.; Petursson, S.; Waley, S.G.; Jaurin, B.; Grundström, T. The inhibition of class C β-lactamases by boronic acids. Biochem. J. 1983, 209, 229–233. [Google Scholar] [CrossRef] [PubMed]
- McKinney, D.C.; Zhou, F.; Eyermann, C.J.; Ferguson, A.D.; Prince, D.B.; Breen, J.; Giacobbe, R.A.; Lahiri, S.; Verheijen, J.C. 4,5-Disubstituted 6-Aryloxy-1,3-dihydrobenzo[c][1,2]oxaboroles Are Broad-Spectrum Serine β-Lactamase Inhibitors. ACS Infect. Dis. 2015, 1, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Cahill, S.T.; Cain, R.; Wang, D.Y.; Lohans, C.T.; Wareham, D.W.; Oswin, H.P.; Mohammed, J.; Spencer, J.; Fishwick, C.W.G.; McDonough, M.A.; et al. Cyclic boronates inhibit all classes of β-lactamases. Antimicrob. Agents Chemother. 2017, 61, e02260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brem, J.; Cain, R.; Cahill, S.; McDonough, M.A.; Clifton, I.J.; Jiménez-Castellanos, J.C.; Avison, M.B.; Spencer, J.; Fishwick, C.W.G.; Schofield, C.J. Structural basis of metallo-β-lactamase, serine-β-lactamase and penicillin-binding protein inhibition by cyclic boronates. Nat. Commun. 2016, 7, 12406. [Google Scholar] [CrossRef] [Green Version]
- Jorgensen, S.C.J.; Rybak, M.J. Meropenem and Vaborbactam: Stepping up the Battle against Carbapenem-resistant Enterobacteriaceae. Pharmacotherapy 2018, 38, 444–461. [Google Scholar] [CrossRef]
- Griffith, D.C.; Sabet, M.; Tarazi, Z.; Lomovskaya, O.; Dudley, M.N. Pharmacokinetics/pharmacodynamics of vaborbactam, a novel beta-lactamase inhibitor, in combination with meropenem. Antimicrob. Agents Chemother. 2019, 63, e01659. [Google Scholar] [CrossRef] [Green Version]
- Tsivkovski, R.; Lomovskayaa, O. Biochemical activity of vaborbactam. Antimicrob. Agents Chemother. 2020, 64, e01935-19. [Google Scholar] [CrossRef] [Green Version]
- Hamrick, J.C.; Docquier, J.D.; Uehara, T.; Myers, C.L.; Six, D.A.; Chatwin, C.L.; John, K.J.; Vernacchio, S.F.; Cusick, S.M.; Trout, R.E.L.; et al. VNRX-5133 (Taniborbactam), a broad-spectrum inhibitor of serine- And metallo-β-lactamases, restores activity of cefepime in enterobacterales and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2020, 64, e01963-19. [Google Scholar] [CrossRef] [Green Version]
- Tsivkovski, R.; Totrov, M.; Lomovskaya, O. Biochemical Characterization of QPX7728, a New Ultrabroad-Spectrum Beta-Lactamase Inhibitor of Serine and Metallo-Beta-Lactamases. Antimicrob. Agents Chemother. 2020, 64, e00130-20. [Google Scholar] [CrossRef] [Green Version]
- Plescia, J.; Moitessier, N. Design and discovery of boronic acid drugs. Eur. J. Med. Chem. 2020, 195, 112270. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.K.; Liu, Y.; Ding, C.Z.; Li, Q.; Zhou, Y.; Plattner, J.J.; Baker, S.J.; Qian, X.; Fan, D.; et al. Synthesis and evaluation of novel α-amino cyclic boronates as inhibitors of HCV NS3 protease. Bioorg. Med. Chem. Lett. 2010, 20, 3550–3556. [Google Scholar] [CrossRef]
- Baldwin, A.G.; Rivers-Auty, J.; Daniels, M.J.D.; White, C.S.; Schwalbe, C.H.; Schilling, T.; Hammadi, H.; Jaiyong, P.; Spencer, N.G.; England, H.; et al. Boron-Based Inhibitors of the NLRP3 Inflammasome. Cell Chem. Biol. 2017, 24, 1321–1335.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faria, R.X.; de Jesus Hiller, N.; Salles, J.P.; Resende, J.A.L.C.; Diogo, R.T.; von Ranke, N.L.; Bello, M.L.; Rodrigues, C.R.; Castro, H.C.; de Luna Martins, D. Arylboronic acids inhibit P2X7 receptor function and the acute inflammatory response. J. Bioenerg. Biomembr. 2019, 51, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Maeda, D.Y.; Peck, A.M.; Schuler, A.D.; Quinn, M.T.; Kirpotina, L.N.; Wicomb, W.N.; Fan, G.H.; Zebala, J.A. Discovery of 2-[5-(4-fluorophenylcarbamoyl)pyridin-2-ylsulfanylmethyl]phenylboronic acid (SX-517): Noncompetitive boronic acid antagonist of CXCR1 and CXCR2. J. Med. Chem. 2014, 57, 8378–8397. [Google Scholar] [CrossRef] [PubMed]
- Maeda, D.Y.; Peck, A.M.; Schuler, A.D.; Quinn, M.T.; Kirpotina, L.N.; Wicomb, W.N.; Auten, R.L.; Gundla, R.; Zebala, J.A. Boronic acid-containing CXCR1/2 antagonists: Optimization of metabolic stability, in vivo evaluation, and a proposed receptor binding model. Bioorg. Med. Chem. Lett. 2015, 25, 2280–2284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuler, A.D.; Engles, C.A.; Maeda, D.Y.; Quinn, M.T.; Kirpotina, L.N.; Wicomb, W.N.; Mason, S.N.; Auten, R.L.; Zebala, J.A. Boronic acid-containing aminopyridine- and aminopyrimidinecarboxamide CXCR1/2 antagonists: Optimization of aqueous solubility and oral bioavailability. Bioorg. Med. Chem. Lett. 2015, 25, 3793–3797. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.P.; Windsor, I.W.; Forest, K.T.; Raines, R.T. Stilbene Boronic Acids Form a Covalent Bond with Human Transthyretin and Inhibit Its Aggregation. J. Med. Chem. 2017, 60, 7820–7834. [Google Scholar] [CrossRef] [Green Version]
- Ge, Y.; Li, A.; Wu, J.; Feng, H.; Wang, L.; Liu, H.; Xu, Y.; Xu, Q.; Zhao, L.; Li, Y. Design, synthesis and biological evaluation of novel non-peptide boronic acid derivatives as proteasome inhibitors. Eur. J. Med. Chem. 2017, 128, 180–191. [Google Scholar] [CrossRef]
- Mandal, S.M.; Pegu, R.; Porto, W.F.; Franco, O.L.; Pratihar, S. Novel boronic acid derivatives of bis(indolyl) methane as anti-MRSA agents. Bioorg. Med. Chem. Lett. 2017, 27, 2135–2138. [Google Scholar] [CrossRef]
- Albers, H.M.H.G.; Van Meeteren, L.A.; Egan, D.A.; Van Tilburg, E.W.; Moolenaar, W.H.; Ovaa, H. Discovery and optimization of boronic acid based inhibitors of Autotaxin. J. Med. Chem. 2010, 53, 4958–4967. [Google Scholar] [CrossRef]
- Fontaine, F.; Héquet, A.; Voisin-Chiret, A.S.; Bouillon, A.; Lesnard, A.; Cresteil, T.; Jolivalt, C.; Rault, S. Boronic species as promising inhibitors of the Staphylococcus aureus NorA efflux pump: Study of 6-substituted pyridine-3-boronic acid derivatives. Eur. J. Med. Chem. 2015, 95, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, K.; Tambov, K.V.; Gundla, R.; Manaev, A.V.; Yarovenko, V.; Traven, V.F.; Neamati, N. Discovery of 3-acetyl-4-hydroxy-2-pyranone derivatives and their difluoridoborate complexes as a novel class of HIV-1 integrase Inhibitors. Bioorg. Med. Chem. 2008, 16, 8988–8998. [Google Scholar] [CrossRef] [PubMed]
- Grimes, N. Carboranes, 3rd ed; Academic Press: London, UK, 2016; ISBN 9780128019054. [Google Scholar]
- Fanfrlík, J.; Lepsík, M.; Horinek, D.; Havlas, Z.; Hobza, P. Interaction of Carboranes with Biomolecules: Formation of Dihydrogen Bonds. ChemPhysChem 2006, 7, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Adamska, A.; Rumijowska-Galewicz, A.; Ruszczynska, A.; Studziska, M.; Jaboaska, A.; Paradowska, E.; Bulska, E.; Munier-Lehmann, H.; Dziadek, J.; Lesnikowski, Z.J.; et al. Anti-mycobacterial activity of thymine derivatives bearing boron clusters. Eur. J. Med. Chem. 2016, 121, 71–81. [Google Scholar] [CrossRef]
- Goszczyński, T.M.; Fink, K.; Boratyński, J. Expert Opinion on Biological Therapy Icosahedral boron clusters as modifying entities for biomolecules. Expert Opin. Biol. Ther. 2018, 18, 205–213. [Google Scholar] [CrossRef]
- Goszczyński, T.M.; Fin, K.; Kowalski, K.; Leśnikowski, Z.J.; Boratyński, J. Interactions of Boron Clusters and their Derivatives with Serum Albumin. Sci. Rep. 2017, 7, 9800. [Google Scholar] [CrossRef] [Green Version]
- Ali, F.; Hosmane, N.S.; Zhu, Y. Boron Chemistry for Medical Applications. Molecules 2020, 25, 828. [Google Scholar] [CrossRef] [Green Version]
- Satapathy, R.; Dash, B.P.; Maguire, J.A.; Hosmane, N.S. New Developments in the Medicinal Chemistry of Carboranes. Collect. Czechoslov. Chem. Commun. 2010, 75, 995–1022. [Google Scholar] [CrossRef]
- Eberhardt, W.H.; Crawford, B.; Lipscomb, W.N. The Valence Structure of the Boron. J. Chem. Phys. 1954, 22, 989–1001. [Google Scholar] [CrossRef]
- Lundeen, A. The Isomerization of Trialkylacetic Acids in Sulfuric Acid. J. Am. Chem. Soc. 1960, 82, 3228–3229. [Google Scholar] [CrossRef]
- Heying, T.L.; Ager, J.W.; Clark, S.L.; Mangold, D.J.; Goldstein, H.L.; Hillman, M.; Polak, R.J.; Szymanski, J.W. A New Series of Organoboranes. I. Carboranes from the Reaction of Decaborane with Acetylenic Compounds. Inorg. Chem. 1963, 2, 1089–1092. [Google Scholar] [CrossRef]
- Endo, Y.; Yoshimi, T.; Iijima, T.; Yamakoshi, Y. Estrogenic antagonists bearing dicarba-closo-dodecaborane as a hydrophobic pharmacophore. Bioorg. Med. Chem. Lett. 1999, 9, 3387–3392. [Google Scholar] [CrossRef]
- Iijima, T.; Endo, Y.; Tsuji, M.; Kawachi, E.; Kagechika, H.; Shudo, K. Dicarba-closo-dodecaboranes as a Pharmacophore. Retinoidal Antagonists and Potential Agonists. Chem. Pharm. Bull. 1999, 47, 398–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawthorne, M.F. Advances at the interface of polyhedral borane chemistry and medicine. Comments Inorg. Chem. 2010, 31, 153–163. [Google Scholar] [CrossRef]
- Tachikawa, S.; Miyoshi, T.; Koganei, H.; El-Zaria, M.E.; Viñas, C.; Suzuki, M.; Ono, K.; Nakamura, H. Spermidinium closo-dodecaborate-encapsulating liposomes as efficient boron delivery vehicles for neutron capture therapy. Chem. Commun. 2014, 50, 12325–12328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyajima, Y.; Nakamura, H.; Kuwata, Y.; Lee, J.D.; Masunaga, S.; Ono, K.; Maruyama, K. Transferrin-loaded nido-carborane liposomes: Tumor-targeting boron delivery system for neutron capture therapy. Bioconjug. Chem. 2006, 17, 1314–1320. [Google Scholar] [CrossRef]
- Nakamura, H.; Ueda, N.; Ban, H.S.; Ueno, M.; Tachikawa, S. Design and synthesis of fluorescence-labeled closo-dodecaborate lipid: Its liposome formation and in vivo imaging targeting of tumors for boron neutron capture therapy. Org. Biomol. Chem. 2012, 10, 1374–1380. [Google Scholar] [CrossRef]
- Koganei, H.; Ueno, M.; Tachikawa, S.; Tasaki, L.; Ban, H.S.; Suzuki, M.; Shiraishi, K.; Kawano, K.; Yokoyama, M.; Maitani, Y.; et al. Development of high boron content liposomes and their promising antitumor effect for neutron capture therapy of cancers. Bioconjug. Chem. 2013, 24, 124–132. [Google Scholar] [CrossRef]
- Zhang, T.; Li, G.; Li, S.; Wang, Z.; He, D.; Wang, Y.; Zhang, J.; Li, J.; Bai, Z.; Zhang, Q.; et al. Asialoglycoprotein receptor targeted micelles containing carborane clusters for effective boron neutron capture therapy of hepatocellular carcinoma. Colloids Surfaces B Biointerfaces 2019, 182, 110397. [Google Scholar] [CrossRef]
- Wang, D.; Meng, Y.; Wang, X.; Xia, G.; Zhang, Q. The endocytic mechanism and cytotoxicity of boron-containing vesicles. Chem. Pharm. Bull. 2020, 68, 618–627. [Google Scholar] [CrossRef]
- Hoppenz, P.; Els-Heindl, S.; Kellert, M.; Kuhnert, R.; Saretz, S.; Lerchen, H.G.; Köbberling, J.; Riedl, B.; Hey-Hawkins, E.; Beck-Sickinger, A.G. A Selective Carborane-Functionalized Gastrin-Releasing Peptide Receptor Agonist as Boron Delivery Agent for Boron Neutron Capture Therapy. J. Org. Chem. 2020, 85, 1446–1457. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Iijima, T.; Yamakoshi, Y.; Kubo, A.; Itai, A. Structure-activity study of estrogenic agonists bearing dicarba-closo- dodecaborane. Effect of geometry and separation distance of hydroxyl groups at the ends of molecules. Bioorg. Med. Chem. Lett. 1999, 9, 3313–3318. [Google Scholar] [CrossRef]
- Endo, Y.; Iijima, T.; Yamakoshi, Y.; Fukasawa, H.; Miyaura, C.; Inada, M.; Kubo, A.; Itai, A. Potent estrogen agonists based on carborane as a hydrophobic skeletal structure: A new medicinal application of boron clusters. Chem. Biol. 2001, 8, 341–355. [Google Scholar] [CrossRef] [Green Version]
- Hirata, M.; Inada, M.; Matsumoto, C.; Takita, M.; Ogawa, T.; Endo, Y.; Miyaura, C. A novel carborane analog, BE360, with a carbon-containing polyhedral boron-cluster is a new selective estrogen receptor modulator for bone. Biochem. Biophys. Res. Commun. 2009, 380, 218–222. [Google Scholar] [CrossRef]
- Sakuma, W.; Nakagawasai, O.; Nemoto, W.; Odaira, T.; Ogawa, T.; Ohta, K.; Endo, Y.; Tan-No, K. Antidepressant effect of BE360, a new selective estrogen receptor modulator, activated via CREB/BDNF, Bcl-2 signaling pathways in ovariectomized mice. Behav. Brain Res. 2020, 393, 112764. [Google Scholar] [CrossRef]
- Nakagawasai, O.; Nemoto, W.; Onogi, H.; Moriya, T.; Lin, J.R.; Odaira, T.; Yaoita, F.; Ogawa, T.; Ohta, K.; Endo, Y.; et al. BE360, a new selective estrogen receptor modulator, produces antidepressant and antidementia effects through the enhancement of hippocampal cell proliferation in olfactory bulbectomized mice. Behav. Brain Res. 2016, 297, 315–322. [Google Scholar] [CrossRef]
- Ohta, K.; Ogawa, T.; Kaise, A.; Endo, Y. Enhanced estrogen receptor beta (ERβ) selectivity of fluorinated carborane-containing ER modulators. Bioorg. Med. Chem. Lett. 2013, 23, 6555–6558. [Google Scholar] [CrossRef]
- Ohta, K.; Goto, T.; Fijii, S.; Suzuki, T.; Ohta, S.; Endo, Y. Design and synthesis of carborane-containing androgen receptor (AR) antagonist bearing a pyridine ring. Bioorg. Med. Chem. 2008, 16, 8022–8028. [Google Scholar] [CrossRef]
- Watanabe, K.; Hirata, M.; Tominari, T.; Matsumoto, C.; Endo, Y.; Murphy, G.; Nagase, H.; Inada, M.; Miyaura, C. BA321, a novel carborane analog that binds to androgen and estrogen receptors, acts as a new selective androgen receptor modulator of bone in male mice. Biochem. Biophys. Res. Commun. 2016, 478, 279–285. [Google Scholar] [CrossRef]
- Kaise, A.; Ohta, K.; Fujii, S.; Oda, A.; Goto, T.; Endo, Y. Novel androgen receptor full antagonists: Design, synthesis, and a docking study of glycerol and aminoglycerol derivatives that contain p-carborane cages. Bioorg. Med. Chem. 2018, 26, 3805–3811. [Google Scholar] [CrossRef]
- Mori, S.; Tsuemoto, N.; Kasagawa, T.; Nakano, E.; Fujii, S.; Kagechika, H. Development of Boron-Cluster-Based Progesterone Receptor Antagonists Bearing a Pentafluorosulfanyl (SF5) Group. Chem. Pharm. Bull. 2019, 67, 1278–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bednarska, K.; Olejniczak, A.B.; Wojtczak, B.A.; Sułowska, Z.; Leśnikowski, Z.J. Adenosine and 2′-deoxyadenosine modified with boron cluster pharmacophores as new classes of human blood platelet function modulators. ChemMedChem 2010, 5, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Bednarska, K.; Olejniczak, A.B.; Piskala, A.; Klink, M.; Sulowska, Z.; Lesnikowski, Z.J. Effect of adenosine modified with a boron cluster pharmacophore on reactive oxygen species production by human neutrophils. Bioorg. Med. Chem. 2012, 20, 6621–6629. [Google Scholar] [CrossRef] [PubMed]
- Białek-Pietras, M.; Olejniczak, A.B.; Paradowska, E.; Studzińska, M.; Suski, P.; Jabłońska, A.; Leśnikowski, Z.J. Synthesis and in vitro antiviral activity of lipophilic pyrimidine nucleoside/carborane conjugates. J. Organomet. Chem. 2015, 798, 99–105. [Google Scholar] [CrossRef]
- Żołnierczyk, J.D.; Olejniczak, A.B.; Mieczkowski, A.; Błoński, J.Z.; Kiliańska, Z.M.; Robak, T.; Leśnikowski, Z.J. In vitro antileukemic activity of novel adenosine derivatives bearing boron cluster modification. Bioorg. Med. Chem. 2016, 24, 5076–5087. [Google Scholar] [CrossRef]
- Scholz, M.; Blobaum, A.L.; Marnett, L.J.; Hey-Hawkins, E. Ortho-Carbaborane derivatives of indomethacin as cyclooxygenase (COX)-2 selective inhibitors. Bioorg. Med. Chem. 2012, 20, 4830–4837. [Google Scholar] [CrossRef] [Green Version]
- Neumann, W.; Xu, S.; Sárosi, M.B.; Scholz, M.S.; Crews, B.C.; Ghebreselasie, K.; Banerjee, S.; Marnett, L.J.; Hey-Hawkins, E. Nido-Dicarbaborate Induces Potent and Selective Inhibition of Cyclooxygenase-2. ChemMedChem 2016, 11, 175–178. [Google Scholar] [CrossRef] [Green Version]
- Buzharevski, A.; Paskas, S.; Sárosi, M.B.; Laube, M.; Lönnecke, P.; Neumann, W.; Mijatovic, S.; Maksimovic-Ivanic, D.; Pietzsch, J.; Hey-Hawkins, E. Carboranyl Analogues of Celecoxib with Potent Cytostatic Activity against Human Melanoma and Colon Cancer Cell Lines. ChemMedChem 2019, 14, 315–321. [Google Scholar] [CrossRef]
- Buzharevski, A.; Paskaš, S.; Sárosi, M.B.; Laube, M.; Lönnecke, P.; Neumann, W.; Murganić, B.; Mijatović, S.; Maksimović-Ivanić, D.; Pietzsch, J.; et al. Carboranyl Derivatives of Rofecoxib with Cytostatic Activity against Human Melanoma and Colon Cancer Cells. Sci. Rep. 2020, 10, 4827. [Google Scholar] [CrossRef]
- Couto, M.; Garcia, M.F.; Alamon, C.; Cabrera, M.; Cabral, P.; Merlino, A.; Teixidor, F.; Cerecetto, H.; Vinas, C. Discovery of Potent EGFR Inhibitors through the Incorporation of a 3D-Aromatic-Boron-Rich-Cluster into the 4-Anilinoquinazoline Scaffold: Potential Drugs for Glioma Treatment. Chemistry 2018, 24, 3122–3126. [Google Scholar] [CrossRef]
- Yin, Y.; Ochi, N.; Craven, T.W.; Baker, D.; Takigawa, N.; Suga, H. De Novo Carborane-Containing Macrocyclic Peptides Targeting Human Epidermal Growth Factor Receptor. J. Am. Chem. Soc. 2019, 141, 19193–19197. [Google Scholar] [CrossRef] [PubMed]
- Brynda, J.; Mader, P.; Šícha, V.; Fábry, M.; Poncová, K.; Bakardiev, M.; Grüner, B.; Cígler, P.; Řezáčová, P. Carborane-based carbonic anhydrase inhibitors. Angew. Chem.-Int. Ed. 2013, 52, 13760–13763. [Google Scholar] [CrossRef] [PubMed]
- Dvořanová, J.; Kugler, M.; Holub, J.; Šícha, V.; Das, V.; Nekvinda, J.; El Anwar, S.; Havránek, M.; Pospíšilová, K.; Fábry, M.; et al. Sulfonamido carboranes as highly selective inhibitors of cancer-specific carbonic anhydrase IX. Eur. J. Med. Chem. 2020, 200, 11240. [Google Scholar] [CrossRef] [PubMed]
- Minegishi, H.; Matsukawa, T.; Nakamura, H. Synthesis and Biological Evaluation of Diaryl-Substituted Carboranes as Inhibitors of Hypoxia Inducible Factor (HIF)-1 Transcriptional Activity. ChemMedChem 2013, 8, 265–271. [Google Scholar] [CrossRef]
- Kavianpour, P.; Gemmell, M.C.M.; Kahlert, J.U.; Rendina, L.M. Histone Deacetylase 2 (HDAC2) Inhibitors Containing Boron. ChemBioChem 2020, 21, 2786–2791. [Google Scholar] [CrossRef]
- Julius, R.L.; Farha, O.K.; Chiang, J.; Perry, L.J.; Hawthorne, M.F. Synthesis and evaluation of transthyretin amyloidosis inhibitors containing carborane pharmacophores. Proc. Natl. Acad. Sci. USA 2007, 104, 4808–4813. [Google Scholar] [CrossRef] [Green Version]
- Scholz, M.; Kaluerović, G.N.; Kommera, H.; Paschke, R.; Will, J.; Sheldrick, W.S.; Hey-Hawkins, E. Carbaboranes as pharmacophores: Similarities and differences between aspirin and asborin. Eur. J. Med. Chem. 2011, 46, 1131–1139. [Google Scholar] [CrossRef]
- Scholz, M.; Steinhagen, M.; Heiker, J.T.; Beck-Sickinger, A.G.; Hey-Hawkins, E. Asborin Inhibits Aldo/Keto Reductase 1A1. ChemMedChem 2011, 6, 89–93. [Google Scholar] [CrossRef]
- Tse, E.; Houston, S.; Williams, C.M.; Savage, G.P.; Rendina, L.M.; Hallyburton, I.; Anderson, M.; Sharma, R.; Walker, G.S.; Obach, R.S.; et al. Non-Classical Phenyl Bioisosteres as Effective Replacements in a Series of Novel Open Source Antimalarials. J. Med. Chem. 2020, 63, 11585–11601. [Google Scholar] [CrossRef]
- Řezáčová, P.; Pokorná, J.; Brynda, J.; Kožíšek, M.; Cígler, P.; Lepšík, M.; Fanfrlík, J.; Řezáč, J.; Šašková, K.G.; Sieglová, I.; et al. Design of HIV protease inhibitors based on inorganic polyhedral metallacarboranes. J. Med. Chem. 2009, 52, 7132–7141. [Google Scholar] [CrossRef]
- Ohta, K.; Iijima, T.; Kawachi, E.; Kagechika, H.; Endo, Y. Novel retinoid X receptor (RXR) antagonists having a dicarba-closo- dodecaborane as a hydrophobic moiety. Bioorg. Med. Chem. Lett. 2004, 14, 5913–5918. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Masuno, H.; Taoda, Y.; Kano, A.; Wongmayura, A.; Nakabayashi, M.; Ito, N.; Shimizu, M.; Kawachi, E.; Hirano, T.; et al. Boron cluster-based development of potent nonsecosteroidal vitamin D receptor ligands: Direct observation of hydrophobic interaction between protein surface and carborane. J. Am. Chem. Soc. 2011, 133, 20933–20941. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.M.; Gunosewoyo, H.; Barron, M.L.; Boucher, A.; McDonnell, M.; Turner, P.; Morrison, D.E.; Bennett, M.R.; McGregor, I.S.; Rendina, L.M.; et al. The First CNS-Active Carborane: A Novel p2x7 Receptor Antagonist with Antidepressant Activity. ACS Chem. Neurosci. 2014, 5, 335–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kracke, G.R.; VanGordon, M.R.; Sevryugina, Y.V.; Kueffer, P.J.; Kabytaev, K.; Jalisatgi, S.S.; Hawthorne, M.F. Carborane-derived local anesthetics are isomer dependent. ChemMedChem 2015, 10, 62–67. [Google Scholar] [CrossRef]
- Page, M.F.Z.; Jalisatgi, S.S.; Maderna, A.; Hawthorne, M.F. Design and synthesis of a candidate α-human thrombin irreversible inhibitor containing a hydrophobic carborane pharmacophore. Synthesis (Stuttg). 2008, 555–563. [Google Scholar] [CrossRef]
- Peng, X.; Gandhi, V. ROS-activated anticancer prodrugs: A new strategy for tumor- specific damage. Ther. Deliv. 2012, 3, 823–833. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.; Guo, S.; Zhong, Q.; Zhang, C.; Liu, J.; Yang, L.; Zhang, Q.; Wang, G. Biocompatible Boron-Containing Prodrugs of Belinostat for the Potential Treatment of Solid Tumors. ACS Med. Chem. Lett. 2018, 9, 149–154. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, S.; Zhong, Q.; Zhang, Q.; Hossain, A.; Zheng, S.; Wang, G. Metabolism and Pharmacokinetic Study of the Boron-Containing Prodrug of Belinostat (ZL277), a Pan HDAC Inhibitor with Enhanced Bioavailability. Pharmaceuticals 2019, 12, 180. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.; Xu, L.; Ou, S.; Edwards, H.; Luedtke, D.; Ge, Y.; Qin, Z. H2O2/Peroxynitrite-Activated Hydroxamic Acid HDAC Inhibitor Prodrugs Show Antileukemic Activities against AML Cells. ACS Med. Chem. Lett. 2018, 9, 635–640. [Google Scholar] [CrossRef]
- Bhagat, S.D.; Singh, U.; Mishra, R.K.; Srivastava, A. An Endogenous Reactive Oxygen Species (ROS)-Activated Histone Deacetylase Inhibitor Prodrug for Cancer Chemotherapy. ChemMedChem 2018, 13, 2073–2079. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Xie, S.; Ma, L.; Chen, Y.; Lu, W. 10-Boronic acid substituted camptothecin as prodrug of SN-38. Eur. J. Med. Chem. 2016, 116, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Bhuniya, S.; Lee, H.; Kim, H.M.; Cheong, C.; Maiti, S.; Hong, K.S.; Kim, J.S. An activatable prodrug for the treatment of metastatic tumors. J. Am. Chem. Soc. 2014, 136, 13888–13894. [Google Scholar] [CrossRef]
- Bedini, A.; Fraternale, A.; Crinelli, R.; Mari, M.; Bartolucci, S.; Chiarantini, L.; Spadoni, G. Design, Synthesis, and Biological Activity of Hydrogen Peroxide Responsive Arylboronate Melatonin Hybrids. Chem. Res. Toxicol. 2019, 32, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Okuda, T.; Mori, S.; Konno, M.; Eguchi, H.; Asai, A.; Koseki, J.; Iwagami, Y.; Yamada, D.; Akita, H.; et al. A Hydrogen Peroxide Activatable Gemcitabine Prodrug for the Selective Treatment of Pancreatic Ductal Adenocarcinoma. ChemMedChem 2019, 14, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Zhang, C.; Zhang, Q.; Miele, L.; Zheng, S.; Wang, G. Boronic prodrug of 4-hydroxytamoxifen is more efficacious than tamoxifen with enhanced bioavailability independent of CYP2D6 status. BMC Cancer 2015, 15, 625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Zhong, Q.; Zhang, Q.; Zheng, S.; Miele, L.; Wang, G. Boronic prodrug of endoxifen as an effective hormone therapy for breast cancer. Breast Cancer Res. Treat. 2015, 152, 283–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.; McEachon, J.D., 2nd; Pollock, J.A. Synthesis and characterization of hydrogen peroxide activated estrogen receptor beta ligands. Bioorg. Med. Chem. 2019, 27, 2075–2082. [Google Scholar] [CrossRef]
- Luo, L.; Zhong, Q.; Guo, S.; Zhang, C.; Zhang, Q.; Zheng, S.; He, L.; Wang, G. Development of a bioavailable boron-containing PI-103 Bioisostere, PI-103BE. Bioorg. Med. Chem. Lett. 2020, 30, 127258. [Google Scholar] [CrossRef]
- Previtali, V.; Petrovic, K.; Peiro Cadahia, J.; Troelsen, N.S.; Clausen, M.H. Auxiliary in vitro and in vivo biological evaluation of hydrogen peroxide sensitive prodrugs of methotrexate and aminopterin for the treatment of rheumatoid arthritis. Bioorg. Med. Chem. 2020, 28, 115247. [Google Scholar] [CrossRef]
- Liu, M.; Tang, X.; Ding, J.; Liu, M.; Zhao, B.; Deng, Y.; Song, Y. A Sialylated-Bortezomib Prodrug Strategy Based on a Highly Expressed Selectin Target for the Treatment of Leukemia or Solid Tumors. Pharm. Res. 2019, 36, 176. [Google Scholar] [CrossRef]
- Lei, M.; Feng, H.; Bai, E.; Zhou, H.; Wang, J.; Qin, Y.; Zhang, H.; Wang, X.; Liu, Z.; Hai, O.; et al. Discovery of a novel dipeptidyl boronic acid proteasome inhibitor for the treatment of multiple myeloma and triple-negative breast cancer. Org. Biomol. Chem. 2019, 17, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Hasabelnaby, S.; Goudah, A.; Agarwal, H.K.; Abd alla, M.S.M.; Tjarks, W. Synthesis, Chemical and Enzymatic Hydrolysis, and Aqueous Solubility of Amino Acid Ester Prodrugs of 3-Carboranyl Thymidine Analogues for Boron Neutron Capture Therapy of Brain Tumors. Eur. J. Med. Chem. 2012, 55, 325–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodor, N.; Buchwald, P. Soft drug design: General principles and recent applications. Med. Res. Rev. 2000, 20, 58–101. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Plattner, J.J.; Akama, T.; Baker, S.J.; Hernandez, V.S.; Sanders, V.; Freund, Y.; Kimura, R.; Bu, W.; Hold, K.M.; et al. Design and synthesis of boron-containing PDE4 inhibitors using soft-drug strategy for potential dermatologic anti-inflammatory application. Bioorg. Med. Chem. Lett. 2010, 20, 2270–2274. [Google Scholar] [CrossRef]
- Milo, L.J.; Lai, J.H.; Wu, W.; Liu, Y.; Maw, H.; Li, Y.; Jin, Z.; Shu, Y.; Poplawski, S.E.; Wu, Y.; et al. Chemical and biological evaluation of dipeptidyl boronic acid proteasome inhibitors for use in prodrugs and pro-soft drugs targeting solid tumors. J. Med. Chem. 2011, 54, 4365–4377. [Google Scholar] [CrossRef] [PubMed]
- Snow, R.J.; Bachovchin, W.W.; Barton, R.W.; Campbell, S.J.; Coutts, S.J.; Freeman, D.M.; Gutheil, W.G.; Kelly, T.A.; Kennedy, C.A.; Krolikowski, D.A.; et al. Studies on Proline Boronic Acid Dipeptide Inhibitors of Dipeptidyl Peptidase IV: Identification of a Cyclic Species Containing a B-N Bond. J. Am. Chem. Soc. 1994, 116, 10860–10869. [Google Scholar] [CrossRef]
- Ashley, J.D.; Stefanick, J.F.; Schroeder, V.A.; Suckow, M.A.; Kiziltepe, T.; Bilgicer, B. Liposomal Bortezomib Nanoparticles via Boronic Ester Prodrug Formulation for Improved Therapeutic Efficacy in Vivo. J. Med. Chem. 2014, 57, 5282–5292. [Google Scholar] [CrossRef]
- Zhu, J.; Huo, Q.; Xu, M.; Yang, F.; Li, Y.; Shi, H.; Niu, Y.; Liu, Y. Bortezomib-catechol conjugated prodrug micelles: Combining bone targeting and aryl boronate-based pH-responsive drug release for cancer bone-metastasis therapy. Nanoscale 2018, 10, 18387–18397. [Google Scholar] [CrossRef]
- Gao, Y.; Xiao, Y.; Liu, S.; Yu, J. Camptothecin prodrug nanomicelle based on a boronate ester-linked diblock copolymer as the carrier of doxorubicin with enhanced cellular uptake. J. Biomater. Sci. Polym. Ed. 2018, 29, 160–180. [Google Scholar] [CrossRef]
- Ma, R.; Shi, L. Phenylboronic acid-based glucose-responsive polymeric nanoparticles: Synthesis and applications in drug delivery. Polym. Chem. 2014, 5, 1503–1518. [Google Scholar] [CrossRef]
- Chou, D.H.C.; Webber, M.J.; Tang, B.C.; Lin, A.B.; Thapa, L.S.; Deng, D.; Truong, J.V.; Cortinas, A.B.; Langer, R.; Anderson, D.G. Glucose-responsive insulin activity by covalent modification with aliphatic phenylboronic acid conjugates. Proc. Natl. Acad. Sci. USA 2015, 112, 2401–2406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parrott, M.C.; Marchington, E.B.; Valliant, J.F.; Adronov, A. Synthesis and Properties of Carborane-Functionalized Aliphatic Polyester Dendrimers. J. Am. Chem. Soc. 2005, 127, 12081–12089. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, T.; Gao, J.; Gao, L.; Zhou, L.; Cai, M.; Shi, Y.; Xiong, W.; Jiang, J.; Tong, T.; et al. Variation in Carbohydrates between Cancer and Normal Cell Membranes Revealed by Super-Resolution Fluorescence Imaging. Adv. Sci. 2016, 3, 1600270. [Google Scholar] [CrossRef] [PubMed]
- Ellis, G.A.; Palte, M.J.; Raines, R.T. Boronate-mediated biologic delivery. J. Am. Chem. Soc. 2012, 134, 3631–3634. [Google Scholar] [CrossRef]
- Springsteen, G.; Wang, B. A detailed examination of boronic acid-diol complexation. Tetrahedron 2002, 58, 5291–5300. [Google Scholar] [CrossRef]
- Mothana, S.; Grassot, J.M.; Hall, D.G. Multistep phase-switch synthesis by using liquid-liquid partitioning of boronic acids: Productive tags with an expanded repertoire of compatible reactions. Angew. Chem.-Int. Ed. 2010, 49, 2883–2887. [Google Scholar] [CrossRef]
- Andersen, K.A.; Smith, T.P.; Lomax, J.E.; Raines, R.T. Boronic Acid for the Traceless Delivery of Proteins into Cells. ACS Chem. Biol. 2016, 11, 319–323. [Google Scholar] [CrossRef] [Green Version]


| Diazaborine Structure | Name | Reference |
|---|---|---|
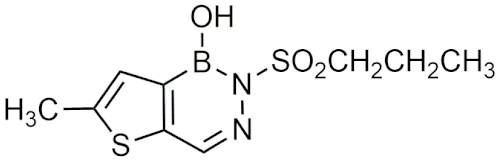 | 1,2-dihydro-1-hydroxy-6-methyl-2-(propanesulphonyl)-thieno (3,2-D) (1,2,3)-diazaborine (Sa 84.474) | [25] |
 | 1,2-dihydro-1-hydroxy-2-phenyl-2,4,1-benzo[e]diazaborin-3(4H) -one | [27] |
 | 1,2-dihydro-1-hydroxy-2-(3-pyridyl)-2,4,1-benzo[e]diazaborin-3(4H)-thione | [27] |
 | AN12855 | [28] |
| Peptidic Boronic Acid Structure | Name | Reference |
|---|---|---|
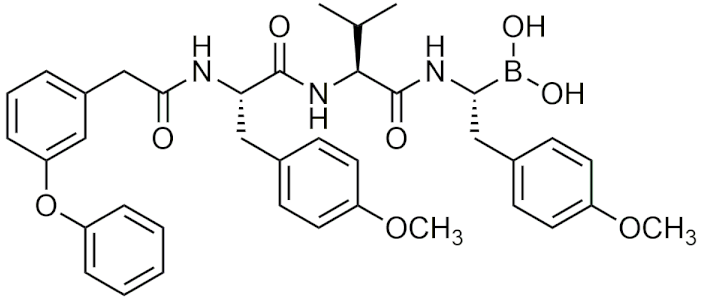 | AS-06 | [46] |
 | AS-29 | [46] |
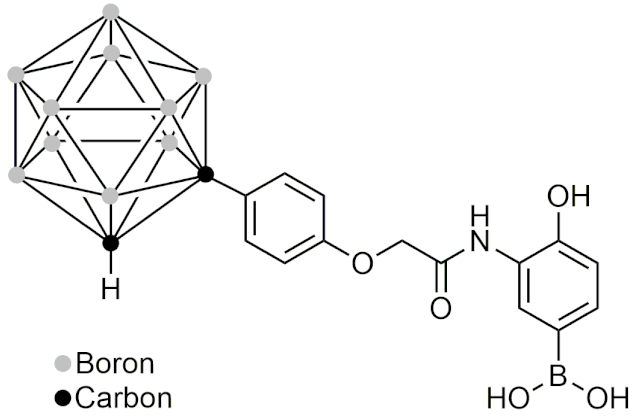 | GN26361 | [54] |
 | PHX1149 (dutogliptin) | [4] |
 | PT100 (Talabostat/Val-boroPro)c | [4] |
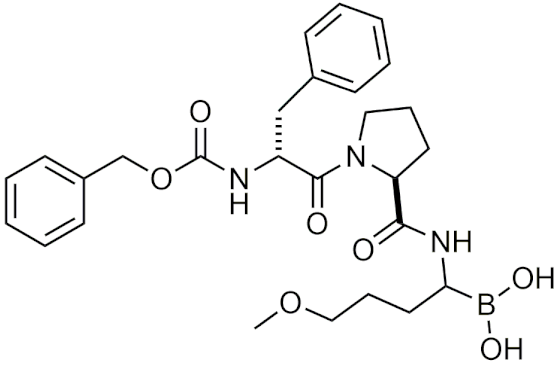 | TRI50c | [4] |
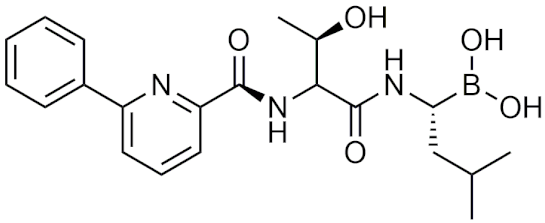 | CEP-18770 (delanzomib) | [64] |
| Benzoxaborole Structure | Name | Reference |
|---|---|---|
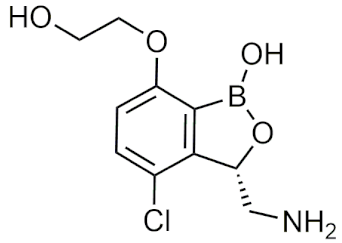 | GSK3036656 | [71,74] |
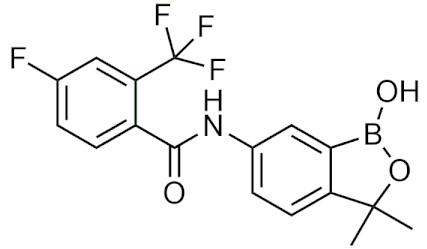 | Acoziborole | [77] |
 | AN3661 | [84] |
| Benzoxaborole Structure | Name | Reference |
|---|---|---|
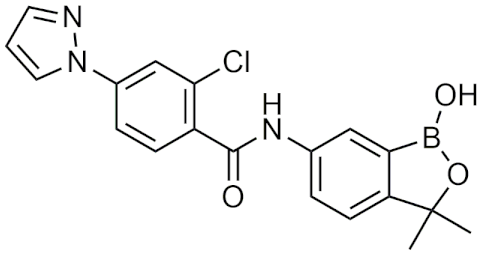 | AN7973 | [102] |
 | AN2898 | [103] |
 | AN2718 | [104] |
 | AN3365 (GSK2551052/epetraborole) | [6] |
| Cyclic Boronic Acid Structure | Name | Reference |
|---|---|---|
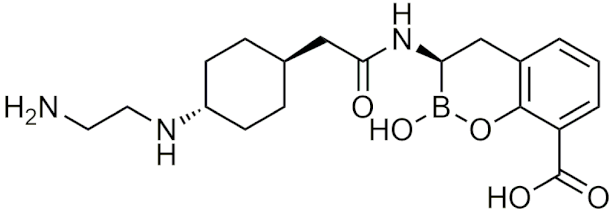 | Taniborbactam (VNRX-5133) | [120] |
 | QPX7728 | [121] |
 | SX-517 | [126] |
 | SX-576 | [127] |
| Carborane Structure | Name | Reference |
|---|---|---|
 | BE120 | [156] |
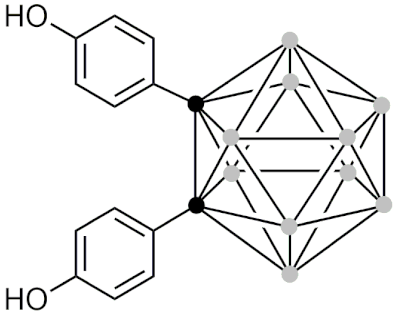 | BE360 | [157] |
 | BA321 | [162] |
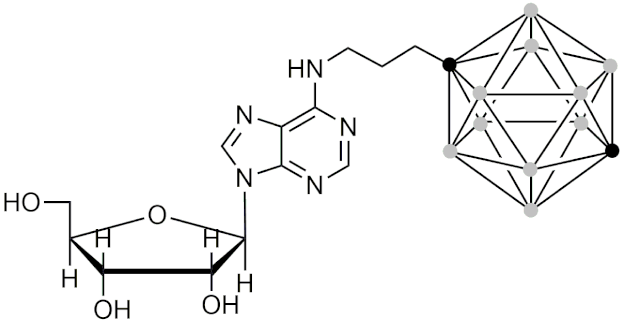 | 6-N-[(1,12-dicarba-closo-dodecaboran-1-yl)propan-3-yl]adenosine | [166] |
 | 2’-O-[(1,12-dicarba-closo-dodecaboran-1-yl)propyleneoxymethyl]adenosine | [166] |
| Boronic Acid Prodrugs | Name | Reference |
|---|---|---|
 | ZL277 (belinostat prodrug) | [191] |
 | ZB497 (4-hydroxytamoxifen prodrug) | [198] |
 | ZB483 (Endoxifen prodrug) | [199] |
 | (4-(((2-amino-4-((((4-boronobenzyl)oxy)carbonyl)amino)pteridin-6-yl)methyl)(methyl)amino)benzoyl)-D-glutamic acid (methotrexate prodrug) | [202] |
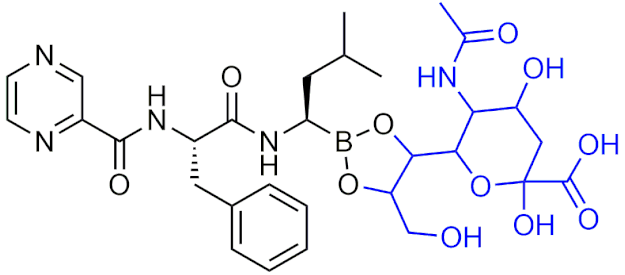 | Bortezomib-sialic acid (BORSA) (Bortezomib prodrug) | [203] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messner, K.; Vuong, B.; Tranmer, G.K. The Boron Advantage: The Evolution and Diversification of Boron’s Applications in Medicinal Chemistry. Pharmaceuticals 2022, 15, 264. https://doi.org/10.3390/ph15030264
Messner K, Vuong B, Tranmer GK. The Boron Advantage: The Evolution and Diversification of Boron’s Applications in Medicinal Chemistry. Pharmaceuticals. 2022; 15(3):264. https://doi.org/10.3390/ph15030264
Chicago/Turabian StyleMessner, Katia, Billy Vuong, and Geoffrey K. Tranmer. 2022. "The Boron Advantage: The Evolution and Diversification of Boron’s Applications in Medicinal Chemistry" Pharmaceuticals 15, no. 3: 264. https://doi.org/10.3390/ph15030264
APA StyleMessner, K., Vuong, B., & Tranmer, G. K. (2022). The Boron Advantage: The Evolution and Diversification of Boron’s Applications in Medicinal Chemistry. Pharmaceuticals, 15(3), 264. https://doi.org/10.3390/ph15030264







